- 1Department of Internal Medicine, Division of Hematology and Oncology, Naef K. Basile Cancer Institute, American University of Beirut Medical Center, Beirut, Lebanon
- 2Department of Internal Medicine, Division of Endocrinology, American University of Beirut Medical Center, Beirut, Lebanon
- 3Department of Radiology, American University of Beirut Medical Center, Beirut, Lebanon
- 4Department of General Surgery, American University of Beirut Medical Center, Beirut, Lebanon
- 5Department of Pathology, American University of Beirut Medical Center, Beirut, Lebanon
Purpose: The aim of this study was to evaluate the diagnostic ability of 2-deoxy-2-[fluorine-18]fluoro-D-glucose (18F-FDG) PET/non-contrast CT compared with those of ultrasound (US)-guided fine needle aspiration (FNA) for axillary lymph node (ALN) staging in breast cancer patients.
Patients and Methods: Preoperative 18F-FDG PET/non-contrast CT was performed in 268 women with breast cancer, as well as ALN dissection or sentinel lymph node (SLN) biopsy. One hundred sixty-four patients underwent US-guided FNA in combination with 18F-FDG PET/CT. The diagnostic performance of each modality was evaluated using histopathologic assessments as the reference standard. The receiver operating characteristic (ROC) curves were compared to evaluate the diagnostic ability of several imaging modalities.
Results: Axillary 18F-FDG uptake was positive in 180 patients, and 125 patients had axillary metastases according to the final pathology obtained by ALN dissection and/or SLN dissection. Of the patients with positive 18F-FDG uptake in the axilla, 21% had false-positive results, whereas 79% were truly positive. Eighty-eight patients had negative 18F-FDG uptake in the axilla, among which 25% were false-negative. 18F-FDG-PET/CT had a sensitivity of 86.59% and a specificity of 63.46% in the assessment of ALN metastasis; on the other hand, US-guided FNA had a sensitivity of 91.67% and a specificity of 87.50%. The mean primary cancer size (p = 0.04) and tumor grade (p = 0.04) in combination were the only factors associated with the accuracy of 18F-FDG PET/CT for detecting metastatic ALNs.
Conclusion: The diagnostic performance of 18F-FDG PET/CT for the detection of axillary node metastasis in breast cancer patients was not significantly different from that of US-guided FNA. Combining 18F-FDG PET/CT with US-guided FNA or SLN biopsy could improve the diagnostic performance compared to 18F-FDG PET/CT alone.
Introduction
Breast cancer is the most common diagnosed cancer and one of the major causes of cancer-related deaths in female patients worldwide (1). Thus, early detection and accurate evaluation of the extent of the disease spread are an ultimate need. Moreover, preoperative evaluation of the axillary lymph node (ALN) status is crucial for reasons such as estimating prognosis and/or deciding on the suitable treatment plan: whether surgery, chemotherapy, or radiation therapy. Axillary lymph node dissection (ALND), previously used as the primary method for detecting lymph node involvement, is considered invasive and is associated with various life-long complications, some of which are lymphedema, seroma, and upper limb movement restrictions (2, 3).
Sentinel lymph node biopsy (SLNB) is currently performed for eligible breast cancer patients with no evidence of clinical or radiological nodal enlargement. The sentinel lymph node (SLN) is the first node that receives lymphatic drainage from the breast tumor. If the SLN is free of metastasis, then the following lymph nodes are expected to have a negative result as well. As a result, SLNB has replaced ALND and is now the standard procedure to stage patients with clinically node-negative early breast cancer. Despite the advantages of SLNB over ALND, this surgical method remains invasive, time-consuming, and has potential complications (4). Consequently, the application of new accurate and noninvasive imaging modalities to preoperatively assess the axillary status is gradually increasing (5). Such imaging modalities include ultrasound (US)-guided fine-needle aspiration (FNA) and positron emission tomography/computed tomography (PET/CT) scan, both of which have more importance in dictating further therapeutic measures.
Preoperative imaging of the axilla with US is the most used noninvasive management approach for the evaluation of regional lymph nodes. US imaging is considered as the standard of reference for noninvasive imaging techniques in the detection of ALN involvement. It can detect changes in the normal morphology of lymph nodes that suggest metastatic disease. The combination of US and fine-needle aspiration biopsy (FNAB) is a highly accurate non-morbid method for ALN staging (6). The study by Oz. et al. has shown that US-guided FNAB has a sensitivity of 76.6% and a specificity of 100% in assessing ALN involvement in cancer patients. When the US-guided FNAB is positive, SLN dissection can be omitted and patients can directly undergo ALND to complete staging and local control (5).
Another alternative to the invasive techniques is 18F-fluorodeoxyglucose positron emission tomography fused with CT (18F-FDG-PET/CT). This modality allows detecting increased glucose metabolism, a feature typical of cancerous cells. It is a widely used imaging technique for initial staging, treatment monitoring, and for detecting distant metastasis (7, 8). Riegger et al. showed that intravenous contrast-enhanced 18F-FDG-PET may be more accurate than US for the detection of ALN metastases; however, due to its low sensitivity, 18F-FDG-PET/CT scan cannot replace SLNB (9). On the other hand, Kim et al. showed that 18F-FDG-PET/CT imaging is a specific imaging modality for predicting ALN metastasis, which in turn is helpful in the selective approach to surgical lymph node dissection (10).
Sohn et al. concluded that combining US-guided FNAB and 18F-FDG-PET/CT resulted in a significantly higher sensitivity but a lower specificity when compared to FNA or 18F-FDG-PET/CT scan alone (3). Nevertheless, a few studies have addressed the diagnostic accuracy of whole-body 18F-FDG-PET/CT for ALN staging in comparison to US-guided FNA with 18F-FDG-PET/CT.
To the best of our knowledge, there has been no report regarding the combination of US-guided FNA and 18F-FDG-PET/CT scan for the preoperative evaluation of the ALN status in breast cancer patients in the Middle East region. Based on all these observations, we aimed to obtain the additional diagnostic performance of US-guided FNA compared with that of an 18F-FDG-PET/CT scan to determine the preoperative ALN status in newly diagnosed breast cancer patients. Subsequently, we would like to demonstrate that US-guided FNA could be omitted in cases where 18F-FDG-PET/CT is positive. This would reduce not only the number of invasive procedures but also the financial, painful, and psychological burdens on patients.
Materials and Methods
Subjects
This was a retrospective chart review-based study that included all women who presented to the American University of Beirut Medical Center between January 1, 2014, and December 31, 2018, with newly diagnosed breast cancer and had invasive ductal carcinoma on excisional or core needle biopsy. Two hundred and sixty-eight patients were evaluated with whole-body 18F-FDG-PET/CT and underwent SLNB and/or ALND. Patients with recurrent breast cancer and patients with ductal carcinoma in situ were excluded. US-guided fine-needle aspiration cytology (FNAC) was done in 164 patients based on the decision of the primary physician (Figure 1).
