- 1Department of Clinical Epidemiology, Shengjing Hospital of China Medical University, Shenyang, China
- 2College of Life and Health Sciences, Northeastern University, Shenyang, China
- 3Clinical Research Center, Shengjing Hospital of China Medical University, Shenyang, China
- 4Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, China
Ovarian cancer (OC) is one of the deadliest gynecological cancers worldwide. Previous observational epidemiological studies have revealed associations between modifiable environmental risk factors and OC risk. However, these studies are prone to confounding, measurement error, and reverse causation, undermining robust causal inference. Mendelian randomization (MR) analysis has been established as a reliable method to investigate the causal relationship between risk factors and diseases using genetic variants to proxy modifiable exposures. Over recent years, MR analysis in OC research has received extensive attention, providing valuable insights into the etiology of OC as well as holding promise for identifying potential therapeutic interventions. This review provides a comprehensive overview of the key principles and assumptions of MR analysis. Published MR studies focusing on the causality between different risk factors and OC risk are summarized, along with comprehensive analysis of the method and its future applications. The results of MR studies on OC showed that higher BMI and height, earlier age at menarche, endometriosis, schizophrenia, and higher circulating β-carotene and circulating zinc levels are associated with an increased risk of OC. In contrast, polycystic ovary syndrome; vitiligo; higher circulating vitamin D, magnesium, and testosterone levels; and HMG-CoA reductase inhibition are associated with a reduced risk of OC. MR analysis presents a2 valuable approach to understanding the causality between different risk factors and OC after full consideration of its inherent assumptions and limitations.
Introduction
Ovarian cancer (OC), the eighth most common type and eighth leading cause of cancer-related mortality in women, is considered the deadliest gynecological cancer. Three main types of OC have been identified, specifically epithelial, germ cell, and sex cord-stromal, with epithelial tumors comprising about 95% of OC cases (1). Epithelial OC is classified into four primary histological subtypes: serous, endometrioid, mucinous, and clear cell carcinoma (1). Serous tumors can be categorized into high-grade serous carcinomas (HGSC) and low-grade serous carcinomas (LGSC) (1, 2), with HGSCs accounting for 70%–80% of all subtypes of epithelial OC and LGSCs for less than 5% cases. Endometrioid, mucinous, and clear cell subtypes account for 10%, 3%, and 10% cases, respectively (2). According to Global Cancer Statistics 2020, the estimated number of new OC cases in 2020 is 313,959, accounting for 3.4% of all new female cancer cases, and the OC death toll in 2018 is estimated as 184,799, representing 4.7% of all female cancer deaths (3). The symptoms of this disease are usually indistinct and diagnosed at the late stages, having spread at the time of clinical diagnosis in 75% of cases (1). The survival rate of patients with OC is related to stage at diagnosis. For instance, in the United States, the 5-year survival rate of a small proportion of women with stage I OC exceeds 90%. The 5-year survival rate of patients with regional disease is 75%–80% while that of patients with distant metastasis is only 25%. Although the prognosis of early OC is good, overall 5-year survival rate is only 48.6%, highlighting the critical need to develop effective prevention strategies to reduce the public health burden of OC.
OC is a multifactorial disorder influenced by both genetic predisposition and modifiable exposures. Identification of causative risk factors amenable to modification is thus essential for prevention of this disease. Randomized controlled trials (RCTs) can be uniformly applied to determine whether certain exposures are causal factors for diseases of public health interest. While RCTs remain the gold standard research design for inferring causality, they are extremely expensive, time-consuming, and associated with a high failure rate (>50% due to lack of efficacy) (4, 5). In addition, RCTs often involve multi-effect interventions (such as drugs that modify multiple biomarkers), which may challenge the causal inferences of any single biomarker. Finally, RCTs are not always feasible or ethical (6, 7). Observational studies provide another opportunity to clarify the relationship between exposure and disease (8). These studies provide a wealth of information on associations between disease exposure and outcome but cannot be interpreted as indicating causality owing to limitations introduced by confounding and reverse causality (9, 10).
To overcome the limitations of observational design, genetic variants have been proposed as potential instrumental variables (IV), usually single-nucleotide polymorphisms (SNPs), to simulate the effects of modifiable environmental exposures on disease susceptibility, referred to as Mendelian randomization (MR) (11). MR offers a number of advantages over observational epidemiology. First, although reverse causality cannot be completely avoided, MR can still avoid the bias caused by reverse causality to a certain extent (12). Second, MR studies are relatively immune to common behavioral, physiological, and socioeconomic confounders owing to random assignment of alleles at meiosis. Third, in most cases, genetic variants are precisely measured and reported and thus not subject to bias and errors, which is especially useful in evaluating risk factors of long-term effects (13). Therefore, MR design resembling RCT can aid in strengthening causal inferences on the roles of modifiable exposures (14), not only with significantly reduced concerns in terms of ethical, applicability, and financial issues but also for examination of causal factors for phenotypes that are not appropriate for RCTs, such as height.
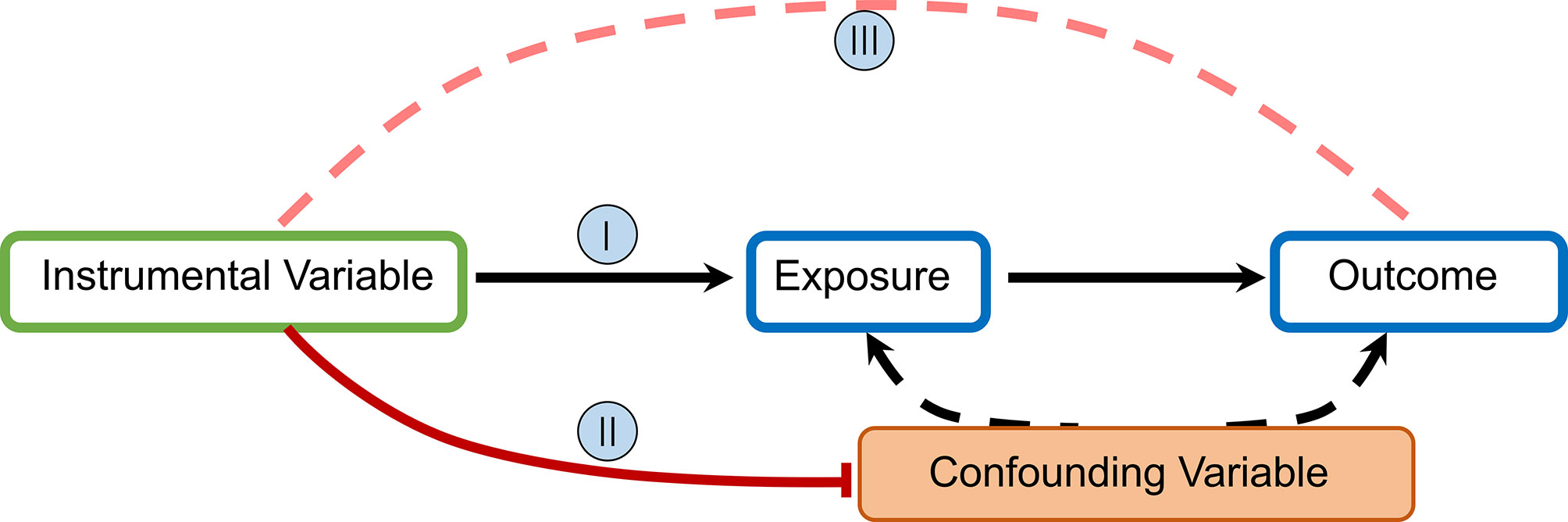
MR uses germline genetic variants as instruments (i.e., proxies) for exposures (e.g., environmental factors, biological traits, or drug pathways) to examine the causal effects of these exposures on health outcomes (disease incidence or progression) (15). Exposure is determined as causal if its association with outcomes is statistically significant and can be explained entirely by the two associations of genetic variants: (1) exposure and (2) outcome (16, 17). The MR technique relies on a number of assumptions for accuracy. The rationale underlying MR and required IV assumptions are as follows:
I. IVs (SNPs being used) should be clearly and quantifiably linked to the exposure(s) in question.
II. IVs should not be linked in any way to confounding variables.
III. IVs should be linked to outcomes only through the exposure(s) in question.
To estimate a causal effect with IV analysis, additional assumptions are required. One such assumption is that:
IV. The associations are linear and not affected by statistical interactions (6).
In MR studies, researchers initially identify and extract information for SNPs associated with exposure at the genome-wide significance level (p = 5×10−8) and subsequently evaluate the relationship between these SNPs and outcomes to obtain odds ratios (OR) and mean differences (Figure 1).
Application of MR in OC
Although epidemiological research has revealed a wealth of biomarkers associated with increased or decreased risk of OC, causality remains largely undefined. Over the past few decades, genome-wide association studies (GWAS) have made an important contribution to the identification of genetic variants associated with numerous potential risk factors for health-related outcomes. GWAS results have facilitated the application of MR in evaluating causal relationships between modifiable exposures and outcomes. During recent years, numerous MR studies focusing on OC have been conducted (18). In addition, development of new methodologies in MR research has challenged the previously reported causality of certain biomarkers. Therefore, it is essential to record research progress and focus on the quality and effectiveness of MR. In this review, we have sorted and analyzed evidence from MR research on OC published in the literature, focused on its advantages and limitations, and designed strict literature retrieval strategies and selection criteria.
Search Strategy and Selection Criteria
Original studies were identified by searching for relevant articles up to February 2, 2021, in the PubMed database. The search algorithms for PubMed database were as follows: “Mendelian randomization” or “genetic instrumental variable” or a related term (e.g., “genetic instrument”) and “Ovarian Cancer” or “Ovarian Neoplasm” or “Ovary Cancer” or “Ovary Neoplasm” or “Cancer, Ovary” or “Neoplasm, Ovary”, with no restriction on subheadings. All retrieved articles were checked for relevant citations and studies not included in the above electronic sources were searched manually. We included studies based on the following criteria: (1) those using MR methodology and instrumental variable analysis to evaluate risk factors of OC and (2) those performed on the basis of observational study design. The search strategy and selection criteria have been checked by two independent authors and, if necessary, the inconsistent part would be judged by third authors. A total of 30 articles were finally included and classified according to type of exposure (Table 1).
