- Department of Surgical Oncology and General Surgery, Key Laboratory of Precision Diagnosis and Treatment of Gastrointestinal Tumors, Ministry of Education, The First Affiliated Hospital of China Medical University, Shenyang, China
Regenerating islet-derived type 4 (REG4), a member of the calcium-dependent lectin gene superfamily, is abnormally expressed in various cancers, such as colorectal, gastric, gallbladder, pancreatic, ovarian, prostate, and lung cancer. REG4 is associated with a relatively unfavorable prognosis and clinicopathologic features in cancers, including advanced tumor and nodal stage, histological differentiation, and liver and peritoneal metastasis. Moreover, REG4-positive cancer cells show more frequent resistance to chemoradiotherapy, especially 5-FU-based chemotherapy. REG4 participates in many aspects of carcinogenesis, including cell proliferation, apoptosis, cell cycle, invasion, metastasis, and drug resistance. The underlying mechanisms are complex and involve a series of signaling mediators and multiple pathways. Thus, REG4 may be a potential diagnostic and prognostic biomarker as well as a candidate therapeutic target in cancer patients. In this review, we systematically summarize the advances about the clinical significance, biological functions, and mechanisms underlying REG4 in cancer to provide new directions for future cancer research.
Introduction
The regenerating islet-derived (REG) family genes belong to the calcium-dependent lectin (C-type lectin) gene superfamily. There are five REG members expressed in humans: REG1A, REG1B, REG3A, REG3G, and REG4. All of these are located on the second chromosome, except REG4, which is located on chromosome 1 (1). REG members are identified to be essential for cell proliferation, regeneration, inflammation, tumor formation, and formation of immune system (1). Of these, REG4 is the most frequently observed member and has been characterized as a key regulator in the initiation, differentiation, and progression of various human cancer cell types.
REG4 was originally identified by a high-throughput sequence analysis of a cDNA library derived from patients with inflammatory bowel disease (2). It is located on the long arm of chromosome 1, contains six introns and seven exons, and encodes 158 amino acids that include a signal peptide of 22 amino acids and a conserved calcium-dependent hydrocarbon recognition domain (CRD). CRD is located at amino acid positions 30–155 in the REG4 protein and is critical for the biological function of REG4, especially in its promotion of invasion and migration abilities (3). Unlike other C-type lectins, REG4, in the absence of calcium, can bind heparin, polysaccharides and mannan mediated by CRDs and shows a potential role in specific carbohydrate recognition (4). These findings may provide clues to understanding the molecular interactions with currently uncertain receptors and the sugar-binding role of REG4 protein.
REG4, a small secretory protein sized about 18-kD, is also referred to as regenerating protein-like protein (RELP) (5). REG4 is expressed in parietal cells of the gastric mucosa and epithelial neuroendocrine cells of the small intestine (5, 6), and inflammatory bowel disease (6–9). REG4 may be involved in the metaplastic responses and inflammation of the gastrointestinal epithelium. The expression levels in cancerous tissues, such as the stomach, pancreatic, colorectal, prostate, gallbladder, ovarian and lung cancers are much higher than that in normal tissues (6, 9–13). As a secretory protein, REG4 shows two mucin-like and perinuclear patterns with immunohistochemical staining (14) and promotes carcinogenesis in tumor cells via both autocrine and paracrine manners (15). The expression of REG4 was associated with clinical characteristics, such as histologic differentiation, invasion depth, and TNM stage in cancer patients and is recommended to be a promising biomarker for predicting metastasis, combined with S100A4 and MACC1 (16). The combination of VEGF-C and REG4 has been characterized as a promising factor for clinical staging to supplement the TNM classification system (17). High expression of REG4 predicts poor prognosis and drug-resistance by promoting cancer cell proliferation, invasion and anti-apoptosis (18).
Kumar et al. reported that REG4 promotes cell proliferation in colon adenocarcinoma cells via the EGFR/Akt/AP-1 pathway (19). The mechanisms involved are far more complex than perceived. The understanding of mechanisms of REG4 in many cancer types has increased in the recent years (Tables 1 and 2). The current review will focus on the clinical significance and underlying mechanisms of REG4 in various human cancers and highlight its potential applicability for diagnostic, prognostic and therapeutic approaches.
REG4 Expression Pattern and Clinical Significance in Human Cancers
Colorectal Cancer
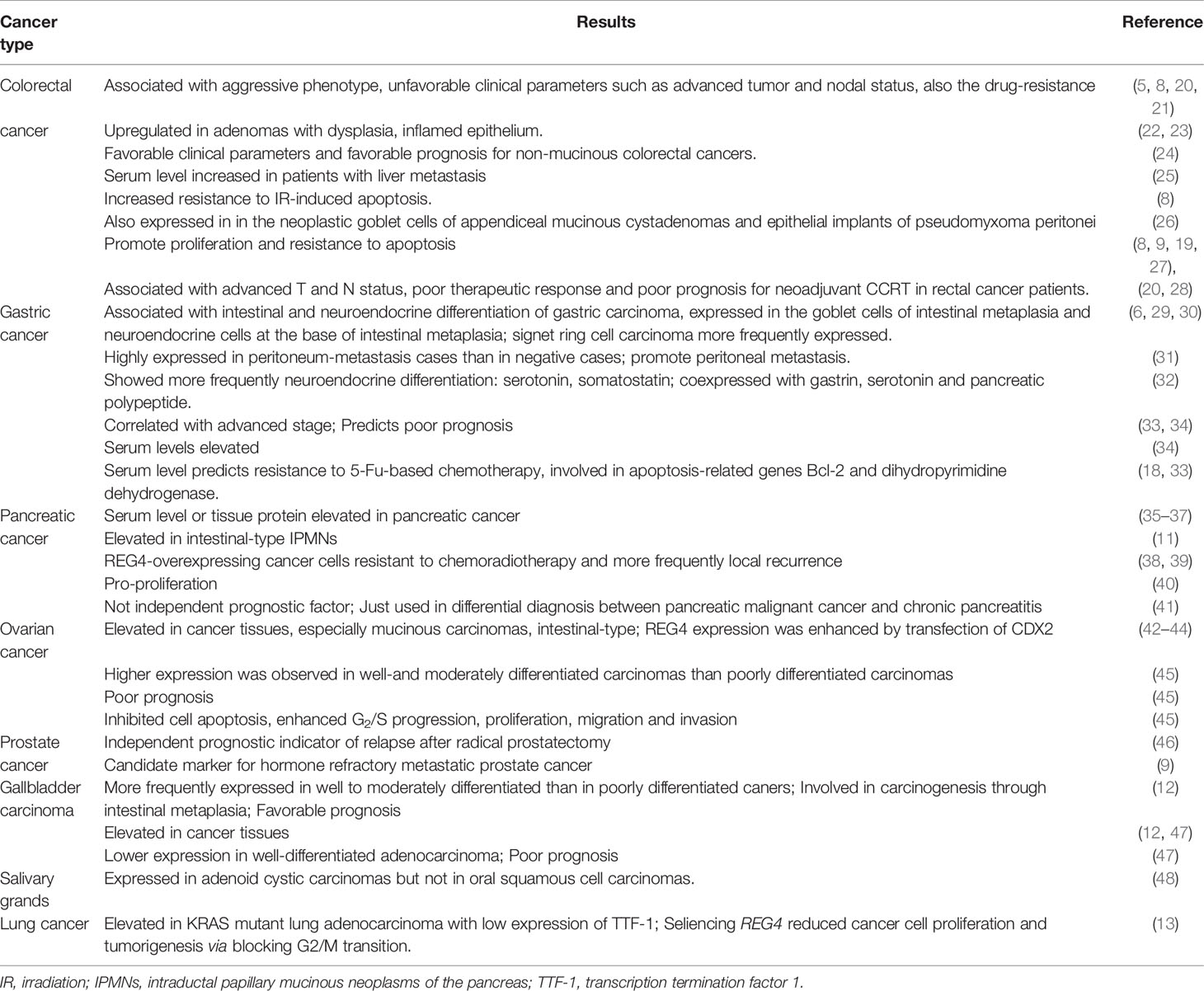
REG4 is expressed in colorectal adenomas with dysplasia (22) or inflamed epithelium (23). Xiao et al. explored the physiological functions of REG4 in intestinal inflammation and found that REG4 altered the colonic bacterial composition and reduced the number of the bacteria adhering to the colonic epithelium in vivo and promoted the growth of colonic organoids via activation of signal transducer and activator of transcription 3 (STAT3) in vitro (56). REG4 was upregulated in colorectal cancer tissues than in adjacent normal mucosa (7, 10), indicating that REG4 overexpression may be an early event in colorectal carcinogenesis. Kukka et al. also observed robust expression of REG4 in the epithelial implants of pseudomyxoma peritonei and neoplastic goblet cells of appendiceal mucinous cystadenomas (26). REG4 overexpression is frequently associated with aggressive phenotypes, unfavorable clinical parameters such as advanced tumor and nodal status, and drug-resistance (5, 8, 20, 21). Moreover, REG4 was useful in predicting response to neoadjuvant chemoradiotherapy in patients with rectal cancer (20, 28). Kumar et al. identified a relationship of REG4 with the increased resistance to irradiation-induced apoptosis (8). Kobunai et al. found that REG4 gene expression was 12-fold higher in radioresistant cells and might be a useful predictor of the sensitivity of rectal cancer patients to radiotherapy (57). Additionally, colorectal cancer patients with metastatic recurrence in the liver showed more frequent REG4 immunostaining and serum levels than in those without recurrence. Serum REG4 levels can be used to predict liver recurrence (25). Survival analysis revealed that high REG4 expression could be correlated with shortened survival time and emerged as an adverse prognostic