- Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Ultrasound, Peking University Cancer Hospital and Institute, Beijing, China
Radiofrequency ablation (RFA) can be a favorable option for patients with colorectal liver metastasis (CRLM). However, current reports about the therapeutic efficacy of liver resection (LR) and RFA for colorectal liver metastasis (CRLM) still remain controversial, especially for solitary CRLM. Therefore, this meta-analysis was performed to evaluate the therapeutic efficacy between LR and RFA for solitary CRLM. First, a comprehensive search for published studies was conducted using PubMed, the Cochrane Library Central, and Web of Science. Each study was reviewed and data extracted. In this meta-analysis, 10 studies (11 study arms) were finally included. The meta-analysis was performed using risk ratio (RR) and random effect model or fixed effect model, in which 95% confidence intervals (95% CI) for RR were calculated. The primary outcomes were disease-free survival (DFS) and overall survival (OS) at 1, 3, or 5 years plus complication rate. The results showed that patients treated by LR achieved better PFS and OS than those by RFA, but subgroup analysis and meta-regression displayed that the efficacy of RFA was equivalent to that of LR in solitary CRLM, when conditions were limited to tumors of ≤ 3 cm and fewer synchronous metastasis in the publication years 2011–2018. Meanwhile, RFA achieved lower complication rates when compared with LR. In conclusion, although patients treated by RFA cannot achieve better PFS and OS than those by LR, RFA can be considered a viable treatment option for solitary CRLM, with potentially lower complication rates.
Introduction
Colorectal cancer has become one of the most common human malignancies, affecting nearly 1 million individuals in the world every year (1, 2). When the colorectal cancer was diagnosed, up to half of the patients developed colorectal liver metastases (CRLM) (3). Colorectal liver metastasis significantly affects overall survival (OS), which has become the leading cause of cancer-related mortality in patients with colorectal cancer; the median OS for patients with untreated CRLM is 4.5–12 months (4, 5). Currently, liver resection (LR) is considered as the most effective treatment approach for CRLMs. However, only 10–30% of the cases are considered eligible for surgical resection because of general health status, anatomical location, disease extent, hepatic function reservation, or comorbidities (6–8).
Radiofrequency ablation (RFA), as a common minimally invasive treatment modality, has been widely used in clinical practice for the local control of liver tumors, and previous reports have demonstrated that thermal ablation had an advantage over surgical resection in being less invasive for hepatocellular carcinoma (HCC) (≤ 3 cm); therefore, it can also be an alternative option for patients with unresectable CRLM (9–14). Although RFA has established its role in the management of HCC as a safe, well-tolerated, and less invasive procedure, there has been no consensus on the therapeutic efficacy of RFA for those patients with CRLM, especially for solitary lesions (15–18).
In recent years, several studies about solitary CRLM reported a comparable OS and complication rates for RFA vs. LR. These results have led to the discussion that RFA should be favored over LR due to its less invasive and easily repeated procedure, yet RFA for patients with unresectable CRLM has been labeled inferior to LR for patients with resectable CRLM according to previous studies (17, 19). However, these results should be interpreted with caution because of the apparent selection bias.
Along this line, this study analyzed the existing literature comparing the therapeutic efficacy and safety of RFA and LR for patients with solitary CRLM by conducting a meta-analysis and analyzed the factors influencing prognosis to evaluate noninferiority or inferiority of RFA for patients with unresectable CRLM.
Methods
Literature Search
The QUOROM guidelines were followed for conducting the meta-analysis. The systematic literature search was performed independently by two of the authors using PubMed, Web of Science, and the Cochrane Library Central. No restriction was set for the date of publication. Only studies on humans and in the English language were considered for inclusion. The following Medical Subject Heading terms (MeSH) search headings were used: “radiofrequency ablation,” “resection,” “surgical treatment,” “surgery,” “hepatectomy,” “colorectal tumor,” “colorectal neoplasm,” “colorectal cancer,” “liver,” “hepatic,” “metastases,” and “metastasis.” The computer program Endnote X7 was used for reference management.
Inclusion Criteria
For inclusion in the meta-analysis, the study had to fulfill the following criteria: (1) the comparative studies of clinical outcomes between RFA and LR for solitary CRLM; (2) the studies reporting at least 1-year disease-free survival and 3- or 5-year overall survival of each treatment group; (3) the studies clearly document indications for RFA and HR; (4) when more than one study were reported by the same research, the one of higher quality or the most recent publication was included.
Exclusion Criteria
The following studies (cohorts) were excluded: (1) the original studies lacking the comparative results about the clinical outcomes of RFA and LR; (2) those studies published in the form of review articles (including meta-analysis), abstracts, comments, letters, editorials, and case reports.
Data Extraction
Data extraction was performed independently by Wu Hao and Jiang Binbin, and in the case of discrepancy, the decision was made by a discussion with a third author (Yan Kun). For literatures with no clear survival data, data extraction was performed in the survival curve from primary literature by the Engauge digitizer software. The following parameters from each study were extracted: (1) the first author, the year of publication, study design; (2) the baseline oncological characteristics of patients including tumor size, tumor count, study period, primary lymph nodes, adjuvant chemotherapy, timing of metastasis; and (3) the outcome of the trials including 1-year disease-free survival (DFS), 3- and 5-year overall survival (OS), and complications.
