- Department of Hematology, The Second Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
Patients with multiple myeloma (MM) rarely present with central nervous system (CNS) involvement as a manifestation of extramedullary disease (EMD), a condition that is associated with poor prognosis. CNS relapse without evidence of systemic involvement is even rarer, and there is no standardized treatment because there are only few case reports. We present a 47-year-old female who was diagnosed with nonsecretory multiple myeloma (NSMM) 9 years previously. She had a complete remission after receiving aggressive therapies, including high-dose chemotherapy and autologous stem cell transplantation (ASCT). However, after 7 years of progression-free survival, she had CNS relapse without evidence of systemic involvement. We switched to a salvage regimen consisting of high-dose methotrexate with lenalidomide. She achieved rapid clinical improvement, with a reduction in cerebrospinal fluid plasmacytosis of more than 80%, and no notable side effects. Our description of this unique case of a patient with MM and isolated CNS relapse after ASCT provides a reference for physicians to provide more appropriate management of these patients. We also reviewed previously reported cases and summarized the outcomes of isolated CNS relapse after ASCT, and discuss the pathogenesis and possible treatment strategies for MM with isolated CNS relapse.
Introduction
Multiple myeloma (MM) is characterized by the monoclonal proliferation of plasma cells (PCs) in bone marrow (1). Despite the use of established treatments followed by autologous stem cell transplantation (ASCT) and improvements in patient outcomes during recent years, MM is still incurable (2). Relapse in most patients is characterized as a medullary monoclonal proliferation, and 3.4% to 35% of these patients present with extramedullary disease (EMD) (3). Central nervous system (CNS) involvement is a very rare aggressive presentation of EMD, and occurs in only about 1% of patients (4). CNS relapse without evidence of systemic involvement is even rarer, with only few case reports, and these patients face a very poor prognosis, with a median survival time less than 6 months (5).
The present study describes a female who had MM with isolated CNS relapse after ASCT, and faced a poor prognosis despite the use of aggressive therapy. There is no standard treatment for CNS localization of multiple myeloma (CNS-MM) (4, 6) due to the rarity of this presentation. Thus, we also conducted a literature review to summarize the outcomes of other MM patients who had isolated CNS relapse after ASCT and examined the pathogenesis and possible treatment strategies for this condition.
Case report
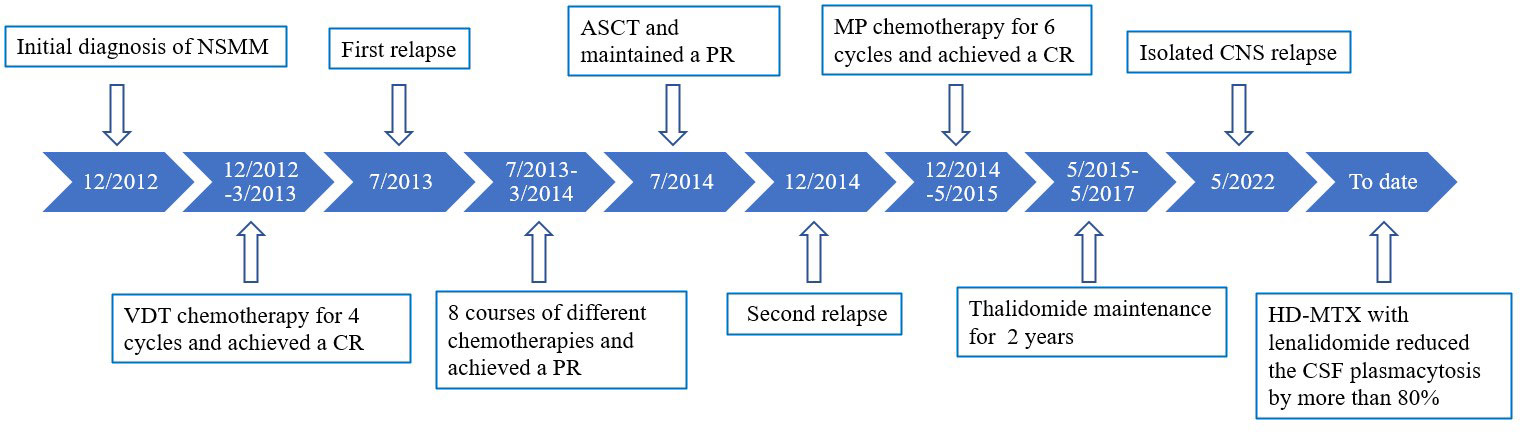
A 38-year-old female with lumbago was diagnosed with nonsecretory multiple myeloma (NSMM) in December 2012. At that time, bone marrow specimens indicated 74% infiltration of plasma cells, and flow cytometry analysis showed abnormal plasma cells, which were positive for CD38, CD56, CD138, and cytoplasmic λ light-chain. Serum immunofixation (IFE) showed no detectable monoclonal component, a blood examination showed no anemia or renal dysfunction, and the levels of lactate dehydrogenase (LDH) and β2 microglobulin (β2-MG) were normal. Whole body bone imaging showed diffuse abnormal signals in the ribs, spinal vertebrae, and ilium. These findings led to a diagnosis of NSMM, with stage I based on the International Staging System (ISS) and stage IIIA based on the Durie-Salmon (DS) staging system. The patient received 4 courses of bortezomib, dexamethasone, and thalidomide (VDT) and achieved a complete response (CR).
