- 1Medical Oncology 2, Veneto Institute of Oncology IOV—IRCCS, Padova, Italy
- 2Department of Surgery, Oncology and Gastroenterology, University of Padova, Padova, Italy
- 3Oncology Unit, Hospital of Montecchio Maggiore, Montecchio Maggiore, Vicenza, Italy
- 4Immunology and Molecular Oncology Unit, Veneto Institute of Oncology IOV—IRCCS, Padova, Italy
- 5Istituto Europeo di Oncologia - IRCCS, Milano, Italy
- 6University of Milano-Bicocca, Milano, Italy
Purpose: We aimed to evaluate the clinico-pathological characteristics and survival outcomes of patients with synchronous or metachronous breast cancer (BC) and ovarian cancer (OC).
Materials and Methods: Patients with synchronous or metachronous BC and OC were retrospectively identified at two large cancer centers. Clinico-pathological characteristics, BRCA1/2 status and follow-up data were gathered. Patients were classified according to the first cancer diagnosis in the following groups: Breast Cancer first, Ovarian Cancer first, Synchronous Breast and Ovarian Cancer. Overall survival (OS) was calculated as the time interval between each cancer diagnosis to death or last follow-up.
Results: Overall, 270 patients were included: n = 194 (72%) in BC first group, n = 51 (19%) in OC first, and n = 25 (9%) in synchronous. BRCA status was available for 182 (67.4%) patients and 112 (62%) harbored pathogenetic mutations. BC first group included more frequently patients with BRCA mutation, triple negative BC phenotype and more aggressive OC features. Median time between the two diagnosis was longer in BC first group vs OC first group (95 vs 68 months, p = 0.021). A total of 105 OS events occurred, mostly related to OC (70.5%). We observed no differences in terms of OS according to the first cancer diagnosis. Age >50 years and advanced OC stage were negative independent prognostic factors for OS from the first diagnosis.
Conclusions: In this cohort of patients with BC and OC, survival was dominated by OC related mortality. These data may be useful to plan and carry out adequate and timely surveillance programs and preventive measures.
Introduction
Breast cancer (BC) is the most frequent cancer and the leading cause of cancer death among females worldwide, with an estimated 1.6 million cases and 521,900 deaths in 2012 (1). Ovarian cancer (OC) is the 7th most frequent cancer diagnosis, with 238,700 new cases in 2012, and the 8th cause of cancer mortality, with 151,900 deaths (1).
When compared with the general population, cancer survivors have generally an increased risk of developing a second primary cancer at a different sites (2, 3). Register-based studies show that women with BC are at increased risk of developing OC and that long term OC survivors are at increased risk of developing BC cancer (4, 5).
In the early 1970s, Lynch provided the first evidence of an autosomal-dominant inherited trait predisposing women to both BC and OC (6, 7). In 1990 Mary-Claire King demonstrated that a single gene on chromosome 17, later known as BRCA1, was responsible for many breast and ovarian cancer (8). Actually, hereditary breast and ovarian cancer syndrome (HBOC) is a well-described hereditary cancer predisposition syndrome caused by mutations in BRCA1 and BRCA2 genes.
The lifetime risk for women with BRCA1 mutations is estimated to be about 72% for BC (95%CI, 65–79%) and 44% for OC (95%CI, 36–53%. The corresponding estimates for BRCA2 are 69% (95%CI,61–77%) and 17% (95%CI, 11–25%) respectively (9). A timely identification of BRCA-mutation carriers is therefore key in order to plan adequate risk-reduction strategies.
However, synchronous or metachronous BC and OC diagnoses have been documented also in the absence of a germline BRCA mutation, suggesting other common etiological factors such as hormonal and reproductive aspects and mutation of other genes involved in tumor suppression (10–12). We nowadays know several other genes whose germline mutations can increase the lifetime risk of breast and/or ovarian cancer, such as CHEK2, BRIP1, BARD1, ATM; RAD51C, RAD51D, PALB2 and the genes associated with familial hereditary non-polyposys colorectal cancer (MSH6, MSH2, and MLH1) (13). There are few studies describing cohorts of patients with synchronous or metachronous BC and OC. In the present paper, we describe clinico-pathological characteristics including BRCA status, treatments and clinical outcome of a multicentric cohort of patients diagnosed with synchronous or metachronous BC and OC.
Materials and Methods
Patients
We reviewed medical reports of patients with synchronous or metachronous BC and OC, diagnosed between 1981 and 2016 at two large Italian cancer centers: the Istituto Oncologico Veneto IRCCS (Padova) and the Istituto Europeo di Oncologia (Milano). Patients with borderline or non-epithelial OC and patients with in situ BC were excluded. According to the sequence of cancer diagnoses, patients were classified into three groups: BC first (BC followed by OC), OC first (OC followed by BC) and synchronous (time between the two diagnoses <4 months). When available, the BRCA-mutational status was also recorded.
Further information including tumor stage (according to the AJCC 7th Edition BC Staging and FIGO 2014 OC staging), histological type, tumor grade, hormonal/HER2 receptors status (BC), surgical and medical treatment were collected. Follow-up data including death cause were also gathered.
Statistical Analysis
Statistical analysis was conducted using IBM SPSS Version 24. The association between variables was evaluated using the χ2 test or t test, as appropriate.
Overall survival (OS) from the first diagnosis was defined as the time interval from the first cancer diagnosis (BC or OC whichever first) to the date of death/last follow-up. We also evaluated the OS after the second diagnosis, calculated from the time of BC or OC diagnosis (whichever last) to death/last follow-up. For survival analyses, the hazard ratio (HR) and 95% confidence interval (95% CI) were calculated with the Cox regression model. Survival curves were estimated using the Kaplan–Meier model, and the log-rank test was used to test the differences between the groups. Level of significance was set at 0.05. The data cut-off for the survival events was May 2017.
