- 1Hepatobiliary and Pancreatic Interventional Treatment Center, Division of Hepatobiliary and Pancreatic Surgery, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Zhejiang Clinical Research Center of Hepatobiliary and Pancreatic Diseases, Hangzhou, China
- 3Zhejiang Provincial Research Center for Diagnosis and Treatment of Hepatobiliary Diseases, Hangzhou, China
- 4Department of Radiology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Introduction: The care for patients with hepatocellular carcinoma (HCC) is challenging. This study is to evaluate the effect of adjuvant transarterial chemoembolization (TACE) for Barcelona Clinic Liver Cancer (BCLC) stage A HCC patients after hepatectomy.
Methods: Consecutive HCC patients with BCLC stage A, treated by hepatectomy alone (HA) or hepatectomy with TACE (HT), were retrospectively enrolled. Propensity score matching (PSM) was used to balance baseline differences. The recurrence-free survival (RFS) and overall survival (OS) were evaluated using the Kaplan-Meier. The impact of TACE on survival outcome was determined by Cox hazard regression.
Results: After PSM, 230 patients (115 HT and 115 HA) were enrolled in the analysis. The 1-, 3-, and 5-year RFS rates were 87.0, 63.5, and 50.4%, respectively, for the HT group, and 87.8, 67.0, and 58.3% for the HA group. The OS rates at 1-, 3-, and 5-year were 99.1, 93.9, and 87%, respectively, for the HT group, and 100, 92.2, and 88.7% for the HA group. No significant differences were seen in either the RFS (log-rank test, χ2 = 0.891, p = 0.345) or OS (log-rank test, χ2 = 0.146, p = 0.702) between the specific pairs of two groups. Cox regression identified that TACE was not the factor affecting RFS or OS (p = 0.399; HR 0.847; 95% CI 0.576–1.245 for RFS vs. p = 0.989; HR 0.995; 95% CI 0.471–2.100 for OS).
Conclusion: Our data indicate that TACE is not an effective intervention in the adjuvant setting for BCLC stage A HCC after hepatectomy.
Introduction
Hepatocellular carcinoma (HCC) is one of the most frequently encountered malignancies globally, with the second-highest cancer-related mortality rate (1). Hepatectomy is the most widely practiced therapy, as it is a potentially curative treatment for HCC (2). However, long-term survival after hepatectomy is unsatisfactory because more than 70% tumor recur during the first 5 years (3). The prevention of tumor recurrences is the key to improve the outcome of liver resections.
The Barcelona Clinic Liver Cancer (BCLC) staging system including is widely used for HCC staging and treatment (4). Current clinical practice guidelines do not endorse any particular adjuvant therapy after hepatectomy but do recommend more and larger studies that undertake lower risks of systematic error (2, 4). Transcatheter arterial chemoembolization (TACE) has recently been reported as a postoperative adjuvant therapy for HCC patients. Previous clinical studies (5–7) showed that postoperative TACE significantly reduce tumor recurrence and improve the overall survival of patients with resectable BCLC stage B HCC or high recurrence risk (exceeding 5 cm in diameter or multiple tumors or vascular invasion) after curative liver resection. Nevertheless, none of these studies cover BCLC stage A HCC (single tumor or up to 3 tumors ≤3 cm), for which the BCLC staging system recommends surgical resection as the best option (8). The efficacy of TACE as adjuvant therapy after hepatectomy for patients with BCLC stage A HCC is not clear.
To further investigate the efficacy of TACE as adjuvant therapy after hepatectomy for patients with BCLC stage A HCC, we conducted a cohort study to follow up the survival outcome of BCLC stage A HCC who underwent hepatectomy alone or had postoperative adjuvant TACE.
Materials and Methods
The study protocol was approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine, and the written informed consent was obtained from all the patients.
Patients
In this retrospective cohort study, consecutive patients with BCLC stage A HCC, who underwent curative hepatectomy, were enrolled from January 2012 to August 2014 at the First Affiliated Hospital, Zhejiang University School of Medicine. The inclusion criteria: (1) the HCC diagnosis was confirmed by pathologic examination; (2) the patients had a stage A HCC using the BCLC staging system; and (3) histologic evidence of tumor-free margins on the resected tissues (defined as the distance between the cancer tissue and resected tissue margins is 1 cm or more). The exclusion criteria: (1) an intrahepatic recurrence within 2 months after curative hepatectomy; (2) the presence of other malignant tumors; and (3) loss of patients to follow-up.
Patients were divided into two groups: (1) HCC underwent hepatectomy with adjuvant TACE (HT group) and (2) HCC underwent hepatectomy alone (HA group). Standard demographic and clinical data potentially related to recurrence and survival were collected: gender, age, hepatitis, cirrhosis, tumor characteristics, surgical margin, and pathologic results.
Adjuvant TACE
Postoperative adjuvant TACE was performed 4–6 weeks after hepatic resection, according to the Eastern Cooperative Oncology Group (ECOG) performance status and patient liver function. A 5-F angiographic catheter (Cook Inc., Bloomington, IN, United States) was introduced into the common hepatic artery through femoral artery, then hepatic angiography was performed to evaluate the arterial blood supply to the liver. 150 mg oxaliplatin (Hengrui Medicine Co., Ltd., Jiangsu, China) was slowly infused into proper hepatic artery, followed by an emulsion of 20 mg pirarubicin (Shenzhen Main Luck Pharmaceuticals Inc., Shenzhen, China) and 2–4 mL lipiodol (Lipiodol Ultrafluide, Guerbet, Aulnay-sous-Bois, France) using the microcatheter (Terumo, Tokyo, Japan). After 4–6 weeks, these patients underwent a complete assessment.
Follow-Up
All patients were followed-up every 2 to 3 months during the first year and then every 3 to 6 months after surgery until death or dropout from the follow-up (4). On the follow-up visits patients tested ECOG, liver function, serum alpha-fetoprotein (AFP), abdominal ultrasonography, and CT or MRI scan. The primary endpoint for this study was recurrence-free survival (RFS), defined as the interval from surgery to the first recurrence. Secondary endpoints were overall survival (OS), defined as the interval from surgery to the date of death.