After a treatment-free period of 4 months, she presented again with low backache. Bone marrow flow cytometry indicated that 6.5% of the plasma cells were abnormal, indicative of medullary recurrence. She then received 8 courses of different chemotherapies: 4 courses of vincristine, doxorubicin, and dexamethasone (VAD); 3 courses of vincristine, dexamethasone, cyclophosphamide, and thalidomide (VDCT); and 1 course of thalidomide, dexamethasone, cis-platin, doxorubicin, cyclophosphamide, and etoposide (DTPACE). After treatment, she achieved a partial response (PR) with regression of bone pain and 1% plasma cells in bone marrow.
In July 2014, she was given ASCT with preconditioning using semustine, busulphan, and etoposide (Me-CCNu + Bu + VP-16) and maintained a PR. However, 5 months after ASCT, she developed right-lower limb pain. Whole body bone imaging at that time showed a new focus in the right femoral region, and the bone marrow had 14% plasma cells with a normal level of the M protein based on immunofixation electrophoresis (IFE). Thus, melphalan and prednisolone (MP) therapy was initiated. There were no detectable myeloma cells in the bone marrow after 6 courses of this therapy. Thalidomide (100 mg orally) maintenance therapy was then administered for 2 years, and she had no further relapse.
In May 2022, she presented again and reported the sudden onset of dizziness, staggering gait, and loss of hearing. Physical examination revealed that she had clear poor hearing. The muscular strength tension of limbs was normal. Physiological reflexes were existent without any pathological ones. No enlargement of lymph nodes, liver, or spleen was found. Brain magnetic resonance imaging (MRI) showed cerebrospinal meninges and auditory nerve thickening (Figure 1A). Positron emission tomography/computerized tomography (PET/CT) showed multiple cerebrospinal meninges with increased 18F-flurodeoxyglucose metabolism, but no other site of disease involvement (Figures 1B, C). Further examination showed she had no abnormalities in the hemogram, M-protein level, renal function, LDH level, and β2-MG level. A bone marrow analysis showed no chromosomal abnormalities and no increased number of abnormal plasma cells. However, her cerebrospinal fluid (CSF) was positive for plasma cells (Figure 2A), and a lumbar puncture showed the CSF had a protein content of 213.8 mg/dL (normal range: 20–40), glucose of 50 mg/dL (normal range: 50–60), and 42×106 nucleated cells/L (normal range: 0–8×106). These findings indicated that the relapse was localized to the CNS.
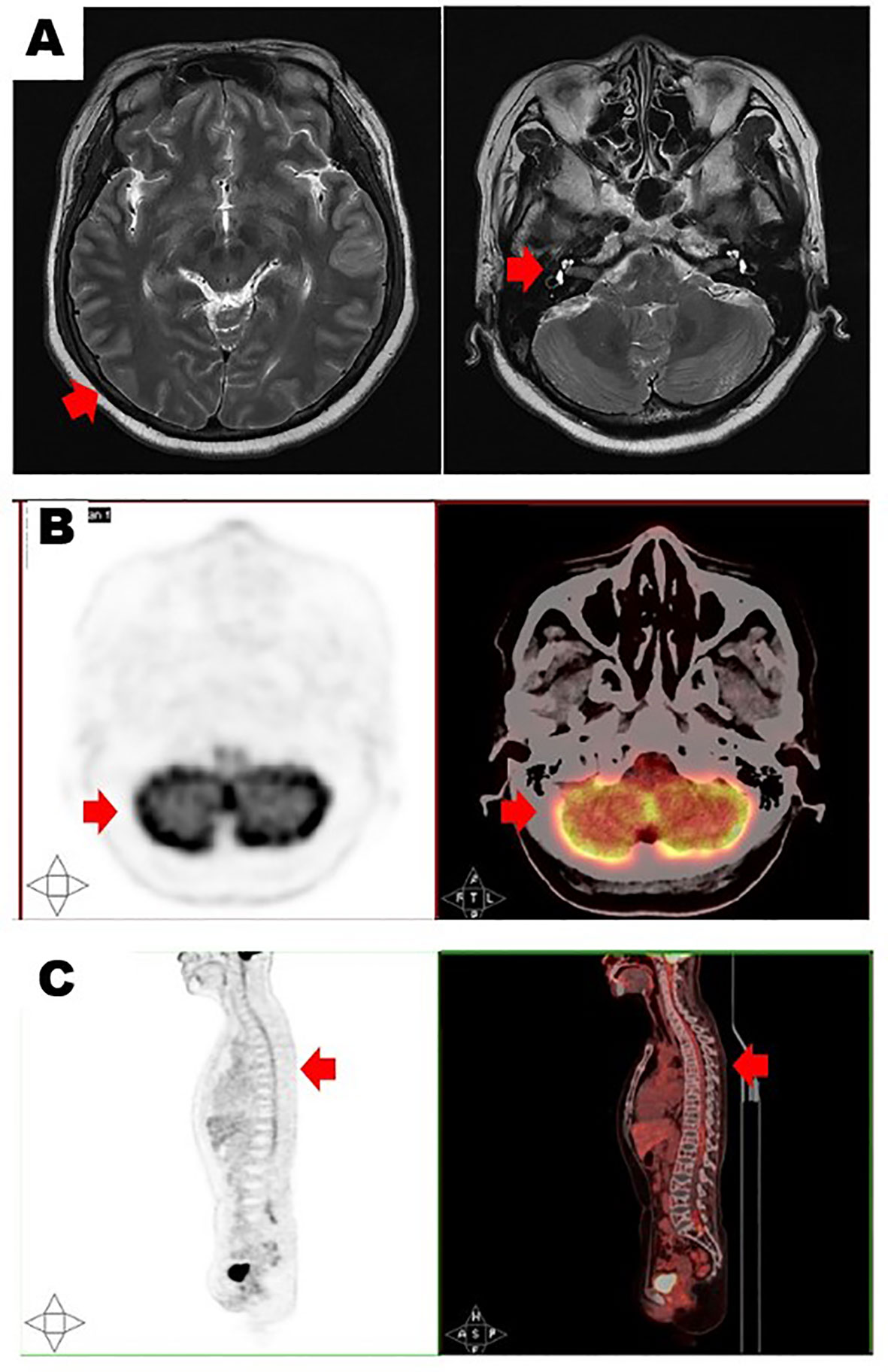
Figure 1 Brain magnetic resonance imaging (A) showed cerebrospinal meninges (left, red arrow) and auditory nerve thickening (right, red arrow). Positron emission tomography/computerized tomography in transverse section (B) and longitudinal section (C) showed multiple cerebrospinal meninges with increased 18F-flurodeoxyglucose metabolism (red arrows).