Results
Patients Characteristics According to the Sequence of Breast Cancer and Ovarian Cancer Diagnoses
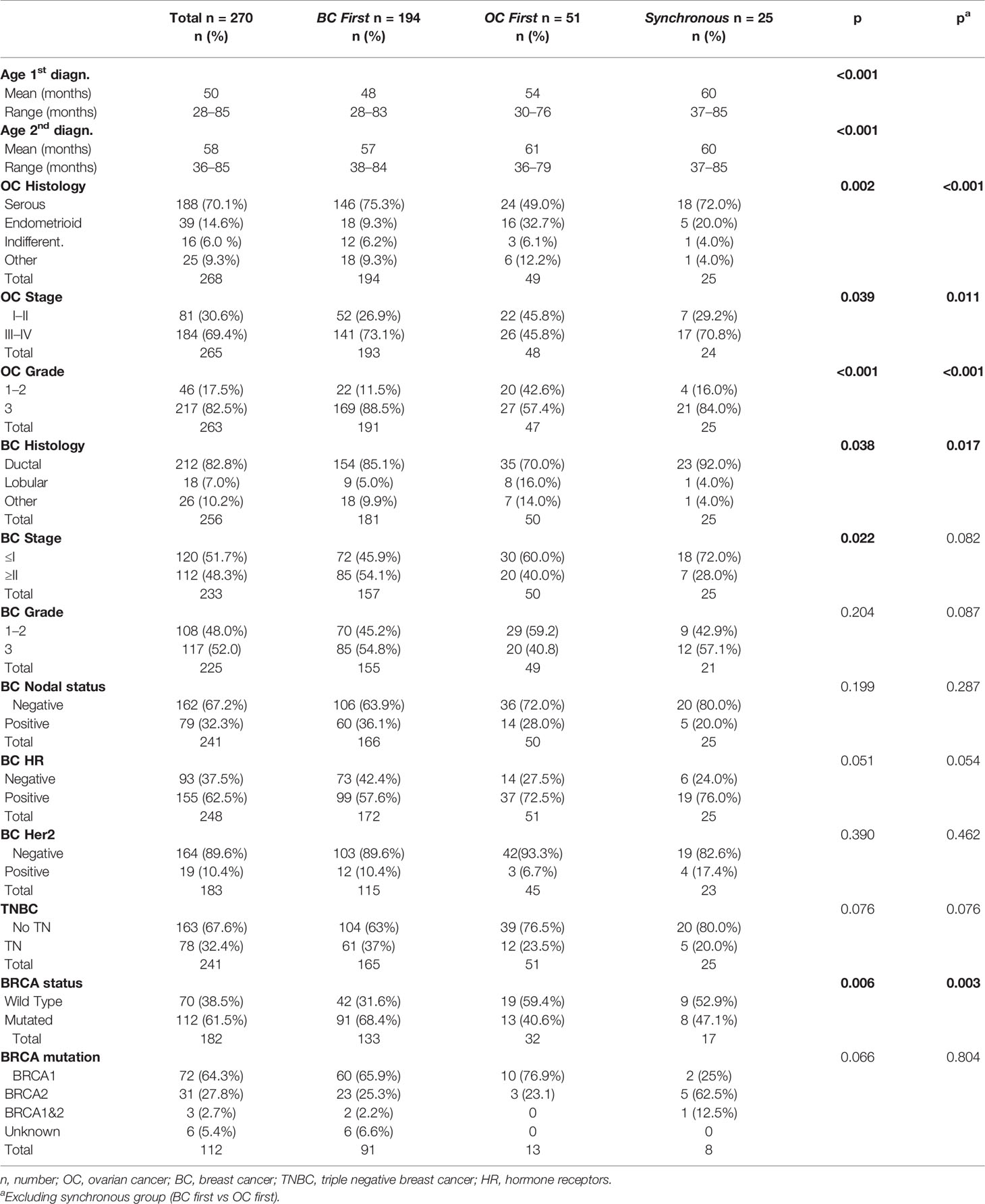
Two hundred and seventy patients were included and classified as follows according to the first cancer diagnosis: BC first n = 194 (72%), OC first n = 51 (19%), and synchronous n = 25 (9%). The clinico-pathological characteristics of the population according to BC/OC diagnosis sequence are summarized in Table 1.
BC first patients presented the youngest age at first and second diagnosis compared to the other groups (p < 0.001 in both cases).
The BC first and synchronous groups, as compared to OC first group, showed a significantly higher frequency of OC of serous histology (75.3 and 72.0 vs 49%, p = 0.002), high grade (88.5 and 84.0 vs 57.4%, p < 0.001) and FIGO stage ≥III (73.1 and 70.8 vs 45.8%, p = 0.039).
The most frequent histotype of BC was ductal infiltrating carcinoma in all groups; however, in the OC first group, lobular histotype was more represented (16.0 vs 5.0% in BC first and 4.0% in synchronous; p = 0.038). More than a half (54.1%) of BC first patients presented stage >II BC at diagnosis as compared to 40.0 and 28.0% of patients in the OC first and synchronous groups, respectively (p = 0.022). BC first patients showed the highest proportion of TNBC (37 vs 23.5% and 20.0% in the OC first and synchronous groups, p = 0.076).
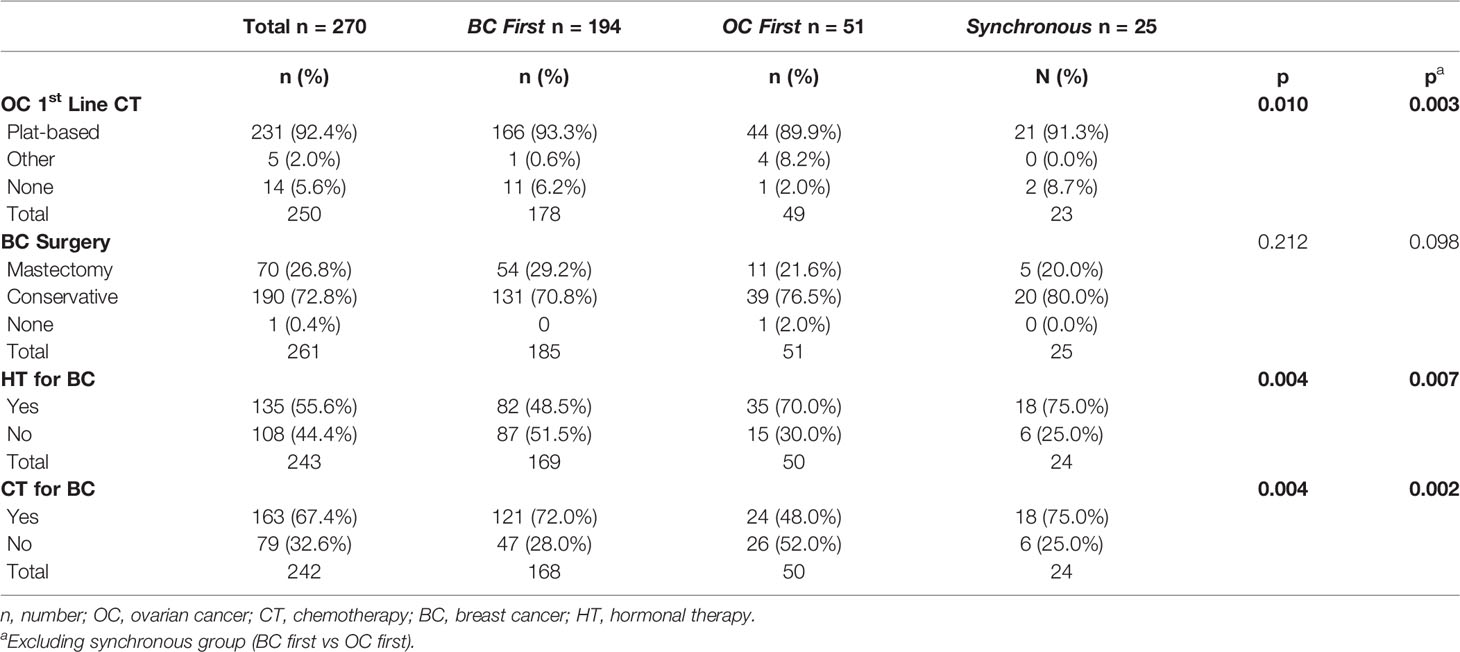
Treatments According to the Sequence of Breast Cancer and Ovarian Cancer Diagnoses
The majority of patients received a platinum-based chemotherapy for OC (92.4%). More patients in the OC first group were treated with other chemotherapy regimens (8.2%), reflecting the different histological patterns.
As expected, since treatment selection for BC is based on tumor phenotype, BC treatment was significantly different among the cancer sequence groups. Indeed, more than 70% of BC first and synchronous patients were treated with adjuvant chemotherapy reflecting the higher prevalence of TNBC subtype in this group. Table 2 shows data regarding medical and surgical treatments.
