- 1Department of Biotherapy, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 2West China School of Medicine, Sichuan University, Chengdu, China
- 3The Center of Gerontology and Geriatrics, West China Hospital, Sichuan University, Chengdu, China
Hepatoid adenocarcinoma of the stomach (HAS) is a rare malignant tumor, accounting for only 0.17–15% of gastric cancers. Patients are often diagnosed at an advanced disease stage, and their symptoms are similar to conventional gastric cancer (CGC) without specific clinical manifestation. Morphologically, HAC has identical morphology and immunophenotype compared to hepatocellular carcinoma (HCC). This is considered to be an underestimation in diagnosis due to its rare incidence, and no consensus is reached regarding therapy. HAS generally presents with more aggressive behavior and worse prognosis than CGC. The present review summarizes the current literature and relevant knowledge to elaborate on the epidemic, potential mechanisms, clinical manifestations, diagnosis, management, and prognosis to help clinicians accurately diagnose and treat this malignant tumor.
Introduction
Hepatoid adenocarcinoma of the stomach (HAS), the Primer's focus, is a scarce primary extrahepatic malignant neoplasm. The estimated annual incidence of HAS is 0.58–0.83 cases per million individuals. Most tumors have metastasized at diagnosis with a poor prognosis due to their aggressive behavior (1, 2). Hepatoid adenocarcinoma(HAC) has been reported to occur in the stomach (3), esophagus (4, 5), duodenum (6), jejunum (2), colon (7), peritoneum (8), pancreas (9–13), lung (14), ovary (15, 16), gallbladder (17), uterus (16, 18) and other sites (19). Of these locations, the stomach is the most common site of HAC. Histologically, HAC has similar morphology and immunohistochemistry to hepatocellular carcinoma (HCC). This is considered to be an underestimation in diagnosis due to its rare incidence, and no consensus is reached regarding therapy (20). Although numerous cases and a small sample of retrospective reports on HAS have been reported over the years, it has not been sufficiently identified. Herein, to deepen the comprehensive understanding of HAS, we elaborate on the epidemic, potential mechanisms, clinical manifestations, diagnosis, management, and prognosis of this neoplasm based on current literature and relevant materials to assist clinicians in diagnosing and treating this disease.
Epidemiology
HAS is a rare neoplasm and the annual incidence of HAS is approximately 0.58–0.83 cases per million people (2, 21). It is also a scare entity with an inconstant reported incidence between 0.17% and 15.0% in all gastric carcinomas across several studies (20, 22). A large number of HAS case reports come from the Asian region, mainly from the Japanese and Chinese cohort (22). According to previously published reports, HAS predominantly occurred in around 65 years old male patients (21, 23). Although no specific risk factors have been reported to influence the occurrence and progression of HAS positively, several cases described patients diagnosed as HAS with HBsAg seropositivity (8, 24).
Pathogenesis
The exact molecular mechanism of HAS remains unclear. A possible hypothesis is that based on the stomach and liver, with a common embryonic and histological origin, originating from the endoderm and the primitive foregut during the development of the embryo (25–27). The major genotypes of gastric malignancy have been defined by The Cancer Genome Atlas (TCGA) Research Network as Epstein–Barr virus-positive (EBV), microsatellite-instable (MSI), genomically stable tumors (GS), and chromosomally instability tumors (CIN): HAS is excluded from any of these due to its scarcity and characteristics of geographical distribution (28). Nevertheless, HASs are genetically heterogeneous groups with a majority of HAC are “CIN” and a small number of HAC with “MSI” (29, 30). It has been speculated that HAS is the result of trans-differentiation, transitioning from the intestinal type to hepatoid phenotypic (31); and the emergence of Alpha-fetoprotein (AFP) leading to hepatoid focus in gastric adenocarcinoma, may as a result of dedifferentiation of cancer cells into HAC progenitor cells. The HAS, obtaining AFP phenotype expression, may evolve into various microscopic histological morphology, including enteroblastic carcinoma and poorly differentiated medullary carcinoma through genetic divergence and evolution (32). Furthermore, HAS appears as invasive cancer with high deletion of alleles and extensive loss of heterozygosity (LOH), where some tumor suppressor genes are located in Ref. (32).
Diagnosis
Pathology
Pathology is the “gold standard” for diagnosing the HAS. Macroscopically, according to Borrmann’s classification, majority of patients were type III with poor differentiation and elevated serum AFP levels. The most common primary locations of these tumors were the antrum and body (26, 33). Microscopically, HAS was defined as a tumor with the resemble features of hepatoid adenocarcinomas with hematoxylin and eosin (H&E) stains, consisting of large eosinophilic cells with a similar morphology to HCC, which exhibiting trabecular or solid nested arrangement, separated by sinusoidal vascular channels (33–35). Assorted degrees differentiation of clear cells imitating embryonic foregut epithelium can also be found, indicating the differentiation of fetal enteroblastic. Nevertheless, precise diagnosis of HAC was difficult based on findings in histology statistics alone, with a low positive rate of 9.3% (36). Further assistance like immunohistochemistry (IHC) stains was regularly performed for diagnosis (37).
Immunohistochemistry
IHC is typically required to establish the diagnosis of HAS. The pathological characteristics and expression of various immunohistochemistry staining for HAS are summarized in Figure 1. HAC had diffuse expression of AFP, HepPar-1, glypican 3(GPC3), and spalt-like transcription factor 4 (SALL4) with a moderate sensitivity (27). IHC staining for Carcinoembryonic proteins (AFP, SALL4, and GCP3) shows strong diffuse staining of the hepatoid element, suggesting both hepatoid and intestinal mucin phenotype differentiation (33). The intestinal component usually stains for CDX-2 (33, 38). HepPar-1 and Arginase-1 immunostainings are regarded as highly sensitive and specific markers of HCC, while the positive staining of these markers can be detected in some HAC, causing certain difficulties in distinguishing HAS from HCC (37, 39). Among epithelial markers, CK8/18, CK19, and AE1/AE3 are always positive for hepatoid adenocarcinoma; nevertheless, the expression of CK7, CK14, CK20 rarely appears in HAS (37). It has been reported that staining for CEA, CK19, and CK20 is detected more frequently in HAS than in HCC. Furthermore, palate, lung, and nasal epithelium clone protein (PLUNC) is a good marker for distinguishing HAS from HCC because it is often positive in the papillary and tubular adenocarcinoma components of HAS. Anecdotally, PLUNC-positive tumor cells cannot be stained by AFP (40). Though LIN28 is not as sensitive as SALL4, it is a particular marker (98% specificity) for distinguishing classic HAS from HCCs when combining with SALL4. Other IHC stains for HAS, such as Her-2, alpha 1-antitrypsin (AAT), and alpha 1-antichymotrypsin (ACT), have been reported to be promising in making the diagnosis (30, 41).
