- Department of Hematology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China
Several tyrosine kinase inhibitors (TKIs) have been developed as targeted therapies to inhibit the oncogenic activity of several tyrosine kinases in chronic myeloid leukemia (CML), acute lymphoid leukemia (ALL), gastrointestinal stromal tumor (GIST), and other diseases. TKIs have significantly improved the overall survival of these patients and changed the treatment strategy in the clinic. However, approximately 50% of patients develop resistance or intolerance to imatinib. For second-generation TKIs, approximately 30%–40% of patients need to change therapy by 5 years when they are used as first-line treatment. Clinical study analysis showed that the T315I mutation is highly associated with TKI resistance. Developing new drugs that target the T315I mutation will address the dilemma of treatment failure. Olverembatinib, as a third-generation TKI designed for the T315I mutation, is being researched in China. Preliminary clinical data show the safety and efficacy in treating CML patients harboring the T315I mutation or who are resistant to first- or second-line TKI treatment. Herein, we review the characteristics and clinical trials of olverembatinib. We also discuss its role in the management of CML patients.
Introduction
Chronic myeloid leukemia (CML) is a clonal hematopoietic stem cell disorder that accounts for approximately 15% of adult leukemia (1). The incidence of CML varies from 0.4/100,000 to 1.75/100,000 persons per year (2). Diagnosis is usually suspected from the complete blood count (CBC) and blood smear. Fluorescence in situ hybridization (FISH) for abnormal chromosome t(9;22)(q34;q11.2) and reverse transcriptase quantitative PCR for the BCR-ABL1 fusion gene help in the final diagnosis (3). CML is clinically staged into the chronic phase (CP), accelerated phase (AP), and blast phase (BP). In untreated patients, progression to BP occurs at a median of 3–5 years after initial diagnosis (4).
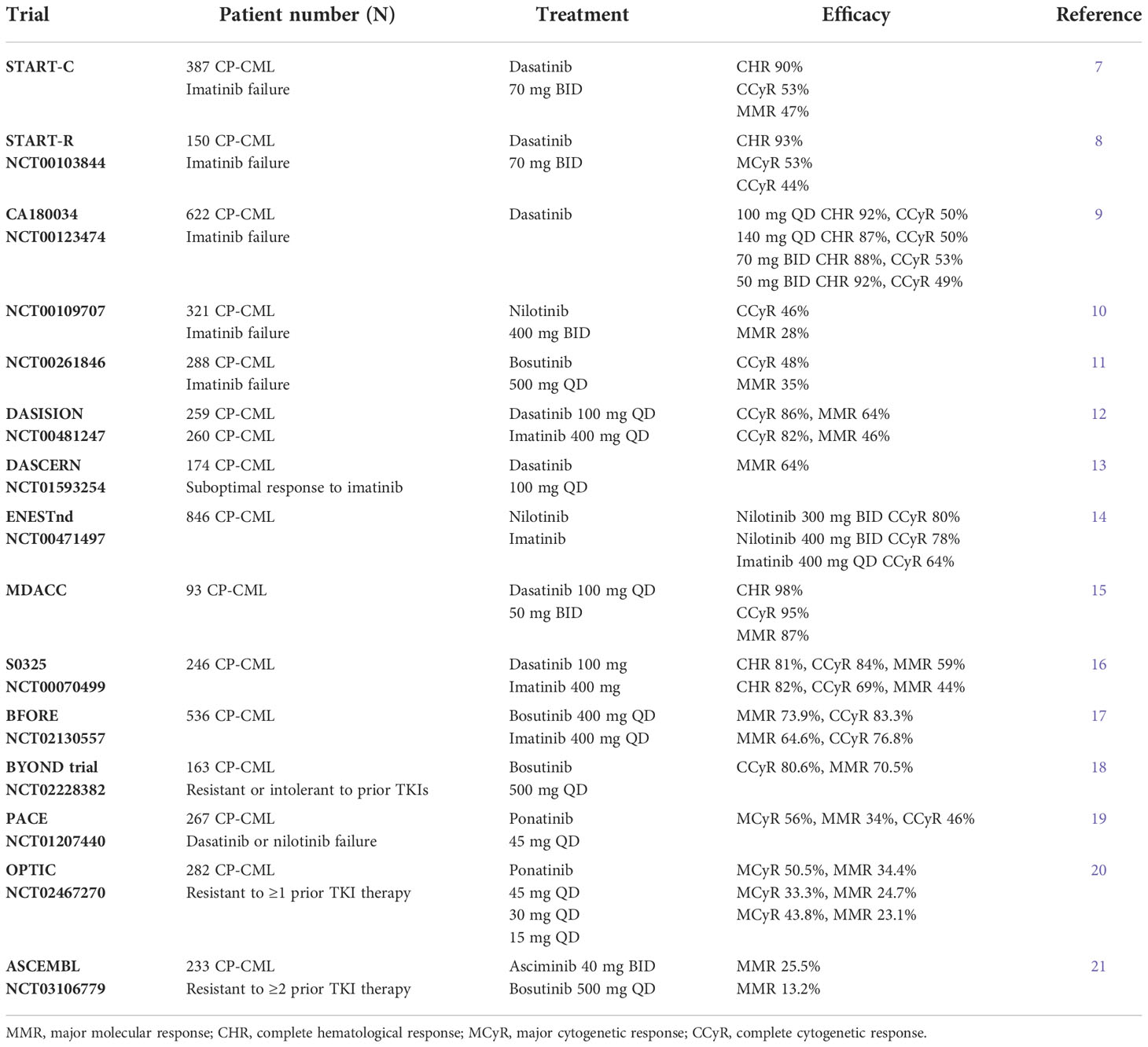
When a patient is diagnosed with CML, the first-line treatment is a TKI. In an open-label, multicenter trial with a crossover design for patients treated with the first-generation TKI imatinib, the estimated overall survival rate at 10 years was 83.3%, and the cumulative rate of major cytogenetic response (MCyR) at the end of the trial was 89.0%. A total of 82.8% of patients had a complete cytogenetic response (CCyR) (5). Despite the excellent results obtained in clinical trials, approximately 40%–45% of patients discontinue imatinib for various reasons, including unsatisfactory therapeutic outcomes in 