- Hepatobiliary Surgery Department, Guangxi Liver Cancer Diagnosis and Treatment Engineering and Technology Research Center, Guangxi Medical University Cancer Hospital, Nanning, China
Background: The relationship between serum prealbumin and the risk of all-cause mortality after hepatectomy in patients with hepatocellular carcinoma (HCC) needs to be evaluated.
Methods: We conducted a retrospective study. A Cox proportional hazards regression model was used to adjust for potential confounders. Prealbumin level was transformed by Z-scores and categorized into quartiles (Q1: <147 mg/L, Q2: 147–194 mg/L, Q3: 194–239 mg/L, Q4: >239 mg/L). We assessed the dose-response relationship between serum prealbumin and the risk of all-cause mortality using a restricted cubic spline model.
Results: Data were included from 2,022 HCC patients who underwent hepatectomy at Guangxi Medical University Cancer Hospital in China between January 2006 and January 2016. The adjusted hazard ratios (HRs) for increasing quartiles of serum prealbumin were 0.78 [95% confidence interval (CI): 0.64–0.95] for Q2, 0.66 (0.53–0.81) for Q3, and 0.51 (0.41–0.64) for Q4 in the Cox model (all P < 0.001). Serum prealbumin showed an L-shaped, non-linear dose-response relationship with the risk of all-cause mortality (P < 0.001). Among patients whose serum prealbumin was below 250 mg/L, risk of all-cause mortality decreased by 27% (95% CI: 18–36%) per increase of one standard deviation (69.8 mg/L) in serum prealbumin.
Conclusions: Levels of serum prealbumin under 250 mg/L may be considered dangerous with respect to all-cause mortality after hepatectomy in HCC patients. Serum prealbumin may be useful as a prognostic marker in HCC patients undergoing hepatectomy.
Highlights
● Low serum prealbumin is associated with higher risk of all-cause mortality after hepatectomy in patients with hepatocellular carcinoma.
● Among these patients, levels of serum prealbumin under 250 mg/L may indicate elevated risk of all-cause mortality.
Introduction
Hepatocellular carcinoma (HCC) is a common malignant neoplasm (1). Hepatectomy is one of the main radical treatments for HCC. The 5-year recurrence rate of HCC is up to 70% (2), and the 5-year overall survival (OS) is only 37% for patients with portal hypertension, 30% for those with multiple tumors, and 18% for those suffering macrovascular invasion after hepatectomy (3). Therefore, it is important to explore reliable preoperative risk markers to predict the OS of such patients. Some preoperative variables, such as tumor number, tumor differentiation, tumor size, alpha-fetoprotein (AFP) level, liver function, and the presence of liver cirrhosis have been associated with OS of HCC patients after hepatectomy (4–7). Preoperative malnutritional status may also contribute to poor prognosis after hepatectomy (8–10).
Serum prealbumin synthesized by the liver is a common laboratory indicator of nutritional status. Serum prealbumin is less affected by liver diseases than other serum proteins (11, 12). In addition, the level of serum prealbumin is not significantly altered by blood transfusion or supplemental infusion of human albumin. Some studies reported a positive association between the level of serum prealbumin and prognosis in various cancers (13–15). However, few studies have investigated the association between the level of serum prealbumin and the risk of mortality after hepatectomy in patients with HCC. In our previous study, we found that serum prealbumin <200 mg/L was associated with mortality after hepatic resection in patients with HCC (16). Other studies found similar results (17, 18). However, these studies were limited to multivariate analysis of serum prealbumin treated as a categorical variable, so they could not determine whether the risk of death of patients with HCC changed to different degrees with slight changes in serum prealbumin level.
In the present study, we examined the dose-response relationship of serum prealbumin level with the risk of all-cause mortality after resection in patients with HCC. In order to capture the relationship in detail and to compensate for the statistical problems of treating serum prealbumin as a continuous or categorial variable (19, 20), we applied a restricted cubic spline (RCS) function. The RCS function has proven effective at characterizing dose-response correlations between a continuous exposure (such as serum prealbumin) and all-cause mortality, regardless of whether the relationship was linear or not (21). It has been used in several single studies and meta-analyses (22–24). Therefore, in this study, we evaluated the dose-response relationships of serum prealbumin level with the risk of all-cause mortality after resection in patients with HCC.
Methods
Patients
We retrospectively reviewed records of patients who underwent hepatectomy for HCC between January 2006 and January 2016 at Guangxi Medical University Cancer Hospital, in Nanning, China. Eligible patients had to be admitted for initial HCC treatment at our hospital and had to have histologically confirmed HCC. Indications for hepatectomy was described as previous (2). No age restriction was applied during enrollment of patients in this study. HCC patients with distant metastasis or with other tumors were excluded, as were patients for whom preoperative serum prealbumin data were missing. Liver function indicators were assayed in blood collected at 6:00–7:00 a.m. on the second morning of hospitalization after overnight fasting. Hepatectomy was performed after 3 to 7 days of hospitalization. This study was conducted in accordance with the Declaration of Helsinki guidelines, and the protocol was approved by the Ethics Commitment of our hospital.
Assessment of Covariates
The following data were extracted from patient records: sex, age, liver function categorized according to Child-Pugh category criteria, Barcelona Clinic Liver Cancer (BCLC) stage, serum prealbumin, hepatitis B surface antigen (HBsAg), albumin (ALB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), AFP, and pathology of HCC involving cirrhosis, tumor size and number, integrity of tumor capsule, and presence or absence of macrovascular invasion. All measurements were obtained before surgery. However, liver cirrhosis and macrovascular invasion were confirmed by postoperative histopathology.
In order to ensure the accuracy and reliability of data, measures for standard quality controls were planned before the start of study, such as a table for collection of patients’ information and a code for replacement of categorical data. Two researchers independently collected and organized data; any discrepancies were corrected by a third reviewer.
Outcomes and Follow-Up
Follow-up was conducted starting from the first month after surgery, every 2 months during the first year, then every 6 months thereafter until May 31, 2019 or death or loss to follow-up. During follow-up appointments, data were collected about time to HCC recurrence, survival or death, and liver function. Follow-up was conducted by telephone and review of the hospital’s data management system. The endpoint of the study was OS, defined as the interval from the date of hepatic resection until the date of last follow-up (May 31, 2019) or death.
