- 1Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China
- 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
Cyclin-dependent kinases (CDKs) are key regulators of cell cycle progression in malignant tumor cells and play an important role through complex molecular interactions. Dysregulation of CDK dependent pathways is often found in non-small cell lung cancer, which indicates its vulnerability and can be used in clinical benefit. CDK4/6 inhibitors can prevent tumor cells from entering the G approved 1 and S phases, which have been studied in a series of explorations and brought great clinical effect to patients and encouragement to both physicians and researchers, thereby showing potential as a new therapeutic agent. A series of preclinical and clinical studies have been carried out on CDK4/6 inhibitors in NSCLC, and have been achieved some results, which may become a new potential treatment in the future. This review focuses on the research progress on CDK4/6 inhibitors in NSCLC, particularly the mechanisms of action, drugs, clinical research progress, and future application.
Introduction
Lung cancer is one of the frequently diagnosed cancer and is among the main causes of cancer death. Non-small cell cancer (NSCLC) accounts for approximately 85% of lung malignancies. Most of newly diagnosed patients are considered incurable because of the presence of metastases at the time of initial presentation (1). Despite a growing number of treatment methods for advanced NSCLC, the overall benefit is limited. Novel therapeutic targets for NSCLC have attracted considerable interest.
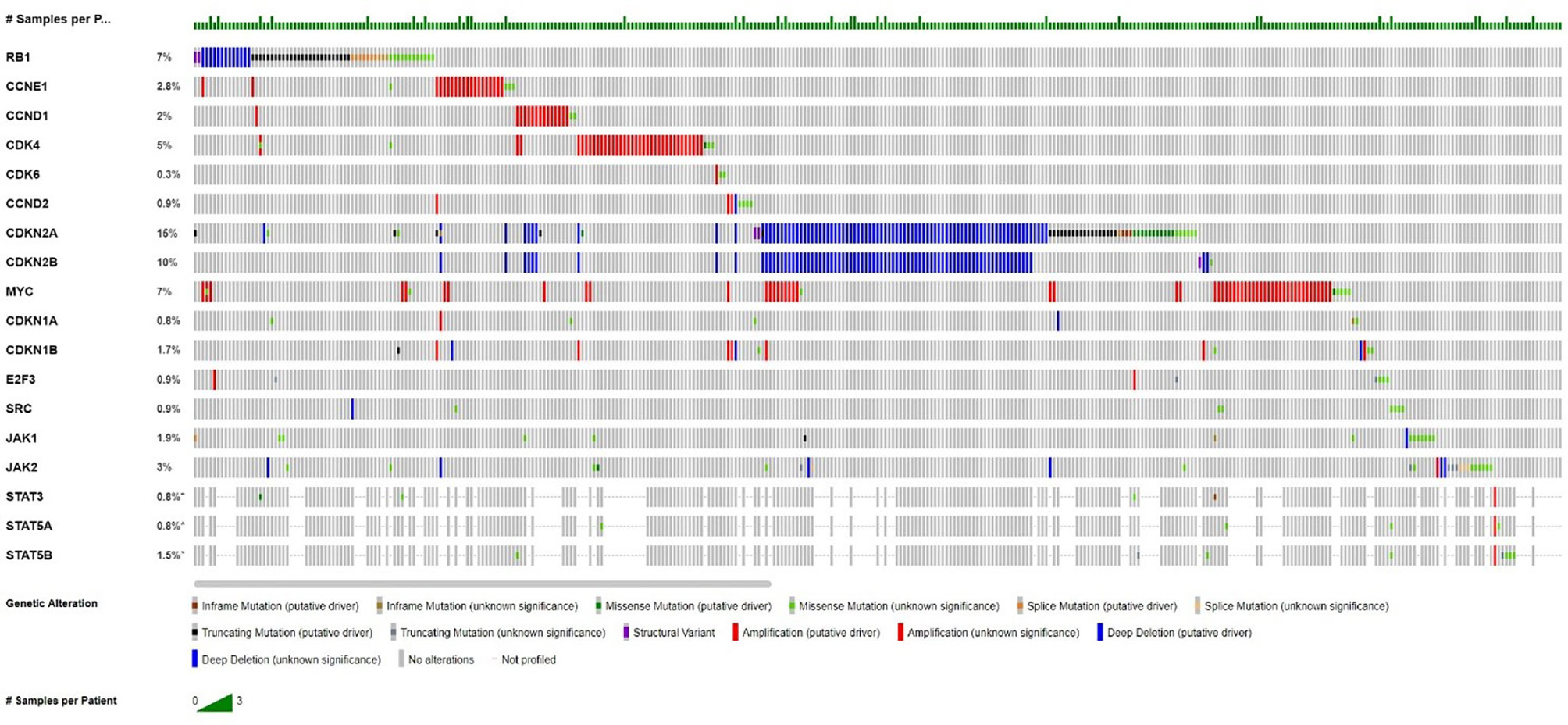
In normal and malignant cells, cyclin dependent kinases (CDKs) are the key regulators, which play roles in multiple points in the cell cycle to drive cellular proliferation through most complex molecular interactions (2). The expression and activation of cell cycle mediators is deranged, especially within the CDK–cyclin–RB pathways, and is involved in malignant transformation and tumor progression in lung cancer (3). In over 90% of lung cancers, the cell cycle occurs as dysregulation, which makes the derangements of cell cycle mediators in the expression and/or activation, especially within the CDK–cyclin–RB pathways, and is integrally involved in malignant transformation and tumor progression, destroying the cell proliferation mechanism controlling the growth of advanced NSCLC (3–5). Cyclin-dependent kinases 4 and 6 (CDK4 and CDK6) form a complex with D-type cyclins, which promote the cell cycle through the G1 restriction point through phosphorylation of the Rb tumor suppressor protein (6). Inhibition of CDK4 and CDK6 can prevent cell cycle progression, prevent tumor growth and promote senescence. Through aberrant retinoblastoma protein (RB) expression and the mutations of cyclin D CDK4 (INK4) proteins (p16INK4A) (7) and K-RAS, these cyclins promote uncontrolled cellular proliferation and drive cell cycle progression in lung carcinogenesis (8). According to the analysis of UALCAN cancer database, the CDK6 gene was moderately expressed in LUAD, and the overall survival rate of patients was negatively correlated with it. Figure 1 shows frequencies of aberrations in the Cyclin D-CDK4/6-Rb pathway related genes in NSCLC from the publicly available cBioportal webpage: Pan-Lung Cancer (TCGA, Nat Genet 2016). Therefore, CDK4/6 has been a key target for the clinical development for cancer therapy (9, 10).
