- Medical Centre for Digestive Diseases, Second Affiliated Hospital, Nanjing Medical University, Nanjing, China
Background: Surgery has been the primary treatment in patients with localized gastrointestinal stromal tumors (GISTs) for many decades, whereas it remains controversial regarding the efficacy of primary tumor resection for metastatic GISTs treated with chemotherapy, and likewise it is unclear who would benefit from the surgical resection.
Methods: GISTs patients with distant metastases were identified from the Surveillance, Epidemiology, and End Results (SEER) database between 2010 and 2016. Cox proportional hazards regression models were used to identify prognostic factors of overall survival (OS) and cancer-specific survival (CSS). Kaplan-Meier analyses and log-rank tests were conducted to assess the effectiveness of surgery on survival.
Results: In total, of 455 patients with metastatic GISTs, 235 patients (51.6%) underwent primary tumor resection and 220 patients (48.4%) did not. Median survival of patients in resection group was 72 (95% CI: 62.90–81.10) months vs. 40 (95% CI: 29.53–50.47) months for those in non-resection group (p < 0.001). Similarly, surgery in conjunction with chemotherapy led to a favorable impact on survival than chemotherapy alone (OS: 72 vs. 40 months, p < 0.001; CSS: 74 vs. 44 months, p < 0.001). Multivariable analysis showed that both OS (HR: 0.581, 95% CI: 0.386–0.874, p = 0.009) and CSS (HR: 0.663, 95% CI: 0.439–0.912, p = 0.042] were dramatically improved in patients with surgical removal of primary site, as well as primary tumor size between 5 and 10 cm, while increasing age was predictive of poorer survival. Stratified analysis revealed that patients with tumor locations in the stomach demonstrated a prolonged survival after surgery, with no significant differential surgical effect between the stomach and small intestine.
Conclusions: Our study preliminarily suggests that carefully selected patients with metastatic GISTs might prolong survival after treatment of surgery, especially those with a primary tumor between 5 and 10 cm and a tumor located in the stomach.
Introduction
Gastrointestinal stromal tumors (GISTs) which derive from the interstitial cells of Cajal or their precursors, are the most frequent mesenchymal neoplasms of the gastrointestinal (GI) tract, with an estimated prevalence of 130 per million and the lowest annual incidence of 4.3 per million (1–3). GISTs usually have morphologically and clinically heterogeneous features, for which they may occur anywhere along the GI tract, ranging from esophagus to rectum, but the most common location is the stomach, approximately accounting for 60–70% (4). It is widely known that up to 80% of GISTs can harbor functional mutations in PDGFRA (platelet-derived growth factor receptor α) or KIT which have the function of encoding receptor tyrosine kinase protein called CD117 antigen. It is these activating mutations that are predominantly responsible for the initiation of the malignant process in GISTs (5). Some studies indicate that the rate of overt metastatic diseases in patients with GISTs is roughly between 15 and 50% with the most common sites for metastases being the liver (6, 7).
Prior to the imatinib era, surgery is the only potentially curative therapy for patients with localized or resectable GISTs, which is also a standard treatment in patients with tumors >2 cm in diameter. Even so, almost three-fifths of patients experience local tumor recurrence and metastasis after radical surgery during follow-up, with small studies suggesting a 5-year survival rate between 28 and 80% (8). Imatinib mesylate (IM), as a tyrosine kinase inhibitor, has been widely demonstrated its efficacy in terms of overall survival (OS) and progression-free survival (PFS), especially for metastatic or advanced GISTs (9, 10). Nevertheless, with the long-term use of IM, most patients will inevitably occur adverse events and secondary resistance to the drug, in part, as a result of secondary molecular alteration (secondary mutations) attributed to high tumor burden (11). Further to this, given the considerable inter-individual variability, some patients may not respond to IM and experience rapid progression, while the beneficial effects of IM can be maintained indefinitely in the other patients (12). For these reasons, as a means of assisted treatment, primary tumor resection is attempted for recurrent and metastatic GISTs on the basis of the theory that cytoreduction can minimize the number of tumor cells exposed to IM, thereby reducing the risk of secondary mutations (13). However, there is no consensus on the efficacy of surgery in patients with metastatic GISTs. Both Mussi et al. and Sato et al. currently do not make any unequivocal recommendations on whether we should perform surgery on the primary site in this clinical scenario since the trials are fraught with multiple limitations (14, 15). As such, additional efforts to ascertain the therapeutic role of surgery for GISTs are needed.
For such a disease process with an insufficient population and relatively low incidence, we use a well-constructed database named SEER (Surveillance, Epidemiology, and End Results) which can potentially offer more detailed and accurate results. The aim of our study was to assess whether and for whom primary tumor resection has a survival benefit on metastatic GISTs patients undergoing chemotherapy, as well as compare the survival outcomes between combined therapy and chemotherapy alone.
Patients and Methods
Database and Patient Selection
We conducted a retrospective review of all GIST patients registered in the SEER database from 2010 to 2016. SEER, sponsored by the National Cancer Institute, records information on patient demographics, cancer incidence and prevalence, tumor characteristics, treatments and mortality, which consists of 19 regional registries and comprises ~34% of the population across the USA (16). SEER*Stat software (Version 8.3.6; National Cancer Institute, Bethesda, MD, USA) is accessible to us to capture detailed information from the database.
A total of 4,714 cases were initially identified based on the specific ICD-O-3 histologic codes (8936, Gastrointestinal stromal tumor). Then, we excluded patients without distant metastases, chemotherapy, complete data on survival time and surgery, and those who lacked microscopic or histologic confirmation. In addition, patients having a history of another primary malignancy or diagnosed at the time of autopsy were also excluded. After multiple rounds of screening, only 455 patients with metastatic GISTs were enrolled in this dataset. The detailed process of study selection were presented in Figure 1.
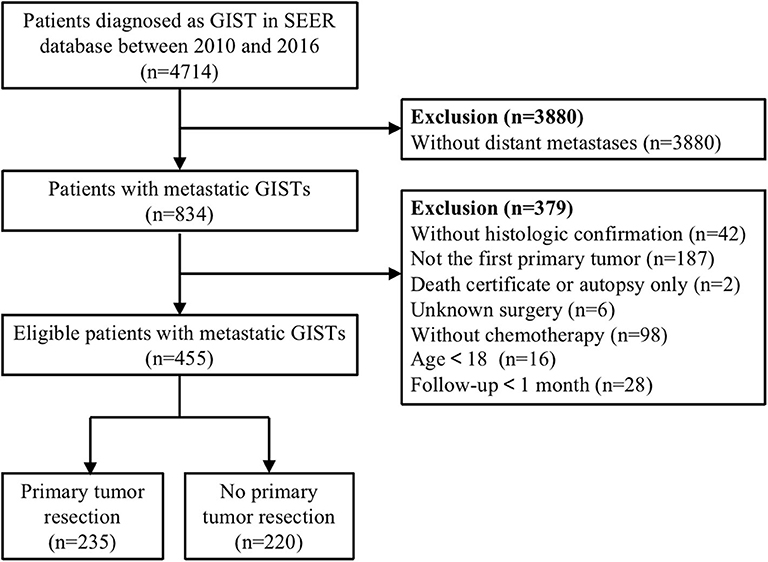
Figure 1. Flow diagram of eligible patients diagnosed with metastatic gastrointestinal stromal tumors (GISTs).
Covariates and Outcomes
Data extraction from the database included the following: demographics (age, race, gender, marital status, and insurance status at diagnosis), cancer characteristics (primary tumor site, tumor size, lymph node status, tumor grade, mitotic index, survival time, vital status, and cause of death), and treatment (primary tumor resection and chemotherapy). Variable “RX Summ—Surg Prim Site” was retrieved to define primary tumor resection, which describes the exhaustive information about surgical removal of the primary lesion by using SEER site-specific codes. Eligible patients were categorized into the resection group and non-resection group. Besides, according to our clinical experience, continuous tumor size variable was grouped into <2, 2–5, 5–10, or>10 cm, with the rest being classified into unknown size. The mitotic index was divided into three groups (≤5/50, >5/50 HPFs or unknown) based on the recognized breakpoint.
OS and CSS were the major endpoint outcomes of our study. We defined OS as the time from a positive diagnosis until death or the last contact date. While CSS was defined as the interval between the date of diagnosis and the date of death attributed to GISTs or the last follow-up. Since the SEER database is public and accessible to applicants, containing unidentifiable patient information, our study was exempted from the approval of the Office of Human Subjects Research of the National Institutes of Health.
