- 1Department of Radiation Oncology, Fujian Cancer Hospital and Fujian Medical University Cancer Hospital, Fuzhou, China
- 2College of Clinical Medicine, Fujian Medical University, Fuzhou, China
Background: Small-cell lung cancer (SCLC) is the most lethal cancer. With the development of chemotherapy and radiotherapy, brain metastasis (BM) emerged as one most predominant treatment failure. However, the factors affecting BM have not been identified completely. The purpose of this study was to investigate the risk factors involved in the development of BM in patients with limited-stage small-cell lung cancer (LS-SCLC) following definitive thoracic radiotherapy (TRT) and to provide a reference for the planning of a clinical treatment strategy.
Methods: The clinical data of patients with LS-SCLC treated with neoadjuvant chemotherapy (NAC) followed by TRT were collected and retrospectively reviewed. The factors affecting BM, BM-free survival (BMFS) and overall survival (OS) rates were analyzed statistically.
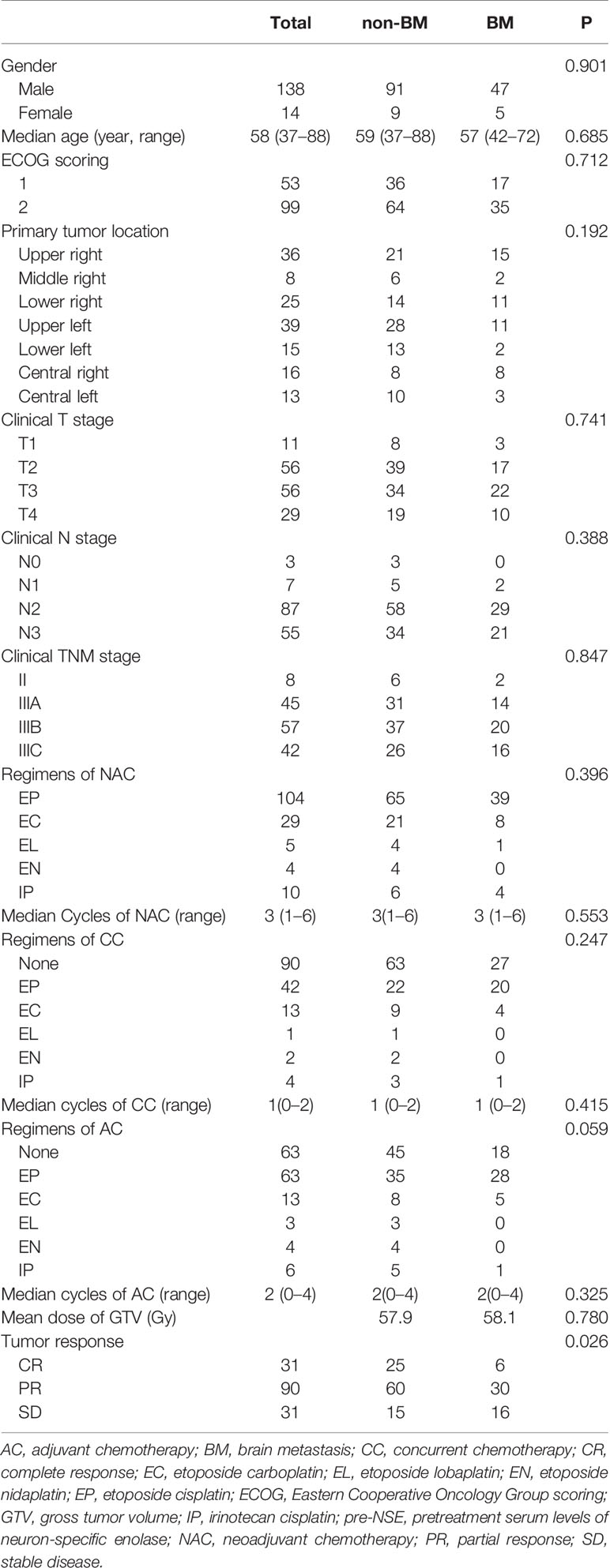
Results: A total of 152 patients with LS-SCLC fulfilled the inclusion criteria were reviewed. Following TRT, 31 (20.4%) patients achieved CR, 90 (59.2%) patients reached PR, 31 (20.4%) patients maintained SD, and no patients developed PD. The OS at 1, 3, and 5 years was 80.6, 34.2, and 19.4%, respectively. Multivariate analyses indicated that the greatest dimension of primary tumor (Dmax-T) and short-term response to TRT were risk factors affecting BM. The clinical N stage (cN), greatest dimension of metastatic nodes (Dmax-N), short-term response to TRT, and adjuvant chemotherapy (AC) were identified as independent factors correlated with OS.
Conclusions: Poor short-term response to TRT and huger Dmax-T were risk factors for BM. AC following TRT improved patient survival, but not decreased BM. However, due to the limitations associated with the retrospective design of the present study, further prospective clinical trials are required to confirm these conclusions.
Introduction
Lung cancer remains the most lethal type of cancer worldwide (1), with small-cell lung cancer (SCLC) accounting for 15–20% of newly diagnosed cases. Due to the characteristics of rapid tumor growth and early dissemination (2), the prognosis of SCLC is dismal, with a 5-year overall survival (OS) rate of 2.8–7.2% (3). With intensive chemotherapy the risk of extracranial progression decreases, as a result, intracranial progression emerges as one of the main types of treatment failure (4, 5). It has been reported that the 2-year cumulative risk of brain metastasis (BM) reached up to 49% for patients with limited disease and 65% for patients with extensive disease post-treatment (6).