Causality Between Life Habits and OC Risk
Alcohol Consumption
Alcohol is hypothesized to promote ovarian carcinogenesis based on its potential to increase circulating levels of estrogen and other hormones through its oxidation by-product acetaldehyde, which may act as a co-carcinogen, induction of cytochrome P450 enzymes involved in activation of liver carcinogens, and depletion of folate (49). In contrast, alcohol is reported to prevent ovarian carcinogenesis by decreasing follicle-stimulating hormone levels (50). Observational studies do not support association of alcohol intake with increased risk of OC (51–53). Interestingly, in a subgroup analysis on multiple subpopulations, low alcohol intake was associated with reduced risk of OC while high alcohol intake had the opposite effect (54). Limited reports to date have focused on the causal associations between alcohol and OC risk.
Alcohol is degraded to acetaldehyde in the liver by alcohol dehydrogenase (ADH1) and then to acetate by acetaldehyde dehydrogenase (ALDH2). Carriers of the A-allele of ADH1B rs1229984 consumed less alcohol per week (48). Therefore, early MR studies often use rs1229984 as an instrumental variable. A two-sample MR study based on participants of European ancestry, single instrument MR using rs1229984 and multiple instrument MR using 34 SNPs on alcohol consumption and epithelial OC showed no causal evidence of association (48). In the other two MR studies, similar results were obtained after eliminating interference of potential confounding factors such as body mass index (BMI), smoking, and education (39, 40).
Cigarette Smoking
A number of epidemiological studies on epithelial OC have shown that smoking increases risk of OC, but only for the mucinous subtype. Significantly increased risk of invasive mucinous and borderline mucinous OC among current smokers has been reported (55), shown to increase with increased duration of smoking and decline with time after smoking cessation (56). In other studies, smoking was not associated with risk of serous OC and current smokers had a 20% lower risk of developing endometrioid and clear cell OC (57, 58).
An MR study using 115 SNPs from participants of European ancestry recruited from 14 countries reported that lifetime smoking exposure was associated with increased risk of invasive epithelial OC. In subtype-specific analyses, evidence for association of smoking with high grade serous cancer (HGSC), but not the mucinous subtype, was obtained (29). Another MR study on smoking and OC risk with subjects of European descent reported no causal evidence (39).
Coffee
Coffee consumption is suggested to be associated with decreased estrogen circulation in pre- and postmenopausal women. Its intake is linked with obesity, metabolic syndrome, and type 2 diabetes as well as liver fibrosis, cirrhosis, and specific types of cancer, including breast, colorectal, lung, endometrial, and prostate cancer. Given that elevated estrogen has been long suspected to increase the risk for OC, coffee consumption may decrease this risk (59). Additionally, risk could be lower because coffee contains flavonoids, and both flavonoids and caffeine have anti-carcinogenic properties. Previous observational studies have shown that coffee intake is potentially associated with reduction of cancer risk. However, prospective studies on the relationship between intake of caffeine and different types of coffee and OC risk have reported conflicting results (60). MR research could aid in clarifying whether this association is causal.
In 2018, Ong et al. (26) conducted MR analysis of moderate coffee consumption and OC risk among subjects of European ancestry. Their results showed no evidence of a strong association between EOC risk and genetically predicted coffee or caffeine levels. In 2019, Ong and co-workers performed a large-scale MR study in a Caucasian British population, with the aim of understanding the causal link between coffee consumption and various cancer types. After several experiments, corrections and meta-analysis, the results of MR remained unchanged. The authors propose that the relationship between coffee intake and disease outcome may have changed due to smoking behavior (33).
Causality Between Anthropometric Characteristics and OC Risk
Previous studies suggest that anthropometric characteristics are related to OC risk and prognosis (55). While several studies have focused on the role of anthropometric characteristics in risk of OC, the findings to date are inconsistent (55).
BMI
Observational studies have revealed an association between BMI and various cancer types. In 2014, fat index was identified as a potential risk factor for OC by World Cancer Research Fund/American Institute for Cancer Research (61). Conversely, according to the US National Cancer Institute, OC is not considered an obesity-related disease. Similarly, the American Cancer Society lists OC as only possibly being linked to overweight or obesity (62). Overall findings from substantial research on adiposity (primarily adult BMI) suggest only a weak positive association, with stronger correlations observed for population-based case–control studies compared to prospective studies. Relatively few studies have conducted detailed examinations of other adiposity-related factors, such as childhood BMI, birth weight, and waist–hip ratio (WHR) (63). The mechanisms by which obesity leads to OC risk remain poorly understood, and the issue of whether associations between obesity and cancer in observational studies are causal is currently unclear.
An MR study published in 2016 with data (all European ancestry) from FOCI and large-scale GWAS of adiposity-related traits comprehensively analyzed the causal relationship between adiposity at different life stages and OC risk. The group reported potential associations of genetic scores for higher adult BMI with increased risk of overall OC but failed to show strong evidence of associations between genetically predicted birth weight, childhood BMI or WHR, and OC risk (21). In 2016, an MR study on the BMI of European adults in relation to risk of different subtypes of OC was published showing that higher genetically predicted BMI was associated with increased risk of non-HGSC but not HGSC cases (22). Secondary analyses stratified by behavior/subtype suggested that consistent with observational data, the strongest association was observed for low-grade/borderline serous OC. Consistent with findings in the general population, MR analysis of height and BMI as modifiers of OC risk in BRCA1 and BRCA2 mutation carriers revealed a positive association between BMI and OC risk in premenopausal BRCA1/2 mutation carriers (32). Subsequent MR analysis showed strong evidence of an association of BMI with invasive epithelial OC. Furthermore, association of BMI with HGSC, endometrioid carcinoma, and low malignant potential tumors but not other subtypes was observed. However, MR-Egger analysis showed little evidence of horizontal pleiotropy (29).
Height
Changes in sex hormones in females during their 20s and 30s are important in the pathogenesis of epithelial OC. Height is strongly influenced by the peripubertal hormonal milieu and reflects pubertal hormonal levels. Observational studies support an association of increased height in adults with higher risk of OC (64). Reports of the 2014 World Cancer Research Project Fund/American Institute for Cancer Research have documented convincing evidence of a correlation between adult height and increased OC risk (55). However, these conventional observational studies are subject to inherent bias, including selection bias, differential and non-differential reporting bias, and confounding.
In contrast, an earlier MR study demonstrated little evidence that height is associated with risk of aggressive epithelial OC. In analyses examining histotypes and low malignant potential tumors, significant association of height with clear cell carcinoma was observed, which was robust in various sensitivity analyses, but not with other subtypes (29). In 2018, Dixon-Suen et al. published an MR study on height and OC risk based on data from 16,395 European women with primary ovarian/fallopian tube/peritoneal cancer and 23,003 controls from 39 OCAC studies. The group concluded that greater genetically predicted height was associated with increased OC risk, both overall and separately for invasive and borderline tumors. Among BRCA1/2 mutation carriers, no causal relationship between height and OC risk was observed (28).
Causality Between Reproductive Factors and OC Risk
Numerous studies have been performed to establish whether reproductive factors are associated with risk of OC as a gynecological tumor. Infertility has been consistently identified as a risk factor for OC and the use of oral contraceptives, parity, and tubal ligation shown to reduce the risk of disease. In addition, risk of OC is related to use of a number of hormone drugs. Taking into account the effects of pregnancy and use of oral contraceptives on risk of OC, it is reasonable to assume that age at menarche and natural menopause are potential risk factors (65, 66).
Age at Menarche
The “incessant ovulation” hypothesis suggests that delaying the age of menarche may reduce the number of ovulations, thereby reducing risk of OC. Moreover, levels of sex hormones (such as progesterone and androgens) show changes during childhood and adolescence, which are thought to play an important role in the etiology of OC. In 2013, a meta-analysis including 22 case–control and 5 cohort studies on age at menarche and OC risk supporting an inverse relationship between menarche and risk of OC was published. An inverse association between menarche age and OC risk has been reported in the majority of subgroups, but limited to invasive and borderline serous OC (65, 67).
Another article showed evidence for association of earlier age at menarche with risk of invasive epithelial OC in inverse-variance-weighted (IVW) models. However, horizontal pleiotropy may bias the IVW estimate. In studies examining invasive epithelial OC histotypes and low malignant potential tumors, evidence for association of earlier age at menarche with endometrioid carcinoma was obtained, which was robust in MR-Egger, weighted median, weighted mode, and leave-one-out analyses (29). MR analysis of women of European descent revealed a strong reverse genetic correlation between age at menarche and BMI. Meanwhile, increasing age at menarche adjusted for genetically predicted BMI was associated with lower risk for OC, in particular, serous OC and endometrial cancer (24). Further MR analysis of Chinese genome-wide association studies and women of European descent revealed a causal relationship between earlier age at menarche and epithelial OC in both Chinese and European populations (34).
Age at Natural Menopause
Menopause is permanent cessation of the menstrual cycle, marking the end of female reproductive life. In addition to changes in related sex hormone levels, the timing of menopause can also be applied to predict future health outcomes, such as risk of hormone-related cancers. Earlier menopause may be related to increased risk of OC. This theory is based on the gonadotropin hypothesis for pathogenesis of OC, which predicts that ovarian aging, accompanied by higher concentrations of follicle-stimulating and luteinizing hormones, increases the risk of OC (68). Previous MR analysis of individuals of European descent showed little evidence that late natural menopause is associated with risk of aggressive epithelial OC. However, in subtype-specific analysis, evidence of a potential association of later age of natural menopause with risk of endometrioid carcinoma was obtained (29).
Parity
Past epidemiological studies have shown that parity is associated with the occurrence of ovarian cancer. Nulliparity and low parity are associated with an increased risk of ovarian cancer. Parous women have a 30%–40% lower risk of developing ovarian cancer, and an additional protective effect is seen with increasing parity (58). Studies have shown that after the first pregnancy, the risk of ovarian cancer is related to the number of pregnancies, and every pregnancy is related to a reduced risk of ovarian cancer (69). Conversely, MR studies show that there is no relationship between parity and ovarian cancer risk (29).