Statistical Analysis
The results were presented as proportions for categorical variables or medians for continuous variables. To identify differences in clinicopathological characteristics among the resection group and the non-resection group, Student's t-tests and chi-square tests (or Fisher's exact test) were used for comparisons of continuous variables and categorical variables, respectively. We used the Kaplan-Meier method and the log-rank test to describe the difference in survival between the groups of study patients. Additionally, survival analyses stratified by age, tumor size, primary tumor site and treatment were also performed. Median survival time was reported by using the Kaplan-Meier method. Interaction term analyses (pint) in the multivariable model were conducted by age (<65 and ≥65), tumor size (5–10 and >10 cm), and primary tumor site (stomach and small intestine). To gain insight on patient selection for surgery, we conducted a logistic regression model, and the results were expressed as adjusted odds ratios (aORs) and the corresponding 95% confidence interval (CI). For regression survival analysis, we initially established a multivariable Cox regression (named Model 1) which included age, race, gender, marital status, insurance status, tumor site, tumor size, LN metastases, and primary tumor resection. Additionally, to verify the authenticity of our results, a second multivariable model (named Model 2) was carried out based on the least absolute shrinkage and selection operator (LASSO) method proposed by Tibshirani, which was an effective technique for shrinkage and selection method for regression (17). Then, Model 2 was built by incorporating the selected variables with non-zero coefficients from the LASSO regression model (18). Furthermore, Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC) were calculated to evaluate the quality of Model 1 in comparison to Model 2, and the best model was defined as the one with the lowest AIC and BIC (19). It is worth noting that we excluded the two variables of mitotic index and tumor grade from the multivariable model because the proportion of unknown data was very high (up to 65%) which would severely reduce the sample size and statistical power for multivariable analysis. In the above analysis, the following software was applied: SPSS 24.0 (IBM Corp, Armonk, NY) for Student's t-test, chi-square test, interaction test, and Cox regression analyses, GraphPad Prism 8.3 (GraphPad Software, San Diego, CA) for Kaplan-Meier survival curves and log-rank test, Stata 14.0 (StataCorp, College Station, TX) for forest plot, AIC and BIC, and R 3.6.2 (Institute for Statistics and Mathematics, Vienna, Austria) for LASSO regression. All P-values were two-tailed, and P < 0.05 was recognized as statistically significant.
Results
Patient and Tumor Characteristics
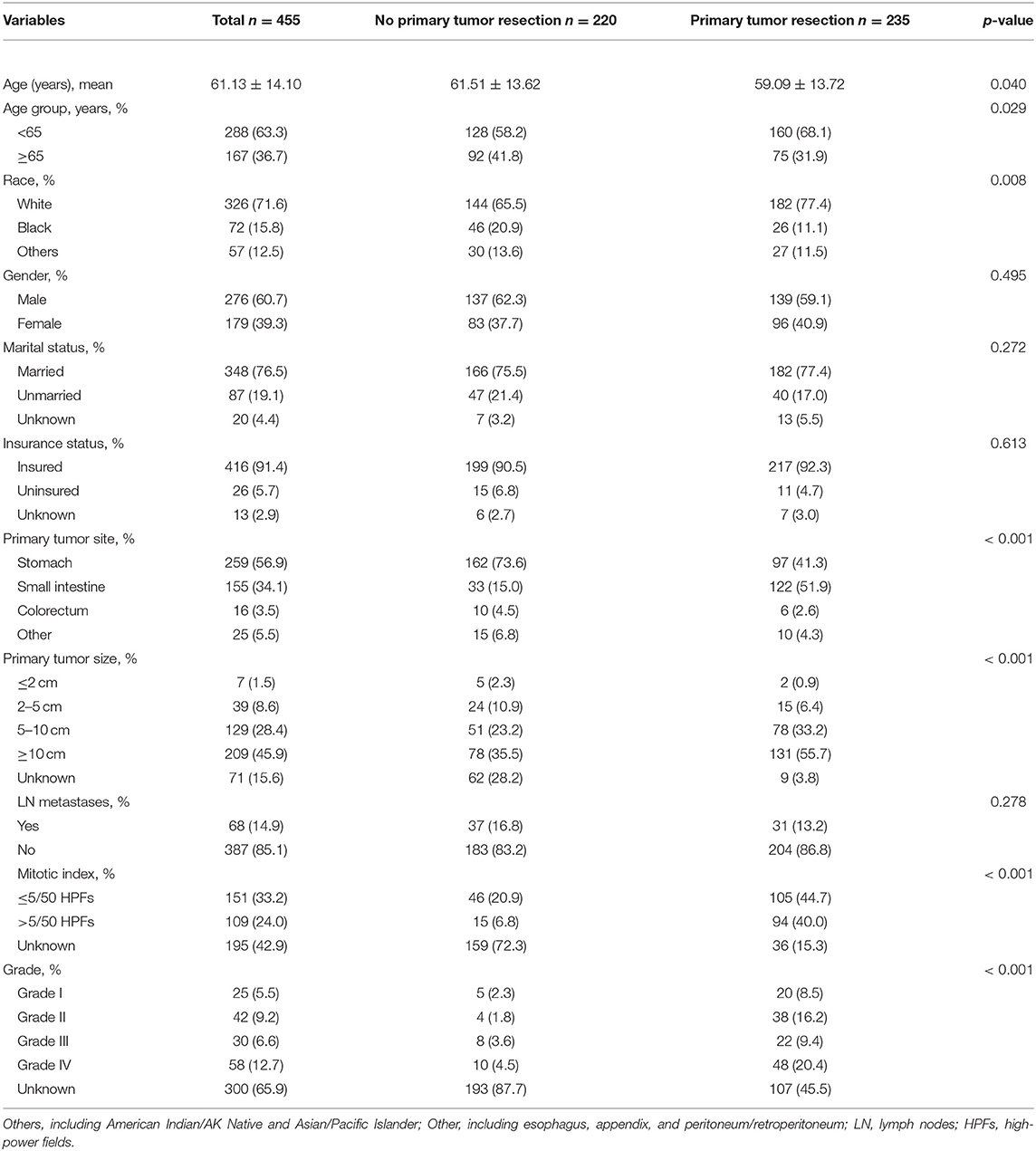
The demographic and clinical features of the participants were summarized in Table 1. In total, 455 patients with metastatic GISTs were considered qualified between 2010 and 2016, of whom 235 (51.6%) underwent primary tumor resection and 220 (48.4%) did not. The mean age at diagnosis was 61 years ranged from 17 to 88 years old. Male patients (60.7%) presented a higher proportion as compared to female (39.3%). The majority of the population were white (71.6%), married (76.5%) and insured (91.4%) individuals. Among these patients, over half were located in the stomach (56.9%), followed by the small intestine (34.1%) and far less frequently in the other sites (5.5%) including esophagus, appendix, peritoneum or retroperitoneum, and colorectum (3.5%). In addition, 14.9% of patients were identified with lymph node metastases. Compared to patients who did not accept surgery, patients in the resection group tended to be younger (59 vs. 62 years, p = 0.040), had a greater percentage of tumors ≥5 cm in diameter (88.9 vs. 58.7%, p < 0.001), and had more common sites in the small intestine (51.9 vs. 15.0%, p < 0.001). Besides, a total of 150 (33%) deaths occurred during follow-up, of which 139 (31%) patients died owing to the metastatic GIST. According to Tables 2, 3 showing the detailed deaths in each group, we can find that the number of deaths was adequate when performing the relevant survival analysis.