The diagnosis of tumor recurrence or metastasis was based on cytologic/histologic evidence or non-invasive diagnostic by the EASL (2). Two senior radiologists independently reviewed images. If any discrepancy in CT or MRI scans, the final diagnosis was made after reviewing all clinical information. After tumor recurrence was confirmed, the patients were treated according to the practice guidelines, which included curative treatments (surgical resection, liver transplantation, or radiofrequency ablation) and/or non-curative treatments (TACE, percutaneous ethanol injection, radiotherapy, or systemic therapy) to improve survival.
Statistical Analysis
Sample sizes were computed using the RFS as the primary endpoint. Based on previous study, the 5-year RFS rate after curative resection was 40% (9). We expected a 5-year RFS rate in the adjuvant TACE group of 60%. Using a two-sided test with 80% power at a significance level of 5%, the minimum sample size in each group was estimated to be 94 patients.
To eliminate potential baseline confounding factors and isolate the effects of adjuvant TACE, propensity score matching (PSM) was used to balance baseline differences and thereby simulate random group allocation. The propensity score model included all variables known to be associated with survival outcomes. A one-to-one nearest-neighbor matching without replacement was used to match patients based on the logistic regression of the propensity score within a caliper of 0.05. That is to say, one patient from the HT group could get matched with one patient from the HA group with a similar propensity score.
Quantitative data were expressed as the mean ± standard deviation or median (range), as appropriate. Categorical data between HT and HA groups were compared using the chi-square or Fisher’s exact test, while quantitative data were compared using the Student’s t-test. Survival curves in this study were analyzed using the Kaplan-Meier to measure RFS and OS and compared using the log-rank test. Multivariable Cox regression analyses were used to identify the prognostic significance of the variables to predict RFS and OS. For subgroup analyses, multiple individual Cox models were used separately from treatment comparisons for each factor. Statistical analysis of the data was performed using SPSS V.25.0 (IBM, Armonk, NY, United States) and R V.3.6.11 with the add-on packages survival, forestplot and survminer. A p < 0.05 was considered statistically significant, and all statistical tests were two-sided.
Results
Patients
From January 2012 to August 2014, 472 patients with BCLC stage A HCC underwent curative hepatectomy. Among them, 147 patients were excluded due to the presence of other malignancies (5 patients), loss to follow-up evaluations (130 patients), or HCC recurrences within 2 months (12 patients). Three hundred and twenty-five patients (157 HA patients and 168 HT patients) were included in the study. Then, 95 patients (42 HA patients and 53 HT patients) were excluded using one-to-one matching of the propensity scores to balance baseline differences between two groups. Finally, 230 patients (115 HA patients and 115 HT patients) were enrolled in the analysis. All patients had a good performance status and liver function (ECOG PS 0 and Child-Pugh A). The flow diagram demonstrating the screening and grouping of participants in the study is shown in Figure 1.
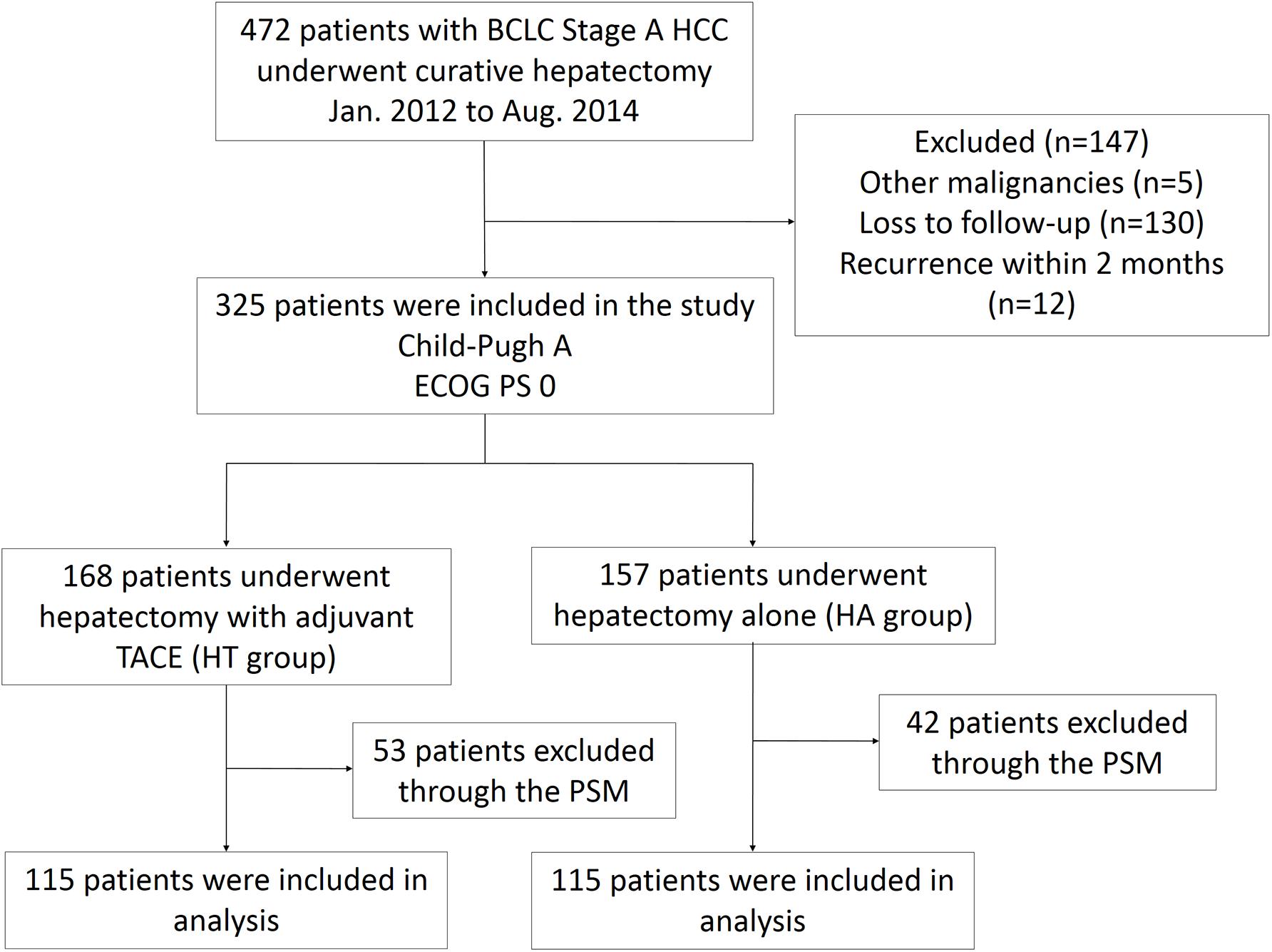
Figure 1. Flow diagram for extracting eligible cases for comparison. BCLC, Barcelona Clinic Liver Cancer; HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; PSM, propensity score matching; ECOG, Eastern Cooperative Oncology Group; PS, performance status.
