- Department of Gastrointestinal Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Purpose: The purpose of the current study was to evaluate the impact of colorectal cancer (CRC) surgery on type 2 diabetes mellitus (T2DM) and to analyze the change in T2DM on overall survival after CRC surgery.
Methods: Patients who underwent CRC surgery were retrospectively enrolled from January 2013 to December 2019. The status of T2DM pre- and 1-year after CRC surgery was recorded, and predictive factors for T2DM remission and overall survival were analyzed.
Results: A total of 296 patients were included in this study. Thirty-eight patients experienced remission of T2DM 1 year after CRC surgery, and the remission rate was 12.8%. Weight loss was significantly higher in the T2DM remission group (p = 0.038), and the T2DM duration was significantly shorter in the T2DM remission group (p = 0.015). In the multivariate logistic regression analysis, higher weight loss (p = 0.046, odds ratio = 1.060, 95% CI = 1.001–1.122) and shorter T2DM duration (p = 0.019, odds ratio = 1007, 95% CI = 1.001–1.014) were predictive factors for remission of T2DM. Furthermore, in multivariate Cox regression analysis, lower TNM stage (p = 0.000, odds ratio = 2.147, 95% CI = 1.474–3.128) and T2DM remission (p = 0.033, odds ratio = 2.999, 95% CI = 1.091–8.243) were the predictive factors for better overall survival.
Conclusion: Patients with concurrent CRC and T2DM had a 12.8% remission 1 year after CRC surgery. Higher weight loss and shorter T2DM duration contributed to T2DM remission, and patients with T2DM remission could improve in terms of their overall survival.
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related death in the world (1). The 5-year overall survival rates of colon cancer and rectal cancer are approximately 60 and 55%, respectively, in nonmetastatic CRC (2), and surgery is currently the most effective treatment for CRC (3).
The prevalence of type 2 diabetes mellitus (T2DM) is rapidly increasing worldwide (4). Currently, 400 million people suffer from T2DM, and it is estimated that 650 million cases of T2DM will be diagnosed by 2040 (5). It is considered to be one of the most challenging public health problems and greatly affects life expectancy (6). A national survey conducted in 2010 stated that the incidence of T2DM had risen to 11.6%, of which only 25.8% had received conventional treatment, and only 39.7% was well controlled in China (7).
Previous studies reported that T2DM could improve the incidence of CRC (8, 9), and mortality and complications would increase when patients with concurrent CRC and T2DM underwent CRC surgery (10). Furthermore, T2DM has been shown to have significant effects on chemoresistance, and pre-existing T2DM patients decreases overall survival and increases the risk of relapse survival after CRC surgery as well (11, 12).
As previously described, gastric cancer surgery could contribute to T2DM remission (13), and the remission rate was 20–50% (14–16). Moreover, recovery from pre-existing T2DM after radical gastrectomy was associated with better overall survival (14). Similarly, CRC surgery is a digest surgery, however, no previous studies have reported the effect of CRC surgery on T2DM. Therefore, the purpose of this study was to evaluate the impact of CRC surgery on T2DM, and to analyze the change in T2DM on overall survival after CRC surgery.
Materials and Methods
Patients
Patients who underwent CRC surgery were retrospectively enrolled from January 2013 to December 2019. Ethical approval from the institutional review board was obtained (2021-046), and informed consent was acquired from all patients.
The inclusion criteria were as follows: 1. patients who were pathologically diagnosed with CRC; 2. patients who underwent radical CRC surgery; and 3. patients diagnosed with concurrent T2DM. The exclusion criteria were as follows: 1. patients who underwent combined organ resection (n = 7); 2. patients with preoperative chemotherapy (n = 11); 3. patients with other endocrine disorders, such as thyroid or adrenal disease (n = 18); 4. Patients who died or were lost to follow-up within one year (n = 17); and 5. patients with incomplete medical records before surgery (n = 43).
A total of 4,627 patients were identified in the database, and according to the inclusion criteria, 392 patients with concurrent CRC and T2DM remained. Finally, 296 patients with completed follow-up of 1 year were involved in this study according to the exclusion criteria (Figure 1).