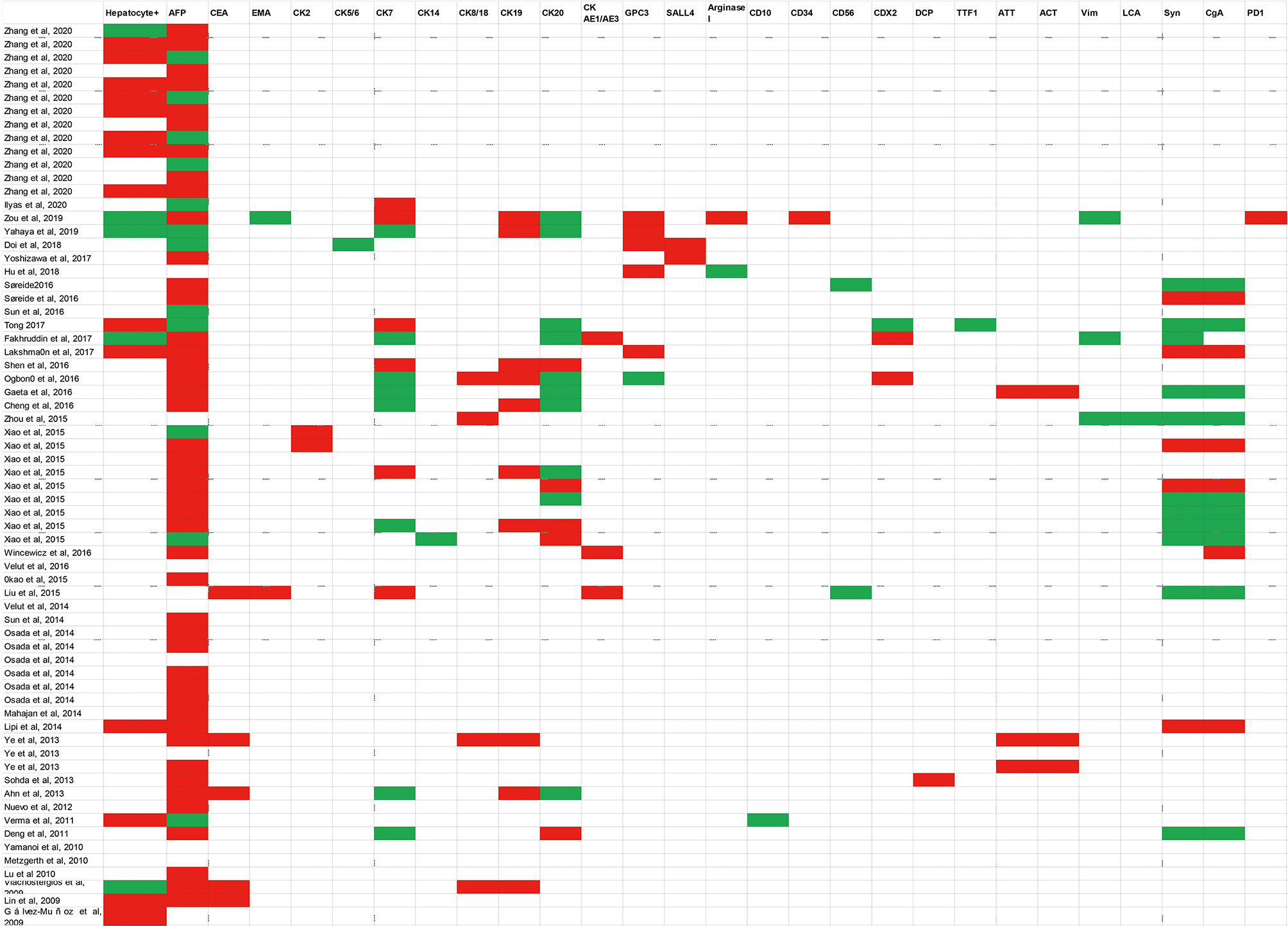
Figure 1 Summarized a variety of immunohistochemistry markers in published case reports. Diagnostic markers include Hepatocyte+, AFP, CEA, EMA, CK2, CK5/6, CK7, CK14, CK8/18, CK19, CK20, CK AE1/AE3, GPC3, SALL4, Arginase I, CD10, CD34, CD56, CDX2, DCP, TTF1, ATT, ACT, Vim, LCA, Syn, CgA, PD1.White blocks mean this examination has not been mentioned in case reports; green blocks represent negative results; red blocks represent positive results. AFP Alpha-fetoprotein; CEA Carcinoembryonic antigen; EMA, Epithelial cell membrane antigen; CK, Cytokeratin 2; GPC3, Glypican 3; SALL4, Sal-like protein 4; DCP, Des-gamma-carboxyprothrombin; TTF1, Thyroid transcription factor-1; ATT, A-1-antitrypsin; ACT, A-1-antichymotrypsin; Vim: Vimentin; LCA, Leucocyte common antigen; Syn Synaptophysin; CgA, Chromogranin A; PD-1 Programmed cell death protein 1.
Molecular Characteristics
Limited information can be found in the existing literature on the molecular features of HAS. Consisting with the TCGA database, previous reports uncovered that the most frequent genetic mutation in both HAS and GC tumor samples was TP53 (31, 42, 43). RPTOR, CD3EAP, CEBPA, WISP3, and MARK1 other than TP53 were high-frequency gene alternations in HAS (29, 43). It is of note that CTNNB1 and KRAS mutation might be detected in HAC, while subsequent researchers surmised that CTNNB1, KRAS, or BRAF mutations do not exist in most HAC. In addition to gene mutation, HAS is a tumor with a remarkable augment of copy number gains (CNGs). Primarily, the HAS patients with CNGs situated in 20q11.21–13.12 of a chromosome, with a trend of increasing serum concentration of AFP, might be related to more adverse bio-behavior than nonamplified tumors, including lower differentiation, greater nerve and vascular invasion, and more significant liver metastasis and is associated with worse prognosis (29, 42, 43). Moreover, the signaling pathway, including ErbB, PI3K-Akt, HIF-1 and p53 pathway regulating the pluripotency of stem cells, were specifically enriched in the mutated genes. In terms of Epigenetic modifications, GATA4 is not responsible for forming and maintaining the hepatocellular carcinoma-like phenotype (44).
Serum Tumor Markers
The majority of cases reported the elevations in AFP concentration in patients with HAS (Figure 2), and the serum AFP concentration was associated with HAC cell component percentage: the higher HAC cell component ratio in a tumor, the more AFP could be secreted by the tumor (22, 42). Although a majority of cases reported the patient had been diagnosed as HAS with the elevation of serum AFP (22), it is of note that there were still patients with HAS whose serum AFP levels were negative despite pathological results that confirmed the presence of Hyaline globule and canalicular structures morphologically (26). Accordingly, HAS's clinicopathological entity was extended, involving adenocarcinomas performing histological patterns of similarity to HCC morphologically regardless of AFP expression/production (36, 39, 45). Other hematological markers, such as the concentration of CA19-9, CA125, CEA, and CA72-4 in the blood, were also elevated in some cases.