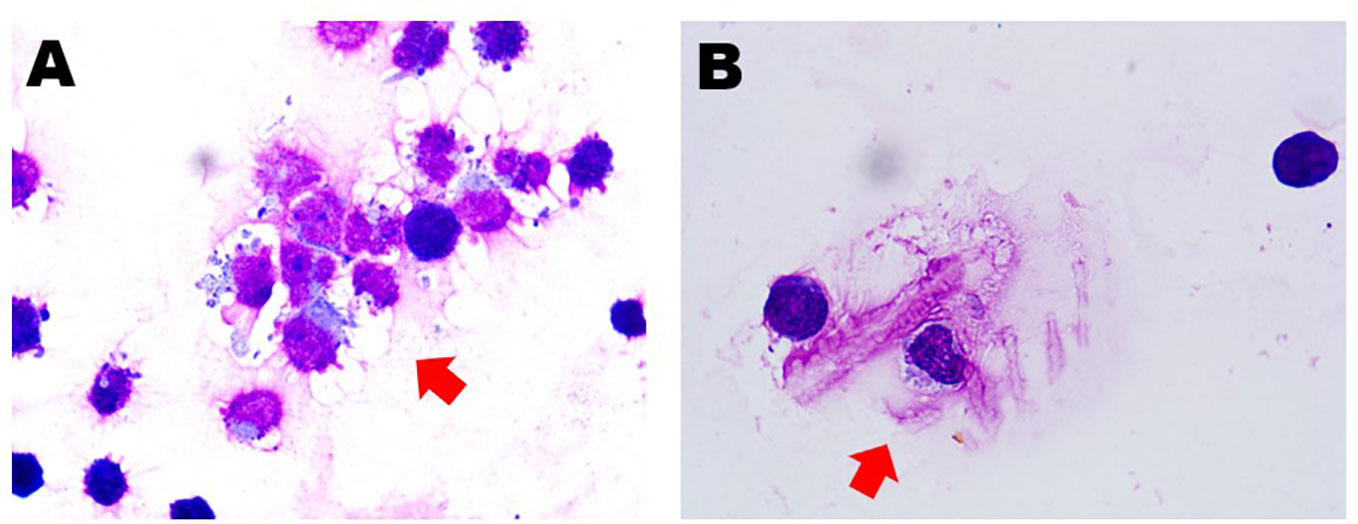
Figure 2 Cerebrospinal fluid smear showed the presence of abnormal plasma cells (red arrows) before (A) and after (B) salvage therapy.
We advised high-dose methotrexate (HD-MTX) therapy with lenalidomide (25 mg orally). After one course of salvage therapy, she achieved rapid clinical improvement without any notable side effects, such as hematological toxicity or peripheral neuropathy. Furthermore, this treatment reduced the CSF plasmacytosis by more than 80% (Figure 2B). The timeline of the patient is summarized in Figure 3.
Discussion
ASCT after induction therapy is a common standard treatment for eligible MM patients because it can induce durable remission and improve long-term survival. Nonetheless, MM is still an incurable disease. Although most patients who experience relapse have proliferation of monoclonal plasma cells, mainly in the bone marrow, about 3.4% to 35% of these patients present with EMD (1, 3). CNS involvement is a specific presentation of extramedullary extraosseous, and occurs in only about 1% of patients (4). The median survival time of these patients is only 4 to 7 months, even when aggressive therapy is given (4, 7). CNS relapse without evidence of systemic involvement after ASCT is even rarer in patients who have MM, and there are only a few case reports in the literature.
