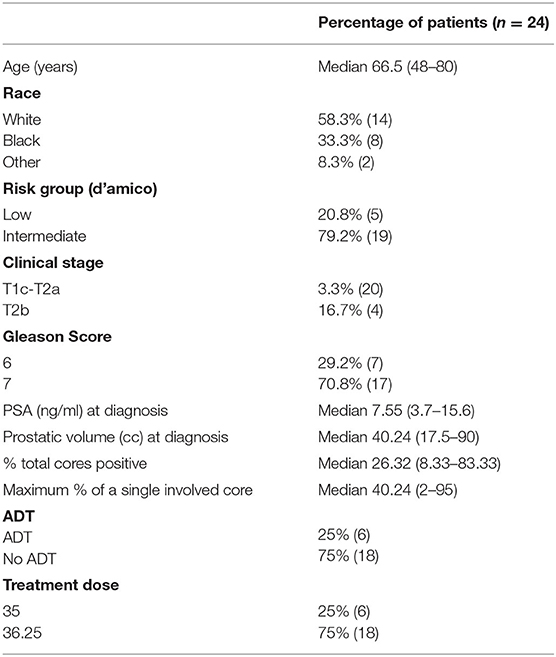
- 1Department of Radiation Medicine, Georgetown University Hospital, Washington, DC, United States
- 2George Washington School of Medicine and Health Sciences, Washington, DC, United States
- 3Georgetown University School of Medicine, Washington, DC, United States
- 4Columbia University Valegos College of Physicians and Surgeons, New York, NY, United States
- 5Department of Radiation Oncology, University of California, Los Angeles, Los Angeles, CA, United States
- 6Geisinger Commonwealth School of Medicine, Scranton, PA, United States
- 7Julius L. Chambers Biomedical/Biotechnology Research Institute, North Carolina Central University, Durham, NC, United States
- 8Department of Radiology, Georgetown University Hospital, Washington, DC, United States
- 9Department of Urology, Georgetown University Hospital, Washington, DC, United States
Background: Stereotactic body radiation therapy (SBRT) is a safe and effective treatment option for patients with low to intermediate risk prostate cancer (1). SBRT results in very low PSA nadirs secondary to the delivery of high biologically effective doses. Studies reporting on the diagnosis, confirmation, and management of salvageable isolated local failures (ILF) are limited. This study aims to determine the incidence and management approach of ILF after SBRT in a large single institution cohort.
Method: All patients with low or intermediate risk localized prostate cancer treated with SBRT at Georgetown University Hospital were eligible for this study. Treatment was delivered using robotic SBRT with doses of 35–36.25 Gy in five fractions. ILF were diagnosed using multiparametric MRI and/or biopsy prompted by rising PSA levels after achieving long-term nadir. Patient's characteristics were extracted from a prospective institutional quality of life trial (IRB 2009-510). Type of salvage therapy and post-salvage PSA were determined on subsequent follow-up and chart review.
Results: Between December 2008 to August 2018, 998 men with low to intermediate risk prostate cancer were eligible for inclusion in this analysis. Twenty-four patients (low risk, n = 5; intermediate risk, n = 19) were found to have ILF within the prostate on either MRI (n = 19) and/or biopsy (n = 20). Median pre-treatment PSA was 7.55 ng/ml. Median time to diagnosis of ILF was 72 months (24–110 months) with median PSA at the time of ILF of 2.8 ng/ml (0.7–33 ng/ml). Median PSA doubling time was 17 months (5–47 months). Thirteen patients with biopsy proven ILF proceeded with salvage therapy (cryotherapy n = 12, HIFU n = 1). Of 12 patients who underwent cryotherapy, 7 had a post-treatment PSA of <0.1 ng/ml. One patient experienced a urethral-cutaneous fistula (grade 3 toxicity).
Conclusion: The incidence of isolated local recurrence is rare in our cohort. Diagnosis and management of isolated local failures post-SBRT continues to evolve. Our report highlights the importance of early utilization of MRI and confirmatory biopsy at relatively low PSA levels and long PSA doubling time (1). Additionally, undetectable PSA levels after salvage therapy supports the role of early treatment in ILF (1). Further research is needed to determine appropriate patient selection and salvage modality in this population.
Introduction
External beam radiation therapy (EBRT) traditionally results in slow prostate specific antigen (PSA) declines that stabilize with time (2). Undetectable PSA levels following treatment are infrequent as EBRT does not fully ablate normal prostate tissue. Although EBRT achieves high early biochemical relapse free survival rates, patients may experience biochemical failure many years after undergoing treatment (3, 4). PSA rises are utilized for the early detection of recurrent disease and commonly occur years prior to clinical failure. Per the Phoenix criteria, biochemical failure is defined as at least 2 ng/mL rise in PSA above the nadir (5). Favorable prostate cancer most commonly recurs at the site of the initial tumor in the prostate bed (6–8). Local failure following definitive radiotherapy indicates a poorer prognosis (9). If left untreated, these local recurrences could lead to distant metastases and a prostate cancer-specific death (10, 11).
Brachytherapy is an ablative procedure with very low PSA nadirs (< 0.01 ng/ml) and excellent long-term outcomes with 7-year biochemical disease-free survival rates at 93–95% (12). It has recently been reported that a PSA of < 0.2 ng/ml following brachytherapy predicts for a very high probability of cure (13). One study found that individuals with a stable PSA had a median nadir of 0.03 ng/ml while those who experienced biochemical failure had a PSA median nadir of 0.5 ng/ml (14). A rising PSA after brachytherapy treatment of favorable prostate cancer by an experienced practitioner is rare.