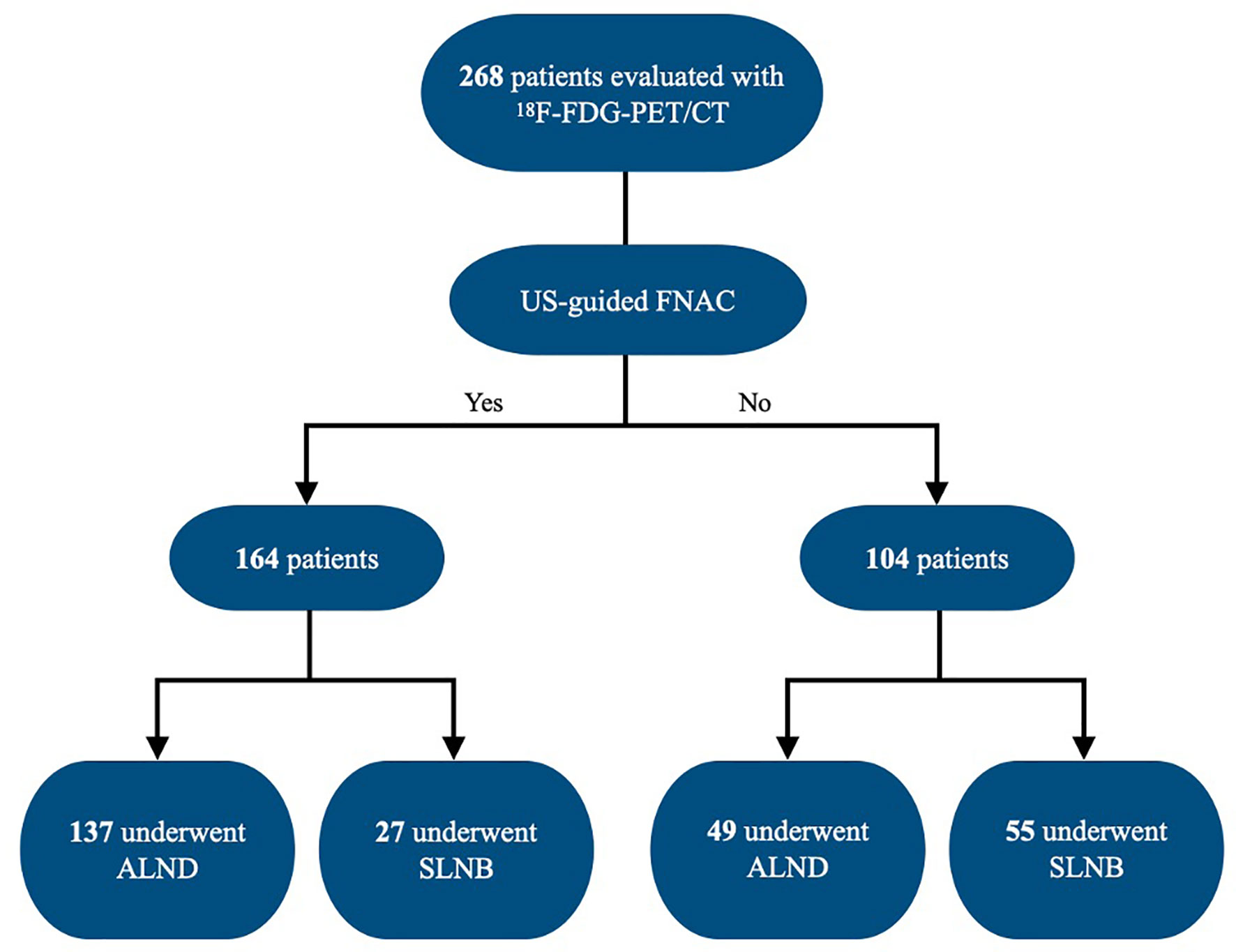
Figure 1 Flowchart of the study group. FNA, fine-needle aspiration; ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy.
Image Acquisition
For the time-of-flight (TOF) FDG-PET imaging protocol, patients were required to fast for at least 6 h prior to scanning. Before intravenous injection of 18F-FDG, the blood glucose levels were measured to ensure a value below 15 mmol/L. Patients received an intravenous injection of 180–296 MBq of FDG in the arm contralateral to the primary tumor. At 50–60 min after the injection of FDG, whole-body emission scans were obtained using the Philips GEMINI TF machine (Philips Medical Systems, Cleveland, OH, USA) with the possibility to reconstruct data in 2-mm voxels instead of 5 mm. Patients were in a prone or supine position, with their arms raised. The PET scans were performed using a whole-body PET/CT acquisition protocol with 50% bed overlap. The acquisition time for each patient was 1 min per bed position, with a total of 18 beds. PET data were reconstructed using a default 3D ordered-subset iterative TOF reconstruction technique. The images were reconstructed in two types of matrices: 144 × 144 matrices with a voxel size of 4 × 4 × 4 mm (standard-voxel reconstruction) and 288 × 288 matrices with a voxel size of 2 × 2 × 2 mm (small-voxel reconstruction). Quantitative measurements of the maximal standardized uptake value (SUVmax) were performed for breast and axillary lesions, when identified. Reconstruction of images in 2 mm centered to the breast and axillary regions was performed for all patients and compared to standard reconstruction.
Image Analysis
FDG-PET images were evaluated by two experienced nuclear radiologists specializing in PET/CT imaging. Lymph nodes with 18F-FDG uptake exceeding that of the surrounding soft tissues were reported as positive. In other words, any focal uptake showing a strong target-to-background ratio compared to the surrounding tissues was considered positive, and any focal uptake with SUVmax greater than 2 and did not correspond to physiologic tracer accumulation was considered positive.
Axillary ultrasounds were performed and read by two radiologists. US was performed using a 5.5- to 18-MHz 18L6 HD linear transducer from the Siemens ACUSON S2000 ultrasound system (Siemens, Malvern, PA, USA). Transverse and longitudinal scans were taken and the diameter and cortical thickness of the lymph node(s) were measured. Suspicious lymph nodes were defined based on their shape, border, and echogenicity. Images were interpreted using a dedicated commercially available software, IntelliSpace Portal 8.0, by Philips Healthcare (Amsterdam, Netherlands). This software allows reviewing PET, CT, and fused imaging data in the axial, coronal, and sagittal planes. US-guided FNAC was performed on suspicious lymph nodes. A 20-ml syringe and a 3.8-cm-long 20-gauge needle or a 5-cm-long 21-gauge needle was used. The needle was inserted at a very shallow angle in order to remain as close to parallel to the pleura as possible for maximum safety, and the aspirate of cellular material was sent to the pathology laboratory for examination. Given the retrospective nature of the study, the decision to obtain an US-guided FNA was left to the primary physician. FDG-avid ALNs from the 18F-FDG-PET/CT scan were correlated with the positive lymph nodes on the US-guided FNAC by our radiologists.
