- 1Department of Hematology, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2School of Medicine, Shandong University, Jinan, China
- 3Department of Hematology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 4Shandong Provincial Engineering Research Center of Lymphoma, Jinan, China
- 5Branch of National Clinical Research Center for Hematologic Diseases, Jinan, China
- 6National Clinical Research Center for Hematologic Diseases, The First Affiliated Hospital of Soochow University, Suzhou, China
Purpose: Although pegylated liposomal doxorubicin (PLD) has been approved in combination with bortezomib for relapsed/refractory multiple myeloma (MM), the antitumor efficacy and tolerability of PLD in different regimens for patients with newly diagnosed MM (NDMM) have not been fully defined.
Methods: A total of 249 NDMM patients diagnosed between January 2008 and October 2019 were included in this retrospective study. Among them, 112 patients received vindesine-based chemotherapy (35 vDD and 77 vAD) and 137 received bortezomib-based chemotherapy (58 VDD and 79 VD).
Results: In bortezomib-containing regimens, the complete response rate (48.3 vs. 30.4%, p = 0.033) and very good partial response or better rate (74.1 vs. 57.0%, p = 0.038) of VDD were significantly higher than those of VD subgroup. While no superior survival was found between VDD and VD subgroup. In vindesine-containing regimens, no statistical significance was identified between vDD and vAD in terms of response rate and survival. The occurrence rates of all cardiac AEs were similar between VDD and VD.
Conclusions: The vDD regimen was similar with vAD in the aspect of response rate, survival, and toxicity in NDMM patients. The addition of PLD to VD brought deeper response without increased toxicity, while no superior survival was found.
Introduction
Multiple myeloma (MM) is a malignant tumor that ranks second among all hematological tumors worldwide (1). It is characteristic of abnormal proliferation of bone marrow plasma cells, production of clonal immunoglobulin, and destruction of the bones (2). Chemotherapy is the main therapeutic strategy for MM. The conventional first-line chemotherapy mostly uses anthracycline containing doxorubicin, which has a certain effect and less damage to stem cells, but the side effects of conventional anthracycline are obvious (3). With the advances in cytogenetic investigations, various chemotherapy regimens based on novel drugs are emerging, which are expected to improve the prognosis of MM patients.
Pegylated liposomal doxorubicin (PLD) is a liposomal form of doxorubicin, with doxorubicin packaged in liposomes with surface-bound methoxypolyethyleneglycol in the course of pegylation (4). It has a pharmacokinetic feature characterized as longer circulation time and diminished volume of distribution to promote tumor uptake (5). On one hand, interactions between diverse circulating plasma components and the liposome surface are decreased by the hydrophilic coating of PLD formulation, thus blocking the uptake of circulating liposomes mediated by reticuloendothelial system. This allows circulating liposomes to better reach tumors which have increased vascular permeability (4). On the other hand, PLD has a particle size window of 20–200 nm, which seems to be the best opportunity to take advantage of the difference in permeability between normal and tumor vessels (6).
Clinical studies have been carried out using PLD in relapsed/refractory multiple myeloma (RRMM). PLD has been approved in combination with bortezomib for RRMM in many countries (7). In patients with RRMM, although PLD and bortezomib combination did not improve the overall survival (OS) in long-term follow-up compared to bortezomib alone (8), the results from the interim analysis showed that PLD and bortezomib significantly reduced the risk of disease progression by 45% and prolonged the median time of progression by 3 months (9). However, in Asian countries like Japan, the tolerability of dose levels which were approved in many other countries of PLD and bortezomib combination was not confirmed in RRMM patients (10). This combination treatment was prematurely discontinued in all three Japanese patients with RRMM in a phase I study due to adverse events (AEs) including Grade 3 bronchiolitis, Grade 3 peripheral sensory neuropathy, and Grade 2 stomatitis with all achieved partial response (PR). A retrospective study of 28 patients with RRMM showed PLD, bortezomib, and intravenous dexamethasone (DVD) appeared to represent a well-tolerated regimen, with only six patients (21%) showed aggravation of their baseline peripheral neuropathy (PN) and a high overall response rate (ORR) of 61%, which included one (4%) complete response (CR), three (11%) very good partial responses (VGPR), eight (29%) PR, and five (18%) minimal responses (11). In addition, DVD combination was safe and effective in elderly patients of a median age of 75 years with RRMM, with the ORR of 80% (20/25) and progression-free survival (PFS) of 8 months (12).
However, in terms of patients with newly diagnosed MM (NDMM), the antitumor efficacy and tolerability of PLD in different chemotherapy regimens have not been fully defined yet. In traditional vincristine combination regimens, compared with VAd (vincristine + doxorubicin + dexamethasone), DVd (PLD + vincristine + dexamethasone) was related to significantly less toxicity like Grade 3/4 neutropenia, a lower occurrence rate of sepsis, and less supportive care like antibiotic use, while similar efficacy, as objective response rates, PFS, and OS were similar (3). In contrary to RRMM, PLD + bortezomib therapy in a phase II study for NDMM patients did not meet the near CR/CR rate specified in the protocol, which was 7% out of 61 patients, and was associated with increased AEs in older patients (13). However, the three drug regimen VDD (bortezomib + PLD + dexamethasone) in patients with NDMM revealed well tolerance and high efficacy for induction treatment followed by HSCT in appropriate MM patients (14).
In this study, we investigated the efficacy and safety of PLD in different combination therapies based on vindesine or bortezomib in NDMM patients.
Patients and Methods
Patients
Patients with NDMM who received at least one cycle of chemotherapy, including PLD (PLD + vindesine + dexamethasone and PLD + bortezomib + dexamethasone) or excluding PLD (epirubicin + vindesine + dexamethasone and bortezomib + dexamethasone), between January 2008 and October 2019 in Shandong Provincial Hospital affiliated to Shandong University (SPHASU) were eligible in this retrospective analysis. The inclusion criteria were as follows: 1) newly diagnosed with symptomatic MM based on International Myeloma Working Group (IWMG) criteria (15); 2) previously untreated patients; 3) complete clinical data available for basic information as well as assessment of response and survival; 4) without clinically cardiac insufficiency (New York Heart Association Class II or greater); 5) without previous or concomitant tumor. These patients were identified through the hospital discharge registry system and electronic medical records. This study was approved by the Medical Ethical Committee of Shandong Provincial Hospital affiliated to Shandong University. All data of the recruited patients were obtained with written informed consent in accordance with the Declaration of Helsinki.
