- 1Division of Colorectal Surgery, Department of Surgery, Chang Gung Memorial Hospital, Kaohsiung, Taiwan
- 2Department of Colorectal Surgery, China Medical University Hospital, Taichung, Taiwan
- 3Division of Colorectal Surgery, Department of Surgery, Kaohsiung Medical University Chung-Ho Memorial Hospital, Kaohsiung, Taiwan
- 4Department of Surgery, College of Medicine, Kaohsiung Medical University, Kaoshiung, Taiwan
- 5Division of Colon and Rectal Surgery, Department of Surgery, Veterans General Hospital, Taipei, Taiwan
- 6Department of Surgery, Veterans General Hospital, Taichung, Taiwan
- 7Division of Hematology and Oncology, Taipei Medical University-Shuang Ho Hospital, Ministry of Health and Welfare, New Taipei City, Taiwan
- 8Division of Medical Oncology, Department of Oncology, Veterans General Hospital, Taipei, Taiwan
- 9Department of Surgery, National Cheng Kung University Hospital, Tainan, Taiwan
- 10Department of Oncology, National Taiwan University Hospital, Taipei, Taiwan
- 11Division of Hematology Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital, Kaohsiung, Taiwan
- 12Division of Hematology Oncology, Chang Gung Memorial Hospital, Linkou, Taiwan
- 13Department of Hematology and Oncology, Chang Gung Memorial Hospital, Chiayi, Taiwan
- 14Division of General Surgery, Department of Surgery, Shin-Kong Wu Ho Su Memorial Hospital, Taipei, Taiwan
- 15Division of Hematology and Oncology, MacKey Memorial Hospital, Taipei, Taiwan
- 16Department of Colorectal Surgery, Changhua Christian Hospital, Changhua, Taiwan
- 17Graduate Institute of Clinical Medicine, College of Medicine, Kaohsiung Medical University, Kaoshiung, Taiwan
- 18Pingtung Hospital, Ministry of Health and Welfare, Pingtung, Taiwan
Therapeutic options for metastatic CRC (mCRC) have changed significantly in recent years, greatly increasing the complexity of therapeutic decision-making. Although oncology guidelines have helped improve the care process, guidelines may also limit the flexibility to individualize in-clinic decision-making. This consensus paper addresses specific gaps in the current international guidelines to assist Taiwanese colon and rectal experts make specific therapeutic choices. Over 3 years and three meetings with selected experts on “real-world” Taiwanese practice patterns for mCRC, consensus was achieved. The experts also discussed specific questions during in-depth one-on-one consultation. Outcomes of the discussion were then correlated with published evidence by an independent medical writer. The final consensus includes clinically implementable recommendations to provide guidance in treating Taiwanese mCRC patients. The consensus includes criteria for defining fit and unfit intensive treatment patients, treatment goals, treatment considerations of molecular profiles, treatment consideration, and optimal treatment choices between different patient archetypes, including optimal treatment options based on RAS, BRAF, and microsatellite instability (MSI) status. This consensus paper is the second in the Taiwan Society of Colon and Rectal Surgeons (TSCRS) Consensus series to address unmet gaps in guideline recommendations in lieu of Taiwanese mCRC management. Meticulous discussions with experts, the multidisciplinary nature of the working group, and the final drafting of the consensus by independent medical professionals have contributed to the strong scientific value of this consensus.
Introduction
Epidemiology of mCRC
Colorectal cancer (CRC) represents 10% of global cancer incidence and 13% of all-cause deaths (1). It is the second most common cancer in women and the third most common cancer in men. It occurs more commonly in developed countries, but the mortality rate is higher in developing countries (2).
In Taiwan, more than 16,400 new CRC cases occurred in 2017 with an incidence rate of 69.61 per 100,000, mortality rate of 24.66 per 100,000 (3), 5-year survival rate of 63.0% (2015), and mean survival time after CRC diagnosis of 71.27 ± 1.27 months (4). Because mCRC incidence is increasing rapidly in Taiwan, it is critical for experts to examine new methods to provide patients with the latest therapy, optimal survival, and acceptable quality of life.
Differences Between NCCN, ESMO, and Pan-Asian ESMO Guidelines
Oncology guidelines have helped improve both cancer care and patient outcomes (5). The most appreciated and widely used comprehensive guidelines include the European Society for Medical Oncology (ESMO) Colorectal Cancer Guidelines, the National Comprehensive Cancer Network (NCCN) Guidelines in Colorectal Cancer, and the Pan- Asian adapted ESMO consensus guidelines for the Asian region. Several differences are noted when comparing these guidelines (Table 1). (1) ESMO and Pan-Asia ESMO guidelines further stratify treatment by different treatment goals, but NCCN guidelines do not. (2) Tumor-sidedness is not considered in ESMO guidelines. (3) Pan-Asia ESMO guidelines recommend doublet CT plus anti-EGFR in all RAS WT left-sided patients; NCCN guidelines recommend both anti-EGFR and anti-VEGF in these patients. (4) Pan-Asia ESMO guidelines consider doublet CT plus anti-EGFR in RAS WT right-sided patients when cytoreduction is the goal, but NCCN guidelines do not. (5) NCCN guidelines have updated encorafenib plus anti-EGFR in 2L BRAF mutant patients. (6) Pan-Asia ESMO guidelines and NCCN guidelines recommend immunotherapy for patients with dMMR/MSI- H mCRC at different levels of recommendation.
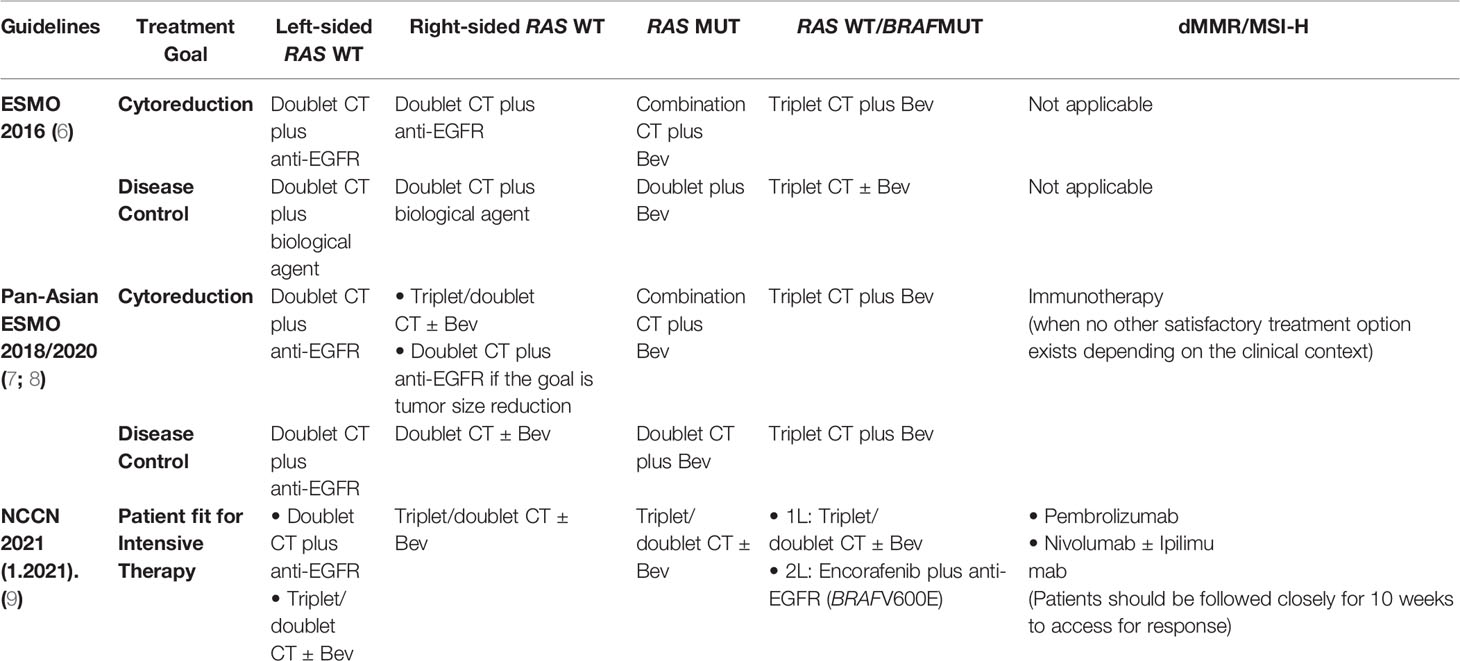
Table 1 Treatment recommendations for first-line management of mCRC in NCCN, ESMO, and Pan-Asian guidelines.
Need for a Taiwanese Expert Consensus
Differences are found between regions of Taiwan regarding issues ranging from patient selection to treatment approaches. Therefore, incorporating local practice must be considered when attempting to standardize and improve treatment for mCRC patients locally. Accordingly, Taiwanese surgical and oncology leaders with extensive experiences in treating mCRC have collaborated to address key areas in the current mCRC treatment landscape and have reached consensus on recommendations for different treatment strategies.
