- 1Breast Surgery Unit, Fondazione IRCCS Ca’ Granda – Ospedale Maggiore Policlinico, Milan, Italy
- 2Division of Pathology, IEO, European Institute of Oncology IRCCS, Milan, Italy
- 3Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy
- 4Division of Radiotherapy, IEO, European Institute of Oncology IRCCS, Milan, Italy
- 5Division of Breast Surgery, IEO, European Institute of Oncology IRCCS, Milan, Italy
- 6Division of Pathology, Fondazione IRCCS Ca’ Granda – Ospedale Maggiore Policlinico, Milan, Italy
- 7Breast Imaging Unit, Fondazione IRCCS Ca’ Granda - Ospedale Maggiore Policlinico, Milan, Italy
- 8Gynecology Unit, Fondazione IRCCS Ca’ Granda - Ospedale Maggiore Policlinico, Milan, Italy
- 9Fertility and Procreation Unit, Division of Gynecologic Oncology, IEO European Institute of Oncology IRCCS, Milan, Italy
Breast cancer is the most common malignancy occurring during gestation. In early-stage breast cancer during pregnancy (PrBC), breast-conserving surgery (BCS) with delayed RT is a rational alternative to mastectomy, for long considered the standard-of-care. Regrettably, no specific guidelines on the surgical management of these patients are available. In this study, we investigated the feasibility and safety of BCS during the first trimester of pregnancy in women with early-stage PrBC. All patients with a diagnosis of PrBC during the first trimester of pregnancy jointly managed in two PrBC-specialized Centers were included in this study. All patients underwent BCS followed by adjuvant radiotherapy to the ipsilateral breast after delivery. Histopathological features and biomarkers were first profiled on pre-surgical biopsies. The primary outcome was the isolated local recurrence (ILR). Among 168 PrBC patients, 67 (39.9%) were diagnosed during the first trimester of gestation. Of these, 30 patients (age range, 23-43 years; median=36 years; gestational age, 2-12 weeks; median=7 weeks; median follow-up time=6.5 years) met the inclusion criteria. The patients that were subjected to radical surgery (n=14) served as controls. None of the patients experienced perioperative surgical complications. No ILR were observed within three months (n=30), 1 year (n=27), and 5 years (n=18) after surgery. Among the study group, 4 (12.3%) patients experienced ILR or new carcinomas after 6-13 years, the same number (n=4) had metastatic dissemination after 3-7 years. These patients are still alive and disease-free after 14-17 years of follow-up. The rate of recurrences and metastasis in the controls were not significantly different. The findings provide evidence that BCS in the first trimester PrBC is feasible and reasonably safe for both the mother and the baby.
Introduction
Breast cancer is the most common malignancy occurring in the course of gestation, with approximately 1,400 new diagnoses every year in Europe (1, 2). In the last decade, it has been observed a steady increase of breast cancer during pregnancy (PrBC) incidence (3–6). Delayed diagnosis is responsible for the worse outcome of PrBC compared to pregnancy-unrelated breast cancer, but stage-normalized survival is not different from that of age-matched non-pregnant controls (7–12). According to most guidelines, PrBC should be managed with the same protocols as breast cancer occurring in young non-pregnant women (1, 2, 13–18). However, the condition of pregnancy adds a layer of complexity to the treatment of PrBC because the benefit for the mother must not harm the fetus (2, 13, 19–22). For example, chemotherapy (CT) is contraindicated during the first trimester of gestation, while it can be safely administered in the second and third trimesters (23). Radiotherapy (RT) should be postponed until the postpartum period because of the risks associated with fetal radiation exposure (24, 25). Surgery is feasible and relatively safe at any stage of gestation, even if it might slightly increase the risk of pregnancy loss in the first trimester (1.0-2.0%) and might lead to premature birth in 1.5-2.0% of cases when performed in the second/third trimester (17, 26–28). Moreover, mastectomy is often proposed during the first trimester of pregnancy, regardless of the tumor stage, to reduce the risks of a delayed RT (14, 25, 26, 29–31). On the other hand, mastectomy is related to higher rates of post-surgical complications, psychological frailty, impairment of health-related quality of life, and increased sanitary costs (19, 30, 32–34). Regrettably, only a handful of studies on the specific outcome of PrBC patients treated with breast-conserving surgery (BCS) are currently available. Thus, the choice of the optimal surgical approach during the first trimester of pregnancy remains a matter of controversy.
In this study, we sought to provide evidence of the clinical feasibility and safety of BCS during the first trimester of pregnancy in women with early-stage PrBC.
Materials and Methods
Study Design
All patients with a diagnosis of PrBC during the first trimester of pregnancy at the European Institute of Oncology IRCCS and Fondazione IRCCS Ca’ Granda – Ospedale Maggiore Policlinico in Milan were included in this study. The first trimester was defined as 12 weeks and 6 days after the first day of the last menstruation. As for internal protocols, surgery followed the same conservative-oriented schemes applied for nonpregnant patients. Only women with early-stage PrBC treated with BCS during pregnancy followed by planned RT to the whole breast after delivery were included. The patients that were subjected to radical surgery (n=14) served as controls. Exclusion criteria for BCS were i) locally advanced disease; ii) history of breast cancer; iii) hereditary breast cancer; iv) multicentric disease; v) diffuse malignant microcalcifications on mammography; vi) inflammatory breast cancer; and vii) connective tissue disease. All cases underwent central pathological review at the Pathology Department of the European Institute of Oncology and were re-classified and re-graded following the latest World Health Organization criteria (35) and the Nottingham grading system (36). The staging was performed according to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (37). This study was approved by the local Institutional Review Board under protocol number #620_2018bis and was fully compliant with The Code of Ethics of the World Medical Association. Women were informed about the possible alternatives, including risks and benefits, and signed written informed consent.