Stereotactic body radiation therapy (SBRT) has been shown to be a safe and effective treatment option for patients with low to intermediate risk prostate cancer (15). SBRT delivers high biologically effective doses in four to five treatment sessions with 7-year biochemical disease-free survival rates in low to intermediate risk prostate cancer of 90–95% (15). PSA nadirs are lower with brachytherapy and SBRT than EBRT (16, 17). The Phoenix criteria was developed for low dose conventionally fractionated EBRT and may not be appropriate for such ablative therapies (18). Studies on the kinetics of PSA after SBRT have shown PSA rises following SBRT may reflect a benign PSA bounce or local and/or distant recurrence (16).
Strategies to optimize the accurate early identification of salvageable isolated local failures (ILF) following SBRT are needed. In general, isolated local failures occur many years post-RT (> 3 years) and are associated with long PSA doubling times (> 12 months) (19, 20). Detecting local recurrence using T2 weighted MRIs poses a challenge secondary to radiation-associated imaging changes (21). The advent of multiparametric magnetic resonance imaging (mpMRI) has enhanced the localization of prostate cancer recurrence by utilizing the combination of multiple imaging modalities (22). Previous studies suggest MRI improves localization of cancer recurrence in the setting of biochemical failure after EBRT and brachytherapy (7, 23–28).
After detection and localization of ILF after radiation therapy, multiple options for salvage therapy are available, including cryoablation and high-intensity focused ultrasound (HIFU) (29–39). Outcomes from the Cryotherapy Online Data (COLD) registry showed a 5-year actuarial biochemical disease-free rates of ~50% with focal/whole gland salvage cryoablation (40, 41). Post-cryotherapy PSA nadir < 0.5 ng/ml is the best predictor of post-cryotherapy cancer control (42). Studies on salvage therapy following SBRT are limited. Given the diagnosis and management of ILF continues to evolve in the era of successful salvage therapy, this study aims to determine the incidence and the approach to diagnosis and management of ILF after SBRT in a large single institution cohort.
Methods
Patient Selection and Data Collection
All individuals diagnosed with low to intermediate risk prostate cancer treated at MedStar Georgetown University Hospital between December 2008 and August 2018 were eligible for inclusion in this study. Eligible patients were required to have documented isolated local failures in the prostate and/or seminal vesicles on either mpMRI and/or TRUS biopsy following rising PSA. Patients were stratified by risk using the D'Amico classification (43). Individuals with high-risk prostate cancer were excluded from our study due to high rates of concurrent metastatic disease. Patients with nodal and/or bone metastases at time of local recurrence were also excluded. Subsequent salvage therapy and post-salvage PSA were determined by chart review. The Georgetown University Institutional Review Board (IRB) approved this single institutional retrospective review (IRB# 2009-510).
SBRT Treatment Planning and Delivery
Simulation, contouring, and treatment planning were conducted from a previously reported institutional protocol (44). One week after placement of 4 to 6 gold fiducial markers into the prostate, patients underwent a CT and MRI for treatment planning. The clinical target volume (CTV) included the prostate and proximal seminal vesicles up to the point where the seminal vesicles separated. The planning target volume (PTV) was equal to the CTV expanded to 3 mm posteriorly and 5 mm in all other directions. The prescription isodose line was limited to >75% to restrict the maximum prostatic urethra dosage to 133% of the prescribed dose. Inverse planning was created with a prescription dose of 35–36.25 Gy in 5 fractions corresponding to an EQD2 of ~85–90 Gy assuming an alpha/beta ratio of 1.5. Treatment was delivered using CyberKnife robotic radiosurgical system (Accuray Inc, Sunnyvale, CA, USA). Given the dose inhomogeneity, it is possible to prescribe up to 40 Gy to the region of the glad with any notable lesion seen on MRI.
Patient Follow-Up
PSA levels were obtained prior to treatment, every 3 months post-SBRT for 1 year, every 6 months for the following 2 years, and then yearly. PSA nadirs were determined and defined as the lowest PSA prior to failure. If PSA levels rose to >1 ng/ml, a digital rectal exam (DRE) was performed and a mpMRI was obtained. If there were abnormalities on the DRE and/or mpMRI, a biopsy was recommended. Following identification of ILF, patients were expectantly managed or underwent salvage therapy including HIFU and cryotherapy. Post-salvage PSA was determined. Toxicities were assessed following salvage therapy and scored using the common terminology for adverse events version 4 (CTCAE v4). This cohort predates the widespread use and availability of PSMA/Choline PET in the USA.
Composite Representation of Localized Failures
To determine the most common locations for isolated local failures following prostate SBRT, post-SBRT imaging reports and prostate biopsies were analyzed. A sample prostate MRI at the time of ILF and the most commonly utilized sequences are represented in Figure 1. Localized failure location was coded into data fields which represented prostate sections (apex, midgland, and base) and prostatic zones (peripheral, central, transitional, and anterior fibromuscular stroma). Data collection occurred in a sequential manner. First, mpMRI reports were examined. If data fields were missing, prostate biopsy data was examined to complete the data fields. If neither mpMRI nor biopsy reports were available, data from other imaging modalities were employed. mpMRI data was sufficient in 14 of 20 patients, biopsy data was used in 5 patients, and ultrasound was used for 1 patient. The degree of involvement of a section and zone combination was determined based on the number of patients with involvement divided by the number of total patients (those with and without involvement). Figure 3 and supplemental data.