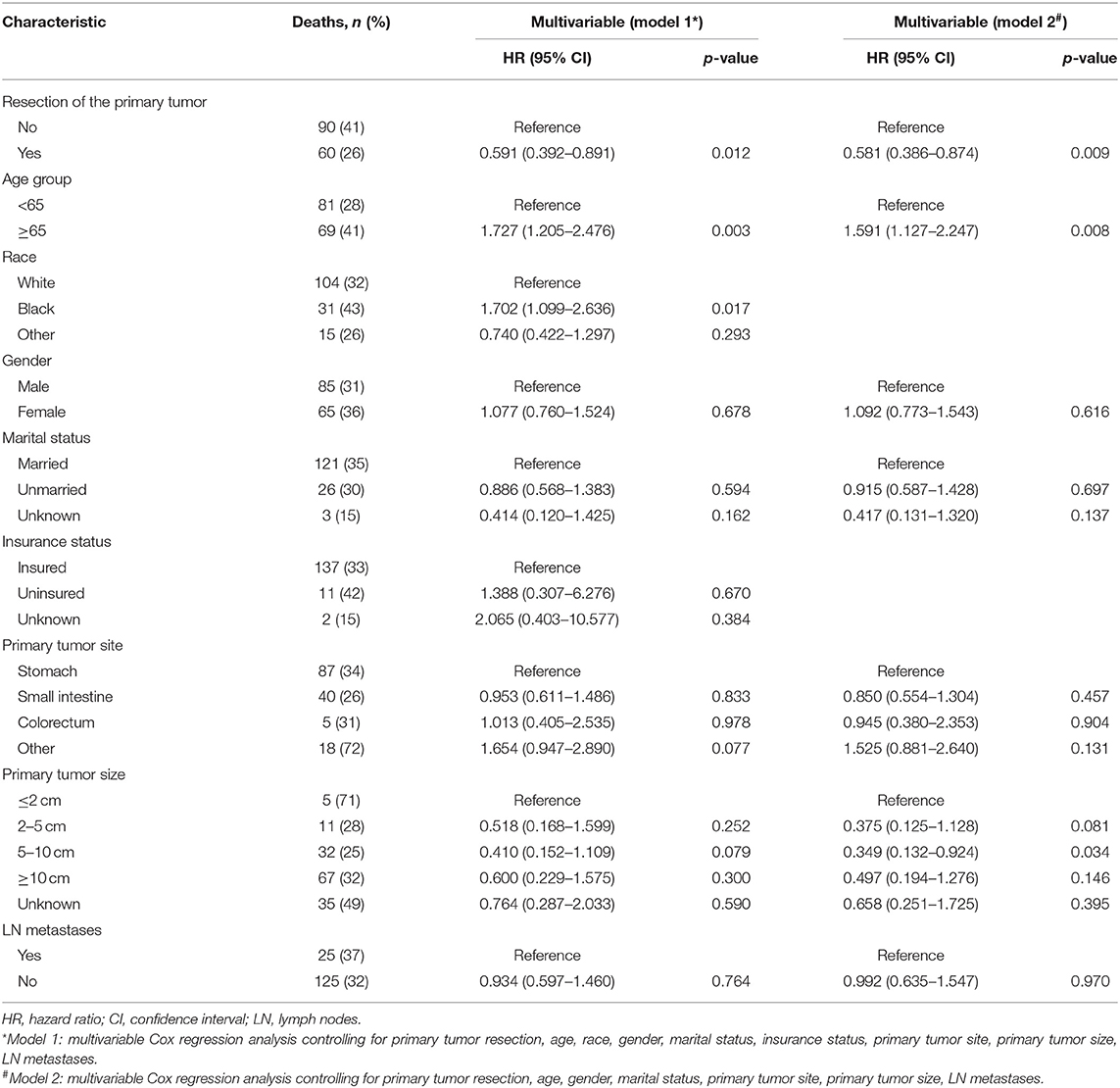
Table 2. Factors associated with overall survival of patients with metastatic gastrointestinal stromal tumors.
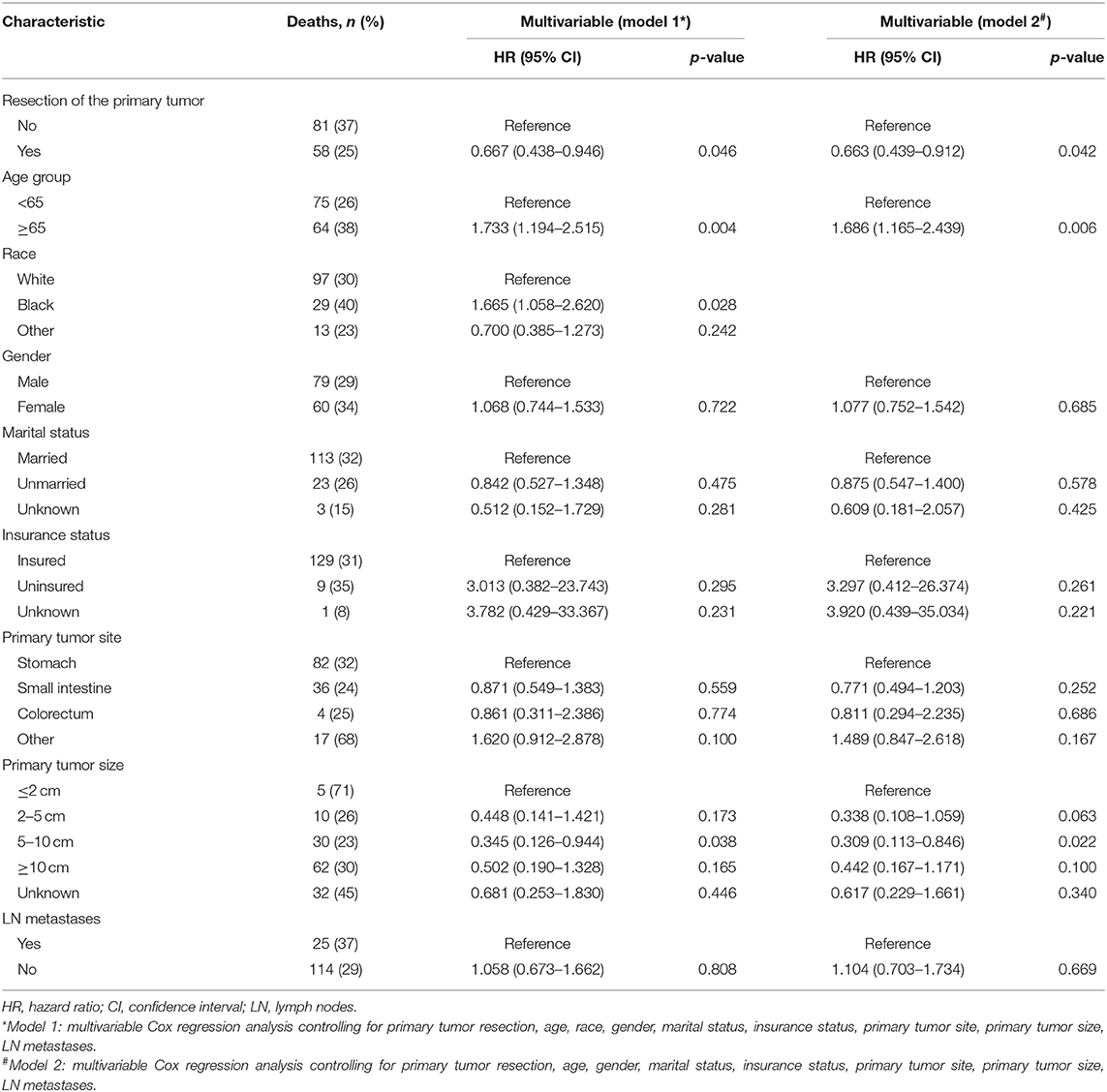
Table 3. Factors associated with cancer-specific survival of patients with metastatic gastrointestinal stromal tumors.
Influence of Primary Tumor Resection on OS and CSS
Patients in the resection group were associated with a significantly high likelihood of longer median survival than those without surgery (72 months, 95% CI: 62.90–81.10 vs. 40 months, 95% CI: 29.53-50.47 months, p < 0.001). Likewise, there was a trend toward a higher 5-year OS (62.2%, 95% CI: 53.2–71.2% vs. 34.3%, 95% CI: 24.1–44.5%) and CSS (63.5%, 95% CI: 54.4–72.5% vs. 36.8%, 95% CI: 26.2-47.4%) in surgery group compared to patients who did not have primary tumors removed (Figure 2). Moreover, patients were divided into multiple subgroups stratified by age, primary tumor size, and primary tumor site. Our stratification analysis indicated that patients in the surgical cohort were connected with improved OS at 5 years (Age <65 years old: 67.9%, 95% CI: 57.2–78.7% vs. 40.5%, 95% CI: 25.2–55.8%; Age ≥65 years old: 49.3%, 95% CI: 30.1–68.5% vs. 26.3%, 95% CI: 12.6–40.0%; Stomach: 53.4%, 95% CI: 37.1–69.6% vs. 34.1%, 95% CI: 21.2–47.0%; Small intestine: 65.7%, 95% CI: 54.0–77.5% vs. 47.1%, 95% CI: 19.3–75.0%; Tumor size between 5 and 10 cm: 68.6%, 95% CI: 51.8–85.5% vs. 41.8%, 95% CI: 19.8-63.7%; Tumor size >10 cm: 59.4%, 95% CI: 46.9–71.9% vs. 44.7%, 95% CI: 27.8–61.5%) (Figure 3). Similarly, when analyses were conducted separately in the above subgroups, the prolonged 5-year CSS rates in resection group were significantly detected among patients with age older than 65 years old (69.8%), as well as in patients with age younger than 65 years old (49.3%) and patients with tumor arising in the stomach (53.4%), while patients in the corresponding non-resection group had 5-year CSS rates of 43.4, 28.3, and 36.1%, respectively (Figure 4). Of importance, our results showed that surgery in conjunction with chemotherapy led to a favorable impact on survival than chemotherapy alone (OS: 72 vs. 40 months, p < 0.001; CSS: 74 vs. 44 months, p < 0.001). However, a log-rank test revealed that there were no survival differences between resection and non-resection groups in the following subtypes: tumor originated from colorectum, tumor originated from other sites, tumor size <2 cm, and tumor size between 2 and 5 cm (P>0.05).