Statistical Analysis
The overall accuracy was calculated as the percentage of all true positives and true negatives compared to the total number of cases. The pathology of ALND or SLNB was considered as the reference standard. Univariate and multivariate logistic regression analyses were conducted to identify the factors affecting the results of 18F-FDG-PET/CT for axillary metastasis. All data were analyzed using SPSS v.23.
Ethics Committee Approval and Consent
This retrospective study was reviewed and approved by the independent Ethical Committee of the American University of Beirut Medical Center (IM.HA.13). All procedures involving human participants were consistent with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The need for informed consent was waived. The demographic data and clinical characteristics of the patients, such as age at diagnosis, histologic type of breast cancer, stage, BMI, and smoking status, were collected from the medical charts. Moreover, morphometric variables such as the number of detected suspicious lymph nodes, PET uptake, and SUVmax were obtained. The sensitivity, specificity, false-negative rate (FNR), and the false-positive rate (FPR) were calculated for 18F-FDG-PET/CT and for US-guided FNAC.
Results
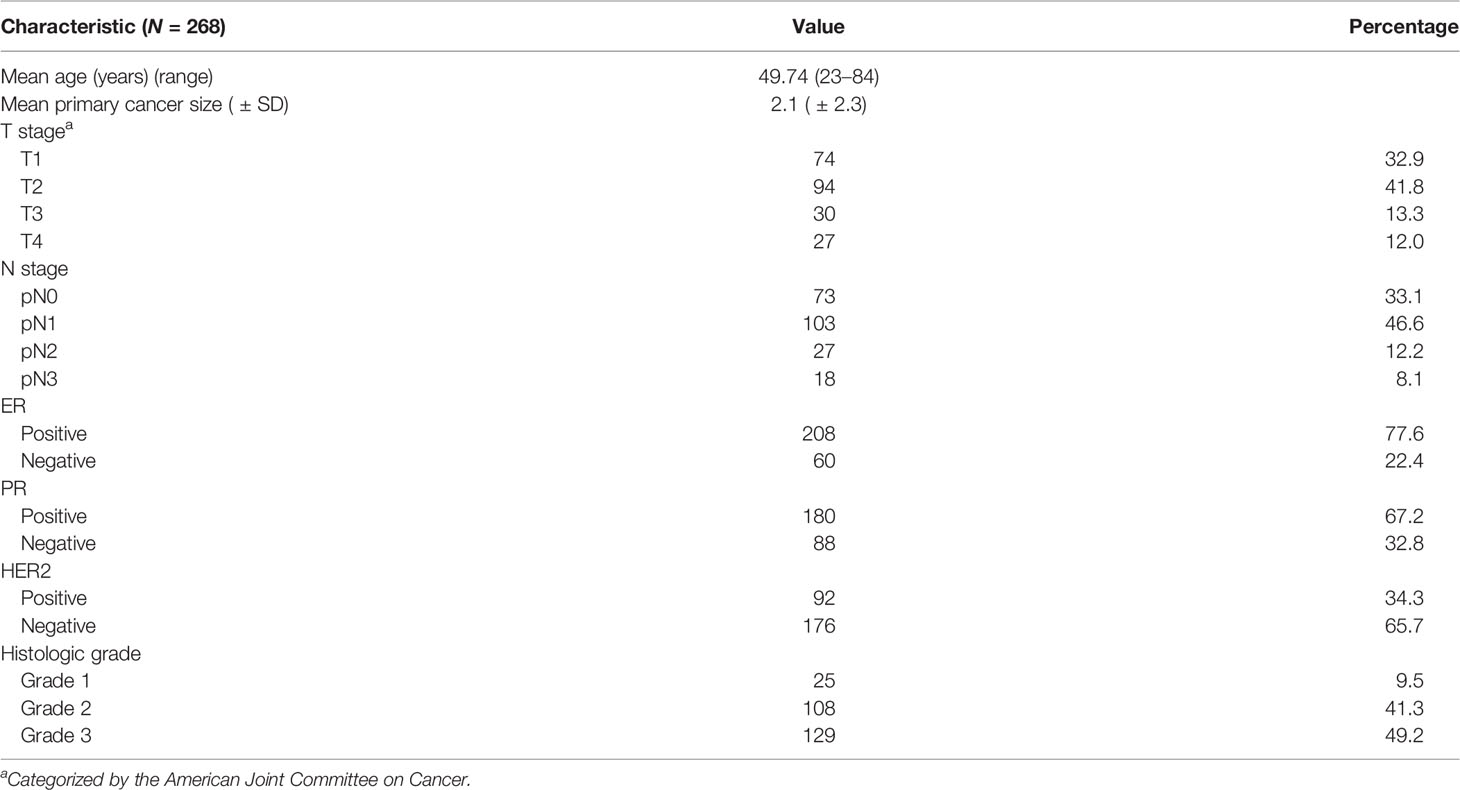
A total of 268 patients were included in this study. The patient demographics and clinical characteristics are listed in Table 1. The mean age of the patients was 49.74 years, with a range between 23 and 84 years. The mean size of the primary breast cancer obtained by surgery was 2.1 ± 2.3 cm. Most patients had T2 stage breast cancer (41.8%), followed by T1 stage (32.9%). The majority had estrogen receptor (ER)-positive (77.6%) and progesterone receptor (PR)-positive breast cancer (67.2%), and only 34.3% of patients had positive HER2 receptors. Regarding the histologic grade, 129 patients had high-grade tumors (grade 3), while only 25 had tumors with histologic grade 1.
Out of the 268 patients who underwent 18F-FDG-PET/CT, 164 patients underwent both 18F-FDG-PET/CT and US-guided FNAC. One hundred twenty-eight patients had positive lymph nodes on the cytology results and 39 patients had negative results. One hundred seventeen patients had concordant results on 18F-FDG-PET/CT and US-guided FNAC (both modalities were positive for lymph node involvement or both were negative); 30 patients had discordant results.
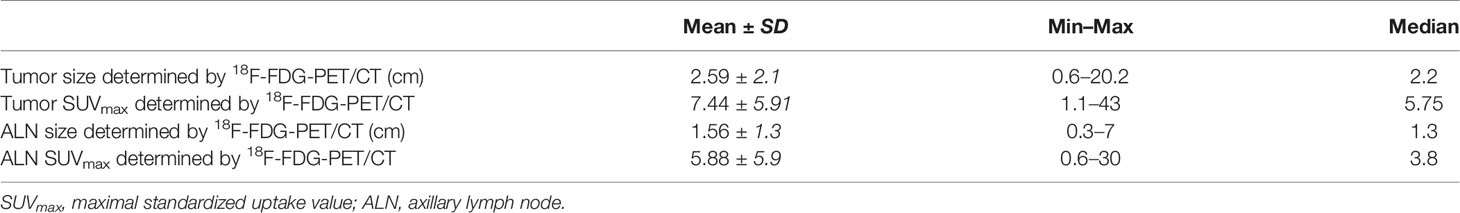
As seen in Table 2, which describes the tumor and lymph node characteristics determined by 18F-FDG-PET/CT, the SUVmax of the ALN was 5.88 and ranged between 0.6 and 30, while the SUVmax of the primary tumor was 7.44, ranging from 1.1 to 43. The mean size of the ALNs by 18F-FDG-PET/CT was 1.56 cm and that of the tumor was 2.59 cm (Figure 2).