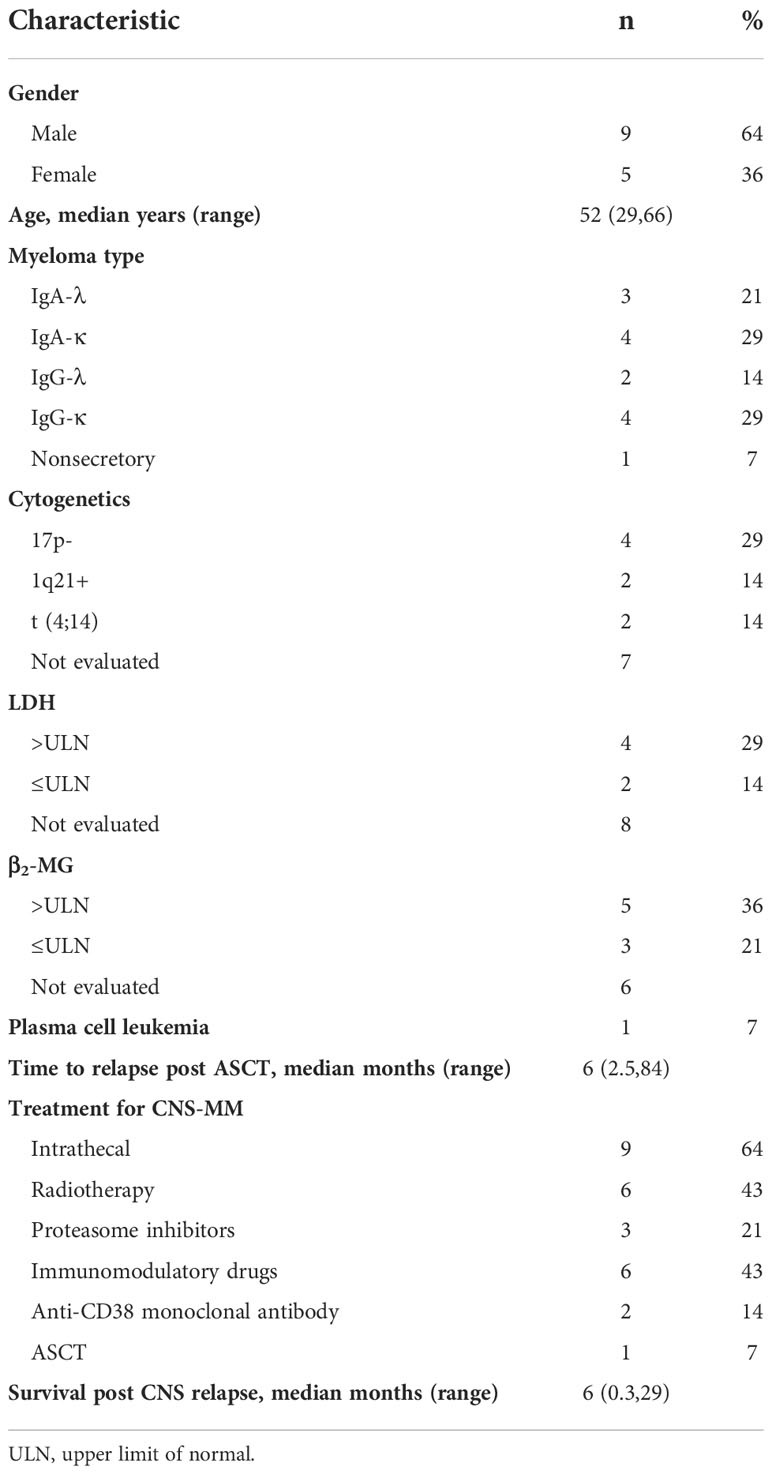
Certain clinical factors are associated with increased risk of CNS-MM, including lambda subtype, elevated LDH, elevated β2-MG, EMD, plasma cell leukemia, and chromosomal abnormalities (deletion of 17p or 13q) (4, 8, 9). We performed a comprehensive search of the literature and identified 14 cases (Tables 1, 2). Most of these patients had ISS stage III disease at diagnosis, but the myeloma subtype was variable. There were more patients with high LDH and β2-MG levels than with normal levels. Only one patient had plasma cell leukemia. The median time from ASCT to CNS disease was 6 months (range: 2.5–84), and most patients died after developing CNS disease, with a median survival post-CNS relapse of 6 months (range: 0.3–29). Cytogenetic results were available in 7 patients: 4 patients had 17p deletion (17p-), 2 patients had 1q21 amplification (1q21+), and 2 patients had translocation (4, 14). These cytogenetic abnormalities may be related to isolated CNS relapse after ASCT for MM. This is consistent with the observations from previous studies (4). One cohort study showed that deletion of chromosome 17p13,1 (p53) was present in 89% of the CNS-MM patients and associated with metastatic features of myeloma cells (20). Moreover, investigators found that amplification of 1q21 was associated with disease progression and poor prognosis in MM despite the use of novel regimens (21). Patients with 1q21+ showed a high incidence of aggressive features, including an unusually high CNS involvement incidence (11%) and early onset of CNS disease (22). Our patient, who had bone marrow expression of CD56 had no EMD or circulating plasma cells at baseline. Our patient differed from other previously described patients in that she had normal levels of LDH and β2-MG and no cytogenetic abnormalities. Because factors that apparently increase the risk for CNS involvement were not present in our patient, we examined the possible reasons why she developed such aggressive disease.