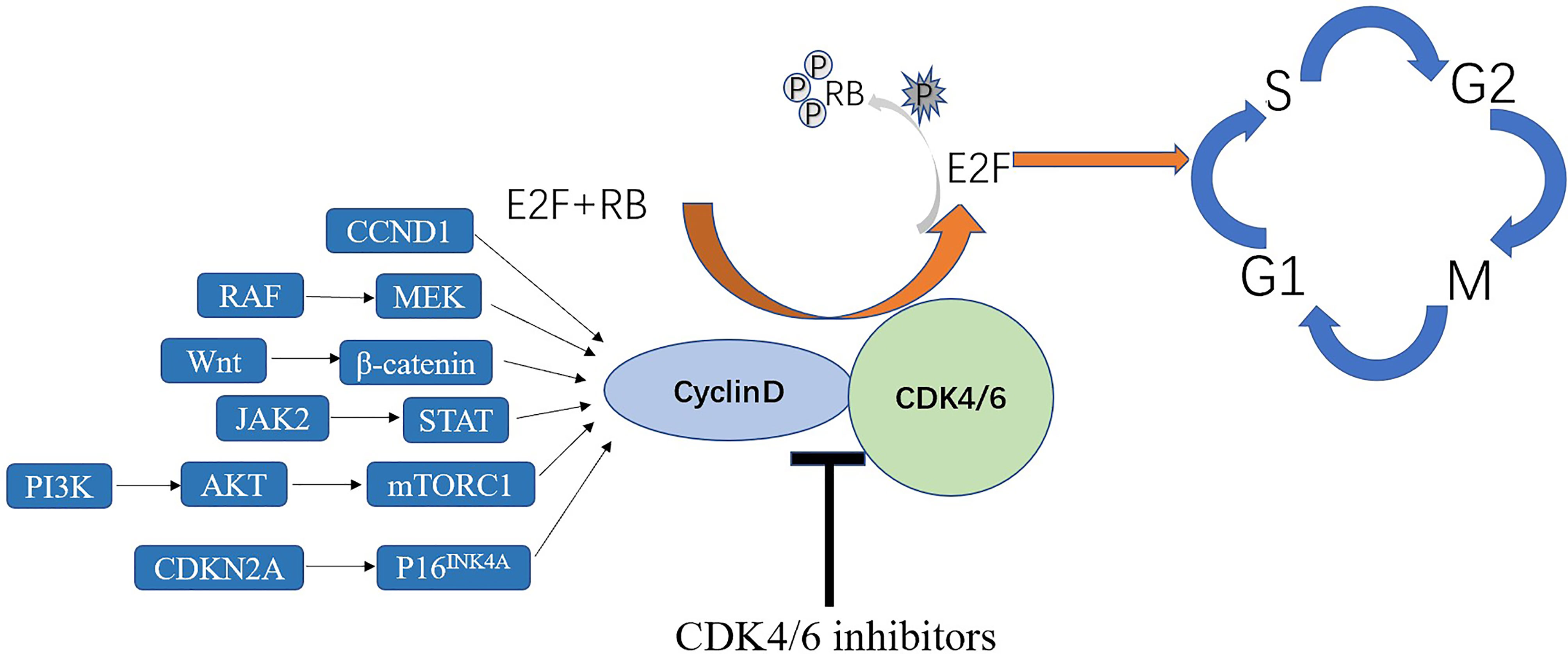
CDK4/6 inhibition has been tested in several clinical trials as a plausible treatment option for lung cancer (11–14). CDK4/6 inhibitors, designed to inhibit uncontrolled cellular proliferation, made tumor types with better efficacy and few adverse effects in which CDK4/6 plays a key role in G1-to-S-phase cell-cycle transition to be targeted (9). It can inhibit tumor growth by decreasing phosphorylation of retinoblastoma (RB) protein and inducing cell cycle arrest at the G1/S phase transition, inducing irreversible growth arrest or cell death when used alone or in combination with other therapies (15, 16) (Figure 2) and also promote anti-tumor immunity (17). Some drugs have been approved by the Food and Drug Administration (FDA) as treatment agents to be combined with letrozole in the treatment of hormone receptor (HR)-positive advanced-stage breast cancer (18, 19). Some clinical trials of CDK4/6 inhibitors in other tumors have achieved initial impressive results (20). CDK4/6 inhibitors are still in the early stage of other cancers, mainly confined to basic experiments and stage I or II clinical trials, such as liposarcoma, lymphoma and many other advanced cancers (21–23). A study indicated that CDK4/6 inhibitors in patients with head and neck squamous cell carcinoma have the objective response rate of 39% (n = 62) (24). Some cell cycle inhibitors also have been used in human clinical trials and achieved success in lung cancer (25, 26). Therefore, this article reviews the mechanisms of CDK4/6 inhibitors in NSCLC, monotherapy using CDK4/6 inhibitors, and the effects of combining them with other drugs in the context of NSCLC treatment.
