- 1Department of Radiation Oncology, Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia, PA, United States
- 2Department of Radiation Oncology, Dana Farber Cancer Institute, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
Radiotherapy (RT) is an effective method of cancer treatment, but like any other method of cancer treatment, there are inherent limitations. While technological advances and a growing understanding of its biological effects have improved its results dramatically, the use of RT is still limited to certain patient populations and by normal tissue toxicities. The harmful side effects of treating patients with radiation can offset its therapy benefits, limiting its use in certain cases. Phyto, or plant-based, medicines offer a way to add to radiation treatment, while also protecting patients from its toxic side effects. Phytomedicines such as cannabinoids (CBD) and bitter melon extract have demonstrated therapeutic properties, including the ability to activate apoptotic death in cancer cells, diminish tumor progression, and generally decrease the incidence of several cancer types. In addition, herbal drugs have been shown to be powerful antioxidants with the ability to decrease toxicity of RT without the adverse side effects found in synthetic drugs. Furthermore, a number of phytomedicines have been shown to mitigate hypoxic conditions within the tumor microenvironment, creating a more radiosensitive disease and preventing tumorigenesis. The purpose of this article is to examine the merits and demerits of employing phytomedicines during RT. Results from studies that have tested the effects of combining radiotherapy with supplemental herbal treatment are discussed along with perspectives on where additional research is needed to advance “Phytoradiotherapy”. Overall, experimental evidence points to the fact that phytomedicines have significant potential to enhance RT, with need for cross-disciplinary collaborations to establish optimal dosing combinations with evidence-base for clinical translation.
Introduction
Phytoradiotherapy is the integration of radiotherapy (RT) with phyto- or plant-based medicines to enhance cancer treatment. Phytomedicines have potential to not only enhance the therapeutic effects of radiotherapy but mitigate the damaging side effects as well.
Phytomedicines are plant-based pharmaceuticals or extracts used to treat different ailments. 2019 data taken from the World Heath Organization (1) shows over 60% of people around the globe rely on natural herbs to provide themselves and their families with healthcare. With an increasing number of publications supporting the effectiveness of phytomedicines in oncology and their antioxidant potential, it is hypothesized that combining them with radiotherapy can significantly impact the overall survival of cancer patients, and their quality of life. This constitutes the cross disciplinary area of Phytoradiotherapy, combining radiotherapy with phyto- or plant-based medicines to enhance cancer treatment and/or minimize toxicities.
Conservative estimates from recent years indicate that more than 50% of cancer patients can benefit from radiation therapy (2) which has been playing an increasingly important role in cancer treatment. In spite of its effectiveness and widespread adoption, radiation therapy use for curative treatment of certain cancers is limited. This is largely due to cancers not being found until they are late stage, as well complex patient heterogeneity. In addition, radiation therapy can lead to secondary cancers later in a patient’s life as well as cause harmful side effects during and after treatment.
In this article, we first examine the merits of using phytomedicines such as cannabinoids in oncology. This is followed by a review of work in the emerging area of Phytoradiotherapy, with perspectives on the promise and limitations of this approach. Future directions and perspective on key areas for research to justify clinical translation and adoption are discussed.
Phytomedicine
Phytomedicines, medicinal plants for prevention and treatment of disease, are of growing importance worldwide (3). For so many of the 4 billion across the world who use phytomedicine, it is their mainstay of health care delivery, and demand for it is increasing. Phytomedicine of proven quality, safety, and efficacy, contributes to the World Health Organization’s global health priority of ensuring that all people have access to quality healthcare. However, the use of phytomedicines in cancer treatment is largely driven by anecdotal evidence (4), and is limited by poor bioavailability. Nature has, in fact, been making biologically active compounds in plants for billions of years. Examples of medicines from plants that have a major impact globally include: morphine from opium Poppy (Papaver somniferum), aspirin from the white willow tree (Salix alba vulgaris), and the anticoagulant coumadin from spoiled sweet clover (Melilotus species). Tropical plants such as the Madagascar Periwinkle (Catharanthus roseus) have yielded vinblastine, which has revolutionized the treatment of Hodgkin’s lymphoma, and vincristine, which has done same for acute childhood leukemia.
Over 33 states in the USA, Canada, and a growing number of countries have now legalized medical cannabis, with studies showing promising approaches for use of cannabinoid and flavonoid components of medical Cannabis in oncology (4, 5). The National Institutes of Health, and World Health Organization encourage research on medical cannabis for treating different diseases including cancer, cancer pain, and managing the side effects of other treatments such as radiotherapy or chemotherapy. Besides Cannabis, it is estimated that of the 300,000 plant species that exist in the world, only 15% have been evaluated to determine their pharmacological potential.