factor (13, 45). Jared et al. showed that REG4-postive tumors, but not at a high risk of recurrence, were associated with decreased survival in established recurrent colon adenocarcinoma, possibly via activation of REG4-CD44/CD44ICD pathway (58). The above evidence indicates that REG4 may be a potential therapeutic target in colorectal cancer. However, Kaprio et al. performed immunohistochemistry analysis in 840 consecutive surgically treated colorectal cancer patients and found that REG4 expression was associated with favorable clinicopathological characteristics. REG4 expression indicates higher overall survival rates in non-mucinous colorectal cancer patients (24). Whereas, studies have suggested that REG4 can promote colorectal cancer cell proliferation and elevate resistance to drug-induced apoptosis, in vivo and in vitro (8, 9, 19, 27). The conflicting results may be attributed to the different cancer phenotypes included in the study or the use of different methods to measure RNA or protein levels, which may result in varied conclusions.
Gastric Cancer
The expression of REG4 is elevated in goblet cells of intestinal metaplasia and neuroendocrine cells at the base of intestinal metaplasia (6). Zheng et al. showed that REG4 mRNA or protein expression was upregulated in the intestinal metaplasia and adenoma than in paired normal mucosa (29). Signet ring cell carcinoma, an aggressive phenotype of gastric cancer, expressed more REG4 than other types of gastric cancer (29, 30). Another study reported that REG4-positive cases showed more frequent neuroendocrine differentiation than REG4-negative cases. Double immunofluorescence staining revealed REG4 may be co-expressed with gastrin, serotonin and pancreatic polypeptide, and REG4-positive cells expressed more neuroendocrine hormones than REG4-negative cells (32). These results suggest that REG4 plays an important role in intestinal metaplasia and neuroendocrine differentiation.
REG4 expression in gastric cancer positively correlates with the cell invasive depth, clinical stages, diffuse type, poor differentiation, distant metastasis and intrinsic drug resistance to 5-FU (33, 34). Moreover, REG4 positivity in metastasized human gastric cancer was significantly higher than that in negative cases (31). REG4-positive group showed significantly less survival time than REG4-negative group (34). Zheng et al. also reported that the serum levels of REG4 in gastric carcinoma patients were significantly higher than those in healthy individuals. Additionally, REG4 may be a better serum marker than carbohydrate antigen 19-9 (CA199) and carcinoembryonic antigen (CEA) for early diagnosis and as a prognostic indicator of gastric cancer (34). Patients with high serum REG4 level were less sensitive to 5-FU-based chemotherapy, possibly due to REG4-induced Bcl-2 and dihydropyrimidine dehydrogenase (18, 33). Zheng et al. showed that as the protein expression of REG4 in intestinal metaplasia, adenoma, carcinoma and gastritis gradually decreased according to combined immunohistochemistry and in situ hybridization on tissue microarray, indicates that REG4 may be suitable to distinguish gastric benign disease and malignant tumors (29).
REG4 expression upregulates SRY-box transcription factor 9 (SOX9) and promotes invasiveness and migration in gastric tumor cells (59). Kuniyasu et al. observed increased number and size of peritoneal tumors and decreased apoptosis in vitro, along with worsened mice survival after transfection with REG4 (31). Antibody against REG4 significantly inhibited proliferation in gastric cancer cells (MKN45 and AGS) and synergistically enhanced the lethal effect of 5-FU via the MAPK/ERK/Bim pathway (54, 60). Zhou et al. also revealed that knockdown of REG4 decreased stemness properties in gastric cancer stem cells and increased the effectiveness of cell death following chemoradiation treatment, indicating that the inhibition of endogenous REG4 may be a promising therapeutic strategy in human gastric cancer (61).
Pancreatic Cancer
REG4 is overexpressed in pancreatic cancer tissues than in adjacent normal tissues at either the mRNA or protein level (35–37). Kohei et al. found that intestinal-type intraductal papillary mucinous neoplasms of the pancreas (IPMNs) showed frequent moderate and severe dysplasia. Of the 125 IPMNs, 43 (34%) were positive for REG4 and almost all of the intestinal-type IPMNs (35/38) expressed REG4, suggesting that REG4 was involved in the ‘intestinal’ carcinogenesis pathway in IPMNs (11). Serum REG4 levels could be correlated with REG4 expression in cancer tissues, and they were elevated in patients with pancreatic cancer than in healthy individuals and those with chronic pancreatitis (35, 41). Patients with higher REG4 levels showed unfavorable histologic response to chemoradiation and experienced more frequent local recurrence postoperatively (38, 39). Akio et al. found that knockdown of REG4 resulted in a significant decrease in cell viability in pancreatic ductal adenocarcinoma. Conversely, treatment with recombinant REG4 enhanced cell growth in a dose-dependent manner, indicating that targeting REG4 may be a potential targeted therapy in pancreatic cancer (40). A 2018 revealed that REG4 was not independent prognostic factor by multivariate analysis, although serum REG4 levels could be used in the differential diagnosis of pancreatic malignant cancers and chronic pancreatitis (41).
Tumor of Reproductive System
REG4 is frequently expressed in mucinous ovarian cancer subtype (42, 43), especially intestinal-type, and is absent in the endocervical-like form (44). Higher expression was observed in well- and moderately- differentiated than poorly-differentiated carcinomas (45). REG4 plays an essential role in early ovarian carcinogenesis and is closely linked with mucinous ovarian carcinomas, histologic differentiation and adverse prognosis (45). REG4, with cytokeratin (CK) 7, contributes to the differential diagnosis between primary and metastatic ovarian mucinous carcinomas (44). REG4 overexpression and treatment with recombinant REG4 both inhibited apoptosis, and enhanced G2/S progression, cell proliferation, migration and invasion in SKOV3 ovarian cancer cells (45).
There are only two studies about the clinical role of REG4 in prostate cancer. Shinya et al. demonstrated that high expression of REG4 predicts relapse risk after radical prostatectomy (46). Another study revealed that REG4 is overexpressed in prostate tumors after neoadjuvant hormone ablation therapy, especially in hormone-refractory metastatic prostate cancer tissues (9). Moreover, high expression of REG4 in prostate cancer correlated with tumor recurrence, metastasis and therapy failure.
