- 1Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Department of Urology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
- 2Department of Radiology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
- 3Department of Urology, Glickman Urological and Kidney Institute, Cleveland Clinic, Cleveland, OH, United States
Objectives: Tumor enucleation (TE) optimizes parenchymal preservation with promising short-term oncologic outcomes compared with standard partial nephrectomy (SPN). However, researches/literatures about long-term oncologic outcomes for TE after minimally invasive surgery are scarce. We aim to analyze long-term oncologic outcomes after laparoscopic and robotic tumor enucleation for renal cell carcinoma (RCC).
Patients and Methods: We retrospectively analyzed 146 patients who underwent TE with either laparoscopic or robotic approach for localized RCC in our center. Local recurrence, cancer specific survival (CSS), recurrence free survival (RFS), and overall survival (OS) were the main outcomes. Survival curves were generated using a Kaplan-Meier method. Perioperative outcomes and pathological outcomes were also analyzed.
Results: Overall, 98 male and 48 female patients were eligible for the study. The median tumor size was 3.4 cm with a median R.E.N.A.L. score of seven. Warm ischemia was used in 143 patients with a median ischemia time of 20 min and three patients had zero ischemia. Five patients (3.4%) had major complications (> Clavien IIIa) and only two were related to urinary system. The median global glomerular filtration rate (GFR) preserved after surgery was 93%. Pseudocapsule invasion was reported in 50 tumors (34%) and positive surgical margins were found in 3/146 (2.1%) tumors. At a median follow-up of 66 months, local recurrence happened in two patients (1.4%), and systemic recurrence happened in six patients (4.2%). The 5-year CSS, RFS, OS were 95.7, 89.6, and 91.9%, and the 10-year CSS, RFS, OS were 93.8, 89.6, and 90.0%, respectively.
Conclusion: This study indicates that tumor enucleation with laparoscopic or robotic approach in experienced hands for the treatment of RCC appears oncologically safe with a median follow-up of more than 5 years. Prospective studies with more patients and longer follow-up will be required to further evaluate oncologic safety after TE.
Introduction
Renal cell carcinoma (RCC) represents approximately 3% of all cancers, with an annual growth rate of 2% in incidence both worldwide and in Europe. As EAU and AUA guidelines recommended, surgery is the only curative treatment for localized RCC (1, 2). Surgery approaches include radical nephrectomy (RN) and nephron sparing surgery (NSS). Many studies have supported comparable cancer specific survival (CSS) for NSS versus RN (1–4). In order to preserve more renal units and general kidney function, NSS is recommended for RCCs with tumor <7 cm (1, 2). Approaches for NSS include standard partial nephrectomy (SPN) as well as tumor enucleation (TE). TE preserves more renal parenchyma by sharp combined with blunt dissection of the renal tumor along the plane between the pseudocapsule and the healthy renal tissue (5, 6). SPN excises about 0.5 to 1 cm healthy renal parenchyma (safety margin) around the tumor (7, 8). Many studies suggested that TE might be a better surgical technique for its preservation of more parenchyma without decreasing CSS and the anatomic landmarks it supplied during surgeries (9, 10). As a result, a growing number of clinical centers prefer to use TE but the trade-off between the advantages of preserving renal parenchyma and the oncologic outcomes remains debatable (6, 11, 12). Some retrospective studies reported the short-term oncologic outcomes of TE were comparable with SPN and RN (10, 11, 13). However, researches/literatures about long-term oncologic outcomes for TE after minimally invasive surgery are scarce. In this study we aim to analyze long-term oncologic outcomes after laparoscopic and robotic TE for RCC in a tertiary medical center, which may offer a good supporting evidence for using TE in localized RCC patients.
Patients and Methods
Patients
From 2008 to 2017, data of pathologically diagnosed RCC patients who received either laparoscopic or robotic TE at Sun Yat-sen Memorial Hospital were collected. All Surgeries were performed by two skilled urologists who have passed the learning curve for both laparoscopic and robotic partial nephrectomy. Study was approved by the local ethics committee and informed consent was obtained from each patient.