Monotherapy of CDK4/6 Inhibitors in NSCLC
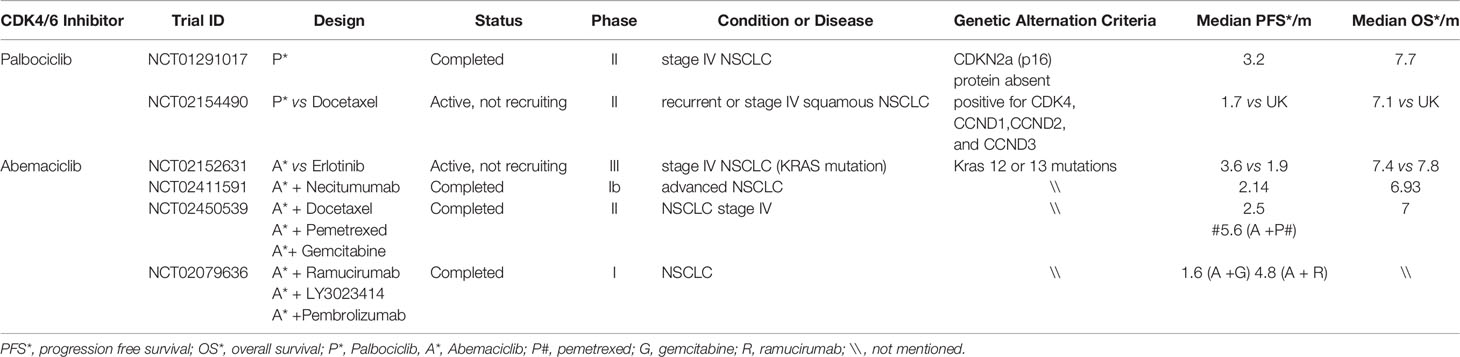
With the recent development of highly specific CDK4/6 inhibitors (Palbociclib, Ribociclib, and Abemaciclib) and the approval of their use by the FDA for advanced metastatic breast cancer, designing multiple clinical trials using these agents for lung cancer have attracted great interest (9). However, effective strategies for formulating appropriate trial designs have not been determined. Thus, proper experiments in suitable animal models and clinical trials are needed. Palbociclib was approved in 2016 in terms of structure, and ribociclib and palbociclib are extremely similar. An in vitro study showed that the inhibitory effects of ribociclib and abemaciclib on CDK4 are stronger than the inhibitory effect of CDK6 and palbociclib is similar (27). Current clinical studies on NSCLC mainly focus on the phases I and II clinical studies of palbociclib and abemaciclib (Table 1).
That NSCLC tumor actively targets the CDKN2a/p16 locus rather than the observed mutational enrichment in this locus due to a selection process during lung carcinogenesis and tumor progression. Hence, several clinical studies have been conducted.
Palbociclib
Palbociclib is a unique selective and promising inhibitor of CDK4 and CDK6 and a cell permeable pyridopyrimidine with oral bioavailability (20, 28). Although CDK4/6 can bind with cyclin D1, resulting in Rb hyperphosphorylation, palbociclib can block Rb phosphorylation and prevent E2F1 release by separating CDK4/6–cyclin D1 complexes, resulting in G1 phase arrest and inhibit tumor growth (29). A phase II clinical study of palbociclib included 19 patients with advanced NSCLC previously treated with p16-null staining and immunohistochemistry, and tumor progression was documented (30). There were 16 evaluable patients who had no objective response, and eight (50%) patients were stable for 4.0–10.5 months. The median progression-free survival (PFS)was 3.2 months, and median overall survival (OS) was 7.7 months. The results showed that palbociclib alone was mainly used as a cell inhibitor inducing aging, but not apoptosis, and the median PFS was equivalent to other available second-line chemotherapeutic drugs (31) and PD-1 inhibitors (32, 33). In addition, the reduction rate of grade 3/4 cytopenia with palbociclib in the treatment of NSCLC was 16%, which was better than that of many effective chemotherapeutic drugs for second- or third-line therapy. A Lung-MAP trial (SWOG S1400) demonstrated the amplification of CDK4 or CCND1/2/3 in patients with squamous NSCLC and tumor. Of the 32 patients included in this study, only two (6%) had a partial response and 38% were stable. The median PFS was 1.7 months, and the median OS was 7.1 months. Unfortunately, in these genomically selected patients, palbociclib did not demonstrate any antitumor activity (12). A phase II pragmatic basket trial demonstrated antitumor activity of palbociclib in patients with NSCLC with CDKN2A alterations. Of the 29 patients who were enrolled, one patient had partial response and six patients with SD were observed, for a disease control rate of 31%. The median PFS was 8.1 weeks, and the median OS was 21.6 weeks. There were 11 patients who had at least one grade 3 or 4 adverse event (AE) or serious AE (SAE) possibly related to palbociclib (most common, cytopenias) (34).