Patients With Known BRCA Status: Baseline Characteristics and Treatment
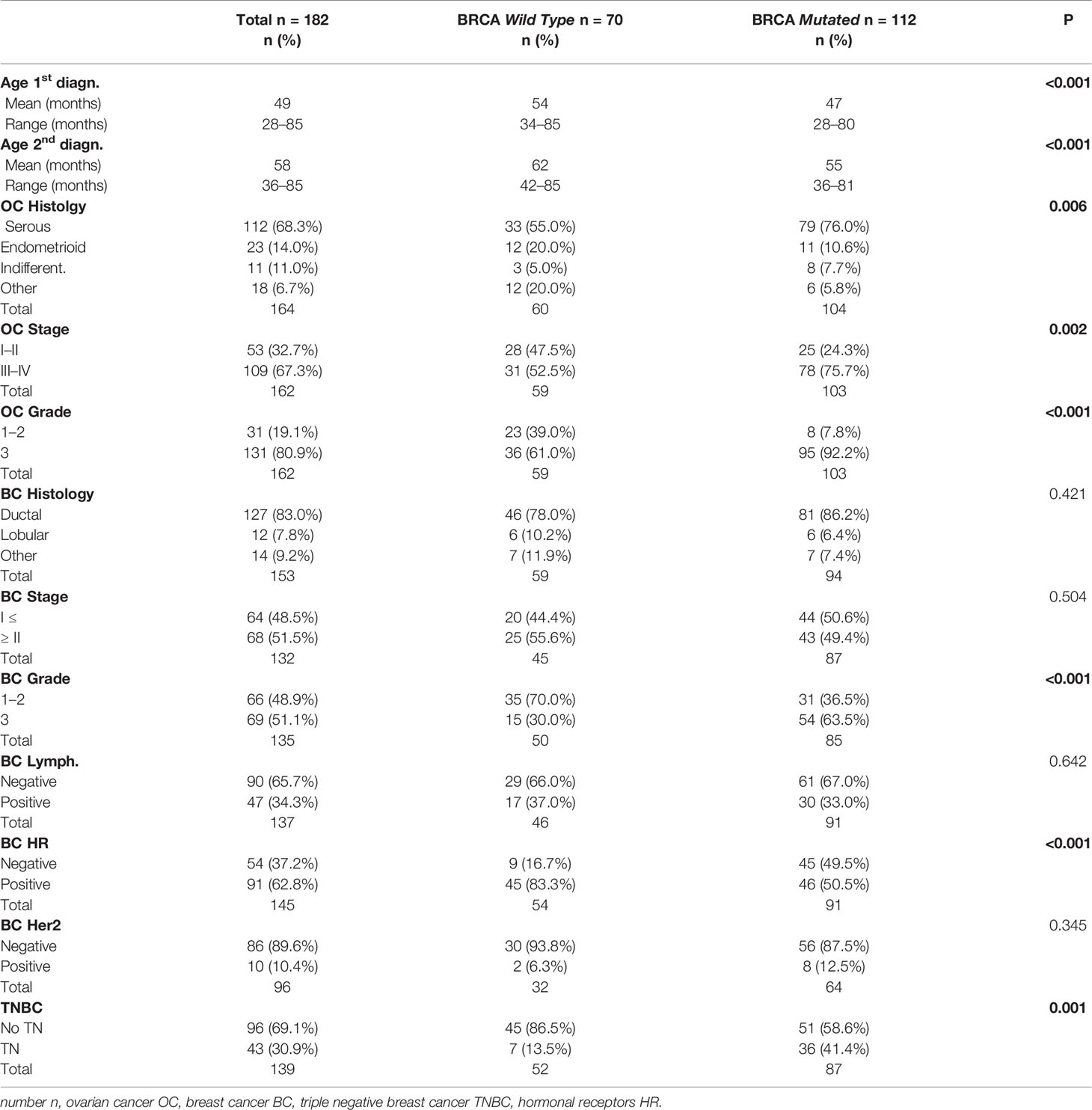
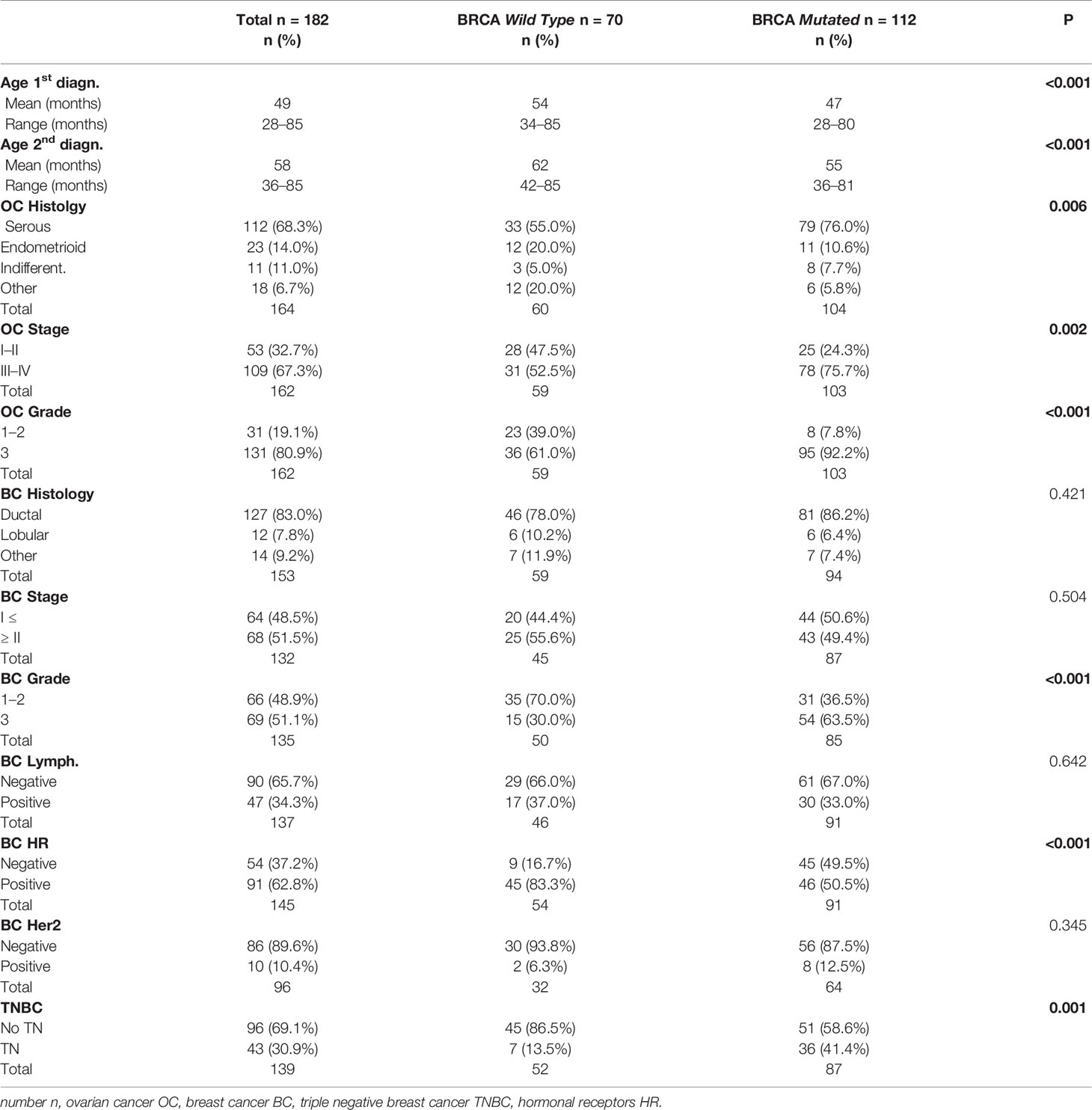
For n = 182 (67.4%) patients, the status of BRCA1/2 genes was available. As shown in Table 1, n = 112 patients (61.5%) were BRCA mutated (64.3% BRCA1, 27.8% BRCA2 and 2.7% BRCA 1&2) and n = 70 (38.5%) were wild type. The frequency of BRCA mutation carriers was significantly different in the three groups: 68.4% of BC first patients, 40.6% of OC first patients and 47.1% of patients in the synchronous group (p = 0.006). For the vast majority of patients with available BRCA status, the genetic test was performed after both tumors had been diagnosed (85.2%), with no difference between the groups (p = 0.312).
