- 1Musculoskeletal Tumor Center, Beijing Key Laboratory of Musculoskeletal Tumor, Peking University People’s Hospital, Beijing, China
- 2Sarcoma Biology Laboratory, Department of Orthopedic Surgery, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States
- 3Department of Orthopedic Surgery, Chulabhorn Hospital, HRH Princess Chulabhorn College of Medical Science, Bangkok, Thailand
Purpose: Chordomas are rare, slow-growing sarcomas without any accepted prognostic biomarkers. Owing to their proximity to critical neurovascular structures, discovering predictive biomarkers in chordoma has been a significant research effort because it may potentially reduce risky therapies in patients with less aggressive tumors. In response, because cyclin E1 overexpression correlates with patient prognosis in several malignancies, we investigated its expression in chordoma and whether it informs patient prognosis.
Methods: Seventy-five chordoma patient specimens were enrolled in a tissue microarray (TMA) to evaluate cyclin E1 expression via immunohistochemical staining. Western blot was used to assess cyclin E1 expression in chordoma cell lines and fresh tissues. We then correlated cyclin E1 staining intensity in the TMA to clinicopathological features and chordoma patient outcomes.
Results: Sixty-three percent of the chordoma patient specimens in the TMA, fifty-six percent of the fresh chordoma tissues, and all chordoma cell lines showed high cyclin E1 expression. In TMA analysis, cyclin E1 expression positively correlated to chordoma patient disease status. By survival analysis, high cyclin E1 expression was an independent prognostic risk factor for chordoma patients along with advanced disease status and positive surgical margin.
Conclusion: Cyclin E1 is a promising biomarker predicting chordoma patient prognosis.
Introduction
Chordomas are rare bone sarcomas that arise from the transformed remnants of notochord, with an incidence of 0.1/100,000 people per year (1). It principally affects elderly patients at the skull base (41.1%), mobile spine (27.4%) and sacrum (31.5%) (2). Surgery is the cornerstone therapeutic modality for chordoma, with adequate surgical margins representing the primary clinical goal for a favorable prognosis (3–5). And although the 5-year survival rate for chordoma patients is an optimistic 70-81%, local recurrence and subsequent metastasis occurs approximately half the time after surgery, with no systemic therapies having shown major benefit (1, 5, 6). And while their diagnosis is made according to clinicopathological features and brachyury expression which is a prominent transcription factor in notochord development, brachyury is a diagnostic biomarker and therapeutic target rather than a prognostic biomarker (7–10). In short, there are no well-recognized biomarkers predictive of chordoma patient prognosis. For the patient, the lack of accurate predictive biomarkers can result in overtreatment of less aggressive chordomas via surgery or adjuvant radiation therapy. In addition, even with aggressive surgery, recurrent rates remain high. There is, therefore, an urgent need for the identification of chordoma biomarkers to better ascertain patient prognosis and whether high-risk surgeries or adjuvant therapies are indicated.
Cyclin E1 was originally described in 1991 as the prototype cyclin E. It is a 47 kDa protein localized within both the nucleus and cytoplasm (11). By complexing with cyclin-dependent kinase 2 (CDK2), it phosphorylates numerous downstream proteins such as Rb and is strong promoter of G1-S transition, a well-known tumorigenic step (12). Cyclin E1 overexpression has been investigated in more common malignancies, and has shown to correlate with therapeutic response and prognosis (13–18). Despite these established findings in other cancers, the expression and clinical significance of cyclin E1 in chordoma is unknown.
In response, we sought to investigate the expression of cyclin E1 in chordoma and its correlation, if any, to clinicopathologic features or prognosis within chordoma patients. To our knowledge, this is the first and only work evaluating cyclin E1 expression in chordoma and its clinical significance.
Materials and Methods
Ethics
The study protocol and the consent of the informed patients were approved by the Partners Human Research Committee (number: 2007-P-002464/5) of Partners HealthCare as previously described (19). All patients provided their written informed consent to participate in this study.
Chordoma Tissue Samples and Tissue Microarray
Seventy-five chordoma patient specimens in formalin fixed paraffin embedded (FFPE) blocks obtained during surgery were used to construct the tissue microarray (TMA) as previously described (19). The inclusion criteria included: 1) patients with chordoma confirmed by histological diagnosis; 2) patients underwent surgical treatment; 3) patients with complete clinical and follow-up data. The exclusion criteria included: 1) peri-operative death; 2) patients with incomplete clinical and follow-up data. The demographic and clinical information of 75 patients was reviewed and documented from a prospective database. In the course of the TMA construction, three sites of each FFPE block were selected to assemble the recipient master block. Representative triplicate 0.5 mm diameter core biopsies of each tissue block were obtained through pathology reports and pathologist read hematoxylin and eosin (HE)-stained slides. In addition, nine fresh chordoma tissue samples were obtained for study.
Immunohistochemistry Staining and Assessment
Immunohistochemistry (IHC) staining was performed to evaluate for cyclin E1 expression. In brief, the paraffin-embedded slide was baked for 1 h at 60°C before xylene deparaffinization. The slide was then rehydrated through graded ethanol (100% and 95%). We then used 3% hydrogen peroxide to quench endogenous peroxidase activity after heated epitope retrieval. After blocking for 1 h with normal goat serum, the slide was incubated with polyclonal rabbit antibody to human cyclin E1 (Cell Signaling Technology, MA, USA. Catalog# 20808S) overnight in a humidified chamber set at 4°C. Afterwards, the bound antibody was detected by SignalStain® Boost Detection Reagent (Cell Signaling Technology, MA, USA) and SignalStain® DAB (Cell Signaling Technology, MA, USA). Finally, all sections were counterstained with Hematoxylin QS (Vector Laboratories, CA, USA), and the slide was mounted with VectaMount AQ (Vector Laboratories, CA, USA) for long-term preservation.
Two independent pathologists blinded to patient clinical information and tumor characteristics assessed and scored the IHC-stained slide. Cyclin E1 expression was scored and categorized according to the staining intensity of chordoma tissues: 0, no staining; 1+, weak staining; 2+, moderate staining; 3+, strong staining. The low cyclin E1 expression subset included group 0 and 1+, while the high cyclin E1 expression subset included group 2+ and 3+ (Figure 1). A Nikon Eclipse Ti-U fluorescence microscope (Diagnostic Instruments Inc., MI, USA) with a SPOT RT™ digital camera (Diagnostic Instruments Inc., MI, USA) was used to obtain cyclin E1 staining images.