Surgical Technique
For laparoscopic TE, as we described before, patients were placed in the flank position with three to four laparoscopic ports placed in standard retroperitoneal fashion (10). After removing extraperitoneal fat, incising the Gerota fascia and resecting the perirenal fat, the tumor and surrounding parenchyma were exposed. Then, the main renal artery was isolated and clamped by bulldog. The resection started from approximately 2 mm away from the tumor margin. When the pseudocapsule was identified, the surgeon took the pseudocapsule as anatomical landmark to enucleate the tumor by blunt together with sharp dissection from the surface to the bottom and then renal renorrhaphy was performed by using running sutures with one layer or two layers. According to the SIB scoring system for standardized reporting of NSS resection techniques, almost all of the tumors had a SIB score of less than 3, which means the tumors were resected mostly by pure enucleation or hybrid enucleation (14). For robotic TE, either transperitoneal approach or retroperitoneal approach was applied based on the surgeon’s preference. For all patients, a four-port technique was used, including a 12 mm trocar for camera, two 8 mm trocars for robotic arms, and a 12 mm trocar for the assistant. Robotic TE was performed similar as laparoscopic TE.
Pathological Assessment
Tumors were classified according to the 2016 World Health Organization histologic classification system (15). The tumor stage and grade were determined according to the 2010 TNM system and the Fuhrman grading system (16). The margin status and pseudocapsule status were also evaluated.
Outcome Parameters
Variables of patient characteristics were collected including age, sex, body mass index, Charlson comorbidity index, tumor size, and R.E.N.A.L. score. The perioperative data included surgical approaches, operative time, ischemia types, warm ischemia time, estimated blood loss, and major complications according to Clavien system. Serum creatinine was also assessed before and after operation and eGFR estimated by the MDRD2 equation for Chinese was calculated for analyzing renal function changes (17, 18).
All patients were regularly followed up in Sun Yat-sen Memorial Hospital. Chest X-ray or computed tomography (CT) scan was performed every year in the first 5 years. Abdominal CT scan was performed every 6 months in the first year and then once per year in the next 4 years. After 5 years of follow-up, CT scan was used every 2 years. Bone scan, brain CT, or magnetic resonance imaging (MRI) might be used in the presence of specific clinical or laboratory signs and symptoms. Oncological outcomes, such as local recurrence, CSS, and OS were also assessed.