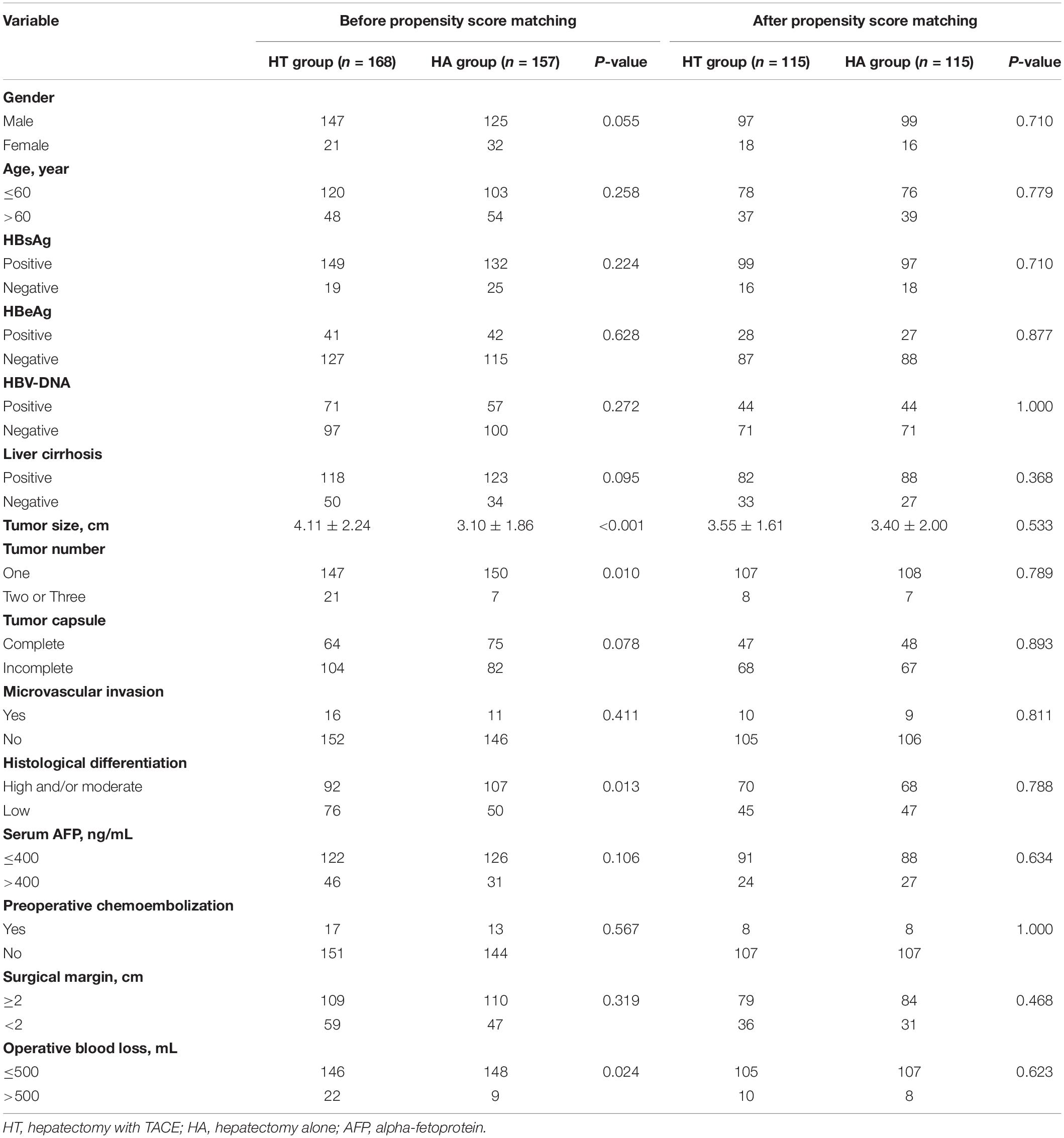
The baseline characteristics of the patients were well-matched between the two groups after PSM. The tumor characteristics, such as the tumor size (3.40 ± 2.00 for HA and 3.55 ± 1.61 for HT), tumor number, AFP level, presence of a tumor capsule, pathologic microvascular invasion, and histologic differentiation also were similar between the two groups (Table 1).
Survival
At the time of censor, 105 patients (45.7%) developed recurrence, 57 patients in the HT group, and 48 patients in the HA group, respectively. 28 patients (12.2%) had died, 15 patients in the HT group, and 13 patients in the HA group died of tumor-related causes.
In the HT group, the 1-, 3-, and 5-year RFS rates were 87.0, 63.5, and 50.4%, respectively. The corresponding figures for the HA group were 87.8, 67.0, and 58.3%, respectively. The RFS difference were not significantly in HT group compared with the HA group (log-rank test, χ2 = 0.891, p = 0.345, Figure 2). The respective 1-, 3-, and 5-year OS rates were 99.1, 93.9, and 87% for the HT group, and 100, 92.2, and 88.7% for the HA group. The OS rates were similar between the HT and HA groups (log-rank test, χ2 = 0.146, p = 0.702, Figure 3).