Causality Between Pathological Conditions and OC Risk
Endometriosis
Endometriosis is a chronic, estrogen-dependent progressive disease characterized by the presence of endometrioid tissue, glands, and interstitium outside the uterine cavity. In addition to serious adverse effects on female health and wellbeing, increased risk of OC development cannot be overlooked. Endometriosis, in particular, ovarian endometriosis, is suggested to increase the risk of malignant tumors. Two main pathways have been proposed to describe the potential association between OC and endometriosis: (1) the two diseases coexist and are the result of common risk factors and their effects and (2) endometriotic cells gradually transform into cancer cells (70). Numerous epidemiological studies have reported a significant increase in incidence of OC in patients with endometriosis. Subsequent retrospective studies consistently demonstrated higher incidence of endometriosis in patients with OC (58). A literature review summarized these findings and indicated that high risk of cancer development was attributable to elevated estrogen concentrations leading to cystic malignant hyperplasia and/or ARID1A gene (SWI/SNF family member) mutations and, consequently, loss of BAF250a expression. Therefore, further exploration of the relationship between endometriosis and OC from a genetic perspective is necessary (70).
Our MR analysis include reports that endometriosis is associated with risk of OC. Strong evidence of an association of genetic liability to endometriosis with increased risk of invasive epithelial OC was obtained in these studies. Subtype-specific analyses further confirmed significant association with clear-cell carcinoma and potential association with endometrioid carcinoma, low malignant potential tumors and HGSC. Findings on invasive epithelial OC and clear-cell carcinoma were reported based on sensitivity analyses examining horizontal pleiotropy whereas somewhat inconsistent effect estimates were found for endometrioid carcinoma, low malignant potential tumors, and HGSC. Analyses employing Steiger filtering provided strong evidence that the causal direction was from genetic liability to endometriosis to invasive epithelial OC whereas the causal direction could not be clearly established for clear-cell carcinoma (29).
Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) is a common hormonal disorder affecting 5%–8% women of reproductive age. A population-based case–control study highlighted the possibility of risk of OC in women with PCOS, which was not supported by other studies (71). Recently, the Ovarian Cancer Association Consortium (OCAC) reported decreased risk of invasive OC among women with self-reported PCOS (71, 72). The conflicting results obtained to date highlight the necessity for further research.
Two recent MR analyses on PCOS and OC risk may contribute to clarification of this issue. The first article provided little evidence that genetic susceptibility to PCOS affects the risk of invasive epithelial OC (58). Further subtype-specific analyses revealed an inverse association of genetic liability to PCOS with endometrioid carcinoma, which remained robust in sensitivity analyses. In contrast, association of PCOS with low-grade serous carcinoma was indicated but not clearly detected across all sensitivity analyses in IVW models, suggesting the presence of horizontal pleiotropy or potentially reflecting limited statistical power in these analyses (29). The second study used 14 SNPs to analyze PCOS and risk of OC in women of European descent and demonstrated an inverse association between genetically predicted PCOS and risk of invasive OC. Subtype-specific analyses disclosed the strongest inverse association between genetically predicted PCOS and endometrioid tumors (30).
Schizophrenia
For more than 100 years, the debate on whether schizophrenia can reduce the risk of cancer has continued. A number of previous studies indicate that schizophrenia contributes to prevention of cancer. Genetic research additionally supports an inverse correlation between schizophrenia and cancer, including evidence of common protein transcription pathways of the two diseases (73). However, epidemiological studies have not validated this correlation, because no significant differences in cancer risk of patients with varying levels of schizophrenia have been identified (74, 75). A number of researchers suggest that the reduction in cancer risk is attributable to protective genetic effects of schizophrenia while others believe that reduced risk is related to the drugs used to treat schizophrenia (73). From this viewpoint, it is necessary to study schizophrenia in relation to risk of cancer from a genetic perspective.
Choline metabolism disorders in association with schizophrenia and epithelial OC are documented. A bidirectional MR analysis of epithelial OC (data from six OCAC and two Consortium of CIMBA projects) and schizophrenia [Schizophrenia Working Group of the Psychiatric Genomics (76)] highlighted an association of schizophrenia with weaker but increased risk of epithelial OC. Moreover, in subtype-specific analyses, schizophrenia was shown to be associated with increased risk of high-grade serous OC (31).
Vitiligo
Vitiligo is an autoimmune disease characterized by selective destruction of melanocytes leading to depigmentation of skin. The association between vitiligo and skin cancer has been discussed previously, but findings to date are inconsistent. The potential correlation between vitiligo and risk of other cancer types has received limited research attention. A recently published MR analysis of vitiligo and cancer risk in European populations suggests a protective role of vitiligo against development of OC (35).
Type 2 Diabetes
Several epidemiological studies support an association between type 2 diabetes and increased risk of some types of gynecologic neoplasms, such as endometrial, cervical, ovarian, and vulvar cancer. Insulin resistance, chronic inflammation, and high levels of free ovarian steroid hormones may be among the potential mechanisms underlying this complex relationship (77). In the MR analyses included, there were two studies that mentioned type 2 diabetes and OC risk and showed no evidence of a causal relationship (29, 45).
Causality Between Nutritional Factors and OC Risk
Nutritional factors are related to OC, and improper lifestyle choices can exacerbate disease progression. Therefore, assessment of the impact of diet on risk of OC is of critical importance to the public, clinicians, and research and health institutions (78). MR research on nutritional factors and OC risk could provide a fundamental understanding of this association.
Vitamin A
Vitamin A activity is important for normal control of cellular differentiation and proliferation and hypothesized to modify cancer risk. Interestingly, a previous study exploring the correlation between vitamin A levels and risk of OC demonstrated no association while a subsequent meta-analysis reached the opposite conclusion (79, 80). A further MR analysis using two SNPs on a Caucasian population showed no causal link between vitamin A levels and risk of OC (37).
Vitamin E
Vitamin E, also designated tocopherol, has strong antioxidant activity that protects cells against oxidative DNA damage and mutagenesis, thereby preventing the onset of specific tumors. Vitamin E also contains putative anti-cancer and anti-mutant compounds and were suggested to play a role in the prevention of cancer. However, conflicting data have been reported showing that vitamin E is not related to OC (80, 81). An MR analysis focusing on three SNPs in a European population showed that this study showed no association between vitamin E and OC risk. However, in a study conducted on invasive epithelial OC and low malignant potential cancers, genetically predicted vitamin E levels were inversely associated with these cancer types (37).
B Vitamins
B vitamins (including folate, thiamin, riboflavin, niacin, vitamin B6, and vitamin B12) are essential micronutrients purported to influence carcinogenesis through regulation of one-carbon metabolism. Women in the highest quintile of folate and vitamin 6 intake were shown to have lower risk of OC than those in the lowest quintile (80, 82). However, an MR analysis of European populations showed that higher vitamin B12 concentration was associated with increased risk of low malignant ovarian tumors while other B vitamins (B6, folate) are not associated with risk of OC (37).
Vitamin D
Vitamin D has attracted widespread scientific interest in cancer prevention research. Data from in vitro and animal model studies support anti-tumor effects of vitamin D (83). Vitamin D functions by activating the nuclear vitamin D receptor, which is ubiquitously expressed and regulates the growth, differentiation, and apoptosis of normal and tumor cells. However, evidence from case–control and cohort studies so far suggests no effect of vitamin D on OC risk and survival (84).
The results of the three earlier MR studies may provide further insights into the potential association of vitamin D with OC. In 2016, Ong et al. failed to find a link between vitamin D and the risk of ovarian cancer (20). In 2017, Dimitrakopoulou et al. conducted an MR study of vitamin D using four SNPs to evaluate multiple cancer risk in women of European ancestry. The group failed to show a causal relationship between circulating vitamin D concentrations and OC risk (23). Similarly, a study published by Ong et al. in 2018 still failed to find a link between vitamin D and the risk of ovarian cancer (27). In 2021, an MR study by Ye et al. (46) using 104 SNPs on women of European descent showed that higher circulating vitamin D concentrations can reduce the risk of OC. The latest study by Ong and co-workers in the face of horizontal pleiotropy involving analysis with 74 SNPs further validated this result. Increase in vitamin D concentration may thus be related to decreased risk of OC (47).
β-Carotene
As a main vitamin precursor, vitamin A carotenoid, β-carotene is metabolized into biologically active retinol and other vitamin A compounds essential for maintenance of normal human physiology and homeostasis. Previous in vitro and in vivo studies have shown that β-carotene is a powerful antioxidant that can neutralize free radicals in cells involved in the development of chronic diseases. However, similar to other active substances with antioxidant properties, variable results have been obtained on potential associations of β-carotene and various cancers (85, 86).
Data from MR studies on β-carotene and OC may provide evidence for related research. In standard IVW analysis, genetically predicted serum β-carotene levels were positively associated with invasive epithelial OC, mucinous carcinoma, and endometrioid carcinoma. Conversely, β-carotene levels were negatively correlated with low-grade serous carcinoma, low malignant potential tumors, and mucinous borderline tumors (37).
Selenium
Selenium is an important trace element in the human body. A lack of trace elements necessary to maintain balance in the body, such as cofactors, and accumulation of specific toxic metals, may destroy resistance of the host to cancer. For example, selenium is a critical component of selenoproteins and plays a key role in resistance to oxidative stress. A number of epidemiological studies support an inverse correlation of selenium levels with cancer, in particular, breast cancer (87). Similar to the results of other epidemiological investigations, no causal relationship between selenium and OC was observed in the MR study (37).
Phosphorus
Phosphorus is one of the main elements that widely affect health of organisms. Almost all natural foods contain phosphorus in the form of inorganic phosphate or organic molecules. Several tumor types are reported to be associated with high phosphorus intake, including lung, colon, breast, ovary, and endometrial cancer, among others (88). In contrast, no causal link between circulating phosphorus concentrations and risk of OC was detected in an MR study (37).