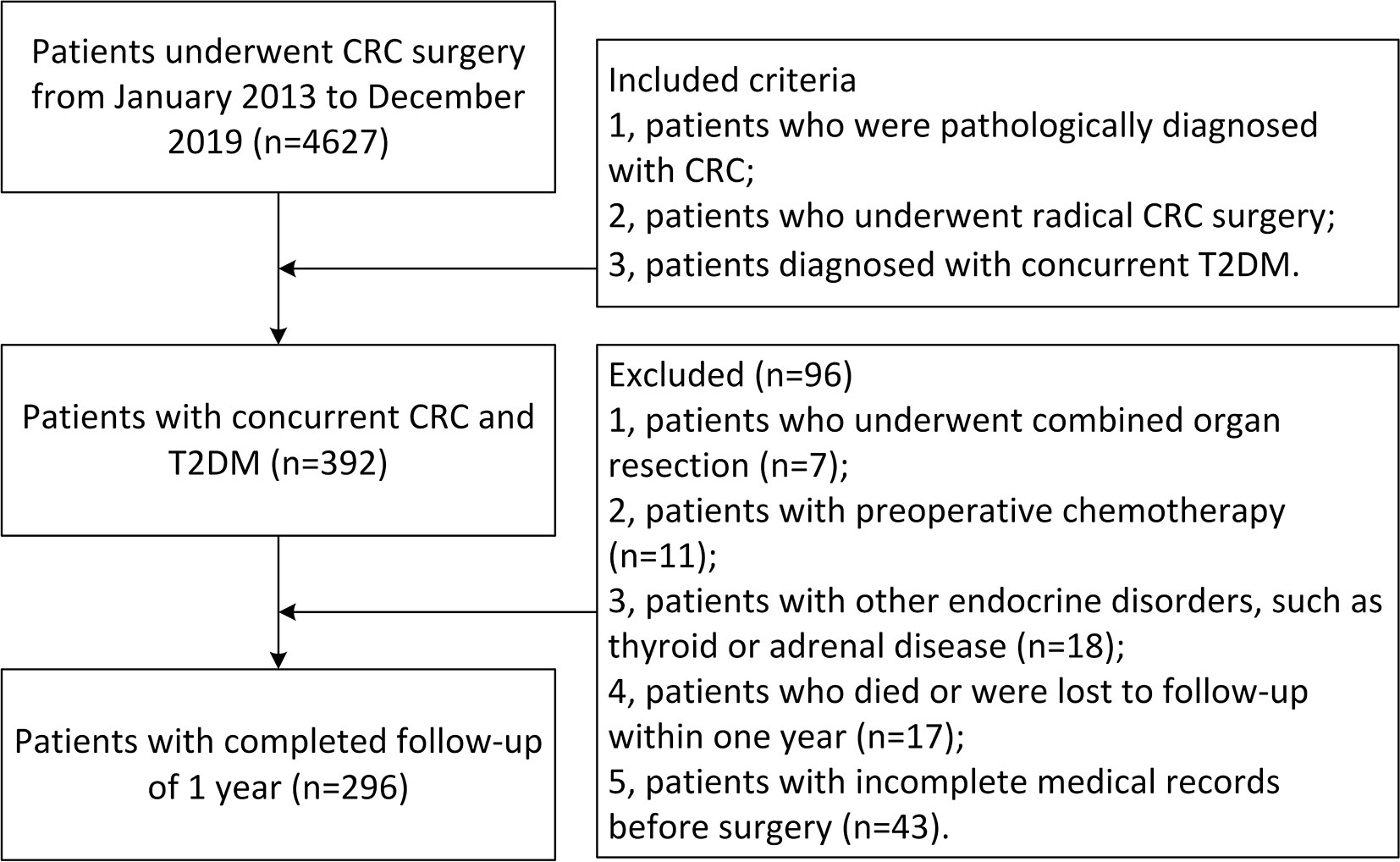
Figure 1 Inclusion and exclusion criteria of patients with concurrent CRC and T2DM. CRC, colorectal cancer; T2DM, type 2 diabetes mellitus.
Definitions and Follow-Up
In this study, we analyzed the status of T2DM in pre- and 1-year after CRC surgery. The remission of T2DM was evaluated following American Diabetes Association criteria (17). The T2DM remission group was defined as follows: a return to a normal fasting blood glucose (FBG) range with no requirement for medication or an improved FBG level with reduced medication. The T2DM no remission group was defined as follows: no changes in medication, or aggravation in FBG levels and more medication requirements after CRC surgery (18).
The tumor node metastasis (TNM) stage of CRC was determined according to the 8th edition of the Japanese Classification of Colorectal Carcinoma (19). Radical CRC surgery was conducted, and pathological examinations confirmed R0 resection.
Weight loss referred to the postoperative weight minus preoperative weight, and the duration of T2DM was defined from the first diagnosis of T2DM to CRC surgery. Long-term survival was defined as the time from CRC surgery to death or the end of the study if the patient was still alive. In addition, patients were followed up every three months for the first two years and every six months thereafter to assess tumor recurrence. Carcinoembryonic antigen, abdominal sonogram, chest radiography, colonoscopy and abdominal computerized tomography were arranged if needed.
Data Collection
The perioperative information was collected from the medical database, and follow-up data were collected from the outpatient service system and through telephone interviews. The extracted clinical data included sex, age, comorbid hypertension, smoking, drinking, weight, body mass index (BMI), T2DM duration, T2DM medication dosage, FBG, tumor site, surgical methods and TNM stage.
Statistical Analysis
Continuous variables are expressed as the mean ± SD, and categorical variables are expressed as n (%). Chi-square tests and independent-samples t tests were used to compare the differences between the remission group and the no remission group. Univariate and multivariate logistic regression analyses were used to identify predictors of T2DM remission, and univariate and multivariate Cox regression analyses were performed to identify predictive factors for overall survival. Data were analyzed using SPSS (version 20.0) statistical software. A bilateral p value of < 0.05 was considered statistically significant.