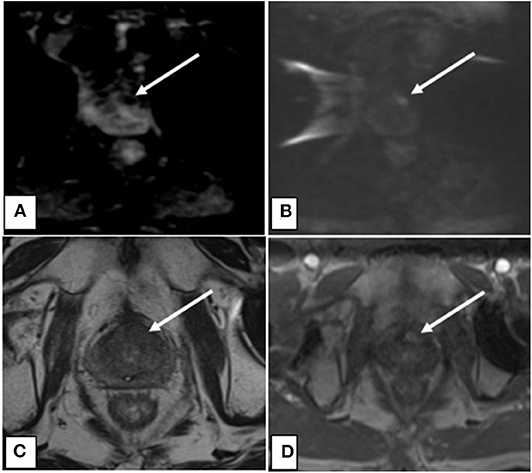
Figure 1. (A) Axial ADC map showing decreased signal intensity associated with lesion indicative of restricted diffusion. (B) Axial DWI showing increased signal intensity associated with lesion indicative of restricted diffusion. (C) Axial T2 showing decreased signal intensity associated with the lesion. (D) Axial DCE with contrast showing increased perfusion to the lesion.
Results
Between December 2008 and August 2018, 998 men with low to intermediate risk prostate cancer were eligible for inclusion in this study. Isolated local failures were identified in 24 patients (2.4%) at a median follow-up of 84 months. Characteristics of the patients who experienced ILF are shown in Table 1. The median age was 66.5 (range 48–80). This patient cohort was diverse. Approximately 58% of the population was white and 33% was black. The median pre-SBRT PSA was 7.55 ng/ml (3.7–15.6 ng/ml). Approximately 21% of individuals were low-risk and 79% of individuals were intermediate risk. Neoadjuvant androgen deprivation therapy was employed in 25% of the cohort. Seventy-five percent were treated with 36.25 Gy in 5 fractions, while the remaining 25% underwent 35 Gy in 5 fractions.
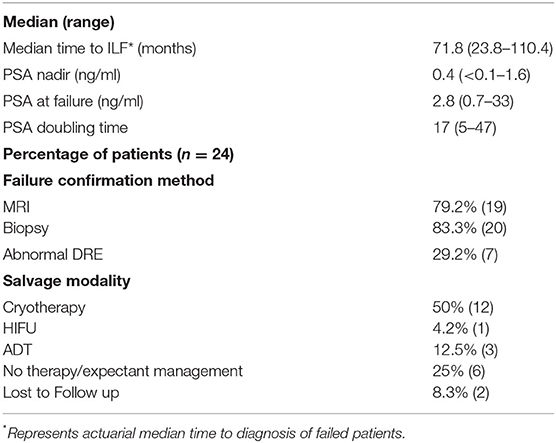
In general, the PSAs declined rapidly and then rose slowly most commonly between 3 and 8 years post-SBRT (Table 2; Figure 2A). The median post-SBRT PSA nadir was 0.4 ng/ml with a median time to diagnosis of ILF was 71.8 months (23.8–110.4 months). The median PSA at failure was 2.8 ng/ml (0.7–33 ng/ml) (Figure 2B) with a median PSA doubling time of 17 months (5–47 months). Approximately 29% of the included individuals were noted to have an abnormal DRE at time of ILF. ILF was confirmed by mpMRI (79.2%) and/or TRUS biopsy (83.3%).
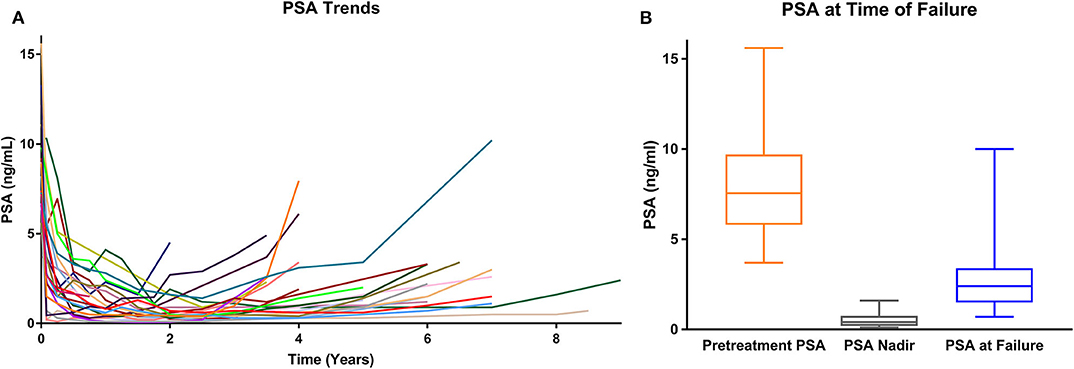
Figure 2. (A) PSA values over time for each isolated local failure. (B) PSA values at time of failure (whisker box plot).
In our series, the location of mpMRI and biopsy confirmed lesions were shown to be concordant (Table 3). The sites of local recurrence documented by mpMRI and/or biopsy did not represent any one region of the prostate more than another (Table 3). The degree of involvement represented was color-coded into a heat map where the most involved areas were represented as red and the least involved were white (Figure 3). As seen previously by others, there was a trend in increased positivity from the base to the apex (45). However, there was no statistical difference in the distribution of the involved areas: apex (74%), midgland (74%) and base (53%). Of note, 25% of the patients had multifocal disease.