Metal Elements
Iron is an essential element for numerous cellular processes. Imbalance in homeostasis attributable to iron overload is harmful to the body (89) and believed to contribute to the onset of cancer. Considering the known functions of oxidative stress, DNA repair, cell cycle regulation, and angiogenesis, trace metal concentrations in the diet (including zinc and copper) can affect cancer risk. Previous studies clearly suggest that circulating zinc and copper status are associated with initiation of OC (90, 91). Increasing evidence supports the synergistic roles of calcium and vitamin D in physiological processes. A recent randomized clinical trial reported that calcium supplementation reduces the risk of all-cause cancer in women and simultaneous supplementation with calcium and vitamin D exerts greater protective effects (92, 93). In addition, accumulating literature indicates that the balance between calcium and magnesium intake (Ca:Mg ratio) may modify the relationship between calcium and magnesium intake and risk of various outcomes (94).
Although various avenues of research on metal elements in relation to OC are ongoing, MR analysis remains an important means to clarify causal relationships. The MR study specified above highlighted an association of increase in magnesium concentration with decreased risk of epithelial OC. However, no causal relationship has been uncovered between other metal ions and risk of OC (37). Notably, a recently published MR study using 21 SNPs as instrumental variables on circulating copper and zinc and risk of OC in subjects of European ancestry showed novel results distinct from previous findings. Their data suggest that the circulating zinc concentration is causally related to risk of OC, in particular, HGSC (43).
Causality Between Biomarkers and OC Risk
C-Reactive Protein
C-reactive protein (CRP) is a highly sensitive and widely used systemic marker of inflammation. The protein is mainly produced by liver cells, together with other acute phase proteins, and released into the circulatory system in response to tissue damage and inflammation. Systematic reviews and meta-analyses have validated the utility of serum CRP levels as an effective indicator of risk of OC (95). However, further research is essential to clarify the causal relationship between CRP and risk of OC and the role of CRP in etiology of disease.
MR analysis conducted on a European population showed that despite no evidence that C-reactive protein affects risk of invasive epithelial OC, analyses examining histotypes and low malignant potential tumors suggested an inverse association of C-reactive protein with endometrioid carcinoma. C-reactive protein was not clearly associated with other histotypes or low malignant potential tumors (29).
Sex Hormone Binding Globulin
The sex hormone binding globulin (SHBG) gene regulates its effect by regulating the bioavailability of sex steroid hormones in target tissues (such as ovary). Hormone stimulation of ovarian epithelial cells is proposed as a mechanism underlying the development of OC. According to animal and in vitro studies as well as epidemiological observations, available evidence that sex steroids play a role in OC is mainly indirect and the precise relationship between circulating levels of sex steroids and risk of OC is yet to be established (96). An earlier MR analysis of a population of European descent showed little evidence of an association of genetic liability to sex hormone binding globulin with OC or its subtypes (29). In 2020, an MR analysis of testosterone and cancer showed the same results (41).
HMG-CoA Reductase
Statins are widely used to treat hypercholesterolemia. These drugs inhibit 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR), an enzyme necessary for synthesis of mevalonate (97). HMGCR is essential for cellular synthesis of cholesterol and various non-steroidal isoprenoid derivatives involved in proliferation, differentiation, and survival (98). Both in vitro and in vivo studies have shown that statins inhibit cancer cell growth by inducing apoptosis and inhibiting cell cycle progression through multiple cell signaling pathways (99). MR studies could be effectively used to explore the causal relationship between HMG-CoA reductase inhibition and risk of OC. An MR study in which all participants were of European descent (median age of the cohort, 41.5 to 59.0 years) showed that genetically proxied HMG-CoA reductase inhibition equivalent to 1 mmol/L (38.7 mg/dl) reduction in LDL cholesterol is associated with lower odds of epithelial OC. Similarly, in BRCA1/2 mutation carriers, genetically proxied HMG-CoA reductase inhibition was associated with lower OC risk (36).
Insulin-Like Growth Factor 1
Due to the increase in cardiovascular, endocrine, and metabolic diseases, such as metabolic syndrome, diabetes and polycystic ovary syndrome, the prevalence of insulin resistance continues to increase. Several studies support a link between insulin resistance and OC. Insulin resistance is reported to be related to ovarian steroid hormone imbalance and inflammation in diabetic patients and gynecological malignancies. Effective control of insulin resistance could therefore prevent various gynecological cancers. However, contrary to these findings, no association between insulin-like growth factor 1 (IGF-1) or binding protein 3 (IGFBP-3) and OC was identified in other studies (100). The causal link between IGF-1 and the risk of OC is also an issue of concern. A previous MR study on insulin-like growth factor-1 and site-specific cancer risk in a population of European descent demonstrated no significant association between genetically predicted IGF-1 levels and 14 other cancers (including OC), with the exception of colorectal cancer (44).
Testosterone
Testosterone is also a key hormone in women. In addition to being an essential precursor for estradiol biosynthesis, testosterone directly acts as an androgen and exerts physiological effects on both reproductive and nonreproductive tissues in women. The role of endogenous androgens in ovarian carcinogenesis is not well understood at present. A number of reports have shown no correlation between androgens and overall risk of invasive epithelial OC while other studies suggest that androgens are both protective and carcinogenic (101, 102). MR research conducted from a genetic perspective may provide constructive perspectives on the relationship between testosterone and OC risk. An MR study on the impact of testosterone on diseases in both sexes highlighted that genetically higher levels of testosterone are harmful to women with metabolic diseases and increase the risk of endometrial cancer but reduce risk and of OC (41).
Arachidonic Acid
Arachidonic acid (AA) is metabolized by cyclooxygenases and lipoxygenases to proinflammatory eicosanoids that modulate tumor cell proliferation, differentiation, and apoptosis according to experimental research. AA is a polyunsaturated fatty acid present at high concentrations in the OC microenvironment and associated with poor clinical outcomes. Several studies support its utility as a therapeutic target for intervention and prognostic indicator of OC (103). However, an MR study on female patients of European descent revealed no association of AA with OC risk (38).
Circulating Adipokine Concentrations
Obesity is considered a chronic inflammatory state characterized by continued infiltration of adipose tissue by macrophages and other immune cells leading to increased or decreased adipose secretion of adipokines [such as adiponectin, leptin, and plasminogen activator inhibitor-1 (PAI-1)] that may be linked to cancer development (104, 105). While accumulating research suggests that obesity presents an important risk factor for development of OC (61, 62), the underlying molecular mechanisms are not fully understood. Obesity is proposed to lead to increased insulin signaling, inflammation, enhanced availability of lipids, and changes in adipokine signaling, resulting in transformation of normal epithelial cells into aggressive tumor cells (106). Conversely, a previous large-scale MR study on circulating adiponectin and five obesity-related cancer types does not support an association of tumor progression with concentrations of circulating adiponectin, leptin, sOB-R and PAI-1. The causal relationship between circulating adipokines and development of obesity-related cancers (including OC) is yet to be established (42).
Telomere Length
Telomeres, which protect the physical integrity of linear chromosomes, are shortened with each cell division, a process that may be accelerated by damage incurred by oxidative stress. Tissue-based studies have revealed a pattern of telomere shortening, genomic instability, and upregulated telomerase expression in many tumor types, including OC. As cells progress from noninvasive precursor lesions to cancer, telomere shortening is a common phenomenon of the early stage of malignant transformation. Prospective studies suggest that greater circulating leukocyte telomere length is associated with lower risk of OC, especially for non-serous and rapid death cases. However, no evidence showing that overall telomere length is causally related to the risk of OC is currently available (107). In 2015, an MR study on telomere length in relation to common cancers in subjects of European descent was published. The study used 11 SNPs as instrumental variables and showed no causal relationship between telomere length and OC and its subtypes (19). However, a more recent MR study published in 2017 using 16 SNPs as instrumental variables on subjects of European descent showed a significant causal relationship of longer telomere length with increased risk of serous low malignant potential OC (25).
Discussion
MR is effective in reducing reverse causality and confounding variables and has gradually become an increasingly useful tool in epidemiological research. Moreover, MR analysis can be effectively used to analyze exposures that are not easy to investigate in some RCT and observational studies (such as height and BMI) (11, 16). The key to MR analysis is use of SNP as an instrumental variable to explore the relationship between exposure and results. Therefore, even under conditions of exploring the same exposure, when different SNPs or different numbers of SNPs are included, the results of MR analysis may differ, which may explain the variable findings discussed above. According to the classification of risk factors, we have sorted out the MR research and research results related to OC from 2015 to the present in detail. Readers can directly and comprehensively understand the application of MR research in the field of OC by reading this article.
A straightforward and common way of performing MR is called the ratio of coefficients or Wald method. The causal effect is triangulated by dividing the coefficients of regression of the outcome on the IV by the regression of the exposure on the IV (108). This method can be performed using summary-level data, without the need for individual-level data (108). Two-stage least-squares method is another method of performing MR analysis. Two-stage least-squares method involves two stages of regression: The first is from the IVs to the exposure, and the second is from the exposure to the outcome (108). However, this method requires individual-level data and becomes biased when at least one invalid IV is used (109).
Despite the fact that the inverse-variance weighting method gives higher weighting to SNPs, it makes the standard errors in the IV-outcome regression smaller (110). A number of limitations must be considered. A common issue is horizontal pleiotropy, which is difficult to avoid in MR research. Horizontal pleiotropy indicates that instrumental variables are not directly related to results through exposure, which violates the third hypothesis of instrumental variables (111). For the horizontal pleiotropy of one-sample MR, the Q test has a good test effect, especially when the data set is large, but the Q test cannot explain the origin of the horizontal pleiotropy (112). Some of the MR studies we included use the Q test, such as Yarmolinsky et al. (36). Another method that serves as a sensitivity analysis is an adaptation of Egger regression called MR-Egger. It can be used to detect bias that results from horizontal pleiotropy based on the assumption that any pleiotropic effects from IVs on the outcome are independent of the exposure (113). This method is widely used in the studies we included. In addition, in recent years, such as Larsson et al. (44), 2020, MR-PRESSO can minimize and correct the level of pleiotropy, but only if the traits that cause horizontal pleiotropy was known a priori (114). On this basis, the weighted median method gives consistent results when at least 50% of the IVs are valid (109) and weighted mode methods can infer a causal effect, even if the majority of IVs are invalid (115).