Results
Baseline Characteristics of Patients With Concurrent CRC and T2DM
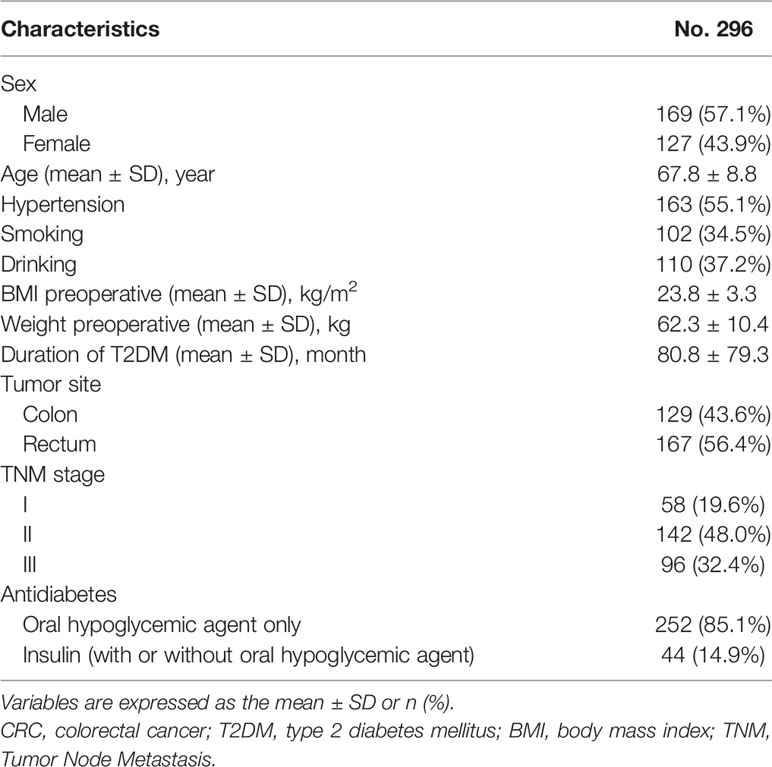
A total of 296 patients who had concurrent CRC and T2DM were included in this study, and the glycemic status in the studied patients was stable before and 1 year after CRC surgery. There were 169 males and 127 females, and the average age was 67.8 ± 8.8 years. A total of 163 patients had concurrent CRC, T2DM and hypertension. The preoperative BMI, weight, duration of T2DM, tumor site, smoking, drinking, TNM stage and antidiabetes therapy are shown in Table 1.
Difference Between Remission and No Remission
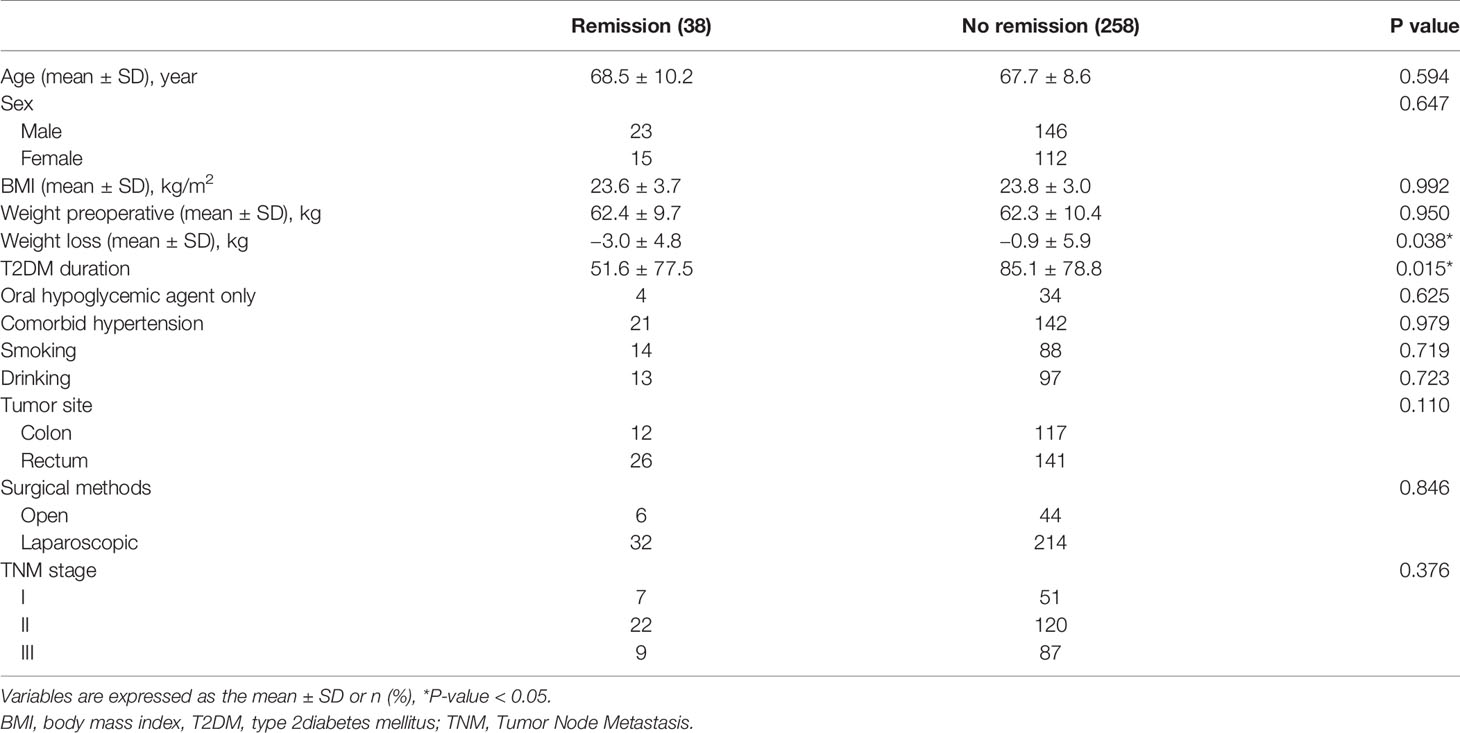
Thirty-eight patients had remission of T2DM 1 year after CRC surgery, and the remission rate was 12.8%. The difference was analyzed between the remission and no remission groups. Weight loss was -3.0 ± 4.8 kg in the remission group, which was significantly lower than −0.9 ± 5.9 kg in the no remission group (p = 0.038), and T2DM duration was 51.6 ± 