- 1Department of Neurosurgery, Chinese People’s Liberation Army General Hospital, Beijing, China
- 2Department of Neurosurgery, Beijing Tian Tan Hospital, Capital Medical University, Beijing, China
- 3China National Clinical Research Center for Neurological Diseases, Beijing, China
- 4Center of Brain Tumor, Beijing Institute for Brain Disorders, Beijing, China
- 5Beijing Key Laboratory of Brain Tumor, Beijing, China
Objective: To investigate the independent risk factors for recurrence in intracranial atypical meningiomas (AMs) treated with gross total resection (GTR) and early external beam radiotherapy (EBRT).
Methods: Clinical, radiological, and pathological data of intracranial AMs treated with GTR-plus-early-EBRT between January 2008 and July 2016 were reviewed. Immunohistochemical staining for Ki-67 was performed. Kaplan–Meier curves and univariate and multivariate Cox proportional hazards regression analyses were used to explore independent predictors of tumor recurrence. Chi square test was performed to compare variables between subgroups.
Results: Forty-six patients with intracranial AMs underwent GTR and early EBRT. Ten (21.7%) recurred and three (6.5%) died during a median follow-up of 76.00 months. Univariate and multivariate Cox analyses revealed that malignant progression (MP) (P = 0.009) was the only independent predictor for recurrence, while Ki-67 was of minor value in this aspect (P = 0.362). MP-AMs had a significantly higher recurrence rate (P = 0.008), a higher proportion of irregularly shaped tumors (P = 0.013) and significantly lower preoperative Karnofsky Performance Scale (KPS) scores (P = 0.040) than primary (Pri) AMs. No significant difference in Ki-67 expression was detected between these subgroups (P = 0.713).
Conclusions: MP was significantly correlated with an increased incidence of recurrence in GTR-plus-early-EBRT-treated intracranial AMs. Significantly higher frequencies of tumor relapse and irregularly shaped tumors and lower preoperative KPS scores were observed in MP-AMs compared with Pri-AMs. Ki-67 expression is of minor value in predicting tumor recurrence or distinguishing tumor origins in AMs.
Introduction
Meningiomas are the most common primary intracranial tumors (1). Among the three World Health Organization (WHO) grades of meningiomas, WHO grade II meningiomas are further classified into three subtypes: AMs, chordoid meningiomas, and clear cell meningiomas (2). Their reported incidence increased as the WHO classification was updated, ranging from 19 to 35.5% of all meningiomas in the literature (3–5). They exhibit a higher recurrence rate (up to 30%) and an unfavorable survival outcome than benign meningiomas (BMs, WHO grade I) (4). Treatment approaches for malignant meningiomas (MMs, WHO grade III) were referenced to improve this unsatisfactory prognosis (6). Surgical resection is the primary treatment, and the extent of resection is considered the most important factor for predicting recurrence and survival (7, 8). Previous studies have also demonstrated that adjuvant radiotherapy significantly improves progression-free survival (PFS) and overall survival (OS) after subtotal resection (STR) of AMs (9). However, its efficiency in those following GTR remains heavily debated (10) and has consequently led to non-uniform clinical decision-making across institutions (6). Confounding effects of different subtypes of WHO grade II meningiomas (11–14), different radiation methods (15, 16), timing of radiation (17–19), etc., in previous studies may have contributed to this uncertainty and complicated the exploration of possible prognostic factors. Therefore, these effects were eliminated in the present study to target the precise reasons for the recurrence of GTR-plus-early-EBRT-treated intracranial AMs.
Materials and Methods
Inclusion Criteria and Clinical Data Collection
Medical records and radiologic data of intracranial AM patients who underwent operations in the Department of Neurosurgery, Beijing Tian Tan Hospital, Capital Medical University from January 2008 to July 2016 were reviewed. All pathology slides were centrally reviewed and graded based on the 2016 revision of the WHO classification of tumors of the central nervous system (20) (independently by two neuropathologists blinded to clinical history, and a senior neuropathologist made the judgment if there was a discrepancy). Patients who underwent GTR as well as adjuvant EBRT at their initial pathological diagnosis of AM were included. The following exclusion criteria were adopted to explore prognostic factors more objectively: 1) pathological diagnosis of chordoid or clear cell meningioma; 2) received any other form of radiotherapy; 3) without explicit documentation of an EBRT plan; 4) lack of timely adjuvant EBRT [which was defined as within 6 months postoperatively in the literature (17)] or EBRT was postponed/terminated early; and 5) diagnosis of neurofibromatosis.
Data of the included patients were compiled from medical records, imaging, and pathological tests, and other records provided by the patients themselves. Follow-ups were performed by postoperative outpatient visits. The extent of resection was based on both the surgeon’s impression during surgery and our review of the first postoperative magnetic resonance imaging (MRI) scans. GTR was defined as Simpson grades I–II. AMs were stratified into the Pri group and the MP group based on tumor origins. MP-AMs refer to AMs who were pathologically diagnosed as BMs in previous surgeries and/or histopathologically confirmed to transform into MMs in subsequent surgeries. Others without any documentation of progression were considered as Pri-AMs. Tumor location was divided into the skull base group (including sphenoidal ridge, petroclival, foramen magnum, middle fossa, olfactory groove and orbital meningiomas) and the non-skull base group (including convexity, parasagittal, falx, cerebellar convexity, lateral ventricular and tentorial meningiomas). Tumor shape was classified as either irregular or regular based on the presence or absence of lobulation at the tumor–brain interface (mushroom-shaped tumors were included in the irregularly shaped group). PFS was defined as the period between the onset of surgery prior to EBRT and the observation of imaging-verified disease progression. OS was defined as the period from the date of surgery prior to EBRT to death or the last follow-up.
Pathological Examination
All AM samples were obtained during the surgery right before EBRT and were formalin-fixed and paraffin-embedded (FFPE) postoperatively. Hematoxylin and eosin (HE) staining and immunohistochemical staining for Ki-67 (the primary Ki-67 antibody was obtained from Abcam, Cambridge, Massachusetts, USA) were performed.