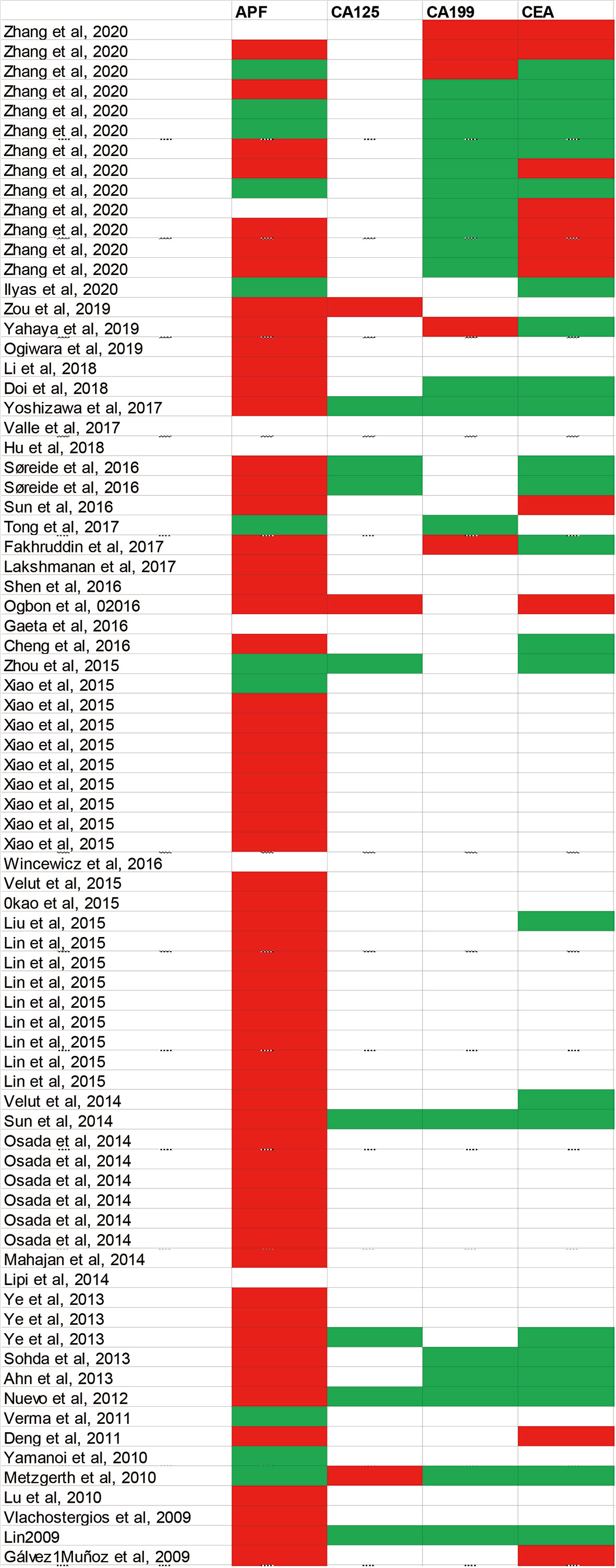
Figure 2 Summarized various of serum tumor markers in published case reports. Diagnostic markers include AFP, CEA, CA19-9 and CA125. White blocks mean this examination has not been mentioned in case reports; green blocks represent negative results; red blocks represent positive results. AFP, Alpha-fetoprotein; CEA, Carcinoembryonic antigen; CA19-9, Carbohydrate antigen 199; CA125, Carbohydrate antigen 125.
Imaging Diagnosis
For primary sites, the findings of computed tomography (CT), covering the longest and mean short diameter of malignancy, the ratio of lesion attenuation to aorta CT attenuation, the ratio of the number of accrete lymph nodes (LNs) on CT to the number of histologically proven metastatic LNs and the strengthening indexes in arterial phase minus portal venous phase, were significant predictors for distinguishing HAS from other gastric cancer (46–48). For HAC liver metastasis, arterial phase hypo-enhancement was more frequently encountered than HCC. Furthermore, the diffusion-weighted magnetic Resonance Imaging (MRI) was performed for a suspected HAS and clarified the diagnosis of HAS (49). The significance of positron emission tomography (PET)/CT had in diagnosing and staging HAS accurately (50–52).
Clinical Presentations
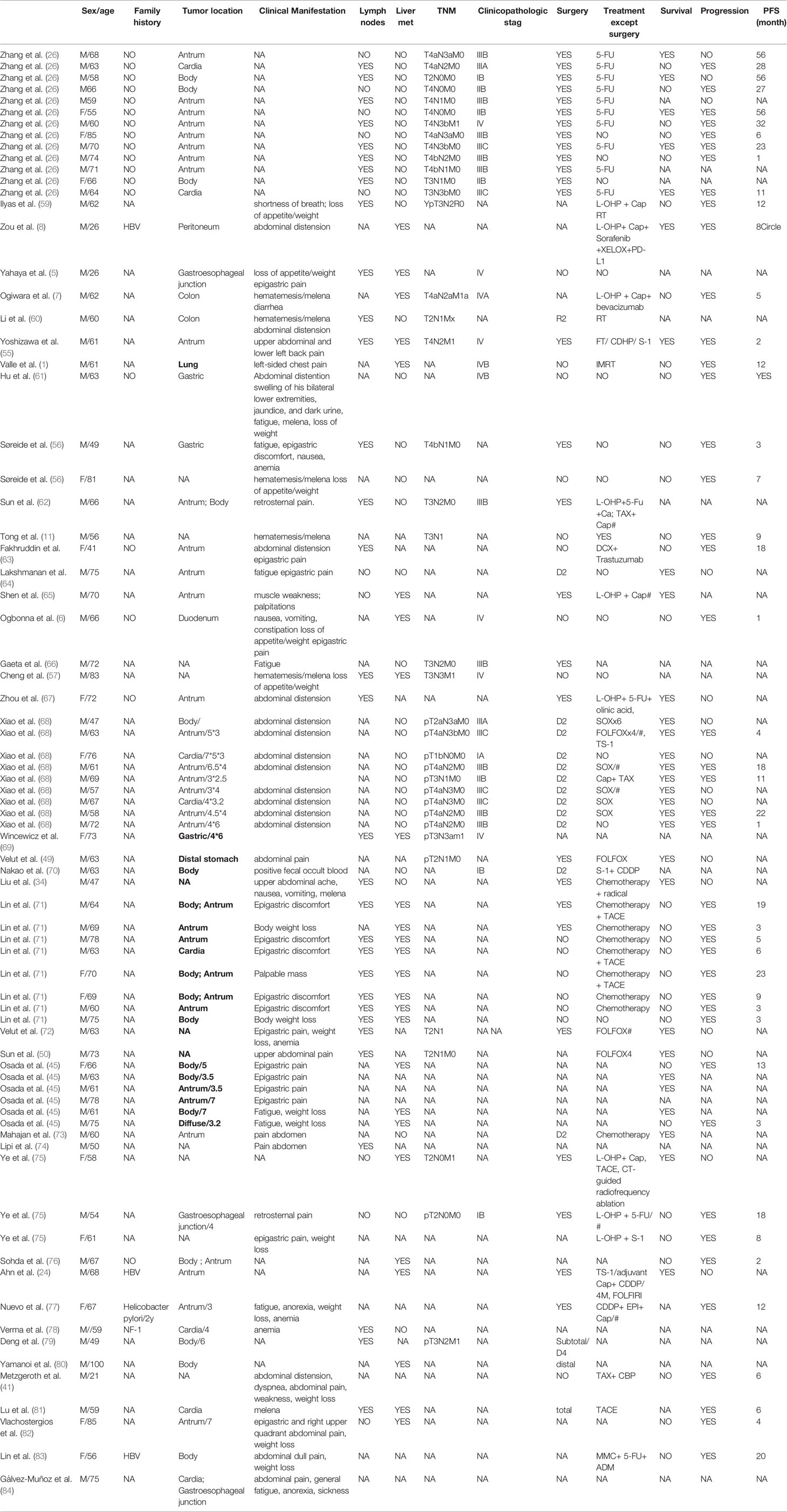
HASs were often diagnosed at an advanced disease stage with lymphatic permeation, blood vessel, and regional lymph node metastasis. Among retrospective analysis, 61.5% of HAC patients were in the III or IV stages at the diagnosis time. The relapse rate of early-stage or locally advanced stage patients was 47% (53, 54). The most common sites in which HAC developed include LNs, liver, lungs, peritoneum, and the spleen from existing literature (2, 37). Lacking specific clinical symptoms, the clinical manifestation of HAS is similar to common gastric cancer with many initial symptoms cover epigastric pain (55), abdominal distention (8), backache (55), fatigue (56), reduced appetite, weight loss (57), hematochezia, hematemesis (57) and shortness of breath (58). The most common presentation of HAS is abdominal pain (Table 1). Moreover, paraneoplastic hypercholesterolemia has been demonstrated in one case of HAS accompanied by liver metastasis (76).