Methodology
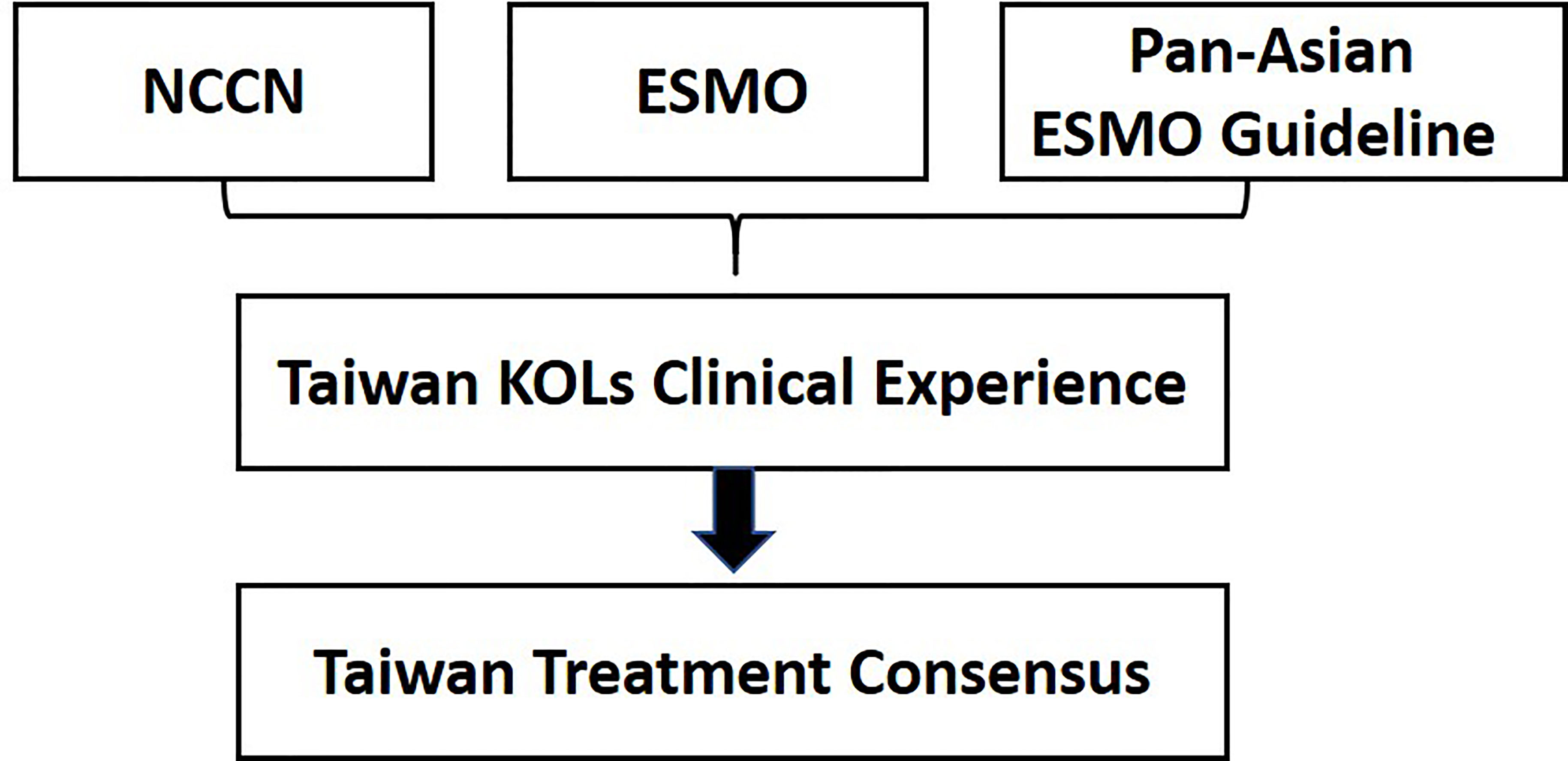
This consensus included synchronous and metachronous colon and rectal cancer patients with any T, any N, and M1. The consensus procedure performed was similar to the Delphi method. The following key characteristics of the Delphi method were applied to help establish this consensus including anonymous voting, structural information, and regular feedback. The participating experts were selected based on the following criteria: (1) demonstrated knowledge/expertise in CRC and (2) geographic representation of the North, Central, and South Taiwan. Consensus was achieved between colorectal experts over the course of three advisory board meetings in 2018, 2019, and 2020 on “real-world” Taiwanese practice patterns for mCRC. During meetings, evidence-based algorithms were generated schematically by incorporating updated scientific evidence and referring to existing NCCN, ESMO, and Pan-Asian ESMO guidelines. Discussion followed a list of questions that had been prepared and selected before the meeting during the one-on-one consultation, and the recommendations were based on the level of consensus achieved after participating experts voted. The voting process was anonymous.
Additional one-on-one consultations lasting 30–40 min each were performed to collect additional opinions and recommendations from individual experts. The experts responded to each recommendation with “agree” or “disagree.” To represent the consensus, each recommendation was required to have the agreement of at least two-thirds of the experts. Outcomes of these meetings were further contextualized by an independent medical writer through reviewing published literature. This consensus paper is an independent report of the expert panel and is not a policy statement of the Taiwan Society of Colon and Rectal Surgeons (TSCRS). This is the second treatment consensus from TSCRS; the preceding development of TSCRS Consensus for Cytoreduction Selection in Metastatic Colorectal Cancer in 2017 has been published on the Annals of Surgical Oncology (10).
The basic approach of consensus formation is shown in Figure 1 and Table 2, respectively. The first meeting was held in August 2018, and six experts deliberated on four key recommendations. The second meeting was held in January 2019 with six experts discussing four more recommendations. The last meeting took place in July 2020 including 6 core members who presented in the first two meetings and additional 12 experts to conclude and revalidate the findings of the previous meetings from the standpoint of the latest guidelines and updated clinical evidence in the new area of microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) and mutated BRAF genes. The expert panel structure and objectives of the meetings held over 4 years are presented in Table 3. The recommendations are based only on the locally approved agents and biologicals in Taiwan.
Recommendations
Definition of Fit and Unfit Intensive Treatment Patients
Defining fit and unfit patients can help to identify appropriate candidates for intensive therapy. Fitness refers to physical condition, health, and wellbeing, and unfitness refers to cumulative impairment of physiological systems. Fitness status affects patients’ treatment tolerance and survival. Because fitness may improve or deteriorate with treatment, reassessing before every line of treatment is essential.