77.5 months in the remission group, which was significantly lower than 85.1 ± 78.8 months in the no remission group as well (p = 0.015). However, no difference was found in terms of age, sex, BMI, weight preoperatively, comorbid hypertension, smoking, drinking, tumor site, surgical methods or TNM stage (p >0.05) (Table 2).
Univariate and Multivariate Analysis of Predictive Factors for Remission of T2DM
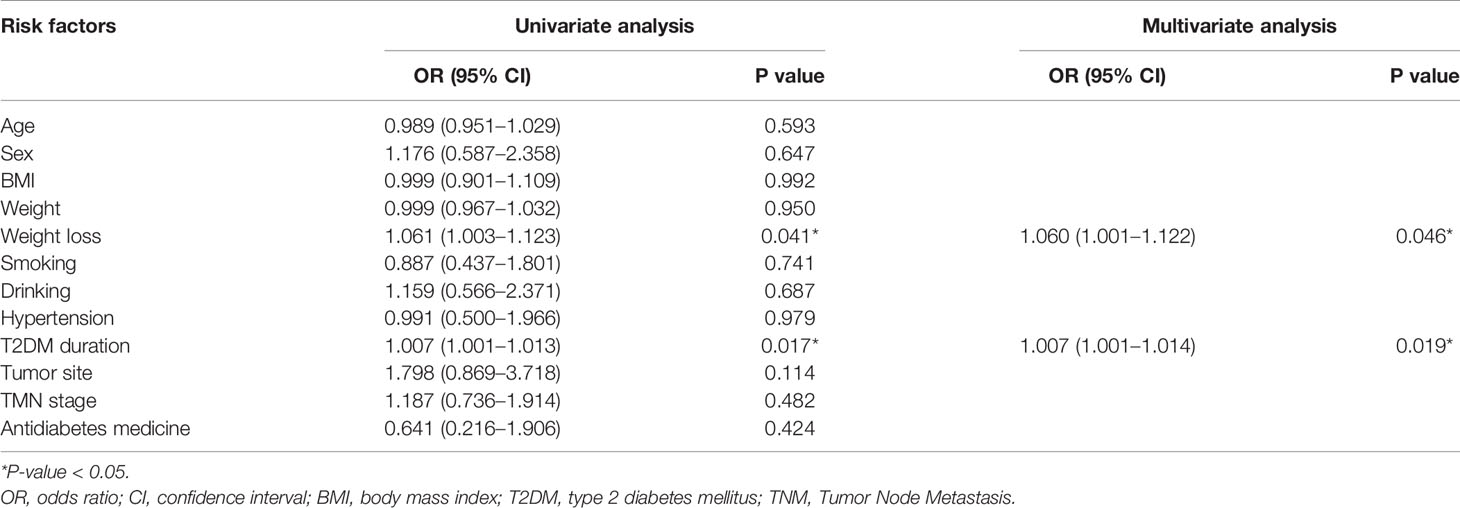
Univariate and multivariate logistic regression analyses were performed to identify predictive factors for remission of T2DM. In univariate analyses, weight loss (p = 0.041, odds ratio = 1.061, 95% CI = 1.003–1.123) and T2DM duration (p = 0.017, odds ratio = 1.007, 95% CI = 1.001–1.013) were predictive factors. Furthermore, in the multivariate analysis, higher weight loss (p = 0.046, odds ratio = 1.060, 95% CI = 1.001–1.122) and shorter T2DM duration (p = 0.019, odds ratio = 1007, 95% CI = 1.001–1.014) were the predictive factors for remission of T2DM (Table 3).