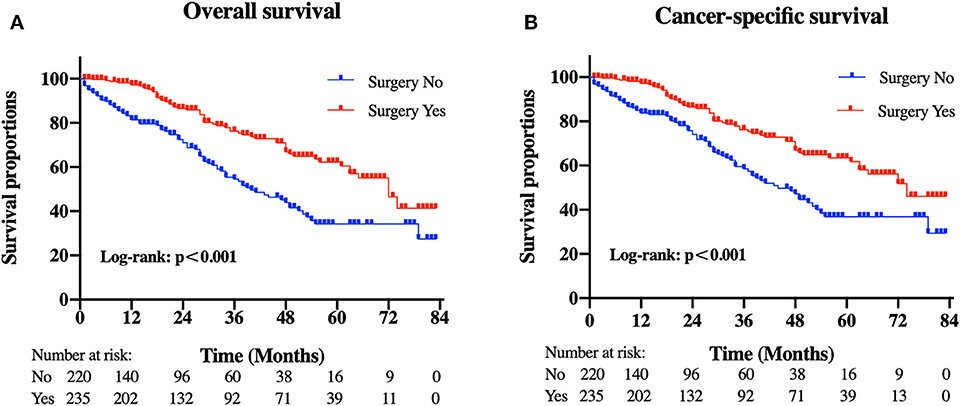
Figure 2. Kaplan-Meier curves of (A) overall and (B) cancer-specific survival according to whether patients underwent primary tumor surgery in the overall cohort.
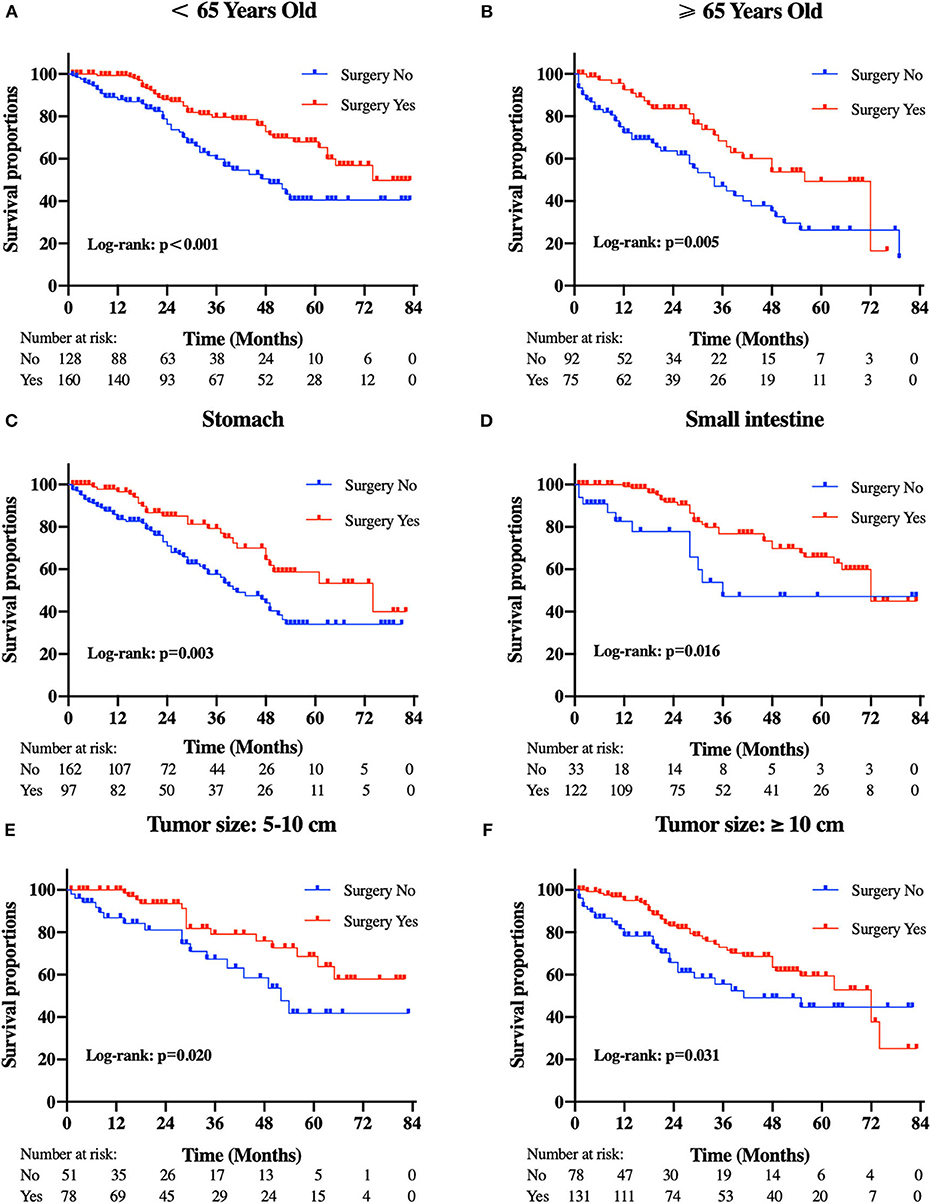
Figure 3. Kaplan-Meier curves of overall survival according to tumor subtype. (A) patients <65 years old, (B) patients ≥65 years old, (C) tumor locations in the stomach, (D) tumor locations in the small intestine, (E) tumor size between 5 and 10 cm, and (F) tumor size ≥10 cm.