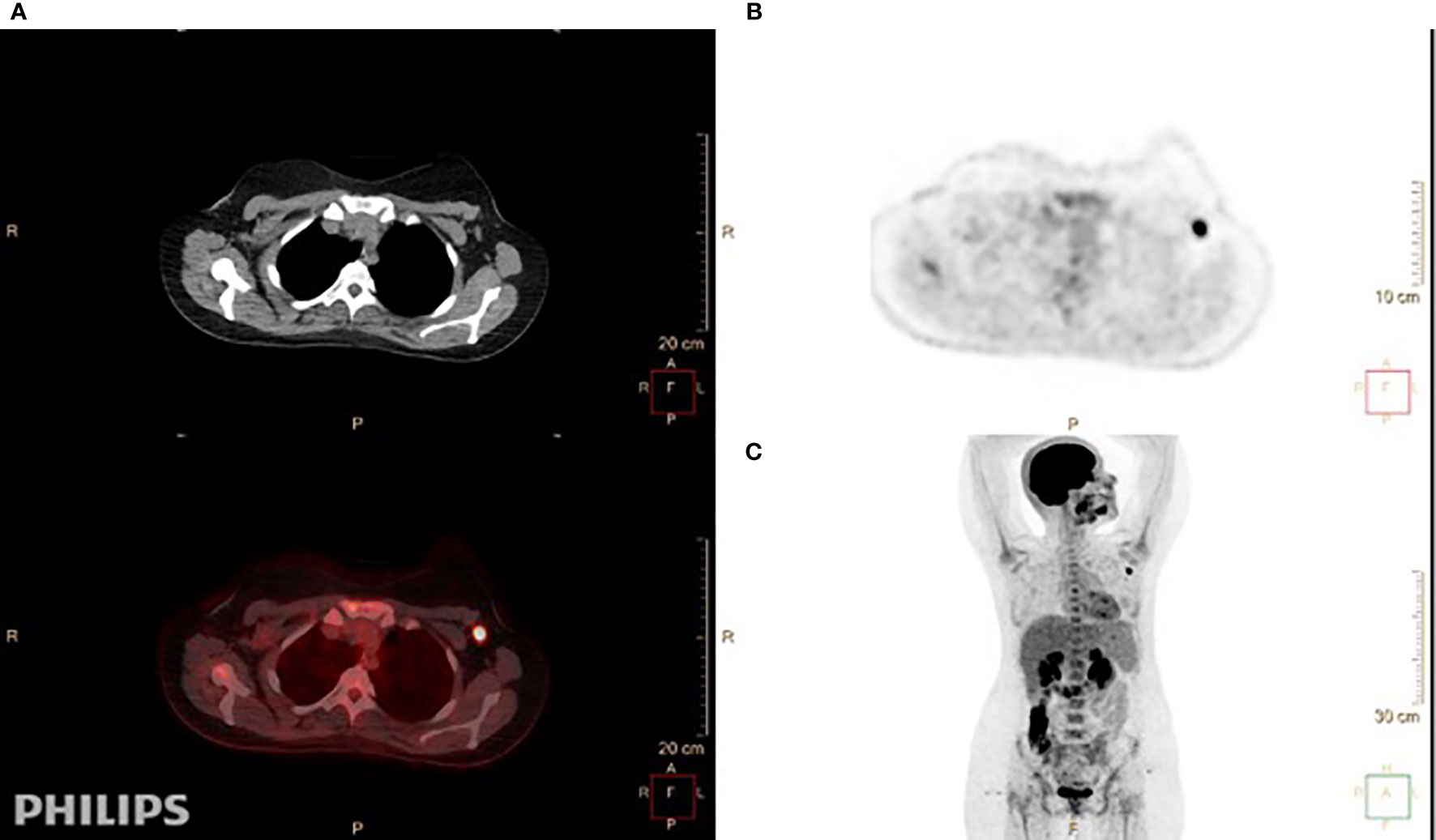
Figure 2 A 24-year-old-female patient with invasive ductal carcinoma in the left breast, grade II, estrogen receptor (ER)-positive, progesterone receptor (PR)-positive, and HER2-negative. She had T1, N1, Mx disease. (A) Axial 18F-FDG-PET/CT showed fluorodeoxyglucose (FDG)-avid left axillary lymph nodes. The largest and most avid measures 1 × 1.6 cm with SUVmax of 9.8. (B, C) Axial (B) and coronal (C) views demonstrate high FDG uptake for the enlarged node in the left axilla.
Axillary 18F-FDG uptake was positive in 180 patients and negative in 88 patients (Table 3). On the other hand, 164 patients had axillary involvement according to the final pathology obtained by ALND and/or SLNB, while the remaining 104 patients had no axillary metastases. In addition, 18F-FDG-PET detected extra-axillary FDG-avid lymph nodes (internal mammary, supraclavicular, and mediastinal) in 19 patients.
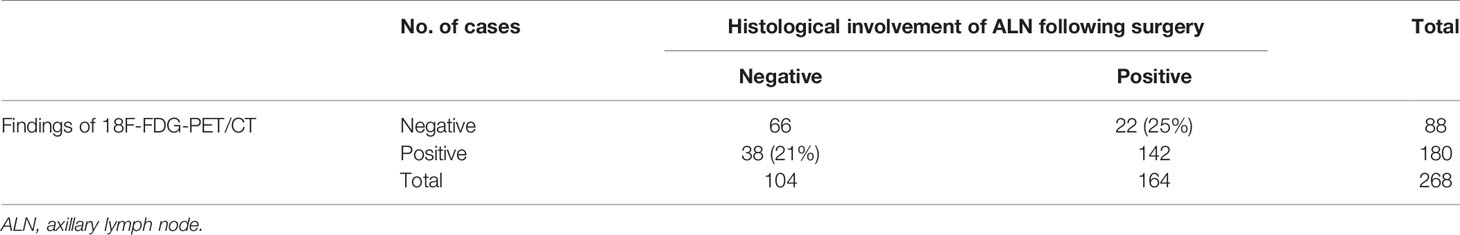
Table 3 Relationship between the 18F-FDG-PET/CT findings and the histological involvement of axillary lymph nodes following surgery.
Thirty-eight patients out of 180 with positive 18F-FDG uptake in the axilla (21%) had a false-positive result, whereas 22 (25%) had a false-negative result (Table 3).
It has to be noted that, upon further subset analysis of the patients who had US-guided FNA before undergoing 18F-FDG-PET/CT (N = 87), six of these patients had a false-positive result. Thus, these six patients out of the 38 with a false-positive result mentioned in Table 3 might have had a false result because of an inflammatory reaction following the FNA.