While some studies have not demonstrated plant-based herbs as effective forms of medicine, especially in oncology (6, 7), there are a plethora of studies, that support phytomedicines as a valuable form of medicine. With many reported studies on natural herbs use in the treatment of cancer and other disease, there is impetus for more research in this area. In addition, the delivery method of phytomedicines may determine their efficacy. Often, modes of phytomedicine delivery like oral administration or intravenous injection may limit their efficacy, even if they demonstrate efficacy in-vitro. Recent advances in technology have led to the development of smart biomaterials that can enable targeted controlled delivery of phytomedicines to their intended targets with sustained bioavailability (8). Experimentations with these materials show their ability to dramatically improve the effects of their phytomedicine payloads. For example, in-vivo studies in mice have shown that smart biomaterials can be developed to administer cannabinoids in combination with radiation therapy for enhanced therapeutic efficacy (9).
Phytomedicines also have palliative applications as in managing pain and have the benefit of being accessible and less expensive compared to other therapeutics as opioids. Cancer treatment can be a very costly endeavor introducing huge amounts of financial pressure on patients. At the University of Pennsylvania, photon intensity modulated radiotherapy (IMRT) and Proton Therapy treatments can cost approximately 20,000 and 30,000 dollars, respectively. It was cited that certain institutions report the cost of their IMRT treatment plans as costing up to over 100,000 US dollars (10). Besides radiotherapy, drugs given to cancer patients are accompanied by an overwhelming price tag. For example, the drug ipilimumab was approved in 2015 by the FDA to help treat metastatic melanoma after it showed the ability to prolong survival of patients by an average of approximately 1.6 months (10). Just four doses of the drug cost 120,000 dollars. Unfortunately, this is not an anomaly; Cancer drugs including Sipuleucel-T, Bevacizumab, and more cost over 80,000 dollars for just a few doses (11). The high cost of such drugs is just one reason the economic burden of cancer in the US is expected to increase substantially in the future. Coupled with the growing population and upward trends in costs of diagnosis/care call attention to finding ways that can help reduce expenses (12). Overall, the tremendous cost of modern-day cancer treatment can be a difficult obstacle to overcome, especially for patients in low/middle income (LMIC) countries. With life expectancy and world population rising, the cancer burden throughout the globe is growing. In many LMICs, phytomedicines constitute a major part of the healthcare system, and it is worth considering evidence-based cost-effective approaches that integrate phytomedicines, which may enhance therapeutic efficacy or enhance safety.
Data reported in the National Business Journal (13) show the growing market in the United States for herbal supplements. From an economic perspective, there may also be benefits for increased adoption of evidence-based phytomedicines in oncology in the USA. The economic impact of phytomedicines is three-fold: reducing the cost of cancer treatment, providing affordable care for patients in developing countries, and boosting the economies of both developing and developed nations.
In a paper reviewing the cost effectiveness of adding phytomedicine to chemotherapy, the cost per annum of phytomedicine per patient was estimated to about $1,000 in India (14). According to Cancer.gov most cancer drugs launched between 2009 and 2014 cost over $100,000 per patient for one year of treatment. It is important to note that the quote of $1,000 for a year’s worth of phytomedicine can be misleading. These two prices are comparing drugs that do not have identical effects and are not used in identical cases therefore, the discrepancy in these two prices is less significant. However, the clear conclusion is that phytomedicines are substantially less costly to patients than synthetic drugs.
Prevalent poverty and limited resources make it difficult to protect the health of citizens (12). Interestingly, many of the plants desirable to be used in medication are found in places near developing countries. The harvesting and sale of such plants delivers economic opportunity. Global statistics indicate that the importance of medicinal plants or plant-based pharmaceuticals is continuously growing (15). For example, in Pakistani communities many citizens often earn additional income by collecting and selling plants used in herbal medicine (16). Further development of phytomedicines as an integral part of cancer treatment will help emphasize these markets in areas like Pakistan. Developing countries can harness these markets and export Phytopharmaceuticals improving their healthcare system and economy. Rwanda, and Uganda are two examples in Africa that have recently approved growth and export of Medical Cannabis. The primary benefit of phytomedicines, however, will be its effectiveness in the clinic.
Phytoradiotherapy
Combining RT and phytomedicines will have the greatest potential impact in improving cancer treatment by (1) enhancing the therapeutic efficacy of RT and (2) minimizing the harmful side effects associated with RT.