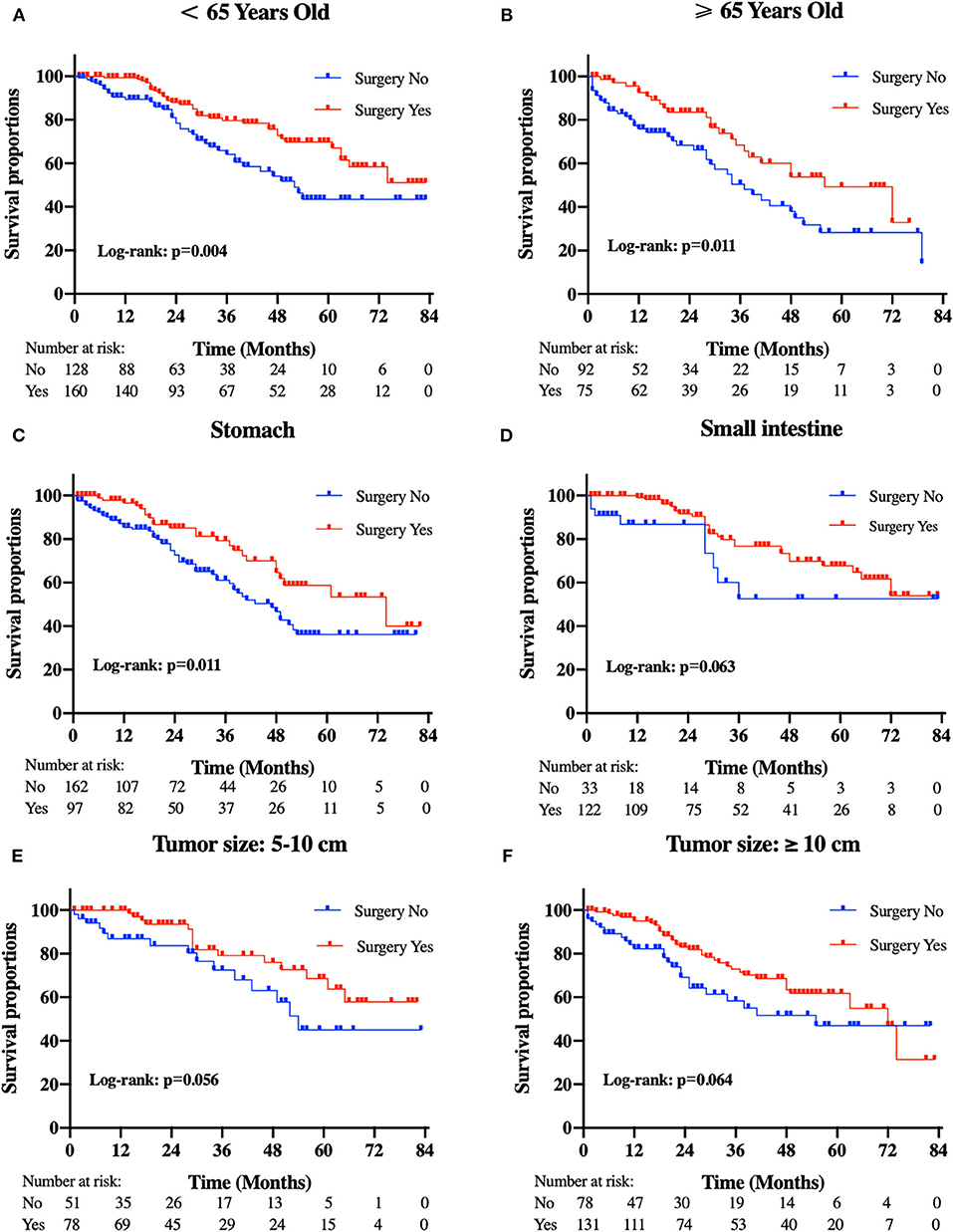
Figure 4. Kaplan-Meier curves of cancer-specific survival according to tumor subtype. (A) patients <65 years old, (B) patients ≥65 years old, (C) tumor locations in the stomach, (D) tumor locations in the small intestine, (E) tumor size between 5 and 10 cm, and (F) tumor size ≥10 cm.
After controlling for confounding factors, removal of primary tumor led to a durable improvement in survival among patients <65 years old (OS HR: 0.476, 95% CI: 0.247–0.872, p = 0.032; CSS HR: 0.578, 95% CI: 0.293–0.924, p = 0.039) and more than 65 years old (OS HR: 0.500, 95% CI: 0.258–0.969, p=0.040; CSS HR: 0.662, 95% CI: 0.384–0.876, p = 0.042) (Figure 5). We also found that, a significant correlation between surgery and improved survival was observed in patients with tumor location in stomach (OS HR: 0.513, 95% CI: 0.310–0.851, p = 0.010; CSS HR: 0.555, 95% CI: 0.332–0.926, p=0.024). A similar improvement was also surprisingly detected across patients with tumor sizes between 5 and 10 cm for OS (HR: 0.430, 95% CI: 0.197–0.938, p = 0.034) after treatment with surgery, but not for CSS (HR: 0.465, 95% CI: 0.207–1.042, p = 0.063). Regardless of the OS and CSS cohort, there was no difference observed in patients with tumor sizes more than 10 cm or tumor location in small intestine. Specifically, our interaction tests showed that patients who presented with tumor sizes between 5 and 10 cm had a stronger association of surgery with the reduction of overall death or cancer-specific death (pint=0.001 for OS; pint=0.002 for CSS). The P values for interactions between surgery and age or primary tumor location were not significant (age pint = 0.818 for OS; pint = 0.964 for CSS; primary tumor location pint=0.494 for OS; pint=0.610 for CSS).
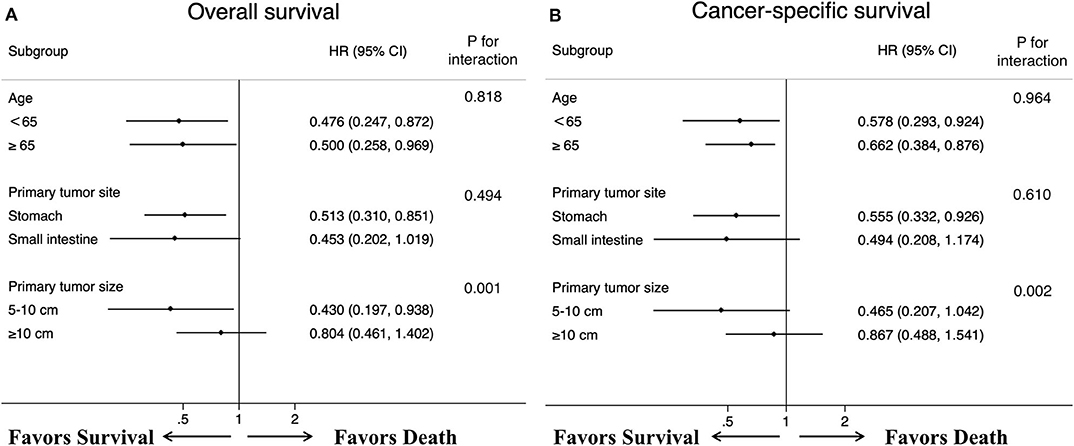
Figure 5. Forest plots summarize the HR and 95% CI of (A) overall and (B) cancer-specific survival according to whether patients underwent primary tumor surgery in subgroup analyses.
Multivariable Predictors of Survival
To gain insight into the association between clinical or tumor characteristics and OS or CSS, a Cox regression was performed. Both in the OS and CSS cohort (Tables 2, 3), multivariable analysis in Model 1 showed that surgery of the primary tumor site was correlated with a decreased risk of overall death (HR: 0.591, 95% CI: 0.392–0.891, p = 0.012, Model 1) and cancer-specific death (HR: 0.667, 95% CI: 0.438–0.946, p = 0.046, Model 1). On the basis of 455 patients in the cohort, variables were reduced to seven and eight potential predictors for OS and CSS (Figure 6), respectively, which were with nonzero coefficients in the LASSO regression model. Model 2 was adjusted for age, gender, marital status, tumor site, tumor size, LN metastases, and primary tumor resection for OS. Model 2 for CSS was adjusted for age, gender, marital status, insurance status, tumor site, tumor size, LN metastases, and primary tumor resection. After adjustment for confounding factors, the multivariable analysis in Model 2 demonstrated that surgery remained an independent prognostic factor of increased OS (HR: 0.581, 95% CI: 0.386–0.874, p = 0.009, Model 2) and CSS (HR: 0.663, 95% CI: 0.439–0.912, p = 0.042, Model 2). Moreover, Model 2 had lower AIC (1571.816) and BIC (1600.658) than the Model 1 (AIC: 1575.536; BIC: 1612.619) for OS, indicating better prediction of the model 2, and the same conclusion can be applied to CSS (AIC: 1463.128 and BIC: 1500.211 for Model 1; AIC: 1461.221 and BIC: 1494.183 for Model 2). Therefore, we took Model 2 as the main result. Furthermore, primary tumor size between 5 and 10 cm was also a predictor of decreased hazard of death, whereas increasing age was predictive of poorer survival.