Statistical Analysis
The baseline patient characteristics are summarized as percentages for categorical variables and as the mean ± standard deviation for continuous variables. Univariate and multivariate Cox proportional hazards regression analyses were used to assess correlations between various factors and recurrence. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. Kaplan–Meier curves were generated to graphically display the associations between variables and PFS. Chi square test was performed to compare variables between different subgroups. All P values are two-sided, and significance was defined using a threshold of 0.05. Statistical analyses were performed with SPSS Statistics software (version 19.0; IBM Corporation, Armonk, NY, USA). The hospital ethics committee approved this study, and all patients provided written consent.
Results
Patient Demographics and Tumor Characteristics
A total of 46 intracranial AM patients met the aforementioned criteria, including 25 (54.3%) males and 21 (45.7%) females. The male-to-female ratio was 1.19:1. The mean age at the first presentation of AM was 49.67 ± 13.15 years (range, 20–77 years). The median surgery-radiation interval was six weeks (range, 2–21 weeks). The median radiation dose of EBRT was 60 Gy (range, 50–63 Gy; delivered to the tumor bed in 1.8- to 2.0-Gy fractions). Ten patients (21.7%) experienced tumor relapse and three patients (6.5%) died before the last follow-up (May 2020). All these three fatalities were due to meningiomas. The median follow-up duration was 76.00 months (range, 48–144 months). The median PFS was 73.50 months (range, 21–144 months). 16 (34.8%) AMs experienced MP. All of these 16 patients progressed from BMs before the combination therapy, and one of them experienced another transformation (from AM to MM) during the follow-up (Table 1).
Univariate and Multivariate Analyses Associated With Tumor Recurrence
The univariate Cox analysis showed that the PFS of GTR-plus-early-EBRT-treated AM patients was significantly influenced by MP (P = 0.012). A high radiation dose (≥60.0 Gy), a Simpson grade II resection and a skull base location were not significant prognostic factors for PFS. Since Ki-67 has been widely correlated with cell proliferation and the degree of malignancy of meningeal tumors (21, 22), both MP and Ki-67 were incorporated in the multivariate analysis. The multivariate Cox analysis revealed that only MP was an independent predictor of tumor recurrence in GTR-plus-early-EBRT-treated AMs (P = 0.009) (Table 2, Figure 1). Regarding OS, three patients died during the follow-up, all of whom experienced MP prior. However, this event number was too small to be used for further exploration of the prognostic factors of OS.
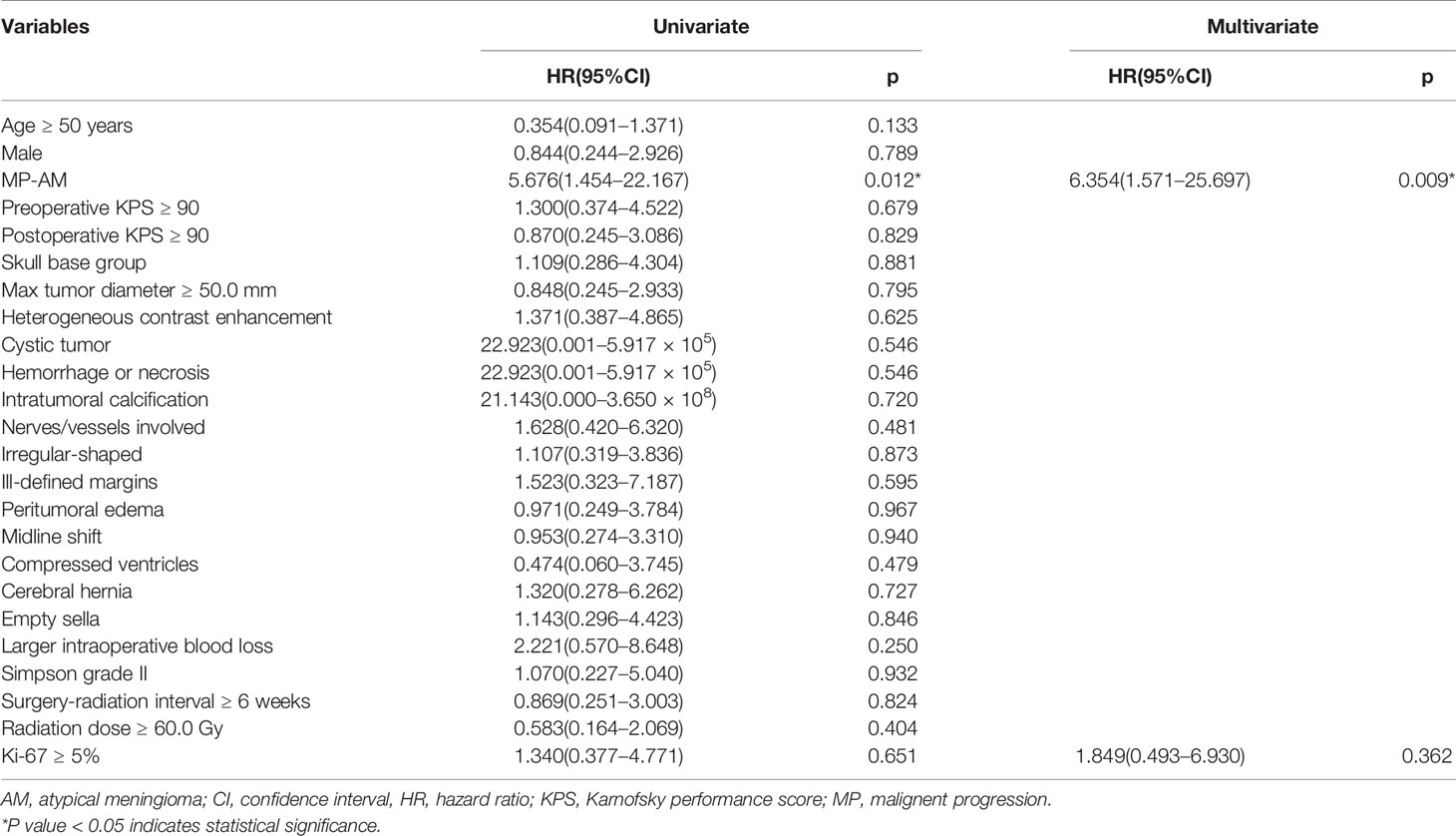
Table 2 Univariate and multivariable Cox regression predicting tumor recurrence in 46 combination-therapy-treated intracranial AM patients.