Statistical Analysis
All analyses were performed using the STATA statistical software package version 12.0 (STATA Corp., College Station, Texas, USA). Calculation for dichotomous variables was carried out using the estimation of risk ratio (RR) with a 95% confidence interval (95% CI). The pooled effect was calculated using either the fixed effects model or the random effects model. The heterogeneity among the included studies was evaluated by the I2 statistics and Chi-squared test. In addition, the heterogeneity was considered to be present if the I2 was more than 50%. Sensitivity analysis was performed to evaluate the stability of the results by subgroup analysis and meta-regression analysis. Evidence of publication bias was evaluated using the Begg's test. Two-sided P < 0.05 was considered statistically significant.
Results
Selection of Trials
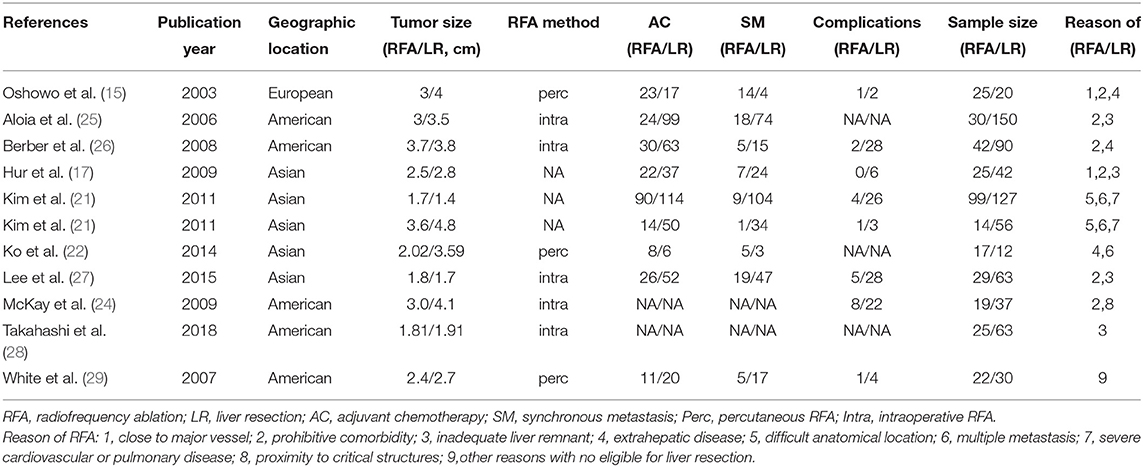
After initial screening, 11 potentially relevant clinical study cohorts were identified. Of them, two study cohorts were from the same medical center, the latest one with the most comprehensive information was enrolled. Thus, a total of 10 study cohorts (11 study arms) with sample size ranging from 29 to 226 have been enrolled (Figure 1) (2, 15–18, 20–24). Among them, 690 patients underwent LR, and 347 patients underwent RFA. A detailed information of the included studies is summarized in Table 1.
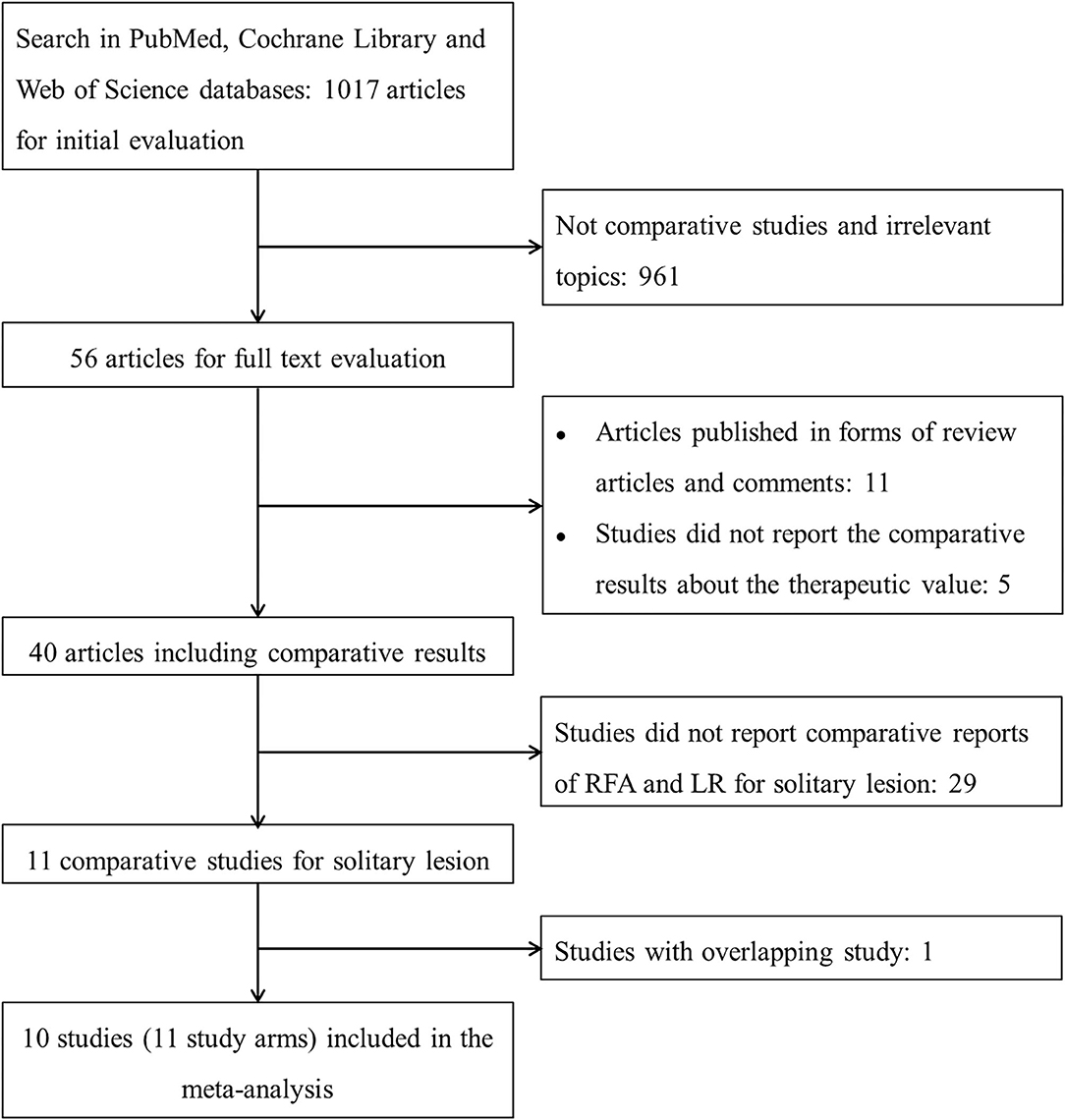
Figure 1. The flowchart describing the selection and exclusion of the existing literature. RFA, radiofrequency ablation; LR, liver resection.