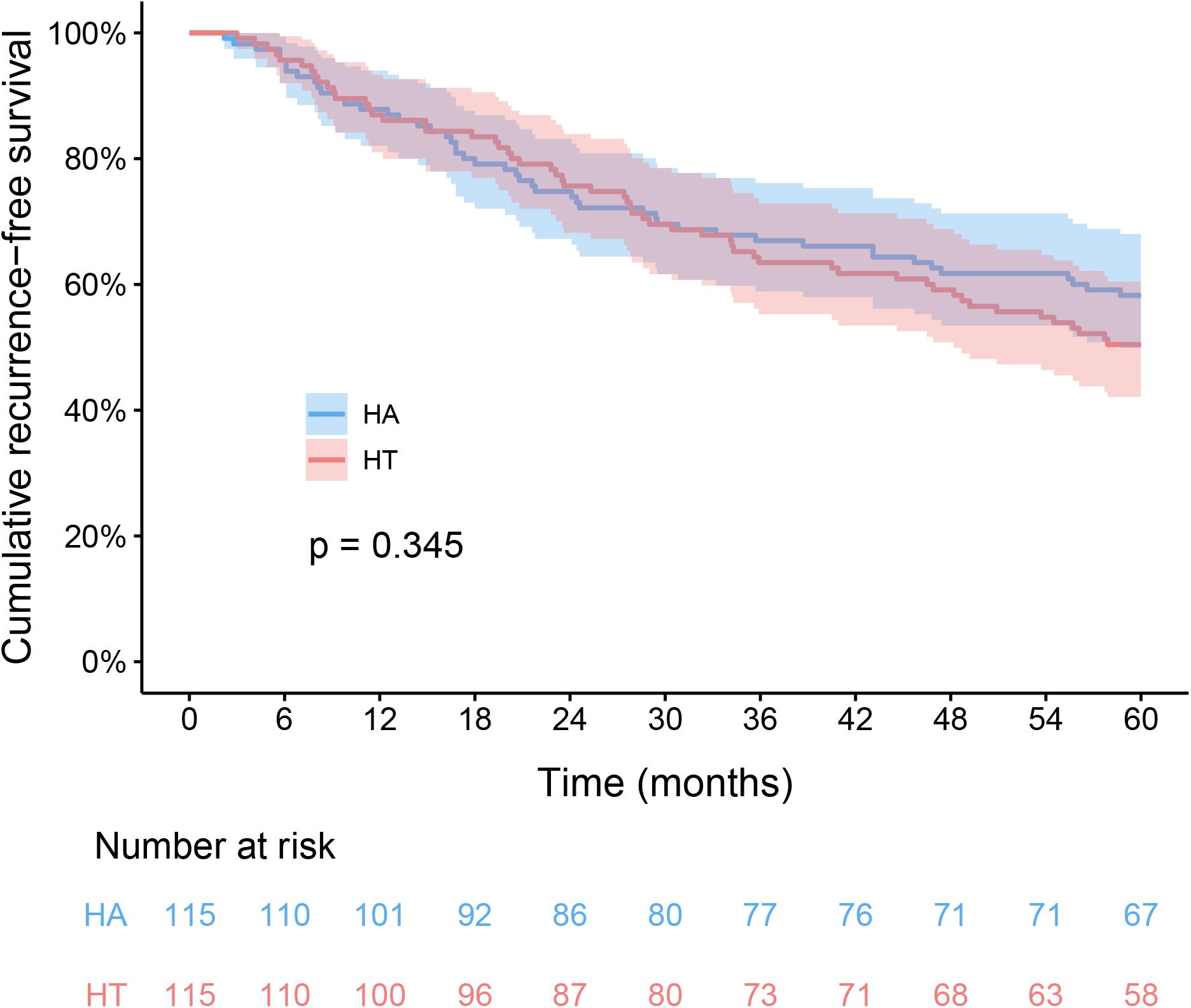
Figure 2. Kaplan-Meier analysis of recurrence-free survival between HT and HA groups. HT, hepatectomy with TACE; HA, hepatectomy alone.
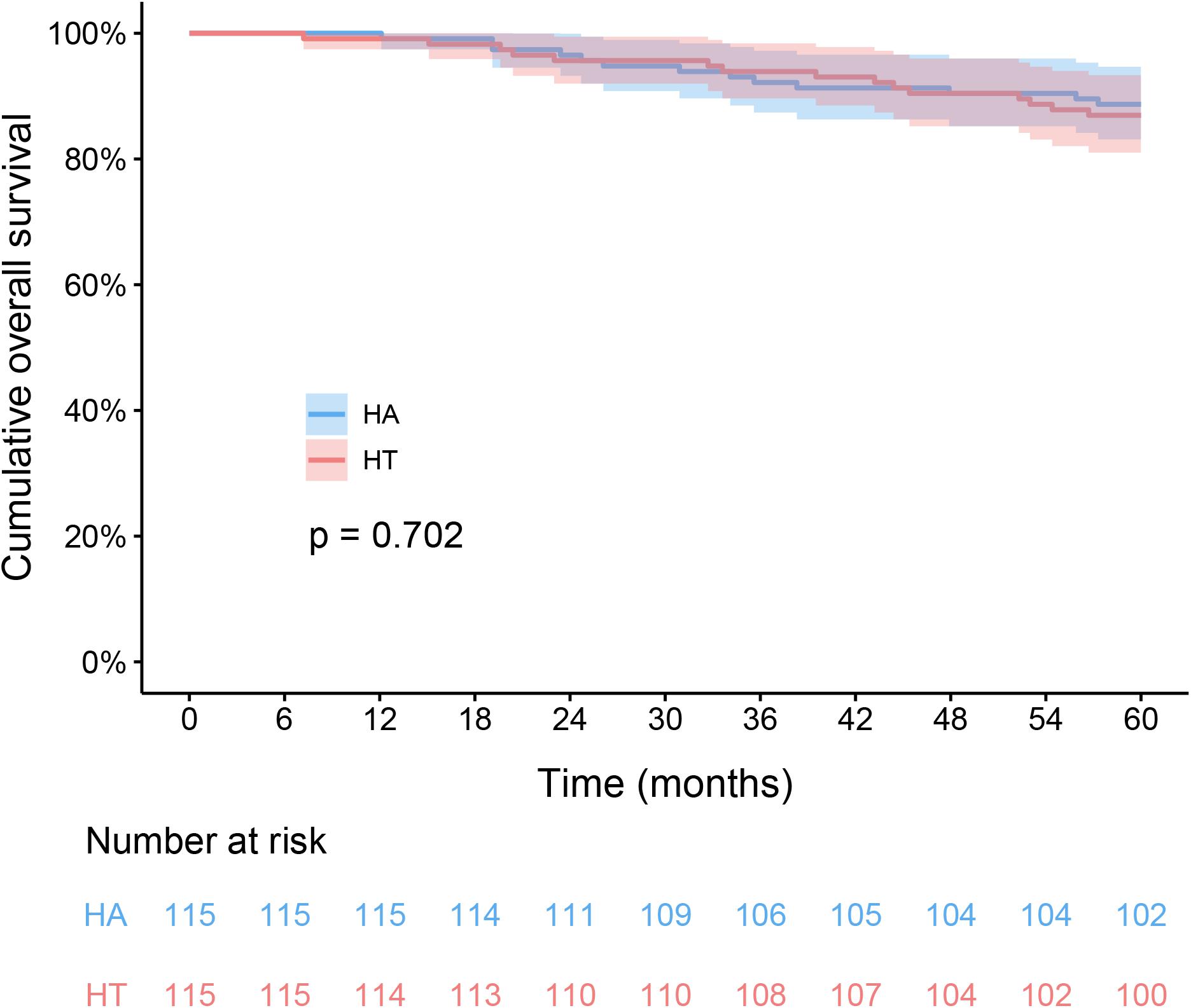
Figure 3. Kaplan-Meier analysis of overall survival between HT and HA groups. HT, hepatectomy with TACE; HA, hepatectomy alone.