The evaluation of clinico-pathological characteristics based on the mutational status of BRCA1/2 genes is reported in Table 3.
The mean age at the first and second diagnoses was lower in the BRCA mutated group (p < 0.001).
BRCA mutated patients had more frequent OC of advanced stage (FIGO stage ≥III 75.7 vs 52.5%; p = 0.002), serous histology (76.0 vs 55.0%, p = 0.006) and high grade (92.2 vs 61.0%, p < 0.001).
Regarding BC, as expected, BRCA mutated patients presented more TN (41.4 vs 13.5%; p = 0.001) and grade 3 (63.5 vs 30.0%; p < 0.001) tumors, reflecting the higher prevalence of BRCA1 mutation (BRCA1 64.3%). Table 4 shows medical and surgical treatments according to BRCA status. No difference was observed in terms of chemotherapy for OC, with the majority of patients being treated with 1st line platinum-based CT (92%). The majority of BRCA wild type patients received HT for BC. CT use was more frequent in BRCA mutated patients (75%) reflecting the higher prevalence of TNBC subtype in this group. Surgical treatment for BC consisted in conservative surgery in most cases (66.4% of BRCA mutated and 66.7% of BRCA wild type), and this finding is consistent with the fact that BRCA status was unknown at time of surgery for most of the patients.
Interval Between Cancer Diagnoses
Median time interval from first to second diagnosis in overall cohort (including synchronous patients) was 78 months (95%CI 67.6–88.4). When comparing BC first group to the OC first group, the time interval was longer in BC first: median 95 months (95%CI 84.0–106.0 months) vs 68 months (95%CI 46.7–89.8), respectively (Figure 1, p = 0.021).
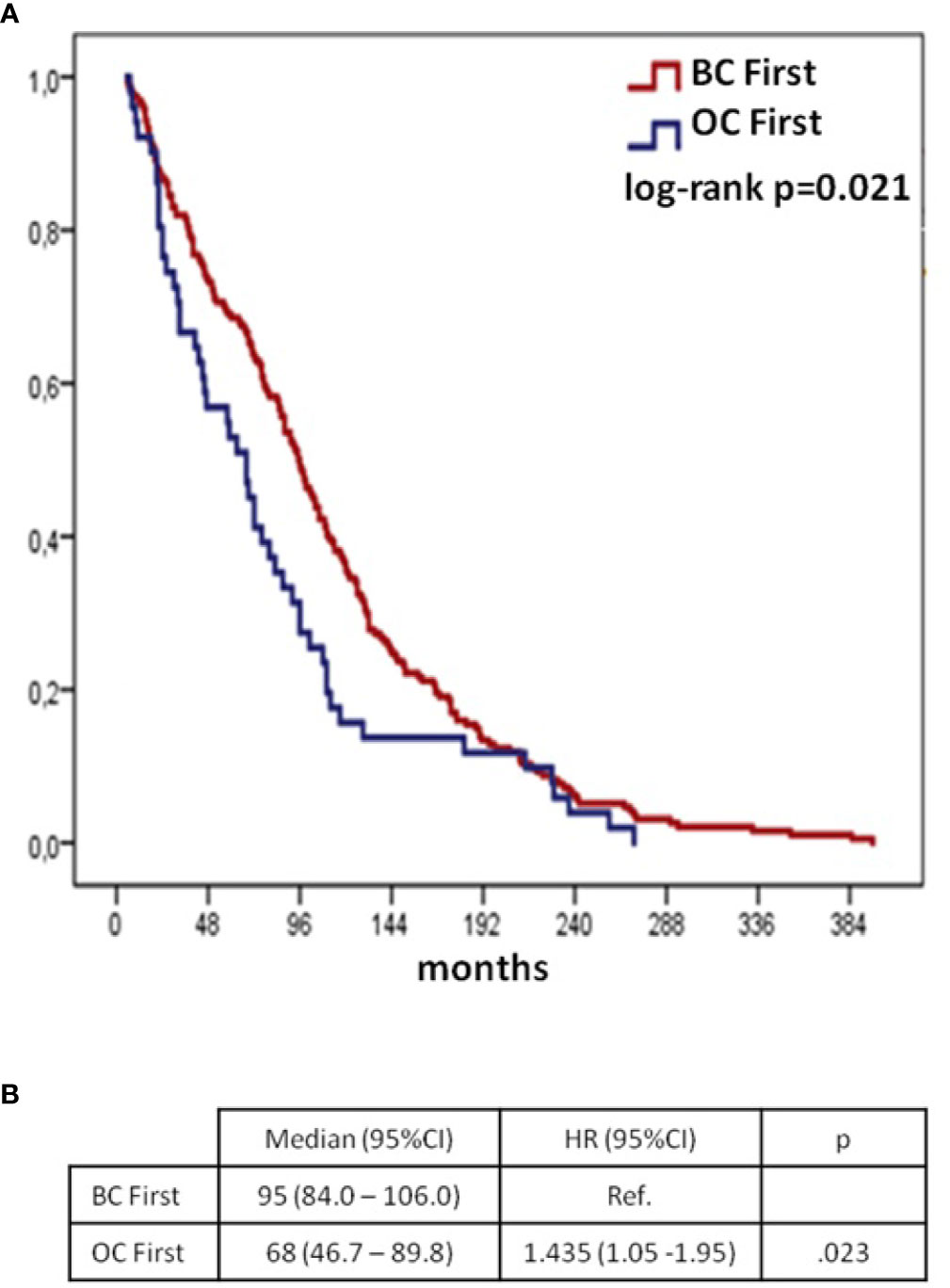
Figure 1 Time from 1st to 2nd cancer diagnoses according to BC/OC diagnosis sequence (synchronous group excluded): Kaplan–Meier curves (A) and Cox regression model (B) BC, breast cancer; OC, ovarian cancer; CI, confidential interval; H, hazard ratio; OS, overall survival.
Median time between diagnoses was 96 months (95%CI 85.6–106.4) for the n=182 patients with available BRCA gene status and was similar in BRCA mutated and wild type patients (Figure 2).