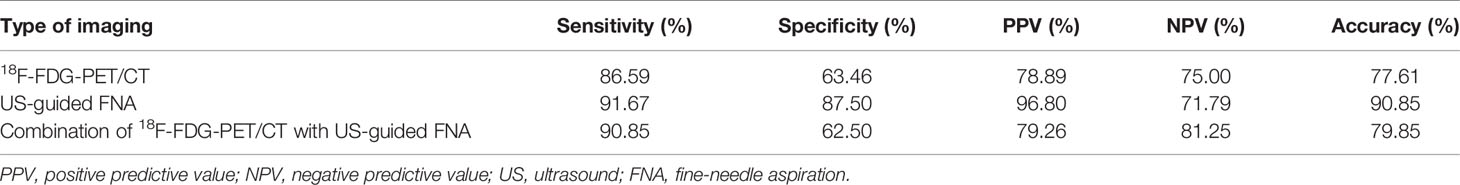
The diagnostic performance of each modality is shown in Table 4. 18F-FDG-PET/CT had a sensitivity of 86.59% and a specificity of 63.46% in the assessment of ALN metastasis; on the other hand, US-guided FNAC had a sensitivity of 91.67% and a specificity of 87.5%. When combining the two modalities, 18F-FDG-PET/CT and US-guided FNAC, we obtained a sensitivity of 62.5% and a specificity of 90.85%.
In addition, the SUVmax values of metastatic lymph nodes were significantly higher than those of benign lymph nodes (p < 0.001). According to the receiver operating characteristic (ROC) curve analysis (Figures 3A, B), the diagnostic performance was significantly better when the cutoff value of SUVmax was 2.55 and the cutoff size of the lymph nodes was 1.05 cm.
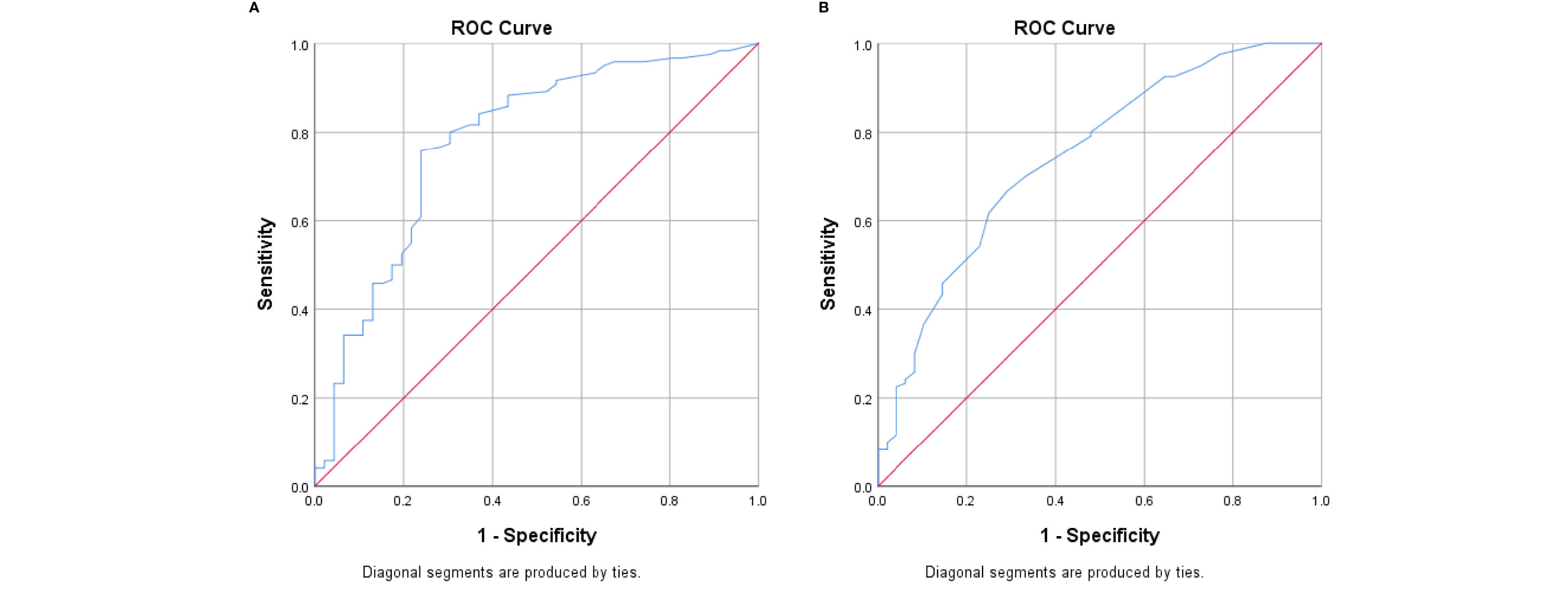
Figure 3 (A) Receiver operating characteristic (ROC) curve analysis of the SUVmax of lymph nodes. (B) ROC curve analysis of lymph node size.
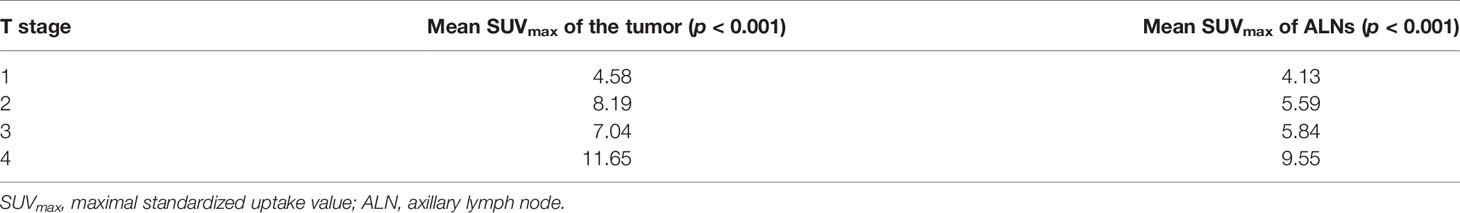
As seen in Table 5, the higher the T stage, the higher the SUVmax values of the tumor (p < 0.001) and that of the lymph nodes (p < 0.001).
A univariate analysis was performed to evaluate the relationship between the true-negative and the false-negative results of the ALNs between 18F-FDG-PET/CT and the final pathology results (Table 6).

Table 6 Univariate analysis of the factors affecting the true-negative and false-negative results of 18F-FDG-PET/CT for axillary metastasis (N = 268).
On multivariate analysis, the mean primary cancer size (p = 0.04) and the tumor grade (p = 0.04) in combination were the only factors associated with the accuracy 18F-FDG-PET/CT for detecting metastatic ALNs. In fact, the higher the mean primary cancer size and the higher the grade of the tumor, the less likely that 18F-FDG-PET/CT will miss the ALNs.