However, the risk factors affecting BM had not been identified so far, the optimal prevention and/or treatment of BM had not been established. In the present study, we attempted to assess the risk factors of BM by retrospectively investigating the clinical data of LS-SCLC patients treated with TRT combined with systemic chemotherapy to provide a reference for treatment strategy planning.
Patients and Methods
Patient Selection and Data Collection
This present retrospective study was approved by the Fujian Province Cancer Hospital Institutional Review Board (approval no. YKT-2019-020-01). All patients’ information of demographics, diagnostic and staging work-up, treatment, and treatment response were extracted from medical records and were anonymized prior to analysis.
The eligibility and exclusion criteria for the current study were summarized as follows: Primary histologically proven SCLC, good performance status [Eastern Cooperative Oncology Group performance status (ECOG PS) score ≤2], efficient pretreatment workup for tumor staging and treatment evaluation, brain magnetic resonance imaging (MRI), complete follow-up data, lack of other concomitant medical conditions that required treatment, initial treatment with TRT combined with systemic chemotherapy, clinical stage I-III (T any, N any, M0, excluding T3-4 due to multiple lung nodules that are not to be encompassed in a tolerable radiation plan) according to the 8th American Joint Committee on Cancer TNM staging system (7). Patients who survived for <1 month after TRT were considered as irradiation fatalities and were excluded from the present study.
Treatment Characteristics
All patients were initially administrated with a ≥45 Gy dose of TRT alone or TRT combined with concurrent chemotherapy (CC) following primary neoadjuvant chemotherapy (NAC). Adjuvant chemotherapy (AC) was executed basing on patient’s treatment intention and short-term treatment response to TRT. The initiation of TRT and AC was no later than 3 weeks after completion of the previous treatment schedule.
The predominant regimens of NAC included a dual-agent combination of etoposide or irinotecan with cisplatin. Carboplatin or lobaplatin or nidaplatin was alternatively used in case of intolerance to cisplatin. Whether CC and AC used was the same drug as NAC, or a different drug was dependent on the short-term response to NAC and TRT. Adjustments to the chemotherapy time intervals and dose intensities were similar to those reported in our previous study (8).
TRT was performed using the intensity-modulated radiotherapy (IMRT) technique. The targets of TRT included the gross tumor volume (GTV) and the clinical target volume (CTV) and, after 2015, the targets only included GTV and without CTV according to the standard practice at the authors’ institution (9). The GTV including the primary tumor (GTV-T) and metastatic lymph nodes (GTV-N) were contoured on the post-NAC simulation computed tomography (CT) scans. The CTV includes the GTV and the initially involved nodal regions (but not their entire pre-NAC volume). Overall irradiation dose of TRT delivered to targets ranged from 45 to 67 Gy once daily with 1.8–2.0 Gy per fraction.
Criteria for Treatment Toxicity and Response to TRT
The toxicities of chemotherapy and TRT were assessed according to the NCI CTC (National Cancer Institute Common Toxicity Criteria) v3.0 (10) and the RTOG (Radiation Therapy Oncology Group) criteria (11), respectively.
The short-term response of the tumor to TRT was first evaluated at the date of TRT completion and was reassessed 4–6 weeks later. The short-term response was defined as clinically complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) according to RECIST1.1 (12).
Surveillance and Statistical Analysis
All patient outcomes were evaluated in May 2019. The primary endpoints were BM incidence, BM-free survival (BMFS) and OS time. The OS was calculated from the date of diagnosis to the date of death or the date of the last follow-up. The BMFS was defined as the duration between the date of BM diagnosis. Patients who were censored at the last follow-up date or who had died without evidence of BM were censored for BM incidence (13).
Data were analyzed using SPSS version 24.0 (IBM Corp., Armonk, NY, USA). The survival curves were constructed using the Kaplan-Meier method and compared with the log-rank test. Univariate and multivariate analyses of the association of clinical baseline characteristics [including sex, age, ECOG, tumor location, clinical TNM (cTNM) stage, greatest dimension of primary tumor (Dmax-T), greatest dimension of metastatic nodes (Dmax-N), thoracic irradiation dose, regimens, and cycles of chemotherapy (NAC, CC, and AC) and short-term response to TRT] with OS and BMFS were performed using the Cox proportional hazards model. Confidence intervals (CIs) represented 95% lower and upper limits.
Receiver operating characteristic (ROC) curve analysis was applied to establish the cut-off value of continuous variables that were most significantly correlated with BM using the area under the curve (AUC). The variables that were statistically significantly correlated with OS or BMFS were entered in the multivariate analysis using logistic regression.
Results
Patient Characteristics
Between February 2012 and August 2018, a total of 152 consecutive patients fulfilled the inclusion criteria, of whom 31 (20.4%) patients achieved CR, 90 (59.2%) patients reached PR, 31 (20.4%) patients maintained SD, and no patients developed PD following TRT. The clinical characteristics of the patients are presented in Table 1.
Overall Survival Analysis
The median follow-up time in the entire cohort and in the surviving patients was 21 (3–82) and 32 (7–86) months, respectively. At the last follow-up, 55 patients remained alive and 97 patients had died, of whom 18 patients had succumbed to extracranial progression (including locoregional or distant recurrence) alone, 52 to BM, 6 to both, and 14 to unascertainable intracranial or/and extracranial progression; a total of 7 patients lost to follow-up and were considered as dead from unknown causes when conducting the survival analysis.