Statistical Analysis
Before testing independent associations of serum prealbumin with the risk of all-cause mortality in patients with HCC, Z-scores were calculated, such that the resulting estimated effect size indicated the change in serum prealbumin in terms of the standard deviation (SD). The Z-score normalizes the mean parameter value to 0 and the parameter value at one SD to 1. In addition, we also categorized serum prealbumin into quartiles based on sample size. Other categorical covariates were classified based on clinical findings and were entered as dummy variables. Continuous covariates were transformed into categorical variables based on cut-off values routinely used in clinical practice.
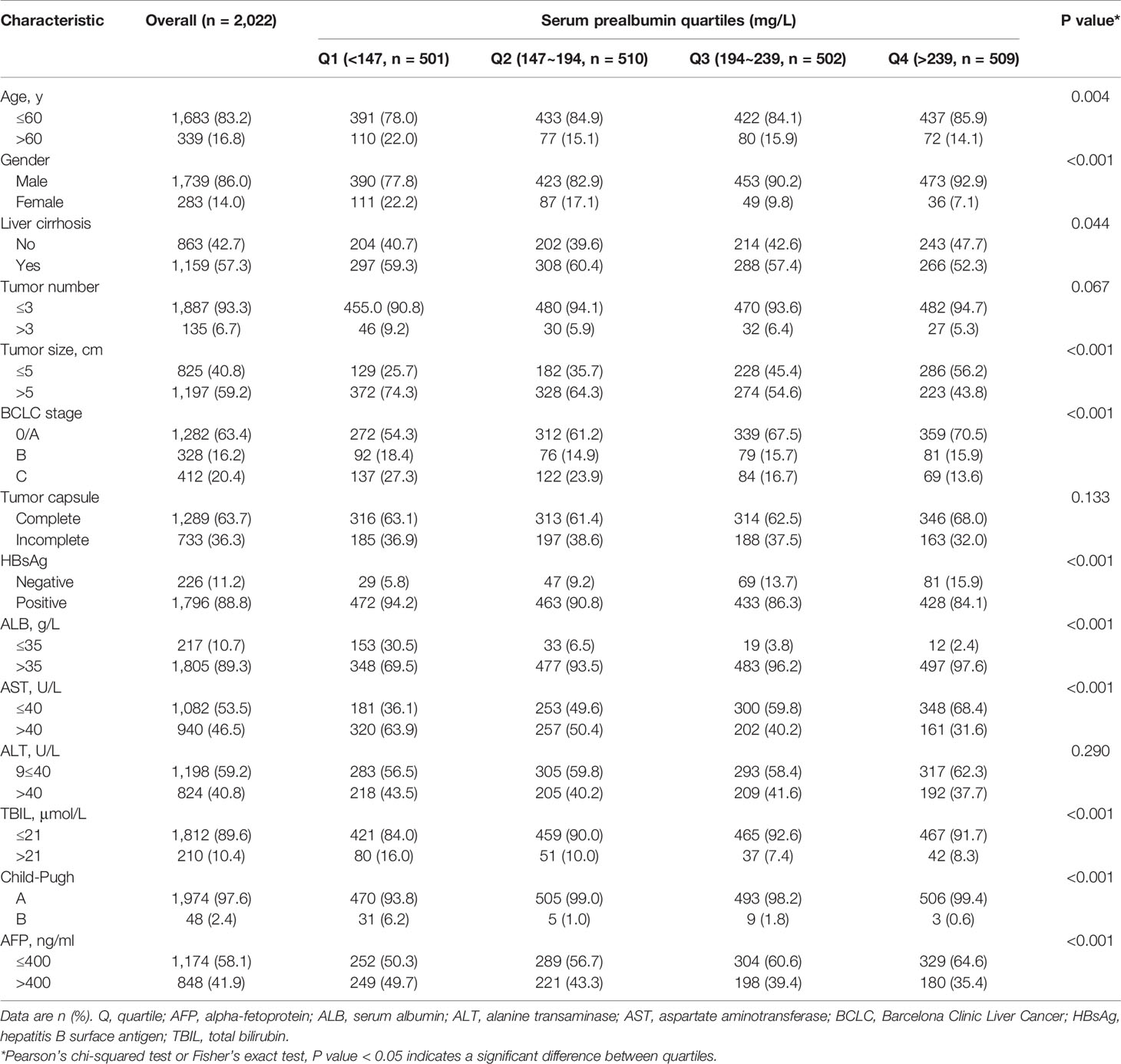
We assessed potential associations between serum prealbumin (as a categorical variable) and other characteristics using a chi-squared test or Fisher’s exact test. Survival curves were generated using the Kaplan-Meier method. The OS curves were compared between groups using the log-rank test. We tested associations of serum prealbumin with the risk of all-cause mortality using two Cox proportional hazards regression models: model 1 was adjusted for age and sex, while model 2 adjusted for age, sex, tumor size and number, tumor capsule, AFP, ALB, AST, ALT, TBIL, HBsAg, liver cirrhosis, liver function, and BCLC stage. From each fitted model, we searched for a linear trend by modeling the median value of each quartile to test ordered relations across the different serum prealbumin quartiles. Interactions between serum prealbumin and other factors were also examined using a likelihood ratio test, with a comparison of the log likelihood of the two models with or without the interaction terms. We calculated hazard ratios (HRs) for risk of all-cause mortality and the corresponding 95% confidence intervals (CIs).
We assessed the dose-response relationship between serum prealbumin (as a continuous variable) and the risk of all-cause mortality using RCS models. RCS assumes that the effect of the exposure on the outcome is a smooth, piecewise, cubic polynomial with linear tails (21). RCS models provide flexibility in fitting highly curved relationships, avoiding significant influences from outlying variables, and may provide better power than dichotomizing continuous variables (21). We used RCS models adjusted for the same potential confounders as in model 2, and plotted smooth curves with five knots at the 5th, 25th (reference level), 50th, 75th, and 95th percentiles of serum prealbumin. We further applied a two-piecewise linear regression model to examine the threshold effect of the serum prealbumin on all-cause mortality using a smoothing function (25, 26).