Enhancing Therapeutic Efficacy
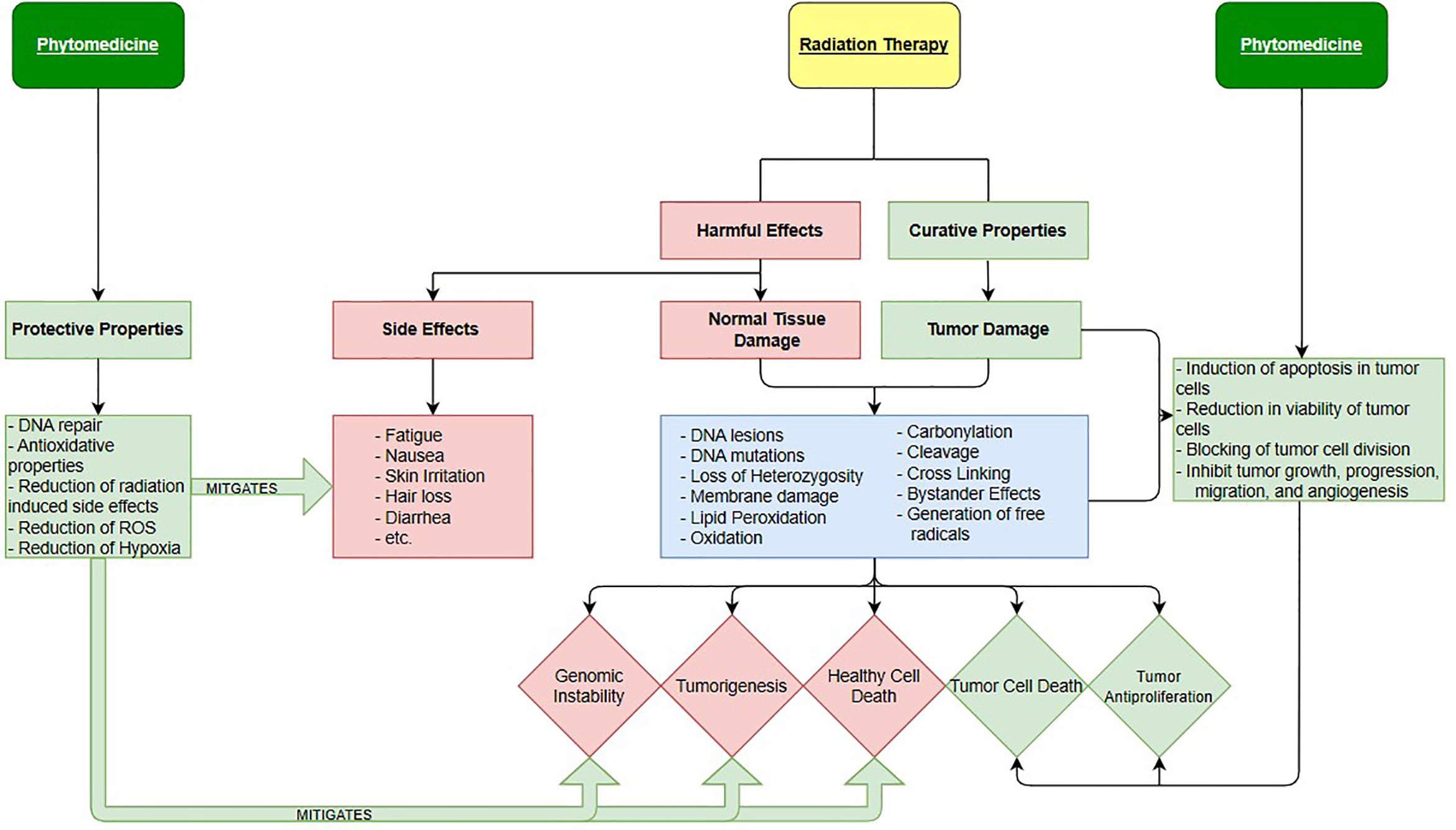
In human cancers, a deficiency in the amount of oxygen reaching tissues, or hypoxia, is common and leads to an increase probability of mortality. Under these conditions, Hypoxia inducible factors (HIF) mediate the body’s physiological response (17). In tumors, HIF transcription is heightened so that cancer cells can survive in hypoxic conditions where healthy cells would have normally died. In addition, in environments lacking oxygen, ionizing radiation is far less lethal, meaning that radiotherapy is less effective. Hypoxia is one of the most important causes of radiotherapy failure due to the hypoxic cells being immune to the reactive oxygen species created by incident ionizing radiation (18, 19). Anemia is a condition in which the victim lacks a sufficient number of healthy red blood cells to adequately supply the body with oxygen (20–23). Since anemia brings on hypoxia-like conditions and it is often a symptom of cancer it presents a serious problem for those who rely on radiation therapy for a cure. There is growing evidence describing how phytomedicines can help reduce the hypoxic resistance of cancer cells by reducing the transcription of the Hypoxia-Inducible factors. This could explain why anti-cancerogenic phytomedicines like Cannabidiol (24–28) displayed synergistic effects against cancer cells in vitro. Studies showing the anti-cancer effects of cannabinoids (29) and their ability to manage cancer related symptoms (25, 29) are plentiful. In fact, there are even a few isolated studies, showing the effectiveness of using cannabinoids (CBD) and other phytomedicines with radiation therapy together. In addition to CBD, some other specific plant-based medicines with therapeutic potential include Bitter Melon Extract, Picrorhiza kurrooa, Justica (20), and many more. Schematic overview of integration of radiation therapy and phytomedicines is shown in Figure 1.