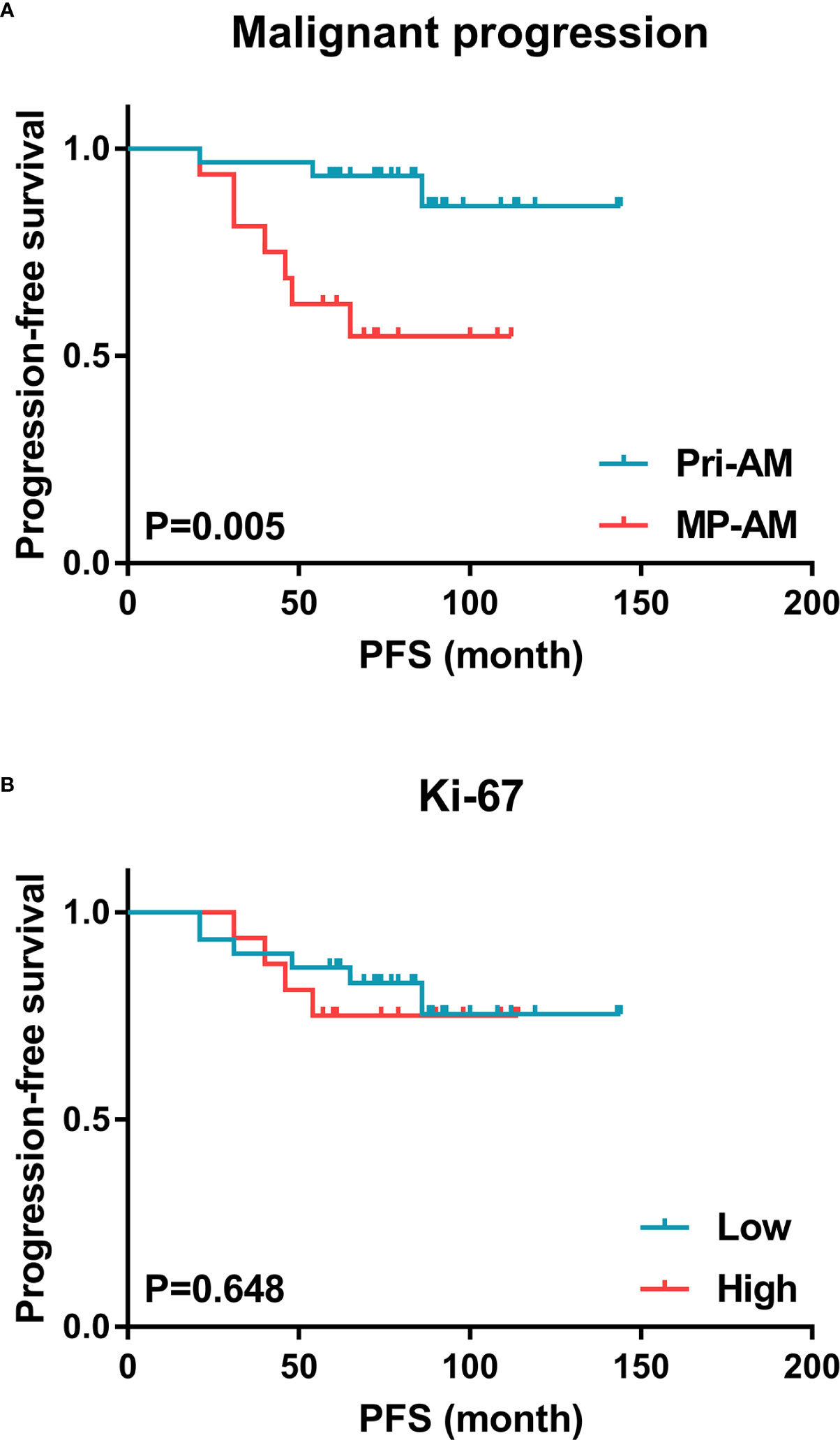
Figure 1 Kaplan–Meier estimates of PFS for combination-therapy-treated AM patients. (A) Malignant progression was a significant predictor of tumor recurrence in GTR-plus-early-EBRT-treated AMs, while (B) the Ki-67 expression level was of minor value in this respect (AM, atypical meningioma; PFS, progression-free survival).
Comparison Between Primary-Atypical Meningiomas and Malignant Progression-Atypical Meningiomas
The characteristics of Pri-AMs and MP-AMs were compared. The recurrence rate (P = 0.008) and the proportion of irregularly shaped tumors were significantly higher in MP-AMs than in Pri-AMs (P = 0.013), while the preoperative KPS score was significantly lower in MP-AMs than in Pri-AMs (P = 0.040). No significant differences in the radiation dose, surgery-radiation interval or Ki-67 expression level were detected between the groups (Table 3).
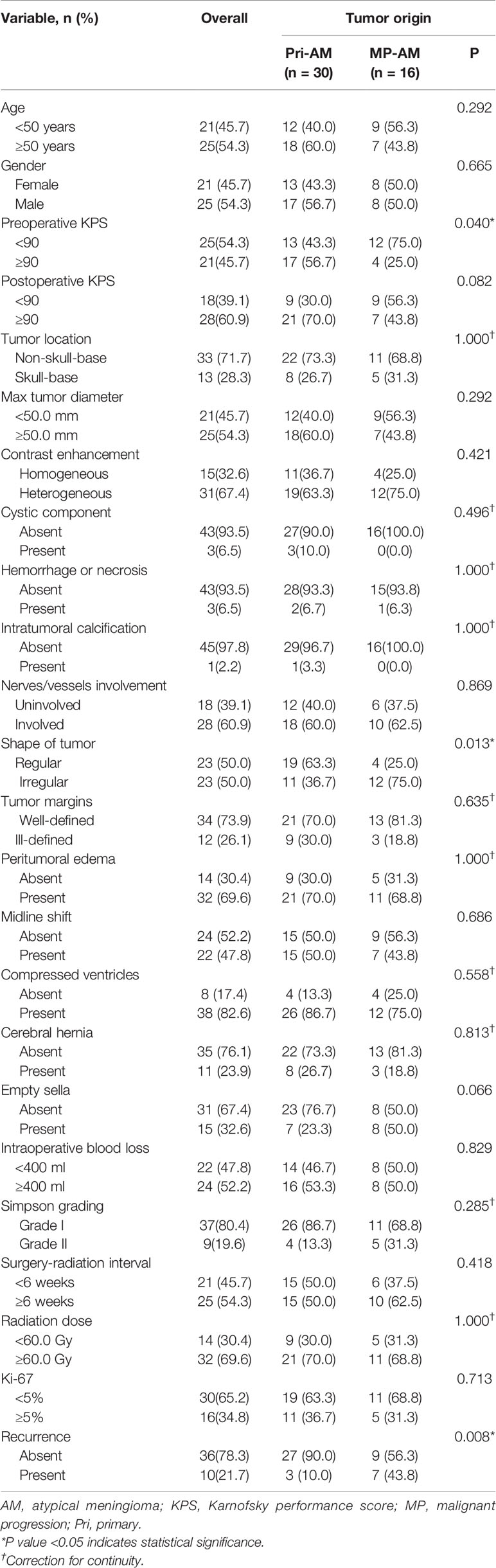
Table 3 Clinical characteristics of different origins of 46 combination-therapy-treated intracranial AMs.