To ensure the stability of the results, we conducted two sensitivity analyses. In one, we repeated the analysis after excluding patients who died 3 months after follow-up began in order to avoid confounding due to premature death. In another, we separately examined patients with or without liver cirrhosis.
We performed all statistical analyses using R version 3.5.1 (https://www.r-project.org/). All P values were two-tailed, with a P value under 0.05 indicating statistical significance.
Results
Baseline Characteristics
The patient records contained details of 2,060 patients with HCC who underwent curative hepatectomy at our institution during January 2006 to January 2016. Based on the inclusion criteria, 2,022 (98.1%) patients were enrolled. The mean age (SD) was 49.5 (11.2) years, and 1,739 patients (86.0%) were male. Among the 2,022 patients, 31% underwent major hepatectomy, while 69% underwent minor hepatectomy. During a median follow-up (range) of 59 months (3–115 months), 385 patients (19%) were lost to follow-up. Among the remaining patients, tumor recurrence and liver failure were the main causes of mortality. The mean level of serum prealbumin level (SD) was 195.5 (69.8) mg/L, and the median (interquartile range) was 193.5 (147.0–239.0) mg/L. Table 1 presents the characteristics of the patients stratified by quartiles of serum prealbumin.
Cox Regression Analysis
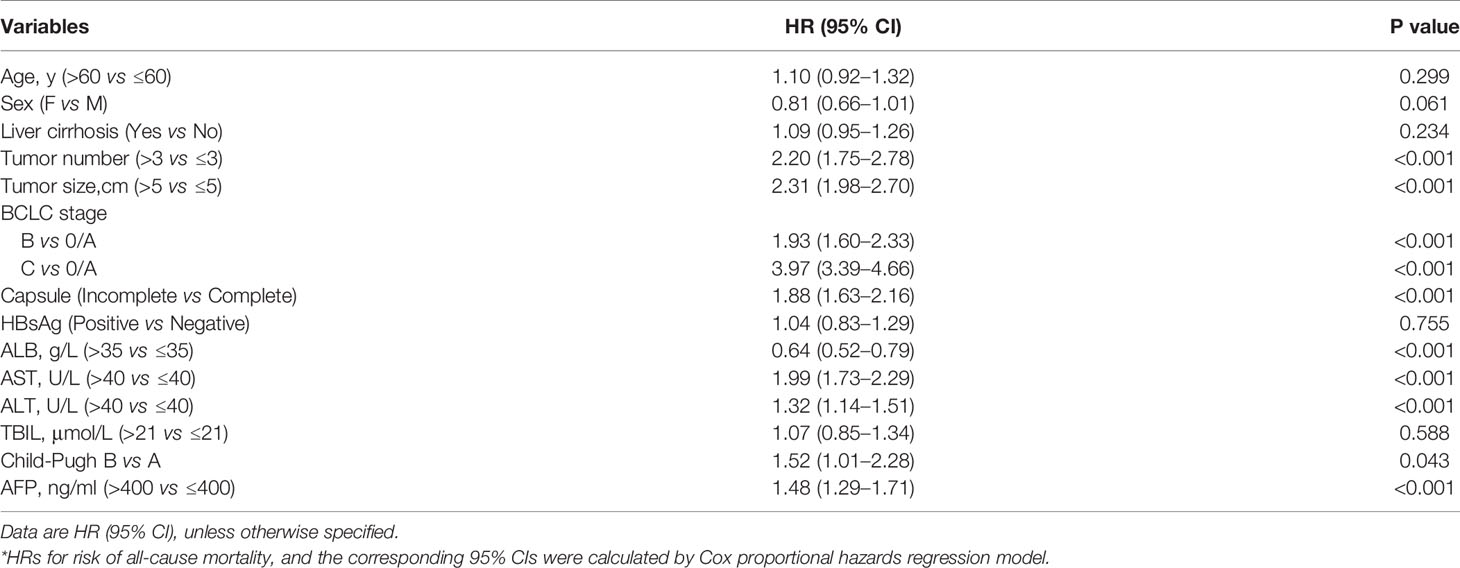
To assess whether clinical variables contributed to the risk of all-cause mortality, we conducted a univariate Cox regression analysis of clinical characteristics (Table 2). We found that tumor size and number, BCLC stage, tumor capsule, ALB, AST, ALT, and AFP levels were related to the risk of all-cause mortality. In contrast, age, sex, liver cirrhosis, HBsAg, TBIL, and Child-Pugh were not related to the risk of all-cause mortality in our cohort. To control for any possible confounding factors affecting the association between serum prealbumin and the risk of all-cause mortality, subsequent multivariate Cox regression and RCS models were adjusted for age, sex, tumor size and number, tumor capsule, AFP, HBsAg, ALB, AST, ALT, TBIL, Child-Pugh, liver cirrhosis, and BCLC stage.