Minimizing the Harmful Effects of Radiotherapy (Antioxidant Properties)
Ionizing radiation mostly causes direct cell killing by damaging the cell’s DNA. Once the DNA is damaged with double-strand breaks beyond correct repair, the cell loses its function and dies (30). Naturally, the aim of radiotherapy is to use ionizing radiation to kill tumor cells while avoiding any healthy cells. Such a goal is a lot easier said than done as the effects of ionizing radiation are dependent on several factors (31). Cells can also be killed indirectly by ionizing radiation due to hydroxyl free radicals which combine with other substances that lead to cell, tissue, and organ damage. The excess of these harmful free radicals created in the target areas of radiation therapy produce conditions that are medically described as “oxidative stress”, causing the onset of several different diseases. Of course, tumor cell death is a positive outcome, but in healthy tissue/organs the oxidative damage they cause can be detrimental (32). The DNA damage caused by oxidative stress can mutate genes leading to the development of cancer (33). These genes are known as Oncogenes and are often described as cancer stem cells.
Oncologists must consider a balance between the reduction/production of Reactive Oxygen Species (ROS) so that tumor cell killing is promoted while dangerous side effects are avoided. Substances with antioxidative properties can help control this balancing act. Many synthetic drugs have been developed to mitigate the toxic effects of these free radicals but introduce health problems of their own. One study from 1993 tested synthetic antioxidants Butylated hydroxyanisole (BHA) and Butylated hydroxytoluene (BHT) and found that they induce both blood clotting impairment and certain types of tumors in animal models. In contrast, the study showed no carcinogenic properties performing the same studies with Vitamin E being used as a natural antioxidant (34).
We see, in Figure 1, for radiotherapy the harmful effects and curative properties are one and the same, both involving the damaging of cells by the same mechanisms. The difference between the two is whether the ionizing radiation interacts with healthy or cancerous cells. However, normal tissues in the human body generally exhibit superior cell repair compared to cancerous cells, attributing to the modern success of radiation therapy. Besides DNA damage, carbonylation, cleavage, loss of Heterozygosity and other events also cause damage, if not death (35). The killing of healthy cells causes the three biological outcomes seen as red diamonds while killing of cancerous cells creates auspicious results represented as the two green diamonds. Notice that the effects of phytomedicines (when used correctly) are only colored green indicating that they only introduce positive effects into cancer treatment. This is not to imply that phytomedicine are never toxic. Certain phytomedicines can be very harmful and even medicinal ones can have toxic effects if a wrong preparation or dose is used. On the right side, we see phytomedicines therapeutic properties at work enhancing tumor cell damage while not contributing to any significant healthy cell death. On the left, the radio-protective properties of phytomedicines are displayed. The protective properties that plant-based pharmaceuticals possess act to mitigate toxic side effects of radiation therapy while also limiting healthy cell death.