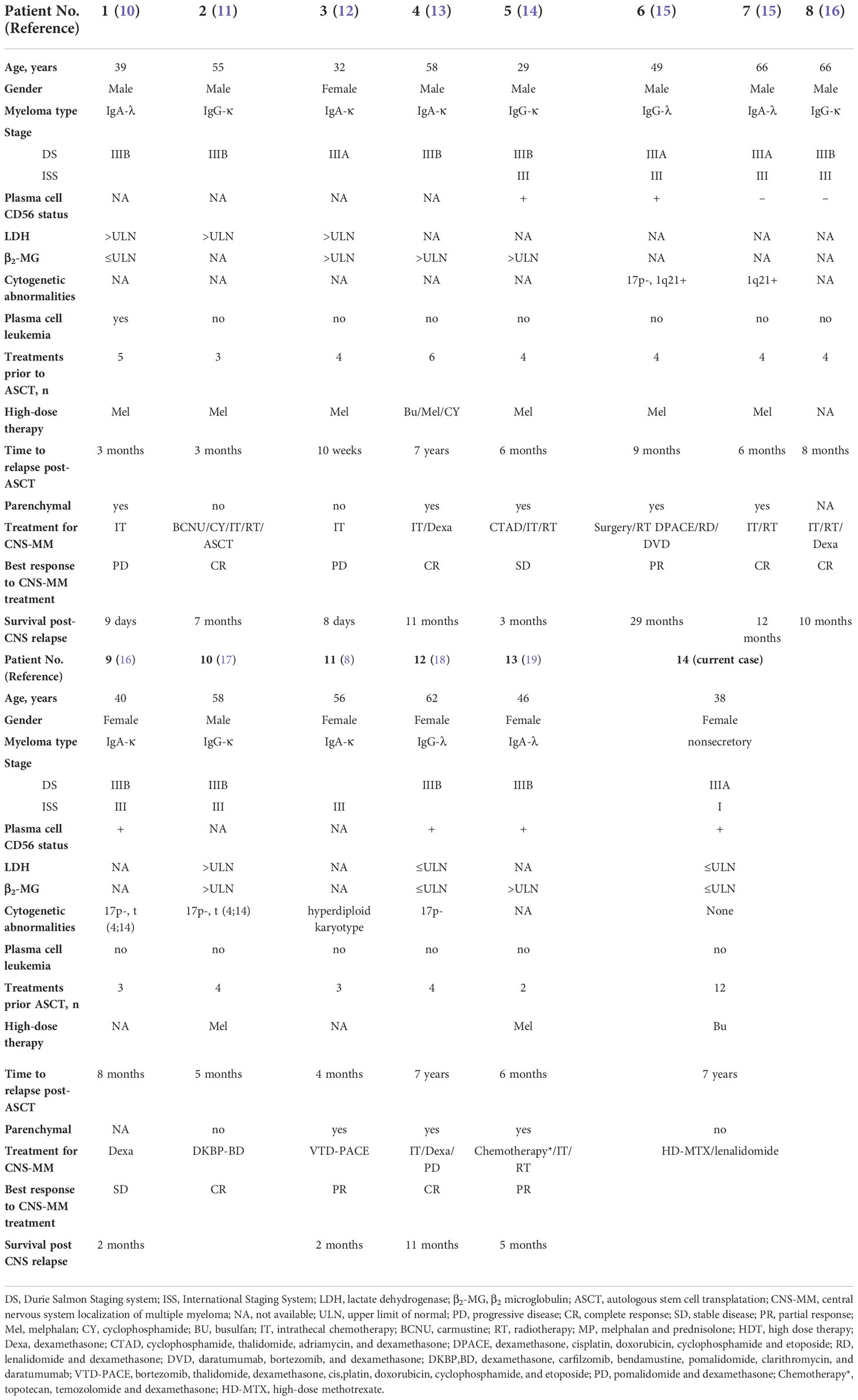
Table 1 Published case reports of patients with multiple myeloma who had isolated CNS relapse after ASCT.
The mechanism leading to isolated CNS relapse post-ASCT is uncertain. One hypothesis is that malignant plasma cells are transmitted by blood or plasma cell precursors, and then spread in the cerebrospinal meninges. In the past decade, therapies using novel agents and ASCT have improved the progression-free survival of MM patients, and it seems likely that this has led to the appearance of new patterns of relapse. The downregulation of CD56 adhesion molecules after first-line therapy could allow MM cells to escape the bone marrow environment and establish distant plasma cell metastasis, including in the CNS (18). Patients with plasma cell leukemia have abnormal plasma cells in the circulating blood, and the presence of these circulating plasma cells increases the risk of hematogenous spread. This supports our first hypothesis that malignant plasma cells are transmitted in the blood, and then spread to the cerebrospinal meninges (23). A second hypothesis is that plasmacytoma infiltrated adjacent skull lytic lesions. These patients mainly have parenchymal infiltration, varying from 39% to 65% in some cohorts (5, 24). Finally, a series of reports showed that clonal heterogeneity could play a role in CNS-MM. In particular, high dose chemotherapy for ASCT might select for extramedullary drug-resistant clonal populations, thus leading to relapse without bone marrow involvement (14, 25, 26). Our patient received first-line ASCT after aggressive therapy, and had none of the factors associated with risk for CNS involvement at baseline. After our patient achieved a 7-year progression-free survival, the selection of plasma cells with an atypical homing behavior and the absence of immunoglobulin secretion may have led to the isolated CNS relapse. We hypothesize that her relapse may have been from a new clone, rather than the clone responsible for the initial diagnosis.
There is currently no standard treatment for CNS-MM. Traditional therapeutic strategies include chemotherapy, surgery, radiotherapy, and intrathecal injection, but evidence supporting their efficacy is limited and durable remission is rare (27). Previous studies of systemic chemotherapy agents (methotrexate, cytarabine, edabixin, azathioprine and thiotepa) that can penetrate the blood-brain barrier (BBB) may provide a rapid therapeutic effect (8, 28). However, due to their CNS toxicity and low efficacy in MM patients who have chromosome 17p-, treatments consisting of traditional chemotherapy drugs alone are insufficient. Given the known radiosensitivity of malignant plasma cells, craniospinal irradiation is frequently used to treat parenchymal CNS-MM lesions (29). Although this treatment modality is associated with a statistically significantly longer survival (9), hematologic toxicity is a potential concern, especially in the cases who prior exposure to several myelosuppressive chemotherapy agents and ASCT (30).
Although novel agents have improved the outcomes of patients with CNS-MM (31), most conventional anti-myeloma drugs have relatively poor CNS penetration. A literature review of the penetration of novel myeloma-active drugs into the CSF reported that some immunomodulatory drugs (IMiDs) entered the CSF. For example, thalidomide can be detected in CSF after oral administration (32) and the lenalidomide and pomalidomide concentrations in CSF can reach 11% to 49% of the peak concentration in blood. Thus, these drugs may have good CSF activity against lymphoma and myeloma when there is CNS involvement (33–36). In addition, similar studies showed that one-third of lenalidomide-resistant patients still responded to pomadodomide, particularly those with MM with chromosome 17p- and/or translocation (4, 14) (37, 38).