Discussion
In the present study, MP was the only independent risk factor for tumor recurrence in GTR-plus-early-EBRT-treated AMs (Table 2). MP-AMs accounted for 34.8% (16/46) of the current series (Table 1). Except for their higher tendency of recurrence as compared with Pri-AMs, their lower preoperative KPS score and higher proportion of irregular-shaped tumors were also presented (Table 3). In addition, three patients died during follow-up, all of whom were MP-AM patients (Table 1).
Malignant Progression Meningiomas
High Proportions of Malignant Progression in Recurrent Meningiomas and Non-Benign Meningiomas
The clinical value of MP has been underestimated due to its low incidence in the entire meningiomas (0.16 to 2%) (23, 24). However, MP-meningiomas account for a large proportion of recurrent meningiomas and non-benign meningiomas. 14 to 28.5% of recurrent BMs transform into atypical or malignant lesions (25–27), and this rate rises to approximately 26 to 33% in recurrent AMs (25, 27, 28). MP-meningiomas have been reported as high a proportion as 38% of AMs and 70% of MMs (29). In the present cohort, 34.8% (16/46) of AMs progressed from BMs (Table 1), consistent with previous literature; 43.8% (7/16) of MP-AMs recurred, which was significantly higher than that of Pri-AMs (10%, 3/30) (p = 0.008) (Table 3); and among these recurrent AMs, MP-AMs accounted for up to 70% (7/10). Due to our strict criteria, the current high proportions of MP-AMs failed to reflect the situations when GTR and/or early EBRT were not achieved. Nevertheless, these high frequencies of MP-meningiomas in recurrent meningiomas and non-benign meningiomas reflect the poor efficacy of the existing therapies on MP-meningiomas. Therefore, MP of meningiomas is of value and should be considered in the prognostic analyses.
Unsatisfactory Therapeutical Efficacy in Malignant Progression Meningiomas
A prognostic benefit associated with Pri-meningiomas has been previously reported in the literature. Krayenbühl et al. demonstrated a statistically significant decrease in the survival time of MP-AMs (average, 1.95 years; range, 1.02–15.95 years) as compared with Pri-AMs (average, 5.36 years; range, 0.07–7.71 years), and they postulated that this difference was caused by the increased technical difficulty of GTR in reoperations and the more aggressive behavior of MP-AMs (29). Moliterno et al. exhibited an OS advantage in patients with Pri-MMs independent of the extent of resection (medium OS: Pri-MM, 3.0 years; MP-MM, 2.4 years), though this finding was prohibited from reaching statistical significance in their multivariate analysis by their small sample size (30). An OS disadvantage in MP-AM patients can also be observed in the present study since three patients died during follow-up and they were all MP-AM patients. Likewise, further analyses were also limited by the small event number, which may be due to the strict criteria applied. Meanwhile, MP was a significant independent predictive factor for tumor recurrence in combination-therapy-treated AMs (Table 2). These findings underscore the value and advantages of exploring an effective identification method of MP-meningiomas.
Even after administration of the combination therapy described herein, the recurrence risk of MP-AMs was still high (43.8%), which may question the necessity of adjuvant radiotherapy (Table 1). It has been demonstrated that ionizing radiation (IR) can enhance cellular invasion and induce malignant transformation in several cancer cells (including breast, lung, and liver cancer and glioma cells) (31–36). Our previous study confirmed that the invasiveness of IOMM-Lee meningioma cells can also be promoted by IR (37). In the context of the unsatisfactory efficacy of combination therapy in MP-AMs and the shortage of effective IR-induced MP-meningioma models (38), whether radiotherapy improves the prognosis of MP-AMs or stimulates them to undergo MP and recur requires further investigation.
Identification of Malignant Progression Meningiomas
At present, the clinical method of identifying MP meningiomas is based on the comparison between former and present pathologic diagnoses. However, for initial treatment, the effectiveness of this method is restricted. Continuous efforts have been made to identify MP-meningiomas cytogenetically and clinically. Accumulated evidences indicated that meningiomas can be classified into two distinct subtypes based on their origins: Pri- and MP-meningiomas (29, 30, 39). Meningiomas with different progression statuses possess variant molecular bases and display distinct clinical characteristics and behaviors (29, 30).