Abemeciclib
As an effective and selective small-molecule inhibitor of CDK4 and CDK6, abemaciclib has a wide range of antitumor activity in preclinical models and acceptable toxicity profile in animals such as mice. Preclinical data showed that the sensitivity of KRAS-mutant NSCLC xenograft models to abemaciclib was higher than that of wild-type KRAS gene expression model (13). Moreover, a JPBA phase I study showed that a single-agent abemaciclib has acceptable tolerability or safety and presented evidence of clinical activity in patients with heavily pretreated metastatic NSCLC (35). In addition, they demonstrated that the combined use of ramucirumab and abemaciclib is consistent with the safety profile of single-agent abemaciclib, with lower hematologic toxicity. The total incidence of neutropenia was 23%, and the incidence of grades 3–4 neutropenia was 10%. A phase III JUNIPER clinical trial was designed according to the result of these studies. In this trial, 453 patients who had stage IV NSCLC with KRAS mutations (codon 12 or 13) and disease progression after two lines of therapy were randomized in a ratio of 3:2 into abemaciclib and erlotinib groups (including a platinum-based regimen). The median OS was similar in both groups (7.4 vs. 7.8 months; HR, 0.97; 95% CI, 0.77–1.22; p = 0.77), and the median PFS was significantly better in the abemaciclib group (3.6 vs. 1.9 months; HR, 0.58; 95% CI, 0.47–0.72; p <0.001). The response rate (8.9% vs. 2.7%; p = 0.01) and disease control rate (54% vs. 32%; p <0.001) were significantly better in the abemaciclib group. In this study, compared with erlotinib, the OS in stage IV NSCLC patients harboring KRAS mutations did not improve. However, the additional studies of abemaciclib in other NSCLC subpopulations or in combination with other drugs are required to increases in response rates and PFS (13).
Combination of CDK4/6 Inhibitors and Other Anti-Lung Cancer Therapies
The disappointing results of palbociclib and abemaciclib in NSCLC clinical trials have prompted studies on the effect of combination of CDK4/6 inhibitions and other therapies. Owing to the unsatisfactory results of single-drug treatments, in-depth study of the pathogenesis of NSCLC, and increasing treatment methods for NSCLC, combinations of CDK4/6 inhibitors have been extensively studied.
Combination of CDK4/6 Inhibitors and Chemotherapy
CDK4/6 inhibitors and chemotherapeutic drugs may have antagonistic effects. For example, CDK4/6 inhibitors in combination with gemcitabine improved antitumor activity without G1 cell cycle arrest in calu-6 xenografts tumor-bearing mice (36). However, another study demonstrated combinations of palbociclib and taxanes at clinically available doses in multiple SqCLC models enhanced antitumor effects by destroying the pRB-E2F signaling pathway (37). Based on preclinical data, a phase Ib clinical study tested abemaciclib in combination with pemetrexed, gemcitabine, or ramucirumab in patients with metastatic NSCLC and confirmed the safety and tolerability of these combinations in previously treated unselected patients with advanced/metastatic NSCLC (38). In these patients, the all-cause high grade (3/4) fatigue occurred in 17–25%.High-grade diarrhea can be well controlled by antidiarrheal treatments and/or dose adjustments.
Combination of CDK4/6 Inhibitors and Immune Checkpoint Inhibitors
The emerge as the times require of immune checkpoint blockade immediately led to the studies of the possible interactions of these therapies with CDK4/6 inhibitors. CDK4/6-targeted therapies have a complex network of immunomodulatory effects on tumor cells and their tumor microenvironment (39). The addition of CDK4/6 inhibitor to chemotherapy/ICI regimens in murine syngeneic tumor models enhanced antitumor response and overall survival compared with chemotherapy, and ICI combinations alone and transient exposure of CDK4/6 inhibition in patients with SCLC during chemotherapy treatment enhanced immune system function by preserving peripheral lymphocyte counts and enhancing T-cell activation (17). These results showed the synergistic antitumor effect of CDK4/6 and immune checkpoint-related inhibitors. The mechanism of CDK4/6 inhibitors combined with immunotherapy may be as follows: First, CDK4/6 inhibitors decrease promoter hypomethylation and inhibit E2F release by inhibiting the proliferation of regulatory T (Treg) cells and the expression of DNA methyltransferase in Treg cells (17). Furthermore, CDK4/6 inhibitors promote tumor cell clearance by enhancing cytotoxic T cells (CTLs) to kill tumor cells (40). Finally, the cyclin D1–CDK4 complex directly phosphorylates speckle-type POZ protein (SPOP), and CDK4/6 inhibitors can enhance the immune escape of tumors by reducing the ubiquitination of SPOP and the degradation of PD-L1 (41). Preclinical research showed that CDK4/6 inhibitors in combination with anti-PDL1 antibodies promote tumor regressions and the effect is accompanied by enhanced antigen presentation, T cell inflamed phenotype, and cytotoxic T cell-mediated clearance of lung cancer cells; moreover, this combination improves the overall survival rates in mouse tumor models (17, 40, 42). All of these mechanisms provide a theoretical basis for the combination therapy of CDK4/6 inhibitors and immune checkpoint inhibitors in NSCLC in the future and related clinical trials (NCT03601598) are ongoing at present (43).
CDK4/6 Inhibitors as Radiosensitizers
Multiple preclinical and small sample clinical studies showed that CDK4/6 inhibitors exhibit a collaborative effect during radiotherapy in vitro and in vivo and show well-tolerated toxicity and promising efficacy in patients (44–47). The potential mechanisms of clinical radiosensitization effects might be apoptosis enhancement, cell cycle progression blockage, and induction of cellular senescence and antitumor immunity (48). A preclinical study showed that abemaciclib and ionizing radiation (IR) had a good radiosensitization effect on tumor cells in proliferative and plateau-phase and tumor xenografts, but had little radiosensitization effect on normal cells, and improve the radiation sensitivity of NSCLC in vitro and in vivo. Abemaciclib inhibited IR-induced DNA damage repair and caused RB-dependent cell cycle arrest; furthermore, the study identified possible predictive biomarkers (p53, RB, and SDF-1) to guide the efficacy and efficacy of the combination therapy, emphasized that CDK4/6 axis is a potential radiation target for NSCLC and warranting the value of abemaciclib as a radiation modifier in clinical trials (49). Therefore, CDK4/6 inhibitors may have different radiosensitization effects in NSCLC, and its mechanisms need to be further assessed. However, most clinical trials of combination therapies are still in the recruitment stage and further work is needed to find the best combination of radiotherapy drugs.