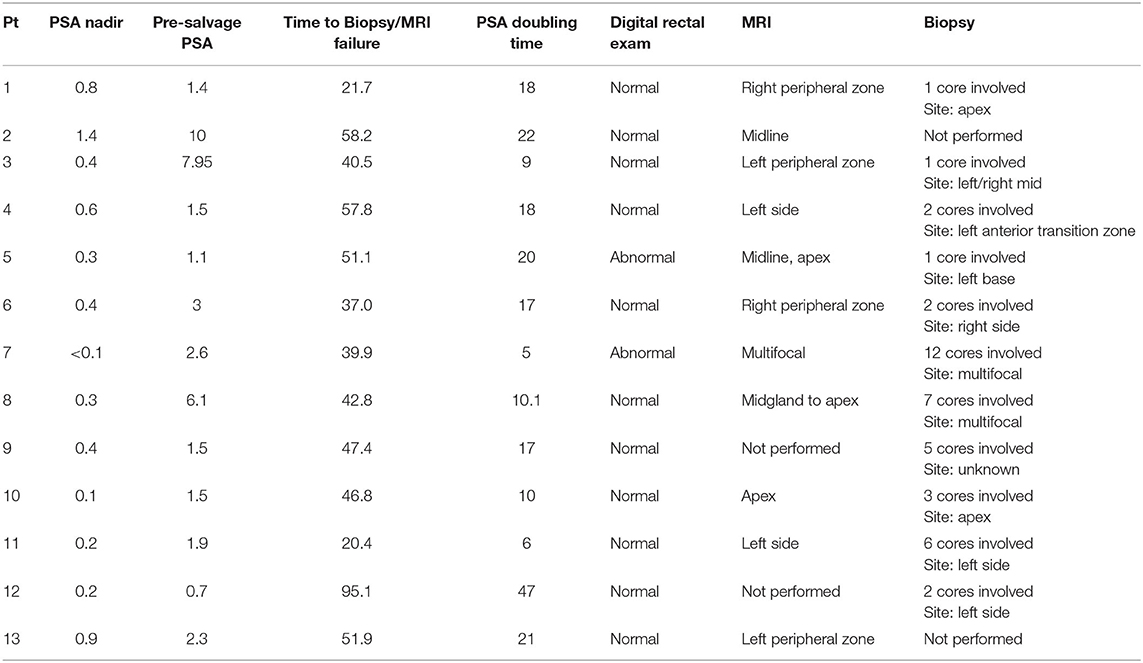
Table 3. Evaluation of individuals who experienced an isolated local failure and later underwent salvage treatment after being treated with SBRT for their prostate cancer.
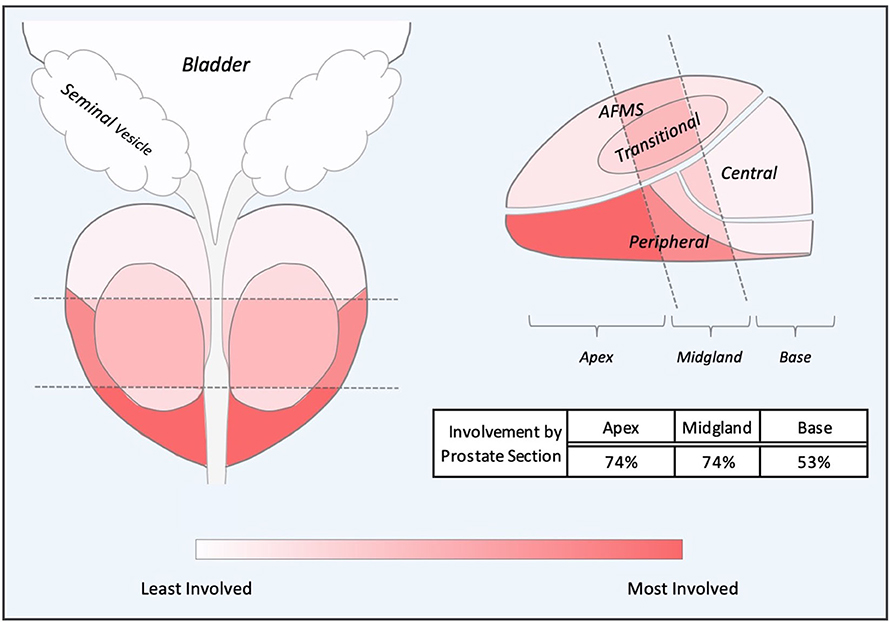
Figure 3. Composite Representations of Localized Failures. A depiction of the prostate, seminal vesicles and bladder shown in coronal and sagittal orientations without laterality. The prostate is subdivided into apex, midgland, and base sections and further subdivided into peripheral, transitional, central and anterior fibromuscular stroma (AFMS) prostatic zones. Sections and zones are overlaid with a color-represented heatmap representing degree of cancer recurrence involvement.
Thirteen patients underwent salvage therapy to treat these lesions including 92% (n = 12) who underwent cryotherapy and 8% (n = 1) who underwent HIFU (Table 2). Of the remaining individuals who chose not to proceed with salvage therapy, three individuals chose ADT, six were expectantly managed, and two were lost to follow up (Table 2). Pre-salvage PSA ranged from 0.7 to 10 ng/ml (Table 3). Post-salvage PSA ranged from <0.1–6.7 ng/ml (Table 4). Of the known post-salvage PSA readings, 58% achieved undetectable limits (Table 4). In general, whole gland salvage produced lower nadirs than focal therapy. One person experienced a urethral-cutaneous fistula (grade 3 toxicity) following whole gland cryotherapy (Table 4).
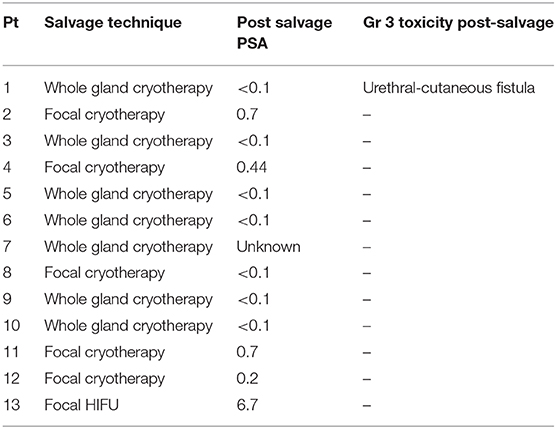
Table 4. Choice of salvage therapy and subsequent PSA and grade 3 toxicities in individuals experiencing an isolated failure after being treated with SBRT for their prostate cancer.