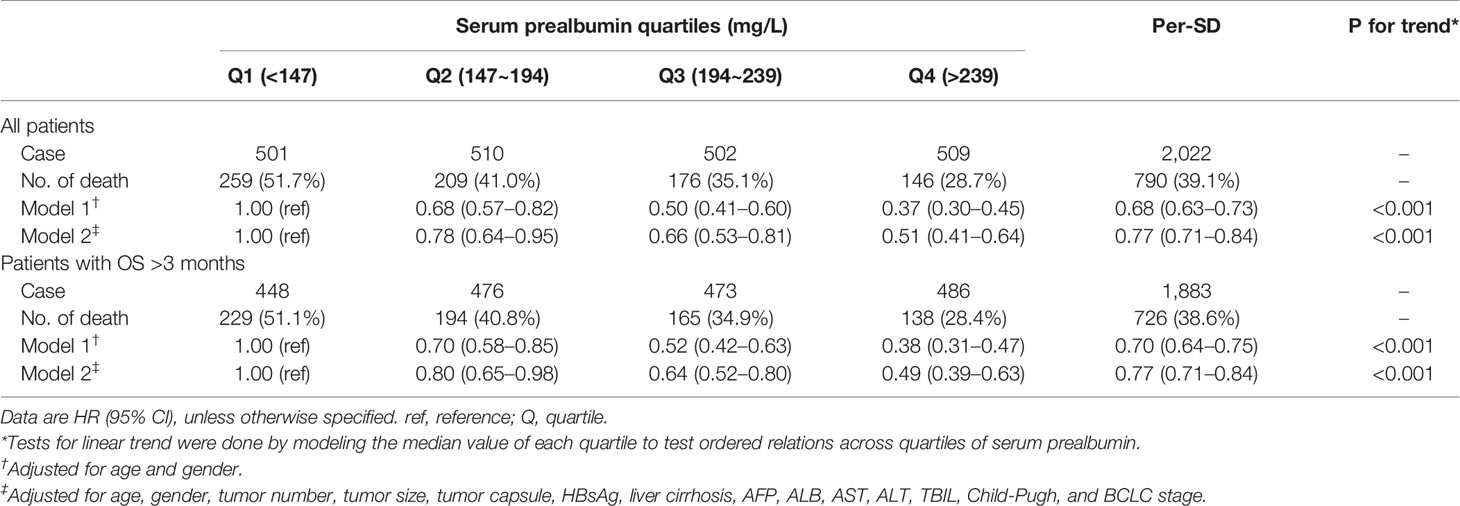
We analyzed the associations between serum prealbumin level and the risk of all-cause mortality (Table 3). In model 1, which was adjusted for age and sex, the HRs (95% CIs) of the risk of all-cause mortality across increasing quartiles of serum prealbumin were 1.00 for Q1, 0.68 (0.57–0.82) for Q2, 0.50 (0.41–0.60) for Q3, and 0.37 (0.30–0.45) for Q4 (P for trend < 0.001). The HR was 0.68 (0.63–0.73) per 1-SD increase in serum prealbumin. According to model 2, the HRs (95% CIs) were 1.00 for Q1, 0.78 (0.64–0.95) for Q2, 0.66 (0.53–0.81) for Q3, and 0.51 (0.41–0.64) for Q4 (P for trend < 0.001). In model 2, the HR was 0.77 (0.71–0.84) per 1-SD increase in serum prealbumin. Inclusion of a quadratic term for serum prealbumin in the Cox proportional hazard models suggested a linear correlation between serum prealbumin and all-cause mortality. However, RCS analysis showed this correlation to be non-linear.
Subgroup Analysis
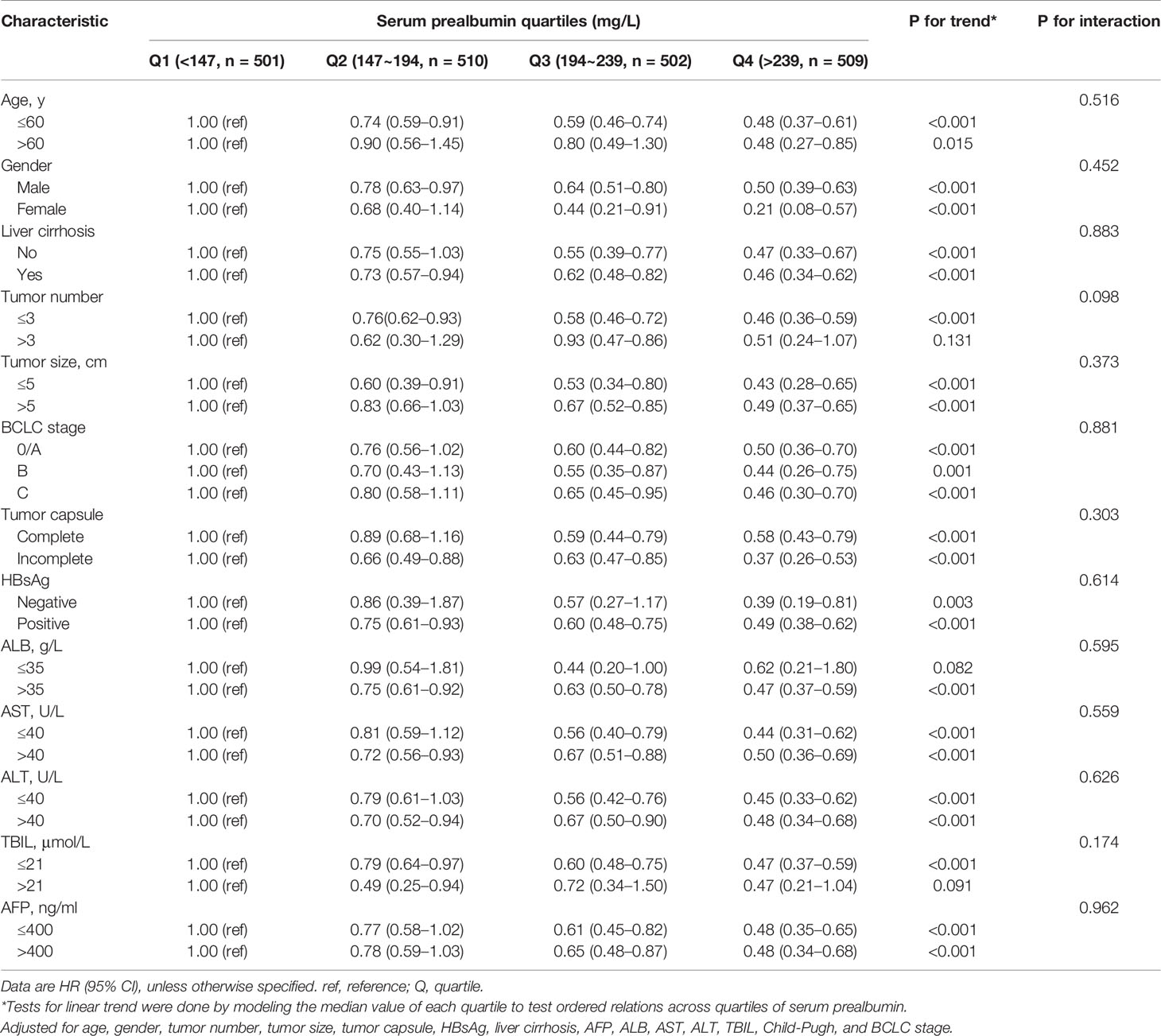
When we analyzed serum prealbumin in correlation with all-cause mortality across subgroups of clinical characteristics (Table 4), we detected no significant subgroup interactions. In analyses stratified by age, sex, tumor capsule, tumor size, liver cirrhosis, HBsAg, AFP, ALT, AST, Child-Pugh, and BCLC stage, inclusion of a quadratic term for serum prealbumin in the Cox proportional hazard models revealed a linear correlation between serum prealbumin and all-cause mortality. However, this correlation was not linear in subsets of patients with tumor number >3, ALB ≤35 g/L, or TBIL >21 μmol/L (Table 4).