Treatment
Surgery
For patients with early-stage HAS, radical surgery is a cornerstone of therapy with curative intent (21, 35). Radical surgery in combination with adjuvant chemotherapy is regarded as the optimal treatment approach (2). Gastric and liver metastasis resection is occasionally performed for palliation in advanced/metastatic HAS patients (85). And it was suggested that salvage surgery following chemotherapy could achieve curative resection of HAS with portal vein tumor thrombus (PVTT) (70).
Chemotherapy
No standard therapies for HAS were recommended by randomized controlled trials currently. Although the feasibility of neoadjuvant or adjuvant therapy for HAS patients and indications and concrete proposals for auxiliary treatments is illegible (21), adjuvant chemotherapy has been reported as one of the independent factors for a better outcome (35, 68) especially for HAS patients diagnosed with LNs or/and distant organ metastasis (2, 68). It was also reported that FOLFOX might be a potential adjuvant therapy for HAS (72). Cisplatin-based chemotherapy is judged as a standard first-line systemic regimen for metastatic HAS (55). Two advanced HAS patients treated with a first-line chemotherapy regimen of cisplatin and etoposide achieved a complete response (21, 86). The effectiveness of other regimens like oxaliplatin, irinotecan, gemcitabine, and 5-FU, as the first- or second-line treatment, either alone or combined, for advanced HAS situations remains obscure (86).
Interventional Therapy
Transcatheter arterial chemoembolization (TACE)/hepatic arterial infusion chemotherapy (HAIC), local intra-arterial chemotherapy for liver metastasis of HAS, has a lower frequency of toxicity reactions than systemic chemotherapy because of high concentrations of the drug injected locally (87). Both are also effective for the remission of the liver nodules of mHAS, accompanied with radical surgery or/and systemic chemotherapy.
Radiotherapy
Radiotherapy (RT) may be an inappropriate therapeutic option for HAS patients due to limited efficacy data. A scarce event reported that one patient with HAC of lung metastasizing to tonsils obtained an extraordinary symptomatic remission after the therapy of intensity-modulated radiation therapy (IMRT) (1). The palliative fractionation of RT was delivered to patients with PS (≧2) purely for symptom control, developing an unusual radiological adverse reaction to RT (59).
Anti-Angiogenesis Drugs
The introduction of anti-angiogenesis drugs has expanded treatment options of HAS. A case demonstrated that a HAS patient's resistance to chemotherapy had an evident clinical response to ramucirumab (RAM) monotherapy (87). The AFP concentration might be a potential marker to predict the response to ramucirumab and other anti-angiogenic drugs in gastric cancer. Besides, the positive Her-2 test rate of HAS patients was around 25%. Combined with chemotherapy, such as capecitabine and cisplatin, Trastuzumab could improve HER2-positive advanced HAS patients' overall survival compared with those who received chemotherapy alone (63, 87–90). Sorafenib, a molecularly targeted drug via the unclear mechanism of its direct pro-apoptotic effects or anti-angiogenic properties, has been administrated in some HAC patients. But it was suspended attributable to early adverse reactions (21). No convincing evidence about the sensitivity of HAS to Sorafenib was reported. In addition, HAC of the ovary and peritoneum were insensitive to Sorafenib (8).
Immunotherapy
Immune checkpoint antibodies have been approved to be administrated in multiple solid tumors, incorporating carcinomas of lungs, liver, esophagus, kidney, and stomach. Currently, immunotherapy applied to HAS is rare to report. Only one case showed that one HAS patient managed with PD-L1 inhibitor represented a low curative effect, which might be related to its low expression of PD-L1. Further experimental verification is expected to be reached in future clinical trials (8).
Prognostic Factors
The prognosis of HAS is poor. HAS patients had notably lower survival rates and disease-free survival (DFS) compared to those with other types. It is revealed that the 5-year DFS of HAS patients was only 20.7% (2, 33, 91). It was concluded that pTNM stage, portal vein thrombosis, vascular invasion, and adjuvant treatments were independent risk factors for DFS and pTNM stage, entirely surgical resection, and adjuvant therapy were independent risk factors for disease-specific survival (DSS) (2). However, some case reports argued that survival was not associated with sex, location, type, the serum AFP level, the degree of differentiation, or the type of therapy received. Although the relationship between neuroendocrine differentiation and the prognosis of HAS remained vague, it was inclined to an unfavorable factor to give rise to low differentiation and prognosis (92).
Morphologically, clear cell histology, more than a threshold of 10% about the ratio of clear cells, harmed prognosis in patients within HAS (33, 38). No evidential relations were deemed between immunohistochemical staining and prognosis in HAC. Among epithelial markers, including CEA, CK7 and CK20 were crucial for survival assessment by immunohistochemistry stains (8). Patients with CEA, CK20, and CK7 staining positive lived a shorter life. Furthermore, the combination of PLUNC, SALL4, and Hep-Par-1 might be a way of a tried prognostic factor in HAS (40).
Also, the patients with higher AFP expression had a significantly more inferior OS (58). AFP was assumed to be adverse to tumor suppression due to inhibiting lymphocyte transformation (27). However, The AFP-positive cases had shown better outcomes than the AFP-negative instances in a series of HAC with enteroblastic differentiation(GAEDs) (43). Meanwhile, It was observed the expression of β-catenin has a significant correlation with survival time (27).
Future Perspectives
Although the standard surgical and systemic chemotherapies have been proved to improve the prognosis of HAS, it still shows a poor clinical outcome. Cisplatin-based chemotherapy regimens are regarded as the first-line treatments for metastatic HAS, while the second-line systemic approaches for optimal management remain unclear. Further researches should be directed at exploring the radiobiological sensibility and radiational therapeutic effects in these patients (59). A significant step toward applying anti-angiogenesis drugs covering RAM combining with chemotherapy, the overall survival of advanced HAS patients has been significantly increased. Of note, the development of molecularly targeted treatments related to Sorafenib should be validated. Immunotherapy as a possible therapeutic means is to be further explored in patients with HAS.