Discussion
Isolated local failures were rare in this study. This is likely secondary to the high biologically effective doses (BED) administered (~200 Gy) and our inhomogeneous treatment plans which provides an intraprostatic dose of >40 Gy (44, 46). This is consistent with a recently reported Phase I dose escalation study in low to intermediate risk patients (47). The 5-year biochemical failure rates were 15, 6, 0, and 0% for 32.5 Gy, 35 Gy, 37.5 Gy, and 40 Gy, respectively (47). Unlike low dose rate brachytherapy, we did not experience any isolated bladder neck/seminal vesicle failures (48). This could be due to the exclusion of high risk patients and inclusion of the proximal seminal vesicles in the high dose volume (44). Notably, our study did not demonstrate any one area of the prostate where recurrence was occurring more frequently (6, 45). In the opinion of the authors, the recurrences identified in this study were likely secondary to innate radiation resistance rather than inadequate treatment margins (49). In the future, it will be important to determine if MRI-guided intraprostatic dose escalation can overcome innate radiation resistance and reduce local recurrences.
Similar to prior studies with alternative forms of radiation, isolated local failures were found to occur several years post-RT with long PSA doubling times (19, 20). In our population, median time to ILF was ~6 years with a median PSA doubling time of 17 months. As we continue to follow this patient cohort, we expect that isolated local recurrences will continue to occur and the median time to recurrence will increase. A recent study has suggested that a PSA nadir of < 0.2 at 4 years post treatment is the definition of cure following brachytherapy (13). In our opinions, this presumption is dangerous. At least two of our patients who were presumably cured developed isolated local failures at a later time.
The Phoenix definition for treatment failure may not be appropriate following SBRT in the era of salvage therapy. Early identification of local recurrences may increase the probability curable of salvage therapy (9). In this study, isolated recurrences were identified by MRI at a PSA of lower than 2 ng/ml in 25% of patients. Additionally, 75% of our patients achieved a post-salvage PSA nadir of < 0.5 ng/ml suggesting that we appropriately selected patients for potentially morbid salvage treatment. Morbidity was acceptable with only one patient experiencing Grade 3 toxicity.
Previous investigations have examined the use of MRI technology in the early detection of localized prostate failure. Detecting local recurrence using T2 weighted MRIs can be a challenge as they frequently show recurrences as a hyposignal in the setting of a diffuse hyposignal post-radiation prostate (21). mpMRI combines several techniques including T1-weighted, T2-weighted, diffusion-weighted imaging, dynamic contrast-enhancing imaging, and magnetic resonance spectroscopy to localize and stage recurrent prostate cancer. The FORECAST trial is currently underway assessing the accuracy of mpMRI-targeted biopsy in radiorecurrent prostate cancer (50). Our study employed a combination of mpMRI and TRUS-biopsies in the detection of isolated local failure in men treated with prostate cancer. We found mpMRI was able to detect lesions with PSA levels as low as 1.1 ng/ml.
In men with very slowly rising PSA values, it may take years for recurrences to develop into metastases and cause a patient's death (8). Many of the men with slowly rising PSA will not survive long enough to experience much of the morbidity and mortality of recurrent prostate cancer. As such, a risks vs. benefits assessment must be made as to whether the patient should undergo salvage treatment vs. expectant management. In our cohort, six men decided to proceed with observation. Mean PSA doubling time in individuals expectantly managed was 17.5 months (range 6–45.6 months). Consideration of doubling time in the context of a patient's age and comorbidities may prove critical to selecting salvage treatment vs. observation.
There are several limitations to our study. Isolated local failures were rare making it difficult to perform robust statistical analyses. In addition, most patients did not undergo fluciclovine/prostate specific membrane antigen (PSMA) PET scans at the time of first failure due to lack of availability in the United States. Additionally brachytherapy and prostatectomy, which is a viable option for patients experiencing local failure, was not offered to our patient cohort (51). This typically reflects patient preference to minimally invasive procedures. Despite this, many of these failures were likely isolated local recurrences due to low PSA nadirs following salvage treatment.
Conclusions
The incidence of isolated local recurrence is rare in our population. Diagnosis and the management of isolated local failures after receiving SBRT for treatment of prostate cancer continues to evolve. Our findings suggest a role for the utilization of mpMRI and confirmatory biopsy in this patient population. Additionally, undetectable PSA post-salvage therapy supports early treatment of isolated local failures.
Data Availability Statement
The datasets presented in this article are not readily available due to patient privacy concerns. Requests to access the datasets should be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Georgetown University IRB#2009-510. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AP and NA were the lead authors, who participated in data collection, data analysis, manuscript drafting, table/figure creation, and manuscript revision. MC aided in figure creation and contributing to data collection. CS and PK aided in figure creation and manuscript drafting. KH and ND aided in data collection. MD contributed to study design and clinical data collection. SL developed the SBRT treatment plans and contributed to data analysis. JL, GB, RH, BC, and JL aided in review of the manuscript. SS is a senior author who organized the data and participated in its analysis. DK participated in data analysis and manuscript review. SC was the principal investigator who initially developed the concept of the study and the design, aided in data collected, and drafted and revised the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
The Department of Radiation Medicine at Georgetown University Hospital receives a grant from Accuracy to support a research coordinator. We gratefully acknowledge the grant R01MD012767 from the National Institute on Minority Health and Health Disparities (NIMHD), NIH to DK and SC. This work was supported by The James and Theodore Pedas Family Foundation. Portions of this research were presented in abstract form at ASCO-GU 2019 (1).