In 2016, a study on Plant Derived Antioxidants in Disease Prevention (36) showed that phytomedicines act successfully as suppressors of oxidation and suggested they should be used in the future as medicinal agents. Extracts from several plants including Green Tea (6, 37–39), Bitter Melon (40, 41) and more display remarkable therapeutic properties. Extracts from these plants were also seen to inhibit growth of malignant melanoma cancers and kill Leukemia cells. The anti-tumorigenesis properties of plants like these would not only enhance a patient’s radiation therapy directly by promoting tumor control, but also minimize side effects, with their antioxidative properties, that are often a contributing factor to an unsuccessful radiotherapy treatment. Table 1 organizes a fraction of the evidence from reviewed literature on using phytomedicines to treat a variety of cancer sites. From the information in this table, we can see a variety of phytomedicines used to treat different sites. For example, Vincea rosea and Pargaum harmala L. were used effectively against cervical cancers while herbs such as Curcumin and Stephania Tetranda were used to battle brain cancers.
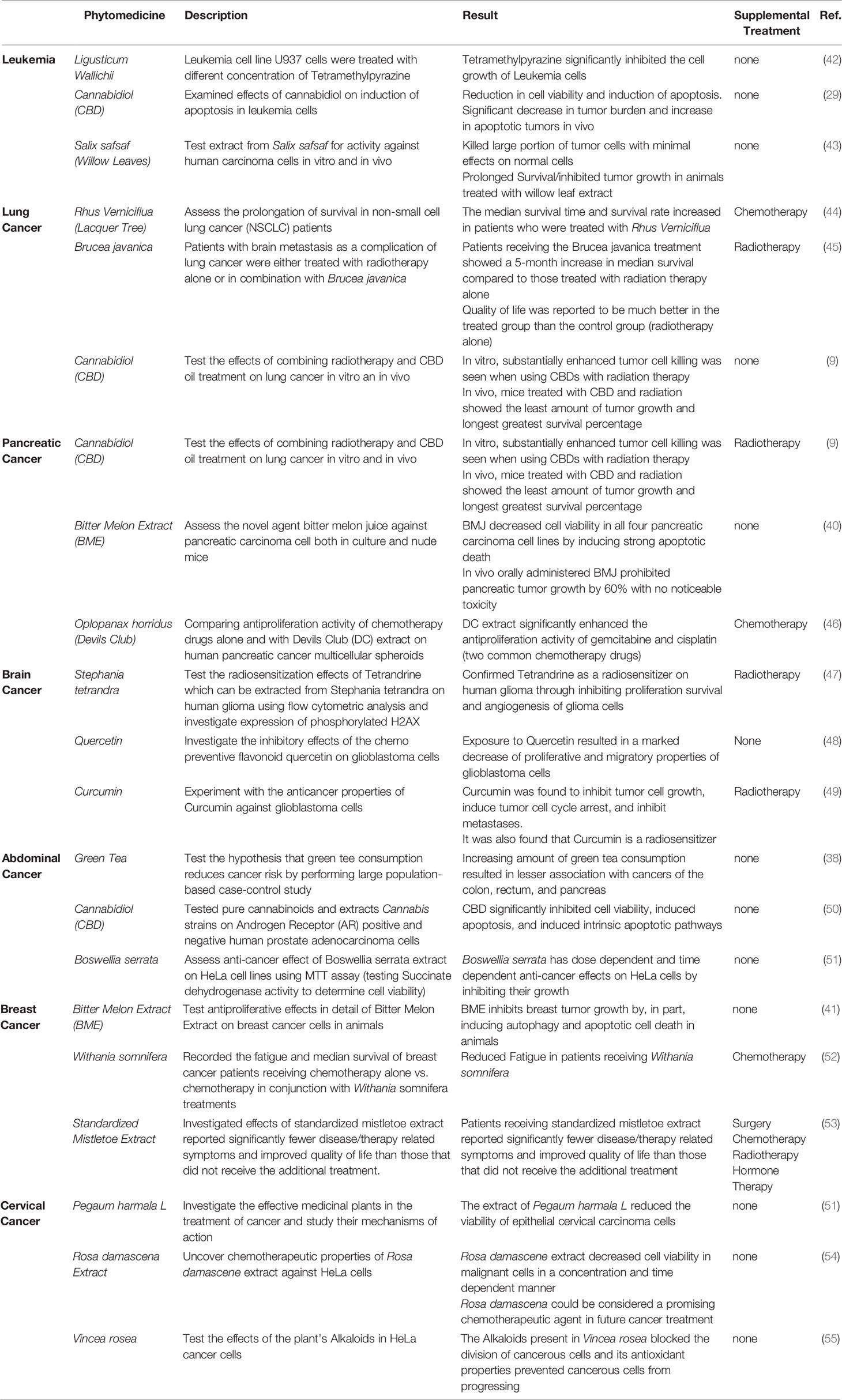
Table 1 Anticancer Properties of Phytomedicines for the Treatment of Leukemia, Pancreatic, Lung, Abdominal, Brain, Cervical and Breast Cancers.
Discussion and Future Perspective
Natural herbs and plants have been an integral part of medicine throughout all human history and despite a relatively recent shift towards more western medicines, many areas of the world still rely heavily on these phytomedicines. The World Health Organization has estimated that 80% of the world population uses herbal or complementary medicine (56) and scientific evidence has already defended the hypothesis that combinations of traditional and western medicines improve prognosis of deadly cancers (57). Upon reviewing a plethora of experimental evidence showing a wide variety of phytomedicines being used to successfully treat different types of cancers, there is overwhelming support for their increased use as an effective part of treatment. This is why organizations like the National Institute of Health support further research on traditional medicines and integrative health practices (58). The organization has placed emphasis on finding ways to curb the crippling opioid problem that is currently devasting the United States of America (59) as well as enhancing pain management for patients suffering from agonizing ailments. Emerging from this line of thought is a promising way to revolutionize radiation therapy to manage a large portion of the world’s cancer. Phytomedicines have potential to not only effectively reduce side-effects and symptoms of cancer but boost the treatment itself.
The pursuit of Phytoradiotherapy is limited by the lack of clinical trials that integrate the two treatments. There is an overwhelming amount of literature discussing phytomedicines and radiotherapy separately, but not nearly enough has been done experimenting with the two used in combination. In addition, a deeper economic analysis should be employed before drawing conclusions about stimulating economic growth that is more quantitative rather than theoretical. More work in this regard, especially cross-disciplinary collaborations are required to draw evidence-based conclusions that will move Phytoradiotherapy forward.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
TA is a student researcher and first author. WN and SA are research mentors and collaborators.WN also contributed funding towards the study of the project. SY-K is a collaborator and has contributed data towards the use of phytomedicines. All authors contributed to the article and approved the submitted version.
Funding
This work was partially funded by the Department of Radiation Oncology Summer Research Grant at the University of Pennsylvania and the Department of Radiation Oncology at Brigham and Women’s Hospital of Harvard University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. World Health Organization. WHO Global Report on Traditional and Complementary Medicine. World Health Organization (2019). Available at: https://apps.who.int/iris/bitstream/handle/10665/312342/9789241515436eng.pdf?ua=1.
2. Rosenblat E, Zubizzarreta E. Radiotherapy in Cancer Care:Facing the Global Challenge. International Atomic Energy Agency (2017). doi: 10.1016/S0168-8278(94)80112-6.