Dose–Response Analysis
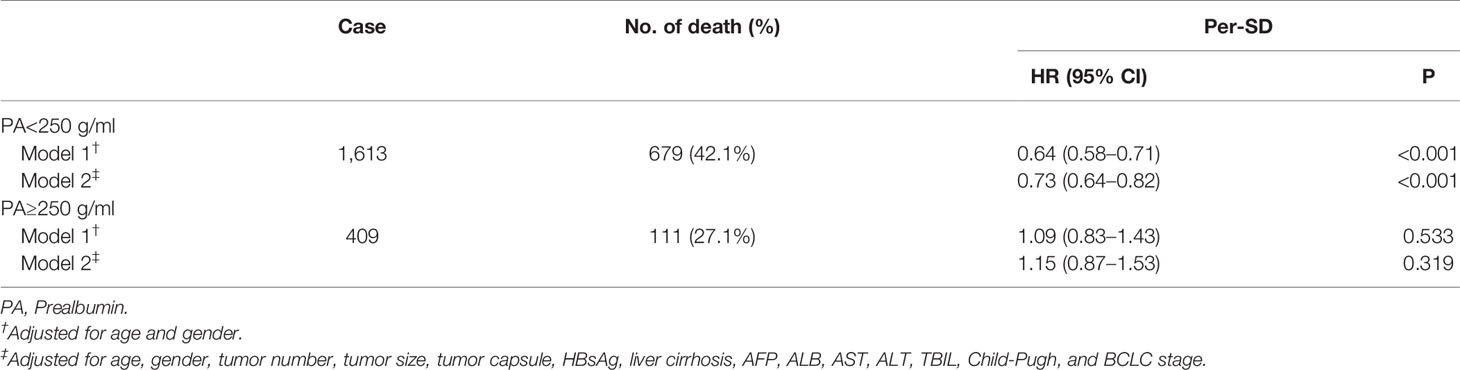
We evaluated the potential dose-response relationship between serum prealbumin and the risk of all-cause mortality using an RCS model adjusted for potential confounders. Serum prealbumin showed an L-shape, non-linear dose-response relationship with the risk of all-cause mortality (P for non-linearity <0.001, Figure 1A). RCS analyses based on a two-piecewise linear regression model included a knot at 250 mg/L, and Kaplan-Meier survival plots showed significantly higher all-cause mortality in patients with serum prealbumin below 250 mg/L than in those with serum prealbumin above this threshold (P < 0.001, Figure 2). The risk of all-cause mortality decreased with serum prealbumin level over the threshold (HR per SD decrease: 0.73, 95% CI: 0.64–0.82, Table 5). Risk was no longer reduced when the serum prealbumin level was above the threshold (HR 1.15, 95% CI: 0.87–1.53).

Figure 1 | Dose–response relationship between serum prealbumin and the risk of mortality after hepatectomy in patients with hepatocellular carcinoma. Graphs show the hazard ratio (HR; solid red lines) and 95% confidence interval (CI, dotted blue lines) describing the association of serum prealbumin with the risk of mortality. Cox regression analysis with a restricted cubic spline approach was conducted to allow flexible, non-linear assessment of the HR for mortality in (A) all patients (n = 2,022), (B) patients with overall survival >3 months (n = 1,883), (C) patients with liver cirrhosis (n = 1,159), or (D) patients without liver cirrhosis (n = 863) (all P < 0.001). All models were adjusted for age, sex, HBsAg, liver cirrhosis, tumor size and number, tumor capsule, AFP, ALB, ALT, AST, TBIL, Child-Pugh, and BCLC stage.