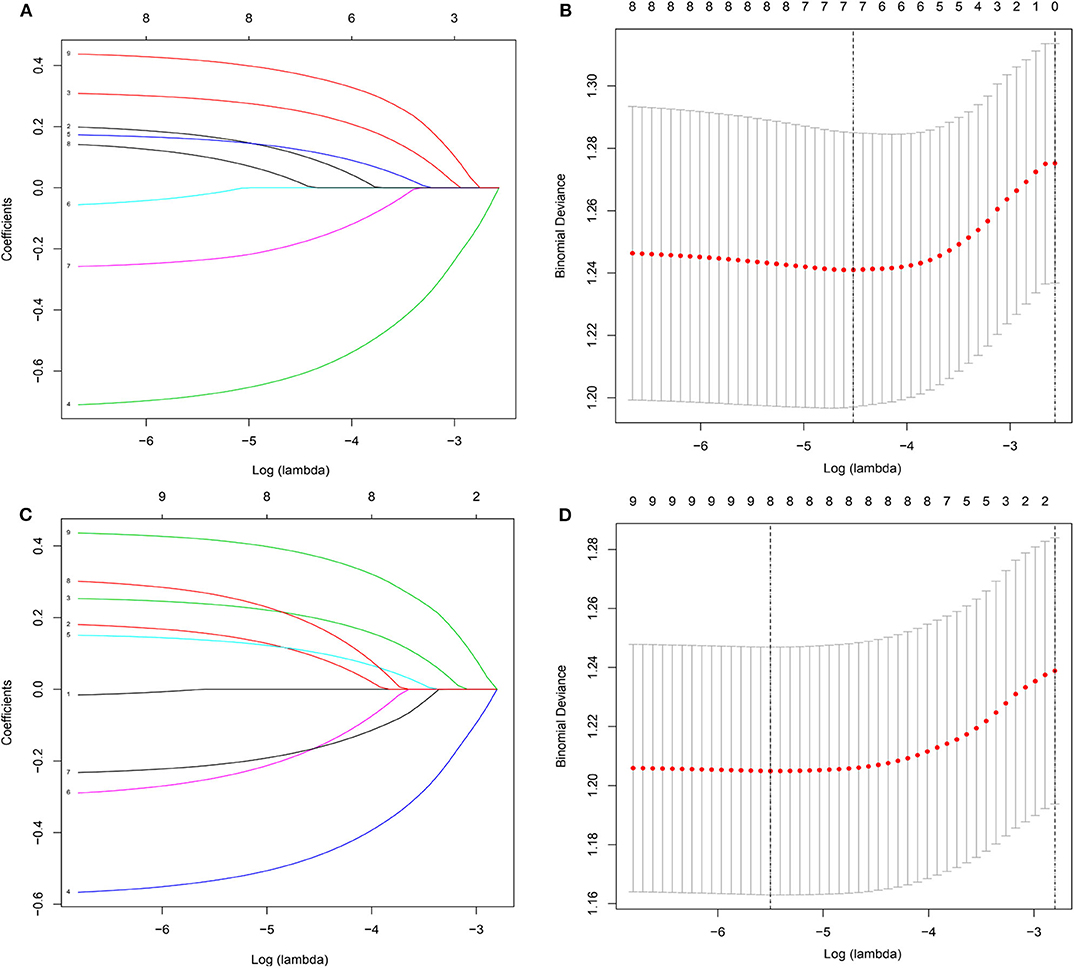
Figure 6. Demographic and clinical feature selection using the LASSO binary logistic regression model. (A,B) for overall survival; (C,D) for cancer-specific survival.
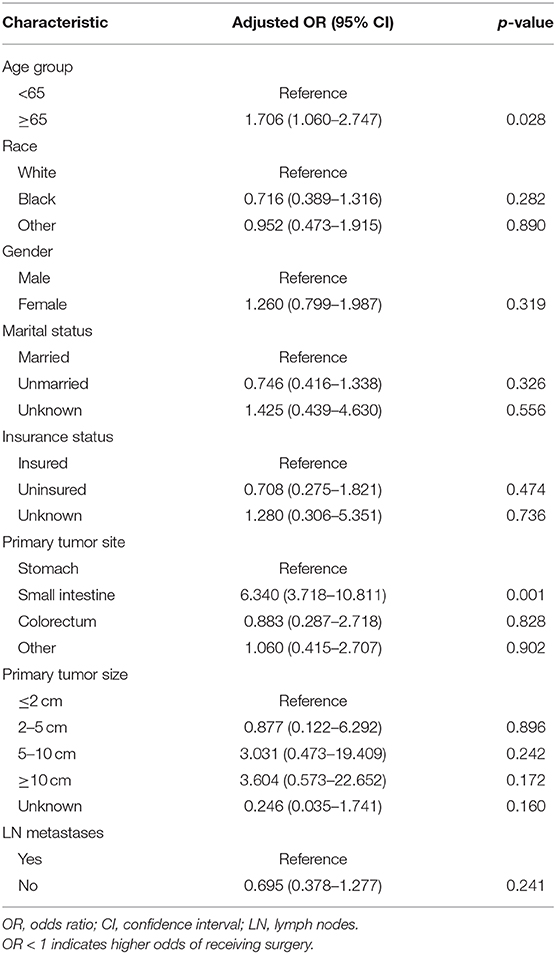
Logistic Regression on Patient Selection for Surgery
The results of the binary logistic regression comparing no surgery vs. surgery as the outcome of interest were shown in Table 4. Specifically, patients older than 65 years with tumors located in the small intestine were much less likely to perform surgery than other patients, but especially compared to younger counterparts with tumors in the stomach.
Discussion
For many decades, GISTs are traditionally regarded as unpredictable and sparse tumors, capable of aggressive behavior. Currently, surgical resection has been established as the sole front-line treatment because of its high insensitivity to conventional standard sarcoma chemotherapy and radiotherapy. For patients with metastatic GISTs, it remains controversial however, if primary tumor removal could confer a survival benefit in the setting of distant metastases (20). With the advances in the cognition of molecular mechanisms, IM has been proposed as principle therapy in the management of metastatic GISTs with a reported overall survival of up to 81 months, representing a paradigm shift in targeted therapy (9, 21). Hence, for the purpose of more intuitively appraising the effectiveness of surgery in the metastatic setting, we conducted the study under the premise that all patients had received chemotherapy.
To our knowledge, this is the largest retrospective study focusing on the survival outcomes of patients who accept surgical resection. Our results provide the initial evidence that primary tumor surgery might yield an association with favorable survival for metastatic GISTs compared with those patients without surgery, and more importantly, this is particularly evident in these patients with a primary tumor between 5 and 10 cm, as well as tumors in the stomach. This information might be beneficial when considering surgical interventions in GISTs patients with distant metastases. Simultaneously, a prospective study taking all potential surgical candidates into account is warranted to confirm our findings.
As for the observed longer survival in patients who had their primary tumors removed, although the exact reasons are not clear, it is mainly because carrying out surgery in primary tumor site for palliative and prognostic aims may contribute to a reduction of tumor load, thereby decreasing secondary gene mutations which confer resistance to IM, ameliorating local tumor-related symptoms and retarding tumor progression (11). From the surgical therapy standpoint, accumulating studies have demonstrated the survival advantage of primary tumor resection in highly selected patients (22, 23). In a retrospective study of 87 patients with advanced GISTs, patients receiving either medical therapy alone or surgical debulking combined with drug therapy had an extension of median OS from 40.4 to 54.8 months (24). Likewise, another single-institution study of 90 advanced GIST patients reached a similar conclusion, emphasizing an increase of overall survival in 38 patients undergoing resection following focal progression with standard doses of IM (53.2 months, CI 36.8–69.6) vs. 52 patients with IM dose escalation alone (35.1 months, CI 25.6–44.7) (25). At the same time, our findings exhibit significantly better survival with median survival time of 72 months in the operation group than previous studies and the discrepancy in outcome seems partially attributed to the different inclusion criteria of patients. It is possible that selection biases may exist in our research because we don't know if these patients receiving resection tend to be relatively good status, which would increase potential confounding effects in the evaluation of the impact of surgery on survival outcomes.
However, consistent with these results, there are other reports, on smaller series, failing to show a benefit for primary tumor resection in the setting of metastatic GIST. We revisit these cohorts to gain further insight into the role of surgical treatment, and this observation could be elucidated by the following. On the one hand, Sato et al. performed their study with small numbers of patients and different frequency of postoperative IM therapy. Only 43% of patients accepting R0/R1 resection and 75% of those who performed R2 resection received this therapy (15). On the other hand, it is worth noting that long-term survival was not observed in advanced patients in whom cytoreductive surgery was carried out prior to IM treatment, which may result in the difference (26). Although few randomized controlled trials (RCTs) concentrating on this point have been conducted, they all ended in failure due to limited patients and poor accrual (27, 28). Hence, from this perspective, there is no doubt that choosing appropriate cases and timing for surgical procedure is the first priority. Analogously, multidisciplinary assessment of these advanced patients using a framework approach should be premeditated before determining different treatment options even if our findings reveal the possible survival benefits of surgery. National Comprehensive Cancer Network (NCCN) guidelines recommend that surgery should be considered for these unresectable or metastatic patients with limited disease progression refractory to IM, advanced tumors responsive to IM, and presented with symptomatic bleeding and obstruction (29). In addition, it is critical to restart chemotherapy as soon as the patient is capable to tolerate oral medication after surgery (30).