Characteristics of the Study Population
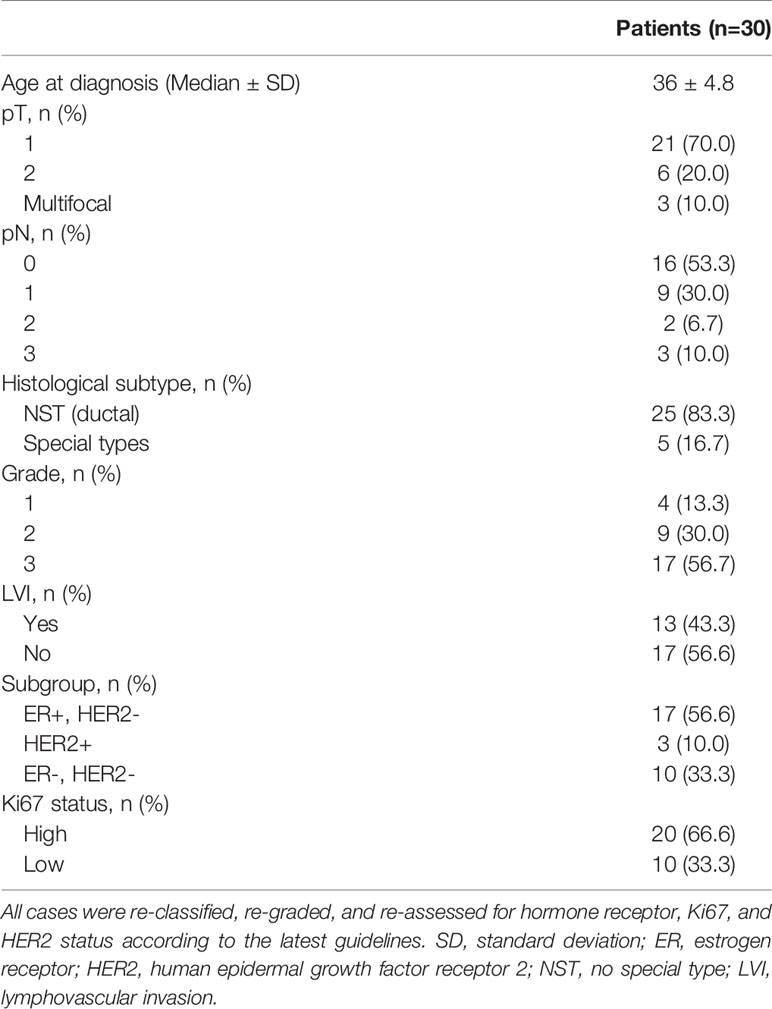
Taken together, 67 out of 168 (39.9%) PrBC patients treated from 2000 to 2020 had a diagnosis during the first trimester of gestation but 20 (29.9%) of them were not eligible for surgery due to locally advanced tumors. Of the remaining patients, 14 (20.9%) women were treated with mastectomy, including 2 that were subjected to a wide excision following a previous mastectomy. Apart from 1 (7.1%) patient who terminated her pregnancy, these no-BCS PrBC patients (n=13) represented our controls. Among the 33 PrBC treated with BCS during the first trimester, 3 were subsequently excluded; 2 because decided to terminate the pregnancy and received RT, and 1 because was enrolled in another study investigating the efficacy of electron intraoperative radiotherapy (ELIOT) (38). Thus, the study group was composed of 30 patients (age range, 23-43 years; median=36 years; gestational age at diagnosis, 2-12 weeks; median=7 weeks; median follow-up time=6.5 years). The study flow chart is portrayed in Figure 1, while the clinicopathological features of the patients included are shown in Table 1.