3. Rashrash M, Schommer JC, Brown LM. Prevalence and Predictors of Herbal Medicine Use Among Adults in the United States. J Patient Exp (2017) 4(3):108–13. doi: 10.1177/2374373517706612
4. Moreau M, Ibeh U, Decosmo K, Bih N, Yasmin-Karim S, Toyang N, et al. Flavonoid Derivative of Cannabis Demonstrates Therapeutic Potential in Preclinical Models of Metastatic Pancreatic Cancer. Front Oncol (2019) 9:66. doi: 10.3389/fonc.2019.0066
5. U.S. Food and Drug Administration. FDA And Cannabis: Research And Drug Approval Process. Food and Drug Administration (2020). Available at: https://www.fda.gov/news-events/public-health-focus/fda-and-cannabis-research-and-drug-approval-process.
6. Jatoi A, Ellison N, Burch PA, Sloan JA, Dakhil SR, Paul N, et al. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer (2003) 97(6):1442–6. doi: 10.1002/cncr.11200
7. Patel KR, Brown VA, Jones DJL, Britton RG, Hemingway D, Miller AS, et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res (2010) 70(19):7392–9. doi: 10.1158/0008-5472.CAN-10-2027
8. Kowalski PS, Bhattacharya C, Afewerki S, R L. Smart Biomaterials: Recent Advances and Future Directions. ACS Sci Eng (2018) 4(11):3809–17. doi: 10.1021/acsbiomaterials.8b00889
9. Yasmin-Karim S, Moreau M, Mueller R, Sinha N, Dabney R, Herman A, et al. Enhancing the therapeutic efficacy of cancer treatment with cannabinoids. Front Oncol (2018), 8(APR):4–11. doi: 10.3389/fonc.2018.00114
10. Rahman F, Seung SJ, Cheng SY, Saherawala H, Earle CC, Mittmann N. Radiation costing methods: A systematic review. Curr Oncol (2016) 23(4):e392–408. doi: 10.3747/co.23.3073
11. Siddiqui M, Rajkumar SV. The high cost of cancer drugs and what we can do about it. Mayo Clin Proc (2012) 87(10):935–43. doi: 10.1016/j.mayocp.2012.07.007
12. Yabroff KR, Lund J, Kepka D, Mariotto A. Economic Burden of Cancer in the US. Cancer Epidemiol Biomarkers Prev (2014) 10.11(301):1–18. doi: 10.1158/1055-9965.EPI-110650.Economic
13. Smith T, Kawa K, Eckl V, Morton C, Stredney R. Herbal Supplement Sales in US increased 8.5% in 2017, Topping $8 Billion. Am Bot Counc (2018) 119):62–71.
14. Chaudhary T, Chahar A, Sharma JK, Kaur K, Dang A. Phytomedicine in the treatment of cancer: A health technology assessment. J Clin Diagn Res (2015) 9(12):XC04–9. doi: 10.7860/JCDR/2015/15701.6913
15. Bukar BB, Dayom DW, Uguru MO. The Growing Economic Importance of Medicinal Plants and The Need For Developing Countries To Harness From it: A Mini Review. IOSR J Pharm www.iosrphr.org (2016) 6(5):2250–3013.
16. Sher H, Aldosari A, Ali A, de Boer HJ. Economic benefits of high value medicinal plants to Pakistani communities: An analysis of current practice and potential. J Ethnobiol Ethnomed (2014) 10(1):1–16. doi: 10.1186/1746-4269-10-71
17. Semenza GL. Targets for cancer therapy. Trends Pharmacol Sci (2012) 33(4):207–14. doi: 10.1016/j.tips.2012.01.005.Hypoxia-inducible
18. Wang H, Jiang H, Van De Gucht M, De Ridder M. Hypoxic radioresistance: Can ROS be the key to overcome it? Cancers (Basel) (2019) 11(1). doi: 10.3390/cancers11010112
19. Nagle D, Zhou Y-D. Natural Product-Based Inhibitors of Hypoxia-Inducible Factor-1 (HIF-1). Curr Drug Targets (2006) 7(3):355–69. doi: 10.2174/138945006776054979
20. Wood J, Yasmin-Karim S, Moreau M, Kumar R, Akwanwi J, Derek A, et al. Characterization of isolated extracts from Justicia plant leaves used as remedy for anemia. Molecules (2020) 25(3):1–10. doi: 10.3390/molecules25030534
21. Mason J, Martorell R, Saldanha L, Shrimpton R. Reduction of anaemia. Lancet Glob Heal (2013) 1(1):e4–6. doi: 10.1016/S2214-109X(13)70009-3
22. Kassebaum NJ, GBD 2013 Anemia Collaborators. The Global Burden of Anemia. Hematol Oncol Clin North Am (2016) 30:247–308. doi: 10.1016/j.hoc.2015.11.002
23. Balarajan Y, Ramakrishnan U, Ozaltin E, Shankar SVS AH. Anaemia in low-income and middle-income countries. Lancet (2011) 378:2123–35. doi: 10.1016/S0140-6736(10)62304-5
24. Velasco G, Sánchez C, Guzmán M. Anticancer mechanisms of cannabinoids. Curr Oncol (2016) 23(March):S23–32. doi: 10.3747/co.23.3080