16% of patients or primary resistance (6). Dose escalation after failure of imatinib treatment is an important option, though it is likely to be effective only in some subsets of patients. However, it is not recommended in the clinic. For such patients, an important salvage treatment strategy is represented by second-generation TKIs including dasatinib, nilotinib, and bosutinib, which allow recovering a CCyR in about 50% of cases (7–11, Table 1). When used as a front-line treatment, second-generation TKIs provide a faster achievement of a higher rate of cytogenetic and molecular responses with respect to imatinib (12–18, 22–24, Table 1). Subsequently, second-generation TKIs have been authorized for the first-line treatment of newly diagnosed Philadelphia chromosome–positive (Ph+) adult CP-CML (25). Some patients experience treatment failure with second-generation TKIs and require a switch to a different TKI. Intolerance and resistance are major causes of treatment failure. Resistance to TKIs can arise from BCR-ABL1-dependent mechanisms, such as mutations in the kinase domain, overexpression, or amplification of BCR-ABL1, or BCR-ABL1-independent mechanisms (26). The well-studied and most common mechanism is point mutation involving the BCR-ABL1 kinase domain (27). In one study, which included 175 patients with newly diagnosed CML or resistance to TKIs, 28 different mutations were detected in 54 (30.86%). A total of 14 (8.0%) patients carried the T315I mutation, accounting for the largest proportion in the mutation group (28). It has been demonstrated that the T315I mutation is insensitive to all first- and second-generation TKIs. Asciminib and ponatinib (19–21, Table 1), as new-generation TKIs, have unique activity against the T315I mutation and are approved to treat patients with resistance or intolerance to prior TKI therapy or the presence of the BCR-ABL1 T315I mutation in all CML phases. Research has shown that the ATP-binding site mutation T315M confers resistance to ponatinib (29). In vitro data indicated that some compound mutations involving T315I also potentially impact ponatinib sensitivity (30). New TKIs have higher sensitivity to the T315I mutation. In November 2021, olverembatinib, as a new third-generation TKI that showed clinical efficacy in CML patients with the T315I mutation, received its first approval in China for the treatment of adult patients with TKI-resistant CP-CML or AP-CML harboring the T315I mutation (31). It is the first and only approved TKI designed for the T315I mutation in China. Here, we review the characteristics and clinical studies of olverembatinib and its application value in the clinic.