On subgroup analysis regarding therapy, as previously shown, patients treated with surgery plus chemotherapy show a tendency of relatively better survival, with 5-year OS rates increasing from 34.3 to 62.2%, when compared with chemotherapy alone, which are supported by recent publications (31, 32). For those who survive to a combination of surgery and chemotherapy, median survival has been demonstrated to be longer in patients with advanced GIST. Notably, contrary to our hypothesis, we found that primary tumor resection seemed to prolong the survival time even among patients with tumor sizes between 5 and 10 cm whereas patients with tumor size smaller than 5 cm did not. This seemingly paradoxical phenomenon might be correlated with the biological behavior of tumors. Generally, patients with smaller tumors are less likelihood of developing metastasis, while once metastases occurring, they would have a higher degree of malignancy and more extensive metastatic diseases over time, which is detrimental to surgery. Besides, the fact that surgical removal supported a negative impact of surgery for patients with tumor sizes >10 cm was detected. Presumably, one possible explanation is that large tumors appear to contain more resistant cells, having a greater chance of recurrence and emerging new resistance mutations during treatment (15). Interaction tests show a differential surgical effect between the two subtypes, further demonstrating a survival benefit of surgery in patients with a tumor size between 5 and 10 cm, which is also perceived as an independent predictive indicator for prolonged OS and CSS in our analyses. Of note, considering the nature of respective analysis, studies in the form of RCTs with balanced characteristics are urgently needed to prove the efficacy of surgical treatment in patients with GISTs and distant metastases.
Despite it is widely recognized that small intestinal GISTs exhibit more aggressive features and portend worse outcomes than gastric GISTs (33), a statistically significant difference regarding OS and CSS was not observed among patients with stomach GIST and patients with small intestine GIST in our study. The growing use of surgery and chemotherapy in patients with small intestine GIST, which could confer a survival improvement, may explain this finding (34). These patients with tumor located in the stomach might have been considered to benefit from their primary tumor resection according to our survival analysis and interaction results. Additionally, younger patients (<65 years old) and older patients (≥65 years old) simultaneously showed a marginal trend toward a favorable survival duration when they perform surgery, and the finding is supported by interaction tests in this subgroup analysis. However, similar to previously published reports (35), our multivariable analysis suggests that older age was an important prognostic factor that could increase the risk of overall death or cancer-specific death. This observation might be the result of poorer physiologic reserves and capability to sustain more invasive treatment in the elderly. Thus, the feasibility of surgery in older patients still needs further verification.
Although the optimal way to reduce or eliminate selection bias is RCTs, such trials always had to be terminated early as a result of lower participant accrual, moral problems, and the rarity of GISTs during the design and implementation of RCTs (36). Accordingly, we did a lot of effort to describe the association and the influence of surgery on survival, including utilizing multiple multivariable Cox regression methods to identify prognostic parameters, performing survival analyses stratified by age, tumor size and tumor site to assess the role of surgery in specific populations, and conducting interaction analysis to explore the stability of our outcomes. Thus, it appeared to be unlikely that such efficacy was completely the result of unadjusted confounding.
There are some limitations that should be acknowledged here. Firstly, given the retrospective design of our study based on the SEER database, it is inevitable to have intrinsic selection bias as clinicians are prone to choose patients for surgery with better performance status, low metastatic extent, and long-lasting good response to therapy, which was also a major limitation. We found that some important factors including age, tumor site, tumor size, mitotic index, and grade were unbalanced between groups, and our binary logistic regression further indicated that younger patients with tumor originated from the stomach were apt to select surgery, thereby weakening the effects of the observed results. Secondly, tumor grade and mitotic index were excluded from our multivariable analysis owing to their large percent of missing data, which were once defined as prognostic factors in previous reports (37). Besides, our study was conducted with a small subset of patients, which might lead to our observations. Hence, larger-scale and well-designed clinical trials are warranted to validate the role of surgery in the multidisciplinary management of GISTs. In addition, some important parameters such as clinical symptoms, KIT gene, exon mutation, detailed chemotherapy information, the timing of surgery, and surgical margin status were not included because they were not available in the SEER registry, and which may have contributed to our findings. Finally, information about local or distant recurrence was not provided; therefore, further analyses are urgently needed before conclusions can be applied to recurrent populations.
We believe that this data does not allow an unequivocal recommendation for surgery in metastatic GISTs patients due to the existence of the above limitations but that it might support a surgical approach in carefully selected patients, especially with tumor sizes between 5 and 10 cm and tumors located in the stomach. Given that it is a preliminary research, our results should be further validated by prospective multicenter collaborative studies with larger samples that examine surgical benefits for patients with metastatic GISTs to obtain high-quality evidence.
Conclusion
Ultimately, our study preliminarily demonstrates that resection of primary tumors might have a beneficial effect in cases of well-selected patients with metastatic GISTs, especially those with a primary tumor between 5 and 10 cm and a tumor located in the stomach. Moreover, surgical resection combined with chemotherapy could dramatically improve the prognosis of patients compared with chemotherapy alone. We hope that individualized treatments in patients with metastatic GISTs should be carefully designed by multidisciplinary teams. Future prospective trials with large samples, however, are needed to validate these findings because we are unable to determine the burden of metastasis or the decision-making factors for tumor removal in these patients.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/).
Author Contributions
SZ and HZ collected and analyzed the data and wrote the paper. RJ and XW performed quality assessment and analyzed the data. GJ and XZ conceived and designed this study. All authors reviewed the paper, read, and approved the final manuscript.
Funding
This research work received the support from the Project of new clinical diagnosis and treatment technology of Jiangsu Province (BE2016799) participated by GJ.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the researchers and study participants for their contributions.
Abbreviations
GISTs, gastrointestinal stromal tumors; SEER, the Surveillance, Epidemiology, and End Results; OS, overall survival; CSS, cancer-specific survival; GI, gastrointestinal; PDGFRA, platelet-derived growth factor receptor α; IM, Imatinib mesylate; PFS, progression-free survival; ICD-O-3, International Classification of Diseases for Oncology, 3rd Edition; aORs, adjusted odds ratios; HR, hazard ratio; CI, confidence interval; LASSO, least absolute shrinkage and selection operator; AIC, Akaike Information Criterion; BIC, Bayesian Information Criterion; RCTs, randomized controlled trials; NCCN, National Comprehensive Cancer Network.
References
1. Burch J, Ahmad I. Cancer, Gastrointestinal Stromal (GIST). Treasure Island, FL: StatPearls (2020).
2. Nilsson B, Bumming P, Meis-Kindblom JM, Oden A, Dortok A, Gustavsson B, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era–a population-based study in western Sweden. Cancer. (2005) 103:821–9. doi: 10.1002/cncr.20862
3. Soreide K, Sandvik OM, Soreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol. (2016) 40:39–46. doi: 10.1016/j.canep.2015.10.031
4. Joensuu H, Vehtari A, Riihimaki J, Nishida T, Steigen SE, Brabec P, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. (2012) 13:265–74. doi: 10.1016/S1470-2045(11)70299-6
5. Quek R, George S. Gastrointestinal stromal tumor: a clinical overview. Hematol Oncol Clin North Am. (2009) 23:69–78. doi: 10.1016/j.hoc.2008.11.006
6. Bauer S, Joensuu H. Emerging agents for the treatment of advanced, imatinib-resistant gastrointestinal stromal tumors: current status and future directions. Drugs. (2015) 75:1323–34. doi: 10.1007/s40265-015-0440-8
7. Zhang D, Wang T, Liu Y, Li L, Li B, Yang X, et al. Clinical features and surgical treatments of rare gastrointestinal stromal tumor spinal metastases. Br J Neurosurg. (2020). doi: 10.1080/02688697.2020.1725438. [Epub ahead of print].