Study Design and Treatment
This single-center, retrospective study investigated the efficacy and safety of PLD in vindesine-based regimens and bortezomib-based regimens as initial treatment for NDMM. The primary objective was CR which was assessed after every cycle of chemotherapy and before HSCT. CR was defined as: immunofixation electrophoresis (IFE) in serum and urine was negative; there was no soft tissue plasmacytoma; and the proportion of bone marrow plasmacytoma was less than 5% (16).
The chemotherapy regimens of included NDMM patients mainly consisted of vindesine-based regimens and bortezomib-based regimens, each of which was with or without PLD. The vindesine-based regimens contained vDD (PLD + vindesine + dexamethasone) and vAD (epirubicin + vindesine + dexamethasone). Patients chose vindesine-based regimens or bortezomib-based regimens mainly for economic reasons. After bortezomib entering China’s medical insurance system in 2017, most patients could use bortezomib regimens without economic pressure. The vDD regimen consisted of PLD 40 mg/m2 intravenously (IV) over 1 h on Day 1, as well as vindesine 1 mg/day and dexamethasone 20 mg/day orally on Days 1–4 of each 28-day cycle. The vAD regimen contained vindesine 1 mg/day and epirubicin 10 mg/day IV on Days 1–4 with dexamethasone 20 mg/day orally on Days 1–4 of each 28-day cycle. The bortezomib-based regimens contained VDD (PLD + bortezomib + dexamethasone) and VD (bortezomib + dexamethasone). The VD regimen was composed of bortezomib 1.3mg/m2 IV on Days 1, 4, 8, 11 and dexamethasone 20 mg/day orally on Days 1, 2, 4, 5, 8, 9, 11, 12 of every 21-day cycle. The VDD regimen consisted of the same VD regimen with PLD 40 mg/m2 IV on Day 1 of each 21-day cycle.
All patients’ subsequent therapies were not limited. After those who completed four to six cycles of the enrolled initial treatment, eligible patients ≤65 years without severe organ dysfunction were offered the opportunity to HSCT. HSCT was not widely used until 2016 limited by transplantation technologies and conditions. Similarly, eligible patients chose HSCT or not as for their own wishes and economic reasons. Those who refused or were not eligible for HSCT progressed to thalidomide, cyclophosphamide, lenalidomide, melphalan, ixazomib, and even cross-group therapies. Genetic abnormalities including gain (1q21), t (4;14), del (17p13) and del (13q14) were detected by fluorescence in situ hybridization (FISH).
Study Assessments
Efficacy
We compared the treatment response and the survival time in vindesine regimens (vDD vs. vAD) and bortezomib regimens (VDD vs. VD), respectively. Response to the treatment was assessed according to the IMWG consensus criteria for response (16). The treatment response was assessed after every cycle during induction chemotherapy and before HSCT and the follow-up was conducted every 3 months during consolidation and maintenance treatment. PFS referred to the time from the beginning of treatment to disease progression or death for any cause. The definition of OS was the time from the beginning of treatment to death for any cause.