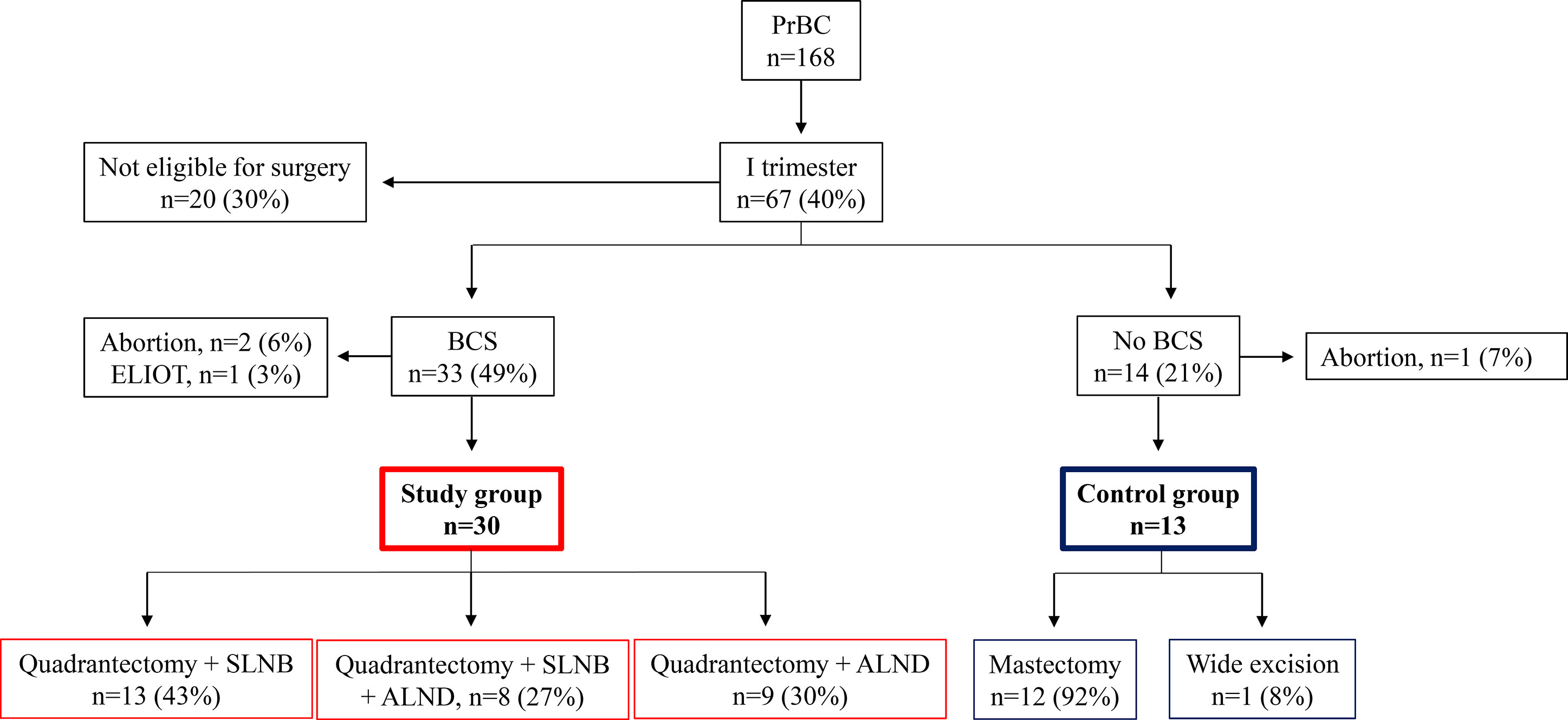
Figure 1 Study flowchart. PrBC, breast cancer during pregnancy. BCS, breast-conserving surgery; ELIOT, electron intraoperative radiotherapy; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection.
Treatment, Follow-Up, and Outcome Evaluation
Before surgery, all participants were investigated with bilateral breast ultrasounds with the axillary examination. Bilateral mammography with a mediolateral-oblique view, +/- cranial-caudal investigation, was performed with fetal shielding. Standard pre-admission testing (e.g. electrocardiogram, blood pressure, laboratory blood work, chest X-ray with shielding, abdomen ultrasound) was performed 2-4 weeks before surgery. Genetic counseling and a thorough obstetrical and neonatological consultation were performed in all patients. After delivery, all patients received conventional 46-60 Gy RT to the ipsilateral breast. Owing to the potential teratogenic effects and increased miscarriage risk, patients were not given CT until the 12th week of pregnancy, when necessary. Both adjuvant endocrine therapy (ET) and target therapies (i.e. Tamoxifen, Aromatase inhibitors, gonadotropin-releasing hormone (GnRH) analogs, trastuzumab, and pertuzumab) were postponed after delivery, if appropriate. The primary outcome was the rate of isolated local recurrences (ILR) after delayed local irradiation of the breast.
Results
Delivery and Clinical Outcome
Thirteen (43.3%) BCS patients were concurrently subjected to sentinel lymph node biopsy (SLNB). Of these, 8 (26.7%) were positive and subsequent axillary lymph node dissection (ALND) was performed, while 9 (30.0%) women had clinically positive axilla and were subjected to ALND right away. Altogether, 21 (70.0%) PrBC were treated with adjuvant CT during gestation (including taxanes in 7 (33.3%) cases). The first RT dose was administered after 202-288 days from the primary surgery (median, 260 days) and after 23-101 days from the childbirth (median, 45 days). A total of 15 (50.0%) women had vaginal delivery; the median gestational age at the delivery was 36 weeks (range, 29-40 weeks). There were not reported fetal deaths nor congenital abnormalities of the newborns in both groups. The 5-year overall survival rate for all patients was 97% (n=29/30), as one patient died of metastatic disease after 33 months from the initial diagnosis.
Isolated Local Recurrence-Free Survival
None of the patients from both groups suffered perioperative surgical complications. No ILR were observed within three months (n=30), 1 year (n=27), and 5 years (n=18) after BCS, while 4 (30.8%) controls had relapses after 3-7 years. Of note, four patients treated with BCS had ILR or new carcinomas after 6-13 years; these patients are still living and disease-free with a median follow-up time of 54 months (range 36-180 months). Their clinical history is detailed below and summarized in Figure 2.
