- 1Clinical School, Weifang Medical University, Weifang, China
- 2Department of Radiation Oncology, Weifang People’s Hospital, Weifang, China
- 3Department of Pathology, Weifang People’s Hospital, Weifang, China
- 4Department of Imaging, Weifang People’s Hospital, Weifang, China
- 5Weifang Key Laboratory of Radiophysics and Oncological Radiobiology, Weifang, China
Background: Glioblastoma (GBM) is the most common primary intracranial tumor and originates from the small pool of adult neural stem and progenitor cells (NSPCs). According to the World Health Organization (WHO) classification of brain tumors, gliomas are classified into grades I–IV, and GBM is defined as the highest grade (IV). GBM can be disseminated by cerebrospinal fluid (CSF), but extracranial metastasis is rare. Additionally, the pathway and mechanism involved remain unclear.
Case Presentation: We report a rare case of left temporal lobe GBM with multiple bone metastases and soft tissue metastasis. This 49-year-old right-handed man who was diagnosed with GBM underwent surgery on May 9, 2017, followed by radiochemotherapy in June 2017. On August 13, 2019, local relapse was found. Then, the patient received a second surgery but not radiochemotherapy. In November 2019, the patient was reported to be suffering from low back pain for nearly 1 month. On December 6, 2019, magnetic resonance imaging (MRI) of the thoracolumbar vertebrae and abdominal computed tomography (CT) confirmed metastases on the ninth posterior rib on the right, the third anterior rib on the left, and the T7 and T10 vertebrae and their appendages. CT-guided rib space-occupying puncture biopsy was performed, and GBM was identified by pathology.
Conclusion: We should pay attention to extracranial metastasis of GBM. Timely detection and early treatment improve overall quality of patients’ life. The extracranial metastasis in this patient may have occurred through the spinal nerve root or intercostal nerve. Further clinical observations are required to clarify the pathway and mechanism involved.
Background
Malignant brain tumors are among the most feared types of cancer, not only because of their poor prognosis but also because of their direct repercussions on quality of life and cognitive function. Glioblastoma (GBM) is the most notorious intracranial tumor in adults (1); after standard treatment (surgery, chemotherapy, and radiation therapy), there is still a high rate of locoregional relapse, and the median survival time of GBM patients is only 15 months (2, 3). Peripheral metastases of GBM are very rare. Usually, they occur in regional lymph nodes (51%) and the lungs and pleura (60%) and occasionally in the bone (31%) and liver (22%) (4); those in soft tissues are extremely unusual (5). In addition, the most common site of bone metastasis is the vertebrae (6). Metastatic diffusion of cerebrospinal fluid (CSF) is observed in some cases. Peripheral metastases of GBM are very rare despite the ability of GBM cells to pass through the blood–brain barrier (BBB) and be disseminated throughout the peripheral blood (7). The mechanism of their diffusion is not well known. We report this rare case of multiple metastases of GBM including bone (vertebrae and rib cage) and soft tissues and discuss a possible new mechanism for metastasis/extracranial metastasis of GBM, which may occur through the nerve root or intercostal nerve.
Case Presentation
On first admission (May 2017), a 47-year-old man presented with a 3-month history of headache, nausea, vomiting, and memory loss. On May 7, 2017, craniocerebral magnetic resonance imaging (MRI) revealed space-occupying lesions in the left temporal lobe (Figure 1). On May 9, 2017, the patient underwent craniotomy for left temporal lobe tumor resection. Intraoperatively, the tumor was found to have a clear capsule and a distinct boundary with the surrounding brain tissue. MRI was performed during the operation to confirm total tumor resection. The pathological diagnosis was GBM, and the patient’s symptoms were relieved. Immunohistochemistry (IHC) demonstrated the following: oligodendrocyte lineage transcription factor-2 (Olig-2) positive, α-thalassemia/mental-retardation-syndrome-X-linked gene (ATRX) positive, glial fibrillary acidic protein (GFAP) positive, isocitrate dehydrogenase 1 (IDH-1) wild-type, O6-methylguanine-DNA-methyltransferase (MGMT) protein 80% positive, P53 10% positive, programmed cell death 1 (PD-1) scattered cells positive, programmed cell death-ligand 1 (PD-L1) partial positive, and Ki-67 30% positive in tumor cell nuclei, indicating high mitotic activity. On May 31, 2017, postoperative reexamination of brain MRI showed that the tumor had been removed (Figure 2). Intensity-modulated radiation therapy (IMRT) (6,000 cGy total in 28 fractions) to the tumor bed was performed from June 5, 2017, to July 13, 2017. The patient was treated with temozolomide (TMZ) chemotherapy (75 mg/m2/day) as an adjunct to radiotherapy and then sequentially (first cycle 150 mg/m2/day, then 200 mg/m2/day for 5 days, per cycle of 28 days for 6 cycles) between June 5, 2017, and December 9, 2017. There were no obvious signs of recurrence or metastasis on craniocerebral MRI every 3 months after the operation.