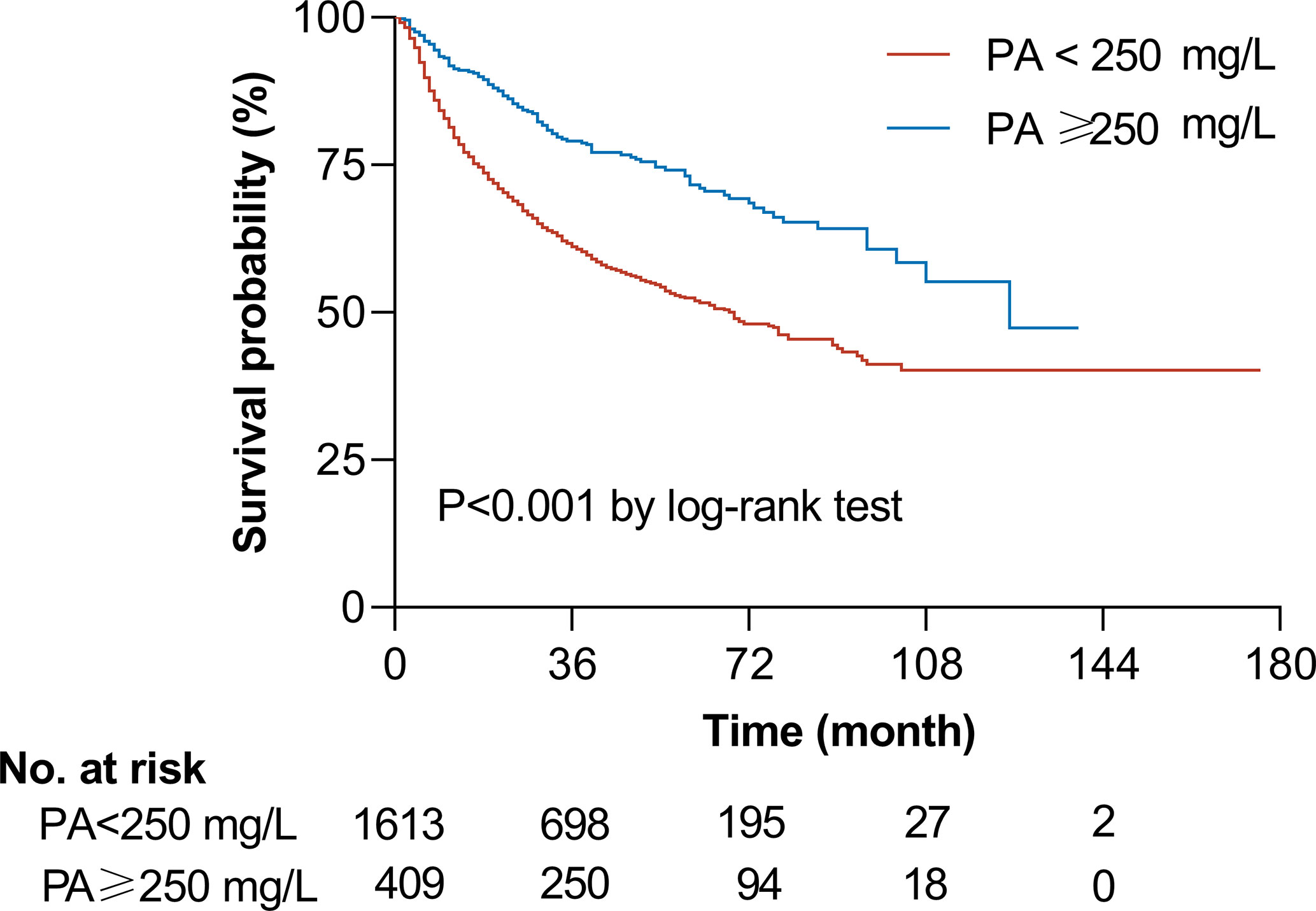
Figure 2 | Unadjusted Kaplan-Meier curve of overall survival according to serum prealbumin (PA) level. The graph shows the survival probability according to serum prealbumin level in patients with a level above a threshold of 250 mg/L (blue line, n = 409) or below the threshold (red line, n = 1,613). The two groups differed significantly in survival, based on the log-rank test. The number of patients indicated in the table is the number of patients at risk at the indicated time.
Sensitivity Analysis
The exclusion of patients who died 3 months prior to follow-up did not substantially alter the results described above, suggesting that the findings are robust (Table 3, Figure 1B). Similarly, excluding patients with or without liver cirrhosis did not substantially affect the results (Figures 1C, D).
Discussion
This is the first epidemiological study to investigate the dose-response association between serum prealbumin level and the risk of all-cause mortality after hepatectomy in patients with HCC. In our large cohort study, we demonstrate that serum prealbumin level is independently associated with the risk of all-cause mortality. The association between serum prealbumin level and all-cause mortality was non-linear: among patients with serum prealbumin below 250 mg/L, risk fell by 27% per SD increase of 69.8 mg/L.
Although some studies have shown that serum prealbumin is associated with the prognosis of HCC patients after hepatectomy (16, 17), no study assessed its association with the risk of all-cause mortality. After controlling for potential confounders, quadratic terms for serum prealbumin in the Cox proportional hazard models suggested a linear association between serum prealbumin and all-cause mortality. However, RCS analysis showed the association to be non-linear. These findings were not substantially affected by potential confounding from premature death or presence of liver cirrhosis. The findings of this study suggest that lower levels of serum prealbumin might be associated with increased risk of all-cause mortality in HCC patients in a non-linear manner.
Since the reference values for what is considered a “normal” serum prealbumin level vary considerably, investigating the appropriate cut-off value of prealbumin for each particular type of cancer is important for predicting OS. Serum prealbumin level using a cut-off value of 170 mg/L could predict long-term OS after hepatectomy for patients with HCC (17). We have previously shown that serum prealbumin <182 mg/L is associated with poor prognosis based on maximally selected rank statistics, and therefore we consider serum prealbumin levels ≥182 mg/L as normal. However, the lower limit of the normal reference range is 200 mg/L in many hospitals (16, 18). Based on the non-linear effects of the serum prealbumin in our study, a serum prealbumin level over 250 mg/L may be considered safe. In our cohort, all-cause mortality was significantly higher among patients with serum prealbumin below this threshold than among patients with levels above this threshold. Under this threshold, the risk of all-cause mortality decreased with increasing serum prealbumin level (HR per SD decrease: 0.73, 95% CI: 0.64–0.82).
Although how prealbumin may influence the prognosis of patients with HCC is not fully understood, a role for immunosuppression in the development of tumors has been widely accepted (27). Prealbumin promotes the production of lymphocytes, with lower serum level associated with reduced lymphocyte number, which leads to a suboptimal immune status (28). Prealbumin is an acute-phase liver protein with a half-life of only 2–3 days and can be used to reflect nutritional and inflammatory status as well as the liver’s ability to synthesize protein (29). The nutritional status is closely related to postoperative recurrence and prognosis (30). Although albumin is more commonly used than prealbumin to detect protein malnutrition and assess liver function in the clinic (11, 31, 32), some studies suggest that prealbumin is more sensitive and specific (31, 33). Therefore, serum prealbumin may be a good indicator of nutritional status and prognosis, and may be preferable to albumin for predicting the risk of death after curative hepatectomy for HCC.