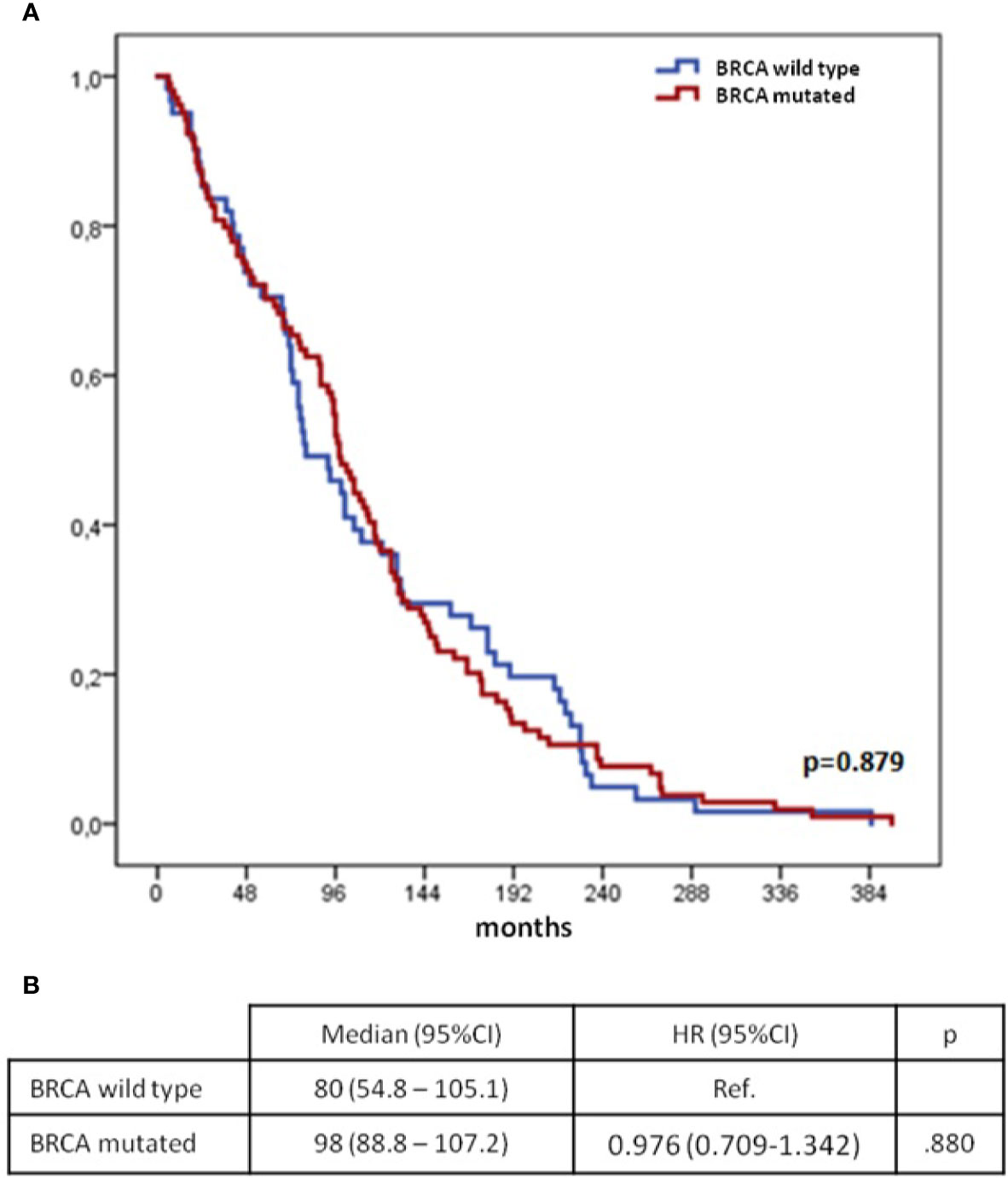
Figure 2 Time from 1st to 2nd cancer diagnoses according to mutational status of BRCA genes (synchronous group excluded): Kaplan–Meier curves (A) and Cox regression model (B) BC, breast cancer; OC, ovarian cancer; CI, confidential interval; H, hazard ratio; OS, overall survival.
Overall Survival Analysis
The median duration of follow-up in the entire cohort of patients was 16 years (95%CI 14.8–17.2) from the first diagnosis and 7.1 years (95%CI 5.6–8.5) from the second; for the BC first group, 16.3 years (95%CI 14.8–17.8) and 6.4 years (95%CI 4.8–8.1); for the OC first group, 16.3 years (95%CI 10.4–22.1) and 8.5 years (95%CI 5.9–11.1); for synchronous group 9.6 years (95%CI 4.6–14.5) and 9.6 years (95%CI 5.1–14.1). At the cut-off date, 105 patients (39.2%) had died. Patients more frequently died from OC-related consequences in all groups (Table 5).
Kaplan–Meier curves and Cox models for OS analyses from first and second diagnoses according to the sequence of cancer diagnoses are shown in Figure 3.
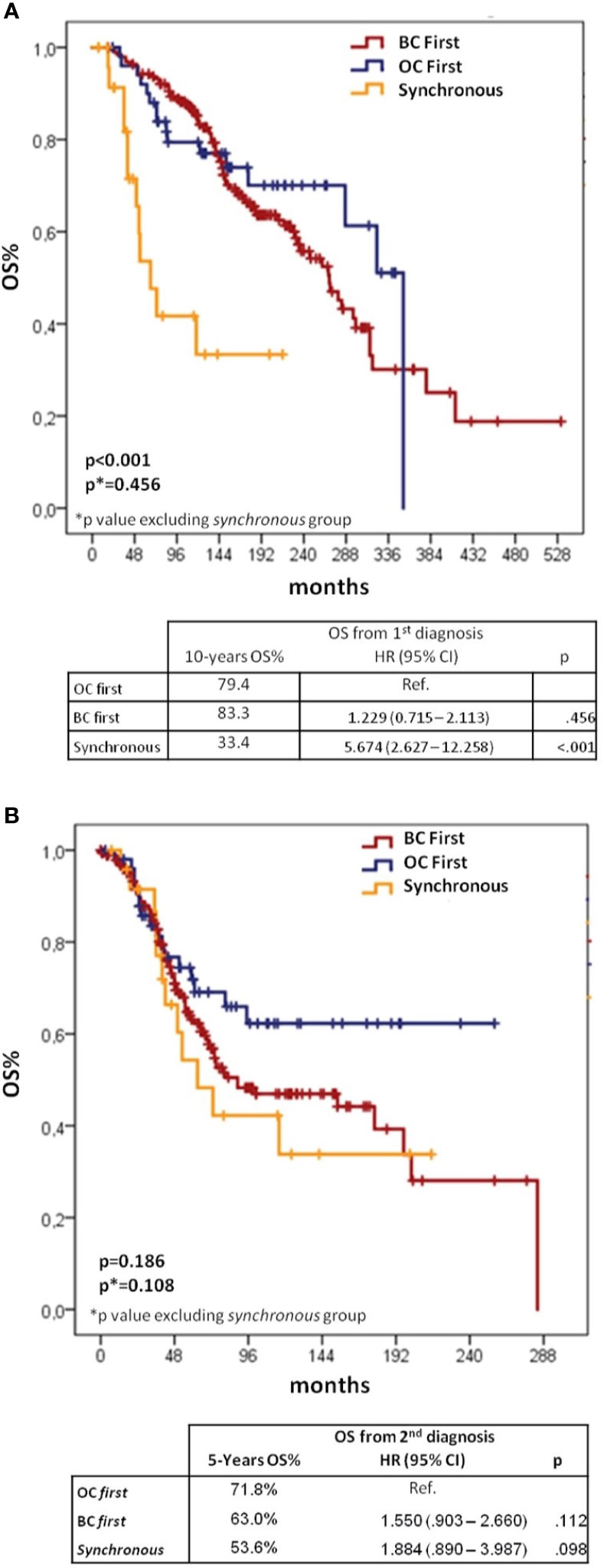
Figure 3 Kaplan–Meier and Cox regression for OS from 1st (A) and 2nd diagnoses (B) according to BC/OC diagnosis sequence. BC, breast cancer; OC, ovarian cancer; CI, confidential interval; H, hazard ratio; OS, overall survival.