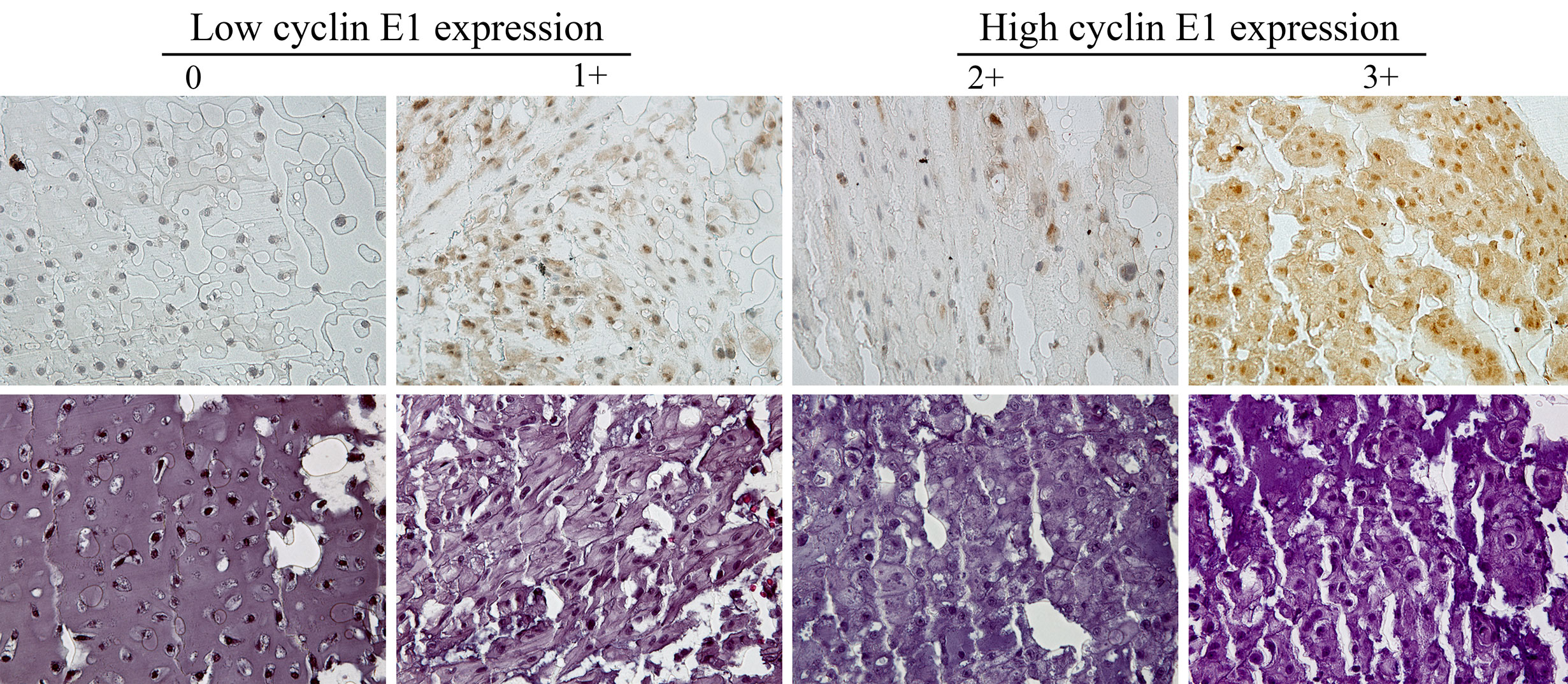
Figure 1 Assessment of cyclin E1 expression in the chordoma tissue microarray by immunohistochemistry. Different immunohistochemistry staining intensities of cyclin E1 and HE in chordoma tissues are shown in representative images. Staining patterns were divided into 4 groups: 0, no staining; 1+, weak staining; 2+, moderate staining; 3+, strong staining (Original magnification, 400×). Low and high cyclin E1 expression groups included chordoma specimens with the staining score of 0 to 1+ and 2+ to 3+, respectively.
Human Chordoma Cell Lines and Cell Culture
The human chordoma cell line UCH2 was established and kindly provided by Dr. Silke Brüderlein (University Hospitals of Ulm, Ulm, Germany) (20). The cell line CH22 was established in our laboratory as previously reported (21). The UCH2 and CH22 cells were incubated at 37°C with 5% CO2 in Dulbecco’s Modified Eagle Medium (DMEM) (Life Technologies Corp., NY, USA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, MO, USA) and 1% penicillin/streptomycin (Life Technologies, CA, USA).
Protein Preparation and Western Blot
We used 1× RIPA lysis buffer (Sigma-Aldrich, MO, USA) combined with protease inhibitor cocktail tablets (Roche Applied Science, IN, USA) to extract protein from cells and fresh tissues. The DC™ protein assay reagents (Bio-Rad, CA, USA) and a spectrophotometer SPECTRA max 340PC (Molecular Devices, LLC., CA, USA) were then used to determine the protein lysate concentrations. Briefly, our Western blot protocol began with an SDSPAGE gel to run the denatured proteins before transfer to nitrocellulose membranes. These membranes were incubated with monoclonal rabbit antibodies to human cyclin E1 (1:1,000 dilution, Cell Signaling Technology, MA, USA) and mouse monoclonal antibody for human β-actin (1:20,000 dilution, Sigma-Aldrich, MO, USA) at 4°C overnight after they were blocked in Odyssey Blocking Buffer (Li-COR Biosciences, NE, USA) for 1 h. Following incubation with the primary antibody, TBST was used as a membrane wash (4 times for 5 min each at room temperature). Next, goat anti-rabbit IRDye 800CW (926–32,211, 1:5,000 dilution) and goat anti-mouse IRDye 680LT secondary antibody (926–68,020, 1:15,000 dilution) (Li-COR Biosciences, NE, USA) were applied for 1 h at room temperature followed by another TBST membrane wash (four times for 5 min each at room temperature). Bands were detected using Odyssey Infrared Fluorescent Western Blot Imaging System from Li-COR Bioscience (NE, USA), and Odyssey software 3.0 was used to quantify the bands.
Statistical Analysis
GraphPad Prism 7 software (GraphPad Software, CA, USA) and SPSS 19.0 software (IBM Corp., Armonk, New York) were used for statistical analyses. Nonparametric testing (Mann-Whitney U test) was performed to compare the two groups and determine statistical significance. One-way analysis of variance and the least-significant difference test were performed for multiple comparisons. As for the survival analysis, overall survival (OS) was defined as the time from surgery (when the tissue was obtained) to death of the patient. Recurrence-free survival (RFS) was defined as the time from the surgery to the first local recurrence after this surgery. Metastasis-free survival (MFS) was defined as the time from surgery to the detection of new onset metastasis. The survival curves were produced by Kaplan-Meier methods. The relation between various key factors and OS, RFS, or MFS was evaluated by Cox regression both univariately and multivariately. Only those factors that were statistically significant (P<0.05) in the univariate analysis were involved in the multivariate analysis. A P-value < 0.05 was considered significant.
Results
Clinicopathological Characteristics of 75 Chordoma Patients Enrolled in TMA
The demographic and clinicopathological characteristics of 75 patients are shown in Table 1. There were 53 (70.7%) males and 22 (29.3%) females, with an average age of 59.5 ± 13.7 years. Thirty-one patients (41.3%) were treated for primary localized lesions while 44 patients (58.7%) had advanced disease, including 39 patients treated for recurrent lesions and 5 patients with metastasis. The majority of the lesions were located at sacrum (44, 58.7%). Surgeries were performed through an anterior and posterior approach in most patients (47, 62.7%), and negative surgical margins were completed in 18 patients (24.0%). Neoadjuvant radiation was performed in 57 patients (76.0%) without detailed information regarding the type and dose of the radiation.
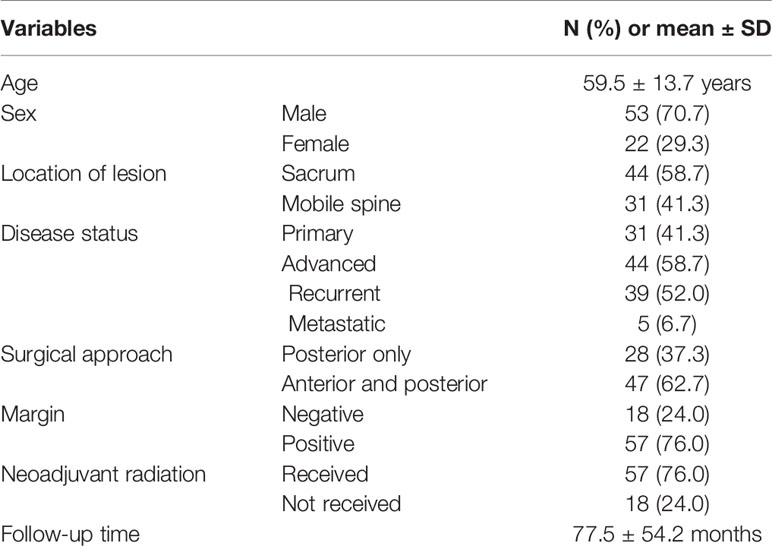
Table 1 Demographic and clinical information of 75 chordoma patients enrolled in a tissue microarray.