Statistical Analysis
Categorical variables were expressed as numbers with percentages and compared using chi-squared test or Fisher exact test. Continuous variables were presented as medians with interquartile ranges (IQRs) and compared using a Mann-Whitney U-test. Survival curves were generated using a Kaplan-Meier method. All values reported were two-sided with statistical significance defined as P < 0.05. All statistical analyses were performed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
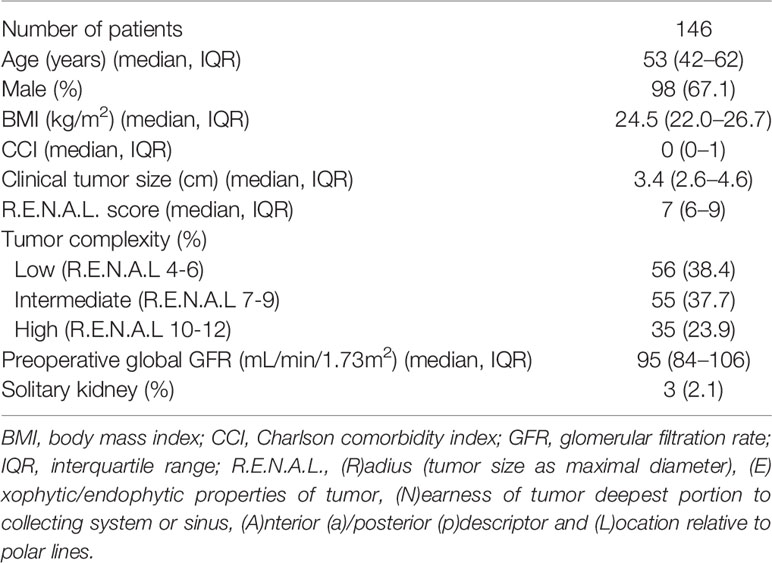
Patient characteristics were summarized in Table 1. Totally, 146 patients with a median preoperative global GFR of 95 ml/min/1.73m2 (IQR: 84–106 ml/min/1.73 m2) were involved. The median age of these patients was 53 y (IQR: 42 y–62 y) and 98 (67.1%) patients were male. Median body mass index was 24.5 kg/m2 (IQR: 22.0–26.7 kg/m2) and median Charlson comorbidity index was 0 (IQR: 0–1). Median clinical tumor size was 3.4 cm (IQR: 2.6–4.6 cm) with a median R.E.N.A.L. score of 7 (IQR: 6–9). Among them, 56 (38.4%) were low tumor complexity, 55 (37.7%) were intermediate, and 35 (23.9%) were high. Three patients had solitary kidney.
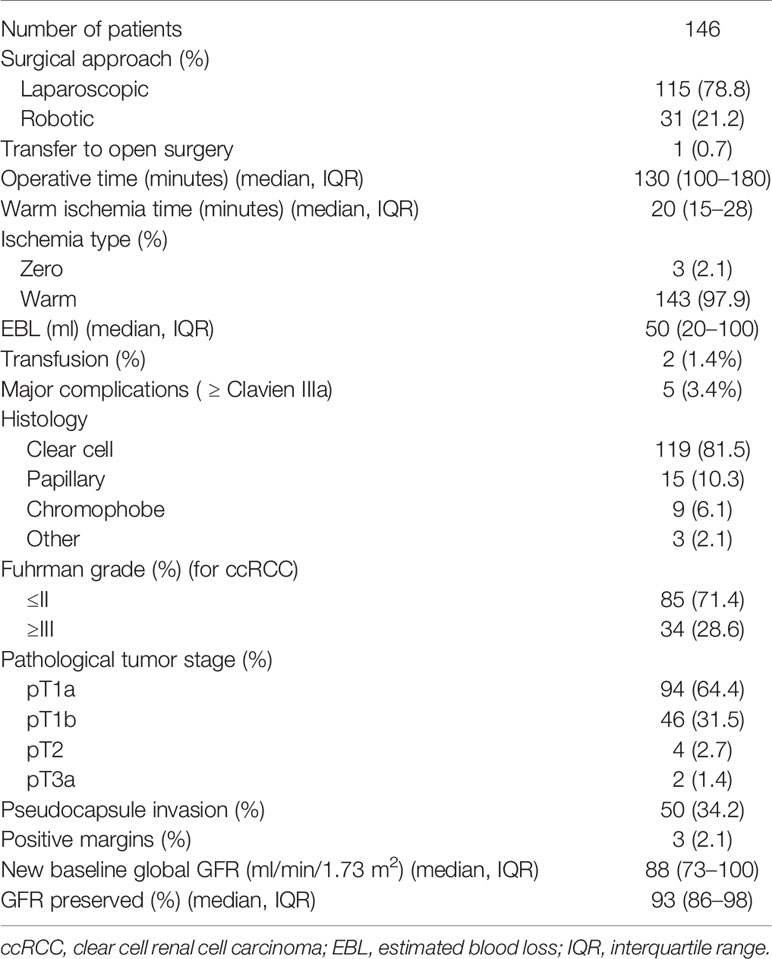
Perioperative, pathological, and functional outcomes were reported in Table 2. With a median operative time of 130 min (IQR: 100–180 min), laparoscopic TE was applied to 115 patients while robotic TE was used for 31 patients. Three patients had zero ischemia while the others had warm ischemia with a median ischemia time of 20 min (IQR: 15–28 min). Median estimated blood loss was 50 ml (IQR: 20–100 ml) and transfusion was required in two patients. According to Clavien system, major complications (≥ Clavien IIIa) happened to five patients and only two were related to urinary system. At pathological assessment, 119 patients were confirmed clear cell carcinoma, 15 were papillary, nine were chromophobe, and three were other types. Fuhrman grade in clear cell carcinoma resulted ≤II in 85 (71.4%) and ≥III in 34 (28.6%). As for pathological tumor stage, 94 (64.4%) patients were pT1a, 46 (31.5%) were pT1b, 4 (2.7%) were pT2, and 2 (1.4%) were pT3a. Pseudocapsule can be seen in each tumor. Pseudocapsule invasion was found in 50 patients and positive margins occurred in three patients. After surgery, median new baseline global GFR was 88 ml/min/1.73 m2 (IQR: 73–100 ml/min/1.73 m2) which meant median global GFR preserved was 93% (IQR: 86–98%).