Safety
Safety assessment included AE monitoring, vital signs, physical examination, and clinical laboratory tests. All AEs were evaluated at each visit and graded based on the National Cancer Institute Common Terminology Criteria of Adverse Events (NCI-CTCAE), version 5.0.
Statistical Analyses
Chi-square test or Fisher exact test was employed for categorical variables. The Kaplan–Meier method was employed to estimate the survival analysis. The log-rank test was used to calculate the PFS and OS. Statistical analyses were performed by SPSS software for Windows Version 25.0 (SPSS Inc, Chicago, IL, USA). P-value of <0.05 was considered statistically significant.
Results
Clinical Characteristics of Patients
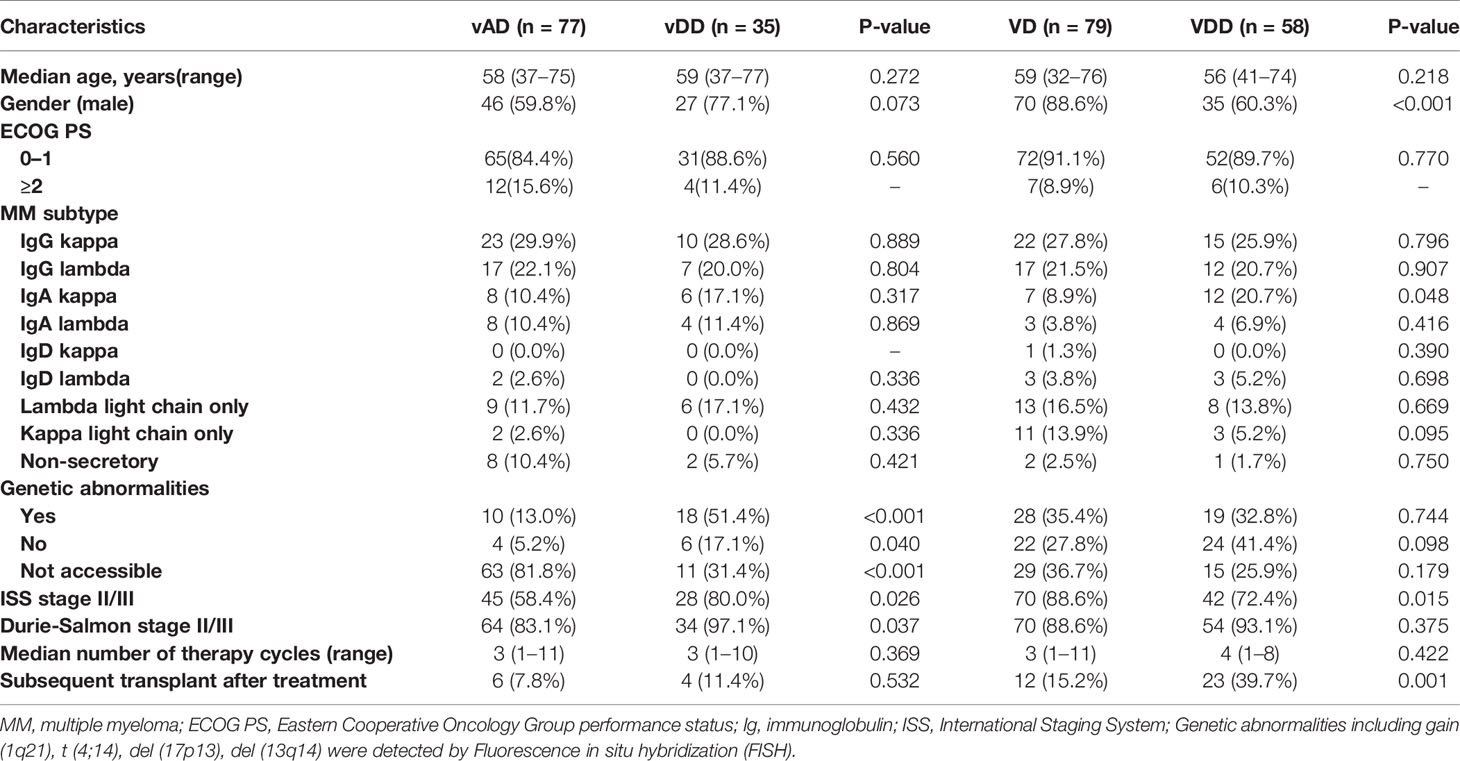
A total of 410 NDMM patients was presented in SPHASU between January 2008 and October 2019. Among them, 309 NDMM patients received at least one cycle of chemotherapy, including vindesine-based regimens (vDD and vAD) and bortezomib-based regimens (VDD and VD). Based on the inclusion criteria, 249 patients were finally included in this study. We excluded patients whose clinical information data were incomplete for assessment, those with clinically cardiac insufficiency (New York Heart Association Class II or greater) and those with previous or concomitant tumor (Figure 1). The median age at diagnosis was 58 years for vAD, 59 years for vDD and VD, and 56 years for VDD subgroup, respectively. 46 (59.8%) patients in vAD subgroup, 27 (77.1%) in vDD subgroup, 70 (88.6%) in VD subgroup and 35 (60.3%) in VDD subgroup were male. Among all, 112 patients received vindesine-based regimens, including 35 in vDD (with PLD) and 77 in the vAD subgroup (without PLD). The median number of treatment cycles patients received was three (range, 1–11). Four patients of vDD (11.4%) and six patients of vAD subgroup (7.8%) received HSCT, respectively. 137 of the included patients were treated with bortezomib-based regimens, 58 with PLD (VDD regimen) and 79 without PLD (VD regimen). The median number of treatment cycles received was four (range, 1–8) in VDD and three (range, 1–11) in the VD subgroup. Among these two subgroups, 23 (39.7%) of the VDD subgroup and 12 (15.2%) of the VD subgroup proceeded to HSCT. The baseline clinical characteristics of patients are presented in Table 1.
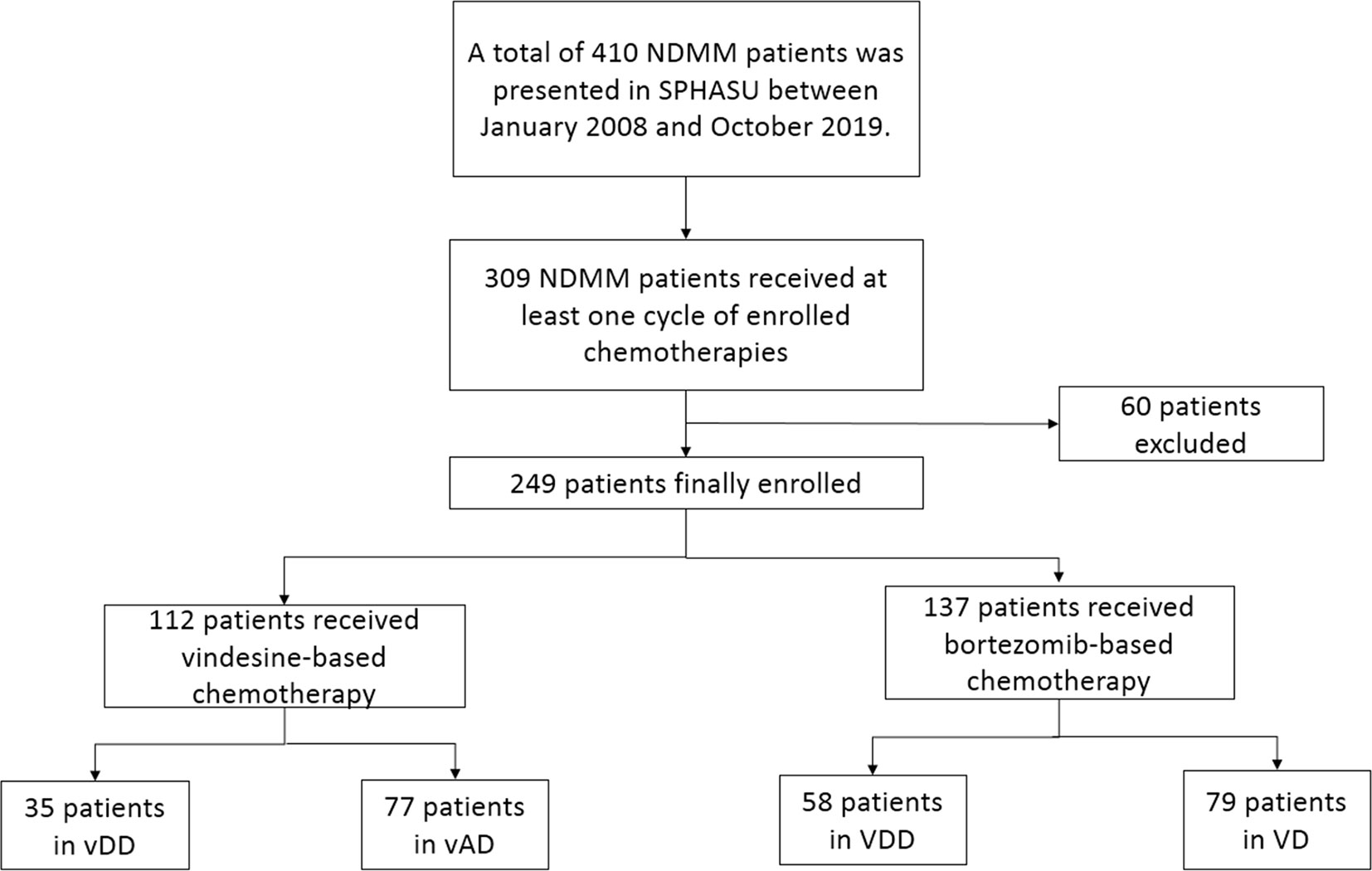
Figure 1 This is a flow diagram of all patients with newly diagnosed multiple myeloma enrolled and their regimen groups in this study.