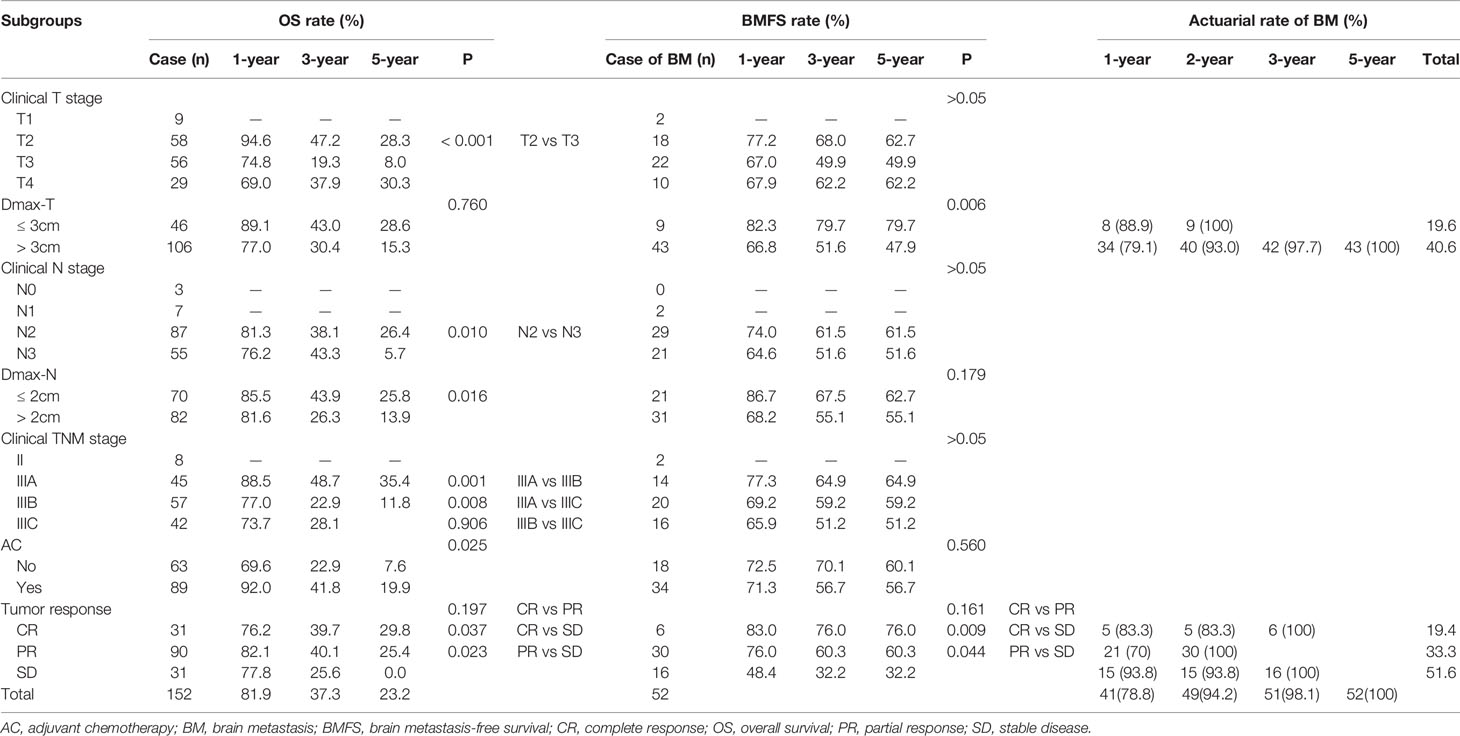
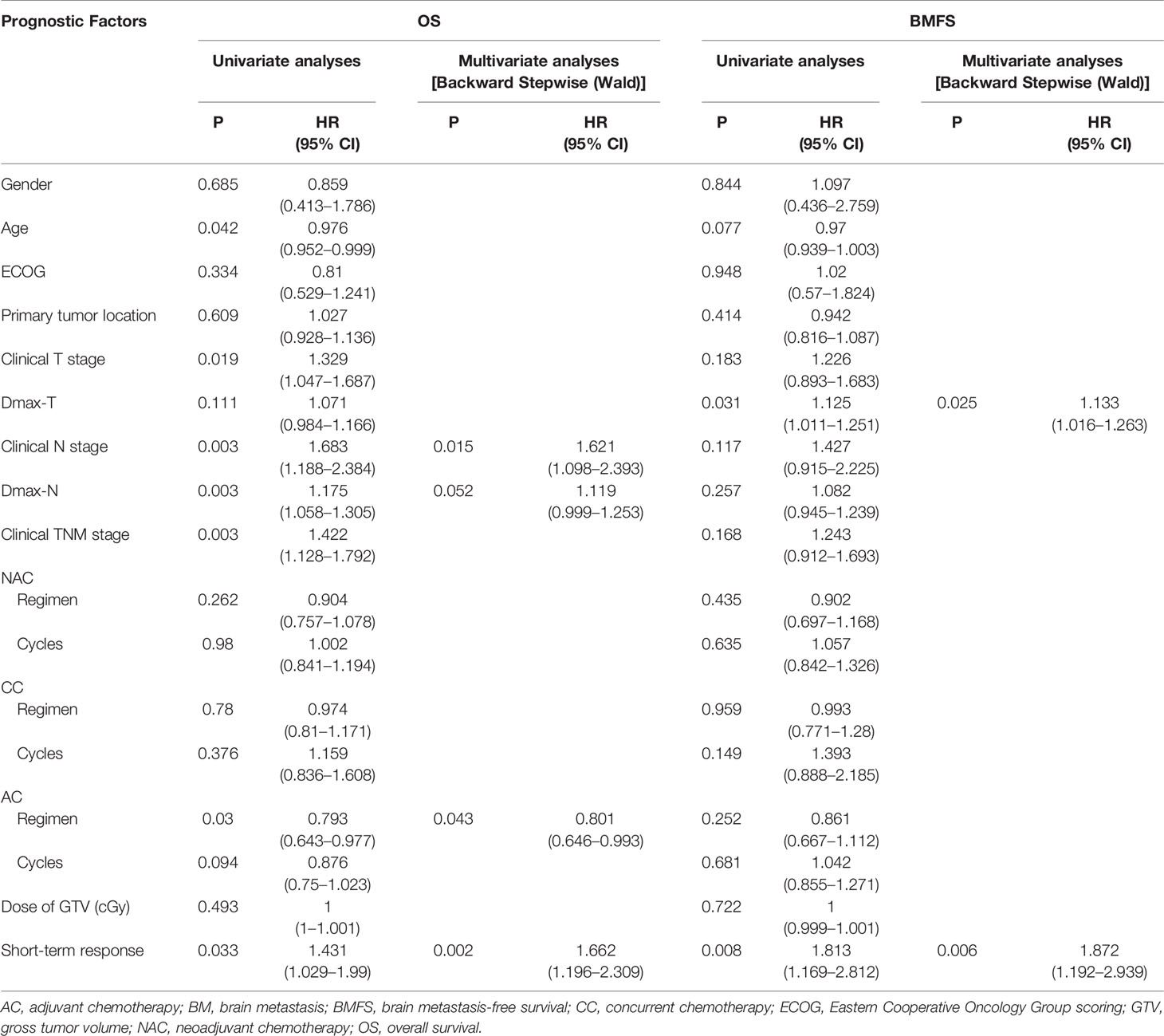
The 1-, 3-, and 5-year OS rates were 80.6, 34.2, and 19.4%, respectively (Table 2). Univariate and multivariate analyses indicated that cN, Dmax-N, the regimen of AC and short-term response to TRT were independent factors affecting OS (Table 3).
Compared with cN0-2 stage, patients with cN3 stage disease exhibited an inferior OS (P = 0.003), whereas the OS difference among cN0-2 stage patients