8. Frankel TL, Chang AE, Wong SL. Surgical options for localized and advanced gastrointestinal stromal tumors. J Surg Oncol. (2011) 104:882–7. doi: 10.1002/jso.21892
9. Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. (2008) 26:620–5. doi: 10.1200/JCO.2007.13.4403
10. Sleijfer S, Wiemer E, Verweij J. Drug insight: gastrointestinal stromal tumors (GIST)–the solid tumor model for cancer-specific treatment. Nat Clin Pract Oncol. (2008) 5:102–11. doi: 10.1038/ncponc1037
11. Raut CP, Gronchi A. Cytoreductive surgery in advanced GIST: timing is everything. Ann Surg Oncol. (2013) 20:4059–60. doi: 10.1245/s10434-013-3281-2
12. Dip Borunda AK, Pimentel Renteria A, Pluma Jimenez M, Perez Martinez M, Martinez Martinez G, Rivera Rivera S, et al. Treatment of non-resectable and metastatic gastrointestinal stromal tumors: experience with the use of tyrosine kinase inhibitors in a third level hospital in Mexico. J Gastrointest Oncol. (2016) 7:632–7. doi: 10.21037/jgo.2016.06.03
13. Bamboat ZM, DeMatteo RP. Metastasectomy for gastrointestinal stromal tumors. J Surg Oncol. (2014) 109:23–7. doi: 10.1002/jso.23451
14. Mussi C, Ronellenfitsch U, Jakob J, Tamborini E, Reichardt P, Casali PG, et al. Post-imatinib surgery in advanced/metastatic GIST: is it worthwhile in all patients? Ann Oncol. (2010) 21:403–8. doi: 10.1093/annonc/mdp310
15. Sato S, Tsujinaka T, Yamamoto K, Takahashi T, Kishi K, Imamura H, et al. Primary surgery as a frontline treatment for synchronous metastatic gastrointestinal stromal tumors: an analysis of the Kinki GIST registry. Surg Today. (2016) 46:1068–75. doi: 10.1007/s00595-015-1282-4
16. Surveillance, Epidemiology, and End Results Program Overview. Available online at: https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf (accessed February 28, 2019).
17. Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. (2007) 26:5512–28. doi: 10.1002/sim.3148
18. Kidd AC, McGettrick M, Tsim S, Halligan DL, Bylesjo M, Blyth KG. Survival prediction in mesothelioma using a scalable lasso regression model: instructions for use and initial performance using clinical predictors. BMJ Open Respir Res. (2018) 5:e000240. doi: 10.1136/bmjresp-2017-000240
19. Marami Milani MR, Hense A, Rahmani E, Ploeger A. Applying least absolute shrinkage selection operator and akaike information criterion analysis to find the best multiple linear regression models between climate indices and components of cow's milk. Foods. (2016) 5:52. doi: 10.3390/foods5030052
20. McCarter MD, Antonescu CR, Ballman KV, Maki RG, Pisters PW, Demetri GD, et al. Microscopically positive margins for primary gastrointestinal stromal tumors: analysis of risk factors and tumor recurrence. J Am Coll Surg. (2012) 215:53–9. doi: 10.1016/j.jamcollsurg.2012.05.008
21. van der Graaf WTA, Tielen R, Bonenkamp JJ, Lemmens V, Verhoeven RHA, de Wilt JHW. Nationwide trends in the incidence and outcome of patients with gastrointestinal stromal tumour in the imatinib era. Br J Surg. (2018) 105:1020–7. doi: 10.1002/bjs.10809
22. Yeh CN, Wang SY, Tsai CY, Chen YY, Liu CT, Chiang KC, et al. Surgical management of patients with progressing metastatic gastrointestinal stromal tumors receiving sunitinib treatment: a prospective cohort study. Int J Surg. (2017) 39:30–6. doi: 10.1016/j.ijsu.2017.01.045
23. Park SJ, Ryu MH, Ryoo BY, Park YS, Sohn BS, Kim HJ, et al. The role of surgical resection following imatinib treatment in patients with recurrent or metastatic gastrointestinal stromal tumors: results of propensity score analyses. Ann Surg Oncol. (2014) 21:4211–7. doi: 10.1245/s10434-014-3866-4
24. Qiu HB, Zhou ZG, Feng XY, Liu XC, Guo J, Ma MZ, et al. Advanced gastrointestinal stromal tumor patients benefit from palliative surgery after tyrosine kinase inhibitors therapy. Medicine. (2018) 97:e9097. doi: 10.1097/MD.0000000000009097
25. Cho H, Ryu MH, Lee Y, Park YS, Kim KH, Kim JH, et al. Role of resection following focal progression with standard doses of imatinib in patients with advanced gastrointestinal stromal tumors: results of propensity score analyses. Oncologist. (2019) 24:e1443–e9. doi: 10.1634/theoncologist.2019-0009
26. An HJ, Ryu MH, Ryoo BY, Sohn BS, Kim KH, Oh ST, et al. The effects of surgical cytoreduction prior to imatinib therapy on the prognosis of patients with advanced GIST. Ann Surg Oncol. (2013) 20:4212–8. doi: 10.1245/s10434-013-3279-9
27. Du CY, Zhou Y, Song C, Wang YP, Jie ZG, He YL, et al. Is there a role of surgery in patients with recurrent or metastatic gastrointestinal stromal tumours responding to imatinib: a prospective randomised trial in China. Eur J Cancer. (2014) 50:1772–8. doi: 10.1016/j.ejca.2014.03.280
28. Gronchi A. A Phase III Randomized Study Evaluating Surgery of Residual Disease in Patients With Metastatic Gastro-Intestinal Stromal Tumor Responding to Imatinib Mesylate. Available online at: https://clinicaltrials.gov/ct2/show/nct00956072.2009
29. von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Ganjoo KN, et al. Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2018) 16:536–63. doi: 10.6004/jnccn.2018.0025
30. Kikuchi H, Hiramatsu Y, Kamiya K, Morita Y, Sakaguchi T, Konno H, et al. Surgery for metastatic gastrointestinal stromal tumor: to whom and how to? Transl Gastroenterol Hepatol. (2018) 3:14. doi: 10.21037/tgh.2018.02.02
31. Guo Y, Liu J, Wang F, Wang Q, Zheng G, Liu S, et al. The role of surgical resection following tyrosine kinase inhibitors treatment in patients with advanced gastrointestinal stromal tumors: a systematic review and meta-analysis. J Cancer. (2019) 10:5785–92. doi: 10.7150/jca.30040
32. Cai Z, Yin Y, Shen C, Tang S, Yin X, Chen Z, et al. Role of surgical resection for patients with recurrent or metastatic gastrointestinal stromal tumors: a systematic review and meta-analysis. Int J Surg. (2018) 56:108–14. doi: 10.1016/j.ijsu.2018.06.016
33. Yang Z, Wang F, Liu S, Guan W. Comparative clinical features and short-term outcomes of gastric and small intestinal gastrointestinal stromal tumours: a retrospective study. Sci Rep. (2019) 9:10033. doi: 10.1038/s41598-019-46520-1
34. Giuliano K, Ejaz A, Reames BN, Choi W, Sham J, Gage M, et al. Comparing the long-term outcomes among patients with stomach and small intestine gastrointestinal stromal tumors: an analysis of the national cancer database. J Surg Oncol. (2018) 118:486–92. doi: 10.1002/jso.25172
35. Yang Z, Feng X, Zhang P, Chen T, Qiu H, Zhou Y, et al. Clinicopathological outcomes and prognosis of elderly patients (>/= 65 years) with gastric gastrointestinal stromal tumors (GISTs) undergoing curative-intent resection: a multicenter data review. J Gastrointest Surg. (2019) 23:904–13. doi: 10.1007/s11605-018-3944-1
36. McCulloch P, Taylor I, Sasako M, Lovett B, Griffin D. Randomised trials in surgery: problems and possible solutions. BMJ. (2002) 324:1448–51. doi: 10.1136/bmj.324.7351.1448
Keywords: gastrointestinal stromal tumors, metastasis, primary tumor resection, survival, SEER
Citation: Zhao S, Zhu H, Jiao R, Wu X, Zhang X and Ji G (2020) Primary Tumor Resection Improves Survival in Patients With Metastatic Gastrointestinal Stromal Tumors: A Preliminary Population-Based Analysis. Front. Oncol. 10:1440. doi: 10.3389/fonc.2020.01440
Received: 23 March 2020; Accepted: 08 July 2020;
Published: 19 August 2020.
Edited by:
Mark Girgis, University of California, Los Angeles, United StatesReviewed by:
Francesco Cavallin, Independent Researcher, Solagna, ItalyCesare Ruffolo, University Hospital of Padua, Italy
Copyright © 2020 Zhao, Zhu, Jiao, Wu, Zhang and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiuhua Zhang, MTEyOXd5QHNpbmEuY29t; Guozhong Ji, amd6emxAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Si Zhao
Si Zhao Hanlong Zhu†
Hanlong Zhu†