Few proteasome inhibitors can penetrate the BBB, limiting their efficacy in patients with CNS-MM (27). Marizomib and carfilzomib are novel next-generation proteasome inhibitors that can pass through the BBB and may be effective in CNS-MM. For example, an animal study of radiolabeled marizomib reported the CNS level was 30% of that in the blood (39). Case reports (40) showed that marizomib provided clinical and radiological improvements, so it may be an effective approach for treatment of CNS-MM. Some case series also reported that carfilzomib was effective in the clearance of myeloma cells from CSF (41).
Some studies examined the ability of monoclonal antibodies to improve the outcomes of patients with CNS-MM. Although the penetration of systemic daratumumab (anti-CD38 monoclonal antibody) into the CNS was limited, it produced durable responses in some case reports. It is possible that the BBB becomes more permeable in certain disease states, such as when there is disruption of the meninges (28, 42).
In addition to monoclonal antibodies, the recently developed B-cell maturation antigen, chimeric antigen receptor T cell (BCMA CAR-T) therapy is a novel treatment strategy for relapsed/refractory(R/R) CNS-involved MM. For example, Wang et al. identified the presence of BCMA CAR-T cells in CSF (43). The mechanisms responsible for the higher CD4/CD8 ratio in CSF than in peripheral blood may regulate the penetration of CD4 + and CD8 + CAR-T cells across the BBB and their proliferation in CSF to kill myeloma cells. Several studies investigated the effects of BCMA CAR-T cells on CNS-MM patients and reported remarkable clinical remissions (43, 44). Closer monitoring of patients may help in the early identification of CAR-T neurotoxicity, thus making immune effector cell-associated neurotoxicity syndrome (ICANS) more predictable and controllable (45). BCMA CAR-T therapy appears to be a safe and effective for treatment for R/R CNS-MM, but the duration of remission is a remaining problem.
Although the optimal therapy for CNS-MM is uncertain because of the rarity of this condition, aggressive management is necessary. Examination of individualized combinations of chemotherapy, targeted drugs, monoclonal antibodies, CAR-T cells, and local therapy could lead to further improvements of outcomes.
Conclusion
Our study describes a case of CNS-MM following ASCT, with no evidence of systemic involvement. High dose methotrexate and lenalidomide (which can cross the BBB) produced a rapid response and effectively cleared myeloma cells from the CSF, but the duration of this remission must be addressed. Isolated CNS relapse after ASCT in MM is extremely rare. Even with novel therapies, the survival time after CNS-MM remains poor, and the optimal method for management of these patients is an open question because of the rarity of this condition. Further studies are required to identify factors associated with CNS relapse after ASCT and the underlying mechanism, and to determine improved methods of prophylaxis and management.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by The Medical Ethics Committee of The Second Affiliated Hospital, College of Medicine, Zhejiang University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XL, WW, and YL contributed to the design and conception of the study; XL and WW contributed to data collection; XL contributed to writing the initial drafting of the manuscript; XZ and YL reviewed and edited the original draft. All authors contributed to manuscript revision and read and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med (2011) 364:1046–60. doi: 10.1056/NEJMra1011442
2. Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos MV, et al. Multiple myeloma. Nat Rev Dis Primers (2017) 3:17046. doi: 10.1038/nrdp.2017.46
3. Gagelmann N, Eikema DJ, Iacobelli S, Koster L, Nahi H, Stoppa AM, et al. Impact of extramedullary disease in patients with newly diagnosed multiple myeloma undergoing autologous stem cell transplantation: A study from the chronic malignancies working party of the EBMT. Haematologica (2018) 103:890–7. doi: 10.3324/haematol.2017.178434
4. Jurczyszyn A, Grzasko N, Gozzetti A, Czepiel J, Cerase A, Hungria V, et al. Central nervous system involvement by multiple myeloma: A multi-institutional retrospective study of 172 patients in daily clinical practice. Am J Hematol (2016) 91:575–80. doi: 10.1002/ajh.24351
5. Chen CI, Masih-Khan E, Jiang H, Rabea A, Cserti-Gazdewich C, Jimenez-Zepeda VH, et al. Central nervous system involvement with multiple myeloma: Long term survival can be achieved with radiation, intrathecal chemotherapy, and immunomodulatory agents. Br J Haematol (2013) 162:483–8. doi: 10.1111/bjh.12414
6. Majd N, Wei X, Demopoulos A, Hormigo A, Chari A. Characterization of central nervous system multiple myeloma in the era of novel therapies. Leuk Lymphoma (2016) 57:1709–13. doi: 10.3109/10428194.2015.1122786