did not reach statistical significance due to the limited number of patients enrolled in the present study (Figure 1A).
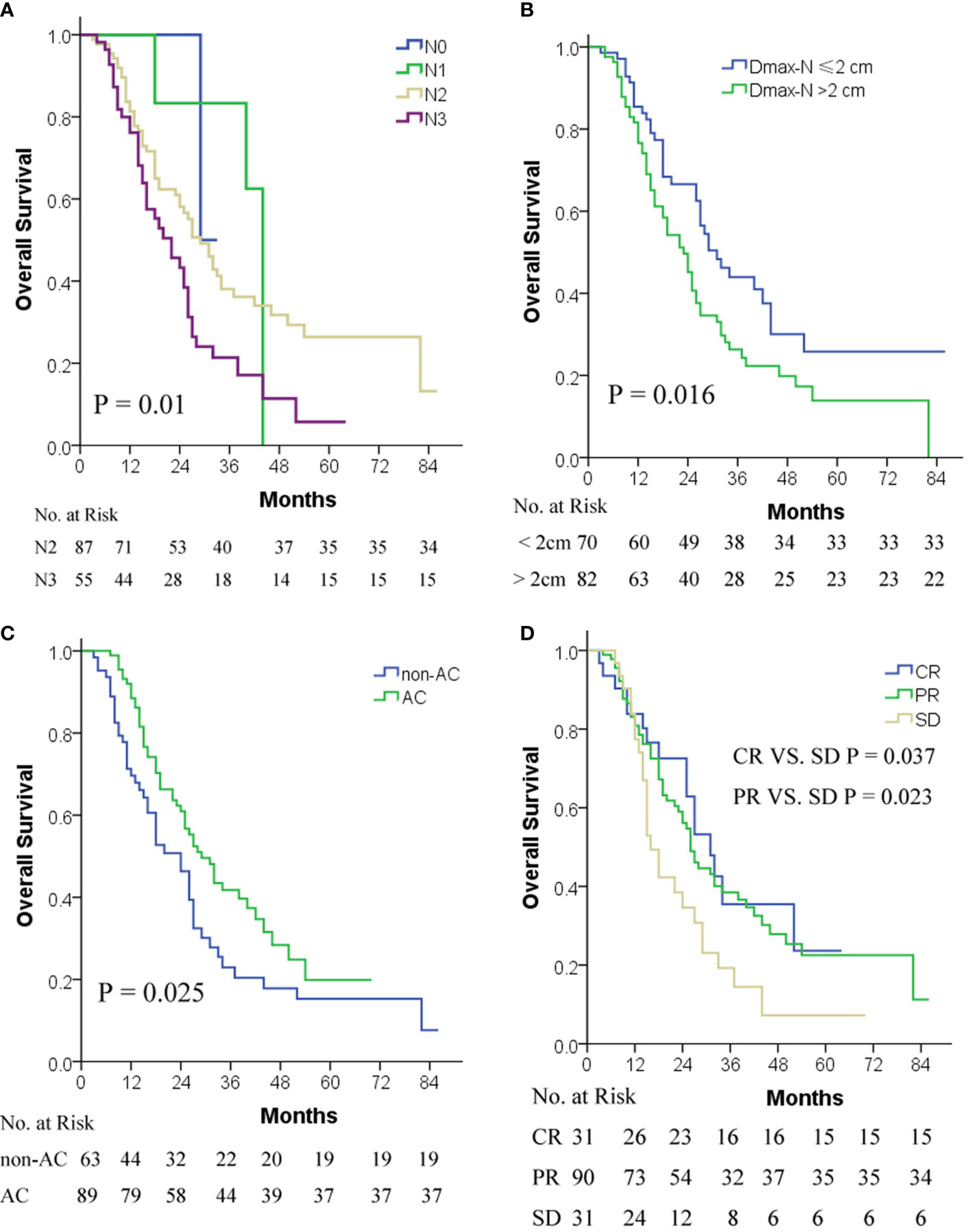
Figure 1 OS by cN stage (A), Dmax-N (B), AC (C), and short-term response to TRT (D). AC, adjuvant chemotherapy; cN, clinical metastatic lymph node; CR, complete response; Dmax-N, greatest dimension of metastatic nodes; OS, overall survival; PR, partial response; SD, stable disease; TRT, thoracic radiotherapy.