Characteristics and inhibitory activity of olverembatinib
Olverembatinib tightly binds to the ATP-binding sites of native BCR-ABL1 and multiple BCR-ABL1 mutants, including Q252H, E255K, F317L, F317I M351T, H396P, and the most refractory gatekeeper mutant T315I (32). It binds to phosphorylated and non-phosphorylated forms of BCR-ABL1 kinase, as well as to several other kinases, including KIT, FLT3, fibroblast growth factor receptor 1 (FGFR1), and platelet-derived growth factor receptor α (PDGFRα). In the predicted binding pose of olverembatinib (GZD824) with activated BCR-ABL1T315I, the 1H-pyrazolo[3,4-b] pyridine core occupies the adenine pocket of BCR-ABL1 kinase to form a hydrogen bond donor-acceptor network. The alkyne moiety of GZD824 presents favorable van der Waals interactions with BCR-ABL T315I (32).
In vitro, olverembatinib strongly inhibits the proliferation of Ba/F3 cells expressing BCR-ABL1T315I, as well as K562/Ku812 CML cells and SUP-B15 ALL cells expressing BCR-ABL1. In mouse allograft leukemia models, it inhibited tumor growth in the allograft model using Ba/F3 cells expressing BCR-ABL1WT or BCR-ABL1T315I in a dose-dependent manner and showed overall survival benefits (33). Moreover, in FLT3-ITD mutant AML and xenograft tumor models, cell growth was significantly inhibited with olverembatinib (34, 35). Olverembatinib also exhibits antileukemic activity in imatinib-resistant/sensitive (GIST) cell lines and a GIST mouse model by inhibiting the phosphorylation of KIT and its downstream proteins, including AKT, ERK1/2, and STAT3 (36). The above studies demonstrate that olverembatinib has wide antitumor activities and exerts a strong inhibitory effect.
Clinical trials of olverembatinib
CP-CML
In an open-label, multicenter phase 1/2 trial, 127 Chinese patients with TKI-resistant CP-CML were enrolled (37). The characteristics of the patients are depicted in Table 2. Forty-one (43.6%) patients had a single T315I mutation, and 15 (16.0%) patients carried T315I and additional mutations. A total of 20.2% of patients had no BCR-ABL1 mutation. Compound mutations were detected in 7 (7.4%) patients. The median follow-up was 37 months. In evaluable patients without baseline responses, MCyR and CCyR were achieved in 79.3% and 69.4% of patients, respectively, at a median of 3 months. The cumulative 3-year incidences of MCyR, CCyR, MMR, MR4.0, and MR4.5 were 78.6%, 69.0%, 55.9%, 43.5%, and 38.6%, respectively. The probabilities of sustained MCyR, CCyR, and MMR at 3 years were 77.3%, 72.2%, and 76.0%, respectively. Seven patients died of disease progression, other diseases, or unknown reasons. The probabilities of PFS and OS at 3 years were 92% and 94%, respectively.