7. Abdallah AO, Atrash S, Shahid Z, Jameel M, Grazziutti M, Apewokin S, et al. Patterns of central nervous system involvement in relapsed and refractory multiple myeloma. Clin Lymphoma Myeloma Leuk (2014) 14:211–4. doi: 10.1016/j.clml.2013.11.004
8. Varga G, Mikala G, Gopcsa L, Csukly Z, Kollai S, Balázs G, et al. Multiple myeloma of the central nervous system: 13 cases and review of the literature. J Oncol (2018) 2018:3970169. doi: 10.1155/2018/3970169
9. Nieuwenhuizen L, Biesma DH. Central nervous system myelomatosis: Review of the literature. Eur J Haematol (2008) 80:1–9. doi: 10.1111/j.1600-0609.2007.00956.x
10. Petersen SL, Wagner A, Gimsing P. Cerebral and meningeal multiple myeloma after autologous stem cell transplantation. a case report and review of the literature. Am J Hematol (1999) 62:228–33. doi: 10.1002/(sici)1096-8652(199912)62:4<228::aid-ajh5>3.0.co;2-3
11. Veinstein A, Brizard A, Randriamalala E, Babin P, Preud'homme JL, Guilhot F. Central nervous system relapses after autologous stem cell transplantation for myeloma. report of two cases. Hematol Cell Ther (1997) 39:327–30. doi: 10.1007/s00282-997-0327-6
12. Ulusakarya A, Youssef A, Bayle C, Vantelon JM, Munck JN. Plasma cell meningitis after an autograft in a patient with multiple myeloma. Leuk Lymphoma (1999) 34:633–4. doi: 10.3109/10428199909058496
13. Seftel MD, Maguire J, Voss N, Woodhurst WB, Dalal BI, Shepherd JD. Intra-cerebral relapse following prolonged remission after autologous stem cell transplantation for multiple myeloma. Leuk Lymphoma (2002) 43:2399–403. doi: 10.1080/1042819021000040125
14. Mittal A, Pushpam D, Kumar L. Isolated central nervous system relapse of multiple myeloma post autologous stem cell transplant- a rare presentation. Leuk Res Rep (2020) 14:100207. doi: 10.1016/j.lrr.2020.100207
15. Bergantim R, Bastos J, Soares MJ, Carvalho B, Soares P, Marques C, et al. Aggressive central nervous system relapse after autologous stem cell transplant in multiple myeloma: Case reports and literature review. Case Rep Hematol (2020) 2020:8563098. doi: 10.1155/2020/8563098
16. Gangatharan SA, Carney DA, Prince HM, Wolf MM, Januszewicz EH, Ritchie DS, et al. Emergence of central nervous system myeloma in the era of novel agents. Hematol Oncol (2012) 30:170–4. doi: 10.1002/hon.1021
17. Mousavi-Fatemi K, Maleki N. Management of central nervous system involvement in multiple myeloma after autologous hematopoietic stem cell transplantation. Leuk Res Rep (2020) 14:100210. doi: 10.1016/j.lrr.2020.100210
18. Marini A, Carulli G, Lari T, Buda G, Lambelet P, Ciancia EM, et al. Myelomatous meningitis evaluated by multiparameter flow cytometry: Report of a case and review of the literature. J Clin Exp Hematop (2014) 54:129–36. doi: 10.3960/jslrt.54.129
19. Annibali O, Nobile C, Greco R, Cellini F, Quattrocchi CC, Tirindelli MC, et al. The combination topotecan, temozolomide and dexamethasone associated with radiotherapy as treatment of central nervous system myeloma relapse. Int J Hematol (2009) 89:513–6. doi: 10.1007/s12185-009-0277-6
20. Chang H, Sloan S, Li D, Keith Stewart A. Multiple myeloma involving central nervous system: high frequency of chromosome 17p13.1 (p53) deletions. Br J Haematol (2004) 127:280–4. doi: 10.1111/j.1365-2141.2004.05199.x
21. Schmidt TM, Fonseca R, Usmani SZ. Chromosome 1q21 abnormalities in multiple myeloma. Blood Cancer J (2021) 11:83. doi: 10.1038/s41408-021-00474-8
22. Biran N, Malhotra J, Bagiella E, Cho HJ, Jagannath S, Chari A. Patients with newly diagnosed multiple myeloma and chromosome 1 amplification have poor outcomes despite the use of novel triplet regimens. Am J Hematol (2014) 89:616–20. doi: 10.1002/ajh.23705
23. Gundesen MT, Lund T, Moeller HEH, Abildgaard N. Plasma cell leukemia: Definition, presentation, and treatment. Curr Oncol Rep (2019) 21:8. doi: 10.1007/s11912-019-0754-x
24. Katodritou E, Terpos E, Kastritis E, Delimpasis S, Symeonidis AS, Repousis P, et al. Lack of survival improvement with novel anti-myeloma agents for patients with multiple myeloma and central nervous system involvement: The Greek myeloma study group experience. Ann Hematol (2015) 94:2033–42. doi: 10.1007/s00277-015-2484-y
25. Keats JJ, Chesi M, Egan JB, Garbitt VM, Palmer SE, Braggio E, et al. Clonal competition with alternating dominance in multiple myeloma. Blood (2012) 120:1067–76. doi: 10.1182/blood-2012-01-405985
26. Zeiser R, Deschler B, Bertz H, Finke J, Engelhardt M. Extramedullary vs medullary relapse after autologous or allogeneic hematopoietic stem cell transplantation (HSCT) in multiple myeloma (MM) and its correlation to clinical outcome. Bone Marrow Transplant (2004) 34:1057–65. doi: 10.1038/sj.bmt.1704713
27. Egan PA, Elder PT, Deighan WI, O'Connor SJM, Alexander HD. Multiple myeloma with central nervous system relapse. Haematologica (2020) 105:1780–90. doi: 10.3324/haematol.2020.248518