The strengths of our study include the relatively large cohort, relatively long follow-up, and our adjustment for HBsAg and liver cirrhosis. Moreover, we applied an RCS model to evaluate the dose-response relationship between serum prealbumin and the risk of all-cause mortality in HCC patients. RCS models are powerful tools that can avoid the loss of information and statistical power caused by discretization of continuous variables (21). Nevertheless, our study has several limitations. First, it was retrospective, and thus, incomplete adherence to the post-resection follow-up protocol and potential confounders for OS are inevitable. In addition, although the multivariable analysis was adjusted for several covariates, we were unable to control for other unknown confounders for lack of data. For example, some inflammatory and nutritional factors such as neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, and sarcopenia were not assessed (10, 34, 35). This may reduce the accuracy of our estimate of the association between serum prealbumin and all-cause mortality. On the other hand, our results are likely to be reliable given that they remained robust to several Cox regression models and sensitivity analyses. Third, we did not measure serum prealbumin in repeated samples in the same patients at different times and thus we could not correct for regression dilution, which could have led to underestimation of the association between serum prealbumin and the risk of all-cause mortality.
Conclusions
We provide evidence that serum prealbumin is non-linearly associated with all-cause mortality after hepatectomy in HCC patients. A serum prealbumin level under 250 mg/L is a risk factor of all-cause mortality in HCC patients after hepatectomy. Our results should be confirmed and extended in pooled analyses across large prospective cohorts of HCC patients, especially in China, where this cancer is highly prevalent.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials; further inquiries can be directed to the corresponding authors.
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki guidelines, and the protocol was approved by the Ethics Commitment of our hospitals. Patients provided written informed consent before their data were analyzed.
Author Contributions
J-HZ and LM conceived the study. R-RH, H-TL, Z-JD, X-ML, W-FG, L-NQ, XMY, and B-DX collected and analyzed the data. Z-JD and J-HZ analyzed the data. R-RH and H-TL drafted the manuscript. B-DX, L-QL, and LM revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was partly supported by the Self-Raised Scientific Research Fund of the Ministry of Health of Guangxi Province (Z2016512, Z2015621, and Z20200923), the Guangxi Natural Science Foundation (2018GXNSFBA138018, 2018GXNSFAA050124, and 2020GXNSFAA159022), the China Postdoctoral Science Foundation (2019M663876XB), the National Natural Science Foundation of China (82060510), the National Major Special Science and Technology Project (2017ZX10203207), and “Guangxi BaGui Scholars” Special Fund (2019AQ20).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The abstract of this study was submitted to the 3rd International Advanced Liver & Pancreas Surgery Symposium (ISLS 2019), October 10–12, 2019, Istanbul, Turkey, and published online (Zhong JH, et al. Dose-response between serum prealbumin and all-cause mortality in patients with hepatocellular carcinoma after hepatectomy. Int J Surg. 2020;75;Suppl:S9).
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin (2018) 68:7–30. doi: 10.3322/caac.21442
2. Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg (2014) 260:329–40. doi: 10.1097/SLA.0000000000000236
3. Zhong JH, Ke Y, Wang YY, Li LQ. Liver resection for patients with hepatocellular carcinoma and macrovascular invasion, multiple tumours, or portal hypertension. Gut (2015) 64:520–1. doi: 10.1136/gutjnl-2014-308139
4. Lu SD, Wang YY, Peng NF, Peng YC, Zhong JH, Qin HG, et al. Preoperative Ratio of Neutrophils to Lymphocytes Predicts Postresection Survival in Selected Patients With Early or Intermediate Stage Hepatocellular Carcinoma. Med (Baltimore) (2016) 95:e2722. doi: 10.1097/MD.0000000000002722
5. Ganslmayer M, Hagel A, Dauth W, Zopf S, Strobel D, Muller V, et al. A large cohort of patients with hepatocellular carcinoma in a single European centre: aetiology and prognosis now and in a historical cohort. Swiss Med Wkly (2014) 144:w13900. doi: 10.4414/smw.2014.13900
6. Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, La Barba G, et al. Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl (2006) 12:966–71. doi: 10.1002/lt.20761
7. Franssen B, Alshebeeb K, Tabrizian P, Marti J, Pierobon ES, Lubezky N, et al. Differences in surgical outcomes between hepatitis B- and hepatitis C-related hepatocellular carcinoma: a retrospective analysis of a single North American center. Ann Surg (2014) 260:650–6; discussion 6-8. doi: 10.1097/SLA.0000000000000917
8. Cornet M, Lim C, Salloum C, Lazzati A, Compagnon P, Pascal G, et al. Prognostic value of sarcopenia in liver surgery. J Visc Surg (2015) 152:297–304. doi: 10.1016/j.jviscsurg.2015.08.001
9. Ji F, Liang Y, Fu S, Chen D, Cai X, Li S, et al. Prognostic value of combined preoperative prognostic nutritional index and body mass index in HCC after hepatectomy. HPB (Oxford) (2017) 19:695–705. doi: 10.1016/j.hpb.2017.04.008
10. Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, et al. Sarcopenia Impacts on Short- and Long-term Results of Hepatectomy for Hepatocellular Carcinoma. Ann Surg (2015) 261:1173–83. doi: 10.1097/SLA.0000000000000743
11. Kuszajewski ML, Clontz AS. Prealbumin is best for nutritional monitoring. Nursing (2005) 35:70–1. doi: 10.1097/00152193-200505000-00056