In addition, the bias in MR can also originate from assortative mating, that is, nonrandom matching between spouses (116). Whether it is a single-trait assortative mating, for example, tall women are more likely to select tall men, or a cross-trait assortative mating (117), for example, women with high intelligence test scores select taller men in research (118), results will be biased due to the non-random nature of this mating. This kind of bias is more common in MR studies where appearance characteristics such as height are used as exposure factors (119). Unfortunately, the two height-related MR studies included in our study did not consider the issue of assortative mating. In these two studies, statistical methods were not used to deal with the bias caused by assortative mating.
Similarly, linkage disequilibrium, defined as a nonrandom association between alleles at a genetic locus on a chromosome, which violates the basic assumption of instrumental variables (6), is a common occurrence. The Bayesian test that can be used to determine whether the association is the result of a colocalized SNP may also reduce the linkage disequilibrium bias in MR analysis (120). As well as setting a maximum pairwise linkage disequilibrium threshold for SNP inclusion, methods such as penalized logistic regression have been described as a means of selecting SNPs based on the knowledge of linkage disequilibrium (121). Some of the MR studies we included use the penalized logistic regression, such as Ong et al. (20). In addition, the winner’s curse is also a situation that has sometimes appeared in past MR studies. In the context of GWAS, the winner’s curse refers to the situation that usually only the main SNP with the smallest P value is reported, and other important SNPs may not even be mentioned (122). This makes the statistical ability of MR analysis insufficient. This situation often occurs in one-sample MR analysis due to chance correlation between instrumental and confounding variables during the discovery stage of the GWAS (123). Two-sample MR analysis can solve this problem well. Most of the MR analyses we have included are two-sample MR analyses.
Weak instrument bias occasionally appears in MR research, such as the IVs explain only a small part of the resulting phenotype (124). This then leads to a bias towards the confounded observational association or the null hypothesis, respectively, depending on whether one- or two-sample MR was used (123). Therefore, the F-statistic regression of the exposure on the IV is generally used to define strength, defining an instrument as being weak with a score lower than 10 (125). The I2 statistic may be used to check for weak instrument bias in MR-Egger analysis; values closer to 0 may be indicative of weak instrument bias (126).
In the context of MR analysis, the collider is a variable, which is the causal downstream of exposure and result (15). When trying to make statistical adjustments or conditioning to the collider, bias may occur (127, 128). This means that sample selection may introduce bias into MR analysis. Selection bias is considered to be a form of collider bias. Inverse probability weighting is a countermeasure to collider/selection bias (127). Inverse probability weighting considers underrepresented cases in the data set and gives them more weight in the analysis, assuming that these cases may be more common in the general population (127).
With the continuous development of GWAS, we should be able to successfully identify further accurate exposure-related SNPs as instrumental variables for continued MR analysis of specific exposures and findings to establish causal relationships. With the enrichment of statistical methods and the deepening of observational research, the results of MR analysis will become more accurate and reliable.
Conclusion
In conclusion, MR analysis plays an important role in etiological research on OC. Overall, higher BMI and height, earlier age of menarche, endometriosis, schizophrenia, and higher circulatory β-carotene and circulatory zinc levels are associated with increased risk of OC. Conversely, PCOS; vitiligo; higher circulatory vitamin D, magnesium, and testosterone levels; and HMG-CoA reductase inhibition are associated with reduced risk of OC. Despite its limitations, MR analysis should provide constructive insights into disease prevention and drug development as well as effective guidance for observational research and RCT.
Author Contributions
J-ZG, Q-JW, and T-TG designed the study and formulated the clinical question. J-ZG, QX, and Q-JW performed the literature search and reviewed the search results for study inclusion. J-ZG, QX, and Q-JW designed the data extraction form and extracted the data. All authors collected, managed, and analyzed the data. J-ZG, QX, and Q-JW drafted the manuscript. All authors prepared, reviewed, revised, and approved the manuscript. Q-JW and T-TG had full access to all data in the study and is responsible for data integrity and the accuracy of data analysis. J-ZG and Q-X contributed equally to this work. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the Natural Science Foundation of China (No. 82073647 to Q-JW), the LiaoNing Revitalization Talents Program (No. XLYC1907102 to Q-JW), the Shenyang High Level Innovative Talents Support Program (No. RC190484 to Q-JW), and the 345 Talent Program to Q-JW (No. M0268).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian Cancer. Lancet (2014) 384:1376–88. doi: 10.1016/S0140-6736(13)62146-7
2. Stewart C, Ralyea C, Lockwood S. Ovarian Cancer: An Integrated Review. Semin Oncol Nurs (2019) 35:151–6. doi: 10.1016/j.soncn.2019.02.001
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
4. Harrison RK, Phase II. And Phase III Failures: 2013-2015. Nat Rev Drug Discov (2016) 15:817–8. doi: 10.1038/nrd.2016.184
5. Fordyce CB, Roe MT, Ahmad T, Libby P, Borer JS, Hiatt WR, et al. Cardiovascular Drug Development: Is It Dead or Just Hibernating? J Am Coll Cardiol (2015) 65:1567–82. doi: 10.1016/j.jacc.2015.03.016
6. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian Randomization: Using Genes as Instruments for Making Causal Inferences in Epidemiology. Stat Med (2008) 27:1133–63. doi: 10.1002/sim.3034
7. Evans DM, Davey Smith G. Mendelian Randomization: New Applications in the Coming Age of Hypothesis-Free Causality. Annu Rev Genomics Hum Genet (2015) 16:327–50. doi: 10.1146/annurev-genom-090314-050016
8. Klungel OH, Martens EP, Psaty BM, Grobbee DE, Sullivan SD, Stricker BH, et al. Methods to Assess Intended Effects of Drug Treatment in Observational Studies Are Reviewed. J Clin Epidemiol (2004) 57:1223–31. doi: 10.1016/j.jclinepi.2004.03.011
9. Smith GD, Lawlor DA, Harbord R, Timpson N, Day I, Ebrahim S. Clustered Environments and Randomized Genes: A Fundamental Distinction Between Conventional and Genetic Epidemiology. PloS Med (2007) 4:e352. doi: 10.1371/journal.pmed.0040352
10. Fewell Z, Davey Smith G, Sterne JA. The Impact of Residual and Unmeasured Confounding in Epidemiologic Studies: A Simulation Study. Am J Epidemiol (2007) 166:646–55. doi: 10.1093/aje/kwm165
11. Bochud M, Rousson V. Usefulness of Mendelian Randomization in Observational Epidemiology. Int J Environ Res Public Health (2010) 7:711–28. doi: 10.3390/ijerph7030711
12. Burgess S, Swanson SA, Labrecque JA. Are Mendelian Randomization Investigations Immune From Bias Due to Reverse Causation? Eur J Epidemiol (2021) 36:253–7. doi: 10.1007/s10654-021-00726-8
13. Hu Q, Hao P, Liu Q, Dong M, Gong Y, Zhang C, et al. Mendelian Randomization Studies on Atherosclerotic Cardiovascular Disease: Evidence and Limitations. Sci China Life Sci (2019) 62:758–70. doi: 10.1007/s11427-019-9537-4
14. Holmes MV, Ala-Korpela M, Smith GD. Mendelian Randomization in Cardiometabolic Disease: Challenges in Evaluating Causality. Nat Rev Cardiol (2017) 14:577–90. doi: 10.1038/nrcardio.2017.78
15. Gala H, Tomlinson I. The Use of Mendelian Randomisation to Identify Causal Cancer Risk Factors: Promise and Limitations. J Pathol (2020) 250:541–54. doi: 10.1002/path.5421
16. Sheehan NA, Didelez V, Burton PR, Tobin MD. Mendelian Randomisation and Causal Inference in Observational Epidemiology. PloS Med (2008) 5:e177. doi: 10.1371/journal.pmed.0050177
17. Cornish AJ, Tomlinson IPM, Houlston RS. Mendelian Randomisation: A Powerful and Inexpensive Method for Identifying and Excluding non-Genetic Risk Factors for Colorectal Cancer. Mol Aspects Med (2019) 69:41–7. doi: 10.1016/j.mam.2019.01.002