Univariate and Multivariate Analysis of Overall Survival
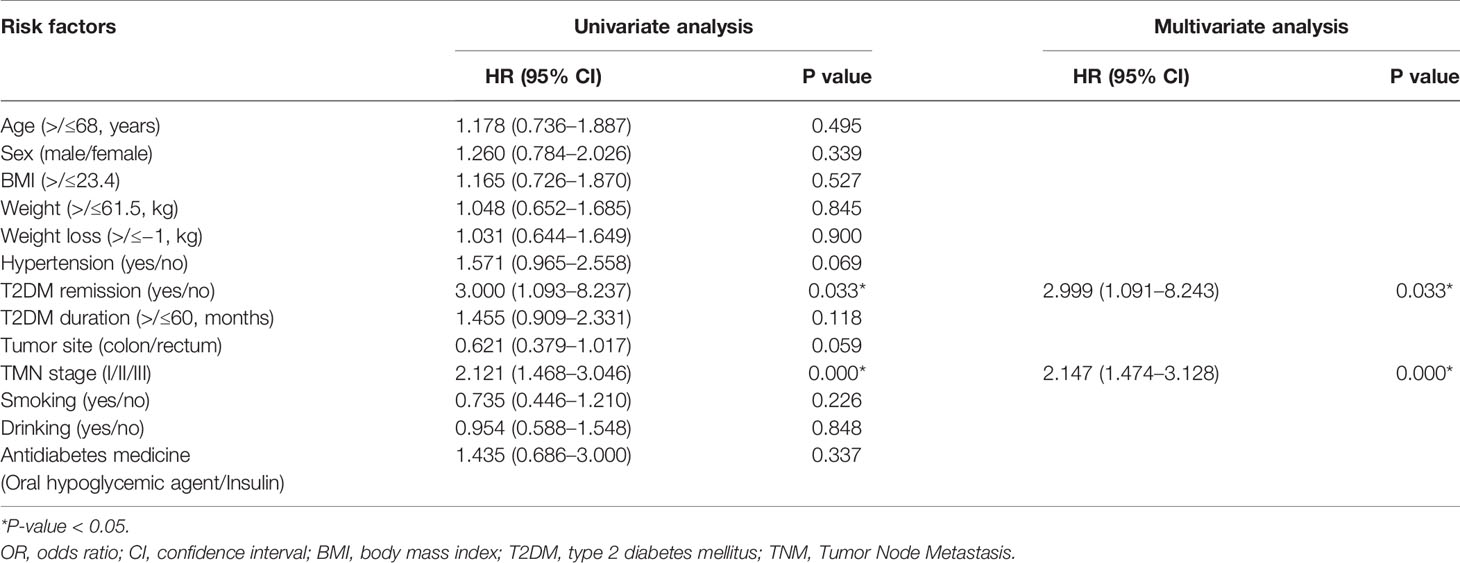
The median follow-up time was 37 (12–99) months. Univariate and multivariate Cox regression analyses were performed to identify predictive factors for overall survival. In univariate analyses, TNM stage (p = 0.000, odds ratio = 2.121, 95% CI = 1.468–3.046) and T2DM remission (p = 0.033, odds ratio = 3.000, 95% CI = 1.093–8.237) were predictive factors. In the multivariate analysis, lower TNM stage (p = 0.000, odds ratio = 2.147, 95% CI = 1.474–3.128) and T2DM remission (p = 0.033, odds ratio = 2.999, 95% CI = 1.091–8.243) were predictive factors for better overall survival (Table 4). The Kaplan–Meier curve between the T2DM remission group and the no remission group is shown in Figure 2.
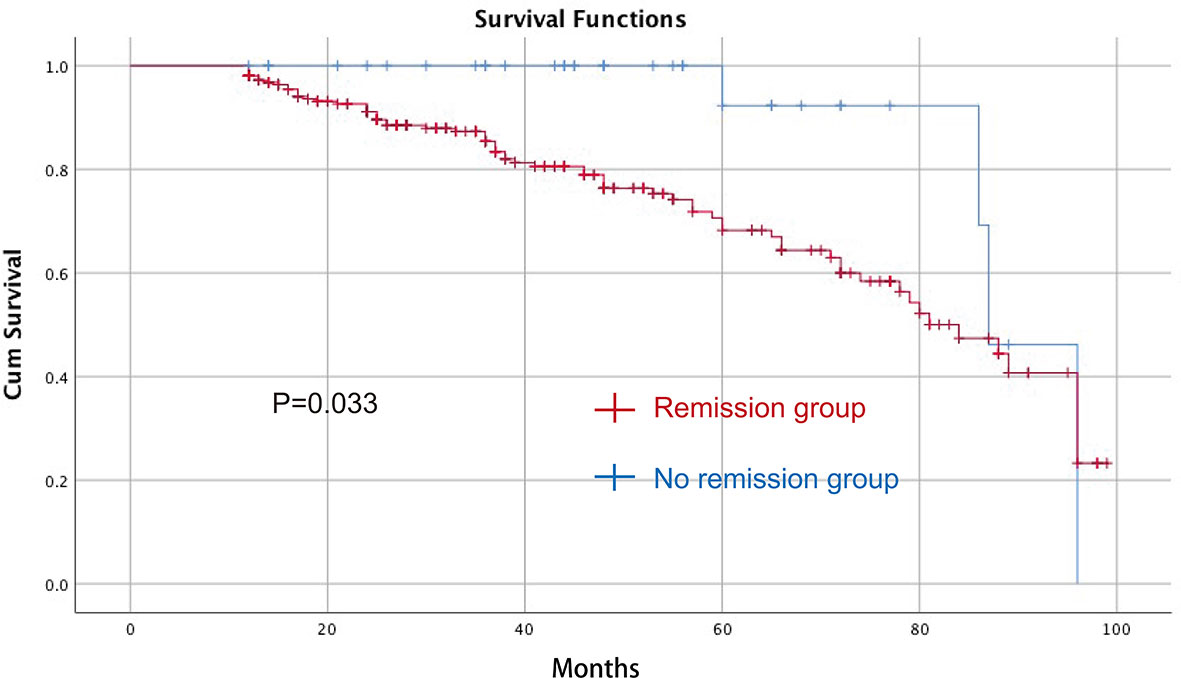
Figure 2 The Kaplan–Meier curve between the T2DM remission group and the no remission group after CRC surgery for OS. CRC, colorectal cancer; T2DM, type 2 diabetes mellitus; OS, overall survival.