ROC curve analysis indicated that 2 cm was a cut-off value of Dmax-N in predicting OS. Patients with Dmax-N ≤2 cm exhibited a significantly superior survival compared with patients with Dmax-N >2 cm (P = 0.016) (Figure 1B).
Patients with AC conferred superior survival to non-AC (P = 0.025) (Figure 1C). However, due to the small sample size, the optimal cycles and regimens of AC were not explored in the present study.
The OS of patients with CR or PR as a short-term response to TRT was superior to those with SD, but the difference between CR and PR was not significant due to the limited number of patients enrolled in the present study (Figure 1D).
Factors Affecting BM Analysis
At the last follow-up, a total of 52 (52/152, 34.2% of the entire cohort) patients encountered BM, of whom 41 (78.8%), 49 (94.2%), 51 (98.1%), and 52 (100%) patients experienced BM within 1-, 2-, 3-, and 5-years post-treatment, respectively. No new BM developed in patients who survived >5 years post-treatment.
The 1-, 3-, and 5-year BMFS rates were 71.7, 58.2, and 58.2%, respectively (Table 2). Different to OS, univariate and multivariate analyses demonstrated that only Dmax-T and short-term response to TRT were two independent factors affecting BM incidences and BMFS (Table 3).
ROC curve analysis indicated that 3 cm was a cut-off value of Dmax-T in predicting BMFS. The BM incidence and BMFS between Dmax-T ≥3 and <3 cm differed significantly (Table 2) (Figure 2A).
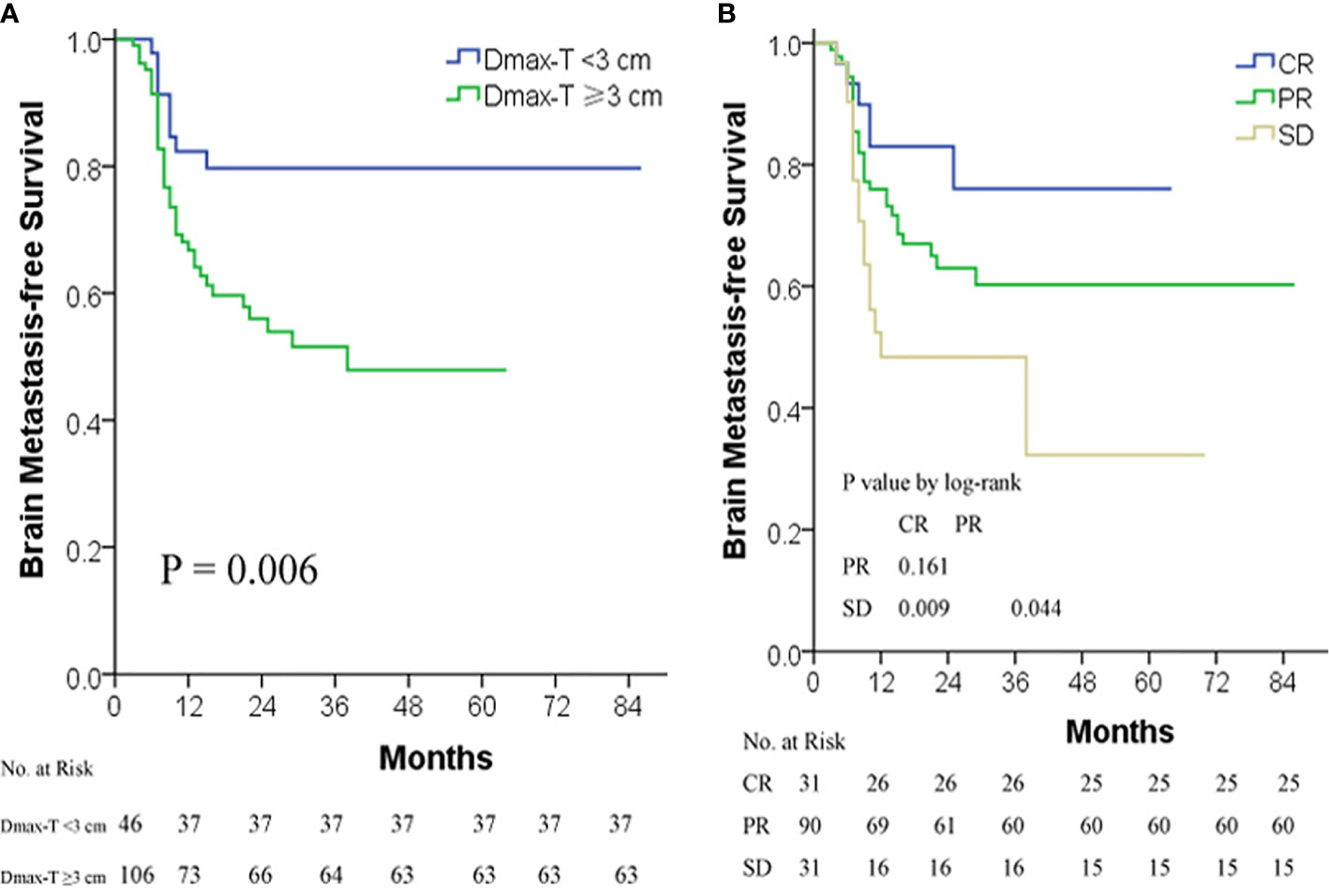
Figure 2 BMFS by Dmax-T (A) and short-term response to TRT (B). BMFS, BM-free survival; CR, complete response; Dmax-T, greatest dimension of primary tumor; PR, partial response; SD, stable disease; TRT, thoracic radiotherapy.