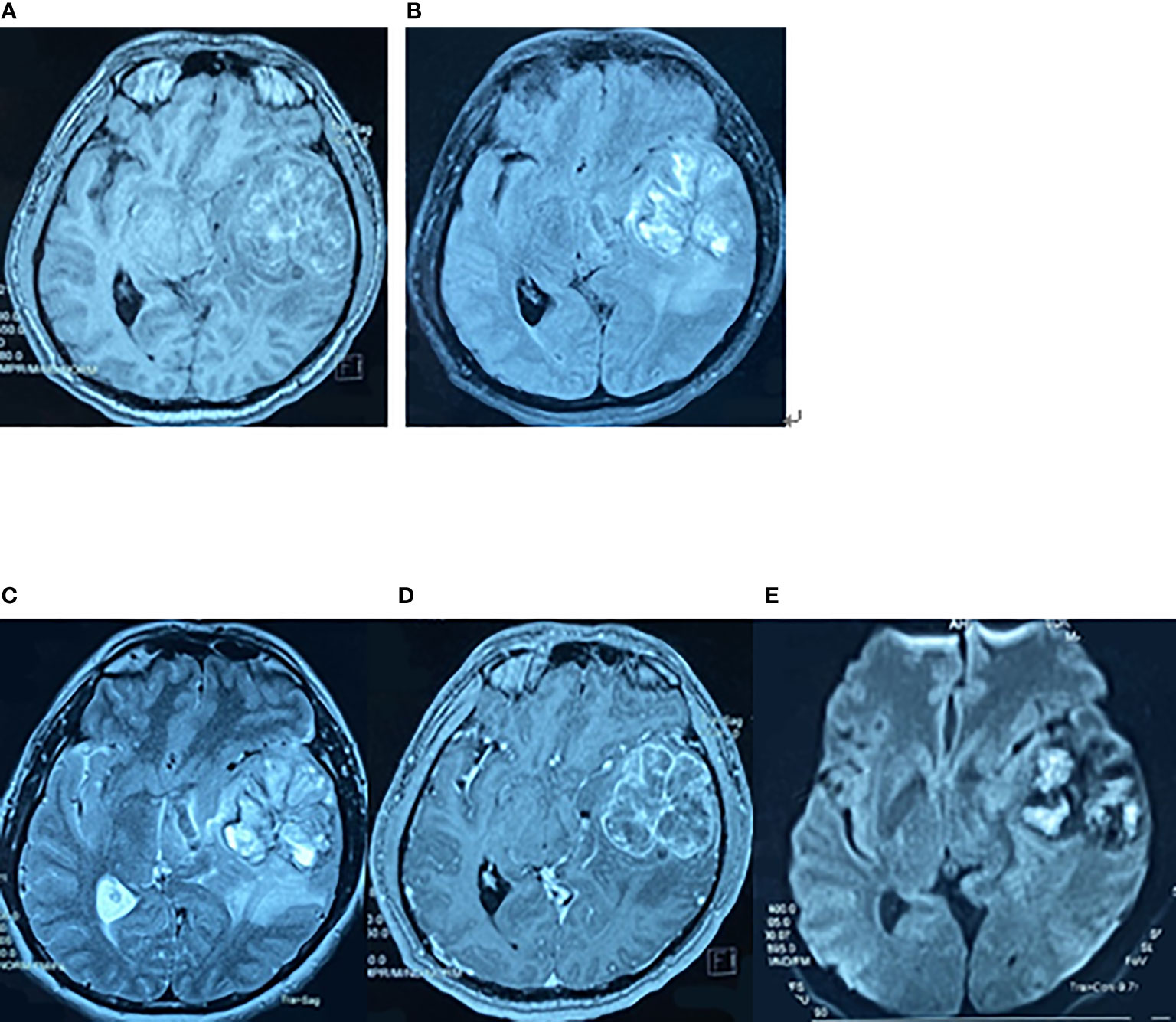
Figure 1 Preoperative brain MRI findings on May 7, 2017. The axial planes of preoperative MRI of the brain showing a 6-cm heterogeneous mass in the left superior temporal lobe. (A) T1-weighted MRI. (B) T2-weighted fluid-attenuated inversion recovery (FLAIR) MRI. (C) T2-weighted MRI. (D) Enhanced T1-weighted MRI. (E) Diffusion-weighted MRI.
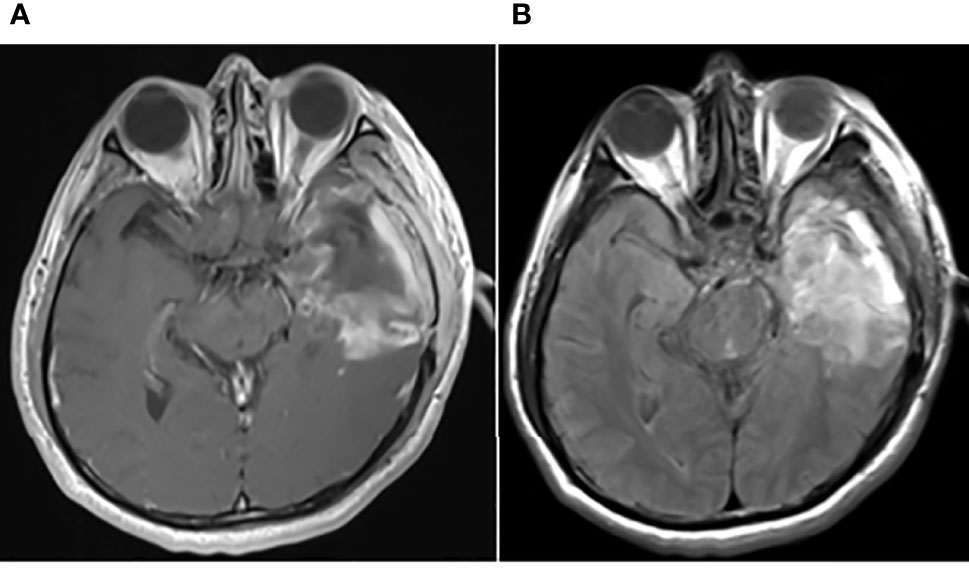
Figure 2 Postoperative brain MRI findings on May 31, 2017. The axial planes of postoperative brain MRI showed that the tumor had been removed. (A) Enhanced T1-weighted MRI. (B) T2-weighted FLAIR MRI.
On second admission (August 13, 2019), MRI demonstrated recurrence of the tumor in the postoperative residual cavity (Figure 3). The tumor in the left frontotemporal lobe was removed on August 21, 2019. Intraoperatively, the tumor was located in the left temporal pole with an unclear boundary and no clear boundary with brain tissues. Postoperative pathology revealed GBM, World Health Organization (WHO) grade IV. IHC demonstrated the following: Olig-2, ATRX and GFAP positive, IDH-1 wild-type, MGMT protein revealed 10% positive, P53 weakly positive in partial cells, and Ki-67 revealed 20% positive. Postoperative genetic tests revealed the following: MGMT promoter was not methylated; 1p/19q genes intact; no mutations in IDH-1 R132, IDH-2 R172, or telomerase reverse transcriptase (TERT) C250T or BRAF V600E; and TERT C228T mutation. Antiepileptic drugs were not given after the two operations, and radiotherapy and chemotherapy were not performed after recurrence.