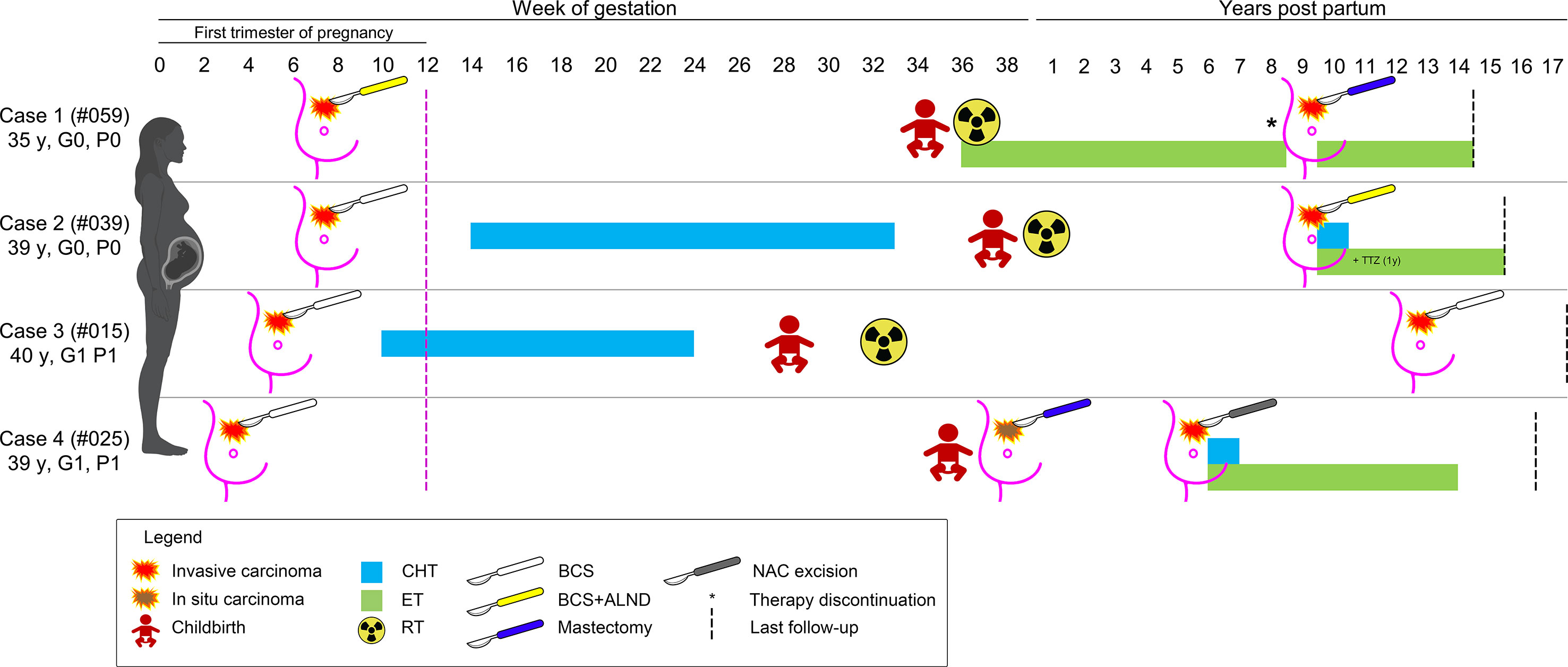
Figure 2 Schematic representation of the clinical history of the four patients with a diagnosis of breast cancer during pregnancy that experienced a secondary event after breast-conserving surgery. The timeline depicts the weeks of gestation and the years after childbirth, as reported on the top; patients are shown as rows, according to their ID and obstetric history on the left; the type of therapy is coded based on the legend on the bottom. CHT, chemotherapy; ET, endocrine therapy; RT, radiation therapy; TTZ, trastuzumab.
Case 1
This patient (#PRBC059) was a 35-year-old Caucasian woman with no family and/or personal history of breast cancer. During the 7th week of gestation, she was diagnosed with a malignant tumor of the left breast and was subsequently subjected to BCS, SLNB (positive), and ALND. Histopathological analyses revealed the presence of a bifocal pure mucinous carcinoma (paucicellular, i.e. type A) with hormone receptors (HR)+/HER2-/Ki67 16% phenotype, with low Ki67 index. BRCA testing revealed a wild-type gene status. The two nodules measured 0.5 cm and 1.5 cm in the greatest dimensions, and the final staging was pT1c(m) pN1(mi,1/34). The surgical margins were free from tumor cells. During the pregnancy period, the patient did not receive adjuvant therapy and gave birth at 34 + 5 weeks. Adjuvant RT and ET were commenced after delivery. Eight years later, the woman received two cycles of ovarian stimulation for a total period of 6 months, resulting in an ectopic pregnancy. New breast cancer in the ipsilateral breast was diagnosed 1 month after this event (ILR-free survival=103 months, 8.6 years). Therefore, the patient underwent a nipple-sparing mastectomy of the left breast and a risk-reducing mastectomy of the right breast with plastic reconstruction. The resected tumor was HR+/HER2- poorly differentiated (G3) invasive ductal carcinoma (IDC) measuring 0.7 cm in greatest dimensions (rpT1b) and no mucinous differentiation was observed. To date, the patient is alive, free of disease, and receiving ET.
Case 2
This patient (#PBC039) was a 39-year-old Caucasian woman with no family history of breast cancer. During the 7th week of gestation, she was subjected to quadrantectomy and SLNB for a malignant tumor of the left breast. Histopathological analyses revealed the presence of a poorly differentiated (G3) HR+/HER2-/Ki67 35% IDC (pT1c pN0(sn)). The resection margins were negative. Starting from the 14th week of gestation, CT was performed up to 33 weeks. Adjuvant RT followed the delivery at 38 weeks, but the patient refused the proposed ET. After 117 months, a new tumor was discovered in the ipsilateral breast. Compared with the former neoplasm, this tumor was HER2+. After BCS, combined CT, trastuzumab, and ET were administered. At present, the patient is alive 6 years after delivery, disease-free, and receiving ET.