Conflict of Interest
SC and BC serve as a clinical consultant to Accuray Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ADT, androgen deprivation therapy; BED, biologically effective dose; CCI, Charlson Comorbidity Index; CT, computerized tomography; CTC, Common Toxicity Criteria; CTV, clinical target volume; DVH, dose-volume histogram; EBRT, external beam radiation therapy; EPIC, expanded prostate cancer index composite; GU, genitourinary; HIFU, high intensity focused ultrasound; ILF, isolated local failure; IMRT, intensity modulated radiation therapy; IPSS, international prostate symptom score; mpMRI, multiparametric MRI; MRI, magnetic resonance imaging; PET, positron emission tomography; PSA, prostate specific antigen; PSMA, prostate specific membrane antigen; PTV, planning target volume; SBRT, stereotactic body radiation therapy; TRUS, transrectal ultrasound.
References
1. Drescher N, Aghdam N, Danner M, Ayoob M, Yung TM, Lei S, et al. Management of isolated local failures following stereotactic body radiation therapy for low to intermediate risk prostate. JCO. (2019) 37(Suppl. 7):66. doi: 10.1200/JCO.2019.37.7_suppl.66
2. Ray ME, Thames HD, Levy LB, Horwitz EM, Kupelian PA, Martinez AA, et al. PSA nadir predicts biochemical and distant failures after external beam radiotherapy for prostate cancer: a multi-institutional analysis. Int J Radiat Oncol Biol Phys. (2006) 64:1140–50. doi: 10.1016/j.ijrobp.2005.07.006
3. Rosenbaum E, Partin A, Eisenberger MA. Biochemical relapse after primary treatment for prostate cancer; studies on natural history and therapeutic considerations. J Natl Compr Canc Netw. (2004) 2:249–56. doi: 10.6004/jnccn.2004.0022
4. Spratt DE, Pei X, Yamada J, Kollmeier MA, Cox B, Zelefsky MJ. Long-term survival and toxicitiy in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. (2013) 85:686–92. doi: 10.1016/j.ijrobp.2012.05.023
5. Cornford P, Bellmunt J, Bolla M, Briers E, de Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. (2017) 71:630–42. doi: 10.1016/j.eururo.2016.08.002
6. Zumsteg ZS, Spratt DE, Romesser PB, Pei X, Zhang Z, Kollmeiet M, et al. Anatomical patterns of recurrence following biochemical relapse in the dose escalation era of external beam radiotherapy for prostate cancer. J Urol. (2015) 194:1624–30. doi: 10.1016/j.juro.2015.06.100
7. Pucar D, Hricak H, Shukla-Dave A, Kuroiwa K, Drobnjak M, Eastham J, et al. Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: magnetic resonance imaging and step-section pathology evidence. Int J Radiat Oncol Biol Phys. (2007) 69:62–9. doi: 10.1016/j.ijrobp.2007.03.065
8. Arrayeh E, Westphalen AC, Kurhanewicz J, Roach M, Jung AJ, Carroll PR, et al. Does local recurrence of prostate cancer after radiation therapy occur at the site of primary tumor? Results of a longitudinal MRI and MRSI study. Int J Radiat Oncol Biol Phys. (2012) 82:e787–93. doi: 10.1016/j.ijrobp.2011.11.030
9. Kishan AU, Chu Fl, King CR, Seiferheld W, Spratt DE, Tran P, et al. Local failure and survival after definitive radiotherapy for aggressive prostate cancer: an individual patient-level meta-analysis of six randomized trials. Eur Urol. (2019) 77:201–8. doi: 10.1016/j.eururo.2019.10.008
10. Coen JJ, Zietman AL, Thakral H, Shipley WU. Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol. (2002) 20:3199–205. doi: 10.1200/JCO.2002.01.086
11. Zelefsky MJ, Yamada Y, Fuks Z, Zhang Z, Hunt M, Cahlon O, et al. Long-term results of conformal radiotherapy for prostate cancer: impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. Int J Radiat Oncol Biol Phys. (2008) 71:1028–33. doi: 10.1016/j.ijrobp.2007.11.066
12. Zelefsky MJ. PSA bounce versus biochemical failure following prostate brachytherapy. Nat Clin Pract Urol. (2006) 3:578–9. doi: 10.1038/ncpuro0611
13. Crook JM, Tang C, Thames HD, Blanchard P, Sanders J, Ciezki JP, et al. A biochemical definition of cute following brachytherapy for prostate cancer: a multi-institution international study. Int J Radiat Oncol Biol Phys. (2019) 105:S57–S58. doi: 10.1016/j.ijrobp.2019.06.494
14. Tetreault-Laflamme A, Crook J, Hamm J, Pickles T, Keyes M, McKenzie M, et al. Long-term prostate specific antigen stability and predictive factors of failure after permanent seed prostate brachytherapy. J Urol. (2018) 199:120–25. doi: 10.1016/j.juro.2017.07.089
15. Kishan AU, Dang A, Katz AJ, Mantz CA, Collins SP, Aghdam N, et al. Long-term outcomes of stereotactic body radiotherapy for low-risk and intermediate-risk prostate cancer prostate cancer. JAMA Network Open. (2019) 2:e188006. doi: 10.1001/jamanetworkopen.2018.8006
16. Jiang NY, Dang AT, Yuan Y, Chu FI, Shabsovich D, King CR, et al. Multi-institutional analysis of prostate-specific antigen kinetics after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. (2019) 105:628–36. doi: 10.1016/j.ijrobp.2019.06.1816