Response Rates
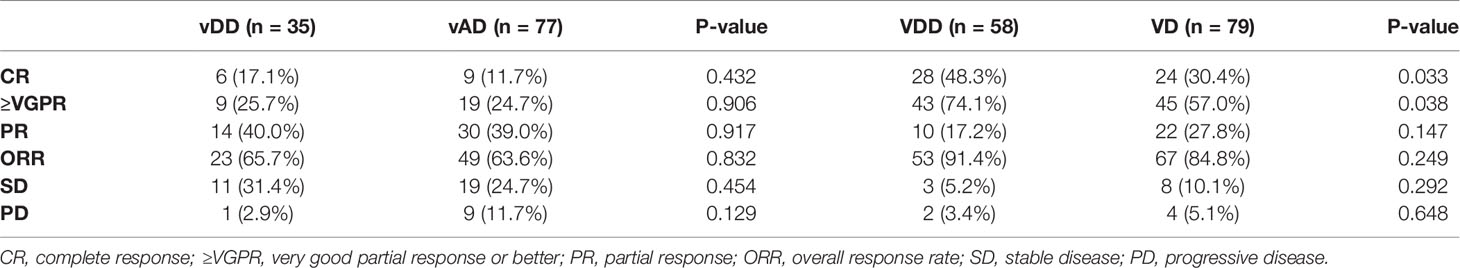
The response rates for each subgroup were summarized in Table 2. In the vindesine-based group, the ORR was 65.7% (23/35) in the vDD subgroup and 63.6% (49/77) in the vAD subgroup, including 17.1% (6/35) patients achieved CR, and 25.7% (9/35) patients achieved ≥VGPR in the vDD subgroup compared to 11.7% (9/77) of CR and 24.7% (19/77) of ≥VGPR in the vAD subgroup, which were all considered not statistically significant.
As for bortezomib-based group, although ORR between the VDD subgroup (91.4%) and VD subgroup (84.8%; p=0.249) had no significant difference, the rates of achieving CR and ≥VGPR were both significantly different between these two subgroups. 48.3% (28/58) of the patients achieved CR and 74.1% (43/58) achieved ≥VGPR in the VDD subgroup, compared to 30.4% (24/79) achieving CR (p = 0.033) and 57.0% (45/79) ≥VGPR in the VD subgroup (p = 0.038).
Survival
The median follow-up time for patients in the vindesine-based group was 25 months (range, 1–125 months), and the median follow-up time for patients in bortezomib-based group was 16 months (range, 1–134 months). Between the vDD and vAD subgroups, the median PFS was 28 months (95% CI, 13–42 months) in the vDD with 25 months (95% CI, 19–30 months) in the vAD. The median OS was 46 months (95% CI, 28–63 months) in the vAD while the median OS not reached in the vDD subgroup partly because of the later use of PLD. However, neither PFS nor OS differed significantly between vDD and vAD subgroup (p = 0.135, p = 0.240, respectively; Figures 2A, B). As for bortezomib regimens, the median PFS was 45 months (95% CI, 23–66 months) and the median OS was 52 months (95% CI, 32–71months) in the VD subgroup; comparing the median PFS and the median OS both did not reach in the VDD subgroup. Similarly, no significant difference was found between the VDD and VD subgroups in either PFS (p = 0.875) or OS (p = 0.448) (Figures 2C, D).
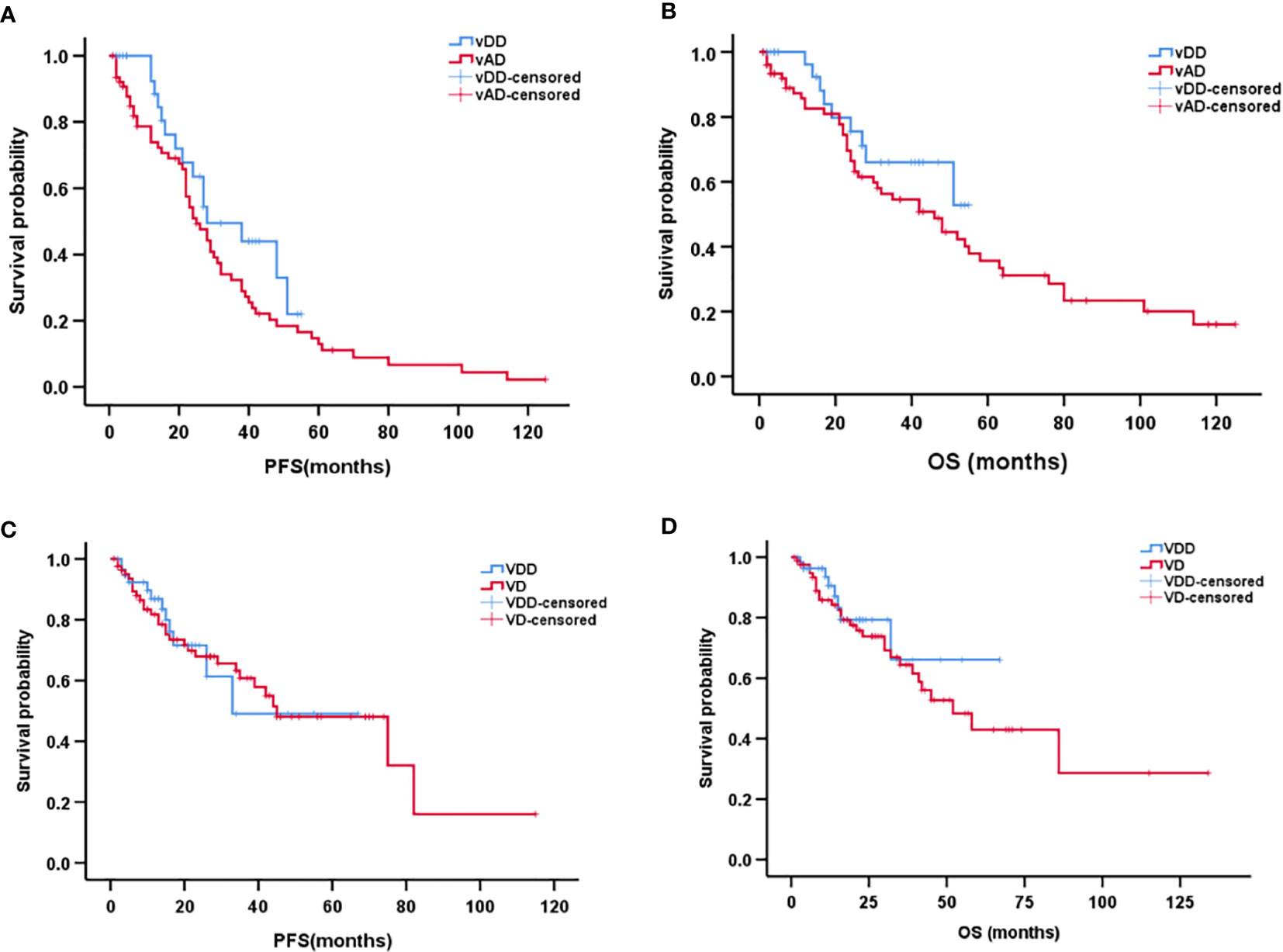
Figure 2 Kaplan–Meier curve of progression-free survival (A) and overall survival (B) between vDD and vAD in patients with newly diagnosed multiple myeloma. Kaplan–Meier curve of progression-free survival (C) and overall survival (D) between VDD and VD in patients with newly diagnosed multiple myeloma.