① Cytogenetical Differences Between Primary- and Malignant Progression Meningiomas
A stepwise clonal evolution model was initially used to explain the MP in meningiomas (40), which states that the malignancy of meningiomas progresses as genetic alterations accumulate (41–44). That is, more aggressive meningiomas tend to present with more complex karyotypes (41). However, this model was proposed based on cytogenetic alterations in large groups of patients with different grades of tumors (39). It is more of a reflection of the difference between WHO grades than a reflection of the difference between prior- and post-status of MP. Moreover, complex karyotypes have been detected in BMs by Perry et al. (45). Based on an analysis of the biological and genetic findings in specimens of successive histological grades of each MP meningioma, a predetermined-progression notion was developed by Al-Mefty and his colleagues (39). They documented that the presence of complex karyotypes in benign tumors preceded the histopathological manifestation of malignancy, which raised the possibility that these tumors were intrinsically malignant and destined to progress. The clonal evolution model states that lower-grade tumors possess lower karyotype complexity, while the predetermined-progression notion states that complex karyotypes already exist in lower-grade statuses of MP-meningiomas. Hence, there is a possibility that, in meningiomas of a same grade, those with higher karyotype complexity may indicate that they are MP-meningiomas, otherwise they are may be Pri-meningiomas. As the only cytogenetic comparison of Pri- and MP-meningiomas to date, Krayenbühl et al. described higher frequencies of combined cytogenetic changes (chromosomes 1, 14 and 22) and monosomy of chromosomes 10 and 18 in MP-AMs and MP-MMs than in their Pri counterparts, respectively (29). Therefore, the Pri- and MP-meningiomas of a same grade may be distinguished by their karyotype differences.
② Clinical Differences Between Primary- and Malignant Progression Meningiomas
The distribution of locations of Pri- and MP-meningiomas has been reported diversely. Based on a research with a high percentage of skull base meningiomas (61.1%, 22/36), Krayenbühl et al. reported that primary grade II-III meningiomas were predominately located in the cranial base (73.7%, 14/19), whereas progressed grade II–III meningiomas displayed a similar distribution in the skull base (47.1%) and non-skull base (52.9%) regions (29). In Moliterno’s study of MMs, the majority of tumors were located along the convexity/parasagittal areas (73.0%, 27/37). In their study, the majority of MP-MMs were located in the skull-base/posterior fossa (57%, 8/14), while Pri-MMs were discovered almost exclusively in the convexity/parasagittal regions (91%, 21/23) (30). In the present study, in which non-skull base AMs accounted for 71.7% of the cohort, a non-skull base predominance in AMs was observed regardless of the progression status (Pri-AMs: 73.3%; MP-AMs: 68.8%) (Table 3).
In addition, Moliterno et al. also detected a slight female predominance in Pri-MMs, and all of the Pri-MMs with metastatic lesions in their series were located along the convexity/parasagittal area (30). In the present study, patients with MP-AMs had lower preoperative KPS scores than those with Pri-AMs, which might be due to their higher frequency of previous surgeries, and the tumors were more likely to be irregular-shaped, which might be attributed to differences in the growth velocity of different regions of the tumor (46) (Table 3).
Minor Value of Ki-67 in the Recurrence Prediction and Origin Identification of Atypical Meningomas
Minor Value of Ki-67 in Predicting Recurrence of Gross Total Resection-Plus-Early-External Beam Radiotherapy-Treated Atypical Meningiomas
Ki-67 has been widely used in studies of the proliferative potential of meningiomas (22). A recent meta-analysis by Liu et al. indicated a significant adverse prognostic value of a high Ki-67 expression level in the prognosis of meningiomas, and 4% was recommended as the appropriate cutoff value (47). Of their 43 included studies (comprising 5012 patients), only seven specifically targeted WHO grade II meningiomas and evaluated the prognostic value of Ki-67 expression in tumor recurrence (11–14, 48–50). Each of these seven studies met at least two of the following situations: 1) inclusion of chordoid and/or clear cell meningiomas; 2) with/without postoperative radiotherapy and/or different radiotherapy modalities; and 3) diverse Ki-67 cutoff values. Based on a relatively short follow-up (1–50 months; median: 10 months), Siegers et al. stated that differences in Ki-67 expression could not be observed between three recurring and 49 non-recurring meningiomas (51). Defining non-recurring meningiomas as those without recurrence at least 8 years postoperatively, Maj-Lis Møller and Otto Brændstrup detected no significant differences in the Ki-67 labeling index between recurring and non-recurring meningiomas, when either totally and subtotally resected tumors were studied or when only radically resected tumors were studied (52). Likewise, our present results suggest that the Ki-67 expression level cannot be used as a predictor of recurrence in GTR-plus-early-adjuvant-EBRT-treated AMs. The possible reasons may be as follows. First, tumor recurrence is not dependent solely on the proliferative status of cells, especially for tumors that have undergone radical GTR. Second, the mitotic index, a proliferation marker, has been utilized as a standard in the WHO classification of meningiomas (49, 53). Therefore, the difference in tumor cell proliferation ability among meningiomas of the same grade is not as obvious as that among meningiomas of different grades. The expression of Ki-67, another proliferation marker, is also associated with cell proliferation (54). Its labeling index determines the growth fraction of tumors in percentages and is widely used to estimate tumor prognoses. The Ki-67 expression level fluctuates throughout the cell cycle, peaks in mitosis (M phase) but is absent in the resting phase (G0 phase) (55, 56). Consequently, the correlation between the peak expression level of Ki-67 in mitosis and the mitotic index leads to a minor difference in Ki-67 expression among meningiomas of the same WHO grade. Third, the abovementioned differential expression of Ki-67 among phases is also related to its role in estimating radioresistance (49). It has been substantiated that meningiomas with a higher Ki-67 labeling index may be more susceptible to adjuvant radiotherapy (49). In the present study, all the samples were obtained before IR, and all the patients received EBRT postoperatively. Hence, it is possible that some of these AMs with higher Ki-67 expression might present higher radiosensitivity to EBRT and obtain better prognoses thereafter. To a certain extent, these aforementioned points might restrict Ki-67’s ability to predict tumor recurrence in GTR-plus-early-adjuvant-EBRT-treated AMs.
Minor Value of Ki-67 in the Origin Identification of Atypical Meningiomas
The positive correlation between the Ki-67 expression level and the degree of malignancy of meningeal tumors has also been reported (21), yet this conclusion was derived mostly from studies including multiple grades of meningiomas. In a study of meningiomas with the same WHO grade yet different origins, Krayenbühl and colleagues explored a statistically significant increase in the number of MP-AM patients with high proliferative indices (a Ki-67 index greater than 5% was considered high) compared with Pri-AM patients. However, it should be noted that only 20 patient samples were stained for Ki-67. Maj-Lis Møller and Otto Brændstrup detected no differences between the Ki-67 labeling index of BMs that recurred as BMs, WHO grade II meningiomas or MMs. In other words, the expression level of Ki-67 cannot be used to judge whether a BM will experience MP. Similarly, it cannot be used to determine whether an AM is primary or malignant progressed based on our results (Table 3). According to Al-Mefty’s theory, some lower-grade meningiomas that harbor complex genetic aberrations are predetermined to histopathological progression to malignancy. They also stated that proliferation indices denoted something that was already occurring in the tumor cells more than they predicted the tumor’s potential behavior. That is to say, MP in meningiomas is a predestined but gradually manifested process. The proliferation index at one certain point in time cannot fully reflect the pre- or post-MP state of these cells. This may explain the current inability to determine the genesis of AMs by the expression level of Ki-67.