Discussion
Various imaging modalities, such as US, MRI, and 18F-FDG-PET/CT, have played a key role in breast cancer staging and management. 18F-FDG-PET/CT has the advantage of allowing examination of the whole body, including the bones, chest, and abdominal organs, in one session (11). The ALN status is a main factor in breast cancer prognosis since preoperative staging of ALNs is essential for surgery guidance and the selection of aggressive local therapy (1). In this study, we retrospectively compared the ability of 18F-FDG-PET/CT against US-guided FNA in detecting lymph node metastases.
The sensitivity and specificity of 18F-FDG-PET/CT in detecting ALNs have varied among many studies, with a wide range of sensitivities between 20% and 100% and specificities between 64% and 97% (12–20). In our study, 18F-FDG-PET/CT had a sensitivity of 86.59% and a specificity of 63.46% in the assessment of ALN metastasis, while US-guided FNAC had a sensitivity of 91.67% and a specificity of 87.50%. Both had comparable sensitivity values, which could open a new pathway for preoperative planning of patients. This would also be beneficial in terms of the financial burden and physical pain from invasive procedures. However, false-negative results can still occur. In our study, 25% of patients had false-negative results, which is similar to that in the study by Nakano et al. showing a false-negative result rate of 22% (4).
Furthermore, the combination of both modalities maintained a sensitivity of 90.85%, but a fall in specificity was marked compared to US-guided FNAC alone (62.50% vs. 87.50%). Compared to the results of Nakano et al., our cohort showed higher sensitivity and accuracy values when combining 18F-FDG-PET/CT and US-guided FNAC (4). With the positive predictive value (PPV) reaching 79.26%, patients could initially undergo 18F-FDG-PET/CT to evaluate regional and distant metastases. A randomized clinical trial is needed to confirm our findings prior to the implementation of new guidelines based on skipping US-guided FNAC as the gold standard in patients with a positive FDG uptake above a certain SUVmax cutoff. On the other hand, going for an US-guided FNAC or US sampling for suspicious lymph nodes is an option in some settings. These settings include situations where there is no hypermetabolic activity in regional lymph nodes or when the specificity and the negative predictive value (NPV) of a modality are low. Therefore, 18F-FDG-PET/CT could be considered as a pretest for invasive locoregional staging procedures.
As shown in Figure 3, the diagnostic performance was significantly better when the cutoff lymph node size was 1.05 cm. In fact, many studies have reported that the inferior sensitivity of 18F-FDG-PET/CT was related to micro-metastasis and considered it as an important limiting factor (21). In a study by Yararbas et al., five patients out of 32 had false-negative ALNs on 18F-FDG-PET/CT; three had millimetric metastatic foci (1). In our study, the higher the T stage, or the higher the grade, the higher the likelihood of the accurate detection of axillary metastasis by 18F-FDG-PET/CT (Table 6).
Moreover, the specificity of 18F-FDG-PET/CT in the present study was shown to be 63.46%, with a PPV of 78.89%. The finding of an FDG-avid axillary node is expected to represent a malignant lymph node. SLNB can be avoided and the surgeon can directly proceed with ALND whenever a patient has a positive 18F-FDG uptake in the axilla. This goes back to the high PPVs of 18F-FDG-PET/CT seen in some studies (20, 22, 23). Nevertheless, false-positive results can occur for several reasons, such as inflammation following a procedure in the axillary area and reactive lymphadenopathy following biopsy of the breast (24, 25). In fact, 87 patients in our study had US-guided FNAC before undergoing 18F-FDG-PET/CT. Six of them had a false-positive result, which could have been attributed to these reasons and could have lowered the overall specificity. At the same time, the higher the mean primary cancer size and the higher the grade of the tumor, the less likely that 18F-FDG-PET/CT will miss the ALNs (Table 7). This is further shown in Table 5; the higher the T stage, the higher is the SUVmax of the lymph nodes (p < 0.001). In this present study, the optimal cutoff value of SUVmax was 2.55. In the study by Kutlutürkhe et al., when the SUVmax of ALNs was higher than 3.2, the likelihood of 18F-FDG-PET/CT being accurate for axillary metastasis was 15.6 higher (26). Thus, the optimal value should be taken into consideration along with visual information when relying on PET/CT.
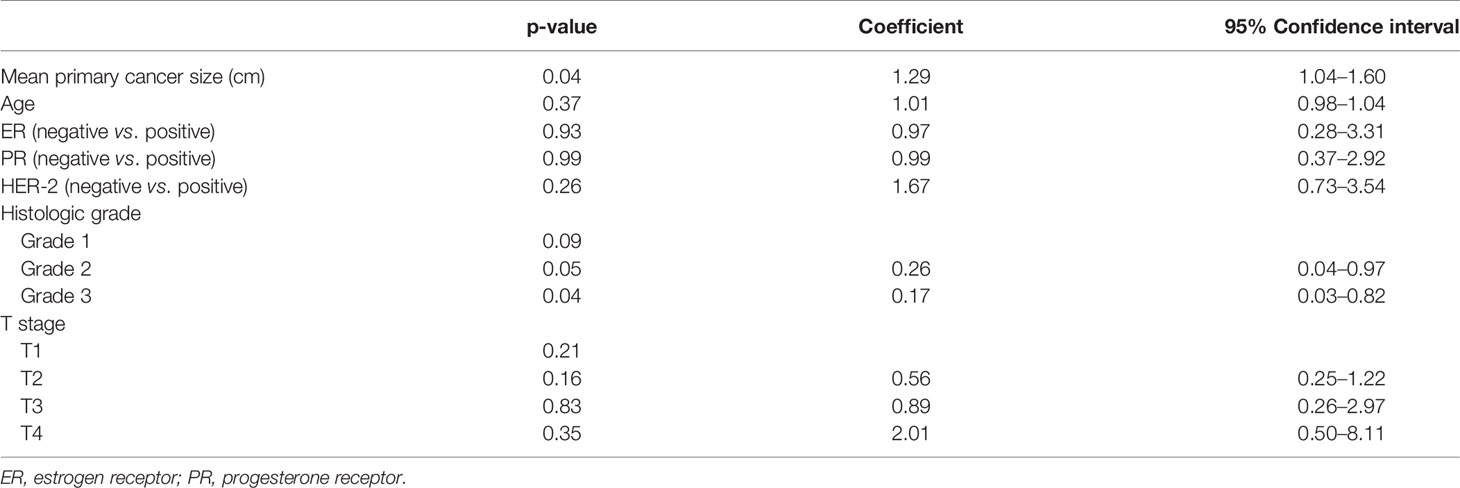
Table 7 Multivariate analysis of the factors affecting the true-positive/true-negative versus false-positive/false-negative results of axillary metastasis.