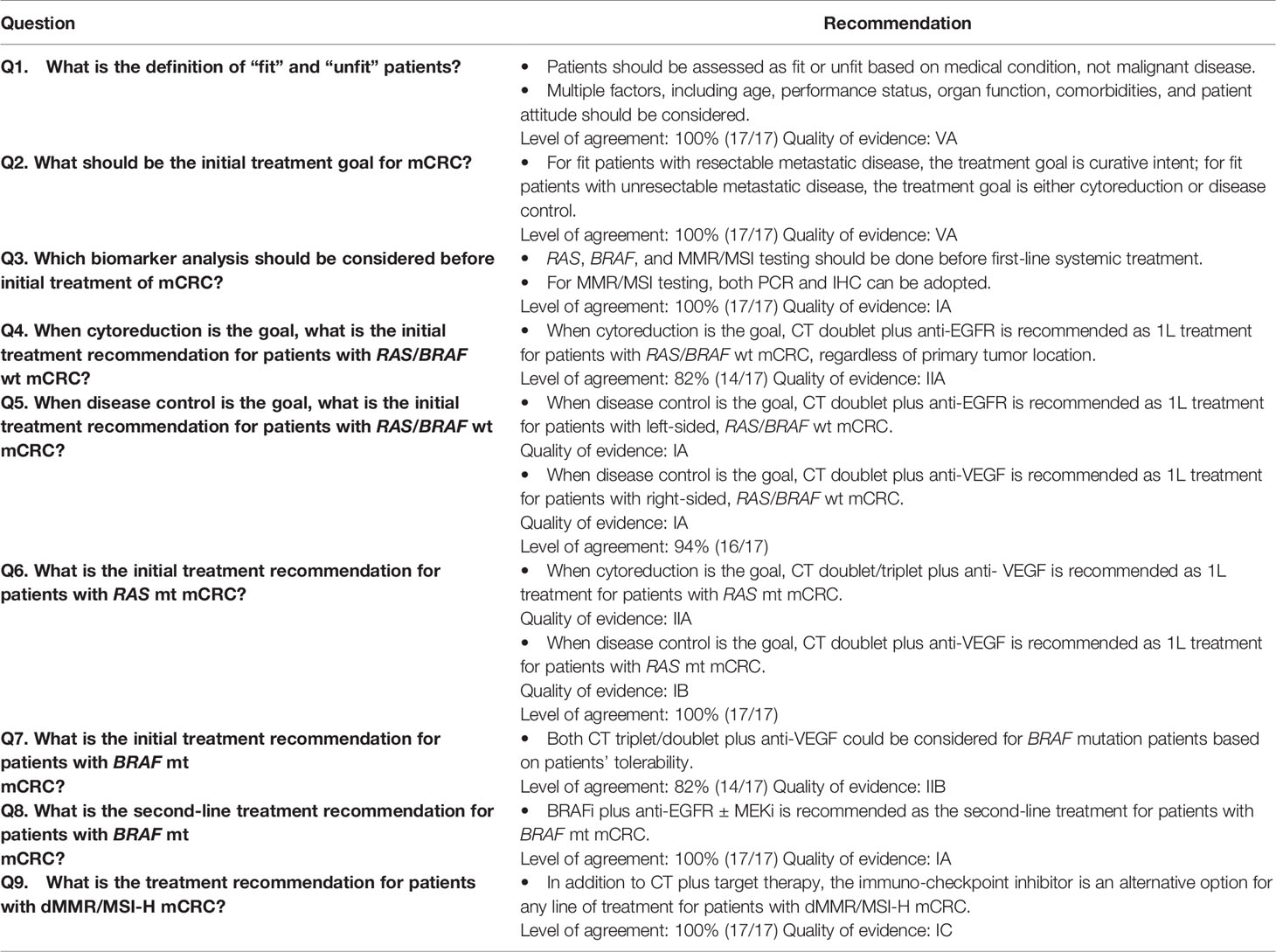
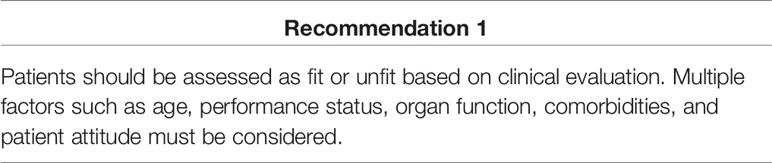
NCCN colon cancer guidelines version 1.202110 classify mCRC patients as “appropriate for intensive therapy” and “not appropriate for intensive therapy.” A patient appropriate for intensive therapy is defined as “one with good tolerance for this therapy and for whom a high tumor response rate would be potentially beneficial.” ESMO and Pan-Asian ESMO guidelines advise to assess patients as “fit” or “unfit” according to their medical condition. The expert advisor discussed whether to identify patients appropriate for intensive treatment based on clinical diagnosis or radiological diagnosis, debating which of the following factors were more likely to influence fit classification: performance status (ECOG), comorbidity, age, tumor size, sites of metastases, and patient attitude. The general opinion was that none of the three guidelines clearly defines fit and unfit patients, and to avoid confusion, defining fit or unfit was not further addressed in the consensus meetings. The experts also suggested that treatment decisions must consider multiple clinical factors, including age, performance status, organ function, comorbidities, and patient attitude, and cannot be defined by any single factor.
Consensus on Treatment Goal
Defining treatment goals for mCRC patients is an individualized process dependent on physician and patient values, judgments, and experience. It is also a dynamic process requiring mutual understanding between patients and physicians with regard to patients’ disease status and treatment expectations.
Variables that define treatment goals for mCRC patients are as follows:
● Patient-related factors, such as age and comorbidities
● Tumor-related factors, such as site of metastasis, grade, and hormone-receptor status
● Ongoing objective measures of disease activity, such as survival and response rates
● Impacts of treatment on subjective improvements in quality of life and symptom palliation
Treatment goals in the ESMO and Pan-Asian ESMO guidelines are cure, cytoreduction, and disease control. Treatment goals in the NCCN guidelines are resectable or unresectable (potentially convertible or unconvertible). Consensus on treatment goals for local Taiwanese mCRC patients was reached through discussion and voting.
The experts discussed treatment as a highly complex, dynamic process, with treatment goals only indicated for initial treatment. In current practice with advanced modalities, after the initial treatment, some patients with disease control may become candidates for resection. The majority of experts concorded with the ESMO treatment goals as cure, cytoreduction, and disease control but mentioned that “cure” should be revised to “curative intent” to more accurately express the treatment goal. Experts agreed that dynamic treatment follows after initial treatment. For instance, cytoreduction patients may undergo surgery, continue cytoreduction, or become disease control patients. Hence, further discussion was needed to address the appropriate treatment flow and was the scope for subsequent consensus statements.
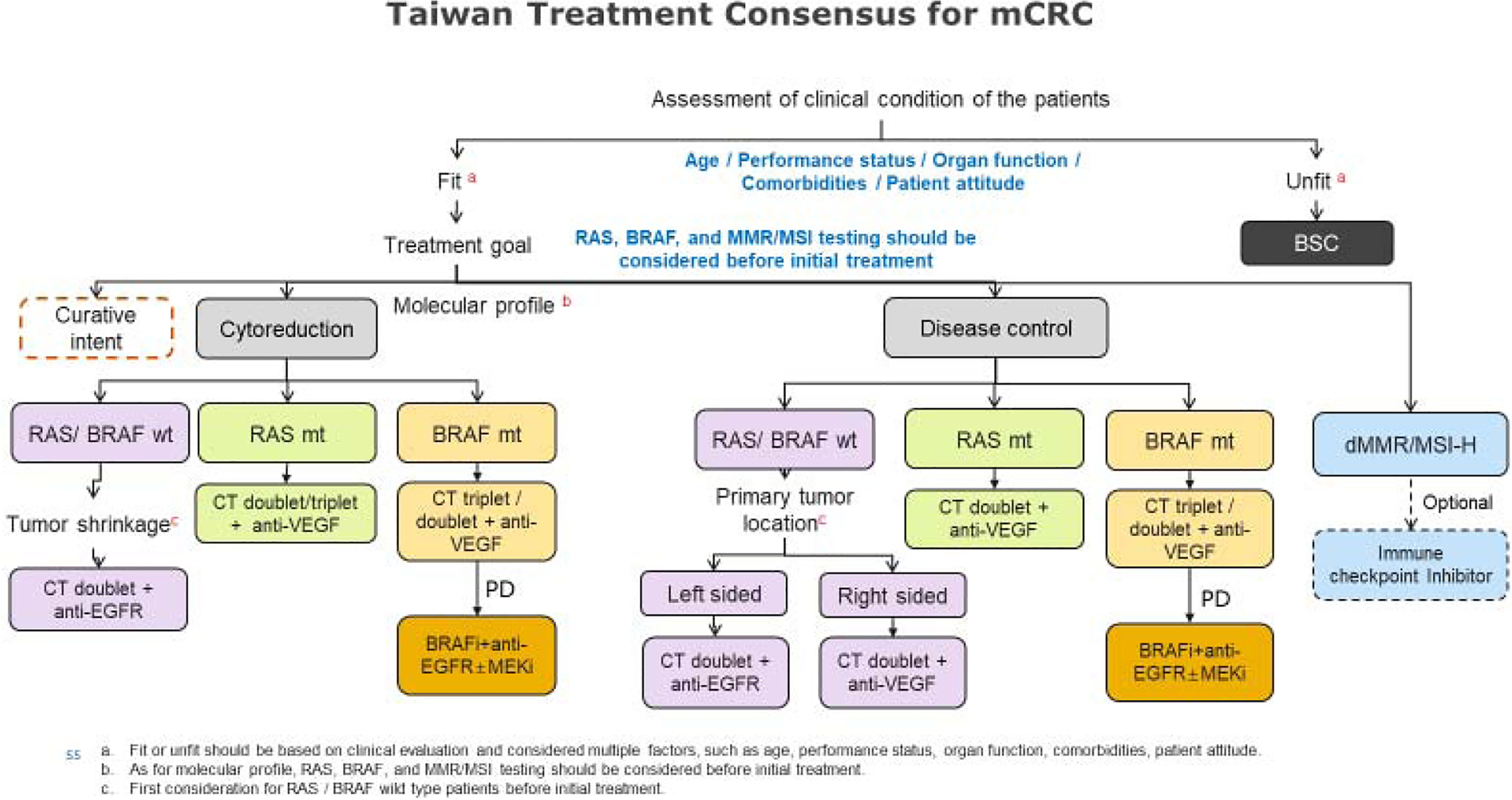
Treatment goals for fit patients with initially unresectable metastases are further categorized into two groups: cytoreduction and disease control. ESMO guidelines recognize two types of patients fit for cytoreduction: (1) those for whom intensive treatment is appropriate with the goal of cytoreduction (tumor shrinkage) and conversion to resectable disease; or 2) those who need intensive treatment but will never receive resection or LAT because rapid reduction in tumor burden is needed due to impending clinical threat, organ dysfunction, or severe symptoms. Patients fit for disease control are those with no impending clinical threat for whom intensive treatment is not necessary. Treatment stratification is presented in Table 4.