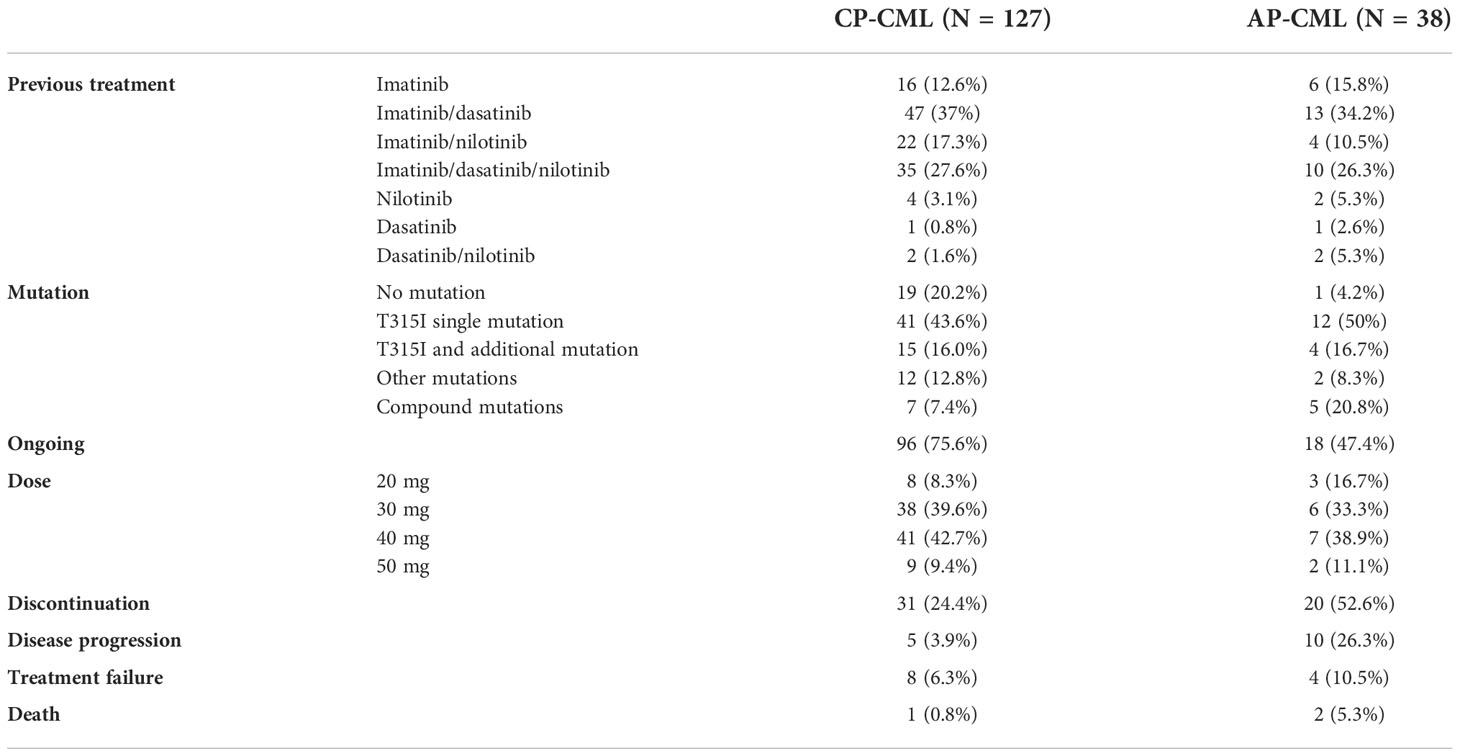
Table 2 Characteristics of patients in the olverembatinib trial (37).
AP-CML
A phase 1/2 study (37) included 38 patients with TKI-resistant BCR-ABL1T315I AP-CML, 12 patients with a single T315I mutation, and four with T315I and additional mutations. Among 37 patients without baseline MaHR, 27 patients experienced CHR at a median of 3 months. The three-year cumulative incidences of achieving MCyR, CCyR, MMR, MR4.0, and MR4.5 were 47.4%, 47.4%, 44.7%, 39.3%, and 32.2%, respectively. The probabilities of PFS and OS at three years were 60% and 71%, respectively. A total of 11 patients had CML that progressed to the blast phase, and 2 patients died. We also summarize some characteristics in Table 2. These studies show that olverembatinib is as efficacious and well tolerated as monotherapy in patients with TKI-resistant BCR-ABL1T315I AP-CML.