Similar to OS, the difference of BM incidence and BMFS was significant between CR/PR and SD but insignificant between CR and PR (Figure 2B).
When Dmax-T and short-term response to TRT were combined for BM prediction, the sensitivity and specificity became the maximum (sensitivity = 80.1%, specificity = 45%). The area under the curve (AUC) was 0.666 (Figure 3).
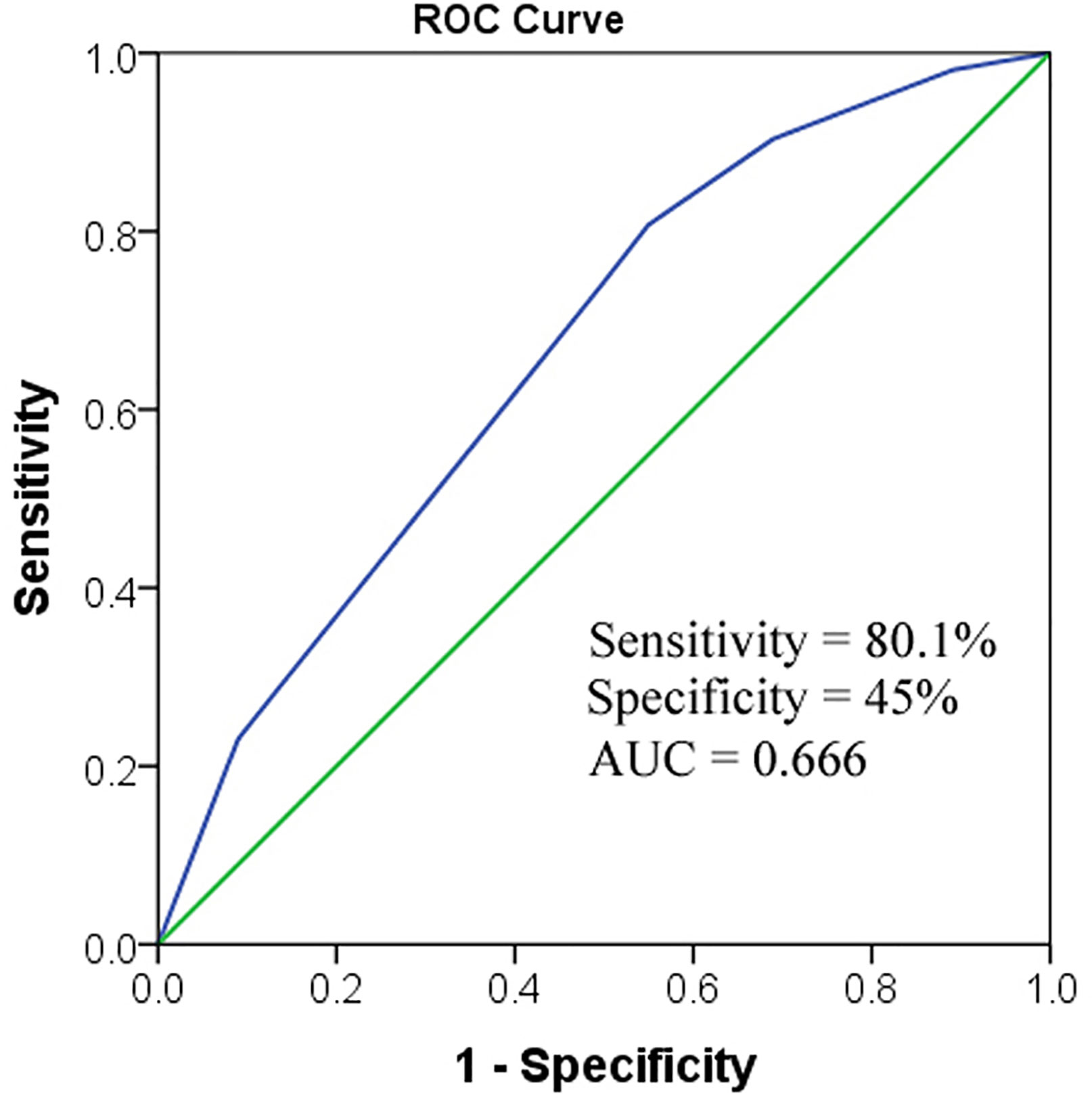
Figure 3 ROC to predict BM. When combining the two factors together, AUC was 0.666. AUC, area under the curve; Dmax-T, greatest dimension of primary tumor; TRT, thoracic radiotherapy.