When considering OS from first cancer diagnosis, 10-yr survival rates were 83.3% for BC first, 79.4% for OC first and 33.4% for synchronous groups (log-rank p < 0.001 overall, p = 0.456 for the comparison between BC first and OC first). The Cox-regression model showed significantly worse OS for synchronous vs OC first group (HR 5.674, 95%CI 2.627–12.258, p < 0.001), but no difference between BC first and OC first groups (HR 1.229, 95%CI 0.715–2.113, p = 0.456).
When assessing OS from the second diagnosis, the OC first group showed the best outcome with a 5-yr survival rate of 71.8%, followed by the BC first group (63.0%) and the synchronous group (53.6%), although differences between groups were not statistically significant.
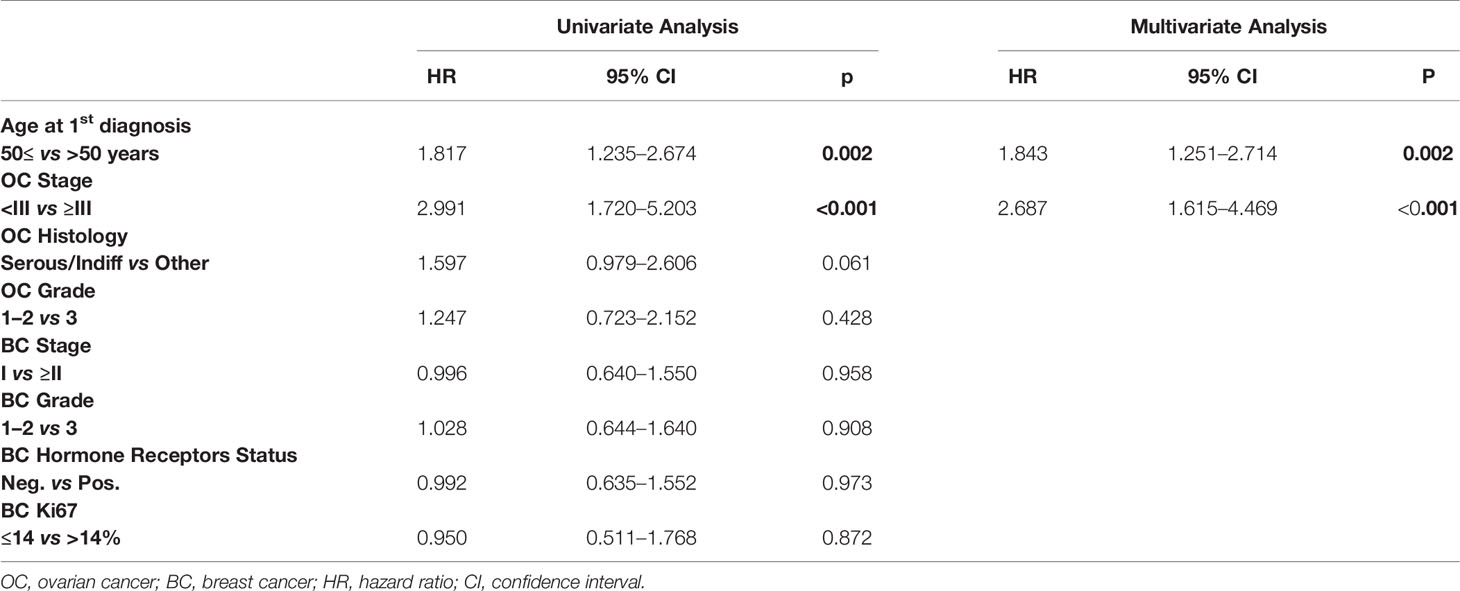
Prognostic Factors for Overall Survival
We investigated other potential prognostic factors for OS after first cancer onset, Table 6 shows univariate and multivariate analyses. Older age (>50 years) at first diagnosis and advanced OC (FIGO stage ≥III) proved to be independent prognostic factors of poorer survival. Comparing BRCA mutation carriers and wild type patients we found no difference in OS from the first diagnosis (10-yr OS of 88.2 and 86.7%, respectively; log-rank p = 0.307) (Figure 4).
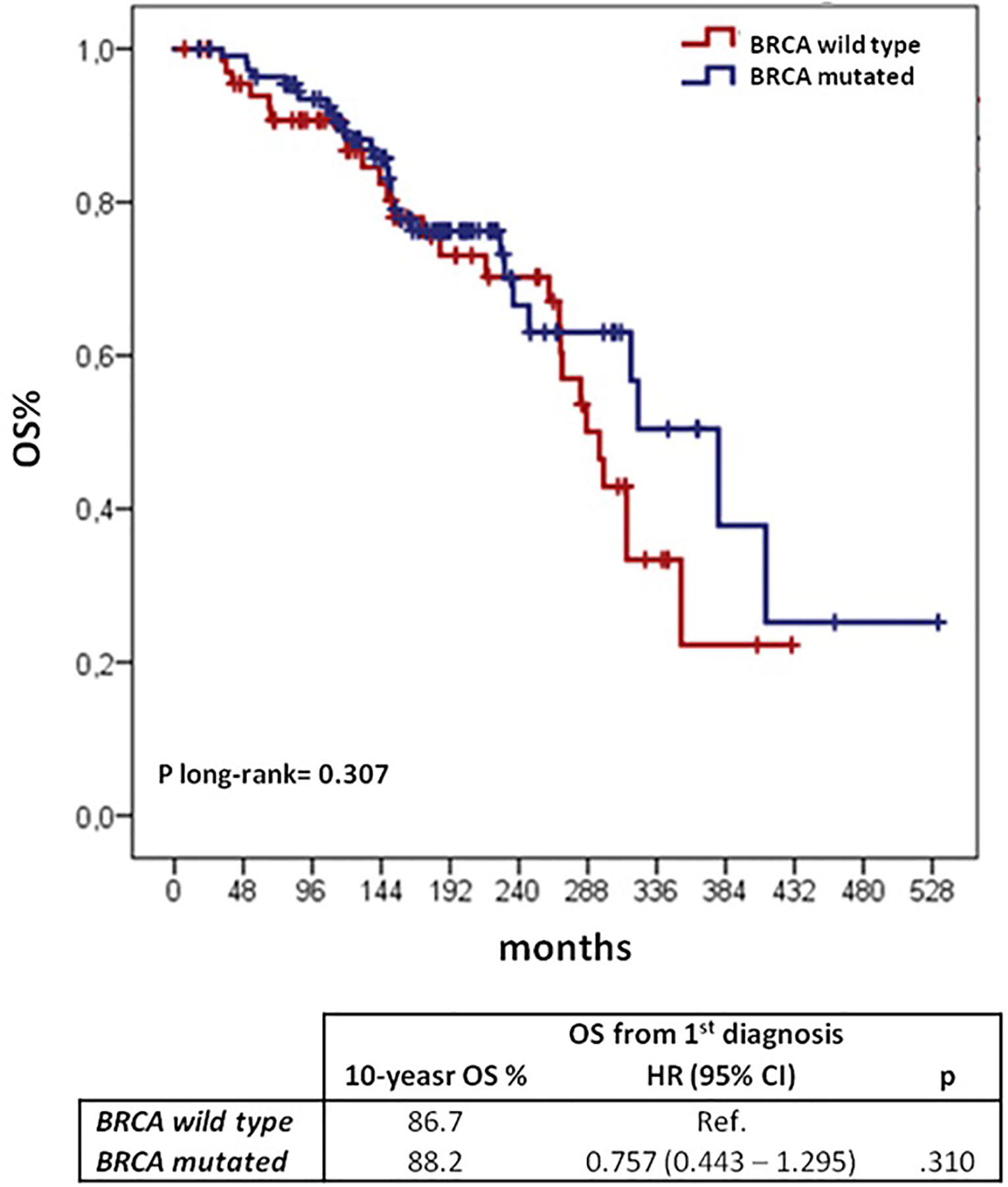
Figure 4 OS from the 1st cancer diagnosis according to BRCA mutational status: Kaplan–Meier curves and Cox regression model. BC, breast cancer; OC, ovarian cancer; CI, confidential interval; H, hazard ratio; OS, overall survival.