Some Other Cancer Types
There are also studies revealing REG4 overexpression in gallbladder adenocarcinomas (12, 47). However, the role and clinical significance of these findings in different studies are controversial. Yang et al. analyzed 108 gallbladder adenocarcinomas samples using immunohistochemical analysis and elucidated that the frequency of REG4-positive cases is lower in well-differentiated adenocarcinoma and that high expression predicts poor prognosis (12). Hidehiko et al. analyzed the mRNA and protein levels in 31 gallbladder carcinoma samples using quantitative reverse transcription-polymerase chain reaction and immunohistochemical staining, and demonstrated that REG4 expression was more frequent in well- and moderately differentiated than in poorly differentiated gallbladder adenocarcinoma samples. REG4 expression in gallbladder adenocarcinoma is associated with a relatively favorable prognosis in patients after surgery (47). However, elucidating the exact role in gallbladder carcinoma requires comprehensive analysis of in a larger cohort.
Further, Sun et al. analyzed 55 clinical samples and combined GEO and TCGA database information, and found that both mRNA and protein levels of REG4 were significantly upregulated in KRAS mutant lung adenocarcinoma samples with low expression of the transcription termination factor 1 (TTF-1) (identified as the KS subgroup). REG4 promotes the progression in KRAS mutant lung adenocarcinoma cells progression and can be used as a novel biomarker in lung adenocarcinoma subtype (13). Another study also reported overexpression of REG4 in invasive mucinous lung adenocarcinoma of gastric differentiation-type (62).
Finally, REG4 was also found to be expressed in adenoid cystic carcinomas in the salivary gland (17/41), but not in oral squamous cell carcinomas. The expression of REG4 could be correlated with nodal metastasis, poor prognosis, and pEGFR levels and that cell growth could be inhibited by anti-REG4 treatment in vitro (48).
Mechanisms Involved in Human Cancers
Promoting Proliferation and Resistance to Apoptosis
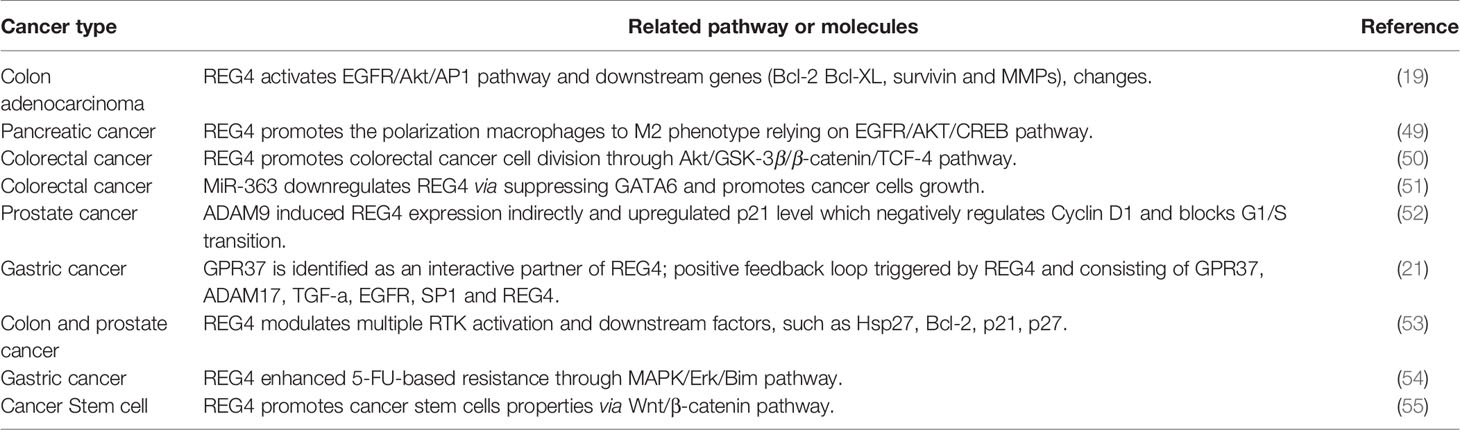
Overexpression and oncogenic role of epidermal growth factor receptor (EGFR) in malignant tumors are commonly identified (63, 64). The activator protein-1 (AP-1) complex, which is predominantly composed of proteins in the Jun and Fos families, is one of the most important transcription factors triggered by EGFR signaling (65). Akt is reported to be a specific upstream kinase regulating AP-1 transcription activity (66, 67). Bishnupuri et al. revealed that REG4 activates EGFR/Akt/AP-1 pathway and contributes to the increased invasiveness and resistance to apoptotic cell death in colon adenocarcinomas. Treatment with recombinant REG4 induced a remarkable increase in the phosphorylation of EGFR at Tyr992 and Tyr1068 and the activation of downstream Akt at Thr308 and Ser473, coupled with increased AP-1 transcriptional activity: quantitative increase in expression of Jun B, Jun D, and Fos B (19). Furthermore, the expression of their downstream anti-apoptotic genes (Bcl-2, Bcl-XL, survivin, and MMPs) was significantly increased (19, 68). Huang et al. also reported that REG4 promotes cell proliferation and migration in gastric cancer via activation of Akt (69).
REG4 can also promote cancer cell proliferation and anti-apoptosis via other mechanisms. Kathryn et al. revealed that REG4 can modulate phosphorylation of multiple additional receptor tyrosine kinases (RTKs), including insulin receptor, insulin-like growth factor receptor, as well as their downstream effectors, EGFR, mitogen-activated protein kinase, and phosphatidylinositol-3-kinase pathways. Knockdown of REG4 affects the ability of insulin and EGF to phosphorylate downstream tyrosine kinase in human colon and prostate cancer cells (53). Jin et al. revealed that REG4 inhibits apoptosis by regulating the MAPK/ERK/Bim signaling pathway, thereby enhancing resistance of gastric cancer cells to 5-FU, based on the western blotting results (54). However, the precise mechanism by which REG4 mediates the phosphorylation of other RTKs and their downstream proteins and the precise role of REG4 in the MAPK pathway is still unclear and requires further research.
Involved in Cell Cycle Regulation
Growth and development of cancer depends on the ability of cancer cells to escape the normal controls and check points of cell division cycle. The division of mammalian cells is mainly regulated at specific points in the cell cycle, particularly at the G1/S and G2/M transitions. Mammalian D-type cyclins and associated cyclin-dependent kinases (CDKs) are essential for driving each cell cycle phase. Misregulated CDKs induce unscheduled proliferation and chromosomal and genomic instability (70). Furthermore, REG4 mediates increased Akt kinase activity and inactivates glycogen synthase kinase 3β (GSK-3β) by increasing phosphorylation of Ser9 residue. Decreased GSK-3β activity induces an increased nuclear translocation of β-catenin by decreasing its phosphorylation at Ser33/37/Thr41 and sequentially increasing TCF-4 transcriptional activity, which promotes the expression of cyclin D1 and D3 coupled with CDK4 and CDK6. REG4 treatment accelerates G1/S and G2/M phase transition, coupled with increased mitotic index of colorectal cancer cells. The use of REG4 antagonists or Akt inhibitors decreased, while GSK-3β antagonist significantly increased mitotic index and proliferation in colorectal cancer cells (50). These results indicated the key role of REG4 in regulating colorectal cancer cell division via the Akt/GSK-3β/β-catenin/TCF-4 signaling pathway (Figure 1). Moreover, the mechanism by which REG4 mediates Akt kinase activity may be attributed to the REG4-mediated phosphorylation of EGFR, as mentioned above.