In addition, locoregional nodes such as the internal mammary and the infra/supraclavicular nodes can be less likely identified through the SLN technique. Therefore, identifying these extraaxillary lymph node metastases using 18F-FDG-PET/CT has an influential value in cancer staging and affecting prognosis. In fact, it can even change the planned therapy (27). The recent 2019 guidelines allow the optional use of 18F-FDG-PET/CT for the initial staging of locally advanced diseases and the evaluation of treatment response, stating that “FDG PET/CT may also be helpful in identifying unsuspected regional nodal disease and/or distant metastases” and that “FDG PET/CT is most helpful in situations where standard staging studies are equivocal or suspicious, especially in the setting of locally advanced or metastatic disease” (28).
Efforts to utilize artificial intelligence (AI) have been prominent in recent years. In a recent study by Li et al. (29), more than 400 patients were retrospectively included as part of a comparative study between two clinicians and AI. Four hundred and fourteen axillae from patients with biopsy-proven breast cancer who had also undergone 18F-FDG-PET/CT before undergoing SLNB and/or ALND were included in the study. Although the AI model, a designed and trained 3D convolutional neural network, did not overtake the clinicians, the accuracies were improved, with sensitivity values from 59.8% and 57.4% to 68.6% and 64.2%, but the specificities remained unchanged (29).
Lastly, tumor markers have been recently utilized in asymptomatic breast cancer patients receiving adjuvant therapies. These tumor markers have been shown to be significantly predictive of distant metastases identified on FDG-PET/CT. In a study by Corso et al., cancer antigen 15-3 (CA 15-3) and carcinoembryonic antigen (CEA) were analyzed in 561 patients (30). The median value of CA 15-3 was 35.0 U/ml in cases where no metastases were detected and was 58.9 U/ml in cases with positive metastases (p < 0.001). Similarly, the CEA values were 6.6 vs. 12.4 U/ml (p < 0.001). Furthermore, CA 15-3 had a significant association with bone/liver metastases compared to other sites of metastasis (30). This opens up other future viable options as staging procedures.
There were several limitations to this study. Firstly, this study was retrospective in nature, and this could have affected the data represented. For this reason, further prospective studies with larger populations are anticipated, with control of 18F-FDG-PET/CT preceding US-guided FNAC to exclude false positivity. Secondly, not all patients underwent US-guided FNAC. Thirdly, most patients had high-risk breast cancers (T2–T4 > T1; N1 > N0). Consequently, early breast cancer with low-risk ALN metastases could not be assessed. Also, the lymph nodes were not marked at the time of ALND or US-guided FNA, which could have ensured that the same node is being studied with each modality.
Conclusion
In conclusion, 18F-FDG-PET/CT has a promising future in staging breast cancer and tailoring the treatment plan. Not only does it help in detecting extra-axillary nodal and metastatic disease, but it also helps in detecting ALNs in a noninvasive way and allows viewing the whole body at a single point of time. However, preoperative ALN staging using 18F-FDG-PET/CT as a single modality is not sufficient. Therefore, a combined evaluation (sonography, FNA, 18F-FDG-PET/CT, and SLNB) is preferred.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the American University of Beirut Medical Center. The patients/participants provided written informed consent to participate in this study.
Author Contributions
IA, JK, VA, and MBZ wrote the manuscript. MC, HA, IA, VA, SB, GB, and MBZ analyzed and interpreted the data. IA, JK, VA, GES, and NS collected and/or assembled the data. HA, ES, SB, NS, GB, and MH provided study materials or patients. HA, VA, FS, ES, and MH conceived and designed the study. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yararbas U, Avci NC, Yeniay L, Argon AM. The Value of 18F-FDG PET/CT Imaging in Breast Cancer Staging. Bosn J Basic Med Sci (2018) 18(1):72–9. doi: 10.17305/bjbms.2017.2179
2. Noguchi M, Morioka E, Ohno Y, Noguchi M, Nakano Y, Kosaka T. The Changing Role of Axillary Lymph Node Dissection for Breast Cancer. Breast Cancer (2013) 20(1):41–6. doi: 10.1007/s12282-012-0416-4
3. Sohn YM, Hong IK, Han K. Role of [18F]Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography, Sonography, and Sonographically Guided Fine-Needle Aspiration Biopsy in the Diagnosis of Axillary Lymph Nodes in Patients With Breast Cancer: Comparison of Diagnostic Performance. J Ultrasound Med (2014) 33(6):1013–21. doi: 10.7863/ultra.33.6.1013
4. Nakano Y, Noguchi M, Yokoi-Noguchi M, Ohno Y, Morioka E, Kosaka T, et al. The Roles of (18)F-FDG-PET/CT and US-Guided FNAC in Assessment of Axillary Nodal Metastases in Breast Cancer Patients. Breast Cancer (2017) 24(1):121–7. doi: 10.1007/s12282-016-0684-5
5. Oz A, Demirkazik FB, Akpinar MG, Soygur I, Baykal A, Onder SC, et al. Efficiency of Ultrasound and Ultrasound-Guided Fine Needle Aspiration Cytology in Preoperative Assessment of Axillary Lymph Node Metastases in Breast Cancer. J Breast Cancer (2012) 15(2):211–7. doi: 10.4048/jbc.2012.15.2.211
6. Pinheiro DJ, Elias S, Nazario AC. Axillary Lymph Nodes in Breast Cancer Patients: Sonographic Evaluation. Radiol Bras (2014) 47(4):240–4. doi: 10.1590/0100-3984.2013.1689
7. Vercher-Conejero JL, Pelegrí-Martinez L, Lopez-Aznar D, Cózar-Santiago Mdel P. Positron Emission Tomography in Breast Cancer. Diagnostics (Basel) (2015) 5(1):61–83. doi: 10.3390/diagnostics5010061
8. Marino MA, Avendano D, Zapata P, Riedl CC, Pinker K. Lymph Node Imaging in Patients With Primary Breast Cancer: Concurrent Diagnostic Tools. Oncologist (2020) 25(2):e231–e42. doi: 10.1634/theoncologist.2019-0427