Discussion
There is limited information in the literature regarding clinical presentation and outcome of patients with synchronous or metachronous OC and BC. This is the second largest cohort, following that reported by Liou et al. in 2006. The majority of our cohort was represented by patients diagnosed with BC followed by OC (72%), consistently with other data (14). Up to 68.5% of patients with available information on BRCA status in this group harbored a BRCA mutation, as compared to 41% of OC first and 47% of synchronous patients. The different prevalence of BRCA mutated patients, especially BRCA1 mutated, may account for some of the differences observed in clinico-pathological characteristics between the groups, with BC first patients showing younger age at 1st and 2nd diagnoses, more aggressive OC features and a higher prevalence of TNBC.
A previous study also described aggressive OC features in patients diagnosed with BC followed by OC (14).
The interval between the two diagnoses was significantly longer in the BC first group as compared to the OC first group. These data are in contrast with previous findings. Olawaiye A et al. found a significantly longer time interval in women who developed OC before BC (7 vs 4 years), but the sample size was limited (49 patients only) (15). To the other hand, Liou et al. did not show any difference in the time from first to second diagnoses (14).
In our cohort, BC first patients presented younger age at both first and second diagnosis as compared to other groups; however, the magnitude of diagnosis anticipation, appeared larger for BC diagnosis than for OC cancer diagnosis, possibly justifying the observation of a longer time to second diagnosis. Indeed, it is well recognized that BRCA carriers, who were more represented in the BC first group, may experience BC at very young age (16). Despite the longer time to second diagnosis in BC first group, we did not detect any difference in OS from first diagnosis between BC first and OC first groups. In the work by Liou et al., where no difference in interval between the two diagnoses was observed, BC first group had a poorer survival from first diagnosis than women in OC first group (14). Data from our work and the one by Lious et al.’s are consistent with the assumption that the subsequent OC diagnosis in BC first group is the main determinant of OS. Indeed, patients in this group had poor prognostic clinico-pathological OC characteristics and OC-related deaths, although being the most frequent death cause in the whole population, accounted for 72.7% of the OS events in BC first patients as compared to 56.3% of the OS events in the OC first group. Therefore, in the BC first group, the potential favorable effect of a long time interval between the two diagnoses was somehow neutralized by the poor prognosis that these patients experienced after OC diagnosis.
Several studies have reported more favorable survival outcomes among BRCA mutated OC patients compared with patients affected by sporadic OC (17–19). Interestingly, Zaaijer LH et al. observed a worse outcome for BRCA carriers vs BRCA non-carriers among patients diagnosed with OC after BC (20). In our paper, we did not find any difference in OS between BRCA mutated and wild type patients with both breast and ovarian cancer. Our data together with those of Zaaijer LH et al., suggest that the evidence of a better prognosis of BRCA mutated patients with OC might not be confirmed in cases with a metachronous BC. Of course, our cohort goes back to a pre-PARPi era and we can assume that because of their high activity in BRCA mutated OC patients they could overwhelm this unfavorable prognostic factor. On the other hand, it will be interesting to observe if after the introduction of PARPi for OC treatment, there will be an increase of BC metachronous diagnoses because of the prolonged survival or if, on the contrary, they could have a sort of “chemo-preventive” effect, reducing the incidence of breast cancer after ovarian cancer.
As already discussed, in our series survival is dominated by OC related mortality. These findings can be useful for adequately counsel BRCA mutated patients with a first diagnosis of breast cancer or ovarian cancer on how to balance potential benefits and harms of subsequent preventive measures. Appropriate surveillance and prophylactic oophorectomy are recommended for BC survivors with BRCA mutation. In case of a first diagnosis of BC in patient with a BRCA mutation who has not yet undergone prophylactic oophorectomy, our data strongly support to recommend this procedure, since subsequent OC was the main determinant of overall survival. This is particularly relevant if we consider that survival of breast cancer patients is constantly improving over time thanks to surveillance and advances in systemic therapies (20). With regard to the optimal timing of prophylactic oophorectomy after a BC diagnosis in a BRCA mutated patient, our data support the same timing as in healthy BRCA carriers. Indeed, the age at OC diagnosis in patients with a previous BC in our study is consistent to the age of ovarian cancer diagnosis in BRCA mutated carriers. On the other hand, counseling in patients with BRCA-associated OC is more complex, because it should address not only the subsequent risk of BC but also the consideration of this risk against the OC prognosis. In our study the rate of BRCA mutated patients was the lowest in the OC first group; this observation is consistent with the results of previous studies, showing that metachronous BC in BRCA carriers with previous OC is infrequent, occurring in around 10% of the patients. Moreover, the same studies also confirmed that survival of this patients is dominated by OC (21, 22). McGee J et al. recently showed that in BRCA mutation carrying patients diagnosed with stage III/IV OC, the chance of dying for all causes was reduced by less than 1% with breast MRI and by less than 2% with mastectomy. The benefits of more aggressive preventive measures, as prophylactic bilateral mastectomy or intensive radiological surveillance, are expected to be small in terms of lives saved in particular in presence of poor prognostic factors like age >50 years and OC ≥III FIGO stage (23). An adequate counseling should account for these aspects.
Finally, the importance of offering genetic test to patients at risk of BRCA1/2 mutation should be underlined. Since the introduction of the BRCA genetic test until recently, the main criteria to allow access to the test was based on family history. This in part explains why in our cohort of patients BRCA status was not available for all patients, and when performed, genetic test occurred most frequently after the second cancer diagnosis. More recently, genetic test eligibility criteria have been expanded. With regard to OC patients, the observation that about 12% of patients with high-grade serous OC were mutated unless there was a family history (24, 25) led to recommend the test for all patients with this diagnosis, irrespectively of familial history. With regard to BC, newly introduced criteria include patients with TNBC diagnosed at the age of 60 or younger, irrespectively of familial history (26, 27).
In conclusion, our study reports data from a large cohort of patients with synchronous and metachronous BC and OC diagnosed in a time span covering recent years. Our data may be useful in order to plan and carry out adequate and timely surveillance programs and preventive measures.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
GT acquired the data, analyzed and interpreted the data, and wrote the original draft. MD developed the methodology, analyzed and interpreted the data, and reviewed the manuscript. ZB, GF, MM, MN, and FP provided the resources. VG conceptualized and designed the study, developed the methodology, reviewed the manuscript, and supervised the study. NC supervised the study and provided the resources. All authors contributed to the article and approved the submitted version.
Conflict of Interest
GT has received fees from AstraZeneca, Tesaro and GSK for consultancy role and participation on advisory boards. MD has received fees from EliLilly for consultancy role and participation on advisory boards, fees from Genomic Health for consultancy role, fees from Celgene for participation on advisory. FP has received remuneration from Ipsen and fees from Roche and AstraZeneca for consultancy role and participation on advisory boards. VG has received fees from EliLilly, Roche, and Novartis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin (2015) 65(2):87–108. doi: 10.3322/caac.21262
2. Curtis RE, Boice JD Jr, Kleinerman RA, Flannery JT, Fraumeni JF Jr. Summary: multiple primary cancers in Connecticut, 1935-82. Natl Cancer Inst Monogr (1985) 68:219–42.