Conclusion
HAS is a scare subtype of gastric cancer. It is often diagnosed with lymph node metastasis and distant organ metastasis and has a poor prognosis, which poses a significant challenge to clinicians' diagnosis and treatment. Several immunohistochemical markers covering AFP, CEA, CK8/18, CK19, glypican 3, SALL4, CDX-2, and HepPar-1 can be performed to assist in pathological confirmation. The level of AFP serum is propitious to the early detection of HAS. The available radical surgery, chemotherapy, radiotherapy, and interventional therapy in HAS patients have achieved a better outcome. The introduction of anti-angiogenesis drugs has expanded the therapeutic boxes of HAS. The prognostic risk factors of HAS are related to infiltrating depth, portal vein thrombosis, vascular invasion, distant metastasis, pTNM stage, serum AFP levels, therapeutic regimen, and immunohistochemical staining. Immunotherapy and radiotherapy need to be further validated in HAS.
Author Contributions
RX collected data, reviewed the literature, and wrote the manuscript. YZ collected data and wrote and revised the manuscript. YW collected data and rechecked the manuscript. JY assisted in drawing. XM designed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Valle L, Thomas J, Kim C, Szabo E, Brown GT, Citrin D, et al. Hepatoid adenocarcinoma of the lung metastasizing to the tonsil. Mol Clin Oncol (2017) 6:705–7. doi: 10.3892/mco.2017.1215
2. Zeng XY, Yin YP, Xiao H, Zhang P, He J, Liu WZ, et al. Clinicopathological Characteristics and Prognosis of Hepatoid Adenocarcinoma of the Stomach: Evaluation of a Pooled Case Series. Curr Med Sci (2018) 38:1054–61. doi: 10.1007/s11596-018-1983-1
3. Gao HY, Zhang YP, Yan YW, Shen HF. [A case report of hepatoid adenocarcinoma of the stomach with liver and spleen metastasis misdiagnosed as advanced liver cancer]. Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chin J hepatology (2019) 27:719–20. doi: 10.3760/cma.j.issn.1007-3418.2019.09.013
4. Nagai Y, Kato T, Harano M, Satoh D, Choda Y, Tokumoto N, et al. [A case of AFP-producing esophagogastric junction cancer with liver metastases with a good response to chemotherapy]. Gan to kagaku ryoho Cancer Chemother (2014) 41:2349–51.
5. Yahaya A, Wa Kammal WS, Abd Shukor N, Osman SS. Oesophageal hepatoid carcinoma with liver metastasis, a diagnostic dilemma. Malaysian J Pathol (2019) 41:59–63.
6. Ogbonna OH, Sakruti S, Sulieman M, Ali A, Shokrani B, Oneal P. Hepatoid Adenocarcinoma of the Duodenum: An Unusual Location. Case Rep Oncol (2016) 9:182–7. doi: 10.1159/000444746
7. Ogiwara S, Furihata M, Fukami K, Yamashita A, Yao T, Osada T. Hepatoid Adenocarcinoma With Enteroblastic Differentiation in the Sigmoid Colon: Lessons From a Rare Case. Am J Gastroenterol (2019) 114:684–5. doi: 10.14309/ajg.0000000000000176
8. Zou M, Li Y, Dai Y, Sun L, Huang T, Yuan X, et al. AFP-producing hepatoid adenocarcinoma (HAC) of peritoneum and omentum: a case report and literature review. OncoTargets Ther (2019) 12:7649–54. doi: 10.2147/OTT.S216501
9. Williams NL, Palmer JD, Bar-Ad V, Anné PR, Sama AR, Weinstein JC, et al. Hepatoid Carcinoma of the Pancreas: A Case Report and Review of the Literature. Case Rep Pancreatic Cancer (2015) 1:3–6. doi: 10.1089/crpc.2015.29001.nlw
10. Soofi Y, Kanehira K, Abbas A, Aranez J, Bain A, Ylagan L. Pancreatic hepatoid carcinoma: a rare form of pancreatic neoplasm. Diagn Cytopathol (2015) 43:251–6. doi: 10.1002/dc.23195
11. Tong L, Pan H, He J, Weng M, Zheng L. Hepatoid adenocarcinoma arising from heterotopic pancreas of the ileum: A case report. Medicine (2016) 95:e4067. doi: 10.1097/MD.0000000000004067
12. Kai K, Nakamura J, Ide T, Masuda M, Kitahara K, Miyoshi A, et al. Hepatoid carcinoma of the pancreas penetrating into the gastric cavity: a case report and literature review. Pathol Int (2012) 62:485–90. doi: 10.1111/j.1440-1827.2012.02814.x
13. Jung JY, Kim YJ, Kim HM, Kim HJ, Park SW, Song SY, et al. Hepatoid carcinoma of the pancreas combined with neuroendocrine carcinoma. Gut Liver (2010) 4:98–102. doi: 10.5009/gnl.2010.4.1.98
14. Cavalcante LB, Felipe-Silva A, de Campos FPF, Martines J. Hepatoid adenocarcinoma of the lung. Autopsy Case Rep (2013) 3:5–14. doi: 10.4322/acr.2013.002
15. Choi W, Cho D, Yim C, Lee NJM. Primary hepatoid carcinoma of the ovary: A case report and review of the literature. Medicine (Baltimore) (2020) 99:e20051. doi: 10.1097/MD.0000000000020051
16. Rotellini M, Messerini L, Stomaci N, Raspollini MR. Hepatoid adenocarcinoma of the ureter: unusual case presenting hepatic and ovarian metastases. Appl Immunohistochem Mol Morphol AIMM (2011) 19:478–83. doi: 10.1097/PAI.0b013e318216af63
17. Devi NR, Sathyalakshmi R, Devi J, Lilly SM. Hepatoid Adenocarcinoma of the Gall Bladder-A Rare Variant. J Clin Diagn Res JCDR (2015) 9:Ed09–10. doi: 10.7860/JCDR/2015/10799.6324
18. Gallego DF, Muñoz C, Jimenez CA, Carrascal E. Hepatoid Adenocarcinoma of the Urachus. Case Rep Pathol (2016) 2016:1871807. doi: 10.1155/2016/1871807
19. Gardiner GW, Lajoie G, Keith R. Hepatoid adenocarcinoma of the papilla of Vater. Histopathology (1992) 20:541–4. doi: 10.1111/j.1365-2559.1992.tb01044.x
20. Lin CY, Yeh HC, Hsu CM, Lin WR, Chiu CT. Clinicopathologial features of gastric hepatoid adenocarcinoma. Biomed J (2015) 38:65–9. doi: 10.4103/2319-4170.126860
21. Søreide JA. Therapeutic Approaches to Gastric Hepatoid Adenocarcinoma: Current Perspectives. Ther Clin Risk Manage (2019) 15:1469–77. doi: 10.2147/TCRM.S204303