Safety
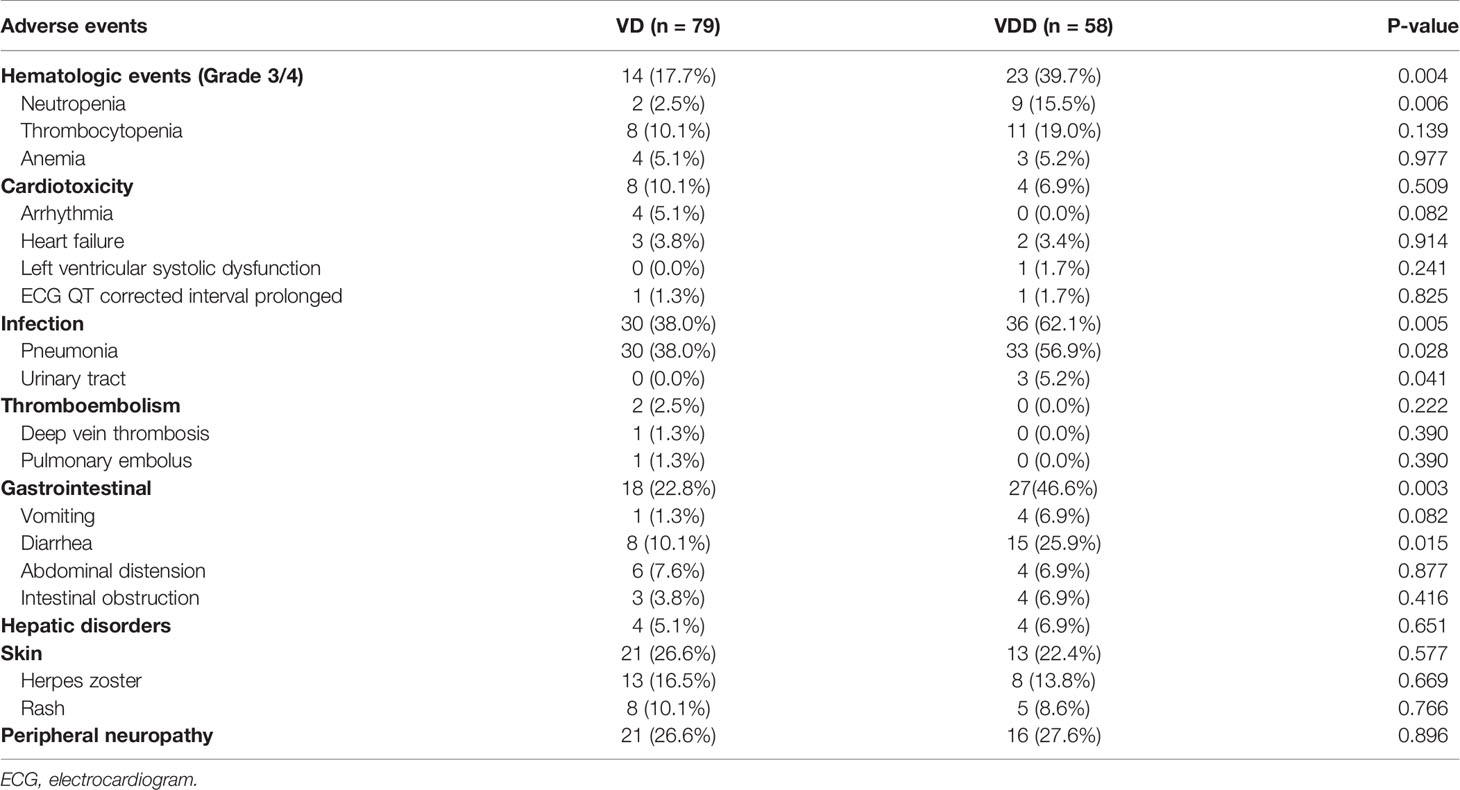
In the bortezomib regimen group, with the addition of PLD, the occurrence rates of Grade 3/4 hematological toxicities, including thrombocytopenia (19.0%), neutropenia (15.5%), and anemia (5.2%), as well as infection, including pneumonia (56.9%) and urinary tract infection (5.2%), in the VDD subgroup were significantly higher than those in the VD subgroup (p = 0.004 and p = 0.005, respectively). Gastrointestinal toxicities including vomiting, diarrhea, abdominal distension, and intestinal obstruction were significantly more frequent in the VDD subgroup (46.6 vs. 22.8%, p = 0.003). While no treatment-related deaths occurred, and these side events can be controlled in the supportive care. The occurrence rates of all cardiac AEs were similar between the VDD and VD subgroups (p = 0.509). Among patients receiving VDD regimen, 3.4% experienced heart failure and 1.7% experienced left ventricular systolic dysfunction. The addition of PLD also did not raise the occurrence rates of herpes zoster and PN. Among all enrolled patients, only one in the VD subgroup developed pulmonary embolism.