Combination of CDK4/6 Inhibitors and Other Anti-Lung Cancer Drugs
Combinations of CDK4/6 inhibitors and other targeted drugs have broad prospects. PI3K-AKT-mTOR and RASRAF-MEK-ERK pathway inhibitors showed synergistic tumor inhibition in many preclinical vitro and vivo models in NSCLC with CDK4/6 inhibitors (50–52). Palbociclib sensitizes lung cancer cells to EGFR-TKI and gefitinib (25). In addition, the combination of MEK inhibitor (trametinib) and palbociclib has significant anti-CDKN2A-mutant and anti-KRAS-mutant NSCLC activities in preclinical models (53). Moreover, in view of the key role of mTOR in cell growth and proliferation, mTOR inhibitors are considered as good candidates for synergism action with CDK4/6 inhibitors. Combinations of CDK4/6 inhibitors and mTOR inhibitors can enhance growth inhibition and induction of apoptotic cell death in p16-null NSCLC cells (30). A recent study also demonstrated that combined treatment with the CDK4/6 inhibitor and a novel distinctive structure PI3Kα inhibitor through arrest enhancing G1-phase and enhancing inhibition of Rb phosphorylation to against KRAS-mutated NSCLC (54). In addition, several on-going clinical trials are studying advanced NSCLC associated with the combination of CDK4/6 inhibitors with ERK, MEK, or mTOR inhibitors (NCT03170206, NCT02065063, NCT02857270, and NCT03454035) based on these and other promising preclinical data (3).
Prospects and Future Application
CDK4/6 inhibitors may exert an essential role in the treatment of NSCLC. Although some phase I/II clinical trials of CDK4/6 inhibitors in patients with advanced/metastatic lung cancer have not yet achieved positive results, which may be related to the small sample size of clinical trials and the lack of effective biomarkers. Given the preclinical benefits of CDK4/6 inhibitors in molecularly selected subsets, CDK4/6 inhibitors may have another role in the treatment of NSCLC in selected populations based on reasonable biomarkers, combined with radiotherapy and other agents, including growth factor pathway inhibitors and immune checkpoint inhibitors. Besides, the mechanism of CDK4/6 inhibitor resistance and the identification of sensitive predictive markers have also been reported, including acquired RB1 mutations, loss of RB1, loss of function mutations of FAT-1, CCNE1 overexpression, CDK6 overexpression, CCNE1/RB1 ratio, interferon β expression (55), CDK4 phosphorylation and tumor cloning kinetics (2, 56, 57). Therefore, the clinical efficacy of CDK4/6 inhibitors in NSCLC depend on the development of predictive biomarkers and biologically rational combination therapy, which might include the addition of growth factor pathway inhibitors in patients with signal transduction pathway mutations or the addition of immune checkpoint inhibitors in patients with immunostimulatory tumor phenotypes. Based on these, more basic and clinical studies are needed explore the precise beneficiaries of CDK4/6 inhibitors in NSCLC treatment in the future.
Author Contributions
JZ, DX, and YZ collected the references and wrote the manuscript. All authors contributed to the article and approved the submitted version. ZZ and XY acquired funding and supervised this study.
Funding
This work was supported by National Natural Science Foundation of China (No. 81872461).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cheng TY, Cramb SM, Baade PD, Youlden DR, Nwogu C, Reid ME. The International Epidemiology of Lung Cancer: Latest Trends, Disparities, and Tumor Characteristics. J Thorac Oncol (2016) 11(10):1653–71. doi: 10.1016/j.jtho.2016.05.021
2. Gong X, Litchfield LM, Webster Y, Chio LC, Wong SS, Stewart TR, et al. Genomic Aberrations That Activate D-Type Cyclins Are Associated With Enhanced Sensitivity to the CDK4 and CDK6 Inhibitor Abemaciclib. Cancer Cell (2017) 32(6):761–76.e6. doi: 10.1016/j.ccell.2017.11.006
3. Qin A, Reddy HG, Weinberg FD, Kalemkerian GP. Cyclin-Dependent Kinase Inhibitors for the Treatment of Lung Cancer. Expert Opin Pharmacother (2020) 21(8):941–52. doi: 10.1080/14656566.2020.1738385
4. Otto T, Sicinski P. Cell Cycle Proteins as Promising Targets in Cancer Therapy. Nat Rev Cancer (2017) 17(2):93–115. doi: 10.1038/nrc.2016.138
6. Weinberg RA. The Retinoblastoma Protein and Cell Cycle Control. Cell (1995) 81(3):323–30. doi: 10.1016/0092-8674(95)90385-2