12. Bernstein L, Pleban W. Prealbumin in nutrition evaluation. Nutrition (1996) 12:255–9. doi: 10.1016/s0899-9007(96)90852-7
13. Kawai H, Ota H. Low perioperative serum prealbumin predicts early recurrence after curative pulmonary resection for non-small-cell lung cancer. World J Surg (2012) 36:2853–7. doi: 10.1007/s00268-012-1766-y
14. Esfahani A, Makhdami N, Faramarzi E, Asghari Jafarabadi M, Ostadrahimi A, Ghayour Nahand M, et al. Prealbumin/CRP Based Prognostic Score, a New Tool for Predicting Metastasis in Patients with Inoperable Gastric Cancer. Gastroenterol Res Pract (2016) 2016:4686189. doi: 10.1155/2016/4686189
15. Mahlck CG, Grankvist K. Plasma prealbumin in women with epithelial ovarian carcinoma. Gynecol Obstet Invest (1994) 37:135–40. doi: 10.1159/000292542
16. Jia RR, Zhong JH, Huo RR, Su QB, Xiang X, Zhao FL, et al. Correlation between serum prealbumin and prognosis of patients with hepatocellular carcinoma after hepatectomy. J Surg Oncol (2019) 119:794–800. doi: 10.1002/jso.25378
17. Li JD, Xu XF, Han J, Wu H, Xing H, Li C, et al. Preoperative prealbumin level as an independent predictor of long-term prognosis after liver resection for hepatocellular carcinoma: a multi-institutional study. HPB (Oxford) (2019) 21:157–66. doi: 10.1016/j.hpb.2018.06.1803
18. Liao YY, Teng CL, Peng NF, Jia RR, Cui J, Chen K, et al. Serum Prealbumin is Negatively Associated with Survival in Hepatocellular Carcinoma Patients after Hepatic Resection. J Cancer (2019) 10:3006–11. doi: 10.7150/jca.30903
19. Zhao LP, Kolonel LN. Efficiency loss from categorizing quantitative exposures into qualitative exposures in case-control studies. Am J Epidemiol (1992) 136:464–74. doi: 10.1093/oxfordjournals.aje.a116520
20. Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ (2006) 332:1080. doi: 10.1136/bmj.332.7549.1080
21. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med (2010) 29:1037–57. doi: 10.1002/sim.3841
22. Lee W, Han HS, Ahn S, Yoon YS, Cho JY, Choi Y. Correlation between Resection Margin and Disease Recurrence with a Restricted Cubic Spline Model in Patients with Resected Hepatocellular Carcinoma. Dig Surg (2018) 35:520–31. doi: 10.1159/000485805
23. Beal EW, Tumin D, Kabir A, Moris D, Zhang XF, Chakedis J, et al. Trends in the Mortality of Hepatocellular Carcinoma in the United States. J Gastrointest Surg (2017) 21:2033–8. doi: 10.1007/s11605-017-3526-7
24. Grosso G, Micek A, Godos J, Pajak A, Sciacca S, Galvano F, et al. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am J Epidemiol (2017) 185:1304–16. doi: 10.1093/aje/kww207
25. Vieth E. Fitting piecewise linear regression functions to biological responses. J Appl Physiol (1985) (1989) 67:390–6. doi: 10.1152/jappl.1989.67.1.390
26. Nakamura T. BMDP program for piecewise linear regression. Comput Methods Programs Biomed (1986) 23:53–5. doi: 10.1016/0169-2607(86)90080-5
27. Qi Q, Geng Y, Sun M, Wang P, Chen Z. Clinical implications of systemic inflammatory response markers as independent prognostic factors for advanced pancreatic cancer. Pancreatology (2015) 15:145–50. doi: 10.1016/j.pan.2014.12.004
28. Delhem N, Carpentier A, Morales O, Miroux C, Groux H, Auriault C, et al. Regulatory T-cells and hepatocellular carcinoma: implication of the regulatory T lymphocytes in the control of the immune response. Bull Cancer (2008) 95:1219–25. doi: 10.1684/bdc.2008.0761
29. Li B, Liu HY, Guo SH, Sun P, Gong FM, Jia BQ. Impact of early enteral and parenteral nutrition on prealbumin and high-sensitivity C-reactive protein after gastric surgery. Genet Mol Res (2015) 14:7130–5. doi: 10.4238/2015.June.29.6
30. Meng J, Zhong J, Zhang H, Zhong W, Huang Z, Jin Y, et al. Pre-, peri-, and postoperative oral administration of branched-chain amino acids for primary liver cancer patients for hepatic resection: a systematic review. Nutr Cancer (2014) 66:517–22. doi: 10.1080/01635581.2013.780628
31. Devoto G, Gallo F, Marchello C, Racchi O, Garbarini R, Bonassi S, et al. Prealbumin serum concentrations as a useful tool in the assessment of malnutrition in hospitalized patients. Clin Chem (2006) 52:2281–5. doi: 10.1373/clinchem.2006.080366
32. Beck FK, Rosenthal TC. Prealbumin: a marker for nutritional evaluation. Am Fam Phys (2002) 65:1575–8.
33. Collins N. The difference between albumin and prealbumin. Adv Skin Wound Care (2001) 14:235–6. doi: 10.1097/00129334-200109000-00009
34. Zhao Z, Liu J, Wang J, Xie T, Zhang Q, Feng S, et al. Platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) are associated with chronic hepatitis B virus (HBV) infection. Int. Immunopharmacol (2017) 51:1–8. doi: 10.1016/j.intimp.2017.07.007
Keywords: all-cause mortality, dose–response relationship, hepatocellular carcinoma, serum prealbumin, prognosis
Citation: Huo R-R, Liu H-T, Deng Z-J, Liang X-M, Gong W-F, Qi L-N, You X-M, Xiang B-D, Li L-Q, Ma L and Zhong J-H (2021) Dose–Response Between Serum Prealbumin and All-Cause Mortality After Hepatectomy in Patients With Hepatocellular Carcinoma. Front. Oncol. 10:596691. doi: 10.3389/fonc.2020.596691
Received: 20 August 2020; Accepted: 25 November 2020;
Published: 11 January 2021.
Edited by:
Nicholas Syn, National University of Singapore, SingaporeReviewed by:
Tian Yang, Eastern Hepatobiliary Surgery Hospital, ChinaMarcus Yeow, National University of Singapore, Singapore
Tousif Kabir, Sengkang General Hospital, Singapore
Copyright © 2021 Huo, Liu, Deng, Liang, Gong, Qi, You, Xiang, Li, Ma and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Ma, bWFsaWFuZ2d4eWRAMTYzLmNvbQ==; Jian-Hong Zhong, emhvbmdqaWFuaG9uZ0BneG11LmVkdS5jbg==, emhvbmdqaWFuaG9uZzY2QDE2My5jb20=
†These authors have contributed equally to this work
 Rong-Rui Huo
Rong-Rui Huo Hao-Tian Liu†
Hao-Tian Liu† Jian-Hong Zhong
Jian-Hong Zhong