Cyclin E1 Is Highly Expressed in Chordoma
Cyclin E1 expression was first evaluated in TMA by IHC staining. Among 75 specimens, no staining was found in 10 specimens (13.3%) with the remaining 65 specimens having various staining intensities, including 1+ staining in 18 specimens (24.0%), 2+ staining in 23 specimens (30.7%), and 3+ staining in 24 specimens (32.0%) (Figure 2A). According to the aforementioned categorization criteria, high and low cyclin E1 expression was found in 47 (62.7%) and 28 (37.3%) specimens, respectively (Figure 2B).
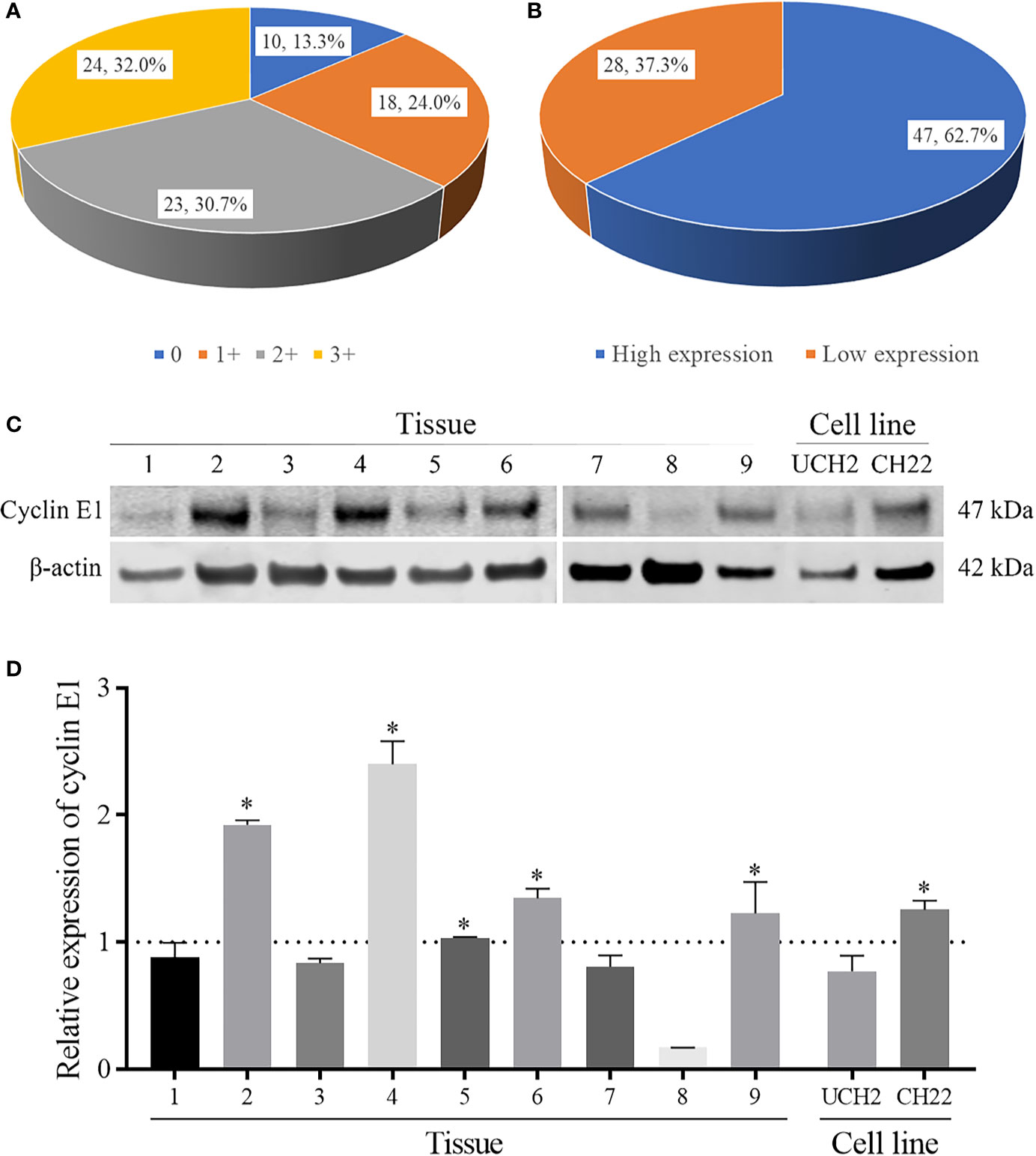
Figure 2 The expression levels of cyclin E1 in chordoma. (A, B) Pie chart showing frequency and percentage of different cyclin E1 expression levels and high/low cyclin E1 expression groups in chordoma tissue microarrays. (C) Western blots showing cyclin E1 expression in nine chordoma fresh tissues and cell lines (UCH2 and CH22). (D) Densitometry quantification of the Western blots of cyclin E1 from Figure 2C, presented as relative to β-actin expression. The data are presented in as mean ± SD of the experiment carried out in triplicate. * means the expression of cyclin E1 is stronger than the β-actin, indicating high cyclin E1 expression.
We further evaluated cyclin E1 expression in nine fresh chordoma tissues and two chordoma cell lines. All chordoma tissue samples showed cyclin E1 expression, including five with high expression (55.6%) and 4 with low expression (44.4%). In chordoma cell lines, Western blots showed cyclin E1 was expressed in both UCH2 and CH22, with CH22 having notably high expression. (Figures 2C, D)
Cyclin E1 Expression Correlated With Disease Status of Chordoma Patients
Cyclin E1 expression did not significantly vary based on patient age, sex, or lesion location (Figures 3A–C). When patients with and without neoadjuvant radiation were compared, cyclin E1 expression was weaker in radiation-treated patients versus those without radiation, but did not meet statistical significance (1.7 ± 1.0 versus 2.0 ± 1.1, P=0.23) (Figure 3D).