Case 3
This patient (#PBC015) was a 40-year-old Caucasian woman, with no family history of breast cancer, who discovered a lump in her left breast. A core biopsy showed invasive breast cancer. During the pre-operative visit, a pregnancy test resulted in positive. Ultrasounds confirmed a gestational sac corresponding to 5 weeks of amenorrhea. Subsequently, she was subjected to left quadrantectomy and ALND and histology confirmed an IDC G3+ with an extensive intraductal component, free margins, negative lymph nodes, with a maximum diameter of 2.5 cm. The tumor had a triple-negative phenotype and the Ki67 index was 35%. During pregnancy, the patient received CT up to the 24th week and had a premature delivery at the 29th gestational week. Subsequently, adjuvant RT was performed one month after delivery. No other medical therapies were performed. After 150 months, the patient developed a new ipsilateral tumor and was subjected to nipple-sparing mastectomy and contralateral reduction mammoplasty. This new tumor was a moderately differentiated triple-negative IDC. The patient has not undergone any type of adjuvant systemic therapy and is presently alive and disease-free after 17 years of follow-up.
Case 4
The patient (#PBC025) was a 39-year-old Caucasian woman with a family history of breast cancer. At the gestational age of 5 weeks, she was treated with BCS and SLNB for an IDC G1 with apocrine features measuring 3 mm in greatest dimensions, with associated ductal carcinoma in situ (DCIS) G2, and pleomorphic lobular carcinoma in situ (LCIS). The pathological staging was pT1a (is) pN0 (sn), and the tumor was a TNBC. The patient did not receive any adjuvant therapy during pregnancy. After delivery at the 36th week, mammography and bilateral breast ultrasound revealed extensive microcalcifications. In the preoperative period, the patient had not performed bilateral mammography as per protocol, and 1 month after delivery, a histological examination confirmed the presence of DCIS G2. She underwent a nipple-areola complex (NAC) sparing mastectomy with radio-immuno-guided SLNB and plastic reconstruction. The pathology examination confirmed the presence of DCIS G2. After 64 months, she underwent removal of the nipple-areola complex due to the presence of CDI G2 with micropapillary aspects and extensive lymph-vascular invasion. Interestingly, the immunophenotype of the tumor was that of luminal breast cancer, with ER 40%, PgR40%, Ki67 15%, and HER2-.
Discussion
PrBC represents an important health issue given its increasing incidence and the necessity of maintaining the balance between maternal and fetal well-being (39). The clinical management of this condition, especially in the first three months of gestation, is fairly challenging with limited therapeutic options (40). In this context, when the tumor is small, a conservative surgical approach could be employed as in the non-pregnant setting, also considering the possible complications after mastectomy, which could be particularly worrisome during pregnancy (41). Most patients received SLNB as part of the axillary investigation. The safety of 99mTechnetium radiotracer for this procedure has been investigated in a dosimetry study, showing a very low dose associated with the lymphoscintigraphy procedure (in the range of 10-100 µGy) (42). Adjuvant RT to the whole breast is avoided throughout the pregnancy due to the risk of fetal radiation exposure. The fatal dose has been estimated to be up to 50 mGy in the first trimester and even higher in the late gestational age (43). This value exceeds the dose which is deemed not to be associated with measurable increased risk of fetal damage by the International Commission of Radiological Protection addressing the biological effects of prenatal irradiation (44). When adjuvant CT is indicated, the risk of delaying RT until the completion of systemic therapy is considered negligible (45). Therefore, it is generally applied the same approach as for non-pregnant, as the standard treatment of non-pregnant patients receiving CT is postponing RT (46). For those not receiving CT, the RT start should ideally be as close as possible to the surgery, shortly after childbirth. Hence, the local disease control is inversely proportional to the RT procrastination time, with a 1.14 relative risk of recurrence per month of delay (47). In the case of BRCA mutation, conservative treatment could be a bridge treatment of a more definitive intervention when the results of the test are available.
In our study, RT, ET, and anti-HER2 treatment were postponed after delivery (48). Local recurrences/second primary tumors were observed in 4 out of 30 patients treated with BCS. Given that patient #4 did not receive postoperative RT, but an after-delivery mastectomy for preoperative diagnostic underestimation during pregnancy, this case does not represent a post-BCS recurrence. On the other hand, cases #1-3 could be considered real relapses. Two of these tumors occurred in patients who received CT during pregnancy, in which the interval between the end of systemic therapies and the onset of RT was not influenced by the pregnancy. In a single patient (not eligible for systemic treatment in pregnancy), the RT was performed with a longer interval than the usual one of the non-pregnant patients. Survival was not affected by local relapse, underlining the efficacy of salvage treatment. Based on their IRC and subsequent salvage surgery, the 4 cases presented in detail here might represent a high-risk group of patients that requires particular attention in the choice of the surgical approach. Additional analyses encompassing not only clinical criteria but also molecular information would be required to precisely identify the early-stage PrBC at increased risk of relapse. Furthermore, due to the relatively small sample size of this feasibility study, and the subsequent impossibility of building a robust multivariable risk model, attention should be paid to the interpretation of our conclusions. It should be noted, however, that this is the first study providing previously unavailable data on BCS feasibility in this extremely rare condition. A randomized controlled multi-institutional trial would be required to address this question after controlling all other factors of the management armamentarium that can have an impact on the short- and long-term outcome, including delayed CT,
Despite these limitations, our results suggest that BCS during the first trimester of pregnancy in early-stage PrBC can be considered reasonably safe, providing the identification of women with low-risk clinical and biological features.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by IRCCS Ca’ Granda Ospedale Maggiore Policlinico. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Study design, CB, MG, ML, and VG. Database curation, CB, NF, ES, KV, BB, and FP. First draft. CB, and MG. Initial review, NF, ES, KV, BB, and FP. Images, NF, ES, and KV. Bibliography, NF, ES, and KV. Supervision, NF, EGR, GV, PV, and VG. Critical review of the final draft, EV, LD, CR, LR, MS, RP, EL, and GS. All authors contributed to the article and approved the submitted version.