Ongoing clinical trials in CML
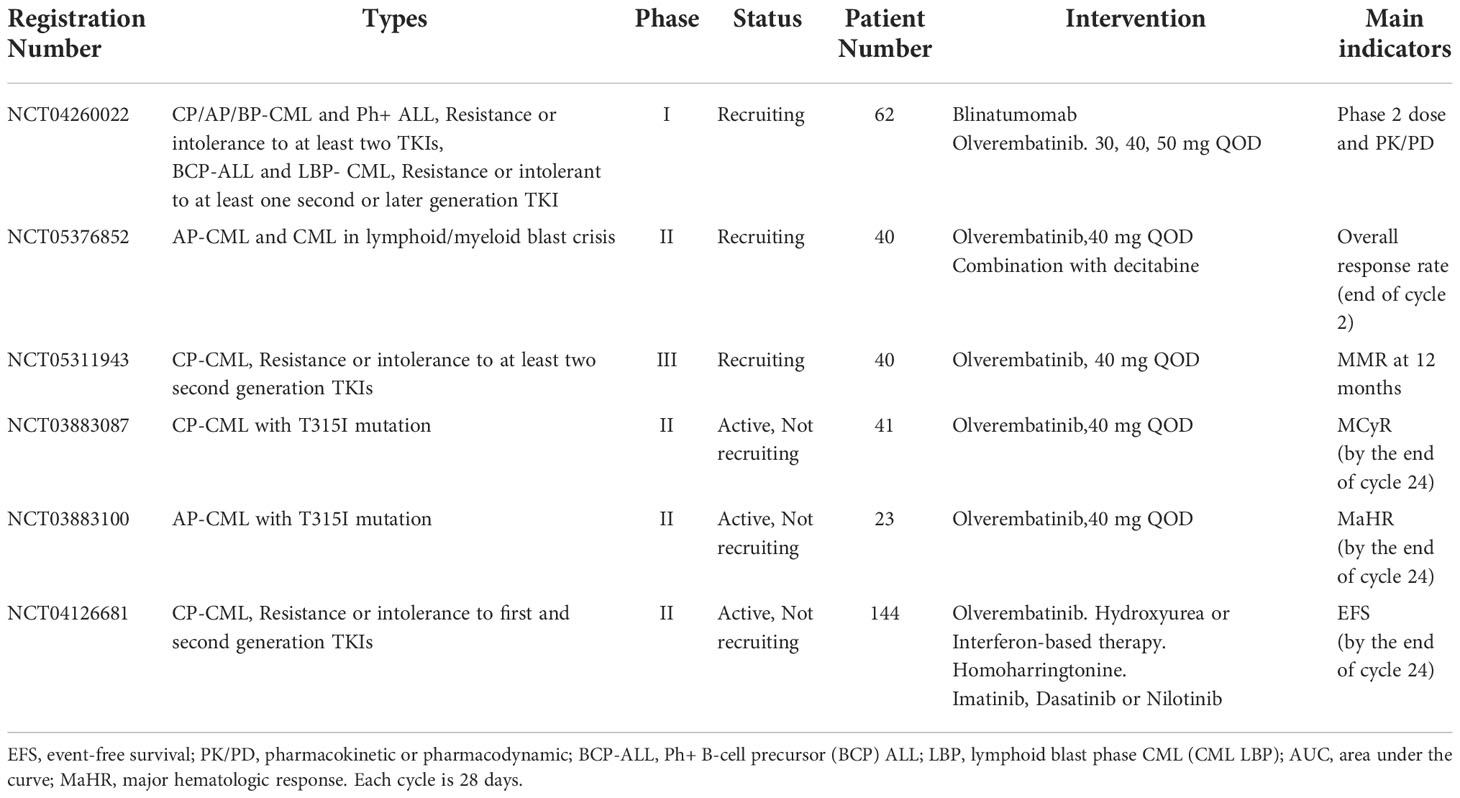
In the phase I dose escalation study (SJ-0002), patients with TKI-resistant CML were enrolled in 11 dose escalation cohorts ranging from 1 mg to 60 mg every other day. The maximum tolerated dose (MTD) has been established as 50 mg (38). Olverembatinib is also being assessed in patients with CP, AP, and BP-CML or with Ph+ ALL who have experienced resistance or intolerance to at least two TKIs or in subjects with Ph+ BCP ALL or LBP CML who have experienced resistance or intolerance to at least one second- or later-generation TKI (NCT04260022). In a phase 2 study, the efficacy and safety of Olverembatinib in CP-CML patients who are resistant or intolerant to first- and second-generation TKI are being evaluated (NCT04126681). In this study, the available treatment including hydroxyurea or interferon-based therapy, homoharringtonine, imatinib, dasatinib, or nilotinib will be selected by the investigator for each participant. The efficacy of olverembatinib will be determined by evaluating the patients’ event-free survival (EFS). In addition, in one phase III study (NCT05311943), 40 patients are recruited to evaluate the efficacy and safety of olverembatinib in patients with CP-CML who are resistant or intolerant to at least two second-generation TKIs. The molecular response (MR), MMR, OS, PFS, and adverse events will be measured. We summarize these trials in Table 3.
Adverse effects in patients treated with olverembatinib
In CP-CML with olverembatinib treatment, the treatment-related adverse events were mainly grade 1 or 2, and the most frequent nonhematologic adverse event was skin hyperpigmentation (85%). Grade 3 or 4 nonhematologic adverse events included hypertriglyceridemia (8.7%), increased creatine phosphokinase (7.9%), and hypertension (5.5%). The most common hematologic treatment-related adverse event was thrombocytopenia (73.2%, including 48.8% of patients with grade 3 or 4). A total of 16.5% of patients had grade 3 or higher leukopenia, while 20.5% of patients had anemia (grade 3 or higher). In AP-CML, the common nonhematologic adverse events included skin