Treatment Consideration of Molecular Profile
Molecular biomarkers play an important role in individualized therapy for mCRC patients. Optimal utilization of molecular biomarker testing is required for patients’ best treatment outcomes.
RAS
RAS protein is the main regulator of growth factor-induced cell proliferation and survival in both cancer and normal cells. RAS is an important gene superfamily, including KRAS, NRAS, and HRAS. KRAS is an oncogene coding for the EGFR signaling pathway. KRAS is associated with increased cell proliferation, migration, angiogenesis, and survival of CRC tissue. The WT KRAS allele is present in 60% of mCRC patients. Determining KRAS wild-type status helps to identify tumors with favorable responses to EGFR inhibitors (11) and predicts long-term prognosis (12).
Bokemeyer et al. (13) studied KRAS exon 2 wild-type patients from the OPUS study for 26 mutations (referred to as new RAS) and additional KRAS and NRAS codons. New RAS mutations were present among 26% of the patients. Patients from the RAS wild-type group showed significant improvement by adding cetuximab to FOLFOX4 therapy. A trend toward worse outcomes was also noted among patients with RAS mutation when adding cetuximab. Tejpar and Köhne (14) presented another set of results from the OPUS study for patients tested for KRAS exons 3 and 4 and NRAS exons 2, 3, and 4. Fewer favorable outcomes resulted, and no benefit from adding cetuximab was found among RAS mutant population. Further studies showed a significant benefit in all end points among RAS wild-type patients by adding cetuximab to the doublet regimen (15, 16).
“Extended RAS wild type” was established as a distinct subgroup with significantly better response to anti-EGFR monoclonal antibody. NCCN, ESMO, and Pan-Asian ESMO guidelines strongly recommend extended RAS biomarker analysis before treatment with anti-EGFR therapy. RAS analysis should include at least KRAS exons 2, 3, and 4 (codons 12, 13, 59, 61, 117, and 146) and NRAS exons 2, 3, and 4 (codons 12, 13, 59, 61, and 117). Sanger sequencing is a standard method for RAS testing but requires at least 10%–25% of RAS mutant neoplastic cells in the sample for reliable detection (17). All experts agreed that RAS testing is essential before considering the initial treatment.
BRAF
The v-Raf murine sarcoma viral oncogene homolog B (BRAF) oncogene encodes a serine/tyrosine-kinase downstream to RAS in the mitogen-activated protein kinase (MAPK) signaling transduction pathway, which plays an important role in regulating cellular proliferation and survival. BRAF mutations are detected almost exclusively in KRAS-wild- type CRC and are present in 8.1% of patients with mCRC (18). It is associated with MSI, multiple sites of metastases, more colon tumors (mainly right-sided), higher grade tumors, mucinous histology, adverse histologic features, older age, ECOG performance status ≥2, female gender, and poor survival (19). BRAF-mutant CRC has emerged in recent years as a distinct biological entity that is refractory to standard therapy and has a poor prognosis. The mOS for such patients without therapy is around 11 months compared with 35 months for patients with BRAF wild-type mCRC (20, 21). Since BRAF and EGFR are on the same signaling pathway, BRAF mutation is considered to be a negative predictive marker for EGRF antibody treatment response (22, 23). Patients with BRAF V600E–mutated metastatic melanoma respond well to BRAF inhibitors; however, BRAF V600E–mutated mCRC patients respond relatively poorly (only 5%) (21). Current clinical evidence supported expert recommendation to consider BRAF testing before initial treatment.
MSI
Germline mutations in MMR genes lead to deficient DNA MMR, resulting in DNA MSI phenotype, which also may result from epigenetic silencing of the MLH1 gene. During DNA synthesis, MMR proteins repair base-pair mismatch errors in tandemly repeated sequences called microsatellites. Deficient MMR results in the production of truncated, nonfunctional protein or protein loss results in the MSI phenotype, which is present in 15% of CRC cases but only 4% in the metastatic setting (24). MMR status provides important prognostic and predictive information in patients with early-stage CRC (particularly stage II). MMR-D is associated with both good prognosis (i.e., significantly lower risk of recurrence) and lack of benefit from fluorouracil-based adjuvant therapy (25, 26). Unlike in early-stage disease, no clear evidence is available regarding the prognostic value of MSI-H/dMMR status in mCRC. Mounting evidence suggests that MSI-H/dMMR tumors are less responsive to conventional chemotherapy, but studies have been inconclusive to date, and chemotherapy remains the standard of care for patients with MSI-H/dMMR colorectal cancer (27, 28).
The prominent predictive value of MSI status in CRC has recently emerged following the unprecedented results of immunotherapy with checkpoint inhibitors in MSI-H/dMMR mCRC, including pembrolizumab (29, 30), nivolumab (31, 32), and ipilimumab (32), which have shown long-term survival benefits in these patients. Thus, MSI status has become a crucial biomarker to define patients’ therapeutic options in the metastatic setting.
Current evidence supports MMR/MSI testing before 1L treatment, either by immunohistochemistry (IHC) of MMR protein or PCR-based assay. A panel of microsatellite markers has been validated and recommended as a reference panel for PCR analyses (33). Since patients can be classified as hypermethylated, PCR is more accurate than IHC, yet IHC is more widely available among Taiwan hospitals. Key opinion leaders unanimously recommend both PCR and IHC for MMR/MSI testing.

Treatment Consideration and Optimal Treatment Choices Among Different Patient Archetypes
Optimize Treatment for RAS/BRAF WT mCRC When Cytoreduction Is the Goal
Cytoreduction (i.e., tumor shrinkage), defined as reduction in tumor volume, correlates highly with prolonged patient survival. When cytoreduction is the goal, the objective response rate (ORR) is considered the primary goal. Better tumor shrinkage allows patients to increase resectability chances, especially to relieve disease-related symptoms in patients with impending threat or severe disease-related symptoms. A meta-analysis by Holch et al. (34) evaluated FIRE-3, CALGB/SWOG 80405, and PEAK. The odds ratio of ORR favored anti-EGFR-based chemotherapy regardless of the primary tumor location (OR: 1.49, 95% CI=1.16–1.0, p=0.002 for left-sided tumor; OR: 1.2, 95% CI= 0.77–1.87, p=0.432 for right-sided tumor) compared to anti-VEGF-based chemotherapy. In addition, anti-EGFR-based chemotherapy showed a higher early tumor shrinkage (ETS) rate (68.2% vs. 49.1% in FIRE-3; 64% vs. 45% in PEAK) and deeper DpR (48.9% vs. 32.3%, p<0.0001 in FIRE-3; 65% vs. 46%, p=0.0007 in PEAK) compared with anti-VEGF-based chemotherapy (35).
After weighing the evidence, experts recommended CT doublet plus anti-EGFR as 1L treatment for patients with RAS/BRAF WT mCRC, regardless of the primary tumor location, when cytoreduction is the goal. As a supportive rationale, experts agreed that data indicated a numerically greater effect from anti-EGFR-based therapy in right-sided tumors. Tumor shrinkage is also much more relevant in liver metastasis to support decisions about the amount of remaining liver tissue to be left. At this stage, tumor shrinkage is more important than tumor location. The experts also agreed that anti-EGFR should be recommended because anti-VEGF is usually withdrawn before surgery.
Due to limitations of Taiwan reimbursement criteria, treatment choices may be different when considering patients’ financial burden. Therefore, for this consensus, financial consideration was excluded from this discussion.

Optimal Treatment for RAS/BRAF Wild-Type mCRC When Disease Control Is the Goal
When disease control is the goal, prolonging survival is considered the primary goal. Four meta-analyses, including the CALGB 80405, FIRE-3, PEAK, and TAILOR studies, have shown significantly better OS, PFS, and ORR with first-line chemotherapy plus anti-EGFR antibodies than with chemotherapy plus anti-VEGF in patients with RAS wt left-sided mCRC (36). In contrast, OS was significantly attenuated when using cetuximab/panitumumab in the RAS WT right-sided subgroup, and such patients seemed to benefit from chemotherapy plus anti-VEGF.
Currently, NCCN and Pan Asian ESMO guidelines have included tumor location as an important consideration within the treatment algorithm. NCCN guidelines recommend using anti-EGFR agents for treating RAS wild-type and left-sided tumors, whereas ESMO guidelines do not consider tumor location. During the meetings, some experts shared that they prefer to consider sidedness for mCRC patients with liver metastases. CT triplet is preferred by some experts for right-sided tumors, and CT doublet is preferred for left-sided tumors.
In summary, when disease control is the treatment goal, most experts suggest that the primary tumor location should be considered, and anti-EGFR shows better treatment outcomes in left-sided RAS/BRAF WT mCRC because1L treatment is based on current clinical evidence.