18. Jones MR, Kamara D, Karlan BY, Pharoah PDP, Gayther SA. Genetic Epidemiology of Ovarian Cancer and Prospects for Polygenic Risk Prediction. Gynecol Oncol (2017) 147:705–13. doi: 10.1016/j.ygyno.2017.10.001
19. Zhang C, Doherty JA, Burgess S, Hung RJ, Lindstrom S, Kraft P, et al. Game-On Network: Corect, and Tricl, Genetic Determinants of Telomere Length and Risk of Common Cancers: A Mendelian Randomization Study. Hum Mol Genet (2015) 24:5356–66. doi: 10.1093/hmg/ddv252
20. Ong JS, Cuellar-Partida G, Lu Y, Australian Ovarian Cancer S, Fasching PA, Hein A, et al. Association of Vitamin D Levels and Risk of Ovarian Cancer: A Mendelian Randomization Study. Int J Epidemiol (2016) 45:1619–30. doi: 10.1093/ije/dyw207
21. Gao C, Patel CJ, Michailidou K, Peters U, Gong J, Schildkraut J, et al. Mendelian Randomization Study of Adiposity-Related Traits and Risk of Breast, Ovarian, Prostate, Lung and Colorectal Cancer. Int J Epidemiol (2016) 45:896–908. doi: 10.1093/ije/dyw129
22. Dixon SC, Nagle CM, Thrift AP, Pharoah PD, Pearce CL, Zheng W, et al. Adult Body Mass Index and Risk of Ovarian Cancer by Subtype: A Mendelian Randomization Study. Int J Epidemiol (2016) 45:884–95. doi: 10.1093/ije/dyw158
23. Dimitrakopoulou VI, Tsilidis KK, Haycock PC, Dimou NL, Al-Dabhani K, Martin RM, et al. Circulating Vitamin D Concentration and Risk of Seven Cancers: Mendelian Randomisation Study. BMJ (2017) 359:j4761. doi: 10.1136/bmj.j4761
24. Day FR, Thompson DJ, Helgason H, Chasman DI, Finucane H, Sulem P, et al. Genomic Analyses Identify Hundreds of Variants Associated With Age at Menarche and Support a Role for Puberty Timing in Cancer Risk. Nat Genet (2017) 49:834–41. doi: 10.1038/ng.3841
25. Telomeres Mendelian Randomization C, Haycock PC, Burgess S, Nounu A, Zheng J, Okoli GN, et al. Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA Oncol (2017) 3:636–51. doi: 10.1001/jamaoncol.2016.5945
26. Ong JS, Hwang LD, Cuellar-Partida G, Martin NG, Chenevix-Trench G, Quinn MCJ, et al. Assessment of Moderate Coffee Consumption and Risk of Epithelial Ovarian Cancer: A Mendelian Randomization Study. Int J Epidemiol (2018) 47:450–9. doi: 10.1093/ije/dyx236
27. Ong JS, Gharahkhani P, An J, Law MH, Whiteman DC, Neale RE, et al. And Overall Cancer Risk and Cancer Mortality: A Mendelian Randomization Study. Hum Mol Genet (2018) 27:4315–22. doi: 10.1093/hmg/ddy307
28. Dixon-Suen SC, Nagle CM, Thrift AP, Pharoah PDP, Ewing A, Pearce CL, et al. Adult Height Is Associated With Increased Risk of Ovarian Cancer: A Mendelian Randomisation Study. Br J Cancer (2018) 118:1123–9. doi: 10.1038/s41416-018-0011-3
29. Yarmolinsky J, Relton CL, Lophatananon A, Muir K, Menon U, Gentry-Maharaj A, et al. Appraising the Role of Previously Reported Risk Factors in Epithelial Ovarian Cancer Risk: A Mendelian Randomization Analysis. PloS Med (2019) 16:e1002893. doi: 10.1371/journal.pmed.1002893
30. Harris HR, Cushing-Haugen KL, Webb PM, Nagle CM, Jordan SJ, Australian Ovarian Cancer Study G, et al. Association Between Genetically Predicted Polycystic Ovary Syndrome and Ovarian Cancer: A Mendelian Randomization Study. Int J Epidemiol (2019) 48:822–30. doi: 10.1158/1557-3265.OVCASYMP18-DP-007
31. Adams CD, Neuhausen SL. Bi-Directional Mendelian Randomization of Epithelial Ovarian Cancer and Schizophrenia and Uni-Directional Mendelian Randomization of Schizophrenia on Circulating 1- or 2-Glycerophosphocholine Metabolites. Mol Genet Metab Rep (2019) 21:100539. doi: 10.1016/j.ymgmr.2019.100539
32. Qian F, Rookus MA, Leslie G, Risch HA, Greene MH, Aalfs CM, et al. Mendelian Randomisation Study of Height and Body Mass Index as Modifiers of Ovarian Cancer Risk in 22,588 BRCA1 and BRCA2 Mutation Carriers. Br J Cancer (2019) 121:180–92. doi: 10.1038/s41416-019-0492-8
33. Ong JS, Law MH, An J, Han X, Gharahkhani P, Whiteman DC, et al. Association Between Coffee Consumption and Overall Risk of Being Diagnosed With or Dying From Cancer Among >300 000 UK Biobank Participants in a Large-Scale Mendelian Randomization Study. Int J Epidemiol (2019) 48:1447–56. doi: 10.1093/ije/dyz144
34. Yang H, Dai H, Li L, Wang X, Wang P, Song F, et al. Age at Menarche and Epithelial Ovarian Cancer Risk: A Meta-Analysis and Mendelian Randomization Study. Cancer Med (2019) 8:4012–22. doi: 10.1002/cam4.2315
35. Wen Y, Wu X, Peng H, Li C, Jiang Y, Liang H, et al. Cancer Risks in Patients With Vitiligo: A Mendelian Randomization Study. J Cancer Res Clin Oncol (2020) 146:1933–40. doi: 10.1007/s00432-020-03245-3
36. Yarmolinsky J, Bull CJ, Vincent EE, Robinson J, Walther A, Smith GD, et al. Association Between Genetically Proxied Inhibition of HMG-CoA Reductase and Epithelial Ovarian Cancer. JAMA (2020) 323:646–55. doi: 10.1001/jama.2020.0150
37. Guo Y, Lu Y, Jin H. Appraising the Role of Circulating Concentrations of Micro-Nutrients in Epithelial Ovarian Cancer Risk: A Mendelian Randomization Analysis. Sci Rep (2020) 10:7356. doi: 10.1038/s41598-020-63909-5
38. Larsson SC, Carter P, Vithayathil M, Mason AM, Michaelsson K, Baron JA, et al. Genetically Predicted Plasma Phospholipid Arachidonic Acid Concentrations and 10 Site-Specific Cancers in UK Biobank and Genetic Consortia Participants: A Mendelian Randomization Study. Clin Nutr (2020) 40:3332–7. doi: 10.1016/j.clnu.2020.11.004
39. Larsson SC, Carter P, Kar S, Vithayathil M, Mason AM, Michaelsson K, et al. Smoking, Alcohol Consumption, and Cancer: A Mendelian Randomisation Study in UK Biobank and International Genetic Consortia Participants. PloS Med (2020) 17:e1003178. doi: 10.1371/journal.pmed.1003178
40. Zhu J, Jiang X, Niu Z. Alcohol Consumption and Risk of Breast and Ovarian Cancer: A Mendelian Randomization Study. Cancer Genet (2020) 245:35–41. doi: 10.1016/j.cancergen.2020.06.001
41. Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A, et al. Using Human Genetics to Understand the Disease Impacts of Testosterone in Men and Women. Nat Med (2020) 26:252–8. doi: 10.1038/s41591-020-0751-5
42. Dimou NL, Papadimitriou N, Mariosa D, Johansson M, Brennan P, Peters U, et al. Circulating Adipokine Concentrations and Risk of Five Obesity-Related Cancers: A Mendelian Randomization Study. Int J Cancer (2020) 148:1625–36. doi: 10.1002/ijc.33338
43. Lin S, Yang H. Ovarian Cancer Risk According to Circulating Zinc and Copper Concentrations: A Meta-Analysis and Mendelian Randomization Study. Clin Nutr (2021) 40:2464–8. doi: 10.1016/j.clnu.2020.10.011
44. Larsson SC, Carter P, Vithayathil M, Kar S, Mason AM, Burgess S. Insulin-Like Growth Factor-1 and Site-Specific Cancers: A Mendelian Randomization Study. Cancer Med (2020) 9:6836–42. doi: 10.1002/cam4.3345
45. Yuan S, Kar S, Carter P, Vithayathil M, Mason AM, Burgess S, et al. Is Type 2 Diabetes Causally Associated With Cancer Risk? Evidence From Two-Sample Mendelian Randomization Study. Diabetes (2020) 69:1588–96. doi: 10.2337/db20-0084
46. Ye Y, Yang H, Wang Y, Zhao H. A Comprehensive Genetic and Epidemiological Association Analysis of Vitamin D With Common Diseases/Traits in the UK Biobank. Genet Epidemiol (2021) 45:24–35. doi: 10.1002/gepi.22357
47. Ong JS, Dixon-Suen SC, Han X, An J, Esophageal Cancer C, Me Research T, et al. A Comprehensive Re-Assessment of the Association Between Vitamin D and Cancer Susceptibility Using Mendelian Randomization. Nat Commun (2021) 12:246. doi: 10.1038/s41467-020-20368-w
48. Ong JS, Derks EM, Eriksson M, An J, Hwang LD, Easton DF, et al. Evaluating the Role of Alcohol Consumption in Breast and Ovarian Cancer Susceptibility Using Population-Based Cohort Studies and Two-Sample Mendelian Randomization Analyses. Int J Cancer (2021) 148:1338–50. doi: 10.1002/ijc.33308
49. Poschl G, Seitz HK. Alcohol and Cancer. Alcohol Alcohol (2004) 39:155–65. doi: 10.1093/alcalc/agh057
50. Gavaler JS, Van Thiel DH. The Association Between Moderate Alcoholic Beverage Consumption and Serum Estradiol and Testosterone Levels in Normal Postmenopausal Women: Relationship to the Literature. Alcohol Clin Exp Res (1992) 16:87–92. doi: 10.1111/j.1530-0277.1992.tb00642.x
51. Wu D, Yang H, Winham SJ, Natanzon Y, Koestler DC, Luo T, et al. Mediation Analysis of Alcohol Consumption, DNA Methylation, and Epithelial Ovarian Cancer. J Hum Genet (2018) 63:339–48. doi: 10.1038/s10038-017-0385-8