7. Kong T, Xue Y, Cencic R, Zhu X, Monast A, Fu Z, et al. Eif4a Inhibitors Suppress Cell-Cycle Feedback Response and Acquired Resistance to CDK4/6 Inhibition in Cancer. Mol Cancer Ther (2019) 18(11):2158–70. doi: 10.1158/1535-7163.MCT-19-0162
8. Fang H, Huang D, Yang F, Guan X. Potential Biomarkers of CDK4/6 Inhibitors in Hormone Receptor-Positive Advanced Breast Cancer. Breast Cancer Res Treat (2018) 168(2):287–97. doi: 10.1007/s10549-017-4612-y
9. O’Leary B, Finn RS, Turner NC. Treating Cancer With Selective CDK4/6 Inhibitors. Nat Rev Clin Oncol (2016) 13(7):417–30. doi: 10.1038/nrclinonc.2016.26
10. Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov (2016) 6(4):353–67. doi: 10.1158/2159-8290.CD-15-0894
11. Besse B, Barlesi F, Demedts I, Fuentes Pradera J, Robinet G, Gazzah A, et al. A Phase 1b Study of Necitumumab in Combination With Abemaciclib in Patients With Stage IV non-Small Cell Lung Cancer. Lung Cancer (2019) 137:136–43. doi: 10.1016/j.lungcan.2019.09.002
12. Edelman MJ, Redman MW, Albain KS, McGary EC, Rafique NM, Petro D, et al. SWOG S1400C (NCT02154490)-A Phase II Study of Palbociclib for Previously Treated Cell Cycle Gene Alteration-Positive Patients With Stage IV Squamous Cell Lung Cancer (Lung-MAP Substudy). J Thorac Oncol (2019) 14(10):1853–9. doi: 10.1016/j.jtho.2019.06.027
13. Goldman JW, Mazieres J, Barlesi F, Dragnev KH, Koczywas M, Goskel T, et al. A Randomized Phase III Study of Abemaciclib Versus Erlotinib in Patients With Stage IV Non-Small Cell Lung Cancer With a Detectable KRAS Mutation Who Failed Prior Platinum-Based Therapy: JUNIPER. Front Oncol (2020) 10:578756. doi: 10.3389/fonc.2020.578756
14. Goldman JW, Shi P, Reck M, Paz-Ares L, Koustenis A, Hurt KC. Treatment Rationale and Study Design for the JUNIPER Study: A Randomized Phase III Study of Abemaciclib With Best Supportive Care Versus Erlotinib With Best Supportive Care in Patients With Stage IV Non-Small-Cell Lung Cancer With a Detectable KRAS Mutation Whose Disease Has Progressed After Platinum-Based Chemotherapy. Clin Lung Cancer (2016) 17(1):80–4. doi: 10.1016/j.cllc.2015.08.003
15. Thangavel C, Boopathi E, Liu Y, McNair C, Haber A, Perepelyuk M, et al. Therapeutic Challenge With a CDK 4/6 Inhibitor Induces an RB-Dependent SMAC-Mediated Apoptotic Response in Non-Small Cell Lung Cancer. Clin Cancer Res (2018) 24(6):1402–14. doi: 10.1158/1078-0432.CCR-17-2074
16. Tempka D, Tokarz P, Chmielewska K, Kluska M, Pietrzak J, Rygielska Z, et al. Downregulation of PARP1 Transcription by CDK4/6 Inhibitors Sensitizes Human Lung Cancer Cells to Anticancer Drug-Induced Death by Impairing OGG1-Dependent Base Excision Repair. Redox Biol (2018) 15:316–26. doi: 10.1016/j.redox.2017.12.017
17. Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, et al. CDK4/6 Inhibition Triggers Anti-Tumour Immunity. Nature (2017) 548(7668):471–5. doi: 10.1038/nature23465
18. Bilgin B, Sendur MAN, Sener Dede D, Akinci MB, Yalcin B. A Current and Comprehensive Review of Cyclin-Dependent Kinase Inhibitors for the Treatment of Metastatic Breast Cancer. Curr Med Res Opin (2017) 33(9):1559–69. doi: 10.1080/03007995.2017.1348344
19. Syed YY. Ribociclib: First Global Approval. Drugs (2017) 77(7):799–807. doi: 10.1007/s40265-017-0742-0
20. Beaver JA, Amiri-Kordestani L, Charlab R, Chen W, Palmby T, Tilley A, et al. FDA Approval: Palbociclib for the Treatment of Postmenopausal Patients With Estrogen Receptor-Positive, HER2-Negative Metastatic Breast Cancer. Clin Cancer Res (2015) 21(21):4760–6. doi: 10.1158/1078-0432.CCR-15-1185
21. Dickson MA, Schwartz GK, Keohan ML, D’Angelo SP, Gounder MM, Chi P, et al. Progression-Free Survival Among Patients With Well-Differentiated or Dedifferentiated Liposarcoma Treated With CDK4 Inhibitor Palbociclib: A Phase 2 Clinical Trial. JAMA Oncol (2016) 2(7):937–40. doi: 10.1001/jamaoncol.2016.0264
22. Leonard JP, LaCasce AS, Smith MR, Noy A, Chirieac LR, Rodig SJ, et al. Selective CDK4/6 Inhibition With Tumor Responses by PD0332991 in Patients With Mantle Cell Lymphoma. Blood (2012) 119(20):4597–607. doi: 10.1182/blood-2011-10-388298
23. Fujiwara Y, Tamura K, Kondo S, Tanabe Y, Iwasa S, Shimomura A, et al. Phase 1 Study of Abemaciclib, an Inhibitor of CDK 4 and 6, as a Single Agent for Japanese Patients With Advanced Cancer. Cancer Chemother Pharmacol (2016) 78(2):281–8. doi: 10.1007/s00280-016-3085-8