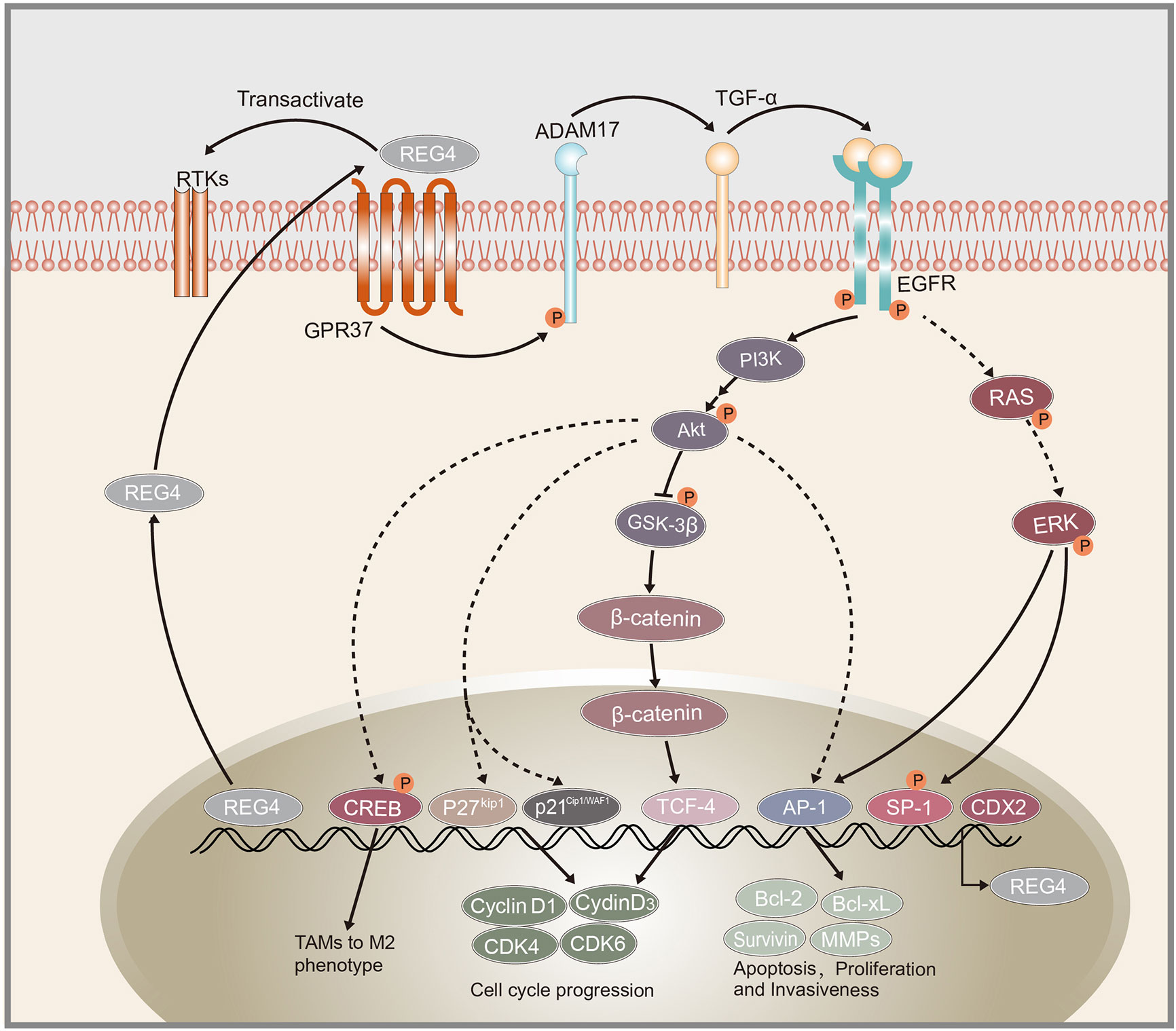
Figure 1 Schematic representation of REG4 signaling pathway. GPR37 as the interactive partner of REG4 complex.REG4 can transactivate RTKs including EGFR. EGFR phosphorylates Akt and activates downstream AP-1, GSK-3β/β-catenin/TCF-4, p21Cip1/WAF1/p27Kip1 pathway regulating cancer cells apoptosis, proliferation and invasiveness. EGFR and Akt can also induced the phosphorylation of CREB and promote TAMs polarization to M2 phenotype. REG4 can amplify itself by a positive feedback loop consisting of GPR37, ADAM17, TGF-α, EGFR, SP1 and REG4. CDX2 was identified as the transcription factor of REG4. REG4, Regenerating islet-derived type 4; GPR37, G protein-coupled receptor 37; RTKs, receptor tyrosine kinases; EGFR, Epidermal growth factor receptor; Akt, serine/threonine kinase 1; AP-1, activator protein-1; GSK-3β, glycogen synthase kinase 3 beta; TCF-4, transcription factor 4; CREB, cAMP response element-binding protein; TAMs, tumor-associated macrophages; ADAM17, a disintegrin and metallopeptidase domain 17; TCF-α,transforming growth factor alpha; CDX2, caudal type homeobox 2.
Mutations in both adenomatous polyposis coli (APC) and KRAS synergistically increase tumorigenesis and enhance the induction of colorectal stem cells (71). As per the microassay-based transcriptional analysis and knockout of all the representative KRAS-inducible genes, knockout of REG4 showed the most significant reduction in spheroid-forming capability in stem cells harboring mutations in both KRAS and APC. Expression of REG4 was significantly upregulated in a mutant KRAS-dependent manner in both colorectal stem cells and cancer tissues harboring APC mutation, consistent with another study with REG4 overexpression in KRAS mutant lung adenocarcinoma (13). Protein levels of p-LRP6, β-catenin, and p-GSK-3β were increased upon treatment with recombinant REG4 in a dose-dependent manner. REG4-induced activation of the GSK-3β/β-catenin signaling pathway promotes colorectal stem cell properties induced by KRAS mutation with loss of APC (55). Another study also indicated that targeting REG4 in aldehyde dehydrogenase 1 (ALDH1) positive cancer-initiating cells regulates the tumorigenic capacity of diffuse-type gastric carcinoma-initiating cells inhibited by GSK-3β (72). Moreover, REG4 was also upregulated in KRAS-mutant lung carcinoma and thus, is a novel biomarker in the lung adenocarcinoma subtype. Silencing REG4 reduced cancer cell proliferation and tumorigenesis in vivo and in vitro by blocking G2/M transition (13), suggesting an important role of REG4 in KRAS-driven lung cancer pathogenesis. However, further studies are needed to clarify the role and underlying mechanisms of REG4 in cell proliferation and division and its potential therapeutic value in lung cancer.
A disintegrin and metalloproteinase 9 (ADAM9) encoded protein regulates prostate cancer proliferation and invasion by interacting with a variety of cell surface proteins in prostate cancer (73–75). Expression of ADAM9 correlates with poor prognosis, recurrence risk and therapy-resistance (75, 76). Radioactive and chemical pharmaceutics or the tumor microenvironment itself can induce endogenous oxidative responses which induce ADAM9 expression (76). Liu et al. found that knockdown of ADAM9 decreases expression of REG4 and upregulates expression of p21Cip1/WAF1 and p27Kip1 which negatively regulates the expression of cyclin D1 and blocks the G1/S transition (52). Radiochemotherapy could induce the endogenous superoxide and upregulation of ADAM, followed by activation of REG4/p21Cip1/WAF1 pathway activation. The ADAM9/REG4/p21Cip1/WAF1 pathway contributes to cancer cell division and drug resistance. Furthermore, Liu et al. also reported that ADAM9 may indirectly induce REG4 expression via activation of EGFR by cleaving HB-EGF (52). Further investigation of the correlation between ADAM9 and REG4 may help to understand the underlying mechanism of therapy-resistance in prostate cancer. Additionally, Wang et al. revealed that REG4 promotes the phosphorylation of ADAM17 and amplifies itself via a positive feedback (21) which indicates that ADAM family members may be involved in the progression of REG4-induced pathological changes.
Promoting the Polarization Macrophages to M2 Phenotype
Another study demonstrated that REG4-induced EGFR/Akt pathway activation promotes cancer cell progression directly and polarization of macrophages to M2 phenotype. Several reports suggest that M2 tumor-associated macrophages (TAMs) can provide a favorable microenvironment to promote tumor angiogenesis, progression and suppress adaptive immunity (77–79). Ma et al. demonstrated that treatment with recombinant REG4 and the culture medium of REG4-positive pancreatic cancer cells induced the expression of some M2-related genes in macrophages, such as IL10 and CD163 (49). TAMs are often recruited to tumors by growth factors or chemokines produced by tumor cells themselves (80). EGFR and cAMP response element-binding protein (CREB) are reported to contribute to M2 polarization of macrophages (81). Further study showed that overexpression of REG4 promotes phosphorylation-mediated activation of EGFR and Akt, which subsequently induce the phosphorylation of CREB at Ser133. However, knockdown of CREB blocked the M2 macrophage polarization mediated by REG4 (49). Tumor-secreted REG4 can change the tumor microenvironment to facilitate cancer cell growth and metastasis by promoting macrophage polarization to M2 via activation of the EGFR/Akt/CREB pathway.