In the all-exploratory subgroup analyses, adjuvant TACE did not provide a clinical benefit for RFS [hazard ratio (HR) 1.202; 95% CI 0.819–1.765; p = 0.347]. Despite adjuvant TACE enhanced RFS for operative blood losses >500 ml, a significant difference was not detected in the HT group compared with that of the HA group (HR 0.449; 95% CI 0.136–1.482; p = 0.189, Figure 4). Similarly, in the OS subgroup analyses, a significant benefit from adjuvant TACE was not seen in patients with the following characteristics: ages >60 years (HR 0.581; 95% CI 0.170–1.986; p = 0.387) and operative blood losses >500 ml (HR 0.118; 95% CI 0.014–1.020; p = 0.052) (Figure 5).
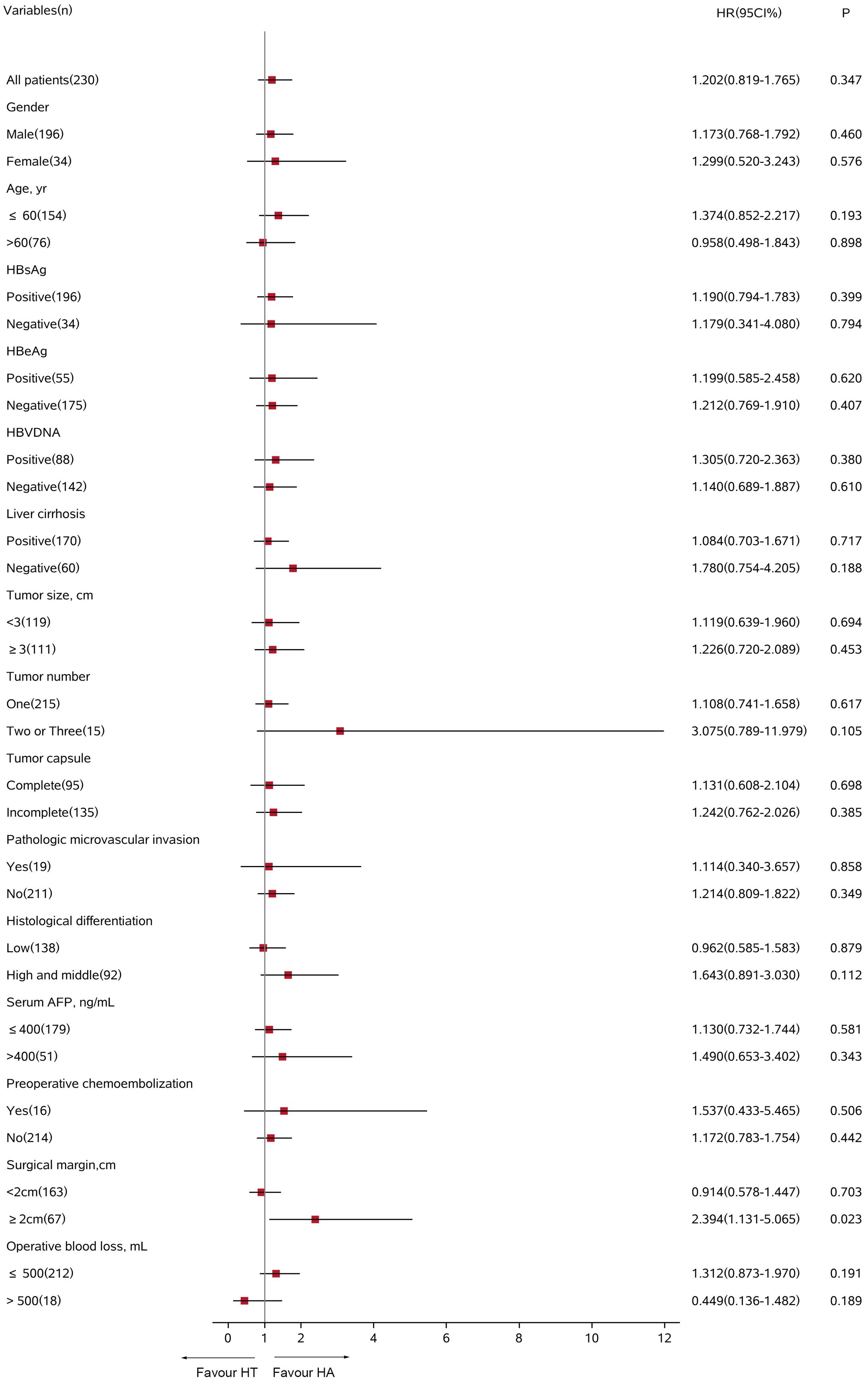
Figure 4. Subgroup analysis for recurrence-free survival between HT and HA groups using Cox regression analysis. HT, hepatectomy with TACE; HA, hepatectomy alone; CI, confidence interval; HR, hazard ratio; AFP, alpha-fetoprotein.