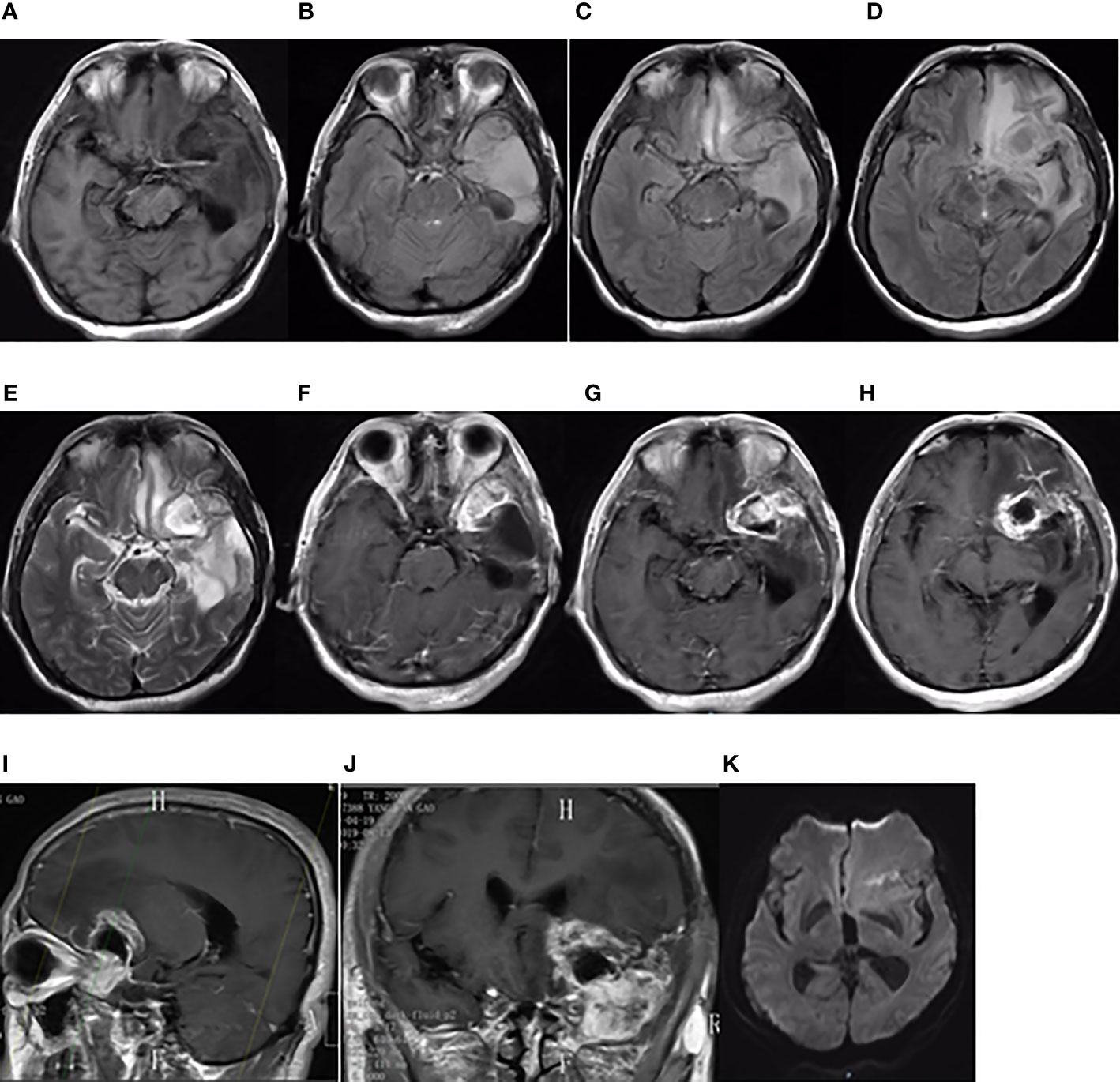
Figure 3 Brain MRI findings on August 13, 2019. Brain MRI showing a new focus in the postoperative residual cavity of the left temporal lobe. (A) The axial plane of T1-weighted MRI. (B–D) The axial planes of T2-weighted FLAIR MRI. (E) The axial plane of T2-weighted MRI. (F–H) The axial planes of enhanced T1-weighted MRI. (I, J) The sagittal and coronal planes of enhanced T1-weighted MRI. (K) The axial plane of diffusion-weighted MRI.
On third admission (November 2019), the patient presented with a 1-month history of back pain affecting sleep and aggravating in the recumbent position, with a numerical rating scale (NRS) score for the first 24 h of 4. On December 4, 2019, he underwent brain MRI. The intracranial conditions are shown in Figure 4. To identify postoperative changes and residual enhancing disease, an enhanced MR scan proved to be extremely valuable for assessing gross residual tumor when performed on postoperative 72 h; this timing avoided surgically induced contrast enhancement (8–10). The diffusion-weighted imaging (DWI) can be helpful in determining whether new enhancement developing in the subsequent weeks or months is postoperative changes or tumor recurrence (11, 12). Since craniocerebral MRI was not performed 72 h after the second surgery, it is difficult to identify the postoperative changes, residual tumors, or recurrence. MRI of the whole spine showed metastases of the T7 and T10 vertebrae and right posterior chest wall (Figures 5, 6), while thoracic and abdominal computed tomography (CT) on December 6, 2019, showed the metastases of the ninth posterior ribs on the right, third anterior ribs on the left, and T7 and T10 vertebrae and their appendages (Figure 7). To determine the nature of the ninth posterior costal mass, CT-guided fine needle biopsy of the ninth posterior costal mass was performed on December 6, 2019. Postoperative pathology and IHC revealed the following: GBM (Figure 8), WHO grade IV, Olig-2, ATRX and GFAP positive, IDH-1 wild-type, P53 revealed 10% positive, Ki-67 revealed 10% positive. Vimentin and S-100 positive, CD31 and CD34 vascular positive, CK widely and Smooth Muscle Actin (SMA) negative. Genetic tests revealed that the MGMT promoter was not methylated, and 1p/19q genes were intact. The IHC and genetic test results are summarized in Table 1. On December 20, abscission cytological examination of the lumbar puncture was negative. To relieve pain and control the disease, IMRT (6145 cGy total in 28 fractions) was performed on the target areas (right ninth posterior costal soft tissue metastases; T7, T8, and T9 prevertebral soft tissue lesions; and T10 right vertebral soft tissue metastases), and the patient received TMZ synchronous chemotherapy (75 mg/m2/day). Oral analgesics were recommended during treatment from December 2019 to January 2020. At the end of treatment, the patient’s symptoms were slightly relieved, but there was still pain, with an NRS score of 2. The patient did not come for further consultation for personal reasons. According to a telephone follow-up, the patient’s pain worsened again in August 2020, and he received analgesic treatment at a local hospital. He eventually developed cachexia and died of systemic failure in December 2020. The progression-free survival (PFS) period was 27 months, and the overall survival (OS) period was 43 months.