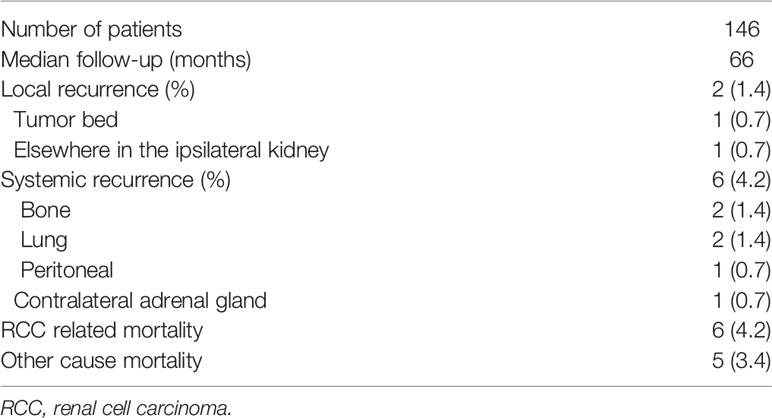
At a median follow-up of 66 months, local recurrence happened to two patients and systemic recurrence happened to six patients (Table 3). Among patients with local recurrence, one happened at tumor bed while the other one happened in elsewhere in the ipsilateral kidney. As for systemic recurrence, two were detected in bone, two in lung, one in peritoneal, and one in contralateral adrenal gland. Besides, five patients died due to non-cancer causes. Figure 1 shows CSS, RFS, and OS in patients after laparoscopic and robotic tumor enucleation for localized RCC according to time after surgery. The 5-year CSS, RFS, OS were 95.7, 89.6, and 91.9%, and the 10-year CSS, RFS, OS were 93.8, 89.6, and 90.0%, respectively.

Figure 1 Kaplan-Meier curves depicting overall survival (blue), cancer specific survival (red) and recurrence free survival (green) in 146 patients treated with laparoscopic and robotic enucleation for renal cell carcinoma.
Discussion
TE has gained growing acceptance among urologists for excellent perioperative results and short-term oncologic outcomes (5, 6, 19). Studies also imply that TE can preserve more normal renal parenchyma which is associated with lower risk of post-operative kidney diseases (20, 21). It is widely accepted that better renal function is beneficial for cardiovascular system and associated with longer overall survival (1–3).
In this study, we had 146 patients performed laparoscopic or robotic TE, and to our knowledge our study is the longest median follow-up to evaluate long-term oncologic outcomes of TE by minimally invasive surgeries. The 5-year and 10-year OS were 91.9 and 90.0% respectively. The 5-year CSS in our study was 95.7% which was comparable to some other studies (13, 22, 23). The 10-year CSS in our study was also up to 93.8% which means we seldom found patients died of cancer more than 5 years after TE. At a median follow-up of 66 months, two patients (1.4%) had local recurrence and only one of them had recurrence at the tumor bed, and six patients (4.1%) had systemic recurrences.
