- 1Deming Department of Medicine, Tulane University Medical School, New Orleans, LA, United States
- 2Department of Medicine, University of California, San Francisco, San Francisco, CA, United States
- 3Department of Internal Medicine, University of Alabama at Birmingham, Birmingham, AL, United States
- 4Department of Medicine, Medical College of Wisconsin Cancer Center, Milwaukee, WI, United States
- 5Department of Medicine, University of Wisconsin Carbone Cancer Center, Madison, WI, United States
- 6Department of Hematology and Medical Oncology, Emory University School of Medicine, Atlanta, GA, United States
Introduction: The treatment landscape of metastatic renal cell carcinoma has advanced significantly with the approval of combination regimens containing an immune checkpoint inhibitor (ICI) for patients with treatment-naïve disease. Little information is available regarding the activity of single-agent ICIs for patients with previously untreated mRCC not enrolled in clinical trials.
Methods: This retrospective, multicenter cohort included consecutive treatment-naïve mRCC patients from six institutions in the United States who received ≥1 dose of an ICI outside a clinical trial, between June 2017 and October 2019. Descriptive statistics were used to analyze outcomes including objective best response rate (ORR), progression-free survival (PFS), and tolerability.
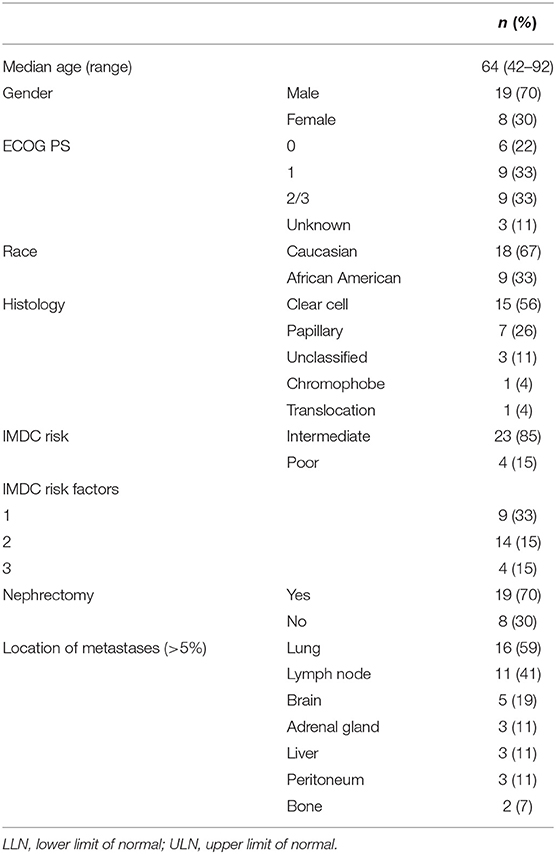
Results: The final analysis included 27 patients, 70% men, median age 64 years (range 42–92), 67% Caucasian, and 33% with ECOG 2 or 3 at baseline. Most patients had intermediate risk (85%, IMDC) with clear cell (56%), papillary (26%), unclassified (11%), chromophobe (4%), and translocation (4%) RCC. All patients had evidence of metastatic disease involving the lungs (59%), lymph node (41%), CNS (19%), liver (11%), adrenal gland (11%), and bone (11%). The median time on ICI was 3.1 (0.1–26.8) months, and the median PFS was 6.3 (95% CI, 0–18.6) months. Among the 21 patients with an evaluable response, the best ORR was 33%, including two complete responses and five partial responses. The ORR was 29% (n = 1 complete response, n = 5 partial response) in clear cell and 5% (n = 1 complete response) in non-clear cell RCC. Adverse events (AEs) of any cause were reported in 37% and included fatigue (11%), dermatitis (11%), diarrhea (7%), and shortness of breath (7%). Significant AEs (30%) included shortness of breath (7%), acute kidney injury (4%), dermatitis (4%), Clostridium difficile infection (4%), cerebrovascular accident (4%), and fatigue (7%). Three patients discontinued therapy due to grade 4 AEs.