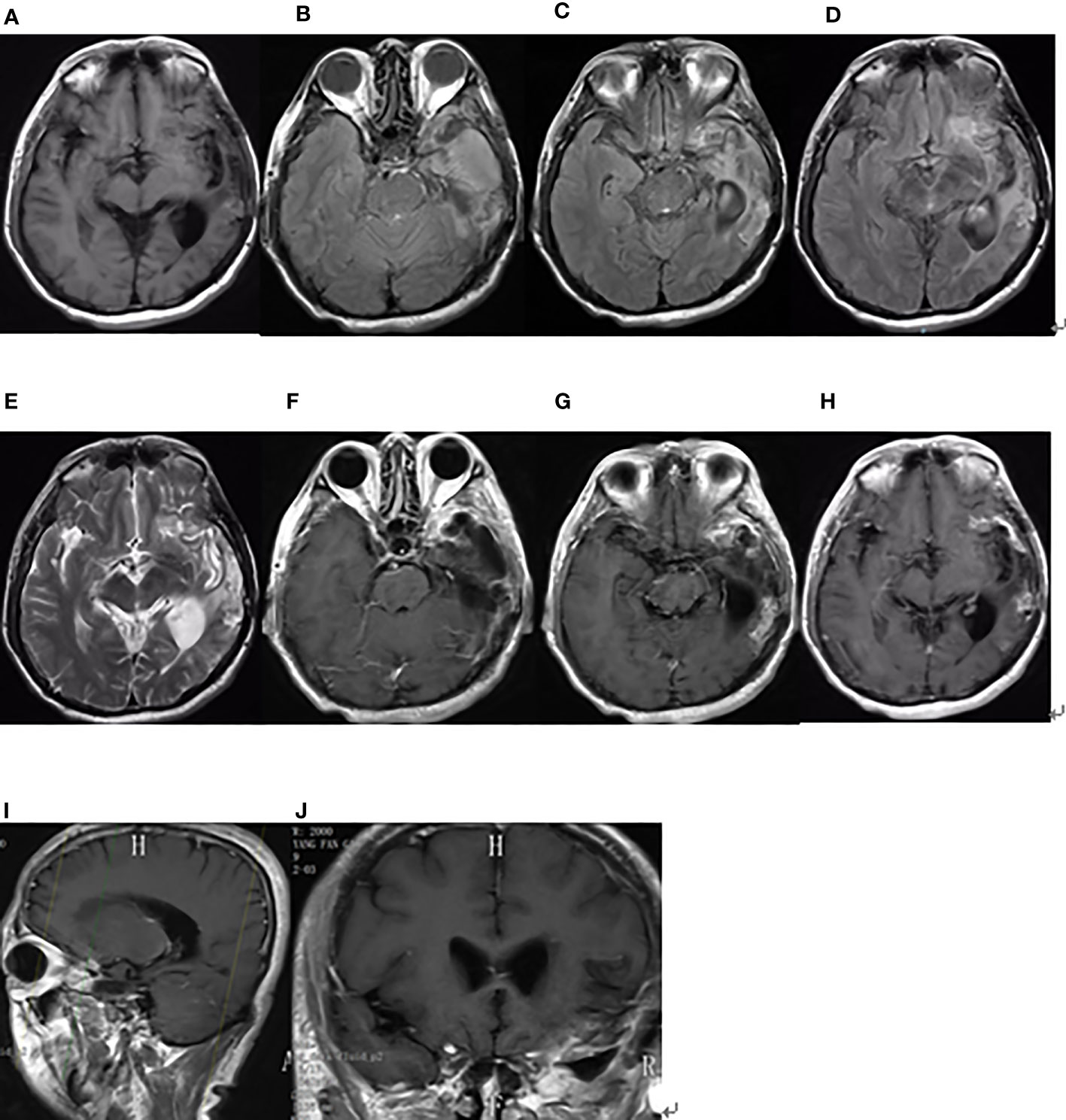
Figure 4 Brain MRI findings after the second surgery on December 4, 2019. (A) The axial plane of T1-weighted MRI. (B–D) The axial planes of T2-weighted FLAIR MRI. (E) The axial plane of T2-weighted MRI. (F–H) The axial planes of enhanced T1-weighted MRI. (I, J) The sagittal and coronal planes of enhanced T1-weighted MRI.

Figure 5 Whole spinal MRI findings on December 12, 2019. Sagittal planes of T1-weighted MRI of the whole spine showed metastases of the T7 and T10 vertebrae. (A) T1-weighted MRI. (B) Enhanced T1-weighted MRI.
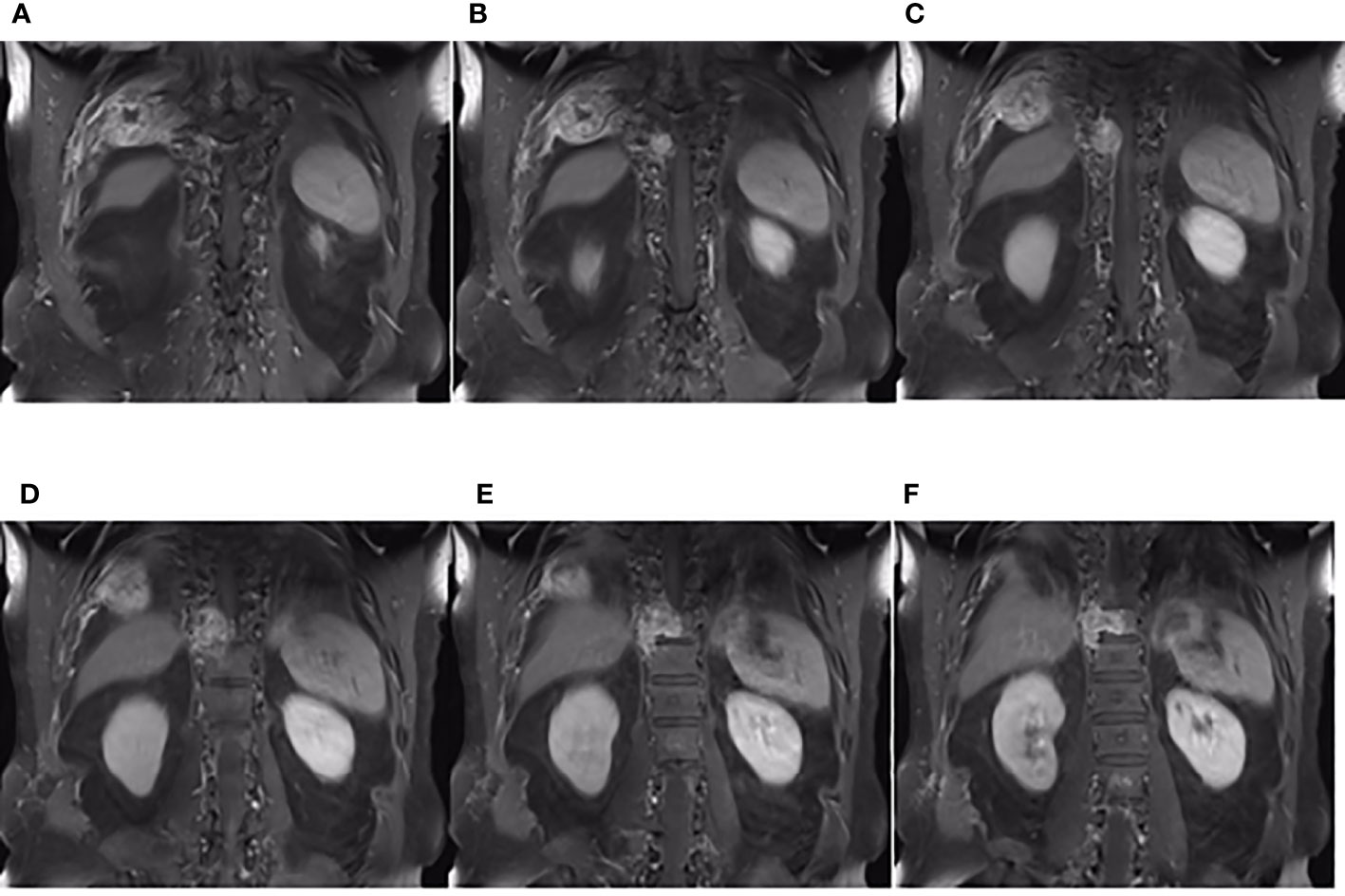
Figure 6 The coronal plane of MRI findings on December 6, 2019. (A–F) Metastasis of the thoracic vertebrae and chest wall on the coronal planes of enhanced T1-weighted MRI.