22. Inagawa S, Shimazaki J, Hori M, Yoshimi F, Adachi S, Kawamoto T, et al. M.J.G.c.o.j.o.t. IGCA Itabashi, and t. JGC Association. Hepatoid Adenocarcinoma Stomach (2001) 4:43–52. doi: 10.1007/s101200100016
23. Qu B, Bi W, Qu B, Qu T, Han X, Wang H, et al. PRISMA-Compliant Article: Clinical Characteristics and Factors Influencing Prognosis of Patients With Hepatoid Adenocarcinoma of the Stomach in China. Medicine (Baltimore) (2016) 95:e3399. doi: 10.1097/MD.0000000000003399
24. Ahn JS, Jeon JR, Yoo HS, Park TK, Park CK, Sinn DH, et al. Hepatoid adenocarcinoma of the stomach: an unusual case of elevated alpha-fetoprotein with prior treatment for hepatocellular carcinoma. Clin Mol hepatology (2013) 19:173–8. doi: 10.3350/cmh.2013.19.2.173
25. Kinjo T, Taniguchi H, Kushima R, Sekine S, Oda I, Saka M, et al. Histologic and immunohistochemical analyses of α-fetoprotein–producing cancer of the stomach. Am J Surg Pathol (2012) 36:56–65. doi: 10.1097/PAS.0b013e31823aafec
26. Zhang ZR, Wu J, Li HW, Wang T. Hepatoid adenocarcinoma of the stomach: Thirteen case reports and review of literature. World J Clin cases (2020) 8:1164–71. doi: 10.12998/wjcc.v8.i6.1164
27. Wang N, Kong R, Han W, Lu J. Aberrant β-catenin Activity in Hepatoid Adenocarcinoma of the Stomach. Curr Mol Med (2020). doi: 10.2174/0929867327666200522215607
28. Arora K, Bal M, Shih A, Moy A, Zukerberg L, Brown I, et al. Fetal-type gastrointestinal adenocarcinoma: a morphologically distinct entity with unfavourable prognosis. J Clin Pathol (2018) 71:221–7. doi: 10.1136/jclinpath-2017-204535
29. Bass AJ, Laird PW, Shmulevich I, Thorsson V, Thorsson V, Schultz N, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature (2014) 513:202–9. doi: 10.1038/nature13480
30. Tsuruta S, Ohishi Y, Fujiwara M, Ihara E, Ogawa Y, Oki E, et al. Gastric hepatoid adenocarcinomas are a genetically heterogenous group; most tumors show chromosomal instability, but MSI tumors do exist. Hum Pathol (2019) 88:27–38. doi: 10.1016/j.humpath.2019.03.006
31. Akiyama S, Tamura G, Endoh Y, Fukushima N, Ichihara Y, Aizawa K, et al. Histogenesis of hepatoid adenocarcinoma of the stomach: molecular evidence of identical origin with coexistent tubular adenocarcinoma. Int J Cancer (2003) 106:510–5. doi: 10.1002/ijc.11246
32. Fujii H, Ichikawa K, Takagaki T, Nakanishi Y, Ikegami M, Hirose S, et al. Genetic evolution of alpha fetoprotein producing gastric cancer. J Clin Pathol (2003) 56:942–9. doi: 10.1136/jcp.56.12.942
33. Zhou K, Wang A, Ao S, Chen J, Ji K, He Q, et al. The prognosis of hepatoid adenocarcinoma of the stomach: a propensity score-based analysis. BMC Cancer (2020) 20:671. doi: 10.1186/s12885-020-07031-9
34. Liu XM, Chen GQ, Li SL, Zai TS. Hepatoid adenocarcinoma of the stomach: A case report and literature review. Exp Ther Med (2015) 9:2133–6. doi: 10.3892/etm.2015.2393
35. Zhang JF, Shi SS, Shao YF, Zhang HZ. Clinicopathological and prognostic features of hepatoid adenocarcinoma of the stomach. Chin Med J (2011) 124:1470–6. doi: 10.3760/cma.j.issn.0366-6999.2011.10.006
36. Nagai E, Ueyama T, Yao T, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. A clinicopathologic and immunohistochemical analysis. Cancer (1993) 72:1827–35. doi: 10.1002/1097-0142(19930915)72:6<1827::AID-CNCR2820720606>3.0.CO;2-8
37. Su JS, Chen YT, Wang RC, Wu CY, Lee SW, Lee TY. Clinicopathological characteristics in the differential diagnosis of hepatoid adenocarcinoma: a literature review. World J Gastroenterol (2013) 19:321–7. doi: 10.3748/wjg.v19.i3.321
38. Kwon MJ, Byeon S, Kang SY, Kim KM. Gastric adenocarcinoma with enteroblastic differentiation should be differentiated from hepatoid adenocarcinoma: A study with emphasis on clear cells and clinicopathologic spectrum. Pathol Res Pract (2019) 215:152525. doi: 10.1016/j.prp.2019.152525
39. Chandan VS, Shah SS, Torbenson MS, Wu TT. Arginase-1 is frequently positive in hepatoid adenocarcinomas. Hum Pathol (2016) 55:11–6. doi: 10.1016/j.humpath.2016.04.008
40. Sentani K, Oue N, Sakamoto N, Arihiro K, Aoyagi K, Sasaki H, et al. Gene expression profiling with microarray and SAGE identifies PLUNC as a marker for hepatoid adenocarcinoma of the stomach. Modern Pathol (2008) 21:464–75. doi: 10.1038/modpathol.3801050
41. Metzgeroth G, Ströbel P, Baumbusch T, Reiter A, Hastka J. Hepatoid adenocarcinoma - review of the literature illustrated by a rare case originating in the peritoneal cavity. Onkologie (2010) 33:263–9. doi: 10.1159/000305717
42. Wang Y, Sun L, Li Z, Gao J, Ge S, Zhang C, et al. Hepatoid adenocarcinoma of the stomach: a unique subgroup with distinct clinicopathological and molecular features. Gastric Cancer (2019) 22:1183–92. doi: 10.1007/s10120-019-00965-5
43. Akazawa Y, Saito T, Hayashi T, Yanai Y, Tsuyama S, Akaike K, et al. Next-generation sequencing analysis for gastric adenocarcinoma with enteroblastic differentiation: emphasis on the relationship with hepatoid adenocarcinoma. Hum Pathol (2018) 78:79–88. doi: 10.1016/j.humpath.2018.04.022
44. Yamamura N, Kishimoto T. Epigenetic regulation of GATA4 expression by histone modification in AFP-producing gastric adenocarcinoma. Exp Mol Pathol (2012) 93:35–9. doi: 10.1016/j.yexmp.2012.03.012
45. Osada M, Aishima S, Hirahashi M, Takizawa N, Takahashi S, Nakamura K, et al. Combination of hepatocellular markers is useful for prognostication in gastric hepatoid adenocarcinoma. Hum Pathol (2014) 45:1243–50. doi: 10.1016/j.humpath.2014.02.003
46. Fu Y, Zhu H, Peng WJ. Gastric Hepatoid Adenocarcinoma: Differentiation From Gastric Adenocarcinoma With Dynamic Contrast-Enhanced Computed Tomographic Findings. J Comput Assisted Tomogr (2019) 43:887–91. doi: 10.1097/RCT.0000000000000924