Conclusions: In this multi-institutional case series, single-agent ICI demonstrated objective responses and was well tolerated in a heterogeneous treatment-naïve mRCC cohort. ICI monotherapy is not the standard of care for patients with mRCC, and further investigation is necessary to explore predictive biomarkers for optimal treatment selection in this setting.
Introduction
Kidney cancer incidence has been increasing, and in 2020, it is estimated that there will be 73,750 new cases in 2020 in the United States (1). Renal cell carcinoma (RCC) is the most common form of kidney cancer, and the overall prognosis is particularly poor for patients who present with metastatic RCC (mRCC) (1, 2).
The treatment of mRCC has advanced significantly with the emergence of targeted agents and immunotherapies. After the approval of the programmed cell death 1 (PD-1) checkpoint inhibitor nivolumab for previously treated mRCC patients based on the phase 3 CheckMate025 study (3), combination regimens that include an immune checkpoint inhibitor (ICI) targeting PD-1, programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte antigen 4 (CTLA-4) have been investigated in treatment-naïve mRCC, in large phase 3 trials. The combination of nivolumab and ipilimumab has an established role in the treatment of intermediate- and poor-risk patients (CheckMate 214), and different combinations of ICIs with angiogenic therapies such as axitinib with pembrolizumab (KEYNOTE-426) and axitinib with avelumab (Javelin 101), among others, have shown significant activity in patients with mRCC (4–7). While these combinations offer a significant clinical improvement vs. sunitinib in the frontline space, adverse events (AEs) are observed in most cases and treatment interruptions, dose reductions, and permanent discontinuation are not uncommon.
The activity of single-agent ICIs in the frontline setting is under investigation. In a single-arm phase 2 study (KEYNOTE-427), the PD-1 inhibitor pembrolizumab has shown encouraging antitumor activity in patients with treatment-naïve mRCC with both clear cell and non-clear cell histology (8, 9). Two different phase 2 studies (HCRN GU16-260; OMNIVORE) testing nivolumab followed by salvage ipilimumab in patients with clear cell mRCC have also shown clinical activity of nivolumab monotherapy in this setting (10, 11).
Yet, little information is available regarding the activity of single-agent ICIs for patients with previously untreated mRCC outside of a clinical trial. We aimed to describe the utilization and outcomes with monotherapy ICI for first-line treatment of mRCC patients.
Methods
Patients diagnosed with treatment-naïve mRCC from six academic centers in the United States (Emory University, Tulane University, University of Alabama, Medical College of Wisconsin, University of Wisconsin and University of California San Francisco) and treated with a frontline ICI between June 2017 and October 2019 were included in this retrospective analysis. Patient data were collected in compliance with the IRB guidelines of each participating institution. All consecutive patients with advanced RCC that met eligibility criteria were included in this study, based on the institutional databases at all participating sites.
Patient eligibility criteria for this analysis included pathologic confirmation of RCC, evidence of unresectable or metastatic disease using conventional CT scans, treatment with at least one dose of any PD-1 or PD-L1 drug (nivolumab, pembrolizumab, atezolizumab, and avelumab), and available baseline patient and disease information. Patients previously treated with an anti-angiogenic drug for more than 2 weeks were excluded as well as prior treatment with any PD-1/PD-L1 targeting drug, anti-CTLA-4, IL-2, or interferon-alpha. Patients treated with frontline PD-1 or PD-L1 drug as part of a clinical trial were excluded from final analysis.
Patients were followed from treatment start until death or last follow-up. Demographic, clinical, and treatment data for each patient were obtained from retrospective chart review by investigators at each institution. Patients' comorbidities considered clinically significant by the investigator at each institution were collected.
Objective best response rate (ORR) was defined by the investigator at each investigator site based on available radiographic information per RECIST. Progressive disease included radiographic and clinical progression. Clinical progression was defined by deterioration of performance status leading to best supportive care/hospice or death in patients without restaging scans available at time of analysis. Duration of response was defined for patients achieving complete or partial response from the initial documentation of response until documentation of progressive disease or until patient was lost to follow-up. Progression-free survival (PFS) was defined as the time from initiation of treatment to the time of progression or death, while overall survival (OS) was defined as the time from initiation of treatment until time of death or date of last follow up. Data on AEs were collected from clinical annotations during routine visits. Reported PD-L1 status (qualitative) data were based on the tumor tissue next-generation sequencing CLIA-certified assay available at each center; a PD-L1 status was considered positive if at least one assay detected PD-L1 expression. Significant AEs included grade 3 or above per treating investigator's assessment. The Kaplan–Meier method was used for survival analyses and two-sided p < 0.05 was considered significant. Statistical analysis was performed using SPSS Statistics version 23 (IBM Corp).