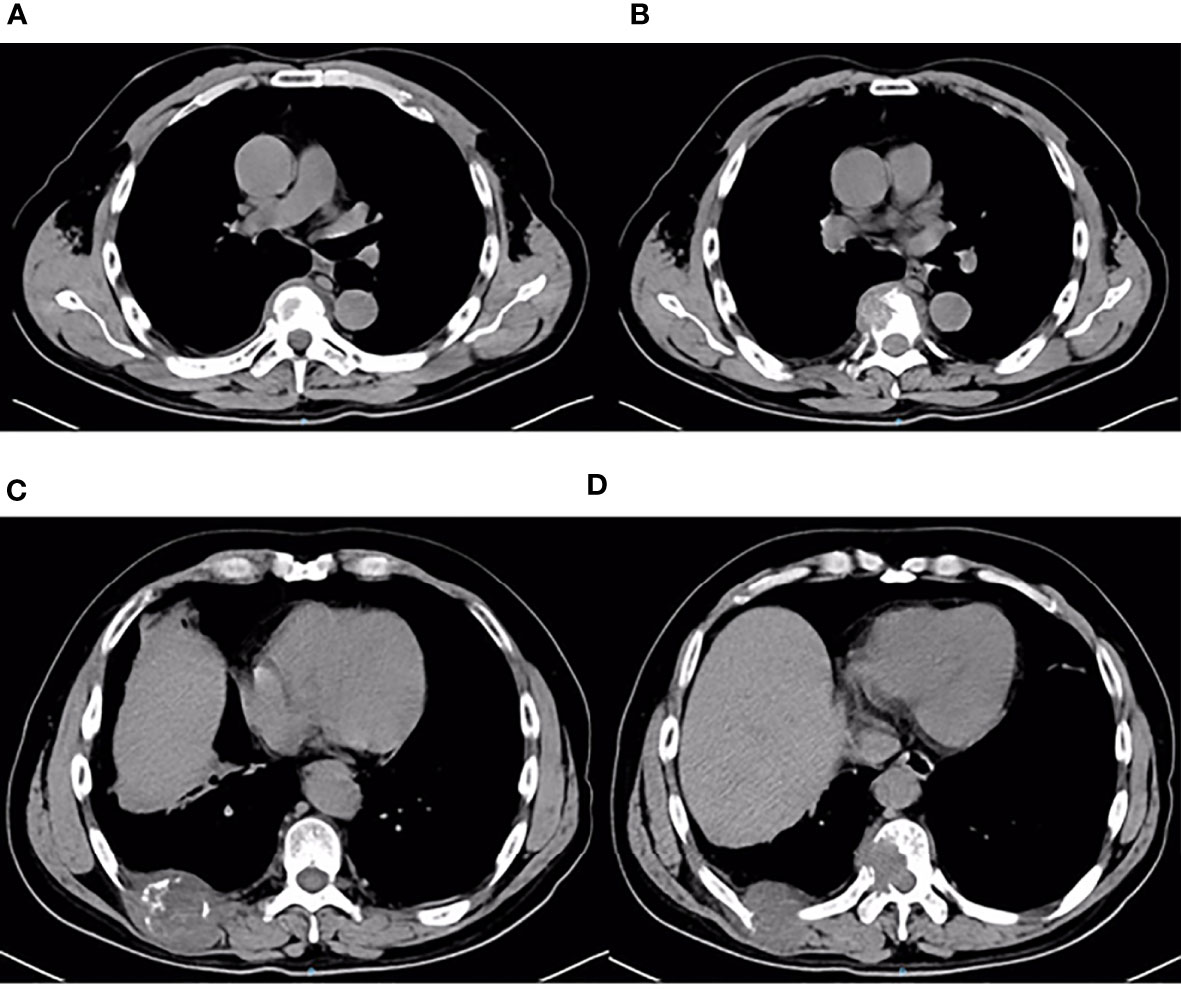
Figure 7 Thoracic and abdominal CT findings on December 6, 2019. Thoracic and abdominal CT revealed multiple metastases of the chest wall and thoracic vertebrae (A–D). Metastases of the third anterior ribs on the left, T7 vertebrae and its appendages, ninth posterior ribs on the right, and T10 vertebrae and its appendages.
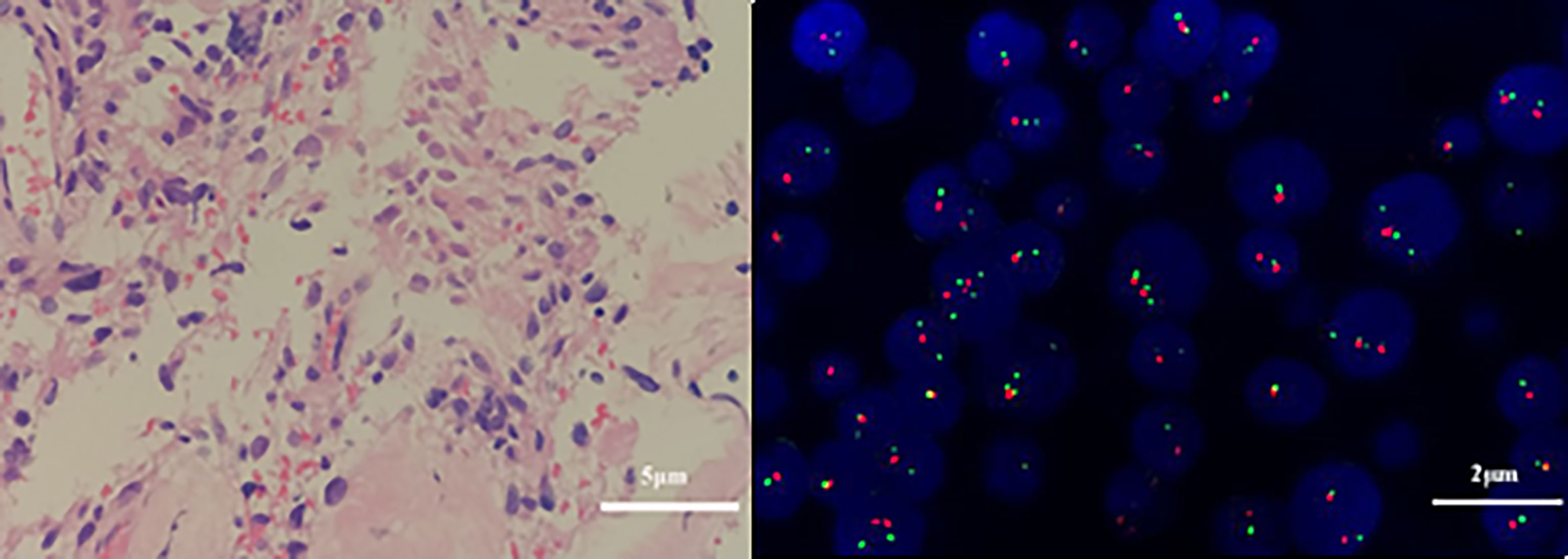
Figure 8 Pathological and genetic tests results of the fibrous tissue obtained on December 10, 2019. Atypical cells in the fibrous tissue, necrosis, and obvious cell atypia, in accordance with GBM, WHO grade IV. (A) Glial fibrillary acidic protein (GFAP) positivity. (hematoxylin and eosin, original magnification × 400). (B) Genetic tests revealed that the 1p/19q genes were intact (× 1000).