Limitations
Potential limitations of this study should be taken into consideration. First, selection bias is inevitable due to the single-center-based retrospective design and the selection of GTR-plus-early-EBRT-treated AMs as the research object. Second, the present rigorous criteria restricted the sample size and the statistical power, and the small event number of death further restricted the exploration of the prognostic factors for OS in GTR-plus-early-EBRT-treated AMs and its difference between Pri-AMs and MP-AMs. Third, the present identification method of MP in meningiomas was based on the comparison between former and present pathologic diagnoses. Hence, there still exist uncertainties that some Pri-AMs in the present study may arise from BMs before any surgery or progress to MMs in the future even though the shortest follow-up period in the current cohort exceeded the reported mean period for MP-AM progression to MM (39.8 months) (57).
Conclusions
MP is the only independent predictor of tumor recurrence in GTR-plus-early-EBRT-treated AMs. Satisfactory efficacy was not achieved in MP-AMs even after radical combination therapy. Significant higher frequencies of tumor relapse and irregularly shaped tumors as well as lower preoperative KPS scores were observed in MP-AMs than in Pri-AMs. The Ki-67 expression level is of minor value in predicting tumor recurrence or distinguishing tumor origins in AMs. More accurate and effective methods to distinguish MP-AMs from Pri-AMs are required. Further comparisons between MP-AMs with or without adjuvant radiotherapy after GTR, and the construction of effective IR-induced MP-meningioma models will be helpful to assess the necessity of radiotherapy in preventing the recurrence of MP-AMs.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials; further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of Beijing Tian Tan Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors conceptualized and made the experimental design. QZ, ZWe, MN, DL, and KW acquired the data. QZ, ZWe, MN, DL, and KW analyzed and interpreted the data. QZ drafted the article. All authors critically revised the article. WJ, JZ, and LW provided administrative/technical/material support. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 81472370 to JZ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Gui-Lin Li, Jiang Du, and Yun Cui from the Department of Neuropathology, Beijing Neurosurgical Institute, Capital Medical University, for their contributions to tissue preparation and immunohistochemical studies.
Abbreviations
AM, atypical meningioma; BM, benign meningioma; CI, confidence interval; EBRT, external beam radiotherapy; FFPE, formalin-fixed and paraffin-embedded; GTR, gross total resection; HE, hematoxylin and eosin; HR, hazard ratio; KPS, Karnofsky Performance Scale; MM, malignant meningioma; MP, malignant progression; MRI, magnetic resonance imaging; OS, overall survival; PFS, progression-free survival; Pri, primary; STR, subtotal resection; IR, ionizing radiation.
References
1. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol (2010) 99(3):307–14. doi: 10.1007/s11060-010-0386-3
2. Wen PY, Huse JT. World Health Organization Classification of Central Nervous System Tumors. Continuum (Minneap Minn) (2017) 23(6, Neuro-oncology):1531–47. doi: 10.1212/CON.0000000000000536
3. Backer-Grøndahl T, Moen BH, Torp SH. The histopathological spectrum of human meningiomas. Int J Clin Exp Pathol (2012) 5(3):231–42.
4. Mair R, Morris K, Scott I, Carroll TA. Radiotherapy for atypical meningiomas. J Neurosurg (2011) 115(4):811–9. doi: 10.3171/2011.5.JNS11112
5. Pearson BE, Markert JM, Fisher WS, Guthrie BL, Fiveash JB, Palmer CA, et al. Hitting a moving target: evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus (2008) 24(5):E3. doi: 10.3171/FOC/2008/24/5/E3
6. Sahm F, Schrimpf D, Stichel D, Jones DTW, Hielscher T, Schefzyk S, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol (2017) 18(5):682–94. doi: 10.1016/S1470-2045(17)30155-9
7. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry (1957) 20:22–39. doi: 10.1136/jnnp.20.1.22
8. Hammouche S, Clark S, Wong AH, Eldridge P, Farah JO. Long-term survival analysis of atypical meningiomas: survival rates, prognostic factors, operative and radiotherapy treatment. Acta Neurochir (Wien) (2014) 156:1475–81. doi: 10.1007/s00701-014-2156-z
9. Kaur G, Sayegh ET, Larson A, Bloch O, Madden M, Sun MZ, et al. Adjuvant radiotherapy for atypical and malignant meningiomas: a systematic review. Neuro Oncol (2014) 16:628–36. doi: 10.1093/neuonc/nou025
10. Pollock BE. Defining the best management for patients with intracranial World Health Organization grade II meningiomas. World Neurosurg (2014) 81(5-6):712–3. doi: 10.1016/j.wneu.2013.08.051
11. Champeaux C, Houston D, Dunn L. Atypical meningioma. A study on recurrence and disease-specific survival. Neurochirurgie (2017) 63(4):273–81. doi: 10.1016/j.neuchi.2017.03.004
12. Klinger DR, Flores BC, Lewis JJ, Hatanpaa K, Choe K, Mickey B, et al. Atypical Meningiomas: Recurrence, Reoperation, and Radiotherapy. World Neurosurg (2015) 84(3):839–45. doi: 10.1016/j.wneu.2015.04.033