Results
Baseline Patient and Disease Characteristics
A total of 34 patients with mRCC were initially identified. Seven patients received immunotherapy as part of a clinical trial and were excluded from this dataset. The final analysis included 27 patients with median age 64 (range 42–92), 70% male, 67% Caucasian, and 33% with ECOG 2 or 3. Most patients (81%) were reported to have some type of coexisting comorbidities, which most commonly included hypertension (n = 7, 26%), obesity (n = 4, 15%), and diabetes (n = 5, 19%). Germline testing results were available in only four patients and detected a pathogenic alteration in SMARCB1 in one patient with unclassified RCC and negative family history of cancer, and a variant of unknown significance in one patient with clear cell RCC (ccRCC).
The study cohort consisted of patients with clear cell (n = 15), papillary (n = 7 including 3 patients with clear cell component), unclassified (n = 3), chromophobe (n = 1), and translocation (n = 1). One patient with papillary tumor had incipient sarcomatoid features. Four patients (4/10) had PD-L1+ tumors. Most patients (85%) had intermediate risk disease (IMDC prognostic score) and 19 underwent nephrectomy prior to initiation of any systemic treatment, including five patients who underwent cytoreductive nephrectomy. One patient with ccRCC underwent a nephrectomy after approximately 7 months after starting on frontline nivolumab with stable tumor regression. All patients had evidence of metastatic disease, most commonly involving the lungs (59%), lymph node (41%), brain (19%), liver (11%), adrenal gland (11%), and bone (11%). The most common reasons for considering a PD-1/PD-L1 monotherapy were physician's choice (41%), ECOG performance status (30%), and patients' comorbidities (15%). In four cases (15%), a combination regimen was offered, but the patient opted to start on single-agent PD-1 inhibitor due to safety concerns. Table 1 summarizes the baseline patient and disease characteristics.
Frontline ICI Response Rates and Outcomes
Patients received an ICI (n = 20 nivolumab, n = 6 pembrolizumab, and n = 1 avelumab) for their first line of systematic treatment for the initial diagnosis of stage IV disease. Median time from diagnosis of metastatic disease to starting ICI was 2.0 (0.1–24.4) months. One patient with ccRCC was previously treated with pazopanib for a total duration of 1 week prior to initiation of immunotherapy and discontinued due to AEs.
With a median follow up of 10.7 months, the median time on immunotherapy was 3.1 (0.1–26.8) months (Figure 1).