25. Guzmán M. Cannabis for the management of cancer symptoms: THC version 2.0? Cannabis Cannabinoid Res (2018) 3(1):117–9. doi: 10.1089/can.2018.0009
26. Dariš B, Verboten MT, Knez Ž, Ferk P. Cannabinoids in cancer treatment: Therapeutic potential and legislation. Bosn J Basic Med Sci (2019) 19(1):14–23. doi: 10.17305/BJBMS.2018.3532
27. Massi P, Vaccani A, Ceruti S, Colombo A, Abbracchio MP, Parolaro D. Antitumor Effects of Cannabidiol, a Nonpsychoactive Cannabinoid, on Human Glioma Cell Lines. J Pharmacol Exp Ther (2004) 308(3):838–45. doi: 10.1124/jpet.103.061002
28. Martinez SR, Maresha SG, L Z. The antitumor activity of plant-derived non-psychoactive cannabinoids. Physiol Behav (2016) 176(1):139–48. doi: 10.1016/j.physbeh.2017.03.040
29. McKallip RJ, Jia W, Schlomer J, Warren JW, Nagarkatti PS. Cannabidiol-induced Apoptosis in Human Leukemia Cells: A Novel Role of Cannabidiol in th. Mol Pharmacol (2006) 70:897–908. doi: 10.1124/mol.106.023937
30. Borrego-Soto G, Ortiz-López R, Rojas-Martínez A. Ionizing radiation-induced DNA injury and damage detection in patients with breast cancer. Genet Mol Biol (2015) 38(4):420–32. doi: 10.1590/S1415-475738420150019
31. Baskar R, Dai J, Wenlong N, Yeo R, Yeoh KW. Biological response of cancer cells to radiation treatment. Front Mol Biosci (2014) 1:24(NOV). doi: 10.3389/fmolb.2014.00024
32. Yaeesh S, Jamal Q, Khan AU, Gilani AH. Free Radicals Antioxidants, diseases and phytomedicines: Current Status and Future Prospoect. Phyther Res (2006) 20(7):546–51. doi: 10.1002/ptr.1897
33. Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcorac V, et al. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev (2017) 2017 doi: 10.1155/2017/8416763
34. Kahl R, Kappus H. Toxicology of the Synthetic Antioxidants BHA and BHT in Comparison With the Natural Antioxidant Vitamin E. Int J Food Sci Technol (1993) 196:329–38. doi: 10.1007/BF01197931
35. Fischer N, Seo EJ, Efferth T. Prevention from radiation damage by natural products. Phytomedicine (2018) 47(2017):192–200. doi: 10.1016/j.phymed.2017.11.005
36. Szymanska R, Pospisil P, Kruk J. Corrigendum to "Plant-derived antioxidants in disease prevention". Oxid Med Cell Longev (2017) 2017:5092754. doi: 110.1155/2017/5092754
37. Gao YT, McLaughlin JK, Blot WJ, Ji BT, JFF QD. Reduced Risk of Esophageal Cancer Associated With Green Tea Consumption. JNCI Journa Natl Cancer Inst (1994) 86(11):855–8. doi: 10.1093/jnci/86.11.855
38. Ji BT, Chow WH, Hsing AW, McLaughlin JK, Dai Q, Gao YT, et al. Green tea consumption and the risk of pancreatic and colorectal cancers. Int J Cancer (1997) 70(3):255–8. doi: 10.1002/(SICI)1097-0215(19970127)70:3<255::AID-IJC1>3.0.CO;2-W
39. Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with highgrade prostate intraepithelial neoplasia: A preliminary report from a one-year proof-ofprinciple study. Cancer Res (2006) 66(2):1234–40. doi: 10.1158/0008-5472.CAN-05-1145
40. Kaur M, Deep G, Jain AK, Raina K, Agarwal C, Wempe MF, et al. Bitter melon juice activates cellular energy sensor AMPactivated protein kinase causing apoptotic death of human pancreatic carcinoma cells. Carcinogenesis (2013) 34(7):1585–92. doi: 10.1093/carcin/bgt081
41. Muhammad N, Steele R, Scott Isbell T, Philips N, Ray RB. Bitter melon extract inhibits breast cancer growth in preclinical model by inducing autophagic cell death. Oncotarget (2017) 8(39):66226–36. doi: 10.18632/oncotarget.19887
42. Li N, Xiu-hong Jia JW. Effects of tetramethylpyrazine on apoptosis of human leukemia cells and the expressions of apoptotic-relevant proteins. Herbal Gram (2014).
43. El-Shemy HA, Aboul-Enein AM, Aboul-Enein KM, Fujita K. Willow leaves’ extracts contain anti-tumor agents effective against three cell types. PLoS One (2007) 2(1). doi: 10.1371/journal.pone.0000178
44. Cheon SH, Kim KS, Kim S, Jung HS, Choi WC, Eo WK. Efficacy and Safety of Rhus verniciflua Stokes Extracts in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer. Forsch Komplementmed (2011) 18:77–83. doi: 10.1159/000327306
45. Wang Z, Chen Y, Jin G, Lin S. Combined therapy of brain metastasis in lung cancer. Chin J Integr Tradit West Med (1995) 1:36–8.