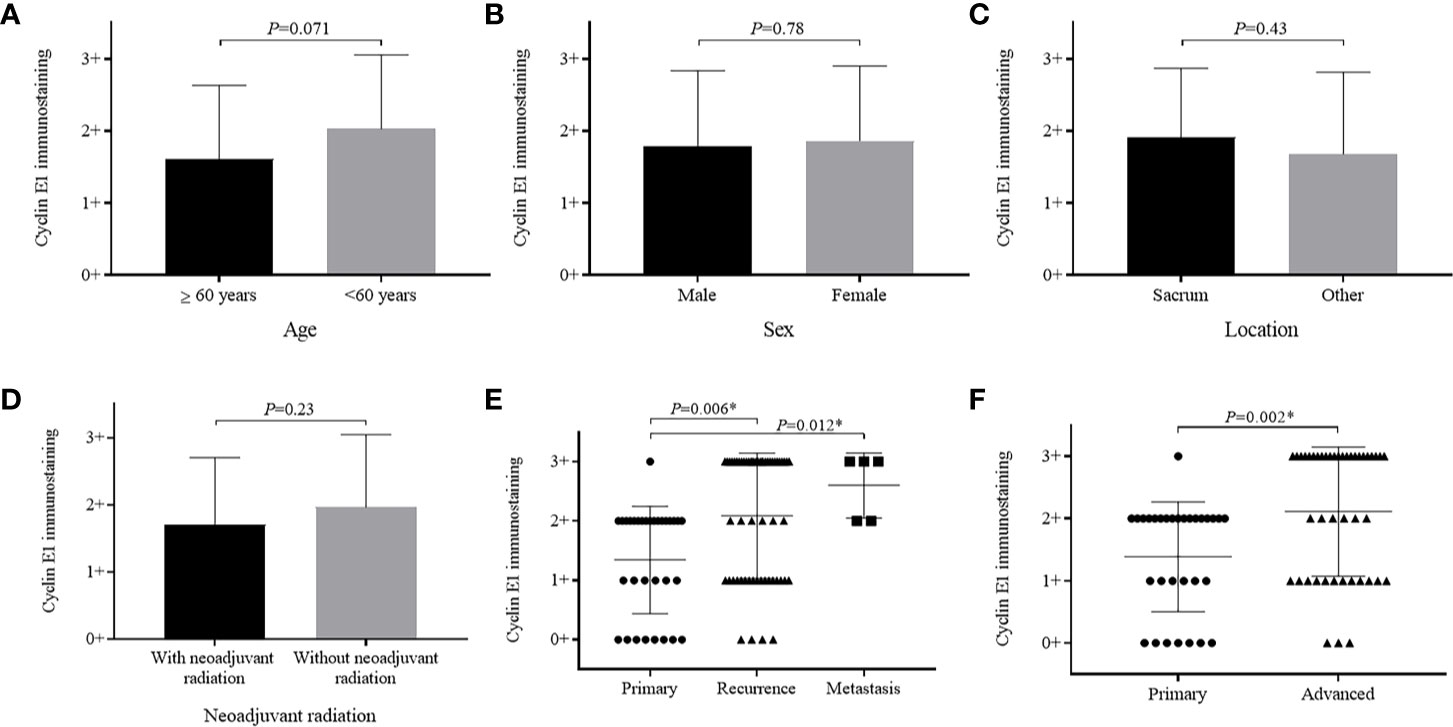
Figure 3 Relationship between cyclin E1 expression and clinicopathological characteristics of chordoma patients. Graphics illustrate no significant differences in cyclin E1 expression level among patients based on (A) age group, (B) sex, (C) tumor location or (D) with and without neoadjuvant radiation. Significant differences were observed in cyclin E1 expression based on patient disease status (E, F). *Statistical significance.
Importantly, patients who had recurrent and metastatic chordomas prior to surgery showed significantly higher cyclin E1 expression compared to those patients with primary lesions (recurrence versus primary, 2.1 ± 1.1 versus 1.4 ± 0.9, P=0.006; metastasis versus primary, 2.6 ± 0.5 versus 1.4 ± 0.9, P=0.012) (Figure 3E). Essentially, a more advanced chordoma staging correlated to higher cyclin E1 expression (advanced versus primary, 2.1 ± 1.0 versus 1.4 ± 0.9, P=0.002) (Figure 3F).
Cyclin E1 Overexpression Is an Independent Prognostic Factor in Chordoma