Discussion
GBM is a highly aggressive tumor, comprising 56% of gliomas, which account for 14.7% of all primary central nervous system (CNS) tumors and 47.7% of all primary malignant CNS tumors (2). Surgery is the first-line treatment for GBM, and gross total resection improves survival (13). Currently, GBM is considered one of the most difficult tumors to treat via neurosurgical management (14). Postoperative radiotherapy plus concomitant and adjuvant chemotherapy with TMZ has also been the standard treatment (15). GBM has a very poor outcome, as the estimated median survival period is 12–15 months (2, 16), and the 5-year survival rate is 5.6% (17).
Although GBM cells can pass through the BBB and be disseminated throughout the peripheral blood (7), extracranial metastasis of GBM is rare, with a reported incidence of approximately 0.4% to 2% (6, 18, 19). This rare phenomenon is also attributed to the absence of lymphatics in the brain (20). Extraneural metastasis of primary CNS neoplasms typically occurs after a median interval of 2 years from diagnosis (21); patients with GBM do not survive long enough to develop such metastases (16, 22), in which not enough time is available for neoplastic cells to metastasize to extracranial organs (19, 23). The most common metastatic sites include the lungs, pleura, lymph nodes, soft tissues, glands, and bones (19, 21, 24, 25). There may be several mechanisms through which GBM escapes the CNS; hence, the locations of the metastases widely vary and include the following: vascular invasion, cranial nerve perineural spread, lymphatic spread, direct invasion, or iatrogenic spread into soft tissue (19). Extracranial GBMs are most commonly found in patients who previously underwent invasive surgery or biopsy or received ventriculoperitoneal shunts, which create iatrogenic access to extracranial structures and disrupt the BBB (19); one clear example of this possible pathway is the migration of GBM cells via the movement of CSF through shunts to the peritoneum or pleura (26). Previous studies have highlighted that, presumably, a tumor gains access to lymphatics by dural or scalp extension through a surgical defect (27, 28). Additionally, the spontaneous trafficking of GBM cells out of the brain may occur via the CSF, lymph drainage that ends in the lymph nodes or blood, and invasive procedures increase the access of tumor cells to metastasize (23). However, this does not explain lymph node metastasis without any invasive manipulation, in which case, intrinsic factors of the tumor may play a role (29). Later studies indicated a lymphatic pathway of GBM metastasis (30). Malte Mohme et al. described that extracranial metastasis of GBM may also be related to genetic drivers and immune escape (7). GBM metastasizing to the distal limbs has not been reported. This indicates that the main vascular route of GBM metastasis is reflux into the systemic venous system, as valves in the peripheral veins prevent such reflux, whereas arteries have no valves and reach the distal limbs (19).
Approximately 10.5% of extracranial metastases of GBM involve multiple metastases, with or without intracranial recurrence (31). The most common sites of bone metastases are the vertebral spine and the thoracic cage (6, 25). Bone metastases of malignant gliomas should show lytic or sclerotic features on neuroimaging, and they are usually reported as multiple lesion spread (21, 32), as observed on an image from the patient described herein. The GBM in our patient could have metastasized via multiple possible pathways, including those described above. However, interestingly, the two metastases that were seen on imaging in December 2019 and the soft tissue metastasis in the ninth posterior costal ribs and T10 metastasis (Figure 6) in our case were consistent with the shape of the ninth intercostal nerve; therefore, we made the bold assumption that GBM could migrate through the nerve root or intercostal nerve. Multiple metastases of the T7 and T10 vertebrae and their appendages may have been caused by tumor invasion into the dura mater. As current studies have shown that there are few cases of nerve root metastasis of malignant tumors (33), so the metastasis in our case is a coincidence or a fact. More clinical cases are needed to confirm this finding.
The survival time of GBM patients is related to many factors. Research has shown that GBM with extracranial metastases most often involves the temporal lobe, either coincidentally or factually (31). For IDH-1 wild-type GBM, which accounts for almost 90% of GBMs, the median survival period of patients receiving the Stupp protocol is 15 months (34). MGMT promoter methylation, P53 and EGFR negativity and IDH 1/2 mutations have shown to be associated with improved OS and PFS in patients with GBM; ATRX and VEGF positivity are associated with worse outcome (34–40). At present, there are still many unknown molecular pathological mechanisms; thus, treatment has not progressed much over recent decades (41). Recently, a multitude of novel therapies including PD-L1/PD-1 inhibitors to maximize survival benefits and move treatment towards precision medicine have achieved effective responses (42–45). We report this case of GBM with multiple metastases of the left temporal lobe, with which wild-type IDH-1, ATRX and P53 positivity, affected the outcomes.
Conclusion
Although the number of reports of GBM metastases has increased over time, the pathogenesis of such metastases remains elusive, and this diagnosis remains rare (41, 46). Our case report indicates a new pathway of metastasis. The extracranial metastasis in this patient may have occurred through the nerve root or intercostal nerve, but more reports of GBM metastases are needed to confirm this finding.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Weifang People’s Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
WZ, YYC, and FRH performed the chemoradiotherapy in this case. WZ, XLW, and XXW drafted the manuscript and performed the literature review. YL and YJC retrieved the clinical and the image information, GYH and YXZ retrieved and analyzed the pathological information. FRH and YXZ conceived, designed, and supervised this study. The paper properly credits the meaningful contributions of the coauthors and coresearchers. All authors have been personally and actively involved in substantial work leading to the publication of paper and take public responsibility for its content. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to Ya-ping Zhang, department of imaging, Weifang People's Hospital, for her support in image processing.