47. Ren A, Cai F, Shang YN, Ma ES, Huang ZG, Wang W, et al. Gastric hepatoid adenocarcinoma: a computed tomography report of six cases. World J Gastroenterol (2014) 20:15001–6. doi: 10.3748/wjg.v20.i40.15001
48. Chang MY, Kim HJ, Park SH, Kim H, Choi DK, Lim JS, et al. CT features of hepatic metastases from hepatoid adenocarcinoma. Abdominal Radiol (New York) (2017) 42:2402–9. doi: 10.1007/s00261-017-1150-3
49. Velut G, Mary F, Aflalo V, Aparicio T. Magnetic resonance imaging diffusion-weighted imaging for diagnosis of a gastric hepatoid adenocarcinoma. Digestive Liver Dis (2015) 47:174. doi: 10.1016/j.dld.2014.08.044
50. Sun X, Li Y, Dong M, Li W, Xing L. Hepatoid adenocarcinoma of the stomach: dual-time-point (18)F-FDG PET/CT findings. Japanese J Radiol (2014) 32:721–4. doi: 10.1007/s11604-014-0366-1
51. Seo HJ, Chung JK, Go H, Cheon GJ, Lee DS. A hepatoid adenocarcinoma of the stomach evaluated with (18)F-FDG PET/CT: intense (18)F-FDG uptake contrary to the previous report. Clin Nucl Med (2014) 39:442–5. doi: 10.1097/RLU.0000000000000275
52. Pan JH, Dong MJ, Ouyang XB. A hepatoid adenocarcinoma of the stomach with liver metastasis mimicking hepatocellular carcinoma detected by F-18 FDG PET/CT imaging. Clin Nucl Med (2011) 36:1137–9. doi: 10.1097/RLU.0b013e3182335ef9
53. Kumashiro Y, Yao T, Aishima S, Hirahashi M, Nishiyama K, Yamada T, et al. Hepatoid adenocarcinoma of the stomach: histogenesis and progression in association with intestinal phenotype. Hum Pathol (2007) 38:857–63. doi: 10.1016/j.humpath.2006.10.020
54. Baek SK, Han SW, Oh DY, Im SA, Kim TY, Bang YJ. Clinicopathologic characteristics and treatment outcomes of hepatoid adenocarcinoma of the stomach, a rare but unique subtype of gastric cancer. BMC Gastroenterol (2011) 11:56. doi: 10.1186/1471-230X-11-56
55. Yoshizawa J, Ishizone S, Ikeyama M, Nakayama J. Gastric hepatoid adenocarcinoma resulting in a spontaneous gastric perforation: a case report and review of the literature. BMC Cancer (2017) 17:368. doi: 10.1186/s12885-017-3357-7
56. Søreide JA, Greve OJ, Gudlaugsson E, Størset S. Hepatoid adenocarcinoma of the stomach–proper identification and treatment remain a challenge. Scand J Gastroenterol (2016) 51:646–53. doi: 10.3109/00365521.2015.1124286
57. Cheng CY, Wu IC, Chen YT, Hu HM. A rare hepatoid adenocarcinoma from the gastric remnant. Kaohsiung J Med Sci (2016) 32:482–3. doi: 10.1016/j.kjms.2016.04.012
58. Chen EB, Wei YC, Liu HN, Tang C, Liu ML, Peng K, et al. Hepatoid Adenocarcinoma of Stomach: Emphasis on the Clinical Relationship with Alpha-Fetoprotein-Positive Gastric Cancer. BioMed Res Int (2019) 2019:6710428. doi: 10.1155/2019/6710428
59. Ilyas W, Jain P, Goody R, Swinson D, Hingorani M. The Potential Role of Radiotherapy in the Management of Hepatoid Carcinomas of the Stomach: A Case Report. Oncol Res Treat (2020) 43:170–4. doi: 10.1159/000505375
60. Li T, Liu T, Wang M, Zhang M. α-fetoprotein producing hepatoid gastric adenocarcinoma with neuroendocrine differentiation: A case report. Medicine (2018) 97:e12359. doi: 10.1097/md.0000000000012359
61. Hu M, Liu W, Yin F, Zhang D, Liu X, Lai J. Liver Metastasis of Hepatoid Colonic Adenocarcinoma: A Rare and Unusual Entity Wih Poor Prognosis and Review of the Literature. Gastroenterol Res (2018) 11:430–5. doi: 10.14740/gr1097
62. Sun N, Sun Q, Liu Q, Zhang T, Zhu Q, Wang W, et al. Zang, α-fetoprotein-producing gastric carcinoma: A case report of a rare subtype and literature review. Oncol Lett (2016) 11:3101–4. doi: 10.3892/ol.2016.4372
63. Fakhruddin N, Bahmad HF, Aridi T, Yammine Y, Mahfouz R, Boulos F, et al. Hepatoid Adenocarcinoma of the Stomach: A Challenging Diagnostic and Therapeutic Disease through a Case Report and Review of the Literature. Front Med (2017) 4:164. doi: 10.3389/fmed.2017.00164
64. Lakshmanan A, Kurian A, Subramanyan A, Srinivasan A. An Alpha Fetoprotein Producing Gastric Tumor with Yolk Sac, Hepatoid and Papillary Adenocarcinoma Components. J Clin Diagn Res JCDR (2017) 11:Ed03–ed05. doi: 10.7860/jcdr/2017/29454.10546
65. Shen Z, Liu X, Lu B, Ye M. Hepatoid adenocarcinoma of the stomach: A case report of a rare type of gastric cancer. Oncol Lett (2016) 11:1077–80. doi: 10.3892/ol.2015.4023
66. Gaeta R, Ugolini C, Castagna M. Case Report of an Hepatoid Adenocarcinoma of the Stomach. Appl Immunohistochem Mol Morphol AIMM (2016) 24:e6–8. doi: 10.1097/pai.0000000000000286
67. Zhou RU, Cai Y, Yang YI, Xiang J, Chen Z. Hepatoid adenocarcinoma of the stomach: A case report and review of the literature. Onco Lett (2015) 9:2126–8. doi: 10.3892/ol.2015.2979
68. Xiao C, Wu F, Jiang H, Teng L, Song F, Wang Q, et al. Hepatoid adenocarcinoma of the stomach: Nine case reports and treatment outcomes. Oncol Lett (2015) 10:1605–9. doi: 10.3892/ol.2015.3430
69. Wincewicz A, Kowalik A, Zięba S, Lewitowicz P, Góźdź S, Sulkowski S. α-Fetoprotein-Producing Hepatoid Gastric Adenocarcinoma With Osteoclast-Like Giant Cells and Neuroendocrine Differentiation: A Case Study With Molecular Profiling. Int J Surg Pathol (2015) 23:537–41. doi: 10.1177/1066896915586807
70. Nakao S, Nakata B, Tendo M, Kuroda K, Hori T, Inaba M, et al. Salvage surgery after chemotherapy with S-1 plus cisplatin for α-fetoprotein-producing gastric cancer with a portal vein tumor thrombus: a case report. BMC Surg (2015) 15:5. doi: 10.1186/1471-2482-15-5
71. Lin YY, Chen CM, Huang YH, Lin CY, Chu SY, Hsu MY, et al. Tseng, Liver metastasis from hepatoid adenocarcinoma of the stomach mimicking hepatocellular carcinoma: Dynamic computed tomography findings. World J Gastroenterol (2015) 21:13524–31. doi: 10.3748/wjg.v21.i48.13524