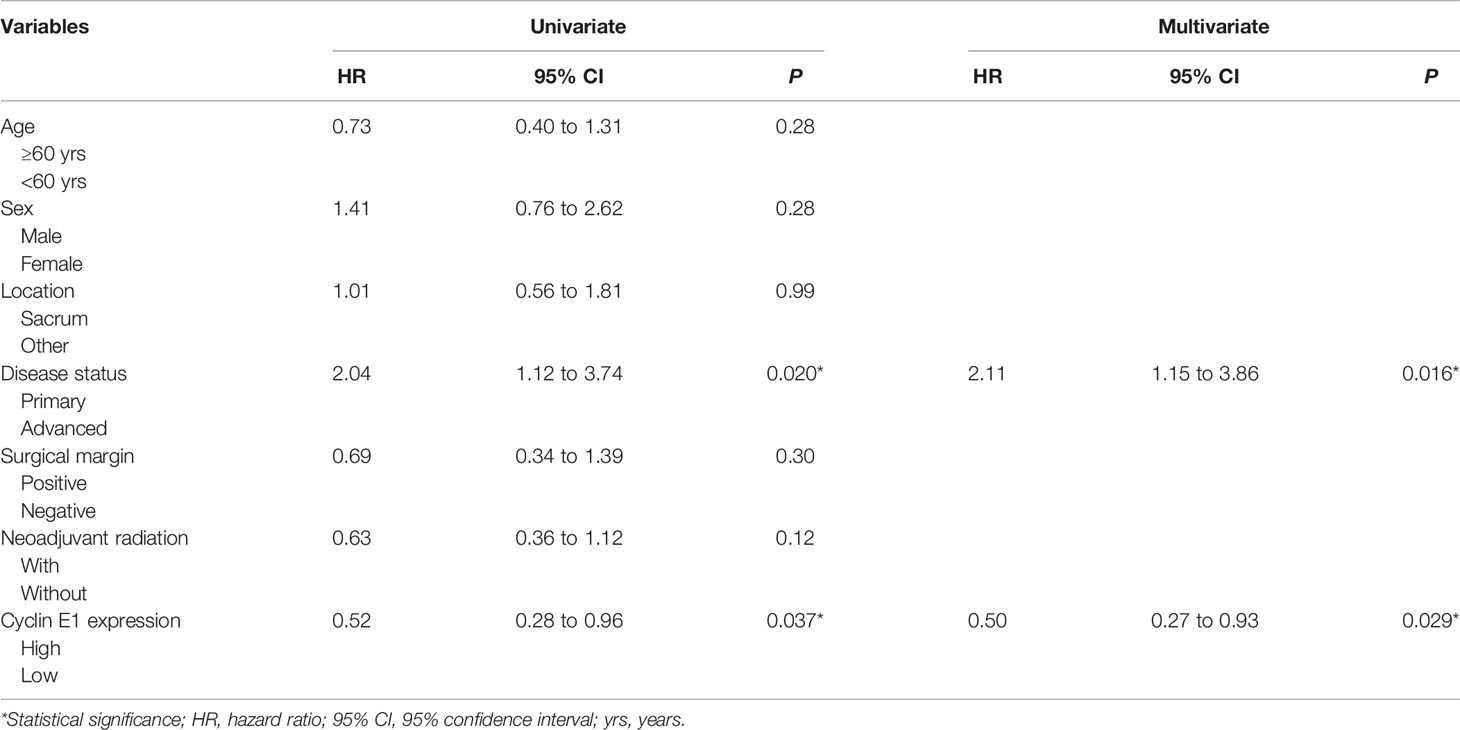
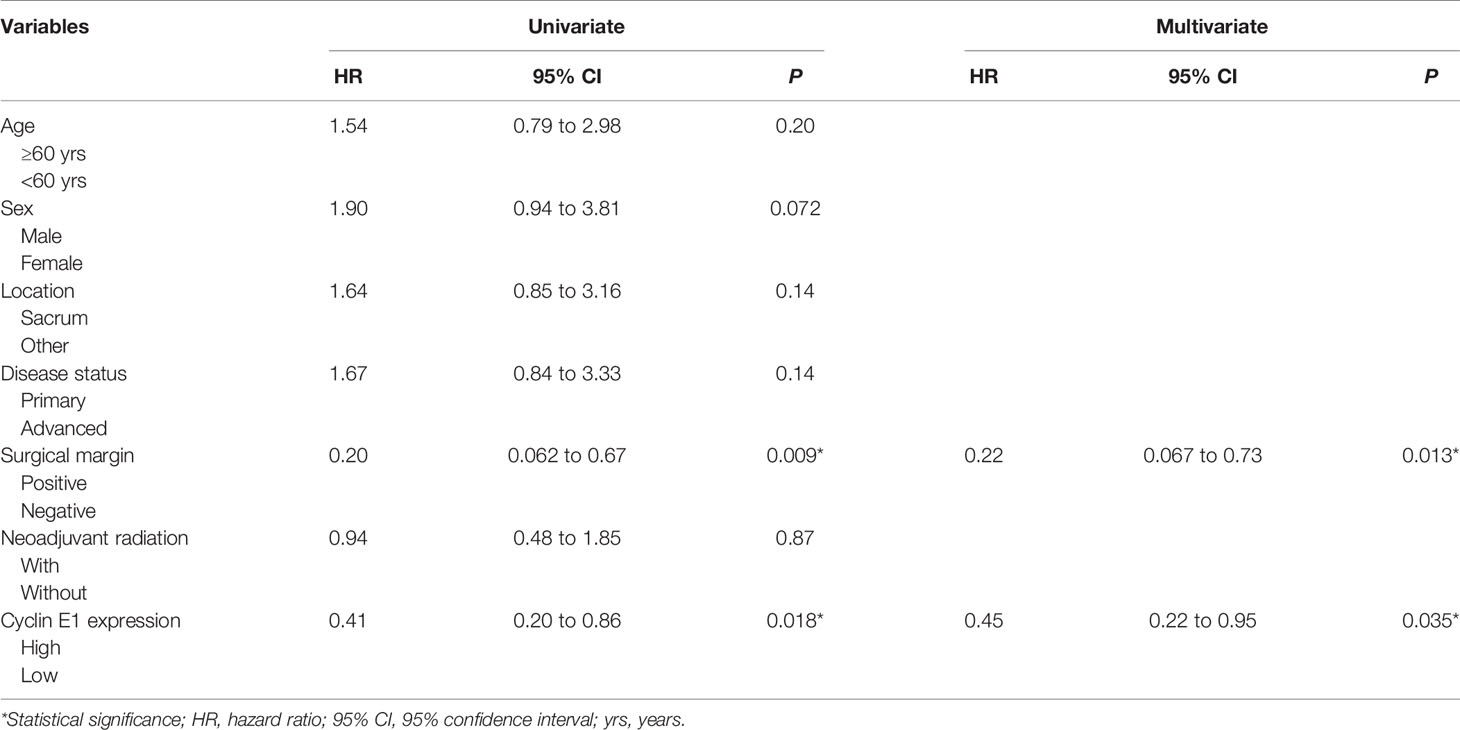
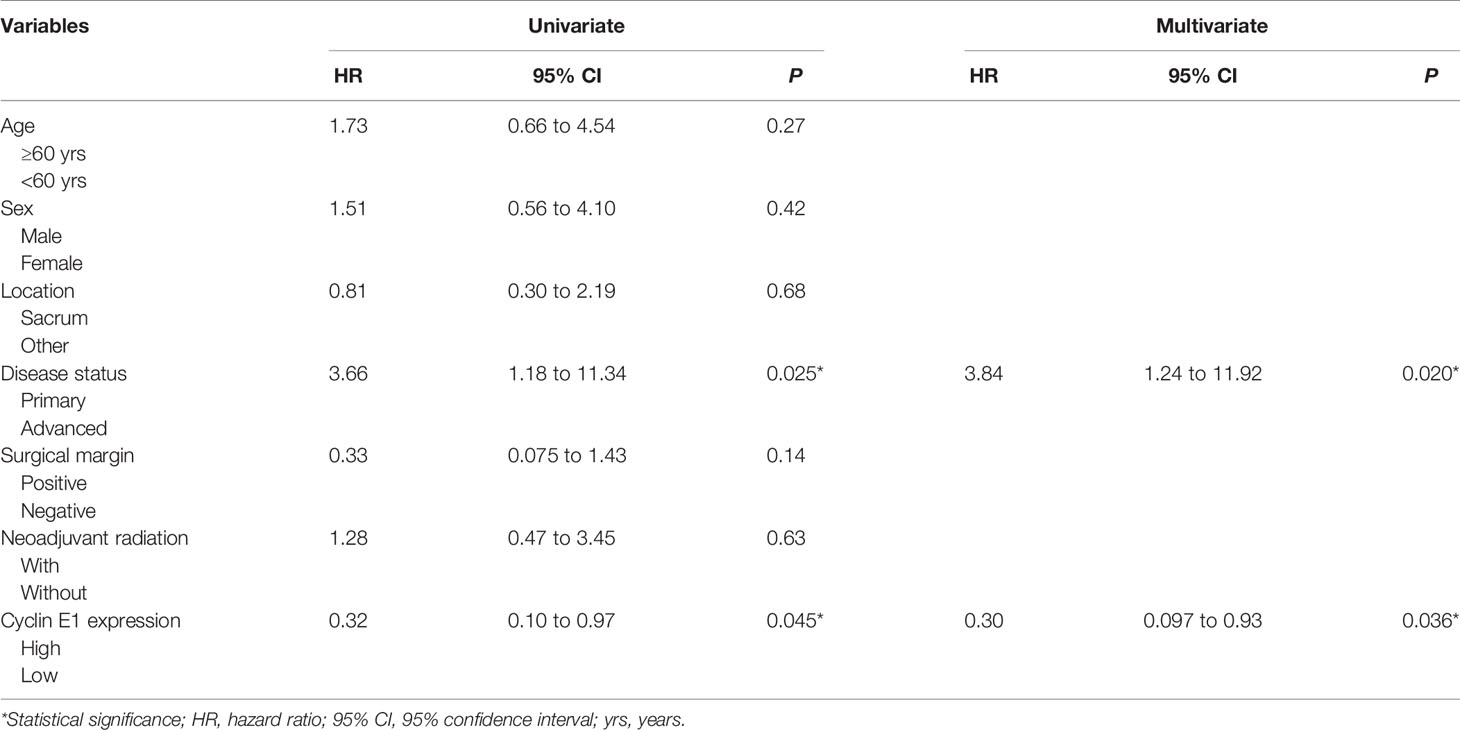
To identify whether cyclin E1 expression correlates to the prognosis of chordoma patients, survival analysis was performed on OS, RFS, and MFS of the 75 chordoma patients. In summary, after the tissue samples were obtained post-surgery, death occurred in 49 patients (65.3%), local recurrence was found in 37 patients (49.3%) and new onset metastasis was found in 17 patients (22.7%). Of the 75 chordoma patients, the mean OS in months was 102.3 (95% CI 83.7 to 121.0), RFS was 102.4 (95% CI 79.5 to 125.3) and MFS was 180.2 (95% CI 151.5 to 208.9). Five-year rates included OS of 63.1% (95% CI 51.7% to 74.5%), RFS was 52.4% (95% CI 40.2% to 64.6%), and MFS rate was 79.3% (95% CI 69.1% to 89.5%). Finally, the ten-year rates included OS of 38.2% (95% CI 25.3% to 51.1%), RFS of 47.0% (95% CI 33.9% to 60.1%), and MFS of 69.5% (95% CI 55.6% to 83.4%) (Figure 4A).