Similarly, CT doublet plus anti-VEGF was established as the preferred 1L treatment for right-sided RAS/BRAF WT mCRC.

Optimal Treatment Option for RAS Mutation and BRAF Wild-Type Patients
RAS mutations are negative predictors of response to anti-EGFR therapy (37). Several large clinical trials, including CO.17, CRYSTAL, OPUS, and PRIME, have elucidated that KRAS mutations predict lack of response and clinical benefit from anti-EGFR mAbs in patients with mCRC (16, 38–44). The phase III FIRE-3 trial randomized 592 patients with KRAS exon 2 WT in a head-to-head comparison of first-line cetuximab versus bevacizumab in combination with FOLFIRI (16). Of 407 KRAS exon 2 WT tumors that could be sequenced, 65 (16%) harbored other RAS mutations. Analysis of 65 patients with other RAS mutations revealed a lower response rate (38% vs. 58%), PFS (6.1 vs. 12.2 months), and OS (16.4 vs. 20.6months) in the cetuximab arm compared to bevacizumab arm. The phase II PEAK study randomized 285 patients with KRAS exon 2 WT tumors between FOLFOX plus panitumumab and FOLFOX6 plus bevacizumab in the first-line setting (40). Expanded RAS testing showed that 51 patients (23.1%) harbored a non-KRAS exon 2 RAS mutation. Patients with RAS mutant tumors showed negative effects of panitumumab treatment (PFS, 7.8 vs. 8.9months; HR, 1.39) compared to bevacizumab treatment.
The BECOM study was a phase II, randomized controlled trial evaluating the efficacy of bevacizumab plus mFOLFOX6 vs. mFOLFOX6 alone as first-line therapy of RAS mutant unresectable colorectal liver metastases (44). In 121 patients, the bevacizumab plus mFOLFOX6 group demonstrated better R0 resection rates for liver metastases (22.3% vs. 5.8%, P<0.01), objective response rates (54.5% vs. 36.7%, P<0.01), median PFS (9.5 vs. 5.6 months, P<0.01), and median OS (25.7 vs. 20.5 months, P=0.03) than the mFOLFOX6 alone group.
ESMO, NCCN, and Pan-Asian ESMO treatment guidelines recommend CT doublet plus anti-VEGF antibody for RAS mutation patients. Targeted therapy is the preferred treatment and is currently reimbursed by Taiwan National Health Insurance. The experts recommended CT doublet plus anti-VEGF as the 1L treatment for RAS mutant patients, but more intensive CT triplet plus anti-VEGF should be considered for cytoreduction purposes.

Optimal Treatment Option for BRAF Mutation Patients
Optimize Initial Treatment for Patients With RAS WT/BRAF Mutant mCRC
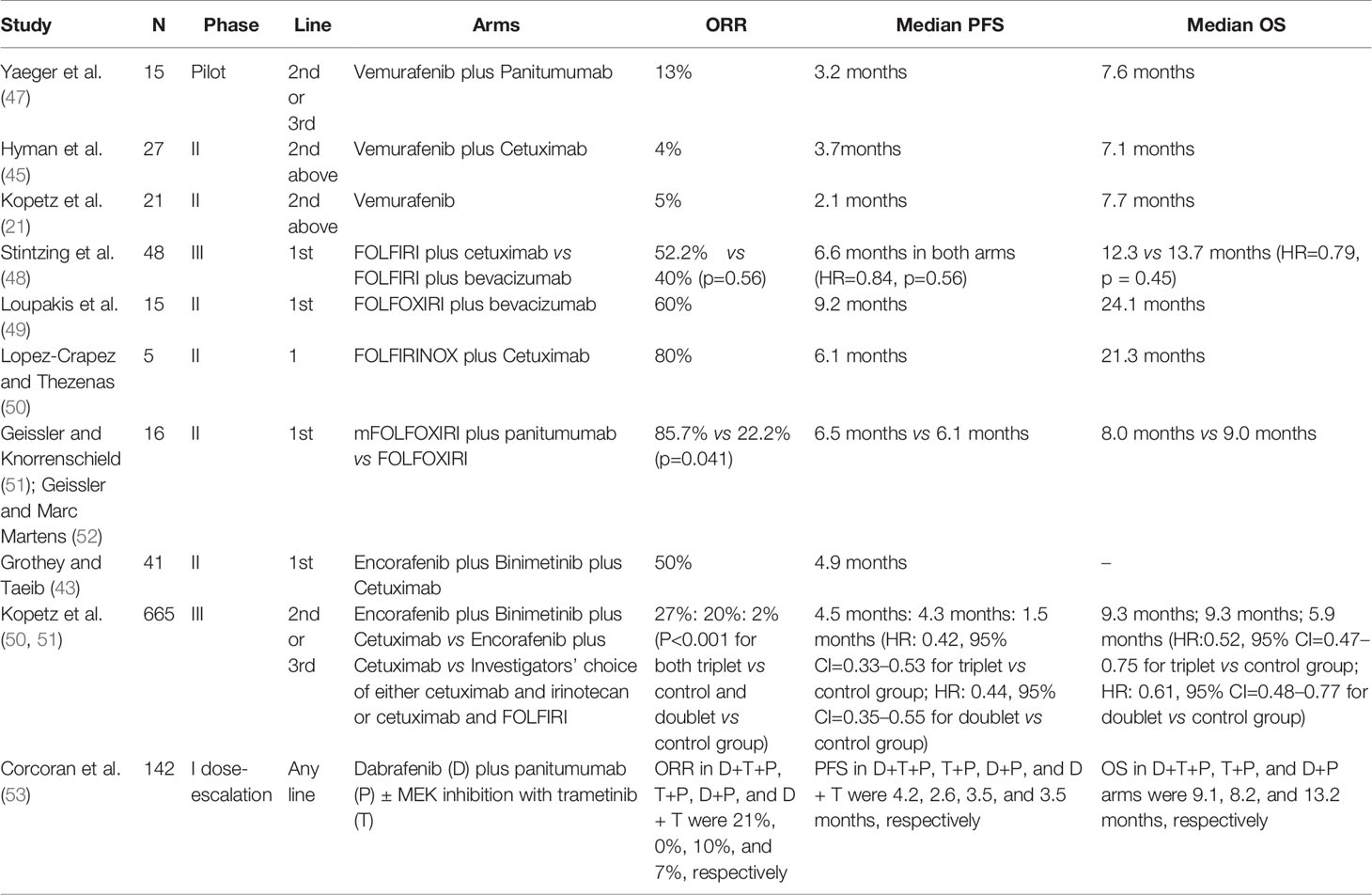
Prognosis is poor for patients with mCRC harboring BRAF mutations (42). Good response rates (90%), median PFS (12.8 months), and OS (30.9 months) were reported in a subgroup of 10 patients with BRAF-mutant tumors treated with FOLFOXIRI plus bevacizumab (post hoc analysis) (39). These results were confirmed in a prospective study in 214 patients, including 15 with BRAF-mutant tumors (45). A 60% response and median PFS and OS of 9.2 and 24.1 months, respectively, were found in a single-arm phase II trial of mCRC patients treated with first-line folinic acid, fluorouracil, oxaliplatin, and irinotecan (FOLFOXIRI)-bevacizumab. Pooling of retrospective and prospective results showed median PFS and OS of 11.8 and 24.1 months, respectively. Similarly, subgroup analysis showed that 16 patients with BRAF-mutant tumors treated with FOLFOXIRI plus bevacizumab had an ORR of 56% and median PFS and OS of 7.5 and 19.0 months, respectively, compared with 12 patients treated with FOLFIRI plus bevacizumab who had slightly lower ORR of 42% (OR: 1.82, 95% CI=0.38–8.78) and shorter median PFS of 5.5 months (HR: 0.57, 95% CI=0.27–1.23) and median OS of 10.7 months (HR: 0.54, 95% CI=0.24–1.20) (41). Based on these results, ESMO and Pan-Asian ESMO guidelines recommended triplet chemotherapy plus bevacizumab as the standard of care for first-line treatment of BRAF-mutant CRC. The ANCHOR CRC single-arm, phase II study (43) in first-line BRAFV600E mCRC patients, recently published at World Congress on GI Cancer 2020, aimed to investigate the efficacy of triplet therapy with BRAF inhibitor encorafenib, MEK inhibitor binimetinib, combined with cetuximab in treatment-naïve patients with RAS WT/BRAF V600E mutant mCRC, in which 51% of the patients had peritoneal metastases. The ORR was 50% (95% CI= 33.8–66.2), and 85% of patients had decreased tumor size. Median PFS was 4.9 months (95%CI, 4.4–8.1). Adverse events were consistent with those observed in prior studies with this triplet combination (46).