9. Riegger C, Koeninger A, Hartung V, Otterbach F, Kimmig R, Forsting M, et al. Comparison of the Diagnostic Value of FDG-PET/CT and Axillary Ultrasound for the Detection of Lymph Node Metastases in Breast Cancer Patients. Acta Radiol (2012) 53(10):1092–8. doi: 10.1258/ar.2012.110635
10. Kim J, Lee J, Chang E, Kim S, Suh K, Sul J, et al. Selective Sentinel Node Plus Additional Non-Sentinel Node Biopsy Based on an FDG-PET/CT Scan in Early Breast Cancer Patients: Single Institutional Experience. World J Surg (2009) 33(5):943–9. doi: 10.1007/s00268-009-9955-z
11. Escalona S, Blasco JA, Reza MM, Andradas E, Gomez N. A Systematic Review of FDG-PET in Breast Cancer. Med Oncol (2010) 27(1):114–29. doi: 10.1007/s12032-009-9182-3
12. Adler LP, Faulhaber PF, Schnur KC, Al-Kasi NL, Shenk RR. Axillary Lymph Node Metastases: Screening With [F-18]2-Deoxy-2-Fluoro-D-Glucose (FDG) PET. Radiology (1997) 203(2):323–7. doi: 10.1148/radiology.203.2.9114082
13. Chae BJ, Bae JS, Kang BJ, Kim SH, Jung SS, Song BJ. Positron Emission Tomography-Computed Tomography in the Detection of Axillary Lymph Node Metastasis in Patients With Early Stage Breast Cancer. Jpn J Clin Oncol (2009) 39(5):284–9. doi: 10.1093/jjco/hyp019
14. Crippa F, Gerali A, Alessi A, Agresti R, Bombardieri E. FDG-PET for Axillary Lymph Node Staging in Primary Breast Cancer. Eur J Nucl Med Mol Imaging (2004) 31 Suppl 1:S97–102. doi: 10.1007/s00259-004-1531-z
15. Kelemen PR, Lowe V, Phillips N. Positron Emission Tomography and Sentinel Lymph Node Dissection in Breast Cancer. Clin Breast Cancer (2002) 3(1):73–7. doi: 10.3816/CBC.2002.n.014
16. Kumar R, Zhuang H, Schnall M, Conant E, Damia S, Weinstein S, et al. FDG PET Positive Lymph Nodes Are Highly Predictive of Metastasis in Breast Cancer. Nucl Med Commun (2006) 27(3):231–6. doi: 10.1097/00006231-200603000-00005
17. Liang X, Yu J, Wen B, Xie J, Cai Q, Yang Q. MRI and FDG-PET/CT Based Assessment of Axillary Lymph Node Metastasis in Early Breast Cancer: A Meta-Analysis. Clin Radiol (2017) 72(4):295–301. doi: 10.1016/j.crad.2016.12.001
18. Utech CI, Young CS, Winter PF. Prospective Evaluation of Fluorine-18 Fluorodeoxyclucose Positron Emission Tomography in Breast Cancer for Staging of the Axilla Related to Surgery and Immunocytochemistry. Eur J Nucl Med (1996) 23(12):1588–93. doi: 10.1007/BF01249621
19. van der Hoeven JJM, Hoekstra OS, Comans EFI, Pijpers R, Boom RPA, van Geldere D, et al. Determinants of Diagnostic Performance of [F-18]Fluorodeoxyglucose Positron Emission Tomography for Axillary Staging in Breast Cancer. Ann Surg (2002) 236(5):619–24. doi: 10.1097/00000658-200211000-00012
20. Veronesi U, De Cicco C, Galimberti VE, Fernandez JR, Rotmensz N, Viale G, et al. A Comparative Study on the Value of FDG-PET and Sentinel Node Biopsy to Identify Occult Axillary Metastases. Ann Oncol (2007) 18(3):473–8. doi: 10.1093/annonc/mdl425
21. Groheux D, Giacchetti S, Espie M, Vercellino L, Hamy AS, Delord M, et al. The Yield of 18F-FDG PET/CT in Patients With Clinical Stage IIA, IIB, or IIIA Breast Cancer: A Prospective Study. J Nucl Med (2011) 52(10):1526–34. doi: 10.2967/jnumed.111.093864
22. Barranger E, Grahek D, Antoine M, Montravers F, Talbot JN, Uzan S. Evaluation of Fluorodeoxyglucose Positron Emission Tomography in the Detection of Axillary Lymph Node Metastases in Patients With Early-Stage Breast Cancer. Ann Surg Oncol (2003) 10(6):622–7. doi: 10.1245/ASO.2003.12.019
23. Bernsdorf M, Berthelsen AK, Wielenga VT, Kroman N, Teilum D, Binderup T, et al. Preoperative PET/CT in Early-Stage Breast Cancer. Ann Oncol (2012) 23(9):2277–82. doi: 10.1093/annonc/mds002
24. Chung A, Liou D, Karlan S, Waxman A, Fujimoto K, Hagiike M, et al. Preoperative FDG-PET for Axillary Metastases in Patients With Breast Cancer. Arch Surg (2006) 141(8):783–8; discussion 8-9. doi: 10.1001/archsurg.141.8.783
25. Ulaner GA. PET/CT for Patients With Breast Cancer: Where Is the Clinical Impact? Am J Roentgenol (2019) 213(2):254–65. doi: 10.2214/AJR.19.21177
26. Kutluturk K, Simsek A, Comak A, Gonultas F, Unal B, Kekilli E. Factors Affecting the Accuracy of (18)F-FDG PET/CT in Evaluating Axillary Metastases in Invasive Breast Cancer. Niger J Clin Pract (2019) 22(1):63–8. doi: 10.4103/njcp.njcp_198_18
27. Groheux D, Moretti JL, Baillet G, Espie M, Giacchetti S, Hindie E, et al. Effect of (18)F-FDG PET/CT Imaging in Patients With Clinical Stage II and III Breast Cancer. Int J Radiat Oncol Biol Phys (2008) 71(3):695–704. doi: 10.1016/j.ijrobp.2008.02.056
28. Network NCC. NCCN Clinical Practice Guidelines in Oncology 2020 . Available at: https://www2.tri-kobe.org/nccn/guideline/breast/english/breast.pdf.
29. Li Z, Kitajima K, Hirata K, Togo R, Takenaka J, Miyoshi Y, et al. Preliminary Study of AI-Assisted Diagnosis Using FDG-PET/CT for Axillary Lymph Node Metastasis in Patients With Breast Cancer. EJNMMI Res (2021) 11(1):1–10. doi: 10.1186/s13550-021-00751-4
Keywords: 18F-FDG PET/CT, axillary lymph node, breast cancer, ultrasonography, FNA (fine needle aspiration)
Citation: Assi HI, Alameh IA, Khoury J, Bou Zerdan M, Akiki V, Charafeddine M, El Saheb GI, Sukhon F, Sbaity E, Baydoun S, Shabb N, Berjawi G and Haidar MB (2021) Diagnostic Performance of FDG-PET/CT Scan as Compared to US-Guided FNA in Prediction of Axillary Lymph Node Involvement in Breast Cancer Patients. Front. Oncol. 11:740336. doi: 10.3389/fonc.2021.740336
Received: 12 July 2021; Accepted: 13 September 2021;
Published: 01 October 2021.
Edited by:
Nicola Fusco, University of Milan, ItalyReviewed by:
Giovanni Corso, European Institute of Oncology (IEO), ItalyKenji Hirata, Hokkaido University, Japan
Copyright © 2021 Assi, Alameh, Khoury, Bou Zerdan, Akiki, Charafeddine, El Saheb, Sukhon, Sbaity, Baydoun, Shabb, Berjawi and Haidar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hazem I. Assi, aGExNTdAYXViLmVkdS5sYg==
 Hazem I. Assi
Hazem I. Assi Ibrahim A. Alameh
Ibrahim A. Alameh Jessica Khoury1
Jessica Khoury1 Maroun Bou Zerdan
Maroun Bou Zerdan Vanessa Akiki
Vanessa Akiki Mohamad B. Haidar
Mohamad B. Haidar