Efficacy
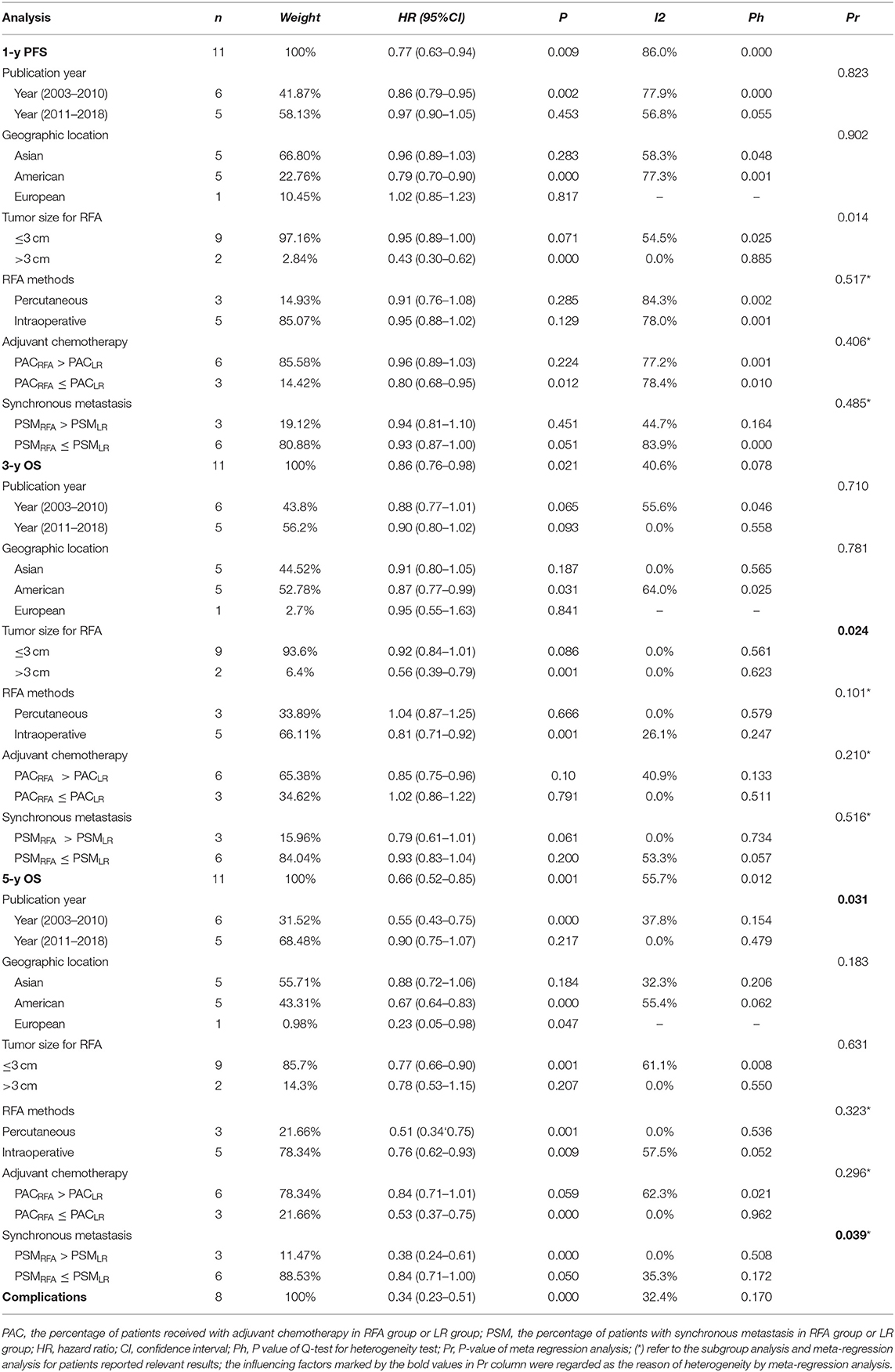
With observable interstudy heterogeneity, patients in the RFA group had slightly inferior 1-year PFS (RR: 0.77, 95% CI: 0.630–0.940, P = 0.009, I2 = 86.0%, Ph = 0.000) (Figure 2A, Table 2), 3-year OS (RR: 0.860, 95% CI: 0.760–0.980, P = 0.021, I2 = 40.6%, Ph = 0.078) (Figure 2B, Table 2), and 5-year OS (RR: 0.66, 95% CI:0.52–0.85, P = 0.001, I2 = 55.7%, Ph = 0.012) (Figure 2C, Table 2) when compared with patients in the LR group.
