- Department of Medical Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China
Background: Systemic inflammation score (SIS) has been verified as a novel prognostic indicator in several cancer types. However, its prognostic value in breast cancer remains unknown. Furthermore, a nomogram based on SIS is yet to be constructed for breast cancer. We conducted this study to explore the association between SIS and prognosis of breast cancer, and to construct a good prognostic nomogram model.
Methods: A total of 1,180 breast cancer patients who underwent curative surgery between December 2010 and January 2013 were recruited. They were randomly assigned to the training set (n = 944) or the validation set (n = 236). All patient blood samples were collected within 1 week prior to operation. According to previous reports, SIS was calculated for all patients, who were then classified into two groups: high-SIS and low-SIS. The Kaplan–Meier method was employed for survival analyses, and univariate and multivariate analyses (Cox proportional hazards regression model) were used for prognostic assessment. A nomogram was constructed based on the results of multivariate analysis. Calibration curves and concordance index (C-index) were compiled to determine predictive and discriminatory capacity.
Results: In the training set, the median follow-up time was 6.07 years. Patients in the high-SIS group had an average OS time of 68.05 months, which is shorter than that of the low-SIS group (72.87 months; P = 0.033). Patients in the high-SIS group had average RFS and DMFS times of 56.04 and 54.46 months, respectively, which are shorter than those of the low-SIS group (60.85 and 59.47 months, respectively; P = 0.247 and P = 0.032). Univariate and multivariate analyses revealed SIS to be an independent prognostic factor for OS and DMFS time. The nomogram for the training set indicated OS and DMFS C-indexes of 0.794 (95% CI, 0.772–0.816) and 0.712 (95% CI, 0.684–0.740), respectively. In the validation set, the OS and DMFS C-indexes were 0.889 (95% CI, 0.845–0.933) and 0.696 (95%. CI, 0.611–0.781), respectively.
Conclusions: SIS was confirmed as an independent prognostic predictor among patients with breast cancer who had undergone surgery with curative intent. Higher preoperative SIS may indicate higher risk of metastasis and shorter overall survival time. The prognostic nomogram based on SIS was dependable for breast cancer patients who underwent curative surgery.
Introduction
Breast cancer is the most common type of cancer in females, with the highest morbidity and mortality rates among all carcinomas in females (1). The diagnosis and management of breast cancer has advanced significantly, yet breast cancer remains a significant threat to female health. Surgery is generally regarded as the optimal therapeutic option when circumstances permit (2). According to estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2) status, breast cancer is divided into different subtypes (3). Each subtype has an associated appropriate postoperative therapy regime, involving chemotherapy, radiotherapy, endocrine therapy or targeted therapy (4, 5). Although a set of standard procedures has been implemented in the clinic, the death rate remains high, and recurrence and metastasis continue to occur. Therefore, the identification of novel prognostic indicators is essential to identify patients with a higher risk of recurrence and metastasis, so that appropriate treatment can be planned in advance. Various prognostic models are widely used for postoperative breast cancer patients, including the TNM staging system (6). However, due variation among individuals, accurate prediction of prognosis remains a challenge. A desirable prognostic predictor is one that considers individualized conditions.
Cancer-related inflammation is regarded as a hallmark of cancer (7, 8). In cancer patients, the systemic inflammatory response induces changes of the peripheral blood and plays a vital role in cancer pathogenesis and progression (9). Thus, several prognostic biomarkers associated with the systemic inflammatory response have been reported in various types of cancer, including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR) (10–13). However, there exists no generally accepted thresholds for peripheral blood-based biomarkers or inflammatory scoring systems. There is a growing consensus that finding a simple, personalized, integrated scoring system based on inflammation is desirable. Recently, a novel systemic inflammatory scoring system, named systemic inflammation score (SIS), had attracted attention. SIS incorporates preoperative serum albumin levels and lymphocyte-to-monocyte ratio (LMR). The baseline LMR at diagnosis seemed to have unfavorable impact on the clinical outcomes in non-hematologic malignancies, including breast cancer, in the previous studies (14–17). Albumin, a classic nutrition index, was also considered as an inflammation related factor, and it was found to have prognostic significance in solid cancer in many studies (18–22). The novel inflammatory index, SIS, is quite promising in the recent studies to better reflect disease change and predict survival outcomes. In theory, SIS, combining LMR and albumin, could better reflect disease change and predict survival outcome. And this assumption has been reported to predict prognosis in several cancer types, including clear cell renal cell carcinoma, gastric cancer and colorectal cancer (23–25). There are no previous reports regarding the use of SIS in breast cancer. We hypothesize that SIS is an independent prognostic indicator in breast cancer.
The nomogram is a widely used medical statistical method, which integrates diverse prognostic and determinant variables to predict event probability (26–28). The purpose of this study is to investigate the association between SIS and other clinical characteristics, and the capacity of SIS as a prognostic predictor for postoperative patients with breast cancer. We present a nomogram based on SIS that provides a reliable prognostic prediction method for patients with breast cancer.
Patients and Methods
Patients
A total of 1,180 patients with breast cancer were enrolled in this study. All patients underwent surgical resection with curative intent at the Sun Yat-sen University Cancer Center (SYSUCC; Guangzhou, China) between December 2010 and January 2013. The inclusion criteria were as follows: (1) histologically confirmed breast cancer; (2) postoperative of resection with curative intent. The exclusion criteria were as follows: (1) synchronal malignancies; (2) ductal carcinoma in situ; (3) lack of associated laboratorial records or missing follow-up data; (4) prior receipt of neoadjuvant or adjuvant chemotherapy; (5) receipt of any medicine within 3 months prior surgery that could induce immune or inflammatory responses; (6) any chronic inflammatory disease (including autoimmune diseases). This study was performed in accordance with the declaration of Helsinki and was approved by the Research Ethics Committee of SYSUCC. All subjects enrolled provided their written informed consent.