Based on currently available evidence, the experts reckoned that data from the TRIBE study were unconvincing as it was subgroup analysis from a small sample. However, HRs at each efficacy endpoint favored the FOLFOXIRI plus bevacizumab arm. Considering the high toxicity of FOLFOXIRI, most Taiwanese patients do not tolerate it, and CT doublet plus bevacizumab is considered another treatment option. Experts agreed that CT triplet/doublet plus anti-VEGF should be recommended for 1L BRAF mutant patients. In the ANCHOR study, encorafenib plusbinimetinib and cetuximab showed promising efficacy and tolerable toxicity in first-line treatment of BRAF mutant mCRC patients, emphasizing that this patient population had high median age (67 years) and disease burden (56% ECOG 1, 78% ≥ 2 metastases site, 51% peritoneal metastasis). Although a phase II, single-arm study has not yet been completed, experts agreed not to include it as a recommendation for first-line treatment for BRAF mutant, and it will be revisited after more data become available.

Optimize Treatment for Patients With Refractory RAS WT/BRAF Mutant mCRC
The open-label, phase 3 trial BEACON CRC study (21, 46) included 665 patients with BRAF V600E–mutated mCRC who experienced disease progression after one or two previous regimens. Patients were randomized to receive encorafenib, binimetinib, and cetuximab (triplet-therapy group); encorafenib and cetuximab (doublet-therapy group); or the investigators’ choice of cetuximab and irinotecan or cetuximab and FOLFIRI (folinic acid, fluorouracil, and irinotecan) (control group) in a 1:1:1 ratio. The mOS was 9.3 months in both the triplet- and doublet-therapy groups vs. 5.4 months in the control group (P<0.001 vs. control). The ORRs were 27% and 20% in the triplet- and doublet-therapy groups, respectively, and 2% in the control group (P<0.001 vs. control). The mOS in the doublet-therapy group was 8.4 months (P<0.001 vs. control). Updated results revealed that the doublet-therapy regimen showed similar overall efficacy and well-tolerated safety profile as the triplet-therapy regimen. Encorafenib in combination with cetuximab was approved by the FDA (https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/210496s006lbl.pdf) and EMA (information_en.pdf) in BRAFV600E-mutant mCRC after prior therapy.
During consensus meetings, encorafenib plus cetuximab ± binimetinib was recommended by the experts as both triplet and doublet regimens with demonstrated superiority compared to controls, and clinicians could select regimens based on each patient’s condition. Different expert opinions toward the doublet or triplet regimen included preference for the doublet regimen because the FDA and NCCN have included this regimen in recent updates, and only limited benefits were seen by adding MEKi to the regimen. Triplet regimens were preferred by other experts because of personal clinical experience with the BRAF and MEK inhibitor in combination with anti-EGFR. BRAF mutant patients were believed to progress rapidly, and triplet therapy may be beneficial in disease control. Because encorafenib and binimetinib were not available in Taiwan, the recommendation was adjusted to BRAFi plus anti-EGFR ± MEKi. Overall, consensus inclined toward using BRAFi plus anti-EGFR ± MEKi as second-line treatment for mCRC patients carrying BRAF mutation.
A brief summary of trials evaluating the role of targeted therapy in patients with BRAF mutated mCRC is shown in Table 5.

Optimal Treatment Option for Patients With MSI-H/dMMR mCRC
Antitumor activity of the checkpoint inhibitor was observed in MSI-H CRC but not in MSS CRC. Based on data from phase II KEYNOTE-016 of pembrolizumab in patients with MSI- H CRC and MSS CRC, the ORR of the CRC MMR-deficient (MSI-H) arms was 57% (95% CI, 39%–73%), whereas the ORR in the MSS CRC arm was 0% (95% CI, 0%–13%).
In the phase II KEYNOTE-16429 (n=124) and Checkmate-142 monotherapy cohort (31) (n=74), pembrolizumab and nivolumab monotherapy demonstrated around 30% ORR in patients with treatment-refractory MSI-H/dMMR mCRC. Results with combination ipilimumab plus nivolumab (n=119) were even more remarkable, with a response rate of 55% (95% CI, 45.2–63.8) and 12-month PFS and survival rates of 71% and 85%, respectively (32).
In Checkmate-142, nivolumab plus low-dose ipilimumab was investigated in first-line MSI- H/dMMR mCRC (54). Two-year follow-up results showed that this combination provided high and durable clinical benefits with 69% ORR (13% CR) without reaching the duration of response. The 24-month PFS and OS rates were 74% and 79%, respectively.
In 2020, the FDA approved pembrolizumab for first-line treatment of unresectable dMMR/MSI-H mCRC patients based on data from the phase 3 KEYNOTE-177 trial, in which 307 treatment-naïve MSI-H/dMMR mCRC patients were enrolled (55). In that study, treatment with pembrolizumab significantly reduced the risk of disease progression or death by 40% (HR, 0.60; 95% CI, 0.45–0.80; P =.0004) versus standard-of-care chemotherapy (2). Additionally, the PD-1 inhibitor had more than double PFS versus chemotherapy at 16.5 months (95% CI, 5.4–32.4) versus 8.2 months (95% CI, 6.1–10.2), respectively. Additional results presented during the 2020 ASCO Virtual Scientific Program (56) showed that the 12-month PFS rate was 55% in the pembrolizumab arm and 37% in the chemotherapy arm; the 24-month PFS rates were 48.3% and 18.6%, respectively. The ORR was 43.8% with pembrolizumab and 33.1% with chemotherapy. Responses were more durable with the PD-1 inhibitor than chemotherapy. The median duration of response had not been reached in the pembrolizumab arm compared with 10.6 months in the chemotherapy arm.
Although pembrolizumab monotherapy has met its primary endpoint of improving PFS compared to chemotherapy in KEYNOTE-177, most experts do not recommend listing the immune checkpoint inhibitor in first-line treatment for MSI-H/dMMR since it lacks OS data and cannot explain having higher PD risk than chemotherapy (29.4% vs. 12.3%). Some experts believe that patients who are both MSI-H and BRAF mutant seem to have better outcomes with the immune checkpoint inhibitor. Overall, immune checkpoint inhibitor monotherapy is an optional choice for first- and second-line treatment for dMMR/MSI-H patients. Experts emphasized that, similar to consensus for BRAF mutant patients, the immune checkpoint inhibitor should be described as better than pembrolizumab.

Conclusion
CRC is recognized as a heterogeneous disease requiring individualized management strategies for its subtypes. This consensus is second in the Taiwan Society of Colon and Rectal Surgeons (TSCRS) Consensus series to address the unmet gaps in guideline recommendations in lieu of Taiwanese mCRC management. It represents the outcome of key opinion leaders’ thought exchange over 3 years on certain unclear aspects in international guidelines. International guidelines were considered as a framework, and discussion addressed gaps in recommendations for advising local Taiwanese clinical practice. The ultimate goal was to improve Taiwanese patient outcomes. As more data emerge and efficacy of newer agents is established, these recommendations will be refined during further meetings.
Author Contributions
Conception and design: H-HC, T-WK, C-WH, J-KJ, C-CC, Y-YH, H-WT, B-WL, Y-HL, Y-LS, H-CH, F-CK, Y-HC, JL, B-RL, Y-YC, and J-YW. Acquisition of data: H-HC, T-WK, C-WH, J-KJ, C-CC, Y-YH, H-WT, B-WL, Y-HL, Y-LS, H-CH, F-CK, Y-HC, JL, B-RL, Y-YC, and J-YW. Analysis and interpretation of data: H-HC, T-WK, C-WH, J-KJ, C-CC, Y-YH, H-WT, B-WL, Y-HL, Y-LS, H-CH, F-CK, Y-HC, JL, B-RL, Y-YC, and J-YW. Drafting of the manuscript: J-YW. Final approval of the manuscript: H-HC, T-WK, C-WH, J-KJ, C-CC, Y-YH, H-WT, B-WL, Y-HL, Y-LS, H-CH, F-CK, Y-HC, JL, B-RL, Y-YC, and J-YW. All authors contributed to the article and approved the submitted version.
Funding
The writing grant was supported by Merck KGaA. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int J Cancer (2015) 136(5):E359–86. doi: 10.1002/ijc.29210
2. Glynne-Jones R, Nilsson PJ, Aschele C, Goh V, Peiffert D, Cervantes A, et al. Anal Cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Radiother Oncol (2014) 111(3):330–9. doi: 10.1016/j.radonc.2014.04.013
3. Health Promotion Administration, M.o.H.a.W. Cancer Registry Annual Report (2017). Available at: https://www.hpa.gov.tw/Pages/ashx/File.ashx?FilePath=%7E/File/Attach/7425/File_6951.pdf.
4. Lee CH, Cheng SC, Tung HY, Chang SC, Ching CY, Wu SF. The Risk Factors Affecting Survival in Colorectal Cancer in Taiwan. Iran J Public Health (2018) 47(4):519–30.
5. Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J. Clinical Guidelines: Potential Benefits, Limitations, and Harms of Clinical Guidelines. BMJ (1999) 318(7182):527–30. doi: 10.1136/bmj.318.7182.527
6. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO Consensus Guidelines for the Management of Patients With Metastatic Colorectal Cancer. Ann Oncol (2016) 27(8):1386–422. doi: 10.1093/annonc/mdw235
7. Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, et al. Pan-Asian Adapted ESMO Consensus Guidelines for the Management of Patients With Metastatic Colorectal Cancer: A JSMO-ESMO Initiative Endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol (2018) 29(1):44–70. doi: 10.1093/annonc/mdx738
8. Yoshino T, Pentheroudakis G, Mishima S, Overman MJ, Yeh KH, Baba E, et al. JSCO-ESMO-ASCO-JSMO-TOS: International Expert Consensus Recommendations for Tumour-Agnostic Treatments in Patients With Solid Tumours With Microsatellite Instability or NTRK Fusions. Ann Oncol (2020) 31(7):861–72. doi: 10.1016/j.annonc.2020.03.299
9. N.C.C.N.C.C.V. (2021). Available at: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
10. Lin CC, Chen TH, Wu YC, Fang CY, Wang JY, Chen CP, et al. Taiwan Society of Colon and Rectal Surgeons (TSCRS) Consensus for Cytoreduction Selection in Metastatic Colorectal Cancer. Ann Surg Oncol (2021) 28(3):1762–76. doi: 10.1245/s10434-020-08914-8
11. Soulières D, Greer W, Magliocco AM, Huntsman D, Young S, Tsao MS, et al. KRAS Mutation Testing in the Treatment of Metastatic Colorectal Cancer With Anti- EGFR Therapies. Curr Oncol (2010) 17 Suppl 1(Suppl 1):S31–40. doi: 10.3747/co.v17is1.614
12. Xu JM, Liu XJ, Ge FJ, Lin L, Wang Y, Sharma MR, et al. KRAS Mutations in Tumor Tissue and Plasma by Different Assays Predict Survival of Patients With Metastatic Colorectal Cancer. J Exp Clin Cancer Res (2014) 33(1):104. doi: 10.1186/s13046-014-0104-7
13. Bokemeyer C, K C, Ciardiello F. Treatment Outcome According to Tumor RAS Mutation Status in OPUS Study Patients With Metastatic Colorectal Cancer (mCRC) Randomized to FOLFOX4 With/Without Cetuximab. Available at: http://meetinglibrary.asco.org/content/127861-144 (Accessed 20th August, 2014).
14. Tejpar S LH, Köhne CH. Effect of KRAS and NRAS Mutations on Treatment Outcomes in Patients With Metastatic Colorectal Cancer (mCRC) Treated First-Line With Cetuximab Plus FOLFOX4: New Results From the OPUS Study. Available at: http://meetinglibrary.asco.org/content/121584-143 (Accessed 20th August, 2014).
15. Ciardiello F, L H, Kohne CH. Effect of KRAS and NRAS Mutational Statusonfirstline Treatment With FOLFIRI Plus Cetuximab in Patients With Metastatic Colorectalcancer (mCRC): New Results From the CRYSTAL Trial. Available at: http://meetinglibrary.asco.org/content/121586-143 (Accessed 20th August, 2014).
16. Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, et al. FOLFIRI Plus Cetuximab Versus FOLFIRI Plus Bevacizumab as First-Line Treatment for Patients With Metastatic Colorectal Cancer (FIRE-3): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2014) 15(10):1065–75. doi: 10.1016/s1470-2045(14)70330-4
17. Carotenuto P, Roma C, Rachiglio AM, Tatangelo F, Pinto C, Ciardiello F, et al. Detection of KRAS Mutations in Colorectal Carcinoma Patients With an Integrated PCR/sequencing and Real-Time PCR Approach. Pharmacogenomics (2010) 11(8):1169–79. doi: 10.2217/pgs.10.86
18. Peeters M, Kafatos G, Taylor A, Gastanaga VM, Oliner KS, Hechmati G, et al. Prevalence of RAS Mutations and Individual Variation Patterns Among Patients With Metastatic Colorectal Cancer: A Pooled Analysis of Randomised Controlled Trials. Eur J Cancer (2015) 51(13):1704–13. doi: 10.1016/j.ejca.2015.05.017
19. Samowitz WS, Sweeney C, Herrick J, Albertsen H, Levin TR, Murtaugh MA, et al. Poor Survival Associated With the BRAF V600E Mutation in Microsatellite- Stable Colon Cancers. Cancer Res (2005) 65(14):6063–9. doi: 10.1158/0008-5472.Can-05-0404
20. Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, et al. Impact of BRAF Mutation and Microsatellite Instability on the Pattern of Metastatic Spread and Prognosis in Metastatic Colorectal Cancer. Cancer (2011) 117(20):4623–32. doi: 10.1002/cncr.26086
21. Kopetz S, Desai J, Chan E, Hecht JR, O'Dwyer PJ, Maru D, et al. Phase II Pilot Study of Vemurafenib in Patients With Metastatic BRAF-Mutated Colorectal Cancer. J Clin Oncol (2015) 33(34):4032–8. doi: 10.1200/jco.2015.63.2497
22. Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, et al. Wild-Type BRAF Is Required for Response to Panitumumab or Cetuximab in Metastatic Colorectal Cancer. J Clin Oncol (2008) 26(35):5705–12. doi: 10.1200/jco.2008.18.0786
23. Morris V, Overman MJ, Jiang ZQ, Garrett C, Agarwal S, Eng C, et al. Progression-Free Survival Remains Poor Over Sequential Lines of Systemic Therapy in Patients With BRAF-Mutated Colorectal Cancer. Clin Colorectal Cancer (2014) 13(3):164–71. doi: 10.1016/j.clcc.2014.06.001
24. Koopman M, Kortman GA, Mekenkamp L, Ligtenberg MJ, Hoogerbrugge N, Antonini NF, et al. Deficient Mismatch Repair System in Patients With Sporadic Advanced Colorectal Cancer. Br J Cancer (2009) 100(2):266–73. doi: 10.1038/sj.bjc.6604867
25. Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective Mismatch Repair as a Predictive Marker for Lack of Efficacy of Fluorouracil-Based Adjuvant Therapy in Colon Cancer. J Clin Oncol (2010) 28(20):3219–26. doi: 10.1200/jco.2009.27.1825
26. Sargent DJ, S Q, Yothers G. Prognostic Impact of Deficient Mismatch Repair (dMMR) in 7,803 Stage II/III Colon Cancer (CC) Patients (Pts): A Pooled Individual Pt Data Analysis of 17 Adjuvant Trials in the ACCENT Database [ASCO Abstract 3507]. J Clin Oncol (2014) 32(suppl):3507. doi: 10.1200/jco.2014.32.15_suppl.3507
27. Innocenti F, Ou FS, Qu X, Zemla TJ, Niedzwiecki D, Tam R, et al. Mutational Analysis of Patients With Colorectal Cancer in CALGB/SWOG 80405 Identifies New Roles of Microsatellite Instability and Tumor Mutational Burden for Patient Outcome. J Clin Oncol (2019) 37(14):1217–27. doi: 10.1200/jco.18.01798
28. Tougeron D, Sueur B, Zaanan A, de la Fouchardiére C, Sefrioui D, Lecomte T, et al. Prognosis and Chemosensitivity of Deficient MMR Phenotype in Patients With Metastatic Colorectal Cancer: An AGEO Retrospective Multicenter Study. Int J Cancer (2020) 147(1):285–96. doi: 10.1002/ijc.32879
29. André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med (2020) 383(23):2207–18. doi: 10.1056/NEJMoa2017699
30. Le DT, Kim TW, Van Cutsem E, Geva R, Jäger D, Hara H, et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J Clin Oncol (2020) 38(1):11–9. doi: 10.1200/jco.19.02107
31. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in Patients With Metastatic DNA Mismatch Repair-Deficient or Microsatellite Instability-High Colorectal Cancer (CheckMate 142): An Open-Label, Multicentre, Phase 2 Study. Lancet Oncol (2017) 18(9):1182–91. doi: 10.1016/s1470-2045(17)30422-9
32. Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol (2018) 36(8):773–9. doi: 10.1200/jco.2017.76.9901
33. Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for Cancer Detection and Familial Predisposition: Development of International Criteria for the Determination of Microsatellite Instability in Colorectal Cancer. Cancer Res (1998) 58(22):5248–57.
34. Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The Relevance of Primary Tumour Location in Patients With Metastatic Colorectal Cancer: A Meta- Analysis of First-Line Clinical Trials. Eur J Cancer (2017) 70:87–98. doi: 10.1016/j.ejca.2016.10.007
35. Heinemann V, Stintzing S, Modest DP, Giessen-Jung C, Michl M, Mansmann UR. Early Tumour Shrinkage (ETS) and Depth of Response (DpR) in the Treatment of Patients With Metastatic Colorectal Cancer (mCRC). Eur J Cancer (2015) 51(14):1927–36. doi: 10.1016/j.ejca.2015.06.116
36. Wang ZX, Wu HX, He MM, Wang YN, Luo HY, Ding PR, et al. Chemotherapy With or Without Anti-EGFR Agents in Left- and Right-Sided Metastatic Colorectal Cancer: An Updated Meta-Analysis. J Natl Compr Canc Netw (2019) 17(7):805–11. doi: 10.6004/jnccn.2018.7279
37. Waring P, Tie J, Maru D, Karapetis CS. RAS Mutations as Predictive Biomarkers in Clinical Management of Metastatic Colorectal Cancer. Clin Colorectal Cancer (2016) 15(2):95–103. doi: 10.1016/j.clcc.2015.10.006
38. Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, et al. K-Ras Mutations and Benefit From Cetuximab in Advanced Colorectal Cancer. N Engl J Med (2008) 359(17):1757–65. doi: 10.1056/NEJMoa0804385
39. Masi G, Loupakis F, Salvatore L, Fornaro L, Cremolini C, Cupini S, et al. Bevacizumab With FOLFOXIRI (Irinotecan, Oxaliplatin, Fluorouracil, and Folinate) as First-Line Treatment for Metastatic Colorectal Cancer: A Phase 2 Trial. Lancet Oncol (2010) 11(9):845–52. doi: 10.1016/s1470-2045(10)70175-3
40. Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Hecht JR, et al. PEAK: A Randomized, Multicenter Phase II Study of Panitumumab Plus Modified Fluorouracil, Leucovorin, and Oxaliplatin (Mfolfox6) or Bevacizumab Plus Mfolfox6 in Patients With Previously Untreated, Unresectable, Wild-Type KRAS Exon 2 Metastatic Colorectal Cancer. J Clin Oncol (2014) 32(21):2240–7. doi: 10.1200/jco.2013.53.2473
41. Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI Plus Bevacizumab Versus FOLFIRI Plus Bevacizumab as First-Line Treatment of Patients With Metastatic Colorectal Cancer: Updated Overall Survival and Molecular Subgroup Analyses of the Open-Label, Phase 3 TRIBE Study. Lancet Oncol (2015) 16(13):1306–15. doi: 10.1016/s1470-2045(15)00122-9
42. Sanz-Garcia E, Argiles G, Elez E, Tabernero J. BRAF Mutant Colorectal Cancer: Prognosis, Treatment, and New Perspectives. Ann Oncol (2017) 28(11):2648–57. doi: 10.1093/annonc/mdx401
43. Grothey A TJ, Taieb J. LBA-5 ANCHOR CRC: A Single-Arm, Phase 2 Study of Encorafenib, Binimetinib Plus Cetuximab In Previously Untreated BRAF V600E- Mutant Metastatic Colorectal Cancer. Ann Oncol (2020) 31:S242–3. doi: 10.1016/j.annonc.2020.04.080
44. Tang W, Ren L, Liu T, Ye Q, Wei Y, He G, et al. Bevacizumab Plus Mfolfox6 Versus Mfolfox6 Alone as First-Line Treatment for RAS Mutant Unresectable Colorectal Liver-Limited Metastases: The BECOME Randomized Controlled Trial. J Clin Oncol (2020) 38(27):3175–84. doi: 10.1200/jco.20.00174
45. Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, et al. Vemurafenib in Multiple Nonmelanoma Cancers With BRAF V600 Mutations. N Engl J Med (2015) 373(8):726–36. doi: 10.1056/NEJMoa1502309
46. Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N Engl J Med (2019) 381(17):1632–43. doi: 10.1056/NEJMoa1908075
47. Yaeger R, Cercek A, O'Reilly EM, Reidy DL, Kemeny N, Wolinsky T, et al. Pilot Trial of Combined BRAF and EGFR Inhibition in BRAF-Mutant Metastatic Colorectal Cancer Patients. Clin Cancer Res (2015) 21(6):1313–20. doi: 10.1158/1078-0432.Ccr-14-2779
48. Stintzing S, Miller-Phillips L, Modest DP, Fischer von Weikersthal L, Decker T, Kiani A, et al. Impact of BRAF and RAS Mutations on First-Line Efficacy of FOLFIRI Plus Cetuximab Versus FOLFIRI Plus Bevacizumab: Analysis of the FIRE-3 (AIO KRK-0306) Study. Eur J Cancer (2017) 79:50–60. doi: 10.1016/j.ejca.2017.03.023
49. Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, et al. Initial Therapy With FOLFOXIRI and Bevacizumab for Metastatic Colorectal Cancer. N Engl J Med (2014) 371(17):1609–18. doi: 10.1056/NEJMoa1403108
50. Lopez-Crapez E AA, Thezenas S. FOLFIRINOX Plus Cetuximab (CET) or Bevacizumab (BEV) in Patients (Pts) With Initially Unresectable Colorectal Liver Metastases (CRLM) With BRAF Mutated (Mut) Tumors: A Subgroup Analysis of the UNICANCER PRODIGE 14- ACCORD 21 (METHEP2) Trial. J Clin Oncol (2018) 36(15_suppl):3548. doi: 10.1200/JCO.2018.36.15_suppl.3548
51. Geissler M MU, Knorrenschield R. 475o-mFOLFOXIRI + Panitumumab Versus FOLFOXIRI as First-Line Treatment in Patients With RAS Wild-Type Metastatic Colorectal Cancer M(CRC): A Randomized Phase II VOLFI Trial of the AIO (AIO- Krk0109). Ann Oncol (2017) 28:v158–208. doi: 10.1200/JCO.2018.36.15_suppl.3509
52. Geissler M R-KJ, Marc Martens U. Final Results and OS of the Randomized Phase II VOLFI Trial (AIO- KRK0109): mFOLFOXIRI + Panitumumab Versus FOLFOXIRI as First-Line Treatment in Patients With RAS Wild- Type Metastatic Colorectal Cancer (mCRC). J Clin Oncol (2019) 37(15_suppl):3511–1. doi: 10.1200/JCO.2019.37.15_suppl.3511
53. Corcoran RB, André T, Atreya CE, Schellens JHM, Yoshino T, Bendell JC, et al. Combined BRAF, EGFR, and MEK Inhibition in Patients With BRAF(V600E)-Mutant Colorectal Cancer. Cancer Discovery (2018) 8(4):428–43. doi: 10.1158/2159-8290.Cd-17-1226
54. Heinz-Josef L SL, Vittorina Z. Nivolumab (NIVO) + Low-Dose Ipilimumab (IPI) as First-Line (1L) Therapy in Microsatellite Instability-High/Mismatch Repair- Deficient (MSI-H/dMMR) Metastatic Colorectal Cancer (mCRC): Two-Year Clinical Update. J Clin Oncol (2020) 38:4040–0. doi: 10.1200/JCO.2020.38.15_suppl.4040
55. FDA Approves Merck’s Keytruda (Pembrolizumab) First-Line Treatment of Patients With Unresectable or Metastatic MSI-H or dMMR Colorectal Cancer, News release. Merck. June 29, A.
Keywords: Taiwan, metastatic colorectal cancer, treatment consensus, molecular biomarker, disease management in real world practice
Citation: Chen H-H, Ke T-W, Huang C-W, Jiang J-K, Chen C-C, Hsieh Y-Y, Teng H-W, Lin B-W, Liang Y-H, Su Y-L, Hsu H-C, Kuan F-C, Chou Y-H, Lin J, Lin B-R, Chang Y-Y and Wang J-Y (2021) Taiwan Society of Colon and Rectal Surgeons Consensus on mCRC Treatment. Front. Oncol. 11:764912. doi: 10.3389/fonc.2021.764912
Received: 26 August 2021; Accepted: 21 October 2021;
Published: 15 November 2021.
Edited by:
Cesare Ruffolo, University Hospital of Padua, ItalyReviewed by:
Tommaso Stecca, ULSS2 Marca Trevigiana, ItalyCatherine Teh, St. Luke’s Medical Center, Philippines
Copyright © 2021 Chen, Ke, Huang, Jiang, Chen, Hsieh, Teng, Lin, Liang, Su, Hsu, Kuan, Chou, Lin, Lin, Chang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaw-Yuan Wang, SmF3eXVhbndhbmdAZ21haWwuY29t; Y3k2MTQxMTJAbXMxNC5oaW5ldC5uZXQ=
†These authors share first authorship
 Hong-Hwa Chen1†
Hong-Hwa Chen1† Hao-Wei Teng
Hao-Wei Teng Yu-Li Su
Yu-Li Su Hung-Chih Hsu
Hung-Chih Hsu Feng-Che Kuan
Feng-Che Kuan Yu-Yao Chang
Yu-Yao Chang Jaw-Yuan Wang
Jaw-Yuan Wang