Conflict of Interest
NF has received honoraria for consulting, advisory role, honoraria, travel, accommodation, and/or speaker bureau from Merck Sharp & Dohme (MSD), Boehringer Ingelheim, and Novartis. ER has received advisory fees from Novartis, Roche, and MSD Italia; honoraria from Thermo Fisher Scientific, AstraZeneca, Roche. GV has received honoraria for consulting, advisory role, speakers’ bureau, travel, accommodation, expenses, and/or research funding from MSD Oncology, Pfizer, Dako, Roche/Genetech, Astellas Pharma, Novartis, Bayer, Daiichi Sankyo, Menarini, Ventana Medical Systems Dako/Agilent Technologies, Cepheid, and Celgene. FP has received honoraria from Ipsen and Roche Diagnostics in the last 3 years.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We wish to thank all the patients, family members, and staff from all the units that made this study possible.
References
1. Peccatori FA, Azim HA Jr, Orecchia R, Hoekstra HJ, Pavlidis N, Kesic V, et al. Cancer, Pregnancy and Fertility: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2013) 24 Suppl 6:vi160–70. doi: 10.1093/annonc/mdt199
2. Alfasi A, Ben-Aharon I. Breast Cancer During Pregnancy-Current Paradigms, Paths to Explore. Cancers (2019) 11(11):1669. doi: 10.3390/cancers11111669
3. Andersson TM, Johansson AL, Hsieh CC, Cnattingius S, Lambe M. Increasing Incidence of Pregnancy-Associated Breast Cancer in Sweden. Obstet Gynecol (2009) 114(3):568–72. doi: 10.1097/AOG.0b013e3181b19154
4. Lethaby AE, O’Neill MA, Mason BH, Holdaway IM, Harvey VJ. Overall Survival From Breast Cancer in Women Pregnant or Lactating at or After Diagnosis. Auckland Breast Cancer Study Group. Int J Cancer (1996) 67(6):751–5. doi: 10.1002/(SICI)1097-0215(19960917)67:6<751::AID-IJC1>3.0.CO;2-Q
5. Azim HA, Santoro L, Russell-Edu W, Pentheroudakis G, Pavlidis N, Peccatori FA. Prognosis of Pregnancy-Associated Breast Cancer: A Meta-Analysis of 30 Studies. Cancer Treat Rev (2012) 38(7):834–42. doi: 10.1016/j.ctrv.2012.06.004
6. Leclère B, Molinié F, Trétarre B, Stracci F, Daubisse-Marliac L, Colonna M. Trends in Incidence of Breast Cancer Among Women Under 40 in Seven European Countries: A GRELL Cooperative Study. Cancer Epidemiol (2013) 37(5):544–9. doi: 10.1016/j.canep.2013.05.001
7. Kakoulidis I, Skagias L, Politi E. Pregnancy Associated Breast Cancer (PABC): Aspects in Diagnosis. Breast Dis (2015) 35(3):157–66. doi: 10.3233/BD-150408
8. Johansson AL, Andersson TM, Hsieh CC, Jirstrom K, Dickman P, Cnattingius S, et al. Stage at Diagnosis and Mortality in Women With Pregnancy-Associated Breast Cancer (PABC). Breast Cancer Res Treat (2013) 139(1):183–92. doi: 10.1007/s10549-013-2522-1
9. Peccatori FA, Azim HA Jr. Managing Pregnancy-Associated Breast Cancer: Is More Really Better? Breast (2016) 30:215–6. doi: 10.1016/j.breast.2016.06.006
10. Azim HA Jr, Botteri E, Renne G, Dell’orto P, Rotmensz N, Gentilini O, et al. The Biological Features and Prognosis of Breast Cancer Diagnosed During Pregnancy: A Case-Control Study. Acta Oncol (2012) 51(5):653–61. doi: 10.3109/0284186X.2011.636069
11. Nettleton J, Long J, Kuban D, Wu R, Shaefffer J, El-Mahdi A. Breast Cancer During Pregnancy: Quantifying the Risk of Treatment Delay. Obstet Gynecol (1996) 87(3):414–8. doi: 10.1016/0029-7844(95)00470-X
12. Waks AG, King TA, Winer EP. Timeliness in Breast Cancer Treatment-The Sooner, the Better. JAMA Oncol (2016) 2(3):302–4. doi: 10.1001/jamaoncol.2015.4506
13. Poggio F, Tagliamento M, Pirrone C, Soldato D, Conte B, Molinelli C, et al. Update on the Management of Breast Cancer During Pregnancy. Cancers (2020) 12(12):3616. doi: 10.3390/cancers12123616
14. Feng C, Yu D, Qian J. Long-Term Results and Predictors of Survival After Conservative Breast Surgery for Breast Cancer During Pregnancy. Med Sci Monit (2019) 25:8587–94. doi: 10.12659/MSM.917288
15. Balaya V, Bonsang-Kitzis H, Ngo C, Delomenie M, Gosset M, Mimouni M, et al. What About Sentinel Lymph Node Biopsy for Early Breast Cancer During Pregnancy? J Gynecol Obstet Hum Reprod (2018) 47(5):205–7. doi: 10.1016/j.jogoh.2018.03.003