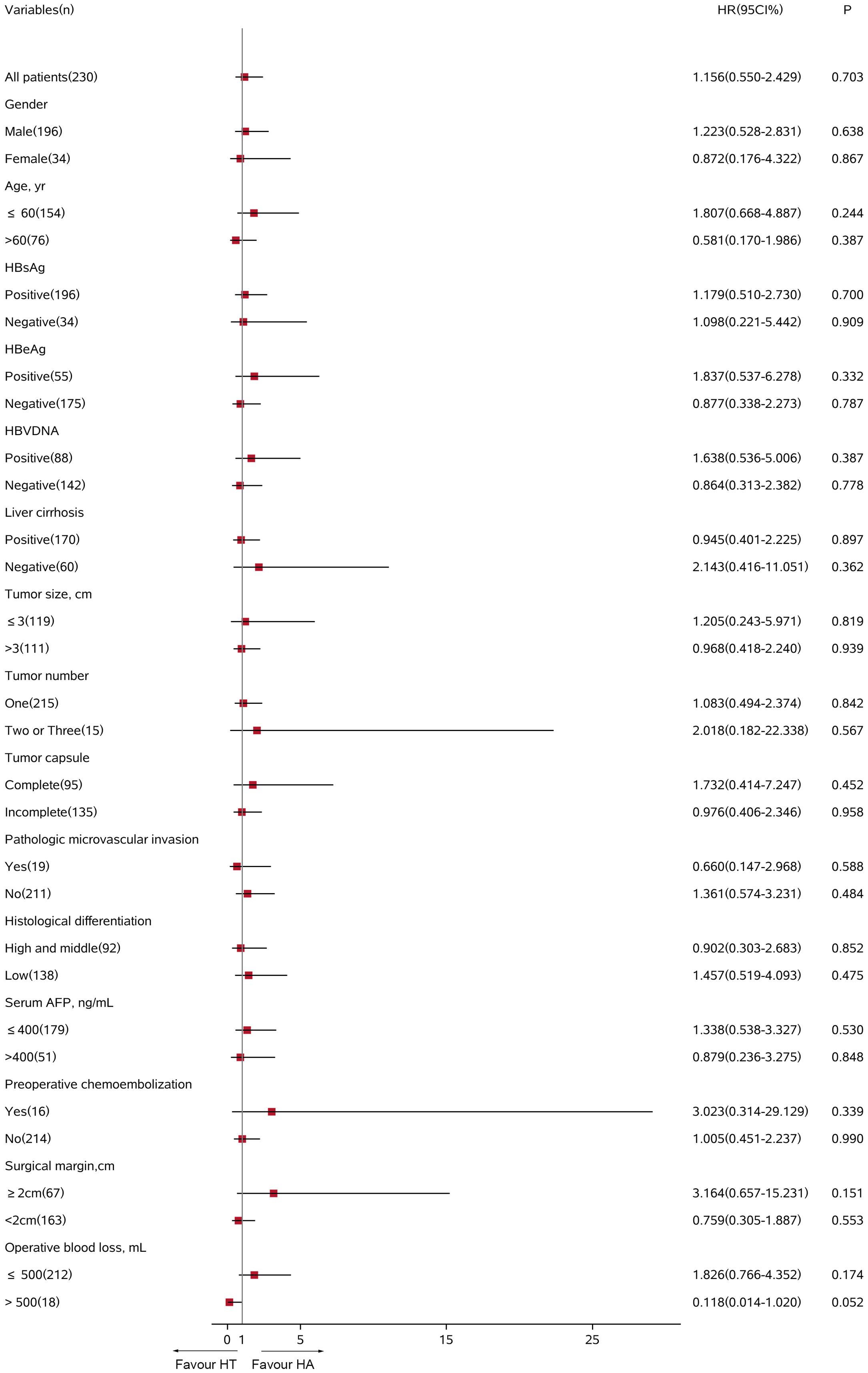
Figure 5. Subgroup analysis for overall survival between HT and HA groups using Cox regression analysis. HT, hepatectomy with TACE; HA, hepatectomy alone; CI, confidence interval; HR, hazard ratio; AFP, alpha-fetoprotein.
Multivariate Cox regression analysis revealed that adjuvant TACE was not the impact factor for RFS or OS (p = 0.399; HR 0.847; 95%CI 0.576–1.245 for RFS vs. p = 0.989; HR 0.995; 95% CI 0.471–2.100 for OS). Tumor size and the presence of microvascular invasion were shown to be significant OS factors (Table 2).