TE preserves more parenchyma, which may contribute to a better renal function after surgery (20, 21). In our study, median global GFR preserved 1 year after surgery was up to 93% (IQR: 86–98%) while it was about 89% in SPN reported in other studies (24, 25). However, a closer distance from the resection plane to tumor makes some urologists uncomfortable because they are worried about the positive margins and local recurrences that might happen in TE patients. Although level 1 evidence is lacking to prove that positive margins in TE patients are comparable to SPN patients, many retrospective studies, review articles, and prospective non-randomized studies imply that TE is as safe as SPN with very few positive margins and local recurrences for localized renal cell carcinomas (10, 26–29).
In the era of open surgery, Carini et al. reported the long-term follow-up of TE for T1a and tumor size between 4 and 7 cm renal cell carcinoma separately (22, 23). At a median follow-up of 61 months and mean follow-up of 76 months for T1a tumors, the 5-year and 10-year cumulative survival rates were 89.5 and 81.4%, and the 5-year and 10-year CSS were 96.7 and 94.7%, respectively. Overall, 6.4% patients had disease progression, three of whom had local recurrences alone (1.5%) elsewhere in the kidney; none had local recurrence at the enucleation bed. At a median follow-up of 51 months and mean follow-up of 74 months for tumor size between 4 and 7 cm, 5-year and 8-year CSS were 85.1 and 81.6%, respectively. Overall 10 patients experienced progressive disease (14.9%), of whom three had local recurrence (4.5%) alone or local recurrence associated with distant metastases. Minervini recently reported a group of 127 robotic TE patients and the positive surgical margins were found in three patients (2.4%). After a median follow-up of 61 months, no recurrence cases at the enucleation site were recorded, and three cases (2.4%) had renal recurrence elsewhere in the ipsilateral kidney. They also found a distinct peritumoral pseudocapsule was presented in 121/127 (95%) tumors, among which partial and complete pseudocapsule invasion was reported in 49/121 (40.5%) and 24/121 (19.8%) cases, respectively (26).
While in our study the rate of positive surgical margin was merely 2.1%, and as we described above, local recurrence was also rarely seen in TE. We could find pseudocapsule in all cases and pseudocapsule invasion in 50 (34%) cases, which was similar to what we had reported several years ago (10). We seldom found complete pseudocapsule invasion in our patients mostly because the T3a patients in our study only accounted for 1.4%. Based on our experience, positive surgical margin and local recurrence are not related to TE technique but the surgeon’s experience. TE is applied to patients after carefully taking into account of the tumor characteristics including growth pattern and interface with normal parenchyma as long as negative surgical margins are prioritized in our center. For beginners, we highly recommend starting with small exophytic tumors surrounded by a distinct pseudocapsule from the images.
Our data also demonstrated that high complexity tumors accounted for about 24% in this study which was higher than data from other studies (19, 26). Our study may have implications for surgical technique as it suggests TE can also be safely used for renal hilar tumors. With the application of robotic surgery and increasing experience in TE, we find TE is the best option to avoid the kidney being completely removed or a large amount of healthy renal parenchyma being devascularized for tumors located in the renal hilum or adjacent to large vessels. Tumor diameter and endophytic status were significantly associated with complete pseudocapsule invasion according to published research (26). In order to avoid positive surgical margins in these complex cases, especially large and endophytic tumors located in the renal hilum, we always clamp the main renal artery and sometimes clamp the renal vein to keep a bloodless field. We also use ultrasound when the boundary of the tumor is difficult to find.