17. Hegde JV, Collins SP, Fuller DB, King CR, Demanes DJ, Wang PC, et al. A pooled analysis of biochemical failure in intermediate-risk prostate cancer following definitive stereotactic body radiotherapy (SBRT) or high-dose-rate brachytherapy (HDR-B) monotherapy. Am J Clin Oncol. (2018) 41:502–7. doi: 10.1097/COC.0000000000000311
18. Abramowitz MC, Li T, Buyyounouski MK, Ross E, Uzzo RG, Pollack A, et al. The phoenix definition of biochemical failure predicts for overall survival in patients with prostate cancer. Cancer. (2008) 112:55–60. doi: 10.1002/cncr.23139
19. Zumsteg ZS, Spratt DE, Romesser PB, Pei X, Zhang Z, Polkinghorn W, et al. The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. Eur Urol. (2015) 67:1009–16. doi: 10.1016/j.eururo.2014.09.028
20. Zeleksky MJ, Ben-Porat L, Scher HI, Chan HM, Fearn PA, Fuks ZY, et al. Outcome predictors for the increasing PSA state after definitive external-beam radiotherapy for prostate cancer. J Clin Oncol. (2005) 23:826–31. doi: 10.1200/JCO.2005.02.111
21. Rouviere, O. Imaging techniques for local recurrence of prostate cancer: for whom, why and how? Diagn Interv Imaging. (2012) 93:279–90. doi: 10.1016/j.diii.2012.01.012
22. Tanaka T, Yang M, Froemming AT, Bryce AH, Inai R, Kanazawa S, et al. Current imaging techniques for imaging spectrum of prostate cancer recurrence and metastasis: a pictorial review. Radiographics. (2020) 20:190121. doi: 10.1148/rg.2020190121
23. Hara T, Inoue Y, Satoh T, Ishiyama H, Sakamoto S, Woodhams R, et al. Diffusion-weighted imaging of local recurrent prostate cancer after radiation therapy: comparison with 22-core three-dimensional prostate mapping biopsy. Magn Reson Imaging. (2012) 30:1091–8. doi: 10.1016/j.mri.2012.04.022
24. Haider MA, Chung P, Sweet J, Toi S, Jhaveri K, Menard C, et al. Dynamic contrast-enhanced magnetic resonance imaging for localization of recurrent prostate cancer after external beam radiotherapy. Int J Radiat Oncol Biol Phys. (2008) 70:425–30. doi: 10.1016/j.ijrobp.2007.06.029
25. Westphalen AC, Reed GD, Vinh PP, Sotto C, Vigneron DB, Kurhanewicz J. Multiparametric 3-tesla endorectal MR imaging after external beam radiation therapy of prostate cancer. J Magn Reson Imaging. (2012) 36:430–37. doi: 10.1002/jmri.23672
26. Ward RD, Purysko AS. Multiparametric magnetic resonance imaging in the evaluation of prostate cancer recurrence. Semin Roentgenol. (2018) 53:234–46. doi: 10.1053/j.ro.2018.04.002
27. Gaur S, Turkbey B. Prostate MRI for post-treatment evaluation and recurrence. Radiol Clin North Am. (2018) 56:263–75. doi: 10.1016/j.rcl.2017.10.008
28. Valle LF, Greer MD, Shih JH, Barrett T, Law YM, Rosenkrantz AB, et al. Multiparametric MRI for detection of local recurrence of prostate cancer in the setting of biochemical recurrence after low dose rate brachytherapy. Diagn Interv Radiol. (2018) 24:46–53. doi: 10.5152/dir.2018.17285
29. Overduin CG, Jenniskens SFM, Sedelaar JPM, Bomers JGR, Fütterer JJ. Percutaneous MR-guided focal cryoablation for recurrent prostate cancer following radiation therapy: retrospective analysis of iceball margins and outcomes. Eur Radiol. (2017) 27:4828–36. doi: 10.1007/s00330-017-4833-9
30. Wenske S, Quarrier S, Katz AE. Salvage cryosurgery of the prostate for failure after primary radiotherapy or cryosurgery: long-term clinical, functional, and oncologic outcomes in a large cohort at a tertiary referral centre. Eur Urol. (2013) 64:1–7. doi: 10.1016/j.eururo.2012.07.008
31. Finley DS, Belldegrun AS. Salvage cryotherapy for radiation-recurrent prostate cancer: outcomes and complications. Curr Urol Rep. (2011) 12:209–15. doi: 10.1007/s11934-011-0182-4
32. de Castro Abreu AL, Bahn D, Leslie S, Shoji S, Silverman P, Desai MM, et al. Salvage focal and salvage total cryoablation for locally recurrent prostate cancer after primary radiation therapy. BJU Int. (2013) 112:298–307. doi: 10.1111/bju.12151
33. Wong WW, Buskirk SJ, Schild SE, Prussak KA, Davis BJ. Combined prostate brachytherapy and short-term androgen deprivation therapy as salvage therapy for locally recurrent prostate cancer after external beam irradiation. J Urol. (2006) 176:2020–24. doi: 10.1016/j.juro.2006.07.008
34. Jereczek-Fossa BA, Rojas DP, Zerini D, Fodor C, Viola A, Fanetti G, et al. Reirradiation for isolated local recurrence of prostate cancer: mono-institutional series of 64 patients treated with salvage stereotactic body radiotherapy (SBRT). Br J Radiol. (2019) 92:20180494. doi: 10.1259/bjr.20180494
35. Fuller D, Wurzer J, Shirazi R, Bridge S, Law J, Crabtree T, et al. Stereotactic body radiation therapy for locally recurrent prostatic carcinoma after prior therapeutic irradiation: prostate-specific antigen response, disease-free survival, and toxicity. Int J Radiat Oncol Biol Phys. (2018) 102:S107–8. doi: 10.1016/j.ijrobp.2018.06.273
36. Janoray G, Reynaud-Bougnoux A, Ruffier-Loubière A, Bernadou Y, Calais G. Stereotactic body re-irradiation therapy for locally recurrent prostate cancer after external-beam radiation therapy: initial report. Cancer Radiother. (2016) 20:275–81. doi: 10.1016/j.canrad.2016.03.005