16. Martinez MT, Bermejo B, Hernando C, Gambardella V, Cejalvo JM, Lluch A. Breast Cancer in Pregnant Patients: A Review of the Literature. Eur J Obstet Gynecol Reprod Biol (2018) 230:222–7. doi: 10.1016/j.ejogrb.2018.04.029
17. Yu CH, Weng SF, Ho CH, Chen YC, Chen JY, Chang YJ, et al. Pregnancy Outcomes Following Nonobstetric Surgery During Gestation: A Nationwide Population-Based Case-Control Study in Taiwan. BMC Pregnancy Childbirth (2018) 18(1):460. doi: 10.1186/s12884-018-2079-4
18. Grizzi G, Ghidini M, Botticelli A, Tomasello G, Ghidini A, Grossi F, et al. Strategies for Increasing the Effectiveness of Aromatase Inhibitors in Locally Advanced Breast Cancer: An Evidence-Based Review on Current Options. Cancer Manag Res (2020) 12:675–86. doi: 10.2147/CMAR.S202965
19. Invernizzi M, Kim J, Fusco N. Editorial: Quality of Life in Breast Cancer Patients and Survivors. Front Oncol (2020) 10(2624). doi: 10.3389/fonc.2020.620574
20. Porrett PM. Biologic Mechanisms and Clinical Consequences of Pregnancy Alloimmunization. Am J Transpl: Off J Am Soc Transplant Am Soc Transplant Surgeons (2018) 18(5):1059–67. doi: 10.1111/ajt.14673
21. Albright CM, Wenstrom KD. Malignancies in Pregnancy. Best Pract Res Clin Obstet Gynaecol (2016) 33:2–18. doi: 10.1016/j.bpobgyn.2015.10.004
22. Pavlidis NA. Coexistence of Pregnancy and Malignancy. Oncologist (2002) 7(4):279–87. doi: 10.1634/theoncologist.2002-0279
23. Peccatori FA, Lambertini M, Scarfone G, Del Pup L, Codacci-Pisanelli G. Biology, Staging, and Treatment of Breast Cancer During Pregnancy: Reassessing the Evidences. Cancer Biol Med (2018) 15(1):6–13. doi: 10.20892/j.issn.2095-3941.2017.0146
24. Kal HB, Struikmans H. Radiotherapy During Pregnancy: Fact and Fiction. Lancet Oncol (2005) 6(5):328–33. doi: 10.1016/S1470-2045(05)70169-8
25. Litton J. Gestational Breast Cancer: Treatment. In: Burtein H, Lockwood C, editors. UpToDate. Waltham, MA: UpToDate, Post TW (Ed) (Accessed on June 2021).
26. Zagouri F, Dimitrakakis C, Marinopoulos S, Tsigginou A, Dimopoulos MA. Cancer in Pregnancy: Disentangling Treatment Modalities. ESMO Open (2016) 1(3):e000016. doi: 10.1136/esmoopen-2015-000016
27. Rojas KE, Bilbro N, Manasseh DM, Borgen PI. A Review of Pregnancy-Associated Breast Cancer: Diagnosis, Local and Systemic Treatment, and Prognosis. J Womens Health (Larchmt) (2019) 28(6):778–84. doi: 10.1089/jwh.2018.7264
28. Amant F, Deckers S, Van Calsteren K, Loibl S, Halaska M, Brepoels L, et al. Breast Cancer in Pregnancy: Recommendations of an International Consensus Meeting. Eur J Cancer (2010) 46(18):3158–68. doi: 10.1016/j.ejca.2010.09.010
29. Becker S. Breast Cancer in Pregnancy: A Brief Clinical Review. Best Pract Res Clin Obstet Gynaecol (2016) 33:79–85. doi: 10.1016/j.bpobgyn.2015.10.013
30. Michelotti A, Invernizzi M, Lopez G, Lorenzini D, Nesa F, De Sire A, et al. Tackling the Diversity of Breast Cancer Related Lymphedema: Perspectives on Diagnosis, Risk Assessment, and Clinical Management. Breast (2019) 44:15–23. doi: 10.1016/j.breast.2018.12.009
31. Pentheroudakis G, Orecchia R, Hoekstra HJ, Pavlidis N. Cancer, Fertility and Pregnancy: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2010) 21 Suppl 5:v266–73. doi: 10.1093/annonc/mdq198
32. Monteiro-Grillo I, Marques-Vidal P, Jorge M. Psychosocial Effect of Mastectomy Versus Conservative Surgery in Patients With Early Breast Cancer. Clin Transl Oncol (2005) 7(11):499–503. doi: 10.1007/BF02717003
33. de Sire A, Losco L, Cisari C, Gennari A, Boldorini R, Fusco N, et al. Axillary Web Syndrome in Women After Breast Cancer Surgery Referred to an Oncological Rehabilitation Unit: Which Are the Main Risk Factors? A Retrospect Case Control Study Eur Rev Med Pharmacol Sci (2020) 24(15):8028–35. doi: 10.26355/eurrev_202008
34. Nardin S, Mora E, Varughese FM, D’Avanzo F, Vachanaram AR, Rossi V, et al. Breast Cancer Survivorship, Quality of Life, and Late Toxicities. Front Oncol (2020) 10:864. doi: 10.3389/fonc.2020.00864
35. WHO Classification of Tumours Editorial Board. Breast Tumours. Fifth Edition Ed. Lyon, France: International Agency for Research on Cancer (2019).