Despite its strengths, our study has some limitations. First, its retrospective and single center design, and the tertiary care patient population could impact generalizability. Second, although laparoscopic and robotic tumor enucleation are both belong to minimally invasive surgery, most studies indicate robotic surgery is superior than laparoscopic surgery in perioperative outcomes (30). It is unclear whether this potential superiority could affect the long-term oncologic outcomes although the surgeons in this study are very good at both approaches. inally, given the natural history of renal cell carcinoma, the relatively small sample size especially only 31 procedures performed with robotic surgery and the median follow-up of 66 months in our study might still be not enough to assess potential delayed recurrences after laparoscopic and robotic TE.
In conclusions, our study indicates that TE for RCC patients either by laparoscopic or robotic approach in experienced hands are oncologically safe with a median follow-up of more than 5 years. TE can preserve more parenchyma and achieve negative surgical margins even in complex cases due to the existence of pseudocapsule in the vast majority of tumors. Prospective studies with more patients and longer follow-up will be required to further evaluate oncologic safety after TE.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Sun Yat-sen Memorial Hospital ethics committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WD and XiC prepared the manuscript, which was reviewed by all authors. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by the National Natural Science Foundation of China (Grant No. 81972383, U1301221, 81702523); Natural Science Foundation of Guangdong (2019A1515010188, 2020A1515010888).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The content of this manuscript has been presented in part at the AUA Annual Meeting Program Abstract 2019 (31).
Abbreviations
CSS, cancer specific survival; CT, computed tomography; EBL, estimated blood loss; GFR, glomerular filtration rate; IQR, interquartile range; MRI, magnetic resonance imaging; NSS, nephron sparing surgery; OS, overall survival; RCC, renal cell carcinoma; R.E.N.A.L., (r)adius (tumor size as maximal diameter), (e)xophytic/endophytic properties of tumor, (n)earness of tumor deepest portion to collecting system or sinus, (a)nterior (a)/posterior (p) descriptor, and (l)ocation relative to polar lines; RFS, recurrence free survival; RN, radical nephrectomy; SPN, standard partial nephrectomy; TE, tumor enucleation; WIT, warm ischemia time.
References
1. Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernandez-Pello S, et al. European association of urology guidelines on renal cell carcinoma: The 2019 update. Eur Urol (2019) 75(5):799–810. doi: 10.1016/j.eururo.2019.02.011
2. Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, Chang A, et al. Renal Mass and Localized Renal Cancer: AUA guideline. J Urol (2017) 198:520–9. doi: 10.1016/j.juro.2017.04.100
3. Van Poppel H, Da Pozzo L, Albrecht W, Matveev V, Bono A, Borkowski A, et al. A Prospective, Randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol (2011) 59(4):543–52. doi: 10.1016/j.eururo.2010.12.013
4. Abdollah F, Arora S, von Landenberg N, Gild P, Sood A, Dalela D, et al. Testing the external validity of the EORTC randomized trial 30904 comparing overall survival after radical nephrectomy vs nephron-sparing surgery in contemporary North American patients with renal cell cancer. BJU Int (2018) 121(3):345–7. doi: 10.1111/bju.14039
5. García AG, León TG. Simple enucleation for renal tumors: indications, techniques, and results. Curr Urol Rep (2016) 17(1):7. doi: 10.1007/s11934-015-0560-4
6. Gupta GN, Boris RS, Campbell SC, Zhang Z. Tumor enucleation for sporadic localized kidney cancer: pro and con. J Urol (2015) 194:623–5. doi: 10.1016/j.juro.2015.06.033
7. Kaouk JH, Khalifeh A, Hillyer S, Haber CP, Stein RJ, Autorino R. Robot-assisted laparoscopic partial nephrectomy: step-by-step contemporary technique and surgical outcomes at a single high-volume institution. Eur Urol (2012) 62(3):553–61. doi: 10.1016/j.eururo.2012.05.021