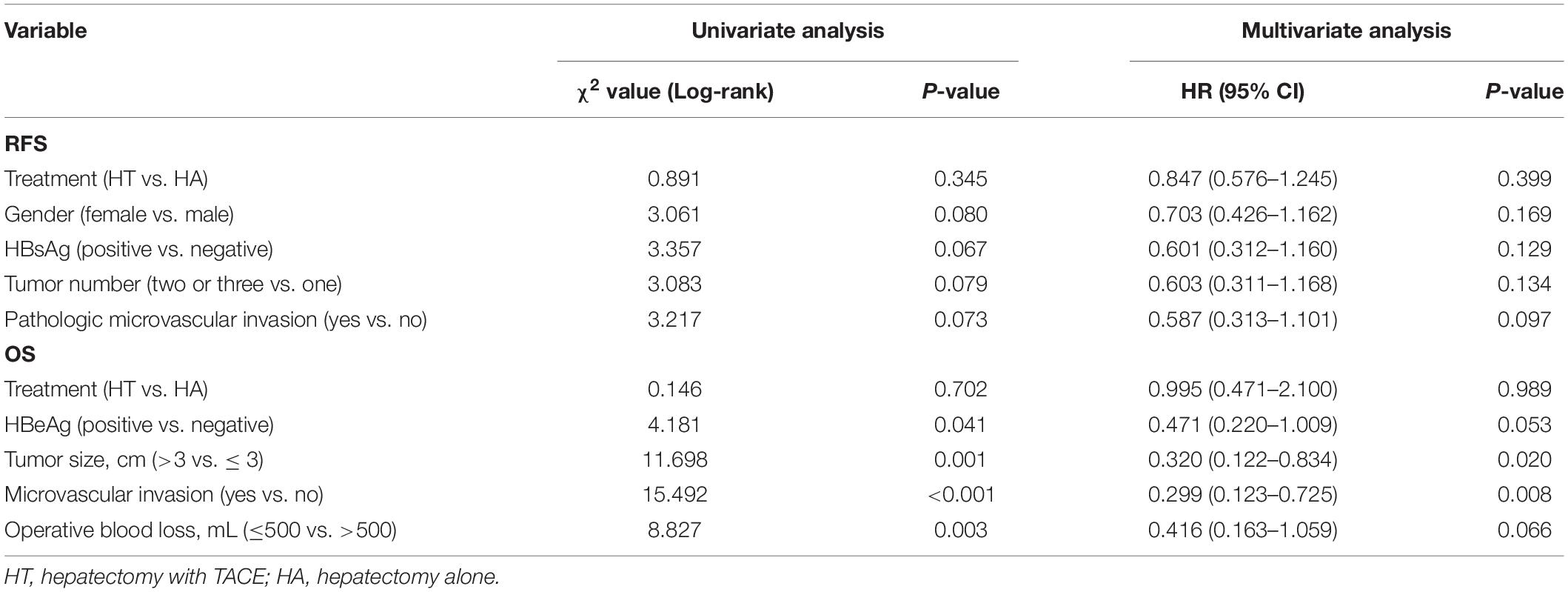
Table 2. Uni- and multivariate analyses of recurrence-free survival (RFS) and overall survival (OS).
Discussion
This study include a large patient cohort. One group received hepatectomy with adjuvant TACE and the other received hepatectomy alone. The result revealed that postoperative adjuvant TACE does not reduce recurrence or improve OS. Although the role of postoperative adjuvant TACE was evaluated to improve the outcomes of resected HCC in several studies, the results have been controversial. Difference in patient selection made the controversial results. A randomized controlled trial revealed that adjuvant TACE after hepatectomy markedly improved the survival outcome of Stage III A HCC patients, including a high proportion of patients with macrovascular invasion (7). However, another prospective, randomized-controlled trial failed to display a significant difference in survival between the two groups, although the main aim of this study was to look into the effect of the dose in the prevention of tumor recurrence (10). Sun et al. (11) retrospectively reported that postoperative adjuvant TACE could prolong the survival of patients with microvascular invasion with 5-year survival rates that increased to 54.0%. However, our study focused on BCLC stage A HCC (single tumor or up to 3 tumors ≤3 cm). Thus, the histopathologic factors of HCC in our study were significantly different from previous studies.
The rationale of adjuvant TACE after curative hepatectomy was to prevent intrahepatic recurrence by killing residual microscopic tumor cells in the remnant live. It can also eliminate tumor cells that might have been shed from tumor masses removed during liver surgery. Our negative results show that adjuvant TACE did not improve the outcomes of BCLC stage A HCC patients are likely related to the following factors. First, immune surveillance to control tumor recurrences and metastases could be responsible. Adjuvant TACE might have depressed host immunity against tumor progression and affected hepatocyte regeneration, resulting in poor overall or recurrence-free survival (12). Second, the recurrent tumors usually have different clones compared with those of the malignant primary tumors (13). By eliminating the subpopulation of drug-sensitive tumor cells, chemotherapy accelerate the formation of new clonal variants from the surviving subpopulation and allow cells to proliferate with higher metastatic capabilities (14).
This study revealed that tumor size and microvascular invasion were both independent prognostic factors of OS, similar to the results of previous studies (15, 16). A large tumor burden is closely associated with increased invasiveness, which was reflected in a higher incidence of microvascular invasion and poor survival (17, 18). Microvascular invasion is present in 20% of tumors 2 cm in size, 30–60% of nodules 2–5 cm in size, and up to 60–90% of nodules larger than 5 cm (19). The proportion of HCC cases with microvascular invasion was only 8.3% in our study, resulting in good long-term survival rates.
There are potential limitations of this study. First, it was a retrospective study with all of the inherent defects of these types of studies and is likely subject to subtle selection biases, even after PSM. Second, this is a single-center study, and the outcome is not the same as patients with BCLC stage A HCC in other countries because of demographics and the underlying causes. The multi-center randomized controlled trials involving patients with BCLC stage A HCC should examine in more detail the effects of postoperative TACE.
Conclusion
Transarterial chemoembolization as adjuvant treatment after hepatectomy for BCLC stage A HCC did not reduce tumor recurrences or improve the overall survival. The adjuvant setting remains an area of high unmet need in HCC management, and further research into strategies to prevent BCLC stage A HCC recurrence is needed.
Data Availability Statement
All datasets presented in this study are included in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Y-LZ and J-HS contributed to conception and design. Y-LZ, C-HN, and FC contributed to development of methodology (provided, acquired, and managed patients, etc.). Y-LZ, T-YaZ, and G-HZ contributed to analysis and interpretation of data (e.g., statistical analysis and computational analysis). Y-LZ, T-YiZ, S-QC, X-HC, H-LW, and J-HS contributed to writing, review, and/or revision of the manuscript. Y-LZ, B-QW, Z-NY, LJ, and Z-MH contributed administrative, technical, or material support (i.e., reporting or organizing the data and constructing databases). All authors contributed to the article and approved the submitted version.