28. Elhassadi E, Murphy M, Hacking D, Farrell M. Durable treatment response of relapsing CNS plasmacytoma using intrathecal chemotherapy, radiotherapy, and daratumumab. Clin Case Rep (2018) 6:723–8. doi: 10.1002/ccr3.1451
29. Tsang RW, Campbell BA, Goda JS, Kelsey CR, Kirova YM, Parikh RR, et al. Radiation therapy for solitary plasmacytoma and multiple myeloma: Guidelines from the international lymphoma radiation oncology group. Int J Radiat Oncol Biol Phys (2018) 101:794–808. doi: 10.1016/j.ijrobp.2018.05.009
30. Kauffmann G, Buerki RA, Lukas RV, Gondi V, Chmura SJ. Case report of bone marrow-sparing proton therapy craniospinal irradiation for central nervous system myelomatosis. Cureus (2017) 9:e1885. doi: 10.7759/cureus.1885
31. Touzeau C, Moreau P. How I treat extramedullary myeloma. Blood (2016) 127:971–6. doi: 10.1182/blood-2015-07-635383
32. Vicari P, Ribas C, Sampaio M, Arantes AM, Yamamoto M, Filho JB, et al. Can thalidomide be effective to treat plasma cell leptomeningeal infiltration? Eur J Haematol (2003) 70:198–9. doi: 10.1034/j.1600-0609.2003.00022.x
33. Bladé J, Fernández de Larrea C, Rosiñol L, Cibeira MT, Jiménez R, Powles R., et al. Soft-tissue plasmacytomas in multiple myeloma: Incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol (2011) 29:3805–12. doi: 10.1200/JCO.2011.34.9290
34. Devoe CE, Li JY, Demopoulos AM. The successful treatment of a recurrent intracranial, dural-based plasmacytoma with lenalidomide. J Neurooncol (2014) 119:217–20. doi: 10.1007/s11060-014-1475-5
35. Li Z, Qiu Y, Personett D, Huang P, Edenfield B, Katz J, et al. Pomalidomide shows significant therapeutic activity against CNS lymphoma with a major impact on the tumor microenvironment in murine models. PloS One (2013) 8:e71754. doi: 10.1371/journal.pone.0071754
36. Selene I, Jose J, Malik MN, Qureshi A, Anwer F. Presentation patterns and management strategies for central nervous system involvement in multiple myeloma: A systematic review of literature. Blood (2018) 132:1951. doi: 10.1182/blood-2018-99-109923
37. Leleu X, Karlin L, Macro M, Hulin C, Garderet L, Roussel M, et al. Pomalidomide plus low-dose dexamethasone in multiple myeloma with deletion 17p and/or translocation (4,14): IFM 2010-02 trial results. Blood (2015) 125:1411–7. doi: 10.1182/blood-2014-11-612069
38. Mussetti A, Dalto S, Montefusco V. Effective treatment of pomalidomide in central nervous system myelomatosis. Leuk Lymphoma (2013) 54:864–6. doi: 10.3109/10428194.2012.718343
39. Di K, Lloyd GK, Abraham V, MacLaren A, Burrows FJ, Desjardins A, et al. Marizomib activity as a single agent in malignant gliomas: Ability to cross the blood-brain barrier. Neuro Oncol (2016) 18:840–8. doi: 10.1093/neuonc/nov299
40. Badros A, Singh Z, Dhakal B, Kwok Y, MacLaren A, Richardson P, et al. Marizomib for central nervous system-multiple myeloma. Br J Haematol (2017) 177:221–5. doi: 10.1111/bjh.14498
41. Espinoza R, Nolasco DB, Alejandro S, Nidia Z, Eduardo C, Candelaria M, et al. Report of 5 cases of extramedullary myeloma with central nervous system involvement treated with a combination of Carfilzomib/Thalidomide/Dexamethasone as a first line treatment at a single institution in Mexico. Blood (2016) 128:5704. doi: 10.1182/blood.V128.22.5704.5704
42. Zajec M, Frerichs KA, van Duijn MM, Nijhof IS, Stege CAM, Avet-Loiseau H, et al. Cerebrospinal fluid penetrance of daratumumab in leptomeningeal multiple myeloma. Hemasphere (2020) 4:e413. doi: 10.1097/HS9.0000000000000413
43. Wang Y, Zu C, Teng X, Yang L, Zhang M, Hong R, et al. BCMA CAR-T therapy is safe and effective for Refractory/Relapsed multiple myeloma with central nervous system involvement. J Immunother (2022) 45:25–34. doi: 10.1097/CJI.0000000000000391
44. Wang Y, Wang L, Zeng Y, Hong R, Zu C, Yin ETS, et al. Successful BCMA CAR-T therapy for multiple myeloma with central nervous system involvement manifesting as cauda equina syndrome-a wandering road to remission. Front Oncol (2021) 11:755584. doi: 10.3389/fonc.2021.755584
Keywords: multiple myeloma, isolated central nervous system relapse, autologous stem cell transplantation, pathogenesis and treatment, case report
Citation: Li X, Wang W, Zhang X and Liang Y (2022) Multiple myeloma with isolated central nervous system relapse after autologous stem cell transplantation: A case report and review of the literature. Front. Oncol. 12:1027585. doi: 10.3389/fonc.2022.1027585
Received: 25 August 2022; Accepted: 07 November 2022;
Published: 25 November 2022.
Edited by:
Ahmad Antar, Almoosa Specialist Hospital, Saudi ArabiaReviewed by:
Yan-Hua Zheng, Fourth Military Medical University (Air Force Medical University), ChinaJoshua Richter, Icahn School of Medicine at Mount Sinai, United States
Copyright © 2022 Li, Wang, Zhang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Liang, bGlhbmd5dW5Aemp1LmVkdS5jbg==
 Xian Li
Xian Li Weiqin Wang
Weiqin Wang Xiaohong Zhang
Xiaohong Zhang Yun Liang
Yun Liang