References
1. Omuro A, DeAngelis L. Glioblastoma and Other Malignant Gliomas: A Clinical Review. JAMA (2013) 310(17):1842–50. doi: 10.1001/jama.2013.280319
2. Alifieris C, Trafalis D. Glioblastoma Multiforme: Pathogenesis and Treatment. Pharmacol Ther (2015) 152:63–82. doi: 10.1016/j.pharmthera.2015.05.005
3. Kuo K, Lieu A, Tsai F, Chen Y, Liang P. Intramedullary Spinal Glioblastoma Metastasis From Anaplastic Astrocytoma of Cerebellum: A Case Report and Review of the Literature. Asian J Neurosurg (2015) 10(3):268–71. doi: 10.4103/1793-5482.161170
4. Cervio A, Piedimonte F, Salaberry J, Alcorta S, Salvat J, Diez B, et al. Bone Metastases From Secondary Glioblastoma Multiforme: A Case Report. J Neuro-Oncol (2001) 52(2):141–8. doi: 10.1023/a:1010629618859
5. Liu J, Shen L, Tang G, Tang S, Kuang W, Li H, et al. Multiple Extracranial Metastases From Glioblastoma Multiforme: A Case Report and Literature Review. J Int Med Res (2020) 48(6):300060520930459. doi: 10.1177/0300060520930459
6. Pasquier B, Pasquier D, N’Golet A, Panh M, Couderc P. Extraneural Metastases of Astrocytomas and Glioblastomas: Clinicopathological Study of Two Cases and Review of Literature. Cancer (1980) 45(1):112–25. doi: 10.1002/1097-0142(19800101)45:1<112::aid-cncr2820450121>3.0.co;2-9
7. Mohme M, Maire C, Schliffke S, Joosse S, Alawi M, Matschke J, et al. Molecular Profiling of an Osseous Metastasis in Glioblastoma During Checkpoint Inhibition: Potential Mechanisms of Immune Escape. Acta Neuropathol Commun (2020) 8(1):28. doi: 10.1186/s40478-020-00906-9
8. Forsyth P, Petrov E, Mahallati H, Cairncross J, Brasher P, MacRae M, et al. Prospective Study of Postoperative Magnetic Resonance Imaging in Patients With Malignant Gliomas. J Clin Oncol (1997) 15(5):2076–81. doi: 10.1200/jco.1997.15.5.2076
9. Albert F, Forsting M, Sartor K, Adams H, Kunze S. Early Postoperative Magnetic Resonance Imaging After Resection of Malignant Glioma: Objective Evaluation of Residual Tumor and its Influence on Regrowth and Prognosis. Neurosurgery (1994) 34(1):45–60; discussion -1. doi: 10.1097/00006123-199401000-00008
10. Wen P, Macdonald D, Reardon D, Cloughesy T, Sorensen A, Galanis E, et al. Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J Clin Oncol (2010) 28(11):1963–72. doi: 10.1200/jco.2009.26.3541
11. Henson J, Ulmer S, Harris G. Brain Tumor Imaging in Clinical Trials. AJNR Am J Neuroradiol (2008) 29(3):419–24. doi: 10.3174/ajnr.A0963
12. Ulmer S, Braga T, Barker F, Lev M, Gonzalez R, Henson J. Clinical and Radiographic Features of Peritumoral Infarction Following Resection of Glioblastoma. Neurology (2006) 67(9):1668–70. doi: 10.1212/01.wnl.0000242894.21705.3c
13. Brown T, Brennan M, Li M, Church E, Brandmeir N, Rakszawski K, et al. Association of the Extent of Resection With Survival in Glioblastoma: A Systematic Review and Meta-Analysis. JAMA Oncol (2016) 2(11):1460–9. doi: 10.1001/jamaoncol.2016.1373
14. Wu W, Zhong D, Zhao Z, Wang W, Li J, Zhang W. Postoperative Extracranial Metastasis From Glioblastoma: A Case Report and Review of the Literature. World J Surg Oncol (2017) 15(1):231. doi: 10.1186/s12957-017-1300-7
15. Stupp R, Hegi M, Mason W, van den Bent M, Taphoorn M, Janzer R, et al. Effects of Radiotherapy With Concomitant and Adjuvant Temozolomide Versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol (2009) 10(5):459–66. doi: 10.1016/s1470-2045(09)70025-7
16. Stupp R, Mason W, van den Bent M, Weller M, Fisher B, Taphoorn M, et al. Radiotherapy Plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med (2005) 352(10):987–96. doi: 10.1056/NEJMoa043330
17. Ostrom Q, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan J. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro-oncology (2018) 20:iv1–iv86. doi: 10.1093/neuonc/noy131
18. Vertosick F, Selker R. Brain Stem and Spinal Metastases of Supratentorial Glioblastoma Multiforme: A Clinical Series. Neurosurgery (1990) 27(4):516–21; discussion 21-2. doi: 10.1097/00006123-199010000-00002
19. Hamilton J, Rapp M, Schneiderhan T, Marcel Schneiderhan T, Sabel M, Hayman A, et al. Glioblastoma Multiforme Metastasis Outside the CNS: Three Case Reports and Possible Mechanisms of Escape. J Clin Oncol (2014) 32(22):e80–4. doi: 10.1200/jco.2013.48.7546
20. Robert M, Wastie M. Glioblastoma Multiforme: A Rare Manifestation of Extensive Liver and Bone Metastases. Biomed Imaging Intervention J (2008) 4(1):e3. doi: 10.2349/biij.4.1.e3
21. Beauchesne P. Extra-Neural Metastases of Malignant Gliomas: Myth or Reality? Cancers (2011) 3(1):461–77. doi: 10.3390/cancers3010461
22. Stupp R, Weber D. The Role of Radio- and Chemotherapy in Glioblastoma. Onkologie (2005) 28:315–7. doi: 10.1159/000085575
23. Lah TT, Novak M, Breznik B. Brain Malignancies: Glioblastoma and Brain Metastases. Semin Cancer Biol (2020) 60:262–73. doi: 10.1016/j.semcancer.2019.10.010
24. Rajagopalan V, El Kamar F, Thayaparan R, Grossbard M. Bone Marrow Metastases From Glioblastoma Multiforme–A Case Report and Review of the Literature. J Neuro-Oncol (2005) 72(2):157–61. doi: 10.1007/s11060-004-3346-y
25. LoRusso P, Tapazoglou E, Zarbo R, Cullis P, Austin D, Al-Sarraf M. Intracranial Astrocytoma With Diffuse Bone Marrow Metastasis: A Case Report and Review of the Literature. J Neuro-Oncol (1988) 6(1):53–9. doi: 10.1007/bf00163541
26. Wakabayashi T, Yoshida J, Kageyama N, Mutsuga N, Takeuchi Y. [Extraneural Metastasis of Malignant Glioma Through a Ventriculo-Peritoneal Shunt: Growth in Peritoneal Cavity as Ascitic Form]. No Shinkei Geka Neurol Surg (1985) 13(5):547–52. doi: 10.1029/2000JD900685
27. González Cámpora R, Otal Salaverri C, Vázquez Ramirez F, Salguero Villadiego M, Galera Davidson H. Metastatic Glioblastoma Multiforme in Cervical Lymph Nodes. Report of a Case With Diagnosis by Fine Needle Aspiration. Acta Cytologica (1993) 37(6):938–42. doi: 10.1007/BF02915141
28. Carvalho J, Barbosa C, Feher O, Maldaun M, Camargo V, Moraes F, et al. Systemic Dissemination of Glioblastoma: Literature Review. Rev Da Associacao Med Bras (1992) (2019) 65(3):460–8. doi: 10.1590/1806-9282.65.3.460
29. Wassati H, Loo S, Low H. Lymphatic Metastasis Due to Glioblastoma. Neurosci (Riyadh Saudi Arabia) (2016) 21(2):168–9. doi: 10.17712/nsj.2016.2.20150497
30. Frank S, Kuhn S, Brodhun M, Mueller U, Romeike B, Kosmehl H, et al. Metastatic Glioblastoma Cells Use Common Pathways via Blood and Lymphatic Vessels. Neurol I Neurochirurgia Polska (2009) 43(2):183–90.