13. Nanda A, Bir SC, Konar S, Maiti T, Kalakoti P, Jacobsohn JA, et al. Outcome of resection of WHO Grade II meningioma and correlation of pathological and radiological predictive factors for recurrence. J Clin Neurosci (2016) 31:112–21. doi: 10.1016/j.jocn.2016.02.021
14. Champeaux C, Dunn L. World Health Organization Grade II Meningioma: A 10-Year Retrospective Study for Recurrence and Prognostic Factor Assessment. World Neurosurg (2016) 89:180–6. doi: 10.1016/j.wneu.2016.01.055
15. Champeaux C, Wilson E, Shieff C, Khan AA, Thorne L. WHO grade II meningioma: a retrospective study for outcome and prognostic factor assessment. J Neurooncol (2016) 129:337–45. doi: 10.1007/s11060-016-2181-2
16. Wu A, Jin MC, Meola A, Wong HN, Chang SD. Efficacy and toxicity of particle radiotherapy in WHO grade II and grade III meningiomas: a systematic review. Neurosurg Focus (2019) 46:E12. doi: 10.3171/2019.3.FOCUS1967
17. Jenkinson MD, Waqar M, Farah JO, Farrell M, Barbagallo GM, McManus R, et al. Early adjuvant radiotherapy in the treatment of atypical meningioma. J Clin Neurosci (2016) 28:87–92. doi: 10.1016/j.jocn.2015.09.021
18. Jenkinson MD, Javadpour M, Haylock BJ, Young B, Gillard H, Vinten J, et al. The ROAM/EORTC-1308 trial: Radiation versus Observation following surgical resection of Atypical Meningioma: study protocol for a randomised controlled trial. Trials (2015) 16:519. doi: 10.1186/s13063-015-1040-3
19. Milosevic MF, Frost PJ, Laperriere NJ, Wong CS, Simpson WJ. Radiotherapy for atypical or malignant intracranial meningioma. Int J Radiat Oncol Biol Phys (1996) 34:817–22. doi: 10.1016/0360-3016(95)02166-3
20. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol (2016) 131(6):803–20. doi: 10.1007/s00401-016-1545-1
21. Amatya VJ, Takeshima Y, Sugiyama K, Kurisu K, Nishisaka T, Fukuhara T, et al. Immunohistochemical study of Ki-67 (MIB-1), p53 protein, p21WAF1, and p27KIP1 expression in benign, atypical, and anaplastic meningiomas. Hum Pathol (2001) 32:970–5. doi: 10.1053/hupa.2001.27119
22. Abry E, Thomassen IØ, Salvesen ØO, Torp SH. The significance of Ki-67/MIB-1 labeling index in human meningiomas: a literature study. Pathol Res Pract (2010) 206:810–5. doi: 10.1016/j.prp.2010.09.002
23. Jellinger K, Slowik F. Histological subtypes and prognostic problems in meningiomas. J Neurol (1975) 208(4):279–98. doi: 10.1007/BF00312803
24. Rohringer M, Sutherland GR, Louw DF, Sima AA. Incidence and clinicopathological features of meningioma. J Neurosurg (1989) 71(5 Pt 1):665–72. doi: 10.3171/jns.1989.71.5.0665
25. Jääskeläinen J, Haltia M, Laasonen E, Wahlström T, Valtonen S. The growth rate of intracranial meningiomas and its relation to histology. An analysis of 43 patients. Surg Neurol (1985) 24(2):165–72. doi: 10.1016/0090-3019(85)90180-6
26. Adegbite AB, Khan MI, Paine KW, Tan LK. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg (1983) 58(1):51–6. doi: 10.3171/jns.1983.58.1.0051
27. Jääskeläinen J, Haltia M, Servo A. Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy, and outcome. Surg Neurol (1986) 25(3):233–42. doi: 10.1016/0090-3019(86)90233-8
28. Palma L, Celli P, Franco C, Cervoni L, Cantore G. Long-term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. J Neurosurg (1997) 86(5):793–800. doi: 10.3171/jns.1997.86.5.0793
29. Krayenbühl N, Pravdenkova S, Al-Mefty O. De novo versus transformed atypical and anaplastic meningiomas: comparisons of clinical course, cytogenetics, cytokinetics, and outcome. Neurosurgery (2007) 61(3):495–503; discussion 503-4. doi: 10.1227/01.NEU.0000290895.92695.22
30. Moliterno J, Cope WP, Vartanian ED, Reiner AS, Kellen R, Ogilvie SQ, et al. Survival in patients treated for anaplastic meningioma. J Neurosurg (2015) 123:23–30. doi: 10.3171/2014.10.JNS14502
31. De Bacco F, Luraghi P, Medico E, Reato G, Girolami F, Perera T, et al. Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. J Natl Cancer Inst (2011) 103:645–61. doi: 10.1093/jnci/djr093
32. Kawamoto A, Yokoe T, Tanaka K, Saigusa S, Toiyama Y, Yasuda H, et al. Radiation induces epithelial-mesenchymal transition in colorectal cancer cells. Oncol Rep (2012) 27:51–7. doi: 10.3892/or.2011.1485
33. Park JK, Jang SJ, Kang SW, Park S, Hwang SG, Kim WJ, et al. Establishment of animal model for the analysis of cancer cell metastasis during radiotherapy. Radiat Oncol (2012) 7:153. doi: 10.1186/1748-717X-7-153
34. Moncharmont C, Levy A, Guy JB, Falk AT, Guilbert M, Trone JC, et al. Radiation-enhanced cell migration/invasion process: a review. Crit Rev Oncol Hematol (2014) 92:133–42. doi: 10.1016/j.critrevonc.2014.05.006
35. Zhang X, Li X, Zhang N, Yang Q, Moran MS. Low doses ionizing radiation enhances the invasiveness of breast cancer cells by inducing epithelial-mesenchymal transition. Biochem Biophys Res Commun (2011) 412:188–92. doi: 10.1016/j.bbrc.2011.07.074
36. Wild-Bode C, Weller M, Rimner A, Dichgans J, Wick W. Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer Res (2001) 61:2744–50.