Compared with non-AC, AC failed to decrease BM incidence, indicating that AC can only eradicate the extracranial, but not the intracranial, occult lesions to improve patient survival (Table 3).
Discussion
Gong et al. had conducted a study to assess the factors affecting BM in LS-SCLC patients treated primarily with surgery. They found that the pathologic stage and complete resection were independent factors affecting BM. Whereas, age, gender, NAC, AC, or adjuvant TRT following surgery were not correlated with BM (13). But, the majority of LS-SCLC patients do not qualify for surgical resection due to advanced cancer stage or concomitant diseases, and definitive TRT was recommended as part of standard treatment. While the factors related to BM in LS-SCLC patients treated with TRT had not yet been identified (14).
Zheng et al. had performed a retrospective small-sample study on factors affecting BM in LS-SCLC patients treated with TRT, which identified that T stage, neutrophil-to-lymphocyte ratio (NLR), TRT, and chemotherapy cycles were risk factors of BM (14). High T stage, high NLR, early TRT, and fewer cycles of chemotherapy were adverse factors correlated with BM. However, the nature of their retrospective study, with single-center, small-sample data, limited the reliability of the conclusions. For example, the study had concluded that earlier TRT increased the BM incidence even though TRT had improved the patients’ survival time.
Different to Zheng’s study, Farooqi et al. had recently reported a retrospective study basing on a larger scale of patients treated with definitive TRT, demonstrating that Dmax-T was the only factor associated with increased risk of BM (15). Similar to Farooqi`s study, the current study identified that Dmax-T was an independent factor correlating to BM. Patients with Dmax-T ≥3 cm developed more frequently BM than patients with Dmax-T <3 cm (19.6 vs. 40.6%).
In addition to the Dmax-T, the current study found that the short-term response to TRT was another factor of BM. The BM incidence increased sequentially in CR, PR, and SD of short-term response to TRT. High up to 51.6% of SD patients developed BM following TRT, while only 19.4% of CR patients encountered BM in the current study.
When Dmax-T and short-term response to TRT were combined for BM prediction, only 8.4% of patients with Dmax-T <3 cm and CR response to TRT developed BM post-TRT. Contrarily, the BM incidence of patients with Dmax-T ≥3 cm and SD response to TRT was as high as 57.1% in the current study.
Although Dmax-T was correlated with BM, it was not an independent factor influencing OS as shown in the current study and Farooqi’s. A reasonable explanation for the discrepancy influence of Dmax-T in BM and OS is that all TRT in the current study was conducted with IMRT technology, which achieved optimal primary tumor radiation dose coverage improving locoregional control and subsequently translated into survival benefits (16).
Numerous clinical trials in various solid tumors have confirmed that the short-term response to RT is an independent prognostic factor of survival. However, no similar studies have been conducted in SCLC to date. To our knowledge, the present study is the first to evaluate the predictive value of short-term response to TRT for survival of SCLC. It was demonstrated that short-term response to TRT was a robust factor predicting OS. Patients with CR had the best survival, followed by PR, whereas SD was associated with the poorest survival.
Chemotherapy is a cornerstone in the treatment of patients with SCLC. Several previous studies evaluated the benefit of AC following surgery in SCLC patients and reported that AC was successful in improving survival (17). Similarly, the present study demonstrated that, compared with non-AC, AC following TRT provided significant survival benefits. However, the incidence of BM in AC patients had not decreased compared to the non-AC in the current study. The reasonable explanation to the results is that AC with current chemotherapy regimens improve patient survival by eradicating the extracranial occult lesions but not the intracranial lesions due to the natural blood-brain barrier. While, limited to the number of patients enrolled in the current study, the optimal regimens and cycles of AC, the optimal sequence of AC and PCI were not explored. This is worthy of further research in future clinical trials.
In addition to the short-term response to TRT and AC, univariate and multivariate analyses in the current study indicated that the cN and Dmax-N were other two independent prognosis factors affecting OS. The higher the cN stage or the huger the Dmax-N, the worse the survival of patients. However, neither cN stage nor Dmax-N was identified correlating with BM in the current study. This finding revealed that cN stage and Dmax-N are only indicators of poor OS, but are not considered as risk factors for BM. Furthermore, the results indicated that for patients with worse N condition more intensive chemotherapy should be administrated to improve patient survival.
Besides risk factors involved BM, the interval time from the completion of TRT to BM may serve as an important reference for treatment decision making. The present study demonstrated that near 95% of BM occurred within 2 years following TRT. In other words, if patients achieved BMFS ≥2 years, the brain was no longer a risk failure site of SCLC.
Conclusions
In conclusion, the short-term response to TRT and Dmax-T were two remarkable predictable factors affecting BM. Compared to patients with Dmax-T <3 cm and CR, patients with Dmax-T ≥3 cm and SD resulted in more frequent BM. AC following TRT improved patient survival, but not decreased BM.
There were certain limitations to the present study, such as the retrospective design from a single institution, the not sufficiently long median follow-up duration and the limited number of patients enrolled, the results of our investigation must be interpreted with caution and further prospective clinical trials are required to confirm the conclusions.