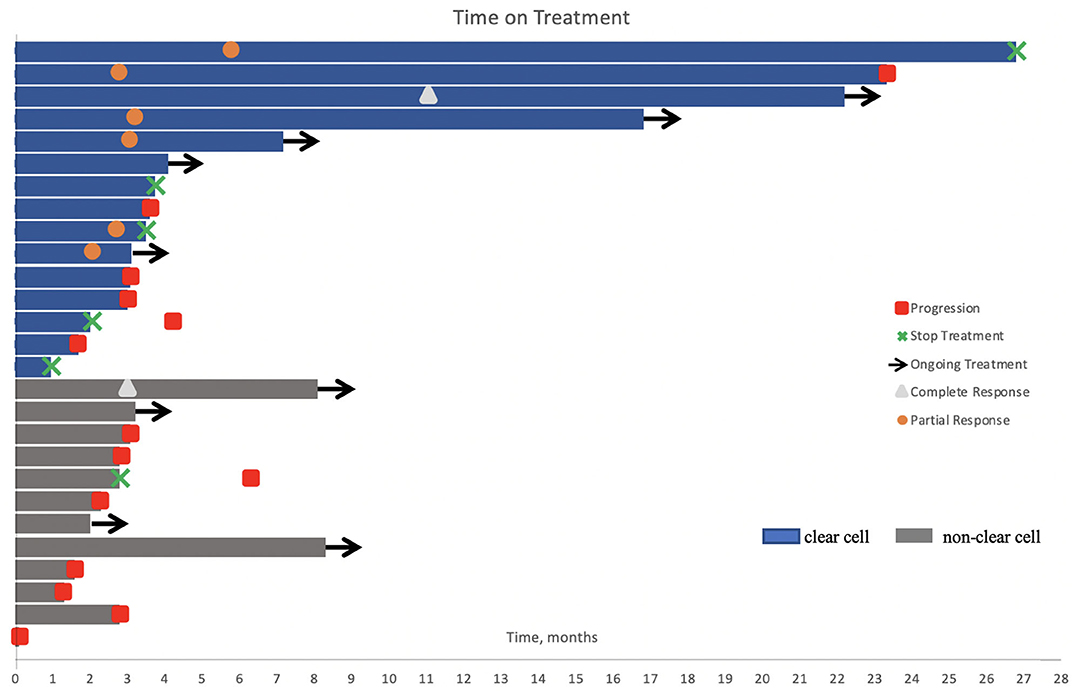
Figure 1. Swimmer's plot of time on treatment for the study cohort (n = 27). One patient received a single dose of immunotherapy. Nine patients were still on treatment at the time of analysis. Six patients had stable disease, including one patient with papillary RCC where axitinib was added to his treatment regimen after three cycles of pembrolizumab. Treatment was held or permanently discontinued in five patients due to adverse events and in one patient after 2 years of treatment.
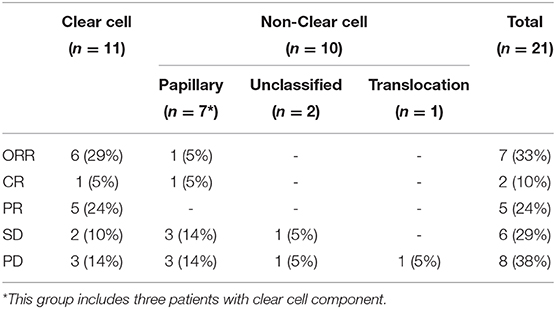
At time of cutoff analysis, nine patients were still on treatment; axitinib was added to pembrolizumab in one patient who was still responding to therapy. Treatment interruption and discontinuation due to AEs occurred in five patients (Figure 1). Among the 21 patients with an evaluable response, the best ORR was 33%, including two complete responses and five partial responses, mostly in patients with ccRCC. The median time to best response was 3.1 (2.1–11.0) months. No responses were observed in patients with unclassified and translocation histology. Table 2 summarizes the best response rate by histology. Among the five patients who were not evaluable for response (n = 3 clear cell, n = 1 chromophobe, and n = 1 unclassified), all had evidence of clinical progression as best treatment response. Additionally, we excluded from the efficacy analysis one patient with prior exposure to pazopanib who achieved partial response as best response. The ORR was 29% (complete response, n = 1; partial response, n = 5) in clear cell and 5% (complete response, n = 1) in non-clear cell RCC (nccRCC). There were six patients with stable disease, including one patient that achieved stable disease on pembrolizumab before adding axitinib.
In the group of patients with CNS metastasis (n = 5) and evaluable response (n = 4), ORR was 25% (1/4 patients). One partial response was observed in ccRCC, while two patients with nccRCC had stable disease and one patient (ccRCC) had progressive disease as best response.
A total of 14 patients had disease progression and the median PFS was 6.3 (95% CI, 0–18.6) months. Patients with a complete or partial response to therapy had a significantly longer PFS compared with those who achieved stable disease or progressive disease (p = 0.030). Patients with ccRCC had a significantly longer PFS compared with non-clear cell histology (p = 0.038).
Four patients with ccRCC have been without progressive disease for more than a year (13.9–36.6 months), including two patients with complete response and two patients with partial response as their best response. On the other hand, most patients who progressed within 3 months of treatment start had non-clear cell histology (n = 6/8).