Discussion
In this study, we analyzed 296 patients who underwent CRC surgery. Thirty-eight patients had remission of T2DM 1 year after CRC surgery, and the remission rate was 12.8%. Weight loss was significantly higher in the T2DM remission group, and T2DM duration was significantly shorter in the T2DM remission group. In multivariate logistic regression analysis, higher weight loss and shorter T2DM duration were the predictive factors for remission of T2DM. Furthermore, in multivariate Cox regression analysis, lower TNM stage and T2DM remission were predictive factors for better overall survival.
As previously described, patients with T2DM have a higher rate of developing CRC (8, 9). It is troublesome for clinicians when performing CRC surgery on patients with T2DM because patients with concurrent CRC and T2DM have greater complications, including infection and anemia, and postoperative mortality increases (10). Better control of T2DM before and after CRC surgery would bring survival benefits for patients (11).
For patients with T2DM, lifestyle changes might also benefit T2DM remission. Patients adhering to low-carbohydrate diets and low-energy diets could experience T2DM remission (20). Increased physical activities could be a predictor for T2DM remission (21). The remission of T2DM reduced the burden of life and the dosage of antidiabetes drugs. Furthermore, the remission of T2DM could reduce DM-related diseases, including diabetic nephropathy, eye disease and peripheral neuropathy (22).
In bariatric surgery, patients had T2DM improvement and apparently lower microvascular and macrovascular disease and mortality after surgery (23). Similarly, patients had remission of T2DM and hypertension after gastric cancer surgery (14, 24), and onco-metabolic surgery occurred. There were some reasons and theories accounting for the remission of T2DM. The foregut theory states that patients undergoing duodenal bypass experience antidiabetic effects (25), whereas the hindgut theory holds that early contact of unabsorbed nutrients with the distal intestine improves T2DM (26). Furthermore, hormones, including ghrelin, GLP-1 and GLP-2, could influence T2DM remission (27).
In this study, patients with a shorter duration of T2DM and greater weight loss had a higher rate of T2DM remission. The possible factor was that a shorter duration of T2DM represented a lower severity of T2DM, and patients with a shorter duration could easily achieve T2DM remission. Higher weight loss is another predictive factor for T2DM remission, and weight loss may be related to lifestyle changes that affected changes of T2DM status (28). However, surgical methods did not have an impact on T2DM remission; therefore, lifestyle change was indicated to be an important factor in T2DM remission (29). Patients might benefit from dietary adjustments, sodium restriction, more exercise and lower alcohol consumption; therefore, T2DM might improve when the lifestyle of patients is modified after CRC surgery.
Pre-existing T2DM patients have a higher rate of developing other second primary malignancies (30), and a worse overall survival after CRC surgery (11, 30). A meta-analysis also reported decreased survival and increased risk of relapse in patients with concurrent CRC and T2DM (11). In the current study, patients with T2DM remission improved in terms of their overall survival after CRC surgery, and T2DM remission improved overall survival in gastric cancer patients (14). The mechanism of improved overall survival remains unclear and might be related to the upregulation of AMP-activated protein kinase, which causes cell cycle arrest in the Gap 1-S (G1-S) phase and inhibits the mTOR pathway (12). Furthermore, modification of lifestyle, dietary restrictions and physical activity are measures for reducing mortality (11).
There are some limitations of this study as well. First, it is a single-center retrospective study, with a total of 296 patients, which is relatively small. Second, postoperative lifestyles, including diet changes and activities, were not included in this study; however, these changes in lifestyle may have influenced T2DM remission. Third, the status of T2DM may change after more than one year, and the specific time of T2DM remission was not documented. Longer follow-up of T2DM change is needed in the future. Finally, we only included patients who survived more than one year in the overall survival analysis, which may have resulted in selection bias, and recurrence-free survival was not documented. Therefore, multicenter and large-sample RCTs concerning the lifestyle changes after CRC surgery are needed in future experiment.