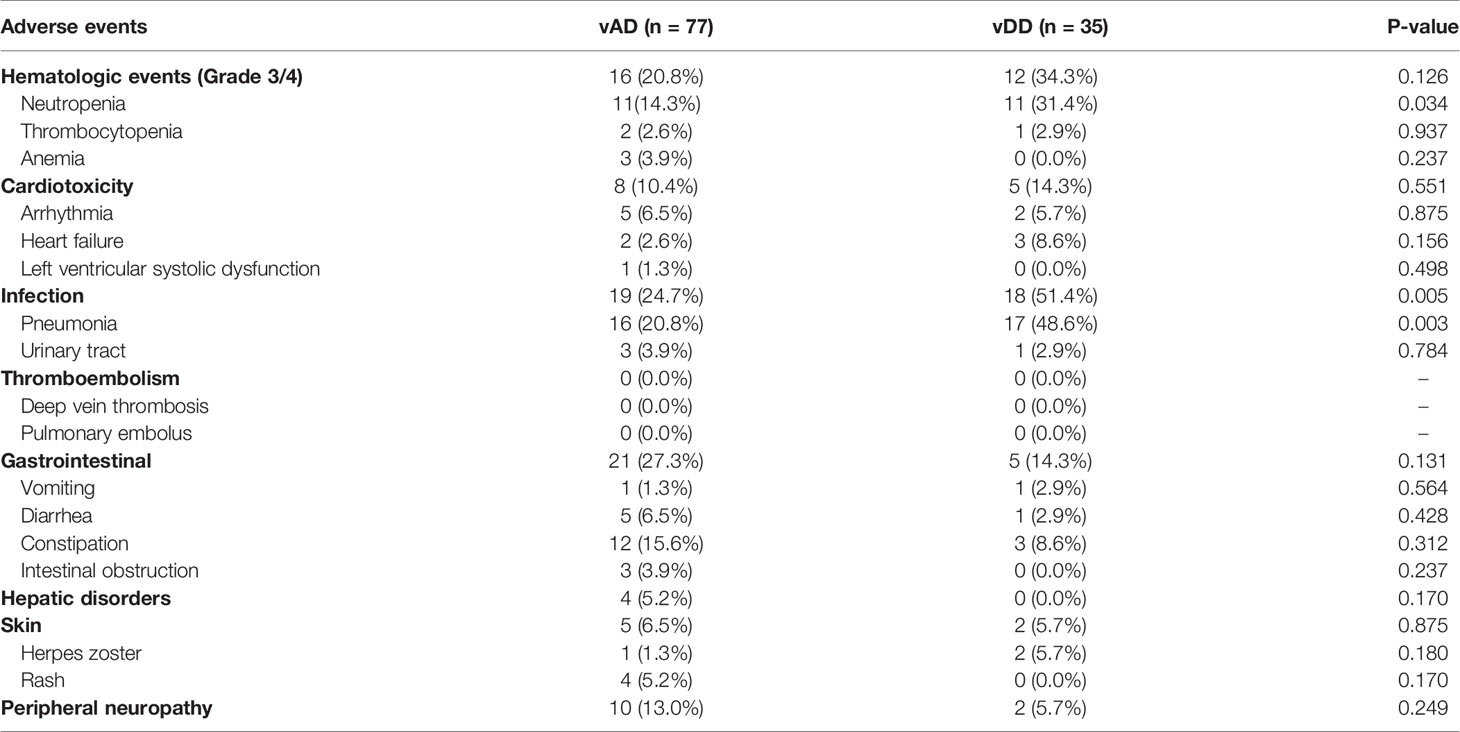
For the vindesine-based group, the occurrence rate of infection in the vDD was significantly higher than in the vAD subgroup (51.4 vs. 24.7%, p = 0.005), partly because of more Grade 3/4 hematological toxicities caused by PLD. Other AEs including gastrointestinal toxicities, cardiac toxicities, herpes zoster, and PN were all without statistical significances. The results of all AEs in each group are shown in Tables 3 and 4.
Discussion
In this present study, we retrospectively analyzed the efficiency and safety of PLD in different combination therapies for patients with NDMM. In therapies based on vindesine, PLD did not show superior antitumor efficacy compared to epirubicin, with similar ORR, CR and ≥VGPR rate. Replacement of epirubicin with PLD combined with vindesine and dexamethasone did not bring about significantly longer PFS or OS.
On the other hand, the addition of PLD to bortezomib and dexamethasone demonstrated a significantly better CR rate of 48.3%, ≥VGPR rate of 74.1%, and a slightly improvement in ORR, which is in accordance with the research by Wang et al. (17), which suggested that addition of PLD resulted in a deeper remission. Preclinical studies demonstrated that the synergistic effect between bortezomib and anthracycline through the caspase-8 pathway and dexamethasone through the caspase-9 pathway provided the rationale for combining PLD to bortezomib and dexamethasone (18).
PLD is an anthracycline compound, which is used as topoisomerase II inhibitor and DNA damaging agent. The upregulation of nuclear factor-κB (NF-κB), which results in transcription of genes involved in oncogenes is one major mechanism that inhibits the effect of PLD. Several researches have verified that bortezomib enhances the anti-MM effects of doxorubicin by suppressing the degradation of the NF-κB inhibitor to inhibit the activation of NF-κB (18). Proteasome inhibitors can also stimulate the MKP-1 to anti-apoptosis. Preclinical studies suggested that anthracyclines attenuated the induction of MKP-1, thus inhibiting its antiapoptotic effect (19). Various tumor model preclinical studies showed a synergy of increased apoptotic activity in combination of doxorubicin and bortezomib, and prevented anti-apoptotic activity which both the transcription factor NF-κB and the MKP-1 involved are observed (4). Thus, the combination of proteasome inhibitors bortezomib and anthracyclines PLD can increase their both efficacy (11).
Clinical data based on therapeutic approaches of MM have already indicated that the quality of response could predict long-term outcomes (20), with deeper responses, such as achievement of ≥VGPR, are associated with longer PFS and OS (14). On one hand, deeper responses of VDD did not show longer PFS or OS than the VD subgroup in our study of the total survival data. It is possible the unlimited subsequent therapy and inadequate follow-up time covered this benefit to some extent. After receiving the observed cycles of specific regimens, different patients progressed to varied therapies including HSCT, thalidomide, cyclophosphamide, lenalidomide, melphalan, ixazomib, and even cross-group therapies.
In terms of safety, PLD showed favorable hematological and non-hematological toxicity profile in clinical studies. A significantly decreased incidence of bone marrow suppression or neutropenic fever, less alopecia and decrease in cardiac function was observed in patients who received vDD regimen compared to those who received vAD (3). Treatment of vDD was significantly related to higher incidence of mostly Grades 1 and 2 hand–foot syndrome (3). Unfortunately, vDD regimen did not present any superior to vAD regimen in terms of safety in our study. Although the addition of PLD to bortezomib increased toxicity compared to bortezomib alone, mostly caused by increased myelosuppression and GI events, these toxicities were predictable and manageable through dose adjustment and supportive treatment (9). The increased non-hematological toxicities were more significant in NDMM patients aged ≥65 years (CALGB (Alliance) 10301) (13). The VDD regimen was associated with frequent grade 3/4 AEs including neutropenia, thrombocytopenia, pneumonitis and a high incidence of PN in several clinical trials (11). Yet PN was proved to be reversible (21) and dose-limiting (22). In our study, with the addition of PLD to VD, in accordance with the above studies, the incidence of Grade 3/4 hematological toxicities, infection, and gastrointestinal toxicities in VDD were significantly higher than those in the VD subgroup, which can be controlled in the supportive care like GSF and antibiotic use. Moreover, the occurrence rates of all cardiac AEs were comparable between VDD and VD subgroups, which means the addition of PLD did not raise the cardiac toxicity.