Molecules Regulating the Expression of REG4
The receptor of REG4 is always a problem that has been confused by researchers. Wang et al. demonstrated a positive feedback loop triggered by REG4, amplifying itself via EGFR, comprising EGFR, ADAM17, G protein-coupled receptor 37 (GPR37), TGF-α, REG4, and transcription factor SP1 (21), as shown in Figure 1. They also demonstrated that GPR37 is a partner of REG4 and promotes peritoneal metastasis in gastric cancer cells by mediating the signal transduction of REG4 (21). However, there is still no study elucidating the exact receptor or the complete complex partners of REG4. Apichat et al. also showed that the expression of REG4 in colon cancer cells can be enhanced by stimulation from transforming growth factor-α (TGF-α), epidermal growth factor (EGF), fibroblast growth factor, and hepatocyte growth factor (23).
The glutamyl-tRNA amidotransferase (GATA) family, a group of evolutionarily conserved zinc finger-containing transcription factors, is essential for proliferation, differentiation and development in many organs (82). Among them, GATA6 is expressed throughout the gastrointestinal epithelium and is essential for the tumorigenicity and cell invasion in colorectal cancer (83). Yoshihiro et al. showed that miR-363 represses transcription of REG4 via suppression of GATA6. GATA6 simultaneously induces expression of leucine-rich repeat containing G-protein-coupled receptor 5 (LGR5) and is presented as a stem cell marker (84, 85). Cooperation between the GATA6/LGR5 and GATA6/REG4 pathways plays an important role in the tumorigenicity in colon cancer cells (51). Yoshihiro et al. also reported that the expression levels of REG4 and LGR5 may not be directly influenced by miR-363 and GATA6. GATA6 usually acts in combination with other transcriptional factors, including TCF-4 and caudal type homeobox 2 (CDX2) (86, 87). CDX2 was frequently found to bind directly to the 5′-flanking promoter of REG4 and positively regulate its expression (11, 42, 44, 88, 89). CDX2 may be involved in the process of inducing upregulation of REG4 via miR-363 and GATA6, which needs further research.
Another study revealed that miR-24 directly downregulated REG4 expression by binding its 3′ untranslated region and restrained gastric cancer progression (90). Moreover, gliotactin (GLI), a transcription factor in the hedgehog signaling pathway, was also identified to bond to REG4 promoter region and induce REG4 expression in pancreatic cancer (36).
Conclusion and Perspective
REG4 is upregulated not only in various human cancers, including colorectal, gastric, pancreatic, ovarian, prostate, gallbladder, and lung cancer (Table 1), but also in some benign diseases, such as ulcerative colitis, intestinal metaplasia, adenoma, and atypical hyperplasia, suggesting a significant role of REG4 in tumorigenesis. Most studies have revealed that REG4 overexpression is positively associated with unfavorable clinical parameters, resistance to therapy and poor prognosis, indicating that REG4 is a promising prognostic biomarker and potential therapeutic target in cancer patients. Serum levels of REG4 were also found to be elevated in several cancer types and could predict metastasis and recurrence, suggesting that serum REG4 levels can potentially be used as a screening and diagnostic serum biomarker similar to carcinoembryonic antigen (CEA).
The mechanism of action of REG4 in human cancers is complex and involves multiple pathways (Table 2). REG4 is upregulated in cancer stem cells and participates in the promotion of colorectal stem cell properties via the Wnt/β-catenin pathway. The REG4/Akt/GSK-3β/β-catenin/TCF-4 pathway was also shown to regulate cell cycle progression and promote colorectal cancer cell proliferation. REG4-induced EGFR/Akt phosphorylation promotes not only cancer cell proliferation directly via increased AP-1 transcriptional activity, but also the polarization of macrophages to M2 phenotype, changing the microenvironment to facilitate cancer cell growth and metastasis via activation of CREB. Additionally, REG4 can amplify its expression via a positive feedback consisting of EGFR, ADAM17, TGF-α, SP1, and GPR37 was identified as an interactive partner of the REG4 complex. Some other molecules such as ADAM9, microRNAs and MAPK pathways were also found to be involved in the process of REG4 promoting cancer cell proliferation and invasion
In this article, we specifically reviewed the expression and role of REG4 in various human cancers. The mechanisms involve promoting proliferation, apoptosis-resistance, cell cycle regulation, and TAMs. However, research on REG4 is still at a preliminary stage, and inhibition of endogenous REG4 or its downstream signaling warrants further investigation to delineate its potential and limits for cancer diagnosis and treatment.
Author Contributions
JZ and ZW searched PubMed about REG4 in human cancers and wrote the draft ZM and XH summarized the different functions in various human cancers ZS and HX searched and classified the complex mechanisms JZ drew the figures attached. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (No 81502101).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Chen Z, Downing S, Tzanakakis ES. Four Decades After the Discovery of Regenerating Islet-Derived (Reg) Proteins: Current Understanding and Challenges. Front Cell Dev Biol (2019) 7:235. doi: 10.3389/fcell.2019.00235
2. Hartupee JC, Zhang H, Bonaldo MF, Soares MB, Dieckgraefe BK. Isolation and characterization of a cDNA encoding a novel member of the human regenerating protein family: Reg IV. Biochim Biophys Acta (2001) 1518(3):287–93. doi: 10.1016/s0167-4781(00)00284-0
3. Guo Y, Xu J, Li N, Gao F, Huang P. RegIV potentiates colorectal carcinoma cell migration and invasion via its CRD domain. Cancer Genet Cytogen (2010) 199(1):38–44. doi: 10.1016/j.cancergencyto.2010.01.011
4. Ho MR, Lou YC, Wei SY, Luo SC, Lin WC, Lyu PC, et al. Human RegIV protein adopts a typical C-type lectin fold but binds mannan with two calcium-independent sites. J Mol Biol (2010) 402(4):682–95. doi: 10.1016/j.jmb.2010.07.061
5. Kamarainen M, Heiskala K, Knuutila S, Heiskala M, Winqvist O, Andersson LC. RELP, a novel human REG-like protein with up-regulated expression in inflammatory and metaplastic gastrointestinal mucosa. Am J Pathol (2003) 163(1):11–20. doi: 10.1016/S0002-9440(10)63625-5
6. Oue N, Mitani Y, Aung PP, Sakakura C, Takeshima Y, Kaneko M, et al. Expression and localization of Reg IV in human neoplastic and non-neoplastic tissues: Reg IV expression is associated with intestinal and neuroendocrine differentiation in gastric adenocarcinoma. J Pathol (2005) 207(2):185–98. doi: 10.1002/path.1827
7. Violette S, Festor E, Pandrea-Vasile I, Mitchell V, Adida C, Dussaulx E, et al. a new member of the regenerating gene family, is overexpressed in colorectal carcinomas. Int J Cancer (2003) 103(2):185–93. doi: 10.1002/ijc.10788
8. Bishnupuri KS, Luo Q, Sainathan SK, Kikuchi K, Sureban SM, Sabarinathan M, et al. Reg IV regulates normal intestinal and colorectal cancer cell susceptibility to radiation-induced apoptosis. Gastroenterology (2010) 138(2):616–26, 26 e1-2. doi: 10.1053/j.gastro.2009.10.050
9. Gu Z, Rubin MA, Yang Y, Deprimo SE, Zhao H, Horvath S, et al. Reg IV: a promising marker of hormone refractory metastatic prostate cancer. Clin Cancer Res (2005) 11(6):2237–43. doi: 10.1158/1078-0432.CCR-04-0356
10. Numata M, Oshima T, Yoshihara K, Watanabe T, Tsuchida K, Tamagawa H, et al. Relationship between RegIV gene expression to outcomes in colorectal cancer. J Surg Oncol (2011) 104(2):205–9. doi: 10.1002/jso.21906
11. Nakata K, Nagai E, Ohuchida K, Aishima S, Hayashi A, Miyasaka Y, et al. REG4 is associated with carcinogenesis in the ‘intestinal’ pathway of intraductal papillary mucinous neoplasms. Modern Pathol (2009) 22(3):460–8. doi: 10.1038/modpathol.2008.205
12. Yang L, Lan S, Liu J, Yang Z. Expression of MK-1 and RegIV and its clinicopathological significances in the benign and malignant lesions of gallbladder. Diagn Pathol (2011) 6:100. doi: 10.1186/1746-1596-6-100
13. Sun S, Hu Z, Huang S, Ye X, Wang J, Chang J, et al. REG4 is an indicator for KRAS mutant lung adenocarcinoma with TTF-1 low expression. J Cancer Res Clin Oncol (2019) 145(9):2273–83. doi: 10.1007/s00432-019-02988-y
14. Li FY, Ren XB, Xu EP, Huang Q, Sheng HQ, Lv BJ, et al. RegIV expression showing specificity to gastrointestinal tract and its potential role in diagnosing digestive tract neuroendocrine tumor. J Zhejiang Univ Sci B (2010) 11(4):258–66. doi: 10.1631/jzus.B0900383
15. Rafa L, Dessein AF, Devisme L, Buob D, Truant S, Porchet N, et al. REG4 acts as a mitogenic, motility and pro-invasive factor for colon cancer cells. Int J Oncol (2010) 36(3):689–98. doi: 10.3892/ijo_00000544
16. Czerniak B, Olszewska-Slonina D, Cwynar A. [S100A4 , MACC - 1 , REG - 4 - promising biomarkers of metastasis in cancers]. Wiadomosci Lekarskie (2017) 70(3 pt 2):604–7.