In conclusion, patients with concurrent CRC and T2DM had a 12.8% remission 1-year after CRC surgery. Higher weight loss and shorter T2DM duration contributed to T2DM remission, and patients with T2DM remission could improve their overall survival.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical approval from the institutional review board was obtained (2021-046). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Wei Luo who helped to collected data from the outpatient service system and make follow-up through telephone interviews, and the authors are grateful to Dr. Zhuozhi Shen, Chongqing Center for Disease Control and Prevention, for the substantial work in the statistical methods.
References
1. Siegel RL, Miller KD, Jemal A. Colorectal Cancer Mortality Rates in Adults Aged 20 to 54 Years in the United States, 1970-2014. JAMA (2017) 318:572–4. doi: 10.1001/jama.2017.7630
2. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-Induced Cancer: Crosstalk Between Tumours, Immune Cells and Microorganisms. Nat Rev Cancer (2013) 13:759–71. doi: 10.1038/nrc3611
3. Dru RC, Curtis NJ, Court EL, Spencer C, El Falaha S, Dennison G, et al. Impact of Anaemia at Discharge Following Colorectal Cancer Surgery. Int J Colorectal Dis (2020) 35:1769–76. doi: 10.1007/s00384-020-03611-0
4. Chatterjee S, Khunti K, Davies MJ. Type 2 Diabetes. Lancet (2017) 389:2239–51. doi: 10.1016/S0140-6736(17)30058-2
5. Cummings DE, Rubino F. Metabolic Surgery for the Treatment of Type 2 Diabetes in Obese Individuals. Diabetologia (2018) 61(2):257–64. doi: 10.1007/s00125-017-4513-y
6. Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of Life Lost Due to Obesity. JAMA (2003) 289:187–93. doi: 10.1001/jama.289.2.187
7. Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and Control of Diabetes in Chinese Adults. JAMA (2013) 310:948–59. doi: 10.1001/jama.2013.168118
8. Ali KU, Fallah M, Tian Y, Sundquist K, Sundquist J, Brenner H, et al. Personal History of Diabetes as Important as Family History of Colorectal Cancer for Risk of Colorectal Cancer: A Nationwide Cohort Study. Am J Gastroenterol (2020) 115:1103–9. doi: 10.14309/ajg.0000000000000669
9. Lee JM, Lee KM, Kim DB, Ko SH, Park YG. Colorectal Cancer Risks According to Sex Differences in Patients With Type II Diabetes Mellitus: A Korean Nationwide Population-Based Cohort Study. Clin Transl Gastroenterol (2019) 10:e00090. doi: 10.14309/ctg.0000000000000090
10. Qiang JK, Sutradhar R, Giannakeas V, Bhatia D, Singh S, Lipscombe LL. Impact of Diabetes on Colorectal Cancer Stage and Mortality Risk: A Population-Based Cohort Study. Diabetologia (2020) 63:944–53. doi: 10.1007/s00125-020-05094-8
11. Petrelli F, Ghidini M, Rausa E, Ghidini A, Cabiddu M, Borgonovo K, et al. Survival of Colorectal Cancer Patients With Diabetes Mellitus: A Meta-Analysis. Can J Diabetes (2021) 45:186–97.e2. doi: 10.1016/j.jcjd.2020.06.009
12. Cheng HC, Chang TK, Su WC, Tsai HL, Wang JY. Narrative Review of the Influence of Diabetes Mellitus and Hyperglycemia on Colorectal Cancer Risk and Oncological Outcomes. Transl Oncol (2021) 14(7):101089. doi: 10.1016/j.tranon.2021.101089
13. Peng D, Cheng YX, Zhang W. Does Roux-en-Y Construction Really Bring Benefit of Type 2 Diabetes Mellitus Remission After Gastrectomy in Patients With Gastric Cancer? A Systematic Review and Meta-Analysis. Diabetes Ther (2020) 11:2863–72. doi: 10.1007/s13300-020-00934-7
14. Wei ZW, Li JL, Wu Y, Xia GK, Schwarz RE, He YL, et al. Impact of Pre-Existing Type-2 Diabetes on Patient Outcomes After Radical Resection for Gastric Cancer: A Retrospective Cohort Study. Dig Dis Sci (2014) 59:1017–24. doi: 10.1007/s10620-013-2965-6
15. Zhu Z, Shan X, Cheng Y, Xu J, Fu H, Wang W, et al. Clinical Course of Diabetes After Gastrectomy According to Type of Reconstruction in Patients With Concurrent Gastric Cancer and Type 2 Diabetes. Obes Surg (2015) 25:673–9. doi: 10.1007/s11695-014-1426-4
16. Choi YY, Noh SH, An JY. A Randomized Controlled Trial of Roux-en-Y Gastrojejunostomy vs. Gastroduodenostomy With Respect to the Improvement of Type 2 Diabetes Mellitus After Distal Gastrectomy in Gastric Cancer Patients. PLoS One (2017) 12:e0188904. doi: 10.1371/journal.pone.0188904
17. Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (Jsccr) Guidelines 2014 for Treatment of Colorectal Cancer. Int J Clin Oncol (2015) 20:207–39. doi: 10.1007/s10147-015-0801-z
18. Buse JB, Caprio S, Cefalu WT, Ceriello A, Del Prato S, Inzucchi SE, et al. How Do We Define Cure of Diabetes? Diabetes Care (2009) 32:2133e2135. doi: 10.2337/dc09-9036
19. Wang KC, Huang KH, Lan YT, Fang WL, Lo SS, Li AF, et al. Outcome After Curative Surgery for Gastric Cancer Patients With Type 2 Diabetes. World J Surg (2014) 38:431–8. doi: 10.1007/s00268-013-2291-3
20. Goldenberg JZ, Day A, Brinkworth GD, et al. Efficacy and Safety of Low and Very Low Carbohydrate Diets for Type 2 Diabetes Remission: Systematic Review and Meta-Analysis of Published and Unpublished Randomized Trial Data. BMJ (2021) 372:m4743. doi: 10.1136/bmj.m4743
21. Dambha MH, Day AJ, Strelitz J, Irving G, Griffin SJ. Behaviour Change, Weight Loss and Remission of Type 2 Diabetes: A Community-Based Prospective Cohort Study. Diabetes Med (2020) 37:681–8. doi: 10.1111/dme.14122
22. Shikata K, Haneda M, Ninomiya T, Koya D, Suzuki Y, Suzuki D, et al. Randomized Trial of An Intensified, Multifactorial Intervention in Patients With Advanced-Stage Diabetic Kidney Disease: Diabetic Nephropathy Remission and Regression Team Trial in Japan (Dnett-Japan). J Diabetes Investig (2021) 12:207–16. doi: 10.1111/jdi.13339
23. Sheng B, Truong K, Spitler H, Zhang L, Tong X, Chen L. The Long-Term Effects of Bariatric Surgery on Type 2 Diabetes Remission, Microvascular and Macrovascular Complications, and Mortality: A Systematic Review and Meta-Analysis. Obes Surg (2017) 27:2724–32. doi: 10.1007/s11695-017-2866-4
24. Peng D, Cheng YX, Tao W, Zou YY, Qian K, Zhang W. Onco-Metabolic Surgery: A Combined Approach to Gastric Cancer and Hypertension. Cancer Manag Res (2020) 12:7867–73. doi: 10.2147/CMAR.S260147
25. Kwon Y, Abdemur A, Lo Menzo ME, Park S, Szomstein S, Rosenthal RJ. The Foregut Theory as a Possible Mechanism of Action for the Remission of Type 2 Diabetes in Low Body Mass Index Patients Undergoing Subtotal Gastrectomy for Gastric Cancer. Surg Obes Relat Dis (2014) 10:235–42. doi: 10.1016/j.soard.2013.09.013
26. Laessle C, Jin K, Seifert GJ, Timme-Bronsert S, Fichtner-Feigl S, Marjanovic G, et al. Putting the Hindgut Hypothesis to the Test in a Diabetic Zucker Rat Model. Obes Surg (2019) 29:4000–7. doi: 10.1007/s11695-019-04079-w
27. Russel SM, Valle V, Spagni G, Hamilton S, Patel T, Abdukadyrov N, et al. Physiologic Mechanisms of Type II Diabetes Mellitus Remission Following Bariatric Surgery: A Meta-Analysis and Clinical Implications. J Gastrointest Surg (2020) 24:728–41. doi: 10.1007/s11605-019-04508-2
28. Kwee LC, Ilkayeva O, Muehlbauer MJ, Bihlmeyer N, Wolfe B, Purnell JQ, et al. Metabolites and Diabetes Remission After Weight Loss. Nutr Diabetes (2021) 11:10. doi: 10.1038/s41387-021-00151-6
29. Dutton GR, Lewis CE. The Look Ahead Trial: Implications for Lifestyle Intervention in Type 2 Diabetes Mellitus. Prog Cardiovasc Dis (2015) 58:69–75. doi: 10.1016/j.pcad.2015.04.002
Keywords: colorectal cancer, surgery, remission, overall survival, type 2 diabetes mellitus
Citation: Peng D, Liu X-Y, Cheng Y-X, Tao W and Cheng Y (2021) Improvement of Diabetes Mellitus After Colorectal Cancer Surgery: A Retrospective Study of Predictive Factors For Type 2 Diabetes Mellitus Remission and Overall Survival. Front. Oncol. 11:694997. doi: 10.3389/fonc.2021.694997
Received: 14 April 2021; Accepted: 31 May 2021;
Published: 06 July 2021.
Edited by:
Alberto Di Leo, Ospedale San Camillo, ItalyReviewed by:
Christos K. Kontos, National and Kapodistrian University of Athens, GreeceJoanna Krajewska, Wroclaw Medical University, Poland
Ahmet Ziya Balta, GATA Haydarpaşa Eğitim Hastanesi, Turkey
Copyright © 2021 Peng, Liu, Cheng, Tao and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Cheng, Y2hlbmd5b25nY3FAMTYzLmNvbQ==
 Dong Peng
Dong Peng Xiao-Yu Liu
Xiao-Yu Liu Yong Cheng
Yong Cheng