8. Russo P. Open partial nephrectomy. Personal technique and current outcomes. Arch Esp Urol (2011) 64(7):571–93.
9. Mukkamala A, Allam CL, Ellison JS, Hafez KS, Miller DC, Montgomery JS, et al. Tumor enucleation vs sharp excision in minimally invasive partial nephrectomy: technical benefit without impact on functional or oncologic outcomes. J Urol (2014) 83:1294–9. doi: 10.1016/j.urology.2014.02.007
10. Dong W, Lin T, Li F, Fang Y, Li K, Jiang C, et al. Laparoscopic partial nephrectomy for T1 renal cell carcinoma: comparison of two resection techniques in a multi-institutional propensity sore-matching analysis. Ann Surg Oncol (2016) 23(4):1395–402. doi: 10.1245/s10434-015-4985-2
11. Minervini A, Ficarra V, Rocco F, Antonelli A, Bertini R, Carmignani G, et al. Simple enucleation is equivalent to traditional partial nephrectomy for renal cell carcinoma: results of a nonrandomized, retrospective, comparative study. J Urol (2011) 185(5):1604–10. doi: 10.1016/j.juro.2010.12.048
12. Ficarra V, Galfano A, Cavalleri S. Is simple enucleation a minimal partial nephrectomy responding to the EAU guidelines’ recommendations? Eur Urol (2009) 55(6):1315–8. doi: 10.1016/j.eururo.2008.08.067
13. Minervini A, Serni S, Tuccio A, Tuccio A, Siena G, Vittori G, et al. Simple enucleation versus radical nephrectomy in the treatment of pT1a and pT1b renal cell carcinoma. Ann Surg Oncol (2012) 19(2):694–700. doi: 10.1245/s10434-011-2003-x
14. Minervini A, Carini M, Uzzo RG, Campi R, Smaldone MC, Kutikov A. Standardized reporting of resection technique during nephron-sparing surgery: the surface-intermediate-base margin score. Eur Urol (2014) 66:803–5. doi: 10.1016/j.eururo.2014.06.002
15. Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol (2016) 70(1):93–105. doi: 10.1016/j.eururo.2016.02.029
16. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol (1982) 6(7):655–63. doi: 10.1097/00000478-198210000-00007
17. Kong X, Ma Y, Chen J, Luo Q, Yu X, Li Y, et al. Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating glomerular filtration rate in the Chinese population. Nephrol Dial Transplant (2013) 28(3):641–51. doi: 10.1093/ndt/gfs491
18. Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int (2014) 85(1):49–61. doi: 10.1038/ki.2013.444
19. Minervini A, Vittori G, Lapini A, Tuccio A, Siena G, Serni S, et al. Morbidity of tumour enucleation for renal cell carcinoma (RCC): results of a single-centre prospective study. BJU Int (2012) 109(3):372–7. 18. doi: 10.1111/j.1464-410X.2011.10360.x
20. Blackwell RH, Li B, Kozel Z, Zhang Z, Zhao J, Dong W, et al. Functional implications of renal tumor enucleation relative to standard partial nephrectomy. J Urol (2017) 99:162–8. doi: 10.1016/j.urology.2016.07.048
21. Dong W, Gupta GN, Blackwell RH, Wu J, Suk-Ouichai C, Sha A, et al. Functional comparison of renal tumor enucleation versus standard partial nephrectomy. Eur Urol Focus (2017) 3(4-5):437–43. doi: 10.1016/j.euf.2017.06.002
22. Carini M, Minvervini A, Masieri L, Lapini A, Semi S. Simple enucleation for the treatment of pT1a renal cell carcinoma: our 20-year experience. Eur Urol (2006) 50:1269–71. doi: 10.1016/j.eururo.2006.05.022