17. Sawada T, Yashiro M, Sentani K, Oue N, Yasui W, Miyazaki K, et al. New molecular staging with G-factors (VEGF-C and Reg IV) by supplementing TNM classification in colorectal cancers. Oncol Rep (2013) 30(6):2609–16. doi: 10.3892/or.2013.2787
18. Mitani Y, Oue N, Matsumura S, Yoshida K, Noguchi T, Ito M, et al. Reg IV is a serum biomarker for gastric cancer patients and predicts response to 5-fluorouracil-based chemotherapy. Oncogene (2007) 26(30):4383–93. doi: 10.1038/sj.onc.1210215
19. Bishnupuri KS, Luo Q, Murmu N, Houchen CW, Anant S, Dieckgraefe BK. Reg IV activates the epidermal growth factor receptor/Akt/AP-1 signaling pathway in colon adenocarcinomas. Gastroenterology (2006) 130(1):137–49. doi: 10.1053/j.gastro.2005.10.001
20. He HL, Lee YE, Shiue YL, Lee SW, Lin LC, Chen TJ, et al. Overexpression of REG4 confers an independent negative prognosticator in rectal cancers receiving concurrent chemoradiotherapy. J Surg Oncol (2014) 110(8):1002–10. doi: 10.1002/jso.23764
21. Wang H, Hu L, Zang M, Zhang B, Duan Y, Fan Z, et al. REG4 promotes peritoneal metastasis of gastric cancer through GPR37. Oncotarget (2016) 7(19):27874–88. doi: 10.18632/oncotarget.8442
22. Zhang Y, Lai M, Lv B, Gu X, Wang H, Zhu Y, et al. Overexpression of Reg IV in colorectal adenoma. Cancer Lett (2003) 200(1):69–76. doi: 10.1016/s0304-3835(03)00460-9
23. Nanakin A, Fukui H, Fujii S, Sekikawa A, Kanda N, Hisatsune H, et al. Expression of the REG IV gene in ulcerative colitis. Lab Investigation J Tech Methods Pathol (2007) 87(3):304–14. doi: 10.1038/labinvest.3700507
24. Kaprio T, Hagstrom J, Mustonen H, Koskensalo S, Andersson LC, Haglund C. REG4 independently predicts better prognosis in non-mucinous colorectal cancer. PLoS One (2014) 9(10):e109600. doi: 10.1371/journal.pone.0109600
25. Oue N, Kuniyasu H, Noguchi T, Sentani K, Ito M, Tanaka S, et al. Serum concentration of Reg IV in patients with colorectal cancer: overexpression and high serum levels of Reg IV are associated with liver metastasis. Oncology (2007) 72(5-6):371–80. doi: 10.1159/000113147
26. Heiskala K, Giles-Komar J, Heiskala M, Andersson LC. High expression of RELP (Reg IV) in neoplastic goblet cells of appendiceal mucinous cystadenoma and pseudomyxoma peritonei. Virchows Archiv Int J Pathol (2006) 448(3):295–300. doi: 10.1007/s00428-005-0105-1
27. Zhu X, Han Y, Yuan C, Tu W, Qiu G, Lu S, et al. Overexpression of Reg4, alone or combined with MMP-7 overexpression, is predictive of poor prognosis in colorectal cancer. Oncol Rep (2015) 33(1):320–8. doi: 10.3892/or.2014.3559
28. Wang XJ, Yu Q, Chi P, Lin HM, Lu XR, Huang Y, et al. [Identification of gene biomarkers to predict responses to neoadjuvant chemoradiotherapy in patients with rectal cancer and pathways enrichment analysis]. Zhonghua Wei Chang Wai Ke Za Zhi Chin J Gastrointestinal Surg (2019) 22(12):1183–7. doi: 10.3760/cma.j.issn.1671-0274.2019.12.015
29. Zheng HC, Xu XY, Yu M, Takahashi H, Masuda S, Takano Y. The role of Reg IV gene and its encoding product in gastric carcinogenesis. Hum Pathol (2010) 41(1):59–69. doi: 10.1016/j.humpath.2009.06.013
30. Sentani K, Oue N, Tashiro T, Sakamoto N, Nishisaka T, Fukuhara T, et al. Immunohistochemical staining of Reg IV and claudin-18 is useful in the diagnosis of gastrointestinal signet ring cell carcinoma. Am J Surg Pathol (2008) 32(8):1182–9. doi: 10.1097/PAS.0b013e318163a8f8
31. Kuniyasu H, Oue N, Sasahira T, Yi L, Moriwaka Y, Shimomoto T, et al. Reg IV enhances peritoneal metastasis in gastric carcinomas. Cell Proliferation (2009) 42(1):110–21. doi: 10.1111/j.1365-2184.2008.00577.x
32. Sentani K, Oue N, Noguchi T, Sakamoto N, Matsusaki K, Yasui W. Immunostaining of gastric cancer with neuroendocrine differentiation: Reg IV-positive neuroendocrine cells are associated with gastrin, serotonin, pancreatic polypeptide and somatostatin. Pathol Int (2010) 60(4):291–7. doi: 10.1111/j.1440-1827.2010.02519.x
33. Ying LS, Yu JL, Lu XX, Ling ZQ. Enhanced RegIV expression predicts the intrinsic 5-fluorouracil (5-FU) resistance in advanced gastric cancer. Digest Dis Sci (2013) 58(2):414–22. doi: 10.1007/s10620-012-2381-3
34. Tao HQ, He XJ, Ma YY, Wang HJ, Xia YJ, Ye ZY, et al. Evaluation of REG4 for early diagnosis and prognosis of gastric cancer. Hum Pathol (2011) 42(10):1401–9. doi: 10.1016/j.humpath.2010.08.023
35. Takayama R, Nakagawa H, Sawaki A, Mizuno N, Kawai H, Tajika M, et al. Serum tumor antigen REG4 as a diagnostic biomarker in pancreatic ductal adenocarcinoma. J Gastroenterol (2010) 45(1):52–9. doi: 10.1007/s00535-009-0114-y
36. Wang F, Xu L, Guo C, Ke A, Hu G, Xu X, et al. Identification of RegIV as a novel GLI1 target gene in human pancreatic cancer. PLoS One (2011) 6(4):e18434. doi: 10.1371/journal.pone.0018434
37. Jiang Y, Liu M, Li Z, Jiang Y. Discovery of novel candidate oncogenes in pancreatic carcinoma using high-throughput microarrays. Hepato-gastroenterology (2013) 60(128):1825–32.