Sample Collection and Classification
All patient records were derived from SYSUCC. Blood samples were collected and analyzed within 1 week prior to surgery. Patients were classified into two groups based on median age. Tumors were staged according to the 8th AJCC (American Joint Committee on Cancer) Tumor-Node-Metastasis (TNM) staging system (6). The expression of ER, PR, HER2, Ki67 were scored using the St. Gallen criteria (3). Overall survival (OS) time was defined as the period from date of surgery to that of death or last follow-up. Recurrence-free survival (RFS) was defined as the period from the date of surgery to that of identification of first recurrence, death from any cause or last follow-up. Distant metastasis-free survival (DMFS) time was defined as the period from the date of surgery to that of identification of metastasis, death from any cause or last follow-up. The SIS score was calculated using two factors: serum albumin level and LMR. Patients with both hypoalbuminemia (<40 gL−1) and low LMR (<4.44) were assigned a score of 2; patients with either a decreased serum albumin level (<40 gL−1) or low LMR (<4.44) were assigned a score of 1; patients with a serum albumin level ≥ 40 gL−1 and LMR ≥ 4.44 were assigned a score of 0.
Follow-Up
Patients were followed up from the day of operation. Long term follow-up was performed by outpatient examination or telephone interview for the first 2 years and every 6 months for the following 3 years. The date of last follow-up date was September 2019.
Statistical Analysis
Statistical analyses were conducted using SPSS software (version 25.0; IBM Corp., Armonk, NY), GraphPad Prism (version 6.0; GraphPad, La Jolla, CA) or R software (“rms” package; version 5.1–0; Vanderbilt University, Nashville, TN). Survival curves were plotted using the Kaplan-Meier method, and significance was determined by the log-rank test. The associations between SIS and other key clinicopathological characteristics were analyzed by Chi-square test or Fisher's exact test. Simple and multivariate regression analyses were performed using the Cox proportional hazards model and multivariate regression analyses model for variables with P < 0.05 in the univariate analysis. Two-tailed P-values < 0.05 were considered to indicate statistically significant differences. A nomogram was created by R software using “rms” package and the nomograms of 3- and 5-year OS and DMFS times were developed. In this study, a validation group was used for internal validation. The concordance index (C-index) and calibration plots were calculated using OS and DMFS times both in training group and validation group. Calibration plots were generated to examine the performance characteristics of the predictive nomogram. The Harrell's Concordance index (C-index) was used to quantify the predictive accuracy (29), which ranges from 0.5 (no predictive power) to 1 (perfect prediction). All statistical tests were two-sided and were performed at a significance level of 0.05.
Results
Grouping by SIS Score
The cutoff of SIS was set at 1 and patients were grouped according to SIS score: group0 (low-SIS), group1 and group2 (high-SIS).
Clinicopathological Features and SIS Scores of the Training Set
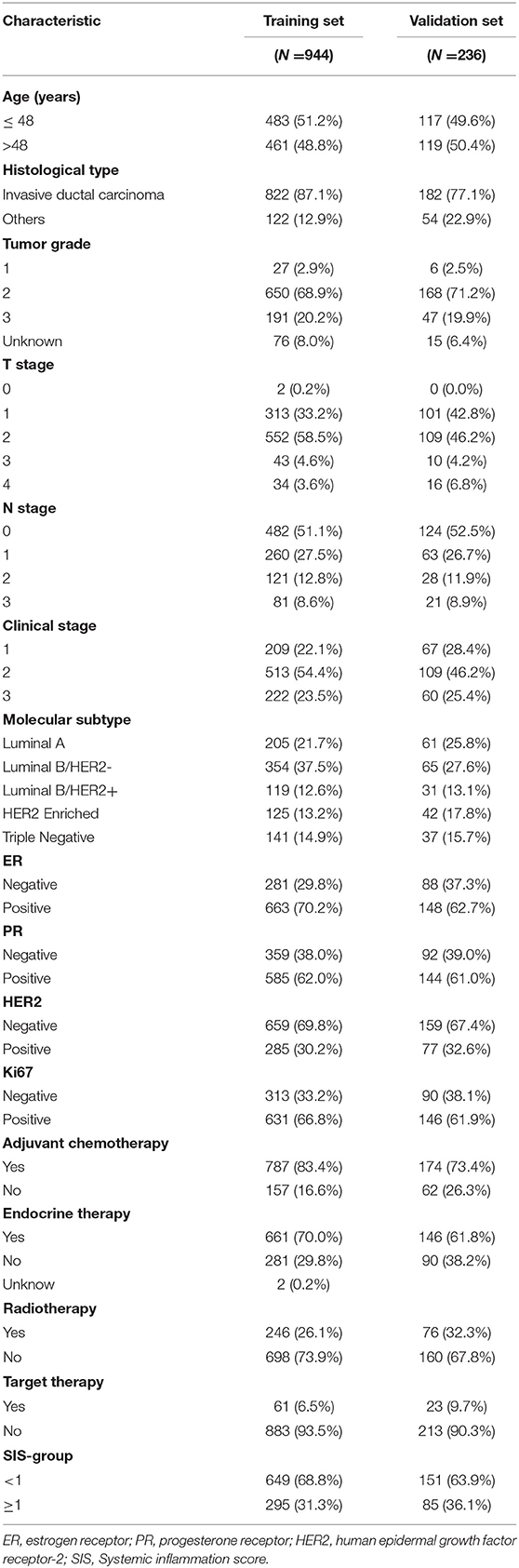
All patient characteristics are listed in Tables 1, 2. The median follow-up time was 6.07 years and the median age at surgery was 48 years old. A total of 822 (87.1%) patients had invasive ductal carcinoma, and 122 (12.9%) patients had other types of carcinoma. There were 209 (22.1%), 513 (54.4%) and 222 (23.5%) patients in stage 1, 2, and 3, respectively, according to the TNM staging system. A total of 205 (21.7%) patients were diagnosed as Luminal A, 354 (37.5%) as Luminal B/HER2–, 119 (12.6%) as Luminal B/HER2+, 125 (13.2%) as HER2 Enriched, and 141 (14.9%) as Triple Negative. ER expression was positive in 663 (70.2%) patients, PR expression was positive in 585 (62.0%) patients and HER2 expression was positive in 285 (30.2%) patients. Ki67 status was positive in 631 (66.8%) patients and negative in 313 (33.2%) patients. According to the aforementioned SIS classification system, 649 (68.7%) patients were stratified to low-SIS, and 295 (31.3%) patients to high-SIS. According to our stratification of SIS, we observed that high-SIS was associated with high T-stage.