Among all patients who progressed on therapy (n = 14), subsequent treatment included a TKI [cabozantinib (n = 4), pazopanib (n = 3), and sunitinib (n = 1)], while six patients transitioned to hospice care or passed away. The estimated median OS was 31 (95% CI, 0–64.1) months with most patients (74%) alive at time of data cutoff.
Safety and Treatment-Related AEs
AEs of any cause were reported in 10 patients (37%). The most common AEs included fatigue (n = 3), dermatitis (n = 3), diarrhea (n = 2), and shortness of breath (n = 2). Significant AEs (n = 8) included shortness of breath (n = 1 grade 4; n = 1 grade 3), acute kidney injury (n = 1 grade 4), dermatitis (n = 1 grade 4), intestinal infection due to Clostridium difficile (n = 1 grade 4, which was not considered immune-mediated), cerebrovascular accident (n = 1 grade 4), and fatigue (n = 2 grade 3).
Two patients had treatment interruption and three patients discontinued therapy due to grade 4 events (dermatitis, intestinal infection due to C. difficile, and cerebrovascular accident) (Figure 1).
Discussion
In this multicenter cohort study, we found that ICI monotherapy was associated with an ORR of 36% in patients with previously untreated mRCC, driven by responses in clear cell tumors. The observed response rate and the mPFS were very similar to the reported efficacy in the prospective, first-line phase II studies KEYNOTE-427 (pembrolizumab) and the HCRN GU16-260 (nivolumab), particularly in the intermediate/poor-risk disease groups (7, 9).
Compared with ccRCC, ICIs might be less active in non-clear cell tumors (9, 12). We observed one complete response in a non-clear cell tumor. The low response rate might be the result of several factors such as the small number of patients with evaluable responses and the low rate of sarcomatoid features, which is associated with higher responses to ICI (13). Additionally, the characteristics of the patient population in this dataset included a poor performance status and brain metastases at presentation compared with other studies, which may help explain the lower clinical efficacy (14, 15). Additionally, no favorable risk patients were included and the median number of adverse risk factors of the patient population was 2, which is associated with worse clinical outcomes (16). Yet, one complete response was observed in a papillary RCC patient that is ongoing at 7 months on therapy, in line with other studies reporting objective responses that tend to last several months (8, 9).
Combination regimens containing ICIs became the standard of care for patients with mRCC based on phase 3 clinical trials conducted mainly in clear cell tumors (17). The same or similar combinations are being investigated in non-clear cell histologies, and emerging data are promising (18). Yet, not all patients will be considered eligible for those regimens. In this real-world dataset, patients' clinical functional status measured by ECOG, number of comorbidities, and concerns with safety impacted the decision to offer an ICI instead of a combination regimen or a TKI monotherapy. Of note, among six academic institutions, the small number of patients offered IO monotherapy reflects the rarity of this strategy.
More patients were treated with nivolumab compared with pembrolizumab and avelumab given the earlier FDA approval of nivolumab/ipilimumab (April 2018) followed by the pembrolizumab/axitinib (April 2019) and avelumab/axitinib regimen (May 2019).
In this cohort, the proportion of significant AEs seemed to be higher compared with prior safety data reported in the clinical studies with single-agent ICIs, which may be explained by the differences of the patient population included. ICI-related attribution was not reported for the AEs observed in our study, but patients on immune therapies who often develop autoimmune dermatitis and colitis with superimposed C. difficile infection have also been observed in such cases (19).
The differential activity of ICIs in this setting supports further investigation on putative predictive biomarkers to help guide treatment selection. While no RNA data were available on this dataset, gene expression signatures from mRCC in the Javelin 101 and IMmotion151 trials have demonstrated an association between the angiogenic or immunogenic signature and responses to targeted and/or immunotherapies, respectively (6, 7, 20, 21). Similarly, T-cell gene expression profile was associated with responses to pembrolizumab in KEYNOTE-427 study (22). This information has the potential to help define the role of upfront TKI or ICI monotherapy vs. combination regimen with ICI/ICI or ICI/TKI.