pigmentation (81.6%), hypocalcemia (60.5%), proteinuria (50%), hyperuricemia (50%), hypertriglyceridemia (34.2%), and hyperphosphatemia (28.9%), of which most were grade 1 or 2. Thrombocytopenia, anemia, leukopenia, and neutropenia were the common hematological adverse events, with incidences of 86.8%, 63.2%, 50%, and 21.1%, respectively; these adverse events were manageable (37).
Role of olverembatinib in CML
The goals of treatment in CML are the prolongation of survival and prevention of progression toward AP and BP. Over time, the goals of treatment have evolved to achieve deep molecular responses. The focus for patients living with CML has shifted toward improving quality of life and saving costs, and the goal of achieving treatment-free remission is becoming more desirable. Some patients show resistance to first-generation TKIs and must switch to second-generation TKIs. Prospective studies report the superiority of second-generation TKIs, particularly in terms of MR rate, speed, and depth (17, 39–41). Actually, four TKIs, namely, imatinib, nilotinib, dasatinib, and bosutinib, are recommended for first-line treatment.
In patients with TKI treatment-naïve BCR-ABL AP/BP-CML, the standard treatment is the abovementioned four TKIs. In the case of intolerance or failure to one TKI, the choice of the subsequent therapy should also consider whether the patients develop ABL1 point mutations (42). The second-line treatment is a switch to any of the other TKIs. If the patients are resistant or intolerant to one of the second-generation TKIs, in such situations, ponatinib is also recommended. If patients are heavily pretreated and resistant to at least two second-generation TKIs, asciminib as the third-line treatment is an important option (43). If disease progresses to AP or BP during TKI treatment, we recommend choosing third-generation TKIs, bone marrow transplantation or experimental treatment (44). In CML patients with the BCR-ABL1 T315I mutation, the third-generation TKIs ponatinib and asciminib are preferentially recommended (45). Nevertheless, there is no consensus about the choice of treatment strategy. According to our previous studies