36. Elston CW, Ellis IO. Pathological Prognostic Factors in Breast Cancer. I. The Value of Histological Grade in Breast Cancer: Experience From a Large Study With Long-Term Follow-Up. Histopathology (1991) 19(5):403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x
37. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. Eight Edition Ed. Springer International Publishing (2017).
38. Leonardi M, Cecconi A, Luraschi R, Rondi E, Cattani F, Lazzari R, et al. Electron Beam Intraoperative Radiotherapy (ELIOT) in Pregnant Women With Breast Cancer: From In Vivo Dosimetry to Clinical Practice. Breast Care (Basel) (2017) 12(6):396–400. doi: 10.1159/000479862
39. Pentheroudakis G, Pavlidis N. Cancer and Pregnancy: Poena Magna, Not Anymore. Eur J Cancer (2006) 42(2):126–40. doi: 10.1016/j.ejca.2005.10.014
40. Zubor P, Kubatka P, Kapustova I, Miloseva L, Dankova Z, Gondova A, et al. Current Approaches in the Clinical Management of Pregnancy-Associated Breast Cancer-Pros and Cons. Epma J (2018) 9(3):257–70. doi: 10.1007/s13167-018-0139-5
41. Veronesi U, Salvadori B, Luini A, Greco M, Saccozzi R, del Vecchio M, et al. Breast Conservation Is a Safe Method in Patients With Small Cancer of the Breast. Long-Term Results of Three Randomised Trials on 1,973 Patients. Eur J Cancer (1995) 31a(10):1574–9. doi: 10.1016/0959-8049(95)00271-J
42. Gentilini O, Cremonesi M, Trifirò G, Ferrari M, Baio SM, Caracciolo M, et al. Safety of Sentinel Node Biopsy in Pregnant Patients With Breast Cancer. Ann Oncol (2004) 15(9):1348–51. doi: 10.1093/annonc/mdh355
43. Mazonakis M, Damilakis J. Estimation and Reduction of the Radiation Dose to the Fetus From External-Beam Radiotherapy. Phys Med (2017) 43:148–52. doi: 10.1016/j.ejmp.2017.09.130
44. Streffer C, Shore R, Konermann G, Meadows A, Uma Devi P, Preston Withers J, et al. Biological Effects After Prenatal Irradiation (Embryo and Fetus). A Report of the International Commission on Radiological Protection. Ann ICRP (2003) 33(1-2):5–206. doi: 10.1016/S0146-6453(03)00021-6
45. Bellon JR, Come SE, Gelman RS, Henderson IC, Shulman LN, Silver BJ, et al. Sequencing of Chemotherapy and Radiation Therapy in Early-Stage Breast Cancer: Updated Results of a Prospective Randomized Trial. J Clin Oncol (2005) 23(9):1934–40. doi: 10.1200/JCO.2005.04.032
46. Balduzzi A, Leonardi MC, Cardillo A, Orecchia R, Dellapasqua S, Iorfida M, et al. Timing of Adjuvant Systemic Therapy and Radiotherapy After Breast-Conserving Surgery and Mastectomy. Cancer Treat Rev (2010) 36(6):443–50. doi: 10.1016/j.ctrv.2010.02.019
47. Chen Z, King W, Pearcey R, Kerba M, Mackillop WJ. The Relationship Between Waiting Time for Radiotherapy and Clinical Outcomes: A Systematic Review of the Literature. Radiother Oncol (2008) 87(1):3–16. doi: 10.1016/j.radonc.2007.11.016
Keywords: breast cancer during pregnancy, pregnancy-associated breast cancer, early-onset breast cancer, early stage, breast-conserving surgery
Citation: Blundo C, Giroda M, Fusco N, Sajjadi E, Venetis K, Leonardi MC, Vicini E, Despini L, Rossi CF, Runza L, Sfondrini MS, Piciotti R, Di Loreto E, Scarfone G, Guerini-Rocco E, Viale G, Veronesi P, Buonomo B, Peccatori FA and Galimberti VE (2021) Early Breast Cancers During Pregnancy Treated With Breast-Conserving Surgery in the First Trimester of Gestation: A Feasibility Study. Front. Oncol. 11:723693. doi: 10.3389/fonc.2021.723693
Received: 11 June 2021; Accepted: 04 August 2021;
Published: 24 August 2021.
Edited by:
Assia Konsoulova, Complex Oncological Center, BulgariaReviewed by:
Hebatallah Gamal El Din Mohamed Mahmoud, Cairo University, EgyptLutfi Dogan, University of Health Sciences, Turkey
Copyright © 2021 Blundo, Giroda, Fusco, Sajjadi, Venetis, Leonardi, Vicini, Despini, Rossi, Runza, Sfondrini, Piciotti, Di Loreto, Scarfone, Guerini-Rocco, Viale, Veronesi, Buonomo, Peccatori and Galimberti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicola Fusco, bmljb2xhLmZ1c2NvQHVuaW1pLml0
†These authors have contributed equally to this work
‡These authors jointly directed this work
 Concetta Blundo1†
Concetta Blundo1† Nicola Fusco
Nicola Fusco Elham Sajjadi
Elham Sajjadi Konstantinos Venetis
Konstantinos Venetis M. Cristina Leonardi
M. Cristina Leonardi Roberto Piciotti
Roberto Piciotti Eugenia Di Loreto
Eugenia Di Loreto Barbara Buonomo
Barbara Buonomo Fedro A. Peccatori
Fedro A. Peccatori