38. Legoffic A, Calvo E, Cano C, Folch-Puy E, Barthet M, Delpero JR, et al. The reg4 gene, amplified in the early stages of pancreatic cancer development, is a promising therapeutic target. PLoS One (2009) 4(10):e7495. doi: 10.1371/journal.pone.0007495
39. Eguchi H, Ishikawa O, Ohigashi H, Takahashi H, Yano M, Nishiyama K, et al. Serum REG4 level is a predictive biomarker for the response to preoperative chemoradiotherapy in patients with pancreatic cancer. Pancreas (2009) 38(7):791–8. doi: 10.1097/MPA.0b013e3181ac5337
40. Takehara A, Eguchi H, Ohigashi H, Ishikawa O, Kasugai T, Hosokawa M, et al. Novel tumor marker REG4 detected in serum of patients with resectable pancreatic cancer and feasibility for antibody therapy targeting REG4. Cancer Sci (2006) 97(11):1191–7. doi: 10.1111/j.1349-7006.2006.00297.x
41. Saukkonen K, Hagstrom J, Mustonen H, Lehtinen L, Carpen O, Andersson LC, et al. Prognostic and diagnostic value of REG4 serum and tissue expression in pancreatic ductal adenocarcinoma. Tumour Biol J Int Soc Oncodevelopmental Biol Med (2018) 40(3):1010428318761494. doi: 10.1177/1010428318761494
42. Koh I, Nosaka S, Sekine M, Sugimoto J, Hirata E, Kudo Y. Regulation of REG4 Expression and Prediction of 5-Fluorouracil Sensitivity by CDX2 in Ovarian Mucinous Carcinoma. Cancer Genomics Proteomics (2019) 16(6):481–90. doi: 10.21873/cgp.20151
43. Lehtinen L, Vesterkvist P, Roering P, Korpela T, Hattara L, Kaipio K, et al. REG4 Is Highly Expressed in Mucinous Ovarian Cancer: A Potential Novel Serum Biomarker. PLoS One (2016) 11(3):e0151590. doi: 10.1371/journal.pone.0151590
44. Huang Q, Chen X, Lu W, Lai M, Lu B. Expression of REG4 in ovarian mucinous tumors. Appl Immunohistochem Mol Morphol AIMM (2014) 22(4):295–301. doi: 10.1097/PAI.0b013e3182936d8e
45. Chen S, Gou WF, Zhao S, Niu ZF, Zhao Y, Takano Y, et al. The role of the REG4 gene and its encoding product in ovarian epithelial carcinoma. BMC Cancer (2015) 15:471. doi: 10.1186/s12885-015-1435-2
46. Ohara S, Oue N, Matsubara A, Mita K, Hasegawa Y, Hayashi T, et al. Reg IV is an independent prognostic factor for relapse in patients with clinically localized prostate cancer. Cancer Sci (2008) 99(8):1570–7. doi: 10.1111/j.1349-7006.2008.00846.x
47. Tamura H, Ohtsuka M, Washiro M, Kimura F, Shimizu H, Yoshidome H, et al. Reg IV expression and clinicopathologic features of gallbladder carcinoma. Hum Pathol (2009) 40(12):1686–92. doi: 10.1016/j.humpath.2009.06.001
48. Sasahira T, Oue N, Kirita T, Luo Y, Bhawal UK, Fujii K, et al. Reg IV expression is associated with cell growth and prognosis of adenoid cystic carcinoma in the salivary gland. Histopathology (2008) 53(6):667–75. doi: 10.1111/j.1365-2559.2008.03188.x
49. Ma X, Wu D, Zhou S, Wan F, Liu H, Xu X, et al. The pancreatic cancer secreted REG4 promotes macrophage polarization to M2 through EGFR/AKT/CREB pathway. Oncol Rep (2016) 35(1):189–96. doi: 10.3892/or.2015.4357
50. Bishnupuri KS, Sainathan SK, Bishnupuri K, Leahy DR, Luo Q, Anant S, et al. Reg4-induced mitogenesis involves Akt-GSK3beta-beta-Catenin-TCF-4 signaling in human colorectal cancer. Mol Carcinogen (2014) 53(Suppl 1):E169–80. doi: 10.1002/mc.22088
51. Kawasaki Y, Matsumura K, Miyamoto M, Tsuji S, Okuno M, Suda S, et al. REG4 is a transcriptional target of GATA6 and is essential for colorectal tumorigenesis. Sci Rep (2015) 5:14291. doi: 10.1038/srep14291
52. Liu CM, Hsieh CL, He YC, Lo SJ, Liang JA, Hsieh TF, et al. In vivo targeting of ADAM9 gene expression using lentivirus-delivered shRNA suppresses prostate cancer growth by regulating REG4 dependent cell cycle progression. PLoS One (2013) 8(1):e53795. doi: 10.1371/journal.pone.0053795
53. Vanderlaag K, Wang W, Fayadat-Dilman L, Wagner J, Bald L, Grein J, et al. Regenerating islet-derived family member, 4 modulates multiple receptor tyrosine kinases and mediators of drug resistance in cancer. Int J Cancer (2012) 130(6):1251–63. doi: 10.1002/ijc.26089
54. Jin J, Lv H, Wu J, Li D, Chen K, Zhang F, et al. Regenerating Family Member 4 (Reg4) Enhances 5-Fluorouracil Resistance of Gastric Cancer Through Activating MAPK/Erk/Bim Signaling Pathway. Med Sci Monitor Int Med J Exp Clin Res (2017) 23:3715–21. doi: 10.12659/msm.903134
55. Hwang JH, Yoon J, Cho YH, Cha PH, Park JC, Choi KY. A mutant KRAS-induced factor REG4 promotes cancer stem cell properties via Wnt/beta-catenin signaling. Int J Cancer (2020) 146(10):2877–90. doi: 10.1002/ijc.32728
56. Xiao Y, Lu Y, Wang Y, Yan W, Cai W. Deficiency in intestinal epithelial Reg4 ameliorates intestinal inflammation and alters the colonic bacterial composition. Mucosal Immunol (2019) 12(4):919–29. doi: 10.1038/s41385-019-0161-5
57. Kobunai T, Watanabe T, Fukusato T. REG4, NEIL2, and BIRC5 gene expression correlates with gamma-radiation sensitivity in patients with rectal cancer receiving radiotherapy. Anticancer Res (2011) 31(12):4147–53.
58. Sninsky J, Bishnupuri K, Gonzalez I, Trikalinos N, Chen L, Dieckgraefe B. The Reg4-CD44ICD pathway and tumor aggression in early-stage colon adenocarcinoma. J Clin Oncol (2020) 38(4_suppl):234–. doi: 10.1200/JCO.2020.38.4_suppl.234
59. Zhang N, Chai D, Du H, Li K, Xie W, Li X, et al. Expression of Reg IV and SOX9 and their correlation in human gastric cancer. BMC Cancer (2018) 18(1):344. doi: 10.1186/s12885-018-4285-x
60. Zhang XQ, Yu LT, Du P, Yin TQ, Zhang ZY, Xu Y, et al. Single-chain Antibody Against Reg4 Suppresses Gastric Cancer Cell Growth and Enhances 5-FU-induced Cell Death in vitro. Anti-cancer Agents Med Chem (2019) 19(5):610–9. doi: 10.2174/1871520619666181122104720
61. Zhou W, Sun M, Wang DL, Wang Y, Jin F, Zhang YY, et al. Silencing of RegIV by shRNA causes the loss of stemness properties of cancer stem cells in MKN45 gastric cancer cells. Oncol Rep (2013) 30(6):2685–90. doi: 10.3892/or.2013.2745
62. Koh MJ, Shin DH, Lee SJ, Hwang CS, Lee HJ, Kim A, et al. Gastric-type gene expression and phenotype in non-terminal respiratory unit type adenocarcinoma of the lung with invasive mucinous adenocarcinoma morphology. Histopathology (2020) 76(6):898–905. doi: 10.1111/his.14077
63. Sorich MJ, Wiese MD, Rowland A, Kichenadasse G, McKinnon RA, Karapetis CS. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol (2015) 26(1):13–21. doi: 10.1093/annonc/mdu378
64. Yarden Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer (2001) 37(Suppl 4):S3–8. doi: 10.1016/s0959-8049(01)00230-1
65. Johnson AC, Murphy BA, Matelis CM, Rubinstein Y, Piebenga EC, Akers LM, et al. Activator protein-1 mediates induced but not basal epidermal growth factor receptor gene expression. Mol Med (2000) 6(1):17–27.