31. Cunha M, Maldaun M. Metastasis From Glioblastoma Multiforme: A Meta-Analysis. Rev Da Associacao Med Bras (1992) (2019) 65(3):424–33. doi: 10.1590/1806-9282.65.3.424
32. Goodwin C, Liang L, Abu-Bonsrah N, Hdeib A, Elder B, Kosztowski T, et al. Extraneural Glioblastoma Multiforme Vertebral Metastasis. World Neurosurg (2016) 89:578–82.e3. doi: 10.1016/j.wneu.2015.11.061
33. Di Sibio A, Romano L, Giuliani A, Varrassi M, De Donato MC, Iacopino A, et al. Nerve Root Metastasis of Gastric Adenocarcinoma: A Case Report and Review of the Literature. Int J Surg Case Rep (2019) 61:9–13. doi: 10.1016/j.ijscr.2019.07.001
34. Louis D, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee W, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol (2016) 131(6):803–20. doi: 10.1007/s00401-016-1545-1
35. Anghileri E, Elena A, Castiglione M, Melina C, Nunziata R, Raffaele N, et al. Extraneural Metastases in Glioblastoma Patients: Two Cases With YKL-40-Positive Glioblastomas and a Meta-Analysis of the Literature. Neurosurg Rev (2016) 39(1):37–45; discussion -6. doi: 10.1007/s10143-015-0656-9
36. Sim J, Nam D, Kim Y, Lee I, Choi J, Sa J, et al. Comparison of 1p and 19q Status of Glioblastoma by Whole Exome Sequencing, Array-Comparative Genomic Hybridization, and Fluorescence in Situ Hybridization. Med Oncol (Northwood London England) (2018) 35(5):60. doi: 10.1007/s12032-018-1119-2
37. Brandner S, von Deimling A. Diagnostic, Prognostic and Predictive Relevance of Molecular Markers in Gliomas. Neuropathol Appl Neurobiol (2015) 41(6):694–720. doi: 10.1111/nan.12246
38. Siegal T. Clinical Impact of Molecular Biomarkers in Gliomas. J Clin Neurosci (2015) 22(3):437–44. doi: 10.1016/j.jocn.2014.10.004
39. Karsy M, Neil J, Guan J, Mahan M, Mark M, Colman H, et al. A Practical Review of Prognostic Correlations of Molecular Biomarkers in Glioblastoma. Neurosurg Focus (2015) 38(3):E4. doi: 10.3171/2015.1.Focus14755
40. Jain R, di Tomaso E, Duda D, Loeffler J, Sorensen A, Batchelor T. Angiogenesis in Brain Tumours. Nat Rev Neurosci (2007) 8(8):610–22. doi: 10.1038/nrn2175
41. Pietschmann S, von Bueren A, Kerber M, Baumert B, Kortmann R, Müller K. An Individual Patient Data Meta-Analysis on Characteristics, Treatments and Outcomes of Glioblastoma/Gliosarcoma Patients With Metastases Outside of the Central Nervous System. PLoS One (2015) 10(4):e0121592. doi: 10.1371/journal.pone.0121592
42. Litak J, Mazurek M, Grochowski C, Kamieniak P, Roliński J. PD-L1/PD-1 Axis in Glioblastoma Multiforme. Int J Mol Sci (2019) 20(21):5347. doi: 10.3390/ijms20215347
43. Tan A, Ashley D, López G, Malinzak M, Friedman H, Khasraw M. Management of Glioblastoma: State of the Art and Future Directions. CA: Cancer J Clin (2020) 70(4):299–312. doi: 10.3322/caac.21613
44. Shen L, Li H, Bin S, Li P, Chen J, Gu H, et al. The Efficacy of Third Generation Anti−HER2 Chimeric Antigen Receptor T Cells in Combination With PD1 Blockade Against Malignant Glioblastoma Cells. Oncol Rep (2019) 42(4):1549–57. doi: 10.3892/or.2019.7263
45. Wang X, Guo G, Guan H, Yu Y, Lu J, Yu J. Challenges and Potential of PD-1/PD-L1 Checkpoint Blockade Immunotherapy for Glioblastoma. J Exp Clin Cancer Res: CR (2019) 38(1):87. doi: 10.1186/s13046-019-1085-3
Keywords: glioblastoma, extracranial metastasis, soft tissue metastasis, nerve root, intercostal nerve, case report
Citation: Zhang W, Cai Y-y, Wang X-l, Wang X-x, Li Y, Han G-y, Chu Y-j, Zhang Y-x and Hao F-r (2021) Bone Metastases of Glioblastoma: A Case Report and Review of the Literature. Front. Oncol. 11:705455. doi: 10.3389/fonc.2021.705455
Received: 05 May 2021; Accepted: 02 September 2021;
Published: 27 September 2021.
Edited by:
Ira Ida Skvortsova, Innsbruck Medical University, AustriaReviewed by:
Francesco Pasqualetti, University of Oxford, United KingdomRocchina Caivano, Oncological Center of Basilicata (IRCCS), Italy
Copyright © 2021 Zhang, Cai, Wang, Wang, Li, Han, Chu, Zhang and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fu-rong Hao, aGtjNTE1QDE2My5jb20=; Yun-xiang Zhang, emhhbmdiaW5nMTk5NTkyQDE2My5jb20=
 Wei Zhang1
Wei Zhang1 Yun-xiang Zhang
Yun-xiang Zhang Fu-rong Hao
Fu-rong Hao