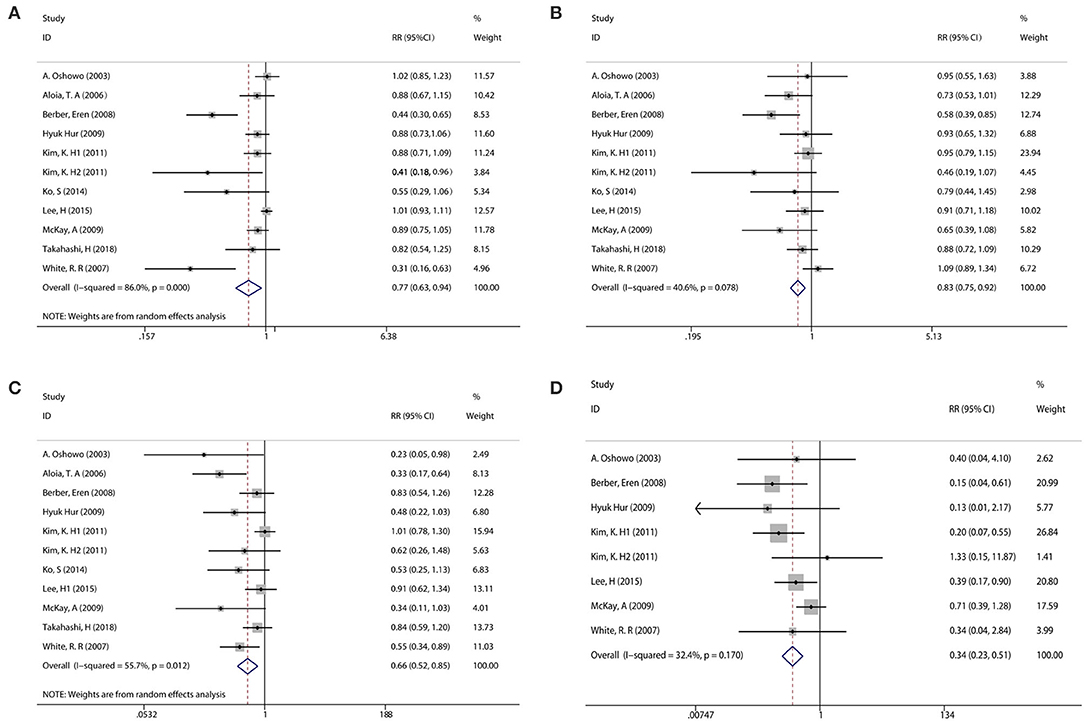
Figure 2. Pooled analysis comparing the survival rate between RFA and LR groups. (A) Pooled analysis comparing the 1-year PFS rate. (B) Pooled analysis comparing the 3-year overall survival (OS) rate. (C) Pooled analysis comparing the 5-year OS rate. (D) Pooled analysis comparing the complication rate.
Complications
Eight of the included studies compared the complications between the RFA group and the LR group. The incidence of postoperative complication was significantly lower in the RFA group than that in the LR group (RR: 0.340, 95% CI: 0.230–0.510, P = 0.000, I2 = 32.4%, Ph <0.170) (Figure 2D, Table 2).
Subgroup Analysis and Meta-Regression
Considering an existing difference among the studies and the differences among participants could contribute to overall heterogeneity among the included studies; the subgroup analysis was used to examine possible relationships between the study characteristics and 1-year PFS, 3-year OS, and 5-year OS. The subgroup analysis underlined several variables that could affect overall heterogeneity and influence the results of this meta-analysis. Namely, these were the publication years 2011–2018, geographic location (Asian), tumor size for RFA ( ≤ 3 cm), and adjuvant chemotherapy [the percentage of patients with adjuvant chemotherapy (PAC); PACRFA > PACLR] in 1-year PFS; the geographic location (Asian), tumor size for RFA (≤ 3 cm), and adjuvant chemotherapy (PACRFA ≤ PACLR) in 3-year OS; the publication years 2011–2018, geographic location (Asian), tumor size for RFA (>3 cm), adjuvant chemotherapy (PACRFA > PACLR), and synchronous metastasis (the percentage of patients with synchronous metastasis, PSM; PSMRFA > PSMLR) in 5-year OS (Figures 3A–D, Table 2). In addition, to further confirm the reason of heterogeneity, a meta-regression analysis was performed with predefined variables. The results of the meta-regression analysis also confirmed the obtained clarification of heterogeneity proposed by the subgroup analysis at several aspects, such as tumor size for RFA (Figures 3A,B, Table 2), publication year (Figure 3C, Table 2), and the percentage of patients with synchronous metastasis (Figure 3D, Table 2).
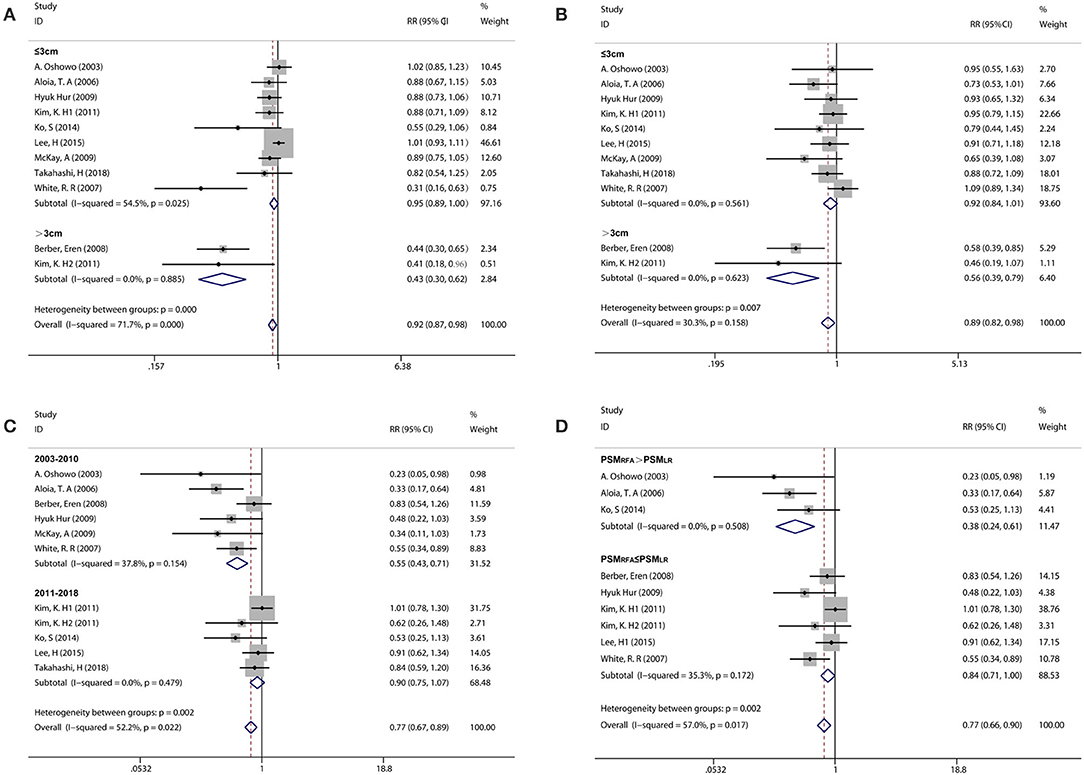
Figure 3. Subgroup analysis comparing the survival rate between RFA and LR groups. (A) Subgroup analysis for tumor size in the 1-year PFS. (B) Subgroup analysis for tumor size in the 3-year OS. (C) Subgroup analysis for publication year in the 5-year OS. (D) Subgroup analysis for the percentage of patients with synchronous metastasis in the 5-year OS.
Publication Bias
The funnel plot did not show significant asymmetry by the Begg's test in 1-year PFS (Pr > |z| = 0.016) (Figure 4A), 3-year OS (Pr > |z| = 0.073) (Figure 4B), 5-year OS (Pr > |z| = 0.016) (Figure 4C), and complication rates (Pr > |z| = 1.000) (Figure 4D).
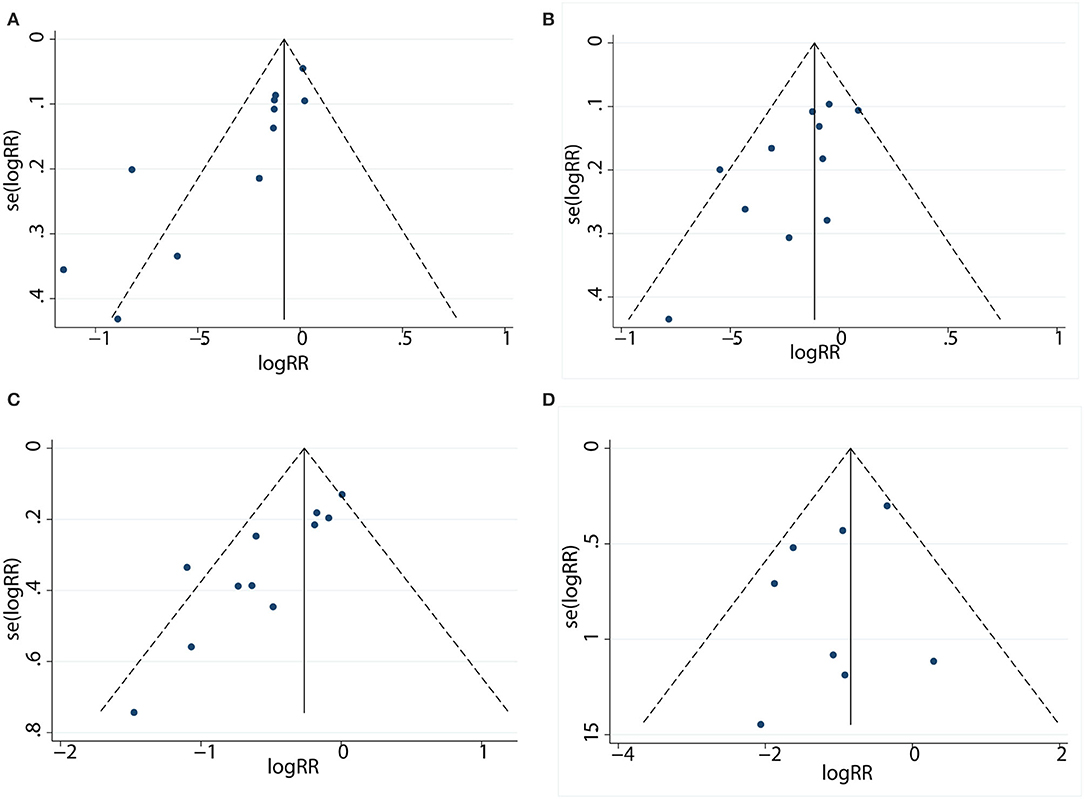
Figure 4. Funnel plot to detect publication bias between patients in the LR and RFA. (A) Funnel plot describing the comparative analysis of 1-year PFS rate. (B) Funnel plot describing the comparative analysis of 3-year OS rate. (C) Funnel plot describing the comparative analysis of 5-year OS rate. (D) Funnel plot describing the comparative analysis of complication rate.
Discussion
This meta-analysis examined published data and evidence obtained from relevant clinical trials to provide pooled estimates regarding the treatment efficacy between RFA and LR in CRLMs. In the present meta-analysis, the results found that patients with CRLMs who were treated by LR achieved better survival outcomes than those who were treated by RFA. However, RFA outperformed LR in terms of fewer perioperative complication rates.
In the meta-analysis, the inferior survival outcomes of RFA could be explained as follows. First, RFA patients in the included studies were not eligible for liver resection because of poor health status, prohibitive comorbidity, extrahepatic disease, multiple metastasis, inadequate liver remnant, etc. The poor basic condition may shorten the overall survival for patients with CRLMs. Second, there were several complex characteristics for tumors treated with RFA, such as close to the major vessel, larger lesion size, and difficult anatomical location. These characteristics increased the possibility of incomplete ablation for tumors, which would further accelerate the risk of tumor recurrence after RFA.
Because of the significant heterogeneity among the included studies, subgroup analysis and meta-regression were carried on. Considering the previously mentioned fact that incomplete ablation resulting from larger tumor size induced the expansion of tumor-initiating cells (16, 30), a subgroup analysis was performed for the influence of tumor size on RFA outcomes. The results showed that RFA patients with no more than a 3-cm tumor can achieve equivalent outcomes when compared with LR patients. In addition, the data from the meta-regression also displayed that the tumor size had an obvious influence on the significant heterogeneity in 1-year PFS and 3-year OS. According to the influence of tumor size on HCC outcomes, the reason for this may be explained by patients with smaller lesions achieving a higher ablation success rate, which can reduce the tumor recurrence attributed to hypoxia-driven acceleration of tumor growth occurring in the transition zone and the stimulated outgrowth of perilesional micrometastases (31, 32).
In contrast to the previous meta-analysis, this study also added the subgroup analysis about the publication year. Subgroup analysis showed that RFA patients in the publication years 2011–2018 had better survival than that in the publication years 2003–2010 and was identical when compared with LR patients in publication years 2011–2018. In addition, the meta-regression process in 5-year OS also further confirmed the subgroup analysis results. The results, to some extent, may be due to continuous improvement in the technical accuracy and performance of RFA in accordance with the learning curve for the treatment of CRLM, and the improvement process may be consistent with that in HCC treatment. In the past decades, RFA techniques have rapidly worked their way into clinical guidelines for the treatment of HCC, especially solitary small HCC. The international guidelines have shifted from surgical resection to minimally invasive percutaneous local ablation for small HCC (14, 33–35). Taken together, it will be possible that the therapeutic efficacy in the future will be better with the maturity of RFA technology in CRLM.
Meanwhile, to validate the effect of synchronous liver metastases on RFA therapeutic efficacy, this study also created an additional subgroup analysis and meta-regression. The results showed that synchronous liver metastases had a negative effect on the RFA therapeutic efficacy through subgroup analysis and meta-regression, which may be explained by cancer biology. According to the previous studies, the results showed that synchronous liver metastases have less favorable cancer biology and expected survival, and data from the corresponding registry showed that 5-year survival rates were shorter with synchronous than with metachronous CRLMs (36, 37). Yet, no biological marker has been identified that distinguishes synchronous metastases from metachronous metastases. Therefore, further research for biological markers is of significant importance for achieving better outcomes.
In addition, one of the results worth considering in the subgroup analysis was the finding that the Asian population can achieve better outcomes than the western population for RFA patients. Although, to the best of our knowledge, no exact evidence could be found to explain this, considering this observation, we propose the existence of a certain genetic variation among different ethnic groups. This was consistent with the fact that genome-wide polymorphism data have clearly established differences in allele frequency among continental regions (38). For the influence of gene mutation on CRLM, previous studies had demonstrated that KRAS mutation was associated with worse disease-free and overall survival following CRLM resection (39–41). However, previous reports showed that CRLM patients in Asian countries have similar KRAS mutation frequency when compared with those in western countries, and the morbidity and mortality of colorectal cancer in Asian countries were different from those in Western countries (42–44). Considering these facts, the result related to certain ethnic groups should only be used to generate a hypothesis that other genes, except the KRAS gene, may affect the outcomes for CRLM patients, which will be investigated in future research.
Of course, this meta-analysis has several limitations. First, all the included studies were retrospectively performed, which were susceptible to several biases. Second, significant heterogeneity was noticed, although the random effect model was used to compensate for part of the interstudy heterogeneity. In addition, the subgroup analysis in the adjuvant chemotherapy and RFA route should be interpreted with caution, which was mainly due to the failure of the meta-regression process to confirm the subgroup analysis results. In addition, the high-quality randomized controlled trails should be needed to resolve this problem and provide us with much more sound clinical evidences. Finally, publication bias remains to be a main concern; the Begg rank correlation for studies that involved comparative studies about therapeutic efficacy between RFA and LR suggested the presence of publication bias in 1-year PFS and 5-year OS. As we all know, articles with negative results were much more difficult to be published, and the majority of the included studies were from the surgery department; thus, the therapeutic efficacy of LR may be overvalued to some extent. In addition, although we tried to search for more relevant studies, the included number of studies may still be insufficient.
In conclusion, although LR was superior to RFA in the treatment of solitary CRLM in the meta-analysis, the subgroup analysis and meta-regression showed that the therapeutic efficacy of RFA was equivalent to that of LR in solitary CRLM, even when conditions were limited to tumors of ≤ 3 cm and fewer synchronous metastases in the publication year 2011–2018. Meanwhile, RFA provided lower rates of morbidities when compared with LR. In addition, further explanation should be interpreted through high-quality RCTs.
Data Availability Statement
All datasets generated for this study are included in the article/ supplementary material.
Author Contributions
The authors were responsible for the study design, data collection and analysis, preparation of the manuscript, and the final publishing decision. YK and YW designed the study, made a critical assessment, and had overall responsibility for this study. WH and JB conducted the literature review and data extraction, performed the statistical analysis, interpreted the statistical results, and wrote the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by Beijing Municipal Science & Technology Commission (Grant no. Z151100004015186), the Capital Health Research and Development of Special (Grant no. 2020-2-2152), and the Fellowship of China postdoctoral science Foundation (Grant no. 2020M670065).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. Cancer J Clin. (2015) 65:87–108. doi: 10.3322/caac.21262
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
3. Ruers T, Van Coevorden F, Punt CJA, Pierie J-PEN, Borel-Rinkes I, Ledermann JA, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. (2017) 109:15. doi: 10.1093/jnci/djx015
4. Adam R, Yi B, Innominato PF, Barroso E, Laurent C, Giuliante F, et al. Resection of colorectal liver metastases after second-line chemotherapy: is it worthwhile? A LiverMetSurvey analysis of 6415 patients. Eur J Cancer. (2017) 78:7–15. doi: 10.1016/j.ejca.2017.03.009
5. Bengmark S, Hafstrom L. The natural history of primary and secondary malignant tumors of the liver. I. The prognosis for patients with hepatic metastases from colonic and rectal carcinoma by laparotomy. Cancer. (1969) 23:198–202. doi: 10.1002/1097-0142(196901)23:1<198::aid-cncr2820230126>3.0.co;2-j
6. Adam R, Hoti E, Folprecht G, Benson AB. Accomplishments in 2008 in the management of curable metastatic colorectal cancer. Gastrointest Cancer Res. (2009) 3:S15–22.
7. Ruers T, Punt C, Van Coevorden F, Pierie JP, Borel-Rinkes I, Ledermann JA, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol. (2012) 23:2619–26. doi: 10.1093/annonc/mds053
8. Song KD, Lim HK, Rhim H, Lee MW, Kim YS, Lee WJ, et al. Repeated hepatic resection versus radiofrequency ablation for recurrent hepatocellular carcinoma after hepatic resection: a propensity score matching study. Radiology. (2015) 275:599–608. doi: 10.1148/radiol.14141568
9. Ahmed M, Brace CL, Lee FT Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. (2011) 258:351–69. doi: 10.1148/radiol.10081634
10. Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Renal cell carcinoma: clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology. (2003) 226:417–24. doi: 10.1148/radiol.2262012062
11. Frericks BB, Ritz JP, Roggan A, Wolf KJ, Albrecht T. Multipolar radiofrequency ablation of hepatic tumors: initial experience. Radiology. (2005) 237:1056–62. doi: 10.1148/radiol.2373041104
12. Chen MH, Yang W, Yan K, Zou MW, Solbiati L, Liu JB, et al. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients–mathematic model, overlapping mode, and electrode placement process. Radiology. (2004) 232:260–71. doi: 10.1148/radiol.2321030821
13. Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. (2006) 243:321–8. doi: 10.1097/01.sla.0000201480.65519.b8
14. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. (2012) 379:1245–55. doi: 10.1016/S0140-6736(11)61347-0
15. Oshowo A, Gillams A, Harrison E, Lees WR, Taylor I. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg. (2003) 90:1240–3. doi: 10.1002/bjs.4264
16. Zhao W, Wang L, Han H, Jin K, Lin N, Guo T, et al. 1B50-1, a mAb raised against recurrent tumor cells, targets liver tumor-initiating cells by binding to the calcium channel alpha2delta1 subunit. Cancer Cell. (2013) 23:541–56. doi: 10.1016/j.ccr.2013.02.025
17. Hur H, Ko YT, Min BS, Kim KS, Choi JS, Sohn SK, et al. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg. (2009) 197:728–36. doi: 10.1016/j.amjsurg.2008.04.013
18. Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. (2007) 445:111–5. doi: 10.1038/nature05384
19. Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. (2004) 239:818–25; discussion 25–7. doi: 10.1097/01.sla.0000128305.90650.71
20. Pattabiraman DR, Bierie B, Kober KI, Thiru P, Krall JA, Zill C, et al. Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science. (2016) 351:aad3680. doi: 10.1126/science.aad3680
21. Kim KH, Yoon YS, Yu CS, Kim TW, Kim HJ, Kim PN, et al. Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J Korean Surg Soc. (2011) 81:25–34. doi: 10.4174/jkss.2011.81.1.25
22. Ko S, Jo H, Yun S, Park E, Kim S, Seo HI. Comparative analysis of radiofrequency ablation and resection for resectable colorectal liver metastases. World J Gastroenterol. (2014) 20:525–31. doi: 10.3748/wjg.v20.i2.525
23. Li N, Zhu Y. Targeting liver cancer stem cells for the treatment of hepatocellular carcinoma. Therap Adv Gastroenterol. (2019) 12:1756284818821560. doi: 10.1177/1756284818821560
24. McKay A, Fradette K, Lipschitz J. Long-term outcomes following hepatic resection and radiofrequency ablation of colorectal liver metastases. HPB Surg. (2009) 2009:346863. doi: 10.1155/2009/346863
25. Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, Wei SH, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg. (2006) 141:460–6; discussion 6–7. doi: 10.1001/archsurg.141.5.460
26. Berber E, Tsinberg M, Tellioglu G, Simpfendorfer CH, Siperstein AE. Resection versus laparoscopic radiofrequency thermal ablation of solitary colorectal liver metastasis. J Gastrointest Surg. (2008) 12:1967–72. doi: 10.1007/s11605–008-0622–8
27. Lee H, Heo JS, Cho YB, Yun SH, Kim HC, Lee WY, et al. Hepatectomy vs radiofrequency ablation for colorectal liver metastasis: a propensity score analysis. World J Gastroenterol. (2015) 21:3300–7. doi: 10.3748/wjg.v21.i11.3300
28. Takahashi H, Akyuz M, Kahramangil B, Kose E, Aucejo F, Fung J, et al. A comparison of the initial cost associated with resection versus laparoscopic radiofrequency ablation of small solitary colorectal liver metastasis. Surg Laparosc Endosc Percutan Tech. (2018) 28:371–4. doi: 10.1097/sle.0000000000000577
29. White RR, Avital I, Sofocleous CT, Brown KT, Brody LA, Covey A, et al. Rates and patterns of recurrence for percutaneous radiofrequency ablation and open wedge resection for solitary colorectal liver metastasis. J Gastrointest Surg. (2007) 11:256–63. doi: 10.1007/s11605-007-0100-8
30. Han Y, Yan D, Xu F, Li X, Cai JQ. Radiofrequency ablation versus liver resection for colorectal cancer liver metastasis: an updated systematic review and meta-analysis. Chin Med J. (2016) 129:2983–90. doi: 10.4103/0366-6999.195470
31. Nijkamp MW, van der Bilt JD, de Bruijn MT, Molenaar IQ, Voest EE, van Diest PJ, et al. Accelerated perinecrotic outgrowth of colorectal liver metastases following radiofrequency ablation is a hypoxia-driven phenomenon. Ann Surg. (2009) 249:814–23. doi: 10.1097/SLA.0b013e3181a38ef5
32. von Breitenbuch P, Kohl G, Guba M, Geissler E, Jauch KW, Steinbauer M. Thermoablation of colorectal liver metastases promotes proliferation of residual intrahepatic neoplastic cells. Surgery. (2005) 138:882–7. doi: 10.1016/j.surg.2005.05.006
33. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. (2018) 69:182–236. doi: 10.1016/j.jhep.2018.03.019
34. Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. (2017) 11:317–70. doi: 10.1007/s12072-017-9799-9
35. Song P, Cai Y, Tang H, Li C, Huang J. The clinical management of hepatocellular carcinoma worldwide: a concise review and comparison of current guidelines from 2001 to 2017. Biosci Trends. (2017) 11:389–98. doi: 10.5582/bst.2017.01202
36. Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. (2006) 244:254–9. doi: 10.1097/01.sla.0000217629.94941.cf
37. Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev. (2015) 41:729–41. doi: 10.1016/j.ctrv.2015.06.006
38. Henn BM, Gravel S, Moreno-Estrada A, Acevedo-Acevedo S, Bustamante CD. Fine-scale population structure and the era of next-generation sequencing. Hum Mol Genet. (2010) 19:R221–6. doi: 10.1093/hmg/ddq403
39. Stremitzer S, Stift J, Gruenberger B, Tamandl D, Aschacher T, Wolf B, et al. KRAS status and outcome of liver resection after neoadjuvant chemotherapy including bevacizumab. Br J Surg. (2012) 99:1575–82. doi: 10.1002/bjs.8909
40. Karagkounis G, Torbenson MS, Daniel HD, Azad NS, Diaz LA Jr, et al. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer. (2013) 119:4137–44. doi: 10.1002/cncr.28347
41. Vauthey JN, Zimmitti G, Kopetz SE, Shindoh J, Chen SS, Andreou A, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. (2013) 258:619–26; discussion 26–7. doi: 10.1097/SLA.0b013e3182a5025a
42. Favoriti P, Carbone G, Greco M, Pirozzi F, Pirozzi RE, Corcione F. Worldwide burden of colorectal cancer: a review. Updates Surg. (2016) 68:7–11. doi: 10.1007/s13304-016-0359-y
43. Li ZZ, Wang F, Zhang ZC, Wang F, Zhao Q, Zhang DS, et al. Mutation profiling in chinese patients with metastatic colorectal cancer and its correlation with clinicopathological features and anti-EGFR treatment response. Oncotarget. (2016) 7:28356–68. doi: 10.18632/oncotarget.8541
44. Modest DP, Ricard I, Heinemann V, Hegewisch-Becker S, Schmiegel W, Porschen R, et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol. (2016) 27:1746–53. doi: 10.1093/annonc/mdw261
Keywords: liver resection, radiofrequency ablation, colorectal liver metastasis, therapeutic efficacy, meta-analysis
Citation: Hao W, Binbin J, Wei Y and Kun Y (2020) Can Radiofrequency Ablation Replace Liver Resection for Solitary Colorectal Liver Metastasis? A Systemic Review and Meta-Analysis. Front. Oncol. 10:561669. doi: 10.3389/fonc.2020.561669
Received: 25 June 2020; Accepted: 24 August 2020;
Published: 17 November 2020.
Edited by:
Aditya Juloori, University of Chicago Medical Center, United StatesReviewed by:
Bilgin Kadri Aribas, Bülent Ecevit University, TurkeySergio Jaramillo, Willis-Knighton Cancer Center, United States
Copyright © 2020 Hao, Binbin, Wei and Kun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Kun, eWRiekB2aXAuc2luYS5jb20=
 Wu Hao
Wu Hao Jiang Binbin
Jiang Binbin Yang Wei
Yang Wei Yan Kun
Yan Kun