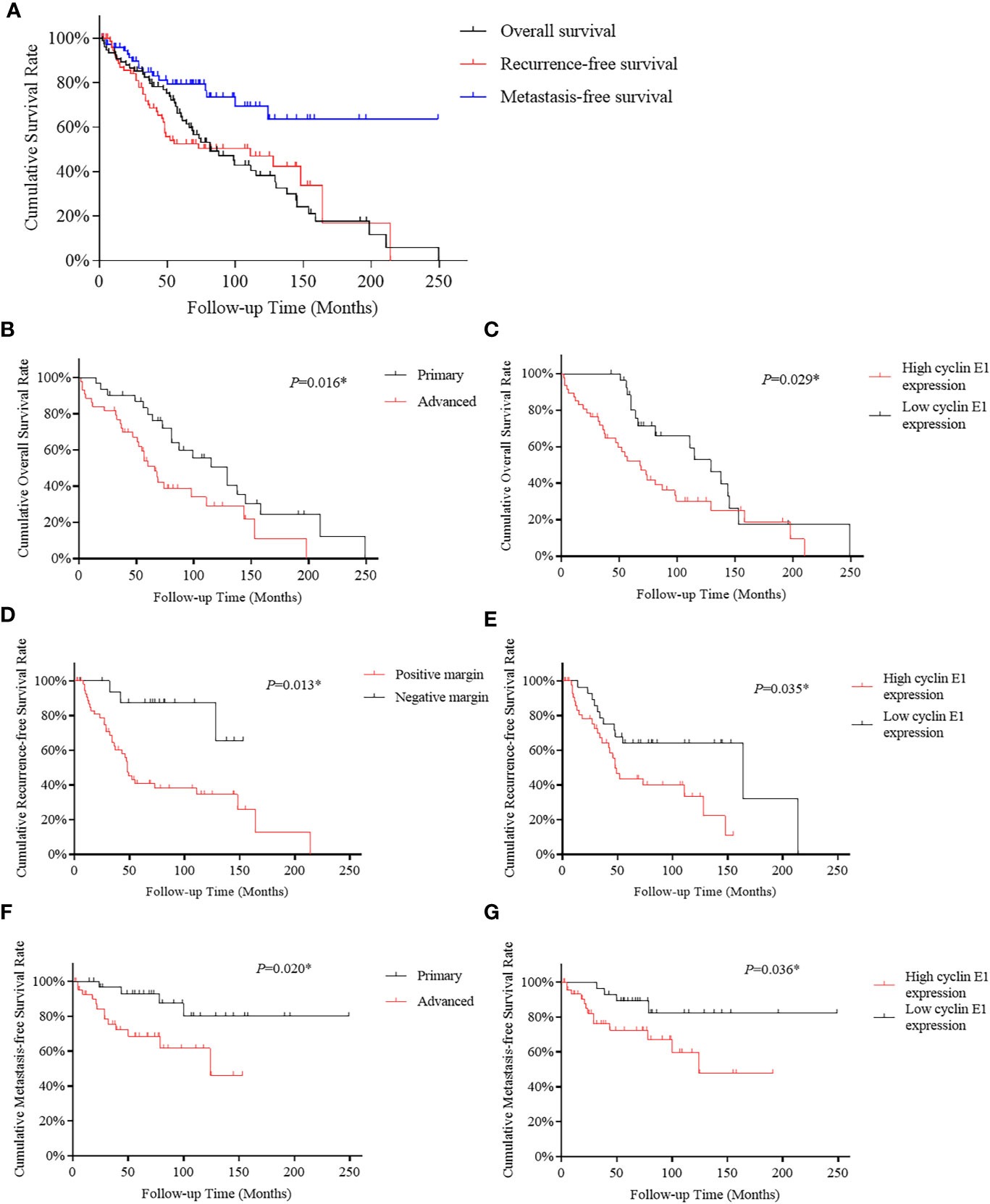
Figure 4 Kaplan-Meier survival curve derived results of survival analysis. (A) Overall, recurrence-free and metastasis-free survival curves of 75 chordoma patients. (B, C) Advanced disease status and high cyclin E1 expression were independent risk factors for overall survival in 75 chordoma patients. (D, E) Positive surgical margins and high cyclin E1 expression were independent risk factors for recurrence-free survival in 75 chordoma patients. (F, G) Advanced disease status and high cyclin E1 expression were independent risk factors for metastasis-free survival in 75 chordoma patients. *Statistical significance.
For OS, both univariate and multivariate analysis suggested advanced disease status and high cyclin E1 expression were independent risk factors (Table 2) (Figures 4B, C). For RFS, positive surgical margins and high cyclin E1 expression were identified by univariate and multivariate analysis to be independent risk factors (Table 3) (Figures 4D, E). And for MFS, advanced disease status and high cyclin E1 expression were independent risk factors (Table 4) (Figures 4F, G). Overall, cyclin E1 overexpression was an independent prognostic risk factor for poor chordoma patient outcomes.
Discussion
Overexpression of cyclin E1 has been recognized in various malignancies including lung cancer (51%–62%), breast cancer (53%–61%), liver cancer (36%–68%) and some sarcomas (29%–61%) (15, 17, 22–28). This elevated cyclin E1 expression has been attributed to gene amplification, transcription upregulation, and disrupted degradation. In terms of frequency, the cyclin E1 gene is one of the most commonly amplified genes in ovarian cancer (19%), anaplastic thyroid cancer (29%) and osteosarcoma (27-33%) (14, 17, 18, 29). Overexpression of transcription factors such as c-MYC and MEIS2 directly up-regulate cyclin E1 expression (30, 31). In addition, inactivation of core units central to the ubiquitin protein ligase complex lead to cyclin E1 overexpression, as this is its principal degradative mechanism (32). In our study, overexpression of cyclin E1 occurred in 62.7% of chordoma patients, and is consistent with findings in more common cancers. In total, our work supports cyclin E1 as a diagnostic biomarker in chordoma.
Our TMA analysis showed cyclin E1 expression positively correlates with chordoma patient disease status. Of note, these results echo works in other malignancies where high cyclin E1 expression is associated with high-staging as well as tumor aggression and therapeutic-resistance (28, 33, 34). Functionally, it is proposed that these carcinogenic features are in part due to the cyclin E1 role in shortening mitosis and facilitating chromosome instability, both of which are known to incite tumorigenesis (35, 36). It therefore follows and is clear that cyclin E1 overexpression advances chordoma.
Cyclin E1 predicts poor prognosis in several malignancies (15, 28, 37). In our study, cyclin E1 showed prognostic significance with respect to OS, RFS and MFS in chordoma patients. Because recurrence and subsequent metastasis are of particular significance in determining chordoma outcomes, the ability of cyclin E1 overexpression to function as a prognostic biomarker is especially noteworthy for correlating with RFS and MFS in our study (4). As a specific prognostic biomarker rather than pure diagnostic biomarker, cyclin E1 has the potential to inform clinical decision-making and therapy selection based on predicted progression and recurrence. This is especially important clinical information, as chordoma surgery and radiation therapy carry significant risks to the patient owing to its anatomic proximity to vital neurovascular structures. Taken together, cyclin E1 expression is a promising prognostic biomarker for chordoma patients that may help guide clinical decision making.
Finally, we assessed the predictive potential of other clinicopathological features for chordoma patients. As expected, an advanced disease status of chordomas engenders more aggressive future tumor behavior. It is an independent risk factor for OS and MFS in chordoma, which is similar to the results of previous studies (2, 5, 38). Moreover, we found a contaminated surgical margin to be an independent risk factor for RFS in chordoma patients. This is consistent to previous studies, and further suggests that an adequate surgical margin is imperative to optimize chordoma treatment and to avoid local progression and recurrence (1, 5). Furthermore, we found no correlation between neoadjuvant radiation with prognosis/cyclin E1 expression level. While the therapeutic efficacy of radiation for chordoma remains controversial, our results, among others, do not clearly support neoadjuvant radiation in chordoma (4, 39).
To our knowledge, ours is the first study evaluating the expression and prognostic role of cyclin E1 specifically in chordoma. While we have identified a significant correlation between cyclin E1 expression and chordoma patient prognosis, future works should focus on the mechanism of the overexpression of cyclin E1 in chordoma and how cyclin E1 overexpression causes chordoma cell proliferation and poor outcomes. This would inform future targeted therapies and clinical trials making use of our initial findings. Moreover, while molecular targeting therapies have been investigated in chordoma but have largely been underwhelming, the cyclin E1 should be investigated as a novel therapeutic strategy for chordoma patients (40–43).