52. Tworoger SS, Gertig DM, Gates MA, Hecht JL, Hankinson SE. Caffeine, Alcohol, Smoking, and the Risk of Incident Epithelial Ovarian Cancer. Cancer (2008) 112:1169–77. doi: 10.1002/cncr.23275
53. Rota M, Pasquali E, Scotti L, Pelucchi C, Tramacere I, Islami F, et al. Alcohol Drinking and Epithelial Ovarian Cancer Risk. A Systematic Review and Meta-Analysis. Gynecol Oncol (2012) 125:758–63. doi: 10.1016/j.ygyno.2012.03.031
54. Yan-Hong H, Jing L, Hong L, Shan-Shan H, Yan L, Ju L. Association Between Alcohol Consumption and the Risk of Ovarian Cancer: A Meta-Analysis of Prospective Observational Studies. BMC Public Health (2015) 15:223. doi: 10.1186/s12889-015-1355-8
55. Minlikeeva AN, Moysich KB, Mayor PC, Etter JL, Cannioto RA, Ness RB, et al. Anthropometric Characteristics and Ovarian Cancer Risk and Survival. Cancer Causes Control (2018) 29:201–12. doi: 10.1007/s10552-017-0997-5
56. Beral V, Gaitskell K, Hermon C, Moser K, Reeves G, Peto R. Ovarian Cancer and Smoking: Individual Participant Meta-Analysis Including 28 114 Women With Ovarian Cancer From 51 Epidemiological Studies. Lancet Oncol (2012) 13:946–56. doi: 10.1016/S1470-2045(12)70322-4
57. Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, et al. Type I and II Endometrial Cancers: Have They Different Risk Factors? J Clin Oncol (2013) 31:2607–18. doi: 10.1200/jco.2012.48.2596
58. Webb PM, Jordan SJ. Epidemiology of Epithelial Ovarian Cancer. Best Pract Res Clin Obstet Gynaecol (2017) 41:3–14. doi: 10.1016/j.bpobgyn.2016.08.006
59. Leung AC, Cook LS, Swenerton K, Gilks B, Gallagher RP, Magliocco A, et al. Tea, Coffee, and Caffeinated Beverage Consumption and Risk of Epithelial Ovarian Cancers. Cancer Epidemiol (2016) 45:119–25. doi: 10.1016/j.canep.2016.10.010
60. Salari-Moghaddam A, Milajerdi A, Surkan PJ, Larijani B, Esmaillzadeh A. Caffeine, Type of Coffee, and Risk of Ovarian Cancer: A Dose-Response Meta-Analysis of Prospective Studies. J Clin Endocrinol Metab (2019) 104:5349–59. doi: 10.1210/jc.2019-00637
61. Calle EE, Kaaks R. Overweight, Obesity and Cancer: Epidemiological Evidence and Proposed Mechanisms. Nat Rev Cancer (2004) 4:579–91. doi: 10.1038/nrc1408
62. Renehan AG, Zwahlen M, Egger M. Adiposity and Cancer Risk: New Mechanistic Insights From Epidemiology. Nat Rev Cancer (2015) 15:484–98. doi: 10.1038/nrc3967
63. Tworoger SS, Huang T. Obesity and Ovarian Cancer. Recent Results Cancer Res (2016) 208:155–76. doi: 10.1007/978-3-319-42542-9_9
64. Jordan SJ, Webb PM, Green AC. Height, Age at Menarche, and Risk of Epithelial Ovarian Cancer. Cancer Epidemiol Biomarkers Prev (2005) 14:2045–8. doi: 10.1158/1055-9965.EPI-05-0085
65. Moorman PG, Alberg AJ, Bandera EV, Barnholtz-Sloan J, Bondy M, Cote ML, et al. Reproductive Factors and Ovarian Cancer Risk in African-American Women. Ann Epidemiol (2016) 26:654–62. doi: 10.1016/j.annepidem.2016.07.004
66. Harris HR, Rice MS, Shafrir AL, Poole EM, Gupta M, Hecht JL, et al. Lifestyle and Reproductive Factors and Ovarian Cancer Risk by P53 and MAPK Expression. Cancer Epidemiol Biomarkers Prev (2018) 27:96–102. doi: 10.1158/1055-9965.EPI-17-0609
67. Gong TT, Wu QJ, Vogtmann E, Lin B, Wang YL. Age at Menarche and Risk of Ovarian Cancer: A Meta-Analysis of Epidemiological Studies. Int J Cancer (2013) 132:2894–900. doi: 10.1002/ijc.27952
68. Dunneram Y, Greenwood DC, Cade JE. Diet, Menopause and the Risk of Ovarian, Endometrial and Breast Cancer. Proc Nutr Soc (2019) 78:438–48. doi: 10.1017/S0029665118002884
69. La Vecchia C. Ovarian Cancer: Epidemiology and Risk Factors. Eur J Cancer Prev (2017) 26:55–62. doi: 10.1097/CEJ.0000000000000217
70. Kralickova M, Lagana AS, Ghezzi F, Vetvicka V. Endometriosis and Risk of Ovarian Cancer: What Do We Know? Arch Gynecol Obstet (2020) 301:1–10. doi: 10.1007/s00404-019-05358-8
71. Barry JA, Azizia MM, Hardiman PJ. Risk of Endometrial, Ovarian and Breast Cancer in Women With Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum Reprod Update (2014) 20:748–58. doi: 10.1093/humupd/dmu012
72. Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, et al. Polycystic Ovary Syndrome. Nat Rev Dis Primers (2016) 2:16057. doi: 10.1038/nrdp.2016.57
73. Brown JS Jr. Cancer Immune Equilibrium and Schizophrenia Have Similar Interferon-Gamma, Tumor Necrosis Factor-Alpha, and Interleukin Expression: A Tumor Model of Schizophrenia. Schizophr Bull (2016) 42:1407–17. doi: 10.1093/schbul/sbw064
74. Ji J, Sundquist K, Ning Y, Kendler KS, Sundquist J, Chen X. Incidence of Cancer in Patients With Schizophrenia and Their First-Degree Relatives: A Population-Based Study in Sweden. Schizophr Bull (2013) 39:527–36. doi: 10.1093/schbul/sbs065
75. Catts VS, Catts SV, O’Toole BI, Frost AD. Cancer Incidence in Patients With Schizophrenia and Their First-Degree Relatives - A Meta-Analysis. Acta Psychiatr Scand (2008) 117:323–36. doi: 10.1111/j.1600-0447.2008.01163.x
76. Schizophrenia Working Group of the Psychiatric Genomics. Biological Insights From 108 Schizophrenia-Associated Genetic Loci. Nature (2014) 511:421–7. doi: 10.1038/nature13595
77. Anastasi E, Filardi T, Tartaglione S, Lenzi A, Angeloni A, Morano S. Linking Type 2 Diabetes and Gynecological Cancer: An Introductory Overview. Clin Chem Lab Med (2018) 56:1413–25. doi: 10.1515/cclm-2017-0982
78. El-Sherif A, El-Sherif S, Taylor AH, Ayakannu T. Ovarian Cancer: Lifestyle, Diet and Nutrition. Nutr Cancer (2021) 73:1092–107. doi: 10.1080/01635581.2020.1792948
79. He C, Wang Q. Dietary Vitamin A Intake and the Risk of Ovarian Cancer: A Meta-Analysis. Biosci Rep (2020) 40. doi: 10.1042/BSR20193979
80. Koushik A, Wang M, Anderson KE, van den Brandt P, Clendenen TV, Eliassen AH, et al. Intake of Vitamins A, C, and E and Folate and the Risk of Ovarian Cancer in a Pooled Analysis of 10 Cohort Studies. Cancer Causes Control (2015) 26:1315–27. doi: 10.1007/s10552-015-0626-0
81. Leng Y, Zhou H, Meng F, Tian T, Xu J, Yan F. Association of Vitamin E on the Risk of Ovarian Cancer: A Meta-Analysis. Biosci Rep (2019) 39. doi: 10.1042/BSR20193311
82. Arthur RS, Kirsh VA, Rohan TE. Dietary B-Vitamin Intake and Risk of Breast, Endometrial, Ovarian and Colorectal Cancer Among Canadians. Nutr Cancer (2019) 71:1067–77. doi: 10.1080/01635581.2019.1597904
83. L’Esperance K, Datta GD, Qureshi S, Koushik A. Vitamin D Exposure and Ovarian Cancer Risk and Prognosis. Int J Environ Res Public Health (2020) 17. doi: 10.3390/ijerph17041168
84. Toriola AT, Surcel HM, Calypse A, Grankvist K, Luostarinen T, Lukanova A, et al. Independent and Joint Effects of Serum 25-Hydroxyvitamin D and Calcium on Ovarian Cancer Risk: A Prospective Nested Case-Control Study. Eur J Cancer (2010) 46:2799–805. doi: 10.1016/j.ejca.2010.05.019
85. Druesne-Pecollo N, Latino-Martel P, Norat T, Barrandon E, Bertrais S, Galan P, et al. Beta-Carotene Supplementation and Cancer Risk: A Systematic Review and Metaanalysis of Randomized Controlled Trials. Int J Cancer (2010) 127:172–84. doi: 10.1002/ijc.25008
86. Middha P, Weinstein SJ, Mannisto S, Albanes D, Mondul AM. Beta-Carotene Supplementation and Lung Cancer Incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: The Role of Tar and Nicotine. Nicotine Tob Res (2019) 21:1045–50. doi: 10.1093/ntr/nty115
87. Canaz E, Kilinc M, Sayar H, Kiran G, Ozyurek E. Lead, Selenium and Nickel Concentrations in Epithelial Ovarian Cancer, Borderline Ovarian Tumor and Healthy Ovarian Tissues. J Trace Elem Med Biol (2017) 43:217–23. doi: 10.1016/j.jtemb.2017.05.003
88. Anderson JJ. Potential Health Concerns of Dietary Phosphorus: Cancer, Obesity, and Hypertension. Ann NY Acad Sci (2013) 1301:1–8. doi: 10.1111/nyas.12208
89. Rockfield S, Raffel J, Mehta R, Rehman N, Nanjundan M. Iron Overload and Altered Iron Metabolism in Ovarian Cancer. Biol Chem (2017) 398:995–1007. doi: 10.1515/hsz-2016-0336
90. Abedini M, Ghaedi E, Hadi A, Mohammadi H, Amani R. Zinc Status and Polycystic Ovarian Syndrome: A Systematic Review and Meta-Analysis. J Trace Elem Med Biol (2019) 52:216–21. doi: 10.1016/j.jtemb.2019.01.002
91. Guo F, Yang Z, Kulbe H, Albers AE, Sehouli J, Kaufmann AM. Inhibitory Effect on Ovarian Cancer ALDH+ Stem-Like Cells by Disulfiram and Copper Treatment Through ALDH and ROS Modulation. BioMed Pharmacother (2019) 118:109371. doi: 10.1016/j.biopha.2019.109371
92. Zhao J, Giri A, Zhu X, Shrubsole MJ, Jiang Y, Guo X, et al. Calcium: Magnesium Intake Ratio and Colorectal Carcinogenesis, Results From the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Br J Cancer (2019) 121:796–804. doi: 10.1038/s41416-019-0579-2