A different treatment strategy based on individual response to upfront ICI monotherapy is being explored (HCRN GU16-260, OMNIVORE) by allowing treatment interruption to responders while adding other ICI as salvage treatment to non-responders (10, 11). Preliminary results in patients with treatment-naïve clear cell mRCC demonstrated some activity of ICI monotherapy with an observed ORR of 29% in the HCRN GU16-260 trial and 12% at 6 months in the OMNIVORE study, respectively. Additionally, the addition of a second agent salvaged a subset of the non-responders.
Finally, the optimal sequence of systemic therapies for patients who progressed on frontline ICIs is still under investigation. In this cohort, cabozantinib was the TKI of choice after progression, which is supported by published data demonstrating the activity of this and other TKIs, such as axitinib, in the post-PD-1/PD-L1 setting (23–25).
The limitations of this case series include the retrospective nature of the analysis and the small sample size including a heterogenous group of patients which limits definitive conclusions. The data on attribution of AEs was largely not available. Nonetheless, these results provide support for ICI monotherapy in patients who are not good candidates for upfront combination regimens in a real-world setting, while we await more data from prospective studies to become available in the near future.
Conclusions
In this multi-institutional, real-world case series, single-agent ICI demonstrated objective responses and was well tolerated in a heterogeneous treatment-naïve mRCC cohort. ICI monotherapy is not the standard of care for patients with mRCC. With the emergence of several active treatment options for patients with treatment-naïve mRCC, further investigation is necessary to explore predictive biomarkers for optimal treatment selection in this setting.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, if requested.
Ethics Statement
The studies involving human participants were reviewed and approved by Tulane University Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
PB: concept. WH, PB, VK, AD, HE, AB, DK, and MB: data collection. All authors: data analysis and interpretation.
Funding
This work was supported by LA CaTS Center Grant Number U54 GM104940.
Conflict of Interest
PB has a consulting/advisory role (Institution) with Exelixis, Caris, Bayer, Janssen, EMD/Serono, Pfizer, Astellas, Dendreon, Clovis, and Sanofi. Contracted Research (Institution) from Seattle Genetics, BlueEarth Diagnostics, Nektar, and AstraZeneca. AD serves as an advisory board member for Dendreon and is also on the NCCN Kidney panel. VK has a consulting/advisory role with Janssen, AstraZeneca, Dendreon, Gerson Lehrman Group, Guidepoint Global, and Seattle Genetics/Astellas. Contracted Research (Institution) from Clovis Oncology, Nektar, and Endocyte. OS has a consulting/advisory role with AAA, Astellas, AstraZeneca, Bayer, Blue Earth Diagnostics, EMD Serono, Endocyte, Pfizer, Progenics, Sanofi, Invitae, Merck, Novartis, Janseen, Constellation, Dendreon, BMS, Bravarin Nordic, Clovis, Myriad, Noria Therapeutics, Noxopharm, Point Biopharma, and Tenebio. Contracted Research from Innocrin, Sotio. He also serves as a consultant on NCI Scientific Board Counselors and is a co-chairman of GU Committee at NRG. AB has a consulting/advisory role with EMD Serono and Dendreon. DK has served as an advisory board member for Exelixis, Pfizer, Sanofi, and Merck and has received institutional grants from Exelixis, Astellas. MB has acted as a paid consultant for and/or as a member of the advisory boards of Exelixis, Bayer, BMS, Eisai, Pfizer, AstraZeneca, Janssen, Genomic Health, Nektar, and Sanofi and has received grants to his institution from Xencor, Bayer, Bristol-Myers Squibb, Genentech/Roche, Seattle Genetics, Incyte, Nektar, AstraZeneca, Tricon Pharmaceuticals, Peleton Therapeutics, and Pfizer for work performed as outside of the current study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with several of the authors PB, VK, and AB.