There are some limitations in our study. First, 75 chordoma cases is comparatively large sample size for this rare disease as compared to other previous studies on investigating prognostic biomarkers in chordoma (7, 41, 42). However, future studies to increase the number of specimens are needed to validate cyclin E1 as a biomarker for chordoma prognosis. Second, no chordoma samples in our study were from skull base. It might be attributed to that all chordoma samples included in this study were surgically treated via orthopedic surgeons, who usually treated chordoma on mobile spine or sacrum. Third, the mechanisms of cyclin E1 overexpression in chordoma remains unknown and need be further investigated. Future investigations to validate and build on the prognostic value of Cyclin E1 in chordoma will be carried out on a larger sample size collaborating with neurosurgeons to include skull base chordoma, and will focus on the detail mechanism and regulation of cyclin E1 expression in chordoma.
Conclusion
In summary, we show cyclin E1 is overexpressed in most chordomas, and its expression positively correlates to disease status and inversely correlates with OS, RFS, and MFS of chordoma patients. Our work suggests cyclin E1 as a prognostic biomarker for chordoma patients and supports future works investigating its mechanism and pathway as a potential drug target.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The study protocol and the consent of the informed patients were approved by the Partners Human Research Committee (number: 2007-P-002464/5) of Partners HealthCare as previously described. All patients provided their written informed consent to participate in this study.
Author Contributions
Research design and data preparation: RW, PT, FH, WG, and ZD. Drafting and revising the paper: RW, DD, and ZD. Approval of the submitted and final versions: WG and ZD. All authors contributed to the article and approved the submitted version.
Funding
This work was supported, in part, by the Musculoskeletal Tumor Center and Beijing Key Laboratory of Musculoskeletal Tumor at Peking University People’s Hospital, Beijing, China, and Department of Orthopaedic Surgery at UCLA, Los Angeles, California, USA. WG is supported, in part, through a grant from Sanming Project of Medicine in Shenzhen, China. ZD is supported, in part, through a grant from Sarcoma Foundation of America (SFA), 222433, a Grant from National Cancer Institute (NCI)/National Institutes of Health (NIH), U01, CA151452-01.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Stacchiotti S, Sommer J. Chordoma Global Consensus G. Building a global consensus approach to chordoma: a position paper from the medical and patient community. Lancet Oncol (2015) 16(2):e71–83. doi: 10.1016/S1470-2045(14)71190-8
2. Zuckerman SL, Bilsky MH, Laufer I. Chordomas of the Skull Base, Mobile Spine, and Sacrum: An Epidemiologic Investigation of Presentation, Treatment, and Survival. World Neurosurg (2018) 113:e618–e27. doi: 10.1016/j.wneu.2018.02.109
3. Wei R, Guo W, Ji T, Zhang Y, Liang H, et al. One-step reconstruction with a 3D-printed, custom-made prosthesis after total en bloc sacrectomy: a technical note. Eur Spine J (2017) 26(7):1902–9. doi: 10.1007/s00586-016-4871-z
4. van Wulfften Palthe ODR, Tromp I, Ferreira A, Fiore A, Bramer JAM, van Dijk NC, et al. Sacral chordoma: a clinical review of 101 cases with 30-year experience in a single institution. Spine J (2019) 19(5):869–79. doi: 10.1016/j.spinee.2018.11.002
5. Ji T, Guo W, Yang R, Tang X, Wang Y, Huang L. What Are the Conditional Survival and Functional Outcomes After Surgical Treatment of 115 Patients With Sacral Chordoma? Clin Orthop Relat Res (2017) 475(3):620–30. doi: 10.1007/s11999-016-4773-8
6. Smoll NR, Gautschi OP, Radovanovic I, Schaller K, Weber DC. Incidence and relative survival of chordomas: the standardized mortality ratio and the impact of chordomas on a population. Cancer (2013) 119(11):2029–37. doi: 10.1002/cncr.28032
7. Vujovic S, Henderson S, Presneau N, Odell E, Jacques TS, Tirabosco R, et al. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol (2006) 209(2):157–65. doi: 10.1002/path.1969
8. Whelan JS, Davis LE. Osteosarcoma, Chondrosarcoma, and Chordoma. J Clin Oncol (2018) 36(2):188–93. doi: 10.1200/JCO.2017.75.1743
9. Heery CR, Singh BH, Rauckhorst M, Marté JL, Donahue RN, Grenga I, et al. Phase I Trial of a Yeast-Based Therapeutic Cancer Vaccine (GI-6301) Targeting the Transcription Factor Brachyury. Cancer Immunol Res (2015) 3(11):1248–56. doi: 10.1158/2326-6066.CIR-15-0119
10. Sharifnia T, Wawer MJ, Chen T, Huang QY, Weir BA, Sizemore A, et al. Small-molecule targeting of brachyury transcription factor addiction in chordoma. Nat Med (2019) 25(2):292–300. doi: 10.1038/s41591-018-0312-3
11. Koff A, Cross F, Fisher A, Human cyclin E. a new cyclin that interacts with two members of the CDC2 gene family. Cell (1991) 66(6):1217–28. doi: 10.1016/0092-8674(91)90044-Y
12. Sauer K, Lehner CF. The role of cyclin E in the regulation of entry into S phase. Prog Cell Cycle Res (1995) 1:125–39. doi: 10.1007/978-1-4615-1809-9_10
13. Ayhan A, Kuhn E, Wu RC, Ogawa H, Bahadirli-Talbott A, Mao TL, et al. CCNE1 copy-number gain and overexpression identify ovarian clear cell carcinoma with a poor prognosis. Mod Pathol (2017) 30(2):297–303. doi: 10.1038/modpathol.2016.160
14. Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature (2015) 521(7553):489–94. doi: 10.1038/nature14410
15. Keyomarsi K, Tucker SL, Buchholz TA, Cyclin E. and survival in patients with breast cancer. N Engl J Med (2002) 347(20):1566–75. doi: 10.1056/NEJMoa021153
16. Scaltriti M, Eichhorn PJ, Cortes J, Prudkin L, Aura C, Jiménez J, et al. Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc Natl Acad Sci U S A (2011) 108(9):3761–6. doi: 10.1073/pnas.1014835108
17. Lockwood WW, Stack D, Morris T, Grehan D, O’Keane C, Stewart GL, et al. Cyclin E1 is amplified and overexpressed in osteosarcoma. J Mol Diagn (2011) 13(3):289–96. doi: 10.1016/j.jmoldx.2010.11.020
18. Sayles LC, Breese MR, Koehne AL, Leung SG, Lee AG, Liu HY, et al. Genome-Informed Targeted Therapy for Osteosarcoma. Cancer Discov (2019) 9(1):46–63. doi: 10.1158/2159-8290.CD-17-1152
19. Chen H, Garbutt CC, Spentzos D, Choy E, Hornicek FJ, Duan Z, et al. Expression and Therapeutic Potential of SOX9 in Chordoma. Clin Cancer Res (2017) 23(17):5176–86. doi: 10.1158/1078-0432.CCR-17-0177