23. Carini M, Minervini A, Lapini A, Semi S. Simple enucleation for the treatment of renal cell carcinoma between 4 and 7 cm in greatest dimension: progression and long-term survival. J Urol (2006) 175(6):2022–6. doi: 10.1016/S0022-5347(06)00275-8
24. Dong W, Wu J, Suk-Ouichai C, Antonio EC, Remer E, Li J, et al. Ischemia and functional recovery from partial nephrectomy: refined perspectives. Eur Urol Focus (2018) 4(4):572–8. doi: 10.1016/j.euf.2017.02.001
25. Dong W, Wu J, Suk-Ouichai C, Antonio EC, Ramer E, Li J, et al. Devascularized parenchymal mass associated with partial nephrectomy: predictive factors and impact on functional recovery. J Urol (2017) 198(4):787–94. doi: 10.1016/j.juro.2017.04.020
26. Minervini A, Campi R, Di Maida F, Mari A, Montagnani I, Tellini R, et al. Tumor-parenchyma interface and long-term oncologic outcomes after robotic tumor enucleation for sporadic renal cell carcinoma. Urol Oncol (2018) 36(12):527.e1–e11. doi: 10.1016/j.urolonc.2018.08.014
27. Longo N, Minervini A, Antonelli A, Bianchi G, Bocciardi AM, Cunico SC, et al. Simple enucleation versus standard partial nephrectomy for clinical T1 renal masses: perioperative outcomes based on a matched-pair comparison of 396 patients (RECORd project). Eur J Surg Oncol (2014) 40:762–8. doi: 10.1016/j.ejso.2014.01.007
28. Minervini A, Campi R, Sessa F, Derweesh I, Kaouk JH, Mari A, et al. Positive surgical margins and local recurrence after simple enucleation and standard partial nephrectomy for malignant renal tumors: systematic review of the literature and meta-analysis of prevalence. Minerva Urol Nefrol (2017) 69(6):523–38. doi: 10.23736/S0393-2249.17.02864-8
29. Schiavina R, Serni S, Mari A, Antonelli A, Bertolo R, Bianchi G, et al. A prospective, multicenter evaluation of predictive factors for positive surgical margins after nephron-sparing surgery for renal cell carcinoma: the RECORd1 Italian project. Clin Genitourin Cancer (2015) 13:165–70. doi: 10.1016/j.clgc.2014.08.008
30. Zhao XZ, Lu Q, Campi R, Ji C, Guo S, Liu G, et al. Endoscopic robot-assisted simple enucleation vs laparoscopic simple enucleation with single-layer renorrhaphy in localized renal tumors: a propensity score-matched analysis from a high-volume centre. J Urol (2018) 121:97–103. doi: 10.1016/j.urology.2018.08.015
Keywords: renal cell carcinoma, nephron sparing surgery, tumor enucleation, survival, follow-up
Citation: Dong W, Chen X, Huang M, Chen X, Gao M, Ou D, Li K, Wang C, Wu S, Liu H, Xie W, Xie W, Campbell SC, Lin T and Huang J (2021) Long-Term Oncologic Outcomes After Laparoscopic and Robotic Tumor Enucleation for Renal Cell Carcinoma. Front. Oncol. 10:595457. doi: 10.3389/fonc.2020.595457
Received: 16 August 2020; Accepted: 18 November 2020;
Published: 14 January 2021.
Edited by:
Kan Gong, Peking University First Hospital, ChinaReviewed by:
Andrea Minervini, University of Florence, ItalySudhir Isharwal, Oregon Health and Science University, United States
Copyright © 2021 Dong, Chen, Huang, Chen, Gao, Ou, Li, Wang, Wu, Liu, Xie, Xie, Campbell, Lin and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianxin Lin, bGludHhAbWFpbC5zeXN1LmVkdS5jbg==; Jian Huang, aHVhbmdqOEBtYWlsLnN5c3UuZWRuLmNu
†These authors have contributed equally to this work
 Wen Dong
Wen Dong Xiong Chen1†
Xiong Chen1† Xu Chen
Xu Chen Chenyang Wang
Chenyang Wang Tianxin Lin
Tianxin Lin Jian Huang
Jian Huang