Abbreviations
AE, adverse events; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; CR, complete response; ICI, immune checkpoint inhibitor; mRCC, metastatic RCC; PD, progressive disease; PD-1, programmed death 1 receptor; PD-L1, programmed death ligand 1; PFS, progression-free survival; PR, partial response; ORR, objective best response rate; OS, overall survival; RCC, renal cell carcinoma; SD, stable disease; TKI, tyrosine kinase inhibitor.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
2. Pal SK, Ghate SR, Li N, Swallow E, Peeples M, Zichlin ML, et al. Real-world survival outcomes and prognostic factors among patients receiving first targeted therapy for advanced renal cell carcinoma: a SEER–medicare database analysis. Clin Genitourin Cancer. (2017) 15:e573–82. doi: 10.1016/j.clgc.2016.12.005
3. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. (2015) 373:1803–13. doi: 10.1056/NEJMoa1510665
4. Motzer RJ, Tannir NM, McDermott DF, Frontera OA, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. (2018) 378:1277–90. doi: 10.1056/NEJMoa1712126
5. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. (2019) 380:1116–27. doi: 10.1056/NEJMoa1816714
6. Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. (2019) 380:1103–15. doi: 10.1056/NEJMoa1816047
7. Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. (2019) 393:2404–15. doi: 10.1016/S0140-6736(19)30723-8
8. McDermott DF, Lee J-L, Szczylik C, Donskov F, Malik J, Alekseev BY, et al. Pembrolizumab monotherapy as first-line therapy in advanced clear cell renal cell carcinoma (accRCC): results from cohort A of KEYNOTE-427. Am Soc Clin Oncol. (2018) 36:4500. doi: 10.1200/JCO.2018.36.15_suppl.4500
9. Lee J-L, Ziobro M, Gafanov R, Matveev VB, Suarez C, Donskov F, et al. KEYNOTE-427 cohort B: first-line pembrolizumab (pembro) monotherapy for advanced non–clear cell renal cell carcinoma (NCC-RCC). J Clin Oncol. (2019) 37:4569. doi: 10.1200/JCO.2019.37.15_suppl.4569
10. Atkins MB, Jegede O, Haas NB, McDermott DF, Bilen MA, Drake CG, et al. Phase II study of nivolumab and salvage nivolumab + ipilimumab in treatment-naïve patients (pts) with advanced renal cell carcinoma (RCC) (HCRN GU16-260). J Clin Oncol. (2020) 38:5006. doi: 10.1200/JCO.2020.38.15_suppl.5006
11. McKay RR, Xie W, McGregor BA, Braun DA, Wei XX, Kyriakopoulos C, et al. Optimized management of nivolumab (Nivo) and ipilimumab (Ipi) in advanced renal cell carcinoma (RCC): a response-based phase II study (OMNIVORE). J Clin Oncol. (2020) 38:5005. doi: 10.1200/JCO.2020.38.15_suppl.5005
12. Koshkin VS, Barata PC, Zhang T, George DJ, Atkins MB, Kelly WJ, et al. Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. J Immunother Cancer. (2018) 6:9. doi: 10.1186/s40425-018-0319-9
13. McDermott DF Choueiri TK Motzer RJ Aren OR George S Powles T . CheckMate 214 post-hoc analyses of nivolumab plus ipilimumab or sunitinib in IMDC intermediate/poor-risk patients with previously untreated advanced renal cell carcinoma with sarcomatoid features. J Clin Oncol. (2019) 37:4513. doi: 10.1200/JCO.2019.37.15_suppl.4513
14. Krishnan M, Teply BA, Yu F, High R. Impact of performance status on response and survival among patients receiving checkpoint inhibitors for advanced solid tumors. Am Soc Clin Oncol. (2020) 38:12028. doi: 10.1200/JCO.2020.38.15_suppl.12028
15. Shuch B, La Rochelle JC, Klatte T, Riggs SB, Liu W, Kabbinavar FF, et al. Brain metastasis from renal cell carcinoma. Cancer. (2008) 113:1641–8. doi: 10.1002/cncr.23769
16. Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. (2009) 27:5794–9. doi: 10.1200/JCO.2008.21.4809