20. Bruderlein S, Sommer JB, Meltzer PS, Li S, Osada T, Ng D, et al. Molecular characterization of putative chordoma cell lines. Sarcoma (2010) 2010:630129. doi: 10.1155/2010/630129
21. Liu X, Nielsen GP, Rosenberg AE, Waterman PR, Yang W, Choy E, et al. Establishment and characterization of a novel chordoma cell line: CH22. J Orthop Res (2012) 30(10):1666–73. doi: 10.1002/jor.22113
22. Hunt KK, Karakas C, Ha MJ, Biernacka A, Yi M, Sahin AA, et al. Cytoplasmic Cyclin E Predicts Recurrence in Patients with Breast Cancer. Clin Cancer Res (2017) 23(12):2991–3002. doi: 10.1158/1078-0432.CCR-16-2217
23. Salon C, Merdzhanova G, Brambilla C, Brambilla E, Gazzeri S, Eymin B, et al. E2F-1, Skp2 and cyclin E oncoproteins are upregulated and directly correlated in high-grade neuroendocrine lung tumors. Oncogene (2007) 26(48):6927–36. doi: 10.1038/sj.onc.1210499
24. Ito Y, Matsuura N, Sakon M, Miyoshi E, Noda K, Takeda T, et al. Expression and prognostic roles of the G1-S modulators in hepatocellular carcinoma: p27 independently predicts the recurrence. Hepatology (1999) 30(1):90–9. doi: 10.1002/hep.510300114
25. Jung YJ, Lee KH, Choi DW, Han CJ, Jeong SH, Kim KC, et al. Reciprocal expressions of cyclin E and cyclin D1 in hepatocellular carcinoma. Cancer Lett (2001) 168(1):57–63. doi: 10.1016/S0304-3835(01)00403-7
26. Molendini L, Benassi MS, Magagnoli G, Merli M, Sollazzo MR, Ragazzini P, et al. Prognostic significance of cyclin expression in human osteosarcoma. Int J Oncol (1998) 12(5):1007–11. doi: 10.3892/ijo.12.5.1007
27. Goto Y, Kawauchi S, Ihara K, Ikemoto K, Ohi R, Kawai S, et al. The prognosis in spindle-cell sarcoma depends on the expression of cyclin-dependent kinase inhibitor p27(Kip1) and cyclin E. Cancer Sci (2003) 94(5):412–7. doi: 10.1111/j.1349-7006.2003.tb01456.x
28. Wei R, Thanindratarn P, Dean DC, Hornicek FJ, Guo W, Duan Z, et al. Cyclin E1 is a prognostic biomarker and potential therapeutic target in osteosarcoma. J Orthop Res (2020) 38(9):1952–64. doi: 10.1002/jor.24659
29. Ravi N, Yang M, Gretarsson S, Jansson C, Mylona N, Sydow SR, et al. Identification of Targetable Lesions in Anaplastic Thyroid Cancer by Genome Profiling. Cancers (Basel) (2019) 11(3):402. doi: 10.3390/cancers11030402
30. Abruzzese MP, Bilotta MT, Fionda C, Zingoni A, Soriani A, Petrucci MT, et al. The homeobox transcription factor MEIS2 is a regulator of cancer cell survival and IMiDs activity in Multiple Myeloma: modulation by Bromodomain and Extra-Terminal (BET) protein inhibitors. Cell Death Dis (2019) 10(4):324. doi: 10.1038/s41419-019-1562-9
31. Deb-Basu D, Karlsson A, Li Q, Dang CV, Felsher DW. MYC can enforce cell cycle transit from G1 to S and G2 to S, but not mitotic cellular division, independent of p27-mediated inihibition of cyclin E/CDK2. Cell Cycle (2006) 5(12):1348–55. doi: 10.4161/cc.5.12.2860
32. Gong Y, Zack TI, Morris LG, Lin K, Hukkelhoven E, Raheja R, et al. Pan-cancer genetic analysis identifies PARK2 as a master regulator of G1/S cyclins. Nat Genet (2014) 46(6):588–94. doi: 10.1038/ng.2981
33. Karakas C, Biernacka A, Bui T, Sahin AA, Yi M, Akli S, et al. Cytoplasmic Cyclin Eand Phospho-Cyclin-Dependent Kinase 2 Are Biomarkers of Aggressive Breast Cancer. Am J Pathol (2016) 186(7):1900–12. doi: 10.1016/j.ajpath.2016.02.024
34. Etemadmoghadam D, deFazio A, Beroukhim R, Mermel C, George J, Getz G, et al. Integrated genome-wide DNA copy number and expression analysis identifies distinct mechanisms of primary chemoresistance in ovarian carcinomas. Clin Cancer Res (2009) 15(4):1417–27. doi: 10.1158/1078-0432.CCR-08-1564
35. Spruck CH, Won KA, Reed SI. Deregulated cyclin E induces chromosome instability. Nature (1999) 401(6750):297–300. doi: 10.1038/45836
36. Aziz K, Limzerwala JF, Sturmlechner I, Hurley E, Zhang C, Jeganathan KB, et al. Ccne1 Overexpression Causes Chromosome Instability in Liver Cells and Liver Tumor Development in Mice. Gastroenterology (2019) 157(1):210–26 e12. doi: 10.1053/j.gastro.2019.03.016
37. Rosen DG, Yang G, Deavers MT, Malpica A, Kavanagh JJ, Mills GB, et al. Cyclin E expression is correlated with tumor progression and predicts a poor prognosis in patients with ovarian carcinoma. Cancer (2006) 106(9):1925–32. doi: 10.1002/cncr.21767
38. Zheng W, Huang Y, Guan T, Lu S, Yao L, Wu S, et al. Application of nomograms to predict overall and cancer-specific survival in patients with chordoma. J Bone Oncol (2019) 18:100247. doi: 10.1016/j.jbo.2019.100247
39. Houdek MT, Rose PS, Hevesi M, Schwab JH, Griffin AM, Healey JH, et al. Low dose radiotherapy is associated with local complications but not disease control in sacral chordoma. J Surg Oncol (2019) 119(7):856–63. doi: 10.1002/jso.25399
40. Stacchiotti S, Morosi C, Lo Vullo S, Casale A, Palassini E, Frezza AM, et al. Imatinib and everolimus in patients with progressing advanced chordoma: A phase 2 clinical study. Cancer (2018) 124(20):4056–63. doi: 10.1002/cncr.31685
41. Yamaguchi T, Imada H, Iida S, Szuhai K. Notochordal Tumors: An Update on Molecular Pathology with Therapeutic Implications. Surg Pathol Clin (2017) 10(3):637–56. doi: 10.1016/j.path.2017.04.008
42. Lebellec L, Chauffert B, Blay JY, Le Cesne A, Chevreau C, Bompas E, et al. Advanced chordoma treated by first-line molecular targeted therapies: Outcomes and prognostic factors. A retrospective study of the French Sarcoma Group (GSF/GETO) and the Association des Neuro-Oncologues d’Expression Francaise (ANOCEF). Eur J Cancer (2017) 79:119–28. doi: 10.1016/j.ejca.2017.03.037
Keywords: chordoma, cyclin E1, prognosis, biomarker, tissue microarray, immunohistochemical staining
Citation: Wei R, Dean DC, Thanindratarn P, Hornicek FJ, Guo W and Duan Z (2020) Prognostic Significance of Cyclin E1 Expression in Patients With Chordoma: A Clinicopathological and Immunohistochemical Study. Front. Oncol. 10:596330. doi: 10.3389/fonc.2020.596330
Received: 19 August 2020; Accepted: 19 October 2020;
Published: 17 November 2020.
Edited by:
Dianwen Song, Shanghai First People’s Hospital, ChinaReviewed by:
Yi Ding, Geisinger Health System, United StatesFrancesca Benedetti, University of Maryland, United States
Copyright © 2020 Wei, Dean, Thanindratarn, Hornicek, Guo and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Guo, Ym9uZXR1bW9yQDE2My5jb20=; Zhenfeng Duan, emR1YW5AbWVkbmV0LnVjbGEuZWR1
 Ran Wei
Ran Wei Dylan C. Dean2
Dylan C. Dean2 Wei Guo
Wei Guo