24. Adkins D, Ley J, Neupane P, Worden F, Sacco AG, Palka K, et al. Palbociclib and Cetuximab in Platinum-Resistant and in Cetuximab-Resistant Human Papillomavirus-Unrelated Head and Neck Cancer: A Multicentre, Multigroup, Phase 2 Trial. Lancet Oncol (2019) 20(9):1295–305. doi: 10.1016/S1470-2045(19)30405-X
25. Liu M, Xu S, Wang Y, Li Y, Li Y, Zhang H, et al. PD 0332991, a Selective Cyclin D Kinase 4/6 Inhibitor, Sensitizes Lung Cancer Cells to Treatment With Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. Oncotarget (2016) 7(51):84951–64. doi: 10.18632/oncotarget.13069
26. He S, Sharpless NE. Senescence in Health and Disease. Cell (2017) 169(6):1000–11. doi: 10.1016/j.cell.2017.05.015
27. Tripathy D, Bardia A, Sellers WR. Ribociclib (LEE011): Mechanism of Action and Clinical Impact of This Selective Cyclin-Dependent Kinase 4/6 Inhibitor in Various Solid Tumors. Clin Cancer Res (2017) 23(13):3251–62. doi: 10.1158/1078-0432.CCR-16-3157
28. Turner NC, Finn RS, Martin M, Im SA, DeMichele A, Ettl J, et al. Clinical Considerations of the Role of Palbociclib in the Management of Advanced Breast Cancer Patients With and Without Visceral Metastases. Ann Oncol (2018) 29(3):669–80. doi: 10.1093/annonc/mdx797
29. Dange Y, Bhinge S, Salunkhe V. Optimization and Validation of RP-HPLC Method for Simultaneous Estimation of Palbociclib and Letrozole. Toxicol Mech Methods (2018) 28(3):187–94. doi: 10.1080/15376516.2017.1388458
30. Gopalan PK, Villegas AG, Cao C, Pinder-Schenck M, Chiappori A, Hou W, et al. CDK4/6 Inhibition Stabilizes Disease in Patients With P16-Null non-Small Cell Lung Cancer and is Synergistic With mTOR Inhibition. Oncotarget (2018) 9(100):37352–66. doi: 10.18632/oncotarget.26424
31. Kawaguchi T, Ando M, Asami K, Okano Y, Fukuda M, Nakagawa H, et al. Randomized Phase III Trial of Erlotinib Versus Docetaxel as Second- or Third-Line Therapy in Patients With Advanced Non-Small-Cell Lung Cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol (2014) 32(18):1902–8. doi: 10.1200/JCO.2013.52.4694
32. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab Versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
33. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab Versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(2):123–35. doi: 10.1056/NEJMoa1504627
34. Eugene R, Ahn M, Pam K, Mangat M, Elizabeth Garrett-Mayer P, Susan Halabi P, et al. Palbociclib in Patients With Non–Small-Cell Lung Cancer With CDKN2A Alterations: Results From the Targeted Agent and Profiling Utilization Registry Study. JCO Precis Oncol (2020) 4:757–66. doi: 10.1200/PO.20.00037
35. Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, et al. Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients With Breast Cancer, Non-Small Cell Lung Cancer, and Other Solid Tumors. Cancer Discov (2016) 6(7):740–53. doi: 10.1158/2159-8290.CD-16-0095
36. Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, Del Prado M, Lallena MJ, et al. Preclinical Characterization of the CDK4/6 Inhibitor LY2835219: In-Vivo Cell Cycle-Dependent/Independent Anti-Tumor Activities Alone/in Combination With Gemcitabine. Invest New Drugs (2014) 32(5):825–37. doi: 10.1007/s10637-014-0120-7
37. Cao J, Zhu Z, Wang H, Nichols TC, Lui GYL, Deng S, et al. Combining CDK4/6 Inhibition With Taxanes Enhances Anti-Tumor Efficacy by Sustained Impairment of pRB-E2F Pathways in Squamous Cell Lung Cancer. Oncogene (2019) 38(21):4125–41. doi: 10.1038/s41388-019-0708-7
38. Kim ES, Kelly K, Paz-Ares LG, Garrido P, Jalal S, Mahadevan D, et al. Abemaciclib in Combination With Single-Agent Options in Patients With Stage IV Non-Small Cell Lung Cancer: A Phase Ib Study. Clin Cancer Res (2018) 24(22):5543–51. doi: 10.1158/1078-0432.CCR-18-0651
39. Alvarez-Fernandez M, Malumbres M. Mechanisms of Sensitivity and Resistance to CDK4/6 Inhibition. Cancer Cell (2020) 37(4):514–29. doi: 10.1016/j.ccell.2020.03.010
40. Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-Cell Activation. Cancer Discovery (2018) 8(2):216–33. doi: 10.1158/2159-8290.CD-17-0915
41. Zhang J, Bu X, Wang H, Zhu Y, Geng Y, Nihira NT, et al. Cyclin D-CDK4 Kinase Destabilizes PD-L1 via Cullin 3-SPOP to Control Cancer Immune Surveillance. Nature (2018) 553(7686):91–5. doi: 10.1038/nature25015