37. Loi M, Di Cataldo V, Simontacchi G, Detti B, Bonomo P, Masi L, et al. Robotic stereotactic retreatment for biochemical control in previously irradiated patients affected by recurrent prostate cancer. Clin Oncol (R Coll Radiol). (2018) 30:93–100. doi: 10.1016/j.clon.2017.11.007
38. Chen CP, Weinberg V, Shinohara K, Roach M 3rd, Nash M, Gottschalk A, et al. Salvage HDR brachytherapy for recurrent prostate cancer after previous definitive radiation therapy: 5-year outcomes. Int J Radiat Oncol Biol Phys. (2013) 86:324–9. doi: 10.1016/j.ijrobp.2013.01.027
39. Crook JM, Zhang P, Pisansky TM, Trabulsi EJ, Amin MB, Bice W, et al. A prospective phase 2 trial of transperineal ultrasound-guided brachytherapy for locally recurrent prostate cancer after external beam radiation therapy (NRG oncology/RTOG-0526). Int J Radiat Oncol Biol Phys. (2019) 103:335–43. doi: 10.1016/j.ijrobp.2018.09.039
40. Pisters, LL, Rewcastle, JC, Donnelly BJ, Lugnani, et al. Salvage prostate cryoablation: initial results from the cryo on-line data registry. J Urol. (2008) 180:559–63. doi: 10.1016/j.juro.2008.04.005
41. Li YH, Elshafei A, Agarwal G, Ruckle H, Powsang J, Jones JS. Salvage focal prostate cryoablation for locally recurrent prostate cancer after radiotherapy: initial results from the cryo online data registry. Prostate. (2015) 75:1–7. doi: 10.1002/pros.22881
42. Green GF, Pisters LL, Scott SM, Von Eschenbach AC. Predictive value of prostate specific antigen nadir after salvage cryotherapy. J Urol. (1998) 160:86–90. doi: 10.1016/S0022-5347(01)63040-4
43. D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitital radiation therapy for clinically localized prostate cancer. JAMA. (1998) 280:969–74. doi: 10.1001/jama.280.11.969
44. Chen LN, Suy S, Uhm S, Oermann EK, Ju AW, Chen V, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the georgetown experience. Radiat Oncol. (2013) 8:58. doi: 10.1186/1748-717X-8-58
45. Huang KT, Stoyanova R, Walker G, Sandler K, Studenski MT, Dogan N, et al. Post-radiotherapy prostate biopsies reveal heightened apex positivity relative to other prostate regions sampled. Radiother Oncol. (2015) 115:101–6. doi: 10.1016/j.radonc.2015.03.006
46. Zaorsky NG, Palmer JD, Hurwitz MD, Keith SW, Dicker AP, Den RB. What is the ideal radiotherapy dose to treat prostate cancer? A meta-analysis of biologically equivalent dose escalation. Radiother Oncol. (2015) 115:295–300. doi: 10.1016/j.radonc.2015.05.011
47. Zelefsky MJ, Kollmeier M, McBride S, Varghese M, Mychalczak B, Gewanter R, et al. Five-year outcomes of a phase 1 dose-escalation study using stereotactic body radiosurgery for patients with low-risk and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. (2019) 104:42–49. doi: 10.1016/j.ijrobp.2018.12.045
48. Stone NN, Unger P, Crawford ED, Stock RG. Diagnosis and management of local recurrence after low-dose-rate brachytherapy. Brachytherapy. (2015) 14:124–30. doi: 10.1016/j.brachy.2014.08.046
49. Chau, MLK, Holgersen, E, Sabelnykova, V, Salcedo A, Meng A, Fraser M, et al. Genomic architecture of prostate cancer at recurrence following radiation therapy. Int J Radiat Oncol Biol Phys. (2018) 35:S132. doi: 10.1016/j.ijrobp.2017.06.308
50. Kanthabalan A, Shah T, Arya M, Punwani S, Bomanji J, Haroon A, et al. The FORECAST study – focal recurrent assessment and salvage treatment for radiorecurrent prostate cancer. Contemp Clin Trials. (2015) 44:175–86. doi: 10.1016/j.cct.2015.07.004
Keywords: isolated local failure, SBRT, prostate cancer, CTC, salvage therapy
Citation: Aghdam N, Pepin AN, Creswell M, Hsieh K, Smith C, Drescher N, Danner M, Ayoob M, Yung T, Lei S, Kumar D, Collins BT, Lischalk JW, Krishnan P, Suy S, Lynch J, Bandi G, Hankins RA and Collins SP (2020) Management of Isolated Local Failures Following Stereotactic Body Radiation Therapy for Low to Intermediate Risk Prostate Cancer. Front. Oncol. 10:551491. doi: 10.3389/fonc.2020.551491
Received: 29 April 2020; Accepted: 31 August 2020;
Published: 29 October 2020.
Edited by:
Susanne Rogers, Aarau Cantonal Hospital, SwitzerlandReviewed by:
Thomas Zilli, Université de Genève, SwitzerlandAlison Claire Tree, Royal Marsden National Health Service Foundation Trust, United Kingdom
Copyright © 2020 Aghdam, Pepin, Creswell, Hsieh, Smith, Drescher, Danner, Ayoob, Yung, Lei, Kumar, Collins, Lischalk, Krishnan, Suy, Lynch, Bandi, Hankins and Collins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sean P. Collins, c3BjOUBndW5ldC5nZW9yZ2V0b3duLmVkdQ==
†These authors share first authorship
 Nima Aghdam
Nima Aghdam Abigail N. Pepin
Abigail N. Pepin Michael Creswell
Michael Creswell Kristin Hsieh1,4
Kristin Hsieh1,4 Clayton Smith
Clayton Smith Malika Danner
Malika Danner Deepak Kumar
Deepak Kumar Brian Timothy Collins
Brian Timothy Collins Jonathan W. Lischalk
Jonathan W. Lischalk Ryan Andrew Hankins
Ryan Andrew Hankins Sean P. Collins
Sean P. Collins