3. Sankila R, Pukkala E, Teppo L. Risk of subsequent malignant neoplasms among 470,000 cancer patients in Finland, 1953-1991. Int J Cancer (1995) 60(4):464–70. doi: 10.1002/ijc.2910600407
4. Bergfeldt K, Nilsson B, Einhorn S, Hall P. Breast cancer risk in women with a primary ovarian cancer–a case-control study. Eur J Cancer (2001) 37(17):2229–34. doi: 10.1016/S0959-8049(01)00282-9
5. Prior P, Waterhouse JA. Multiple primary cancers of the breast and ovary. Br J Cancer (1981) 44(5):628–36. doi: 10.1038/bjc.1981.247
6. Lynch HT, Krush AJ. Carcinoma of the breast and ovary in three families. Surg Gynecol Obstet (1971) 133(4):644–8.
7. Lynch HT, Krush AJ, Lemon HM, Kaplan AR, Condit PT, Bottomley RH. Tumor variation in families with breast cancer. JAMA (1972) 222(13):1631–5. doi: 10.1001/jama.222.13.1631
8. Hall JM, Lee MK, Newman B, Morrow JE, Anderson LA, Huey B, et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science (1990) 250:1684–9. doi: 10.1126/science.2270482
9. Kuchenbaecker KB, Hopper J, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA (2017) 317(23):2402–16. doi: 10.1001/jama.2017.7112
10. Adami HO, Hsieh CC, Lambe M, Trichopoulos D, Leon D, Persson I, et al. Parity, age at first childbirth, and risk of ovarian cancer. Lancet (1994) 344(8932):1250–4. doi: 10.1016/S0140-6736(94)90749-8
11. Lambe M, Hsieh CC, Chan HW, Ekbom A, Trichopoulos D, Adami HO. Parity, age at first and last birth, and risk of breast cancer: a population-based study in Sweden. Breast Cancer Res Treat (1996) 38(3):305–11. doi: 10.1007/BF01806150
12. Riman T, Persson I, Nilsson S. Hormonal aspects of epithelial ovarian cancer: review of epidemiological evidence. Clin Endocrinol (Oxf) (1998) 49(6):695–707. doi: 10.1046/j.1365-2265.1998.00577.x
13. Norquist BM, Herrel M, Brady MF. Inherited mutation in women with ovarian carcinoma. JAMA Oncol (2016) 2(4):482–90. doi: 10.1001/jamaoncol.2015.5495
14. Liou WS, Hamilton CA, Cheung MK, Osann K, Longacre TA, Teng NN, et al. Outcomes of women with metachronous breast and ovarian carcinomas. Gynecol Oncol (2006) 103(1):190–4. doi: 10.1016/j.ygyno.2006.02.022
15. Olawaiye A, Caesar L, Walsh D, Lyman M, Yeh J, Rodabaugh K, et al. Analysis of the time interval between diagnoses in women with double primary breast and ovarian or primary peritoneal cancers. Gynecol Oncol (2004) 94(3):796–802. doi: 10.1016/j.ygyno.2004.06.031
16. Mavaddat N, Barrowdale D, Andrulis IL, Domchek SM, Eccles D, Nevanlinna H, et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev (2012) 21(1):134–47. doi: 10.1016/j.yobg.2012.05.049
17. Vencken PM, Kriege M, Hoogwerf D, Beugelink S, van der Burg ME, Hooning MJ, et al. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann Oncol (2011) 22(6):1346–52. doi: 10.1093/annonc/mdq628
18. Tan DS, Rothermundt C, Thomas K, Bancroft E, Eeles R, Shanley S, et al. “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol (2008) 26(34):5530–6. doi: 10.1200/JCO.2008.16.1703
19. Boyd J, Sonoda Y, Federici MG, Bogomolniy F, Rhei E, Maresco DL, et al. Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA (2000) 283(17):2260–5. doi: 10.1001/jama.283.17.2260
20. Zaaijer LH, van Doorn HC, Mourits MJ, van Beurden M, de Hullu JA, Adank MA, et al. Outcome of ovarian cancer after breast cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer (2016) 115(10):1174–8. doi: 10.1038/bjc.2016.333
21. Gangi A, Cass I, Paik D, Barmparas G, Karlan B, Dang C, et al. Breast cancer following ovarian cancer in BRCA mutation carriers. JAMA Surg (2014) 149(12):1306–13. doi: 10.1001/jamasurg.2014.1081
22. Domchek SM, Jhaveri K, Patil S, Stopfer JE, Hudis C, Powers J, et al. Risk of metachronous breast cancer after BRCA mutation-associated ovarian cancer. Cancer (2013) 119(7):1344–8. doi: 10.1002/cncr.27842
23. McGee J, Giannakeas V, Karlan B, Lubinski J, Gronwald J, Rosen B, et al. Risk of breast cancer after a diagnosis of ovarian cancer in BRCA mutation carriers: Is preventive mastectomy warranted? Gynecol Oncol (2017) 145(2):346–51. doi: 10.1016/j.ygyno.2017.02.032
24. Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet (2001) 358(9291):1389–99. doi: 10.1016/S0140-6736(01)06524-2
25. Schrader KA, Hurlburt J, Kalloger SE, Hansford S, Young S, Huntsman DG, et al. Germline BRCA1 and BRCA2 mutations in ovarian cancer: utility of a histology-based referral strategy. Obstet Gynecol (2012) 120(2 Pt 1):235–40. doi: 10.1097/AOG.0b013e31825f3576
26. Tun NM, Villani G, Ong K, Yoe L, Bo ZM. Risk of having BRCA1 mutation in high-risk women with triple-negative breast cancer: a meta-analysis. Clin Genet (2014) 85(1):43–8. doi: 10.1111/cge.12270
27. Fostira F, Tsitlaidou M, Papadimitriou C, Pertesi M, Timotheadou E, Stavropoulou AV, et al. Prevalence of BRCA1 mutations among 403 women with triple-negative breast cancer: implications for genetic screening selection criteria: a Hellenic Cooperative Oncology Group Study. Breast Cancer Res Treat (2012) 134(1):353–62. doi: 10.1007/s10549-012-2021-9
Keywords: metachronous cancer, synchronous cancer, breast cancer, ovarian cancer, doublet tumors, BRCA mutation
Citation: Tasca G, Dieci MV, Baretta Z, Faggioni G, Montagna M, Nicoletto MO, Peccatori FA, Guarneri V and Colombo N (2020) Synchronous and Metachronous Breast and Ovarian Cancer: Experience From Two Large Cancer Center. Front. Oncol. 10:608783. doi: 10.3389/fonc.2020.608783
Received: 21 September 2020; Accepted: 16 November 2020;
Published: 14 December 2020.
Edited by:
Michael Gnant, Medical University of Vienna, AustriaReviewed by:
Georg Pfeiler, Medical University of Vienna, AustriaMauro Giuseppe Mastropasqua, University of Bari Medical School, Italy
Copyright © 2020 Tasca, Dieci, Baretta, Faggioni, Montagna, Nicoletto, Peccatori, Guarneri and Colombo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valentina Guarneri, dmFsZW50aW5hLmd1YXJuZXJpQHVuaXBkLml0
 Giulia Tasca
Giulia Tasca Maria Vittoria Dieci
Maria Vittoria Dieci Zora Baretta3
Zora Baretta3 Marco Montagna
Marco Montagna Fedro Alessandro Peccatori
Fedro Alessandro Peccatori