42. Schaer DA, Beckmann RP, Dempsey JA, Huber L, Forest A, Amaladas N, et al. The CDK4/6 Inhibitor Abemaciclib Induces a T Cell Inflamed Tumor Microenvironment and Enhances the Efficacy of PD-L1 Checkpoint Blockade. Cell Rep (2018) 22(11):2978–94. doi: 10.1016/j.celrep.2018.02.053
43. Ameratunga M, Kipps E, Okines AFC, Lopez JS. To Cycle or Fight-CDK4/6 Inhibitors at the Crossroads of Anticancer Immunity. Clin Cancer Res (2019) 25(1):21–8. doi: 10.1158/1078-0432.CCR-18-1999
44. Huang CY, Hsieh FS, Wang CY, Chen LJ, Chang SS, Tsai MH, et al. Palbociclib Enhances Radiosensitivity of Hepatocellular Carcinoma and Cholangiocarcinoma via Inhibiting Ataxia Telangiectasia-Mutated Kinase-Mediated DNA Damage Response. Eur J Cancer (2018) 102:10–22. doi: 10.1016/j.ejca.2018.07.010
45. Li F, Xu Y, Liu B, Singh PK, Zhao W, Jin J, et al. YAP1-Mediated CDK6 Activation Confers Radiation Resistance in Esophageal Cancer - Rationale for the Combination of YAP1 and CDK4/6 Inhibitors in Esophageal Cancer. Clin Cancer Res (2019) 25(7):2264–77. doi: 10.1158/1078-0432.CCR-18-1029
46. Whittaker S, Madani D, Joshi S, Chung SA, Johns T, Day B, et al. Combination of Palbociclib and Radiotherapy for Glioblastoma. Cell Death Discov (2017) 3:17033. doi: 10.1038/cddiscovery.2017.33
47. Naz S, Cook JA, Mitchell JB. Abemaciclib: A Multi-Functional Radiation Modifier. Oncotarget (2019) 10(12):1230–2. doi: 10.18632/oncotarget.26652
48. Yang Y, Luo J, Chen X, Yang Z, Mei X, Ma J, et al. CDK4/6 Inhibitors: A Novel Strategy for Tumor Radiosensitization. J Exp Clin Cancer Res (2020) 39(1):188. doi: 10.1186/s13046-020-01693-w
49. Naz S, Sowers A, Choudhuri R, Wissler M, Gamson J, Mathias A, et al. Abemaciclib, a Selective CDK4/6 Inhibitor, Enhances the Radiosensitivity of Non-Small Cell Lung Cancer In Vitro and In Vivo. Clin Cancer Res (2018) 24(16):3994–4005. doi: 10.1158/1078-0432.CCR-17-3575
50. Tao Z, Le Blanc JM, Wang C, Zhan T, Zhuang H, Wang P, et al. Coadministration of Trametinib and Palbociclib Radiosensitizes KRAS-Mutant Non-Small Cell Lung Cancers In Vitro and In Vivo. Clin Cancer Res (2016) 22(1):122–33. doi: 10.1158/1078-0432.CCR-15-0589
51. Wong CH, Ma BBY, Hui CWC, Lo KW, Hui EP, Chan ATC. Preclinical Evaluation of Ribociclib and its Synergistic Effect in Combination With Alpelisib in non-Keratinizing Nasopharyngeal Carcinoma. Sci Rep (2018) 8(1):8010. doi: 10.1038/s41598-018-26201-1
52. Chen SH, Gong X, Zhang Y, Van Horn RD, Yin T, Huber L, et al. RAF Inhibitor LY3009120 Sensitizes RAS or BRAF Mutant Cancer to CDK4/6 Inhibition by Abemaciclib via Superior Inhibition of Phospho-RB and Suppression of Cyclin D1. Oncogene (2018) 37(6):821–32. doi: 10.1038/onc.2017.384
53. Zhou J, Zhang S, Chen X, Zheng X, Yao Y, Lu G, et al. Palbociclib, a Selective CDK4/6 Inhibitor, Enhances the Effect of Selumetinib in RAS-Driven non-Small Cell Lung Cancer. Cancer Lett (2017) 408:130–7. doi: 10.1016/j.canlet.2017.08.031
54. Wang Y, Li X, Liu X, Chen Y, Yang C, Tan C, et al. Simultaneous Inhibition of PI3Kalpha and CDK4/6 Synergistically Suppresses KRAS-Mutated non-Small Cell Lung Cancer. Cancer Biol Med (2019) 16(1):66–83. doi: 10.20892/j.issn.2095-3941.2018.0361
55. Cingoz O, Goff SP. Cyclin-Dependent Kinase Activity is Required for Type I Interferon Production. Proc Natl Acad Sci USA (2018) 115(13):E2950–E9. doi: 10.1073/pnas.1720431115
56. Raspe E, Coulonval K, Pita JM, Paternot S, Rothe F, Twyffels L, et al. CDK4 Phosphorylation Status and a Linked Gene Expression Profile Predict Sensitivity to Palbociclib. EMBO Mol Med (2017) 9(8):1052–66. doi: 10.15252/emmm.201607084
Keywords: cyclin D-dependent kinase 4/6 inhibitor, cell cycle, NSCLC, therapy, drugs
Citation: Zhang J, Xu D, Zhou Y, Zhu Z and Yang X (2021) Mechanisms and Implications of CDK4/6 Inhibitors for the Treatment of NSCLC. Front. Oncol. 11:676041. doi: 10.3389/fonc.2021.676041
Received: 04 March 2021; Accepted: 05 July 2021;
Published: 30 July 2021.
Edited by:
Yueming Sun, Nanjing Medical University, ChinaReviewed by:
Renato Franco, University of Campania Luigi Vanvitelli, ItalyPeng-Chan Lin, National Cheng Kung University, Taiwan
Copyright © 2021 Zhang, Xu, Zhou, Zhu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengfei Zhu, ZnVzY2N6emZAMTYzLmNvbQ==; Xi Yang, bnRnZW9yZ2VAcXEuY29t
†These authors have contributed equally to this work
 Jinmeng Zhang1,2†
Jinmeng Zhang1,2† Yue Zhou
Yue Zhou Zhengfei Zhu
Zhengfei Zhu Xi Yang
Xi Yang