93. Song X, Li Z, Ji X, Zhang D. Calcium Intake and the Risk of Ovarian Cancer: A Meta-Analysis. Nutrients (2017) 9. doi: 10.3390/nu9070679
94. Zhong GC, Peng Y, Wang K, Wan L, Wu YQ, Hao FB, et al. Magnesium Intake and Primary Liver Cancer Incidence and Mortality in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Int J Cancer (2020) 147:1577–86. doi: 10.1002/ijc.32939
95. Li J, Jiao X, Yuan Z, Qiu H, Guo R. C-Reactive Protein and Risk of Ovarian Cancer: A Systematic Review and Meta-Analysis. Med (Baltimore) (2017) 96:e7822. doi: 10.1097/MD.0000000000007822
96. Garcia-Closas M, Brinton LA, Lissowska J, Richesson D, Sherman ME, Szeszenia-Dabrowska N, et al. Ovarian Cancer Risk and Common Variation in the Sex Hormone-Binding Globulin Gene: A Population-Based Case-Control Study. BMC Cancer (2007) 7:60. doi: 10.1186/1471-2407-7-60
97. Robinson E, Nandi M, Wilkinson LL, Arrowsmith DM, Curtis AD, Richardson A. Preclinical Evaluation of Statins as a Treatment for Ovarian Cancer. Gynecol Oncol (2013) 129:417–24. doi: 10.1016/j.ygyno.2013.02.003
98. Liu H, Liang SL, Kumar S, Weyman CM, Liu W, Zhou A. Statins Induce Apoptosis in Ovarian Cancer Cells Through Activation of JNK and Enhancement of Bim Expression. Cancer Chemother Pharmacol (2009) 63:997–1005. doi: 10.1007/s00280-008-0830-7
99. Zhong WB, Liang YC, Wang CY, Chang TC, Lee WS. Lovastatin Suppresses Invasiveness of Anaplastic Thyroid Cancer Cells by Inhibiting Rho Geranylgeranylation and RhoA/ROCK Signaling. Endocr Relat Cancer (2005) 12:615–29. doi: 10.1677/erc.1.01012
100. Gianuzzi X, Palma-Ardiles G, Hernandez-Fernandez W, Pasupuleti V, Hernandez AV, Perez-Lopez FR. Insulin Growth Factor (IGF) 1, IGF-Binding Proteins and Ovarian Cancer Risk: A Systematic Review and Meta-Analysis. Maturitas (2016) 94:22–9. doi: 10.1016/j.maturitas.2016.08.012
101. Whicker M, Black J, Altwerger G, Menderes G, Feinberg J, Ratner E. Management of Sexuality, Intimacy, and Menopause Symptoms in Patients With Ovarian Cancer. Am J Obstet Gynecol (2017) 217:395–403. doi: 10.1016/j.ajog.2017.04.012
102. Davis SR, Wahlin-Jacobsen S. Testosterone in Women—The Clinical Significance. Lancet Diabetes Endocrinol (2015) 3:980–92. doi: 10.1016/S2213-8587(15)00284-3
103. Dietze R, Hammoud MK, Gomez-Serrano M, Unger A, Bieringer T, Finkernagel F, et al. Phosphoproteomics Identify Arachidonic-Acid-Regulated Signal Transduction Pathways Modulating Macrophage Functions With Implications for Ovarian Cancer. Theranostics (2021) 11:1377–95. doi: 10.7150/thno.52442
104. Peng Y, Kajiyama H, Yuan H, Nakamura K, Yoshihara M, Yokoi A, et al. PAI-1 Secreted From Metastatic Ovarian Cancer Cells Triggers the Tumor-Promoting Role of the Mesothelium in a Feedback Loop to Accelerate Peritoneal Dissemination. Cancer Lett (2019) 442:181–92. doi: 10.1016/j.canlet.2018.10.027
105. Parida S, Siddharth S, Sharma D. Adiponectin, Obesity, and Cancer: Clash of the Bigwigs in Health and Disease. Int J Mol Sci (2019) 20. doi: 10.3390/ijms20102519
106. Slomian GJ, Nowak D, Buczkowska M, Glogowska-Gruszka A, Slomian SP, Roczniak W, et al. The Role of Adiponectin and Leptin in the Treatment of Ovarian Cancer Patients. Endokrynol Pol (2019) 70:57–63. doi: 10.5603/EP.a2018.0081
107. Yang M, Prescott J, Poole EM, Rice MS, Kubzansky LD, Idahl A, et al. Prediagnosis Leukocyte Telomere Length and Risk of Ovarian Cancer. Cancer Epidemiol Biomarkers Prev (2017) 26:339–45. doi: 10.1158/1055-9965.EPI-16-0466
108. Burgess S, Small DS, Thompson SG. A Review of Instrumental Variable Estimators for Mendelian Randomization. Stat Methods Med Res (2017) 26:2333–55. doi: 10.1177/0962280215597579
109. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization With Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol (2016) 40:304–14. doi: 10.1002/gepi.21965
110. Burgess S, Butterworth A, Thompson SG. Mendelian Randomization Analysis With Multiple Genetic Variants Using Summarized Data. Genet Epidemiol (2013) 37:658–65. doi: 10.1002/gepi.21758
111. Verbanck M, Chen CY, Neale B, Do R. Detection of Widespread Horizontal Pleiotropy in Causal Relationships Inferred From Mendelian Randomization Between Complex Traits and Diseases. Nat Genet (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
112. Greco MF, Minelli C, Sheehan NA, Thompson JR. Detecting Pleiotropy in Mendelian Randomisation Studies With Summary Data and a Continuous Outcome. Stat Med (2015) 34:2926–40. doi: 10.1002/sim.6522
113. Bowden J, Davey Smith G, Burgess S. Mendelian Randomization With Invalid Instruments: Effect Estimation and Bias Detection Through Egger Regression. Int J Epidemiol (2015) 44:512–25. doi: 10.1093/ije/dyv080
114. Verbanck M, Chen CY, Neale B, Do R. Publisher Correction: Detection of Widespread Horizontal Pleiotropy in Causal Relationships Inferred From Mendelian Randomization Between Complex Traits and Diseases. Nat Genet (2018) 50:1196. doi: 10.1038/s41588-018-0164-2
115. Hartwig FP, Davey Smith G, Bowden J. Robust Inference in Summary Data Mendelian Randomization via the Zero Modal Pleiotropy Assumption. Int J Epidemiol (2017) 46:1985–98. doi: 10.1093/ije/dyx102
116. Hartwig FP, Davies NM, Davey Smith G. Bias in Mendelian Randomization Due to Assortative Mating. Genet Epidemiol (2018) 42:608–20. doi: 10.1002/gepi.22138
117. Tenesa A, Rawlik K, Navarro P, Canela-Xandri O. Genetic Determination of Height-Mediated Mate Choice. Genome Biol (2016) 16:269. doi: 10.1186/s13059-015-0833-8
118. Keller MC, Garver-Apgar CE, Wright MJ, Martin NG, Corley RP, Stallings MC, et al. The Genetic Correlation Between Height and IQ: Shared Genes or Assortative Mating? PloS Genet (2013) 9:e1003451. doi: 10.1371/journal.pgen.1003451
119. Brumpton B, Sanderson E, Heilbron K, Hartwig FP, Harrison S, Vie GA, et al. Avoiding Dynastic, Assortative Mating, and Population Stratification Biases in Mendelian Randomization Through Within-Family Analyses. Nat Commun (2020) 11:3519. doi: 10.1101/602516
120. Giambartolomei C, Vukcevic D, Schadt EE, Franke L, Hingorani AD, Wallace C, et al. Bayesian Test for Colocalisation Between Pairs of Genetic Association Studies Using Summary Statistics. PloS Genet (2014) 10:e1004383. doi: 10.1371/journal.pgen.1004383
121. Ayers KL, Cordell HJ. SNP Selection in Genome-Wide and Candidate Gene Studies via Penalized Logistic Regression. Genet Epidemiol (2010) 34:879–91. doi: 10.1002/gepi.20543
122. Haycock PC, Burgess S, Wade KH, Bowden J, Relton C, Davey Smith G. Best (But Oft-Forgotten) Practices: The Design, Analysis, and Interpretation of Mendelian Randomization Studies. Am J Clin Nutr (2016) 103:965–78. doi: 10.3945/ajcn.115.118216
123. Yarmolinsky J, Wade KH, Richmond RC, Langdon RJ, Bull CJ, Tilling KM, et al. Causal Inference in Cancer Epidemiology: What Is the Role of Mendelian Randomization? Cancer Epidemiol Biomarkers Prev (2018) 27:995–1010. doi: 10.1158/1055-9965.EPI-17-1177
124. Burgess S, Thompson SG. Bias in Causal Estimates From Mendelian Randomization Studies With Weak Instruments. Stat Med (2011) 30:1312–23. doi: 10.1002/sim.4197
125. Burgess S, Thompson SG. Avoiding Bias From Weak Instruments in Mendelian Randomization Studies. Int J Epidemiol (2011) 40:755–64. doi: 10.1093/ije/dyr036
126. Burgess S, Thompson SG. Interpreting Findings From Mendelian Randomization Using the MR-Egger Method. Eur J Epidemiol (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
127. Gkatzionis A, Burgess S. Contextualizing Selection Bias in Mendelian Randomization: How Bad Is It Likely to be? Int J Epidemiol (2019) 48:691–701. doi: 10.1093/ije/dyy202
Keywords: causality, instrumental variables, mendelian randomization, ovarian cancer, risk factors, single-nucleotide polymorphisms
Citation: Guo J-Z, Xiao Q, Gao S, Li X-Q, Wu Q-J and Gong T-T (2021) Review of Mendelian Randomization Studies on Ovarian Cancer. Front. Oncol. 11:681396. doi: 10.3389/fonc.2021.681396
Received: 16 March 2021; Accepted: 16 July 2021;
Published: 11 August 2021.
Edited by:
J. Alejandro Perez-Fidalgo, Institute of Health Research (INCLIVA), SpainReviewed by:
Qian Yang, University of Bristol, United KingdomYang Yang, Shanghai Cancer Institute, China
Copyright © 2021 Guo, Xiao, Gao, Li, Wu and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting-Ting Gong, Z29uZ3R0QHNqLWhvc3BpdGFsLm9yZw==
 Jian-Zeng Guo1,2
Jian-Zeng Guo1,2 Qian Xiao
Qian Xiao Song Gao
Song Gao Qi-Jun Wu
Qi-Jun Wu Ting-Ting Gong
Ting-Ting Gong