46. Tai J, Cheung SSC, Ou D, Warnock GL, Hasman D. Antiproliferation activity of Devil’s club (Oplopanax horridus) and anticancer agents on human pancreatic cancer multicellular spheroids. Phytomedicine (2014) 21(4):506–14. doi: 10.1016/j.phymed.2013.10.003
47. Ma J-W, Zhang Y, Ye J-C, Li R, Wen Y-L, Huang J-X, et al. Tetrandrine exerts a radiosensitization effect on human glioma through inhibiting proliferation by attenuating ERK phosphorylation. Biomol Ther (2017) 25(2):186–93. doi: 10.4062/biomolther.2016.044
48. Michaud-Levesque J, Bousquet-Gagnon N, Béliveau R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp Cell Res (2012) 318(8):925–35. doi: 10.1016/j.yexcr.2012.02.017
49. Ryskalin L, Biagioni F, Busceti CL, Lazzeri G, Frati A, Fornai F. The multi-faceted effect of curcumin in glioblastoma from rescuing cell clearance to autophagy-independent effects. Molecules (2020) 25(20):1–17. doi: 10.3390/molecules25204839
50. De Petrocellis L, Ligresti A, Schiano Moriello A, Iappelli M, Verde R, Stott CG, et al. Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: Pro-apoptotic effects and underlying mechanisms. Br J Pharmacol (2013) 168(1):79–102. doi: 10.1111/j.1476-5381.2012.02027.x
51. Kooti W, Servatyari K, Behzadifar M, Samani MA, Sadeghi F, Nouri B, et al. Effective Medicinal Plant in Cancer Treatment, Part 2: Review Study. J Evidence-Based Complement Altern Med (2017) 22(4):982–95. doi: 10.1177/2156587217696927
52. Biswal BM, Sulaiman SA, Ismail HC, Zakaria H, Musa KI. Effect of withania somnifera (Ashwagandha) on the development of chemotherapy-induced fatigue and quality of life in breast cancer patients. Integr Cancer Ther (2013) 12(4):312–22. doi: 10.1177/1534735412464551
53. Beuth J, Schneider B, Schierholz JM. Impact of complementary treatment of breast cancer patients with standardized mistletoe extract during aftercare: A controlled multicenter comparative Epidemiological Cohort Study. Anticancer Res (2008) 28(1 B):523–7.
54. Zamiri-Akhlaghi A, Rakhshandeh H, Tayarani-Najaran Z, Mousavi SH. Study of cytotoxic properties of Rosa damascena extract in human cervix carcinoma cell line. Avicenna J Phytomed (2011) 1(2):74–7. doi: 10.22038/AJP.2011.124
55. Poul YK, Majd A, Labibi F, Zanjani TM. Cytotoxic effect of methanolic extracts of vegetative and reproductive parts of Vinca rosea on A431, a human skin squamous carcinoma cell line. Physiol Pharmacol (2014) 18(3):364–72.
56. Lu WI, Lu DP. Impact of chinese herbal medicine on american society and health care system: Perspective and concern. Evidence-Based Complement Altern Med (2014) 2014. doi: 10.1155/2014/251891
57. Wong W, Chen BZ, Lee AKY, Chan AHC, Wu JCY, Lin Z. Chinese Herbal Medicine Effectively Prolongs the Overall Survival of Pancreatic Cancer Patients: A Case Series. Integr Cancer Ther (2019) 18. doi: 10.1177/1534735419828836
58. Hopp C. Natural Products Research – Information for Researchers. Maryland: National Center for Complementary and Integrative Health, U.S. Department of Health and Human Services (2020).
Keywords: phytomedicine, radiotherapy, phytoradiotherapy, cannabinoids, bitter melon
Citation: Alfonzetti T, Yasmin-Karim S, Ngwa W and Avery S (2021) Phytoradiotherapy: An Integrative Approach to Cancer Treatment by Combining Radiotherapy With Phytomedicines. Front. Oncol. 10:624663. doi: 10.3389/fonc.2020.624663
Received: 31 October 2020; Accepted: 18 December 2020;
Published: 08 February 2021.
Edited by:
Alessio G. Morganti, University of Bologna, ItalyReviewed by:
Silvia Cammelli, University of Bologna, ItalyMaria Paola Ciliberti, Ospedale Generale Regionale F. Miulli, Italy
Copyright © 2021 Alfonzetti, Yasmin-Karim, Ngwa and Avery. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephen Avery, c3RlcGhlbi5hdmVyeUBwZW5ubWVkaWNpbmUudXBlbm4uZWR1
 Tyler Alfonzetti
Tyler Alfonzetti Sayeda Yasmin-Karim
Sayeda Yasmin-Karim Wilfred Ngwa
Wilfred Ngwa Stephen Avery
Stephen Avery