17. Barata PC, Rini BI. Treatment of renal cell carcinoma. Current status and future directions. CA Cancer J Clin. (2017) 67:507–24. doi: 10.3322/caac.21411
18. McGregor BA, McKay RR, Braun DA, Werner L, Gray K, Flaifel A, et al. Results of a multicenter phase ii study of atezolizumab and bevacizumab for patients with metastatic renal cell carcinoma with variant histology and/or sarcomatoid features. J Clin Oncol. (2020) 38:63–70. doi: 10.1200/JCO.19.01882
19. Babacan NA, Tanvetyanon T. Superimposed clostridium difficile Infection during checkpoint Inhibitor immunotherapy-induced colitis. J Immunother. (2019) 42:350–3. doi: 10.1097/CJI.0000000000000270
20. Rini BI, Huseni M, Atkins MB, McDermott DF, Powles TB, Escudier B, et al. LBA31 Molecular correlates differentiate response to atezolizumab (atezo)+ bevacizumab (bev) vs sunitinib (sun): results from a phase III study (IMmotion151) in untreated metastatic renal cell carcinoma (mRCC). Ann Oncol. (2018) 29:mdy424. 037. doi: 10.1093/annonc/mdy424.037
21. Choueiri TK, Albiges L, Haanen JBAG, Larkin JMG, Uemura M, Pal SK, et al. Biomarker analyses from JAVELIN Renal 101: avelumab + axitinib (A+Ax) versus sunitinib (S) in advanced renal cell carcinoma (aRCC). J Clin Oncol. (2019) 37:101. doi: 10.1200/JCO.2019.37.15_suppl.101
22. McDermott DF, Lee J-L, Donskov F, Tykodi SS, Bjarnason GA, Larkin JMG, et al. Association of gene expression with clinical outcomes in patients with renal cell carcinoma treated with pembrolizumab in KEYNOTE-427. J Clin Oncol. (2020) 38:5024. doi: 10.1200/JCO.2020.38.15_suppl.5024
23. Graham J, Shah AY, Wells JC, McKay RR, Vaishampayan U, Hansen A, et al. Outcomes of patients with metastatic renal cell carcinoma treated with targeted therapy after immuno-oncology checkpoint inhibitors. Eur Urol Oncol. (2019). doi: 10.1016/j.euo.2019.11.001. [Epub ahead of print].
24. Barata PC, De Liano AG, Mendiratta P, Crolley V, Szabados B, Morrison L, et al. The efficacy of VEGFR TKI therapy after progression on immune combination therapy in metastatic renal cell carcinoma. Br J Cancer. (2018) 119:160–3. doi: 10.1038/s41416-018-0104-z
25. Ornstein MC, Pal SK, Wood LS, Tomer JM, Hobbs BP, Jia XS, et al. Individualised axitinib regimen for patients with metastatic renal cell carcinoma after treatment with checkpoint inhibitors: a multicentre, single-arm, phase 2 study. Lancet Oncol. (2019) 20:1386–94. doi: 10.1016/S1470-2045(19)30513-3
Keywords: metastatic renal cell carcinoma, immunotherapy, PD-1/PD-L1 inhibitor, monotherapy, first-line treatment
Citation: Barata P, Hatton W, Desai A, Koshkin V, Jaeger E, Manogue C, Cotogno P, Light M, Lewis B, Layton J, Sartor O, Basu A, Kilari D, Emamekhoo H and Bilen MA (2020) Outcomes With First-Line PD-1/PD-L1 Inhibitor Monotherapy for Metastatic Renal Cell Carcinoma (mRCC): A Multi-Institutional Cohort. Front. Oncol. 10:581189. doi: 10.3389/fonc.2020.581189
Received: 08 July 2020; Accepted: 31 August 2020;
Published: 22 October 2020.
Edited by:
Brian Rini, Vanderbilt University, United StatesReviewed by:
Daniel Y. C. Heng, University of Calgary, CanadaBenjamin Maughan, University of Utah, United States
Copyright © 2020 Barata, Hatton, Desai, Koshkin, Jaeger, Manogue, Cotogno, Light, Lewis, Layton, Sartor, Basu, Kilari, Emamekhoo and Bilen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pedro Barata, cGJhcmF0YUB0dWxhbmUuZWR1
 Pedro Barata
Pedro Barata Whitley Hatton1
Whitley Hatton1 Vadim Koshkin
Vadim Koshkin Oliver Sartor
Oliver Sartor Arnab Basu
Arnab Basu Hamid Emamekhoo
Hamid Emamekhoo Mehmet A. Bilen
Mehmet A. Bilen