Multivariate analysis indicated that PLD could be an effective component in other regimens like bortezomib, cyclophosphamide, PLD, and dexamethasone combination (VCDD) (23, 24). Novel therapies such as the second-generation proteasome inhibitor carfilzomib, PLD, and dexamethasone (KDD), the deacetylase inhibitor vorinostat, PLD and bortezomib, the immunomodulatory drugs lenalidomide (25, 26) or pomalidomide (27) with PLD and bortezomib and so on all have high efficiency and well tolerance for RRMM patients. Especially, KDD demonstrated the ORR of 83% (20/24) and ≥VGPR rate of 54% (13/24), with the median PFS of 13.7 months (7). The vorinostat + PLD + bortezomib regimen produced an ORR of 65%, with the median PFS 13.9 months and the 3-year OS rate of 77% (28).
In this study, the regimens were the initial inductive therapies, and the sequential treatment was not limited, while VDD followed by TD (thalidomide + dexamethasone) or VTD was decided to be active in a clinical study, making more patients including those clinically defined high-risk cohort achieving maximal response before transplant (29). These better-quality responses were maintained following transplant and were related to a trend toward longer TTP and OS.
In conclusion, vDD is similar with vAD in response rate, survival, and toxicity for NDMM. The addition of PLD to VD brings deeper response without increased toxicity. However, superior survival in a long term was not proved in our study. There were still some limitations to our study, which was a retrospective one in a single center, and the amounts of recruited patients were confined. Thus, we included a mixed population of both receiving HSCT and not, which may have led to potential bias that could weaken the results. The heterogeneity in sequential regimens may have contributed to the relative variation in our findings. Moreover, because of the later use of PLD, the follow-up time of the regimens including PLD was inadequate and unequal with that of the regimens excluding PLD. Prospective randomized studies in larger population cohorts and extended follow-up time are necessary for further verifying the prognostic effect of PLD in different regimens.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by the Medical Ethical Committee of Shandong Provincial Hospital affiliated to Shandong University. All data of the recruited patients were obtained with written informed consent in accordance with the Declaration of Helsinki.
Author Contributions
XW designed the study. YZ, DY, XG, and SH collected the clinical data. PL, XF, and YL analyzed the data. YZ wrote the paper. XZ and XW revised the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (no. 82070203, no. 81800194, no. 81770210, no. 81473486, and no. 81270598); Key Research and Development Program of Shandong Province (no. 2018CXGC1213); Shandong Provincial Natural Science Foundation (no. ZR2018BH011); Development Project of Youth Innovation Teams in Colleges and Universities of Shandong Province (no. 2020KJL006); China Postdoctoral Science Foundation (No.2020M672103); Technology Development Projects of Shandong Province (No.2017GSF18189); Translational Research Grant of NCRCH (No.2021WWB02, No.2020ZKMB01); Technology Development Project of Jinan City (No.201805065); Taishan Scholars Program of Shandong Province; Academic promotion programme of Shandong First Medical University (No. 2019QL018, No.2020RC006); and Shandong Provincial Engineering Research Center of Lymphoma.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Liu J, Liu W, Mi L, Zeng X, Cai C, Ma J, et al. Incidence and mortality of multiple myeloma in China, 2006-2016: an analysis of the Global Burden of Disease Study 2016. J Hematol Oncol (2019) 12(1):136. doi: 10.1186/s13045-019-0807-5
2. Cai H, Zhang L, Li N, Zheng B, Liu M. Cost-effectiveness analysis on binary/triple therapy on the basis of ixazomib or bortezomib for refractory or relapsed multiple myeloma. Leuk Lymphoma (2019) 60(12):2951–9. doi: 10.1080/10428194.2019.1620947
3. Rifkin RM, Gregory SA, Mohrbacher A, Hussein MA. Pegylated liposomal doxorubicin, vincristine, and dexamethasone provide significant reduction in toxicity compared with doxorubicin, vincristine, and dexamethasone in patients with newly diagnosed multiple myeloma: a Phase III multicenter randomized trial. Cancer (2006) 106(4):848–58. doi: 10.1002/cncr.21662
4. Duggan ST, Keating GM. Pegylated liposomal doxorubicin: a review of its use in metastatic breast cancer, ovarian cancer, multiple myeloma and AIDS-related Kaposi’s sarcoma. Drugs (2011) 71(18):2531–58. doi: 10.2165/11207510-000000000-00000
5. Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin Pharmacokinet (2003) 42(5):419–36. doi: 10.2165/00003088-200342050-00002
6. Gabizon AA, Patil Y, La-Beck NM. New insights and evolving role of pegylated liposomal doxorubicin in cancer therapy. Drug Resist Updat (2016) 29:90–106. doi: 10.1016/j.drup.2016.10.003
7. Schroeder MA, Fiala MA, Huselton E, Cardone MH, Jaeger S, Jean SR, et al. A Phase I/II Trial of Carfilzomib, Pegylated Liposomal Doxorubicin, and Dexamethasone for the Treatment of Relapsed/Refractory Multiple Myeloma. Clin Cancer Res (2019) 25(13):3776–83. doi: 10.1158/1078-0432.CCR-18-1909
8. Orlowski RZ, Nagler A, Sonneveld P, Blade J, Hajek R, Spencer A, et al. Final overall survival results of a randomized trial comparing bortezomib plus pegylated liposomal doxorubicin with bortezomib alone in patients with relapsed or refractory multiple myeloma. Cancer (2016) 122(13):2050–6. doi: 10.1002/cncr.30026
9. Orlowski RZ, Nagler A, Sonneveld P, Blade J, Hajek R, Spencer A, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol (2007) 25(25):3892–901. doi: 10.1200/JCO.2006.10.5460
10. Kusumoto S, Sunami K, Inagaki M, Iida S. Phase I study of pegylated liposomal doxorubicin in combination with bortezomib for Japanese patients with relapsed or refractory multiple myeloma. Int J Hematol (2015) 101(6):578–84. doi: 10.1007/s12185-015-1773-5