Data Availability Statement
All datasets presented in this study are included in the article/supplementary material.
Ethics Statement
This present retrospective study was approved by the Fujian Province Cancer Hospital Institutional Review Board (approval no. YKT-2019-020-01).
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the present study, and have approved it for publication.
Funding
This study was supported in part by grants from the Fujian Provincial All rivers run into sea of high-end talent fund, the Fujian Provincial Health & Family Planning Commission (Project Number: 2016-ZQN-32), the Fujian Provincial Department of Science & Technology (Project Number: 2017Y9079), and the Science & Technology Program of Fujian Province (Project Number: 2018Y2003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank all patients who participated in the present study.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med (2008) 359:1367–80. doi: 10.1056/NEJMra0802714
3. Lu T, Yang X, Huang Y, Zhao M, Li M, Ma K, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res (2019) 11:943–53. doi: 10.2147/cmar.S187317
4. Paumier A, Cuenca X, Péchoux CL. Prophylactic cranial irradiation in lung cancer. Cancer Treat Rev (2011) 37:261–5. doi: 10.1016/j.ctrv.2010.08.009
5. Yin X, Yan D, Qiu M, Huang L, Yan SX. Prophylactic cranial irradiation in small cell lung cancer: a systematic review and meta-analysis. BMC Cancer (2019) 19:95. doi: 10.1186/s12885-018-5251-3
6. Seute T, Leffers P, ten Velde G, Twijnstra A. Neurologic disorders in 432 consecutive patients with small cell lung carcinoma. Cancer (2004) 100:801–6. doi: 10.1002/cncr.20043
7. Ramón Rami-Porta HA, Travis WD, Rusch VW. Lung. In: MahuI B, Amin M, FCAP, editors. AJCC cancer staging manual. AMERICAN JOINT COMMITTEE ON CANCER Executive Office: Cham, Switzerland: Springer International Publishing AG (2017). p. 431–56.
8. Chen MQ, Lin QL, Chen YG, Guo JH, Xu BH, Tian Y. Neoadjuvant chemotherapy may not benefit esophageal squamous cell carcinoma patients treated with definitive chemoradiotherapy. J Chin Med Assoc (2017) 80:636–43. doi: 10.1016/j.jcma.2017.06.014
9. Su L, Chen M, Su H, Dai Y, Chen S, Li J. Postoperative chemoradiotherapy is superior to postoperative chemotherapy alone in squamous cell lung cancer patients with limited N2 lymph node metastasis. BMC Cancer (2019) 19:1023. doi: 10.1186/s12885-019-6141-z
10. Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol (2003) 13:176–81. doi: 10.1016/s1053-4296(03)00031-6
11. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys (1995) 31:1341–6. doi: 10.1016/0360-3016(95)00060-c
12. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
13. Gong L, Wang QI, Zhao L, Yuan Z, Li R, Wang P. Factors affecting the risk of brain metastasis in small cell lung cancer with surgery: is prophylactic cranial irradiation necessary for stage I-III disease? Int J Radiat Oncol Biol Phys (2013) 85:196–200. doi: 10.1016/j.ijrobp.2012.03.038
14. Zheng Y, Wang L, Zhao W, Dou Y, Lv W, Yang H, et al. Risk factors for brain metastasis in patients with small cell lung cancer without prophylactic cranial irradiation. Strahlenther Onkol (2018) 194:1152–62. doi: 10.1007/s00066-018-1362-7
15. Farooqi AS, Holliday EB, Allen PK, Wei X, Cox JD, Komaki R. Prophylactic cranial irradiation after definitive chemoradiotherapy for limited-stage small cell lung cancer: Do all patients benefit? Radiother Oncol (2017) 122:307–12. doi: 10.1016/j.radonc.2016.11.012
16. Shirvani SM, Juloori A, Allen PK, Komaki R, Liao Z, Gomez D, et al. Comparison of 2 common radiation therapy techniques for definitive treatment of small cell lung cancer. Int J Radiat Oncol Biol Phys (2013) 87:139–47. doi: 10.1016/j.ijrobp.2013.05.040
Keywords: brain metastasis, radiotherapy, small-cell lung cancer, survival, chemotherapy
Citation: Wu S, Wang J, Zhang W, Li J, Wu H, Huang Z, Zhou G, Pan J and Chen M (2020) Analysis of Factors Affecting Brain Metastasis in Limited-Stage Small-Cell Lung Cancer Treated With Definitive Thoracic Irradiation. Front. Oncol. 10:556634. doi: 10.3389/fonc.2020.556634
Received: 28 April 2020; Accepted: 02 October 2020;
Published: 29 October 2020.
Edited by:
Gene A. Cardarelli, Brown University, United StatesReviewed by:
Joshua Silverman, New York University, United StatesZhengfei Zhu, Fudan University, China
Copyright © 2020 Wu, Wang, Zhang, Li, Wu, Huang, Zhou, Pan and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingqiu Chen, ZHJjaGVubWluZ3FpdUB5YWhvby5jb20=; Jiezhong Wang, ZnpqendAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Shuting Wu
Shuting Wu Jiezhong Wang
Jiezhong Wang Wei Zhang
Wei Zhang Jiancheng Li
Jiancheng Li Haishan Wu
Haishan Wu Zhiyu Huang
Zhiyu Huang Guangrun Zhou
Guangrun Zhou Jianji Pan
Jianji Pan Mingqiu Chen
Mingqiu Chen