72. Velut G, Mary F, Wind P, Aparicio T. Adjuvant chemotherapy by FOLFOX for gastric hepatoid adenocarcinoma. Digestive Liver Dis (2014) 46:1135–6. doi: 10.1016/j.dld.2014.08.036
73. Mahajan V, Gupta N, Gupta S, Sharma R. Hepatoid adenocarcinoma of stomach: case report of a rare histological variant. Indian J Pathol Microbiol (2014) 57:116–9. doi: 10.4103/0377-4929.130917
74. Lipi L, Sachdev R, Gautam D, Singh J, Mohapatra I. Triple composite tumor of stomach: a rare combination of alpha fetoprotein positive hepatoid adenocarcinoma, tubular adenocarcinoma and large cell neuroendocrine carcinoma. Indian J Pathol Microbiol (2014) 57:98–100. doi: 10.4103/0377-4929.130912
75. Ye MF, Tao F, Liu F, Sun AJ. Hepatoid adenocarcinoma of the stomach: a report of three cases. World J Gastroenterol (2013) 19:4437–42. doi: 10.3748/wjg.v19.i27.4437
76. Sohda T, Kusuhara H, Egashira Y, Egashira K, Eguchi K, Aoyagi K, et al. Elevated paraneoplastic hypercholesterolemia in a case of hepatoid adenocarcinoma of the stomach with liver metastasis. Clin J Gastroenterol (2013) 6:424–8. doi: 10.1007/s12328-013-0420-z
77. Nuevo-Gonzalez JA, Cano-Ballesteros JC, Lopez B, Andueza-Lillo JA, Audibert L. Alpha-Fetoprotein-Producing Extrahepatic Tumor: Clinical and Histopathological Significance of a Case. J Gastrointestinal Cancer (2012) 43 Suppl 1:S28–31. doi: 10.1007/s12029-011-9310-0
78. Verma M, Loughrey MB. Hepatoid gastric adenocarcinoma in a patient with type 1 neurofibromatosis. Histopathology (2011) 58:799–801. doi: 10.1111/j.1365-2559.2011.03828.x
79. Deng Z, Yin Z, Chen S, Peng Y, Wang F, Wang X. Metastatic splenic α-fetoprotein-producing adenocarcinoma: report of a case. Surg Today (2011) 41:854–8. doi: 10.1007/s00595-010-4336-7
80. Yamanoi K, Kondoh Y, Fujii T, Kurihara N, Mukai M, Sakamoto M. Hepatoid adenocarcinoma of the stomach with multi-nucleated giant cell proliferation in a 100-year-old man. Pathol Int (2010) 60:750–4. doi: 10.1111/j.1440-1827.2010.02588.x
81. Lu CC, De-Chuan C, Lee HS, Chu HC. Pure hepatoid adenocarcinoma of the stomach with spleen and lymph-node metastases. Am J Surg (2010) 199:e42–4. doi: 10.1016/j.amjsurg.2009.05.038
82. Vlachostergios PJ, Voutsadakis IA, Barbanis S, Karasavvidou Papandreou CN. AFP-producing hepatoid adenocarcinoma of the stomach: a case report. cases J (2009) 2:9296. doi: 10.1186/1757-1626-2-9296
83. Lin CW, Hsu CC, Chang HC, Sun YC, Sun PL, Hsu CY, et al. Perng, Hepatoid adenocarcinoma of the stomach with liver metastasis mimicking hepatocellular carcinoma: a case report. cases J (2009) 2:6317. doi: 10.4076/1757-1626-2-6317
84. Gálvez-Muñoz E, Gallego-Plazas J, Gonzalez-Orozco VG-M, Menarguez-Pina F, Ruiz-Maciá JA, Morcillo MA. Hepatoid adenocarcinoma of the stomach - a different histology for not so different gastric adenocarcinoma: a case report. Int Semin Surg Oncol ISSO (2009) 6:13. doi: 10.1186/1477-7800-6-13
85. Wang FQ, Lu Q, Yan J, Peng YY, Xie CR, Su YJ, et al. Ex vivo hepatectomy and partial liver autotransplantation for hepatoid adenocarcinoma: A case report. Oncol Lett (2015) 9:2199–204. doi: 10.3892/ol.2015.3041
86. Simmet V, Noblecourt M, Lizée T, Morvant B, Girault S, Soulié P, et al. Chemotherapy of metastatic hepatoid adenocarcinoma: Literature review and two case reports with cisplatin etoposide. Oncol Lett (2018) 15:48–54. doi: 10.3892/ol.2017.7263
87. Doi Y, Takii Y, Mitsugi K, Kimura K, Mihara Y. The Effectiveness of Hepatic Arterial Infusion Chemotherapy with 5-Fluorouracil/Cisplatin and Systemic Chemotherapy with Ramucirumab in Alpha-Fetoprotein-Producing Gastric Cancer with Multiple Liver Metastases. Case Rep Oncol Med (2018) 2018:5402313. doi: 10.1155/2018/5402313
88. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet (London England) (2010) 376:687–97. doi: 10.1016/S0140-6736(10)61121-X
89. Hayashi K, Nagasaki E, Nakada K, Tamura M, Arakawa Y, Uwagawa T, et al. Chemotherapy for alpha-fetoprotein producing gastric cancers expressing human epidermal growth factor receptor 2. J Fnfection Chemother (2018) 24:298–301. doi: 10.1016/j.jiac.2017.10.019
90. Arakawa Y, Tamura M, Aiba K, Morikawa K, Aizawa D, Ikegami M, et al. Significant response to ramucirumab monotherapy in chemotherapy-resistant recurrent alpha-fetoprotein-producing gastric cancer: A case report. Oncol Lett (2017) 14:3039–42. doi: 10.3892/ol.2017.6514
91. Liu X, Cheng Y, Sheng W, Lu H, Xu X, Xu Y, et al. Analysis of clinicopathologic features and prognostic factors in hepatoid adenocarcinoma of the stomach. Am J Surg Pathol (2010) 34:1465–71. doi: 10.1097/PAS.0b013e3181f0a873
Keywords: hepatoid gastric carcinoma, pathology, diagnosis, prognosis, treatment
Citation: Xia R, Zhou Y, Wang Y, Yuan J and Ma X (2021) Hepatoid Adenocarcinoma of the Stomach: Current Perspectives and New Developments. Front. Oncol. 11:633916. doi: 10.3389/fonc.2021.633916
Received: 26 November 2020; Accepted: 09 March 2021;
Published: 12 April 2021.
Edited by:
Simona Gurzu, George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureş, RomaniaReviewed by:
Bin-Yan Zhong, The First Affiliated Hospital of Soochow University, ChinaFeng Yin, University of Missouri, United States
Dongwei Zhang, University of Rochester, United States
Anastasios Karayiannakis, Democritus University of Thrace, Greece
Copyright © 2021 Xia, Zhou, Wang, Yuan and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuelei Ma, ZHJtYXh1ZWxlaUBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Ruolan Xia1,2†
Ruolan Xia1,2† Yuqing Wang
Yuqing Wang Xuelei Ma
Xuelei Ma