11. Waterman GN, Yellin O, Swift RA, Mapes R, Eades B, Ackerman E, et al. A modified regimen of pegylated liposomal doxorubicin, bortezomib, and dexamethasone is effective and well tolerated in the treatment of relapsed or refractory multiple myeloma. Ann Hematol (2011) 90(2):193–200. doi: 10.1007/s00277-010-1052-8
12. Gozzetti A, Fabbri A, Oliva S, Marchini E, Bocchia M, Defina M, et al. Weekly Bortezomib, Pegylated Liposomal Doxorubicin, and Dexamethasone Is a Safe and Effective Therapy for Elderly Patients With Relapsed/Refractory Multiple Myeloma. Clin Lymphoma Myeloma Leuk (2010) 10(1):68–72. doi: 10.3816/CLML.2010.n.008
13. Voorhees PM, Orlowski RZ, Mulkey F, Watson P, Geyer S, Sanford BL, et al. Long-term outcomes for newly-diagnosed multiple myeloma patients treated with pegylated liposomal doxorubicin and bortezomib: final results of CALGB (Alliance) 10301, a multicentre phase II study. Br J Haematol (2015) 171(3):373–7. doi: 10.1111/bjh.13592
14. Jakubowiak AJ, Kendall T, Al-Zoubi A, Khaled Y, Mineishi S, Ahmed A, et al. Phase II trial of combination therapy with bortezomib, pegylated liposomal doxorubicin, and dexamethasone in patients with newly diagnosed myeloma. J Clin Oncol (2009) 27(30):5015–22. doi: 10.1200/JCO.2008.19.5370
15. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M-V, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol (2014) 15(12):e538–e48. doi: 10.1016/S1470-2045(14)70442-5
16. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol (2016) 17(8):e328–46. doi: 10.1016/S1470-2045(16)30206-6
17. Wang H, Wang L, Lu Y, Chen X, Geng Q, Wang W, et al. Long-term outcomes of different bortezomib-based regimens in Chinese myeloma patients. Onco Targets Ther (2016) 9:587–95. doi: 10.2147/OTT.S97457
18. Mitsiades N, Mitsiades CS, Richardson PG, Poulaki V, Tai YT, Chauhan D, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood (2003) 101(6):2377–80. doi: 10.1182/blood-2002-06-1768
19. Small GW, Shi YY, Edmund NA, Somasundaram S, Moore DT, Orlowski RZ. Evidence That Mitogen-Activated Protein Kinase Phosphatase-1 Induction by Proteasome Inhibitors Plays an Antiapoptotic Role. Mol Pharmacol (2004) 66(6):1478–90. doi: 10.1124/mol.104.003400
20. Harousseau JL, Attal M, Avet-Loiseau H. The role of complete response in multiple myeloma. Blood (2009) 114(15):3139–46. doi: 10.1182/blood-2009-03-201053
21. Berenson JR, Yellin O, Chen CS, Patel R, Bessudo A, Boccia RV, et al. A modified regimen of pegylated liposomal doxorubicin, bortezomib and dexamethasone (DVD) is effective and well tolerated for previously untreated multiple myeloma patients. Br J Haematol (2011) 155(5):580–7. doi: 10.1111/j.1365-2141.2011.08884.x
22. Morawska M, Grzasko N, Kostyra M, Wojciechowicz J, Hus M. Therapy-related peripheral neuropathy in multiple myeloma patients. Hematol Oncol (2015) 33(4):113–9. doi: 10.1002/hon.2149
23. Becker PS, Gooley TA, Green DJ, Burwick N, Kim TY, Kojouri K, et al. A phase 2 study of bortezomib, cyclophosphamide, pegylated liposomal doxorubicin and dexamethasone for newly diagnosed multiple myeloma. Blood Cancer J (2016) 6:e422. doi: 10.1038/bcj.2016.31
24. Nishihori T, Baz R, Shain K, Kim J, Ochoa-Bayona JL, Yue B, et al. An open-label phase I/II study of cyclophosphamide, bortezomib, pegylated liposomal doxorubicin, and dexamethasone in newly diagnosed myeloma. Eur J Haematol (2015) 95(5):426–35. doi: 10.1111/ejh.12509
25. Buda G, Orciuolo E, Galimberti S, Pelosini M, Petrini M. Pegylated liposomal doxorubicin in combination with dexamethasone and bortezomib (VMD) or lenalidomide (RMD) in multiple myeloma pretreated patients. Ann Hematol (2010) 90(9):1115–6. doi: 10.1007/s00277-010-1136-5
26. Berenson JR, Yellin O, Kazamel T, Hilger JD, Chen CS, Cartmell A, et al. A phase 2 study of pegylated liposomal doxorubicin, bortezomib, dexamethasone and lenalidomide for patients with relapsed/refractory multiple myeloma. Leukemia (2012) 26(7):1675–80. doi: 10.1038/leu.2012.51
27. Cohen A, Spektor TM, Stampleman L, Bessudo A, Rosen PJ, Klein LM, et al. Safety and efficacy of pomalidomide, dexamethasone and pegylated liposomal doxorubicin for patients with relapsed or refractory multiple myeloma. Br J Haematol (2018) 180(1):60–70. doi: 10.1111/bjh.14992
28. Voorhees PM, Gasparetto C, Moore DT, Winans D, Orlowski RZ, Hurd DD. Final Results of a Phase 1 Study of Vorinostat, Pegylated Liposomal Doxorubicin, and Bortezomib in Relapsed or Refractory Multiple Myeloma. Clin Lymphoma Myeloma Leuk (2017) 17(7):424–32. doi: 10.1016/j.clml.2017.05.007
29. Landau H, Pandit-Taskar N, Hassoun H, Cohen A, Lesokhin A, Lendvai N, et al. Bortezomib, liposomal doxorubicin and dexamethasone followed by thalidomide and dexamethasone is an effective treatment for patients with newly diagnosed multiple myeloma with Internatinal Staging System stage II or III, or extramedullary disease. Leuk Lymphoma (2012) 53(2):275–81. doi: 10.3109/10428194.2011.606943
Keywords: pegylated liposomal doxorubicin, multiple myeloma, efficacy, survival, toxicity
Citation: Zhai Y, Yuan D, Ge X, Hu S, Li P, Fang X, Li Y, Zhou X and Wang X (2021) Pegylated Liposomal Doxorubicin in Vindesine-Based and Bortezomib-Based Regimens for Patients With Newly Diagnosed Multiple Myeloma: A Retrospective Study of Efficacy and Safety. Front. Oncol. 11:597453. doi: 10.3389/fonc.2021.597453
Received: 21 August 2020; Accepted: 18 January 2021;
Published: 25 March 2021.
Edited by:
Claudio Cerchione, Romagnolo Scientific Institute for the Study and Treatment of Tumors (IRCCS), ItalyReviewed by:
Natalia Paola Schutz, Italian Hospital of Buenos Aires, ArgentinaDinesh Pendharkar, Asian Cancer Institute, India
Copyright © 2021 Zhai, Yuan, Ge, Hu, Li, Fang, Li, Zhou and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Wang, eGludzAwN0AxMjYuY29t; Xiangxiang Zhou, eGlhbmd4aWFuZ3pob3VAc2R1LmVkdS5jbg==
 Yujia Zhai
Yujia Zhai Dai Yuan1,2,3,4,5
Dai Yuan1,2,3,4,5 Peipei Li
Peipei Li