37. Zhang Q, Song LR, Huo XL, Wang L, Zhang GB, Hao SY, et al. MicroRNA-221/222 Inhibits the Radiation-Induced Invasiveness and Promotes the Radiosensitivity of Malignant Meningioma Cells. Front Oncol (2020) 10:1441. doi: 10.3389/fonc.2020.01441
38. Cimino PJ. Malignant progression to anaplastic meningioma: Neuropathology, molecular pathology, and experimental models. Exp Mol Pathol (2015) 99:354–9. doi: 10.1016/j.yexmp.2015.08.007
39. Al-Mefty O, Kadri PA, Pravdenkova S, Sawyer JR, Stangeby C, Husain M. Malignant progression in meningioma: documentation of a series and analysis of cytogenetic findings. J Neurosurg (2004) 101:210–8. doi: 10.3171/jns.2004.101.2.0210
40. Seizinger BR, de la Monte S, Atkins L, Gusella JF, Martuza RL. Molecular genetic approach to human meningioma: loss of genes on chromosome 22. Proc Natl Acad Sci U S A (1987) 84:5419–23. doi: 10.1073/pnas.84.15.5419
41. Simon M, von DA, Larson JJ, Wellenreuther R, Kaskel P, Waha A, et al. Allelic losses on chromosomes 14, 10, and 1 in atypical and malignant meningiomas: a genetic model of meningioma progression. Cancer Res (1995) 55:4696–701.
42. Boström J, Meyer-Puttlitz B, Wolter M, Blaschke B, Weber RG, Lichter P, et al. Alterations of the tumor suppressor genes CDKN2A (p16(INK4a)), p14(ARF), CDKN2B (p15(INK4b)), and CDKN2C (p18(INK4c)) in atypical and anaplastic meningiomas. Am J Pathol (2001) 159:661–9. doi: 10.1016/S0002-9440(10)61737-3
43. Perry A, Banerjee R, Lohse CM, Kleinschmidt-DeMasters BK, Scheithauer BW. A role for chromosome 9p21 deletions in the malignant progression of meningiomas and the prognosis of anaplastic meningiomas. Brain Pathol (2002) 12:183–90. doi: 10.1111/j.1750-3639.2002.tb00433.x
44. Weber RG, Boström J, Wolter M, Baudis M, Collins VP, Reifenberger G, et al. Analysis of genomic alterations in benign, atypical, and anaplastic meningiomas: toward a genetic model of meningioma progression. Proc Natl Acad Sci U S A (1997) 94:14719–24. doi: 10.1073/pnas.94.26.14719
45. Perry A, Jenkins RB, Dahl RJ, Moertel CA, Scheithauer BW. Cytogenetic analysis of aggressive meningiomas: possible diagnostic and prognostic implications. Cancer (1996) 77:2567–73. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2567::AID-CNCR21>3.0.CO;2-P
46. Zhou JL, Liu JL, Zhang J, Zhang M. Thirty-nine cases of intracranial hemangiopericytoma and anaplastic hemangiopericytoma: a retrospective review of MRI features and pathological findings. Eur J Radiol (2012) 81:3504–10. doi: 10.1016/j.ejrad.2012.04.034
47. Liu N, Song SY, Jiang JB, Wang TJ, Yan CX. The prognostic role of Ki-67/MIB-1 in meningioma: A systematic review with meta-analysis. Med (Baltimore) (2020) 99(9):e18644. doi: 10.1097/MD.0000000000018644
48. Kim MS, Kim KH, Lee EH, Lee YM, Lee SH, Kim HD, et al. Results of immunohistochemical staining for cell cycle regulators predict the recurrence of atypical meningiomas. J Neurosurg (2014) 121(5):1189–200. doi: 10.3171/2014.7.JNS132661
49. Choi Y, Lim DH, Yu JI, Jo K, Nam DH, Seol HJ, et al. Prognostic Value of Ki-67 Labeling Index and Postoperative Radiotherapy in WHO Grade II Meningioma. Am J Clin Oncol (2018) 41(1):18–23. doi: 10.1097/COC.0000000000000224
50. Liu H, Li Y, Li Y, Zhou L, Bie L. STMN1 as a candidate gene associated with atypical meningioma progression. Clin Neurol Neurosurg (2017) 159:107–10. doi: 10.1016/j.clineuro.2017.06.003
51. Siegers HP, Zuber P, Hamou MF, van Melle GD, de Tribolet N. The implications of the heterogeneous distribution of Ki-67 labelled cells in meningiomas. Br J Neurosurg (1989) 3(1):101–7. doi: 10.3109/02688698909001031
52. Møller ML, Braendstrup O. No prediction of recurrence of meningiomas by PCNA and Ki-67 immunohistochemistry. J Neurooncol (1997) 34(3):241–6. doi: 10.1023/A:1005794700267
53. Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol (2010) 99(3):379–91. doi: 10.1007/s11060-010-0342-2
54. Lopez F, Belloc F, Lacombe F, Dumain P, Reiffers J, Bernard P, et al. Modalities of synthesis of Ki67 antigen during the stimulation of lymphocytes. Cytometry (1991) 12(1):42–9. doi: 10.1002/cyto.990120107
55. Jonat W, Arnold N. Is the Ki-67 labelling index ready for clinical use. Ann Oncol (2011) 22(3):500–2. doi: 10.1093/annonc/mdq732
56. Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol (2000) 182(3):311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9
Keywords: malignant progression, Ki-67, atypical meningioma, gross-total resection, external beam radiotherapy
Citation: Zhang Q, Wen Z, Ni M, Li D, Wang K, Jia G-J, Wu Z, Zhang L-W, Jia W, Wang L and Zhang J-T (2021) Malignant Progression Contributes to the Failure of Combination Therapy for Atypical Meningiomas. Front. Oncol. 10:608175. doi: 10.3389/fonc.2020.608175
Received: 19 September 2020; Accepted: 01 December 2020;
Published: 15 January 2021.
Edited by:
Hailiang Tang, Fudan University, ChinaReviewed by:
Subhas K. Konar, National Institute of Mental Health and Neurosciences (NIMHANS), IndiaIan Dunn, University of Oklahoma Health Sciences Center, United States
Copyright © 2021 Zhang, Wen, Ni, Li, Wang, Jia, Wu, Zhang, Jia, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wang Jia, and0dHl5QHNpbmEuY29t; Jun-Ting Zhang, emhhbmdqdW50aW5nMjAwM0BhbGl5dW4uY29t; Liang Wang, c2FpbnRhZ2U3QDEyNi5jb20=
 Qing Zhang
Qing Zhang Zheng Wen2,3,4,5
Zheng Wen2,3,4,5 Gui-Jun Jia
Gui-Jun Jia Liang Wang
Liang Wang Jun-Ting Zhang
Jun-Ting Zhang