66. Li J, Chen H, Tang MS, Shi X, Amin S, Desai D, et al. PI-3K and Akt are mediators of AP-1 induction by 5-MCDE in mouse epidermal Cl41 cells. J Cell Biol (2004) 165(1):77–86. doi: 10.1083/jcb.200401004
67. Li J, Tang MS, Liu B, Shi X, Huang C. A critical role of PI-3K/Akt/JNKs pathway in benzo[a]pyrene diol-epoxide (B[a]PDE)-induced AP-1 transactivation in mouse epidermal Cl41 cells. Oncogene (2004) 23(22):3932–44. doi: 10.1038/sj.onc.1207501
68. He XJ, Jiang XT, Ma YY, Xia YJ, Wang HJ, Guan TP, et al. REG4 contributes to the invasiveness of pancreatic cancer by upregulating MMP-7 and MMP-9. Cancer Sci (2012) 103(12):2082–91. doi: 10.1111/cas.12018
69. Huang J, Yang Y, Yang J, Li X. Regenerating gene family member 4 promotes growth and migration of gastric cancer through protein kinase B pathway. Int J Clin Exp Med (2014) 7(9):3037–44.
70. Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer (2009) 9(3):153–66. doi: 10.1038/nrc2602
71. Cristobal I, Rincon R, Manso R, Rojo F, Garcia-Foncillas J. Re: Role of oncogenic K-Ras in cancer stem cell activation by aberrant Wnt/beta-catenin signaling. J Natl Cancer Institute (2014) 106(8). doi: 10.1093/jnci/dju196
72. Katsuno Y, Ehata S, Yashiro M, Yanagihara K, Hirakawa K, Miyazono K. Coordinated expression of REG4 and aldehyde dehydrogenase 1 regulating tumourigenic capacity of diffuse-type gastric carcinoma-initiating cells is inhibited by TGF-beta. J Pathol (2012) 228(3):391–404. doi: 10.1002/path.4020
73. Martin AC, Cardoso AC, Selistre-de-Araujo HS, Cominetti MR. Recombinant disintegrin domain of human ADAM9 inhibits migration and invasion of DU145 prostate tumor cells. Cell Adhesion Migration (2015) 9(4):293–9. doi: 10.4161/19336918.2014.994917
74. Pen CC, Liu CM, Lin CC, Lin CC, Hsieh TF, Josson S, et al. Combined Dynamic Alterations in Urinary VEGF Levels and Tissue ADAM9 Expression as Markers for Lethal Phenotypic Progression of Prostate Cancer. Chin J Physiol (2012) 55(6):390–7. doi: 10.4077/CJP.2012.BAA075
75. Fritzsche FR, Jung M, Tolle A, Wild P, Hartmann A, Wassermann K, et al. ADAM9 expression is a significant and independent prognostic marker of PSA relapse in prostate cancer. Eur Urol (2008) 54(5):1097–106. doi: 10.1016/j.eururo.2007.11.034
76. Sung SY, Kubo H, Shigemura K, Arnold RS, Logani S, Wang R, et al. Oxidative stress induces ADAM9 protein expression in human prostate cancer cells. Cancer Res (2006) 66(19):9519–26. doi: 10.1158/0008-5472.CAN-05-4375
77. Liu JY, Yang XJ, Geng XF, Huang CQ, Yu Y, Li Y. Prognostic significance of tumor-associated macrophages density in gastric cancer: a systemic review and meta-analysis. Minerva Med (2016) 107(5):314–21.
78. Zhang T, Liu L, Lai W, Zeng Y, Xu H, Lan Q, et al. Interaction with tumorassociated macrophages promotes PRL3induced invasion of colorectal cancer cells via MAPK pathwayinduced EMT and NFkappaB signalinginduced angiogenesis. Oncol Rep (2019) 41(5):2790–802. doi: 10.3892/or.2019.7049
79. Gao L, Zhang W, Zhong WQ, Liu ZJ, Li HM, Yu ZL, et al. Tumor associated macrophages induce epithelial to mesenchymal transition via the EGFR/ERK1/2 pathway in head and neck squamous cell carcinoma. Oncol Rep (2018) 40(5):2558–72. doi: 10.3892/or.2018.6657
80. Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J BioMed Sci (2019) 26(1):78. doi: 10.1186/s12929-019-0568-z
81. Lian G, Chen S, Ouyang M, Li F, Chen L, Yang J. Colon Cancer Cell Secretes EGF to Promote M2 Polarization of TAM Through EGFR/PI3K/AKT/mTOR Pathway. Technol Cancer Res Treat (2019) 18:1533033819849068. doi: 10.1177/1533033819849068
82. Tremblay M, Sanchez-Ferras O, Bouchard M. GATA transcription factors in development and disease. Development (2018) 145(20). doi: 10.1242/dev.164384
83. Cai WS, Shen F, Li JL, Feng Z, Wang YC, Xiao HQ, et al. Activated protease receptor-2 induces GATA6 expression to promote survival in irradiated colon cancer cells. Arch Biochem Biophysics (2014) 555–556:28–32. doi: 10.1016/j.abb.2014.05.021
84. Rosiq S, Hammam O, Abdelalim A, Anas A, Khalil H, Amer M. Colonic Stem Cells Expression of Lgr5 and CD133 Proteins as Predictive Markers in Colorectal Cancer among Egyptian Patients. Open Access Macedonian J Med Sci (2018) 6(6):968–74. doi: 10.3889/oamjms.2018.208
85. Tsuji S, Kawasaki Y, Furukawa S, Taniue K, Hayashi T, Okuno M, et al. The miR-363-GATA6-Lgr5 pathway is critical for colorectal tumourigenesis. Nat Commun (2014) 5:3150. doi: 10.1038/ncomms4150
86. Whissell G, Montagni E, Martinelli P, Hernando-Momblona X, Sevillano M, Jung P, et al. The transcription factor GATA6 enables self-renewal of colon adenoma stem cells by repressing BMP gene expression. Nat Cell Biol (2014) 16(7):695–707. doi: 10.1038/ncb2992
87. Verzi MP, Shin H, He HH, Sulahian R, Meyer CA, Montgomery RK, et al. Differentiation-specific histone modifications reveal dynamic chromatin interactions and partners for the intestinal transcription factor CDX2. Dev Cell (2010) 19(5):713–26. doi: 10.1016/j.devcel.2010.10.006
88. Naito Y, Oue N, Hinoi T, Sakamoto N, Sentani K, Ohdan H, et al. Reg IV is a direct target of intestinal transcriptional factor CDX2 in gastric cancer. PLoS One (2012) 7(11):e47545. doi: 10.1371/journal.pone.0047545
89. Oue N, Sentani K, Sakamoto N, Yasui W. Clinicopathologic and molecular characteristics of gastric cancer showing gastric and intestinal mucin phenotype. Cancer Sci (2015) 106(8):951–8. doi: 10.1111/cas.12706
Keywords: REG4, cancer, mechanism, clinical significance, biological function
Citation: Zhang J, Zhu Z, Miao Z, Huang X, Sun Z, Xu H and Wang Z (2021) The Clinical Significance and Mechanisms of REG4 in Human Cancers. Front. Oncol. 10:559230. doi: 10.3389/fonc.2020.559230
Received: 20 August 2020; Accepted: 23 November 2020;
Published: 08 January 2021.
Edited by:
Annamaria Agnes, Catholic University of the Sacred Heart, ItalyReviewed by:
Ram Mohan Ram Kumar, Rajiv Gandhi Centre for Biotechnology, IndiaGopi Sundaram, Central Food Technological Research Institute (CSIR), India
Copyright © 2021 Zhang, Zhu, Miao, Huang, Sun, Xu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenning Wang, am9zaWVvbjgyNkBzaW5hLmNu
 Junyan Zhang
Junyan Zhang Zhi Zhu
Zhi Zhu Xuanzhang Huang
Xuanzhang Huang Huimian Xu
Huimian Xu Zhenning Wang
Zhenning Wang