Funding
The present work was funded by the Zhejiang Provincial Natural Science Foundation of China (Grant No. LZ18H180001), National Natural Science Foundation of China (Grant Nos. 81971713 and 81371658), Grant from Health Commission of Zhejiang Province (JBZX-202004), Research Unit of Collaborative Diagnosis and Treatment for Hepatobiliary and Pancreatic Cancer, Chinese Academy of Medical Sciences (2019RU019), and National S&T Major Project (No. 2018ZX10301201). The funding had no role in designing research and collecting, analyzing, interpreting data, or writing manuscripts.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
All authors appreciate Prof. Xinmei Chen for English proofreading.
Footnotes
References
1. Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. (2012) 62:394–9. doi: 10.3322/caac.21161
2. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. (2018) 69:182–236.
3. Marubashi S, Gotoh K, Akita H, Takahashi H, Ito Y, Yano M, et al. Anatomical versus non-anatomical resection for hepatocellular carcinoma. Br J Surg. (2015) 102:776–84.
4. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the american association for the study of liver diseases. Hepatology. (2018) 68:723–50. doi: 10.1002/hep.29913
5. Dong ZR, Zhang PF, Wang CH, Zhang C, Cai JB, Shi GM, et al. Postoperative adjuvant transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond the Milan criteria: a retrospective analysis. Am J Cancer Res. (2014) 5:450–7.
6. Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res. (2018) 24:2074–81. doi: 10.1158/1078-0432.ccr-17-2899
7. Zhong C, Guo RP, Li JQ, Shi M, Wei W, Chen MS, et al. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for Stage III A hepatocellular carcinoma. J Cancer Res Clin Oncol. (2009) 135:1437–45. doi: 10.1007/s00432-009-0588-2
8. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. (1999) 19:329–38. doi: 10.1055/s-2007-1007122
9. Zou H, Zhu CZ, Wang C, Wang ZS, Ma X, Han B, et al. Recurrence of barcelona clinic liver cancer stage a hepatocellular carcinoma after hepatectomy. Am J Med Sci. (2017) 354:262–7. doi: 10.1016/j.amjms.2017.05.014
10. Kwok PC, Lam TW, Lam PW, Tang KW, Chan SC, Hwang JS, et al. Randomized controlled trial to compare the dose of adjuvant chemotherapy after curative resection of hepatocellular carcinoma. J Gastroenterol Hepatol. (2003) 18:450–5. doi: 10.1046/j.1440-1746.2003.03015.x
11. Sun JJ, Wang K, Zhang CZ, Guo WX, Shi J, Cong WM, et al. Postoperative adjuvant transcatheter arterial chemoembolization after R0 hepatectomy improves outcomes of patients who have hepatocellular carcinoma with microvascular invasion. Ann Surg Oncol. (2016) 23:1344–51. doi: 10.1245/s10434-015-5008-z
12. Mazzola A, Costantino A, Petta S, Bartolotta TV, Raineri M, Sacco R, et al. Recurrence of hepatocellular carcinoma after liver transplantation: an update. Future Oncol. (2015) 11:2923–36.
13. Kwon T, Kwon OS, Cha HJ, Sung BJ. Stochastic and heterogeneous cancer cell migration: experiment and theory. Sci Rep. (2019) 9:16297.
14. McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. (2017) 168:613–28. doi: 10.1016/j.cell.2017.01.018
15. Wang H, Feng LH, Qian YW, Cao ZY, Wu MC, Cong WM. Does microvascular invasion in Barcelona Clinic Liver Cancer stage A multinodular hepatocellular carcinoma indicate early-stage behavior? Ann Transl Med. (2019) 7:428. doi: 10.21037/atm.2019.08.114
16. Zhang Y, Chen SW, Liu LL, Yang X, Cai SH, Yun JP. A model combining TNM stage and tumor size shows utility in predicting recurrence among patients with hepatocellular carcinoma after resection. Cancer Manag Res. (2018) 10:3707–15. doi: 10.2147/cmar.s175303
17. Zhang X, Li J, Shen F, Lau WY. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. (2018) 33:347–54. doi: 10.1111/jgh.13843
18. Truant S, Boleslawski E, Duhamel A, Bouras AF, Louvet A, Febvay C, et al. Tumor size of hepatocellular carcinoma in noncirrhotic liver: a controversial predictive factor for outcome after resection. Eur J Surg Oncol. (2012) 38:1189–96. doi: 10.1016/j.ejso.2012.07.112
Keywords: hepatocellular carcinoma, hepatectomy, recurrence, survival, transarterial chemoembolization
Citation: Zhang Y-L, Nie C-H, Chen F, Zhou T-Y, Zhou G-H, Zhu T-Y, Chen S-Q, Chen X-H, Wang H-L, Wang B-Q, Yu Z-N, Jing L, He Z-M and Sun J-H (2020) Adjuvant Transarterial Chemoembolization for Barcelona Clinic Liver Cancer Stage A Hepatocellular Carcinoma After Hepatectomy. Front. Oncol. 10:1754. doi: 10.3389/fonc.2020.01754
Received: 09 April 2020; Accepted: 05 August 2020;
Published: 02 September 2020.
Edited by:
Marco Scarpa, University Hospital of Padua, ItalyReviewed by:
Xiaowu Li, Shenzhen University General Hospital, ChinaHe Jin Guo, Zhongda Hospital, China
Weijun Fan, Sun Yat-sen University Cancer Center (SYSUCC), China
Copyright © 2020 Zhang, Nie, Chen, Zhou, Zhou, Zhu, Chen, Chen, Wang, Wang, Yu, Jing, He and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun-Hui Sun, MTMwNzAwNUB6anUuZWR1LmNu
 Yue-Lin Zhang
Yue-Lin Zhang Chun-Hui Nie
Chun-Hui Nie Feng Chen
Feng Chen Tan-Yang Zhou
Tan-Yang Zhou Guan-Hui Zhou
Guan-Hui Zhou Tong-Yin Zhu
Tong-Yin Zhu Sheng-Qun Chen
Sheng-Qun Chen Xin-Hua Chen
Xin-Hua Chen Hong-Liang Wang
Hong-Liang Wang Bao-Quan Wang
Bao-Quan Wang Zi-Niu Yu
Zi-Niu Yu Li Jing
Li Jing Zhi-Min He
Zhi-Min He Jun-Hui Sun
Jun-Hui Sun