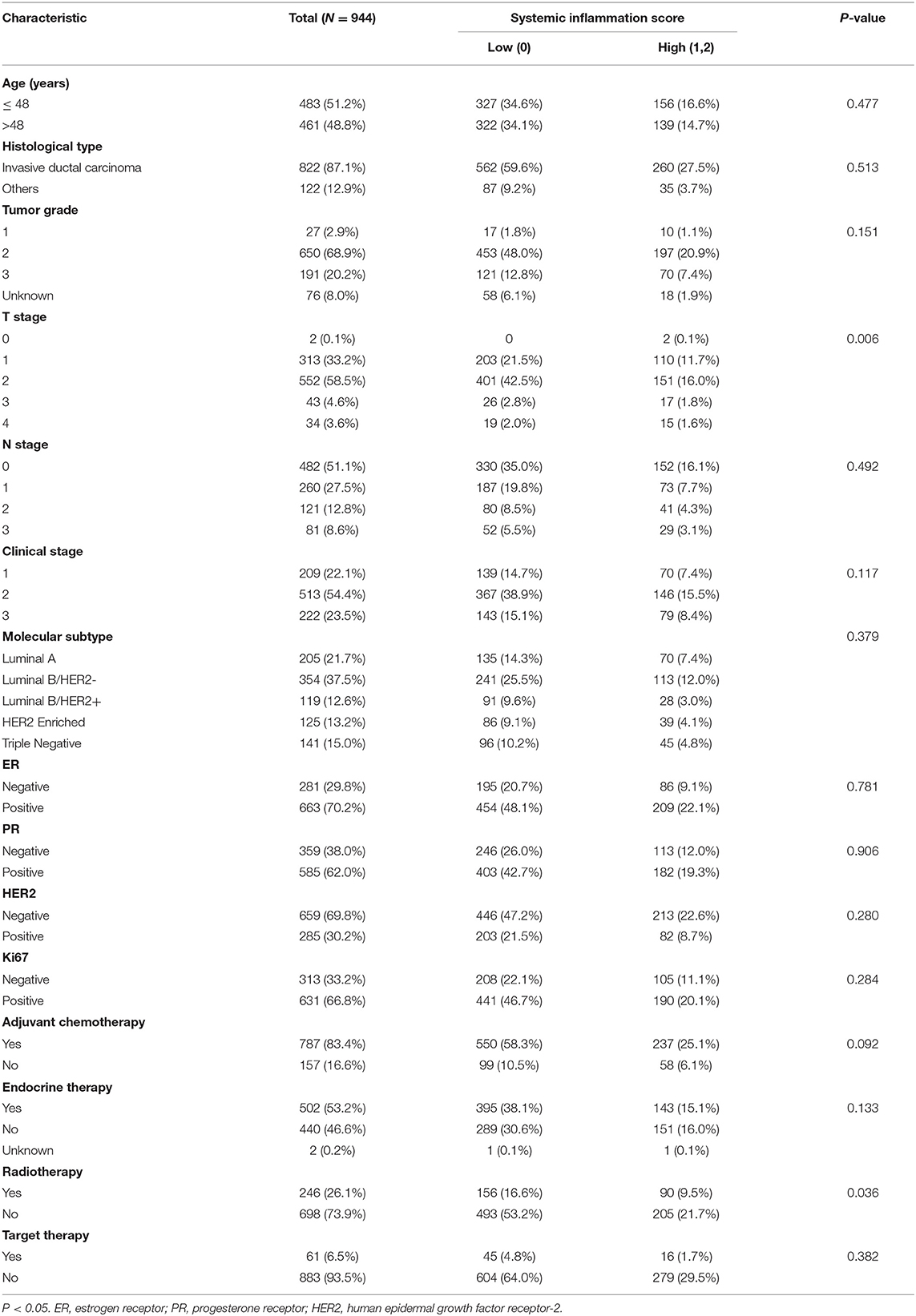
Table 2. The relationship between SIS group and clinicopathological characteristics in the training set.
Survival Outcomes and Predicted Prognosis Based on the SIS Scoring System in the Training Set
As shown in Figures 1A–C there was a correlation between high-SIS and poorer prognosis. The OS times of patients in the high-SIS and low-SIS groups were 68.05 and 72.87 months, respectively (P = 0.033). The RFS times of patients in the high-SIS and low-SIS groups were 56.04 and 60.85 months, respectively (P = 0.247). Finally, the DMFS times for patients in the high-SIS and low-SIS groups were 54.46 and 59.47 months, respectively (P = 0.032).
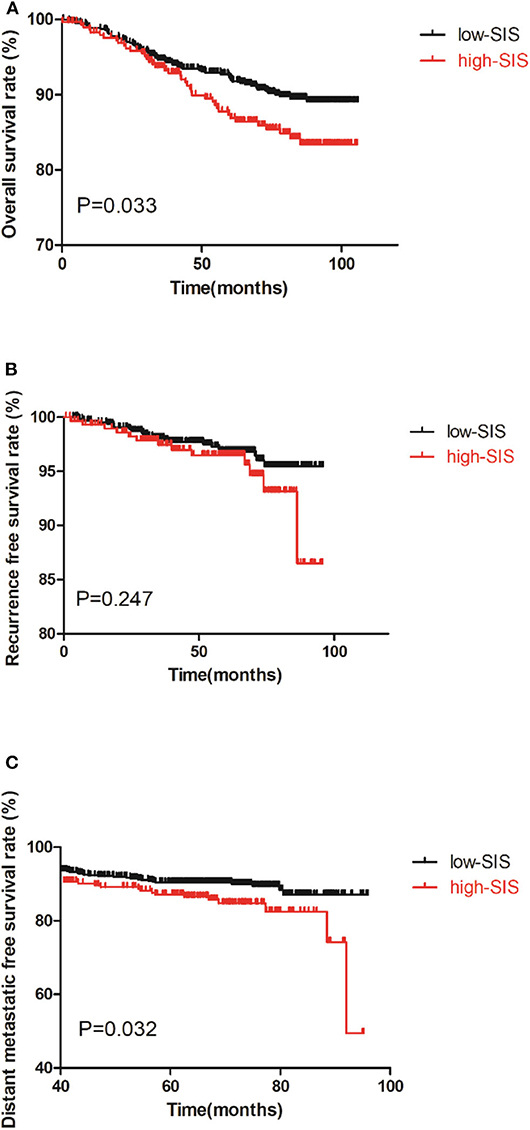
Figure 1. Kaplan-Meier survival curves of the training set of patients with breast cancer patients after surgery. (A) is the survival curves for OS, (B) is the survival curves for RFS, (C) is the survival curves for DMFS.
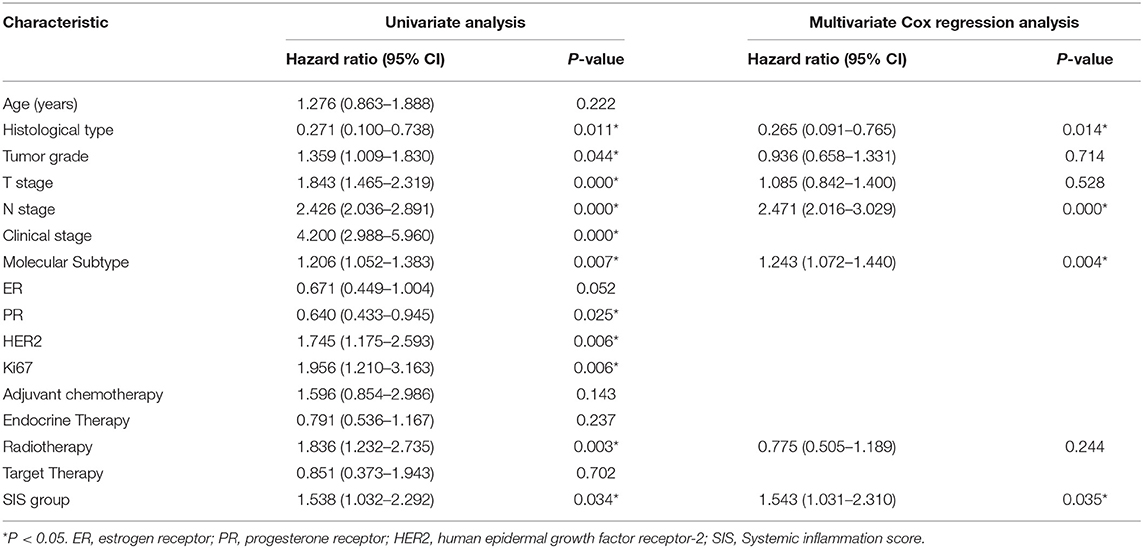
Univariate analysis showed that SIS group was associated with OS time (P = 0.034, Table 3) and DMFS time (P = 0.033, Table 4). Multivariate analysis revealed that SIS group was an independent factor influencing OS time (P = 0.035, Table 3) and DMFS time (P = 0.045, Table 4). Univariate and multivariate analyses for RFS were not performed.
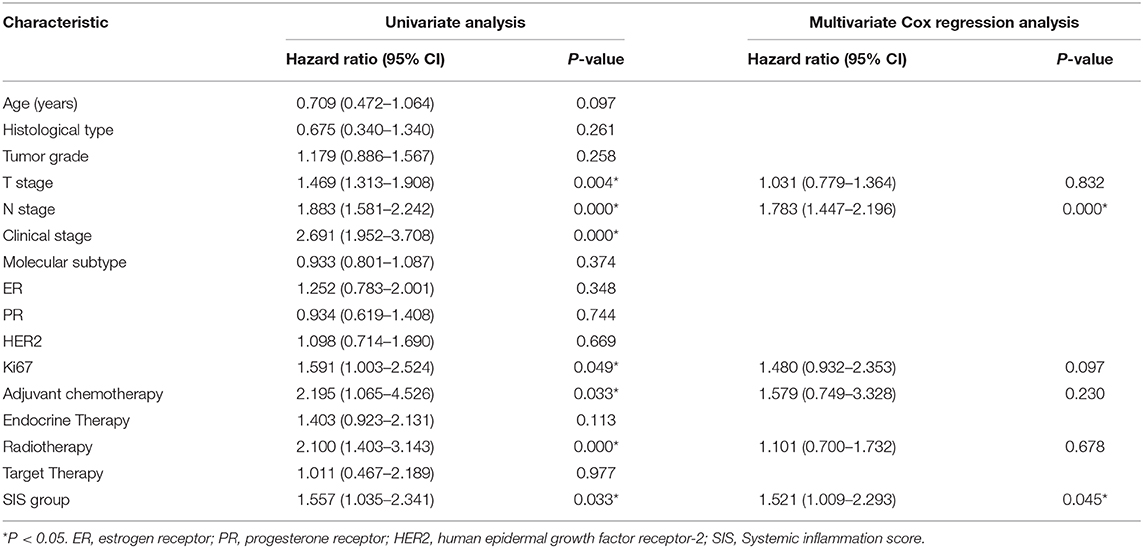
Table 4. Univariate and multivariate analyses of distant metastatic free survival in the training set.
Nomogram Construction and Validation
A nomogram model was constructed based on the results of multivariate analysis. Independent prognostic indicators were integrated into the prediction of OS and DMFS times. With regard to OS time, these factors included N-stage, histological type, molecular subtype and SIS group (Figure 2), and for DMFS, these factors included N-stage and SIS group (Figure 3).
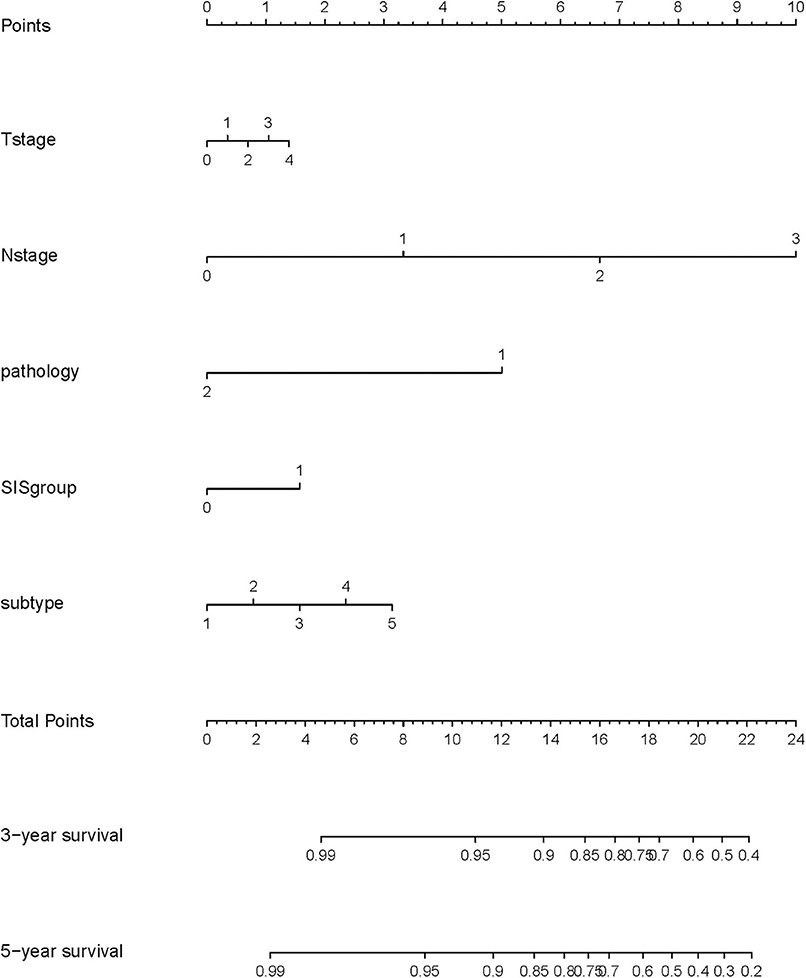
Figure 2. A nomogram predicting the 3- and 5-year overall survival of postoperative breast cancer patients. T stage: 0: T0; 1: T1; 2: T2; 3: T3; 4: T4. N stage: 0: N0; 1: N1; 2: N2; 3: N3. Pathology: 1: invasive ductal carcinoma; 2: others. SIS group: 0: low SIS group; 1: high SIS group. Subtype: 1: Luminal A; 2: Luminal B/HER2-; 3: Luminal B/HER2+; 4: HER2 Enriched; 5: Triple Negative.
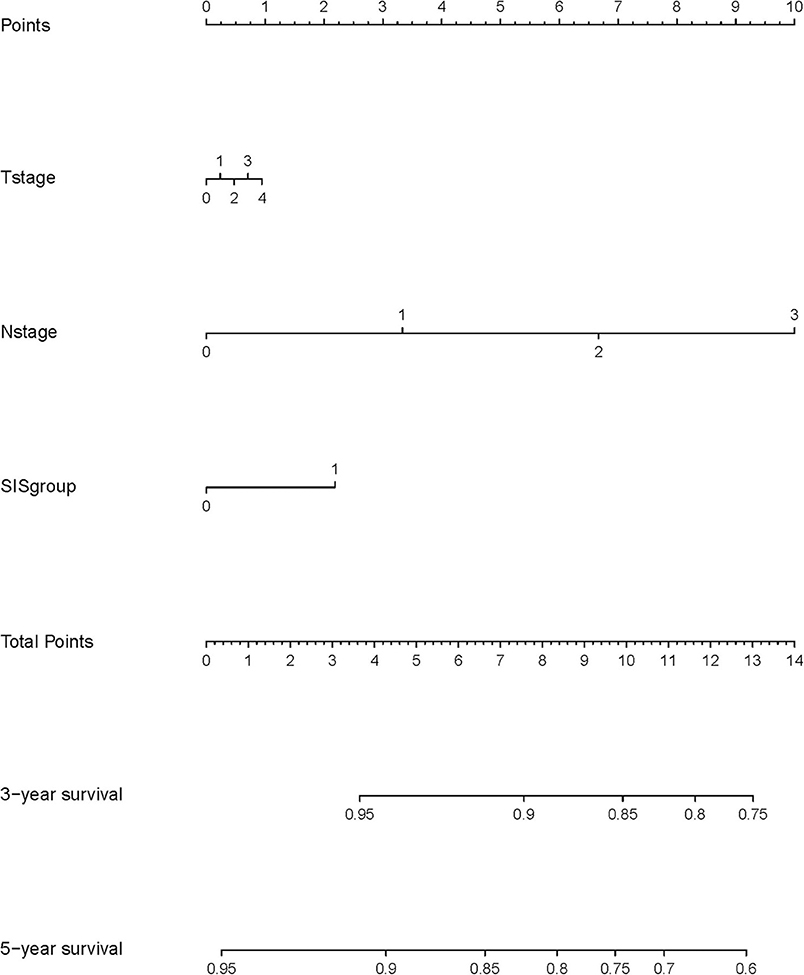
Figure 3. A nomogram predicting the 3- and 5-year distant metastatic free survival of postoperative breast cancer patients. T stage: 0: T0; 1: T1; 2: T2; 3: T3; 4: T4. N stage: 0: N0; 1: N1; 2: N2; 3: N3. SIS group: 0: low SIS group; 1: high SIS group.
The C-index of the nomogram indicated good predictive accuracy for the survival of postoperative patients with breast cancer. The C-index for OS time was 0.794 (95% CI, 0.772–0.816) and for DMFS time, 0.712 (95% CI, 0.684–0.740). For the validation set, the C-index for OS time was 0.889 (95% CI, 0.845–0.933) and for DMFS time, 0.696 (95% CI, 0.611–0.781). The calibration curves indicated good consistency with actual observation in the use of this nomogram in prediction of 3- and 5- year OS and DMFS times in the training and validation cohorts (Figures 4, 5).
Figure 4. Nomogram model calibration curves of 3-year (A) and 5-year (B) overall survival, and 3-year (C) and 5-year (D) disease metastatic free survival in the training cohort.
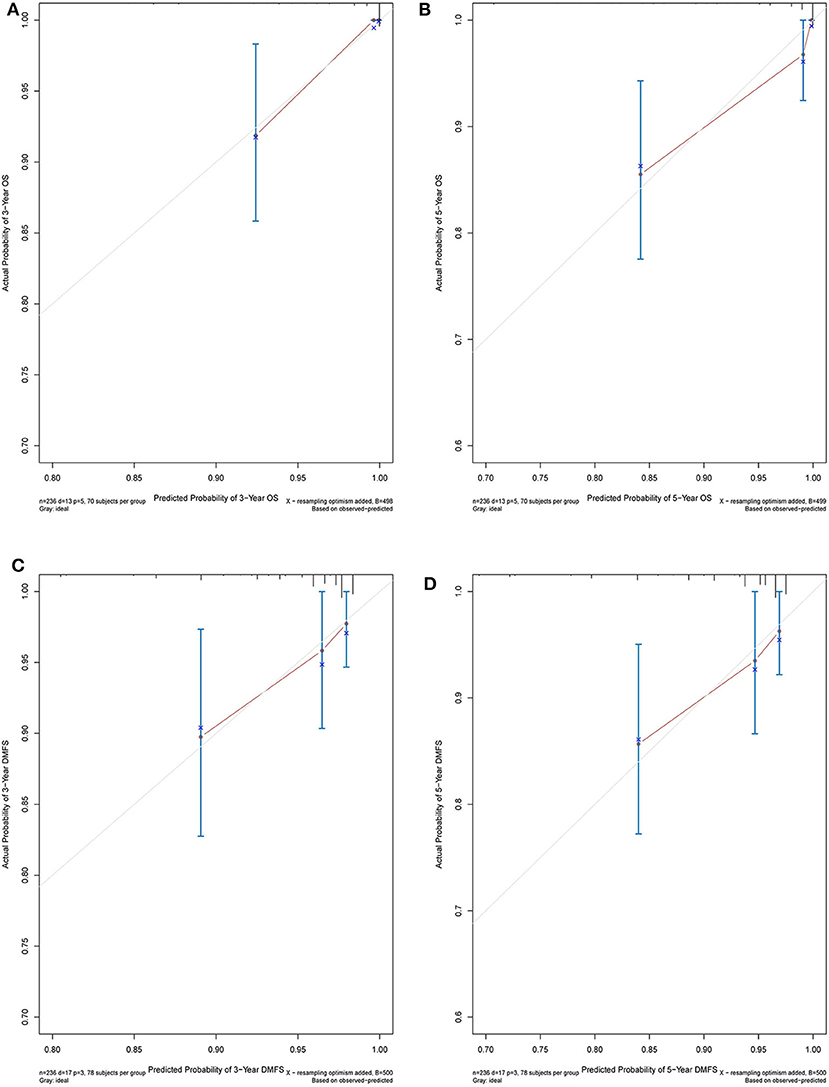
Figure 5. Nomogram model calibration curves of 3-year (A) and 5-year (B) overall survival, and 3-year (C) and 5-year (D) disease metastatic free survival in the validation cohort.
Discussion
In this retrospective study, 994 patients were included in the training set and 236 patients in the validation set. We evaluated the association between SIS and clinicopathological characteristics, the association between SIS group and patient survival, and progressed to the construction of a nomogram prognosis prediction model. In the large training set of patients, we noted that high-SIS was positively associated with poorer prognosis of postoperative patients with breast cancer. High preoperative SIS was found to be indicative of shorter OS and DMFS times. Univariate and multivariate analyses revealed that high-SIS was an independent factor influencing OS and DMFS times of breast cancer patients. Cumulatively, these results demonstrate that high-SIS is strongly associated with tumor progression and shorter survival. Considering independent prognostic factors, we formed a calibrated nomogram model indicating favorable discrimination.
Virchow originally proposed a relationship between cancer and inflammation in 1863, and an increasing number of reports have since been published on this relationship, forming a general consensus that inflammation plays an important role in carcinogenesis (30). Inflammation substantially contributes to the development and progression of various types of cancer (31). Cancer-related inflammation contributes to the tumor microenvironment, therefore, in some types of cancer inflammation is present before a malignant change occurs (32, 33). Inflammation generates not only a cancer-promoting microenvironment, but also systemic changes that are favorable for cancer progression (8). In addition to local reactions, cancer-related inflammation also induces changes of the peripheral blood, including the count of lymphocyte and monocyte (34).
Lymphocytes inhibit tumor development through enhancing cancer immunosurveillance (35). Therefore, a decline in lymphocyte number implies poor tumor monitoring. Moreover, recent evidence has demonstrated that monocytes can be recruited to carcinoma tissues and differentiate into tumor-associated macrophages, which play key roles in stimulating angiogenesis, enhancing tumor cell migration and invasion, and suppressing anti-tumor immunity (36, 37). Therefore, increased monocyte numbers may be indicative of tumor progression. Given these facts, LMR is a good predictor of prognosis.
Serum albumin is a negative acute phase protein, routinely employed as a readout of patient nutritional status (38), but is also indicative of a sustained systemic inflammation response (39). SIS integrates LMR and serum albumin into a scoring system, easily accessible by routine blood test. SIS has been reported as a prognostic indicator in various cancer types, including clear-cell renal cell carcinoma, gastric cancer, colorectal cancer and lung cancer (23–25, 40). However, whether it is predictive of prognosis of patients with breast cancer remains unknown. The user-friendly nomogram has been applied in various oncology studies (28, 41–43), however, no previous studies have constructed a nomogram based on preoperative SIS. The present study aimed to determine the prognostic value of SIS in breast cancer patients after surgery.
It was observed that patients in the high-SIS group always had shorter survival than those in the low-SIS group (OS or DMFS time, all P < 0.05). By univariate analyses, SIS were shown to be associated with OS and DMFS time (all P < 0.05). By multivariate analysis, N-stage (P < 0.001), histological type (P = 0.011), molecular subtype (P = 0.005) and SIS group (P = 0.035) were identified as independent predictors of OS time, and N-stage (P < 0.001) and SIS (P = 0.045) were independent predictors of DMFS time. These results demonstrate that SIS can be used as a prognostic indicator for breast cancer patients after surgery, and the nomogram increases the credibility of this.
There were 101 deaths in the high SIS group. The cause of the death in the high SIS group was mainly related to disease progression (99/101). Besides, the baseline characteristics between the high and low SIS group were also compared. SIS at higher score was found to associate with aggressive behaviors, such as T stage (Table 2). In the multivariate analysis, SIS was shown to be a promising factor to predict the survival outcomes, independent of other clinical parameters. Therefore, the main reason of the worse mortality in the high SIS group was considered to be the progression of breast cancer. Nevertheless, more research is required to exclude the influence of these prognostic factors and to demonstrate that SIS is an independent prognostic factor of for surgically treated patients with breast cancer.
Clinically, SIS is an easy and inexpensive value to access. High preoperative SIS would indicate a higher risk of metastasis and shorter OS time, information which may assist clinicians in formulating more effective treatment plans.
There are some limitations in this study. Firstly, selection bias is difficult to avoid in retrospective studies. And the relationship and comparison between SIS and traditional TNM stage system and other inflammatory indicators, such as NLR, PLR, were not investigated. We concentrated on preoperative SIS status, but it would be beneficial to consider SIS over a disease/treatment time course. The following factors with the potential to affect prognosis were not considered: family history of breast cancer, histological grade, vascular invasion, number of lymphatic invasion events. Finally, our nomogram was validated internally, and external validation would be valuable.
In conclusion, we have demonstrated SIS to be an independent prognostic predictor of OS and DMFS time for patients with breast cancer patients after surgery with curative intent. High preoperative SIS is associated with poorer survival than low SIS. The nomogram derived from SIS grouping indicates satisfactory discrimination and consistency.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number (s) can be found at: https://www.researchdata.org.cn/default.aspx, accession number RDDA2020002632.
Ethics Statement
This study was performed in accordance with the declaration of Helsinki and was approved by the Research Ethics Committee of SYSUCC. All subjects enrolled provided their written informed consent.
Author Contributions
Z-ZH, Z-YH, and J-JH contributed to conception and design of the study. XH organized the database. C-GS performed the statistical analysis. Z-ZH wrote the first draft of the manuscript. WX, X-WB, and Z-YY wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. (2016) 66:7–30. doi: 10.3322/caac.21332
2. Bevers TB, Helvie M, Bonaccio E, Calhoun KE, Daly MB, Farrar WB, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2018) 16:1362–89. doi: 10.6004/jnccn.2018.0083
3. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. (2011) 22:1736–47. doi: 10.1093/annonc/mdr304
4. Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Primers. (2019) 5:66. doi: 10.1038/s41572-019-0111-2
5. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. (2019) 321:288–300. doi: 10.1001/jama.2018.19323
6. Zhang J, Zhao B, Jin F. The assessment of 8th edition AJCC prognostic staging system and a simplified staging system for breast cancer: the analytic results from the SEER database. Breast J. (2019) 25:838–47. doi: 10.1111/tbj.13347
8. Chechlinska M, Kowalewska M, Nowak R. Systemic inflammation as a confounding factor in cancer biomarker discovery and validation. Nat Rev Cancer. (2010) 10:2–3. doi: 10.1038/nrc2782
9. Iftimie S, Escribano A, Diez-Sans A, Albiciuc I, Hernandez-Aguilera A, Fort-Gallifa I, et al. Influence of surgical procedures on serum paraoxonase-1-related variables and markers of inflammation in hospitalized patients. J Invest Surg. doi: 10.1080/08941939.2019.1597223. [Epub ahead of print].
10. Schwartz PB, Poultsides G, Roggin K, Howard JH, Fields RC, Clarke CN, et al. PLR and NLR are poor predictors of survival outcomes in sarcomas: a new perspective from the USSC. J Surg Res. (2020) 251:228–38. doi: 10.1016/j.jss.2020.01.008
11. Seetohul YB, Singh V, Jain RK, Chaudhary AK. Prognostic value of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in head and neck malignancies. Indian J Otolaryngol Head Neck Surg. (2020) 72:128–32. doi: 10.1007/s12070-019-01771-2
12. Xia LJ, Li W, Zhai JC, Yan CW, Chen JB, Yang H. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio and prognostic nutritional index for predicting clinical outcomes in T1-2 rectal cancer. BMC Cancer. (2020) 20:208. doi: 10.1186/s12885-020-6698-6
13. Zhou W, Kuang T, Han X, Chen W, Xu X, Lou W, et al. Prognostic role of lymphocyte-to-monocyte ratio in pancreatic neuroendocrine neoplasms. Endocr Connect. (2020) 9:289–98. doi: 10.1530/EC-19-0541
14. Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev. (2015) 41:971–8. doi: 10.1016/j.ctrv.2015.10.003
15. Wen J, Ye F, Huang X, Li S, Yang L, Xiao X, et al. Prognostic significance of preoperative circulating monocyte count in patients with breast cancer: based on a large cohort study. Medicine. (2015) 94:e2266. doi: 10.1097/MD.0000000000002266
16. Goto W, Kashiwagi S, Asano Y, Takada K, Takahashi K, Hatano T, et al. Predictive value of lymphocyte-to-monocyte ratio in the preoperative setting for progression of patients with breast cancer. BMC Cancer. (2018) 18:1137. doi: 10.1186/s12885-018-5051-9
17. Hu RJ, Liu Q, Ma JY, Zhou J, Liu G. Preoperative lymphocyte-to-monocyte ratio predicts breast cancer outcome: a meta-analysis. Clin Chim Acta. (2018) 484:1–6. doi: 10.1016/j.cca.2018.05.031
18. Stenman M, Laurell A, Lindskog M. Prognostic significance of serum albumin in patients with metastatic renal cell carcinoma. Med Oncol. (2014) 31:841. doi: 10.1007/s12032-014-0841-7
19. Chan AW, Chan SL, Mo FK, Wong GL, Wong VW, Cheung YS, et al. Albumin-to-alkaline phosphatase ratio: a novel prognostic index for hepatocellular carcinoma. Dis Markers. (2015) 2015:564057. doi: 10.1155/2015/564057
20. Nazha B, Moussaly E, Zaarour M, Weerasinghe C, Azab B. Hypoalbuminemia in colorectal cancer prognosis: Nutritional marker or inflammatory surrogate? World J Gastrointest Surg. (2015) 7:370–7. doi: 10.4240/wjgs.v7.i12.370
21. Fujii T, Tokuda S, Nakazawa Y, Kurozumi S, Obayashi S, Yajima R, et al. Implications of low serum albumin as a prognostic factor of long-term outcomes in patients with breast cancer. In Vivo. (2020) 34:2033–6. doi: 10.21873/invivo.12003
22. Yang H, Wang K, Liang Z, Guo S, Zhang P, Xu Y, et al. Prognostic role of pre-treatment serum albumin in patients with nasopharyngeal carcinoma: A meta-analysis and systematic review. Clin Otolaryngol. (2020) 45:167–76. doi: 10.1111/coa.13454
23. Chang Y, An H, Xu L, Zhu Y, Yang Y, Lin Z, et al. Systemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinoma. Br J Cancer. (2015) 113:626–33. doi: 10.1038/bjc.2015.241
24. Suzuki Y, Okabayashi K, Hasegawa H, Tsuruta M, Shigeta K, Kondo T, et al. Comparison of preoperative inflammation-based prognostic scores in patients with colorectal cancer. Ann Surg. (2018) 267:527–31. doi: 10.1097/SLA.0000000000002115
25. Ma M, Weng M, Chen F, Hu Y, Lai J, Wang Y, et al. Systemic inflammation score is a prognostic marker after curative resection in gastric cancer. ANZ J Surg. (2019) 89:377–82. doi: 10.1111/ans.15103
26. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. (2008) 26:1364–70. doi: 10.1200/JCO.2007.12.9791
27. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. (2015) 16:e173–180. doi: 10.1016/S1470-2045(14)71116-7
28. Lu Z, Yan W, Liang J, Yu M, Liu J, Hao J, et al. Nomogram based on systemic immune-inflammation index to predict survival of tongue cancer patients who underwent cervical dissection. Front Oncol. (2020) 10:341. doi: 10.3389/fonc.2020.00341
29. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in develop ing models, 361 evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. (1996) 15:362–87.
30. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. (2001) 357:539–45. doi: 10.1016/S0140-6736(00)04046-0
31. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. (2014) 15:e493–503. doi: 10.1016/S1470-2045(14)70263-3
32. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
33. Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. (2015) 12:584–96. doi: 10.1038/nrclinonc.2015.105
34. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. (2019) 51:27–41. doi: 10.1016/j.immuni.2019.06.025
35. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. (2004) 21:137–48. doi: 10.1016/j.immuni.2004.07.017
36. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. (2010) 141:39–51. doi: 10.1016/j.cell.2010.03.014
37. Aras S, Zaidi MR. TAMeless traitors: macrophages in cancer progression and metastasis. Br J Cancer. (2017) 117:1583–91. doi: 10.1038/bjc.2017.356
38. Esper DH, Harb WA. The cancer cachexia syndrome: a review of metabolic and clinical manifestations. Nutr Clin Pract. (2005) 20:369–76. doi: 10.1177/0115426505020004369
39. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. (2009) 12:223–6. doi: 10.1097/MCO.0b013e32832a7902
40. Li S, Wang Z, Zhang W, Li J, Zhou K, Che G. Systemic inflammation score: a novel risk stratification tool for postoperative outcomes after video-assisted thoracoscopic surgery lobectomy for early-stage non-small-cell lung cancer. Cancer Manag Res. (2019) 11:5613–28. doi: 10.2147/CMAR.S206139
41. Liu J, Geng Q, Chen S, Liu X, Kong P, Zhou Z, et al. Nomogram based on systemic inflammatory response markers predicting the survival of patients with resectable gastric cancer after D2 gastrectomy. Oncotarget. (2016) 7:37556–65. doi: 10.18632/oncotarget.8788
42. Cho U, Park HS, Im SY, Yoo CY, Jung JH, Suh YJ, et al. Prognostic value of systemic inflammatory markers and development of a nomogram in breast cancer. PLoS ONE. (2018) 13:e0200936. doi: 10.1371/journal.pone.0200936
Keywords: SIS, breast cancer, surgery, nomogram, survival
Citation: Huang Z-Z, Hua X, Song C-G, Xia W, Bi X-W, Yuan Z-Y, He Z-Y and Huang J-J (2020) The Prognostic Prediction Value of Systemic Inflammation Score and the Development of a Nomogram for Patients With Surgically Treated Breast Cancer. Front. Oncol. 10:563731. doi: 10.3389/fonc.2020.563731
Received: 28 May 2020; Accepted: 11 September 2020;
Published: 20 October 2020.
Edited by:
Gianluca Vanni, University of Rome Tor Vergata, ItalyReviewed by:
Alfonso Recordare, Ospedale dell'Angelo, ItalyGilles Houvenaeghel, Aix Marseille Université, France
Copyright © 2020 Huang, Hua, Song, Xia, Bi, Yuan, He and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen-Yu He, aGV6aHlAc3lzdWNjLm9yZy5jbg==; Jia-Jia Huang, aHVhbmdqaWFqQHN5c3VjYy5vcmcuY24=
†These authors have contributed equally to this work
 Zhang-Zan Huang
Zhang-Zan Huang Xin Hua
Xin Hua Chen-Ge Song†
Chen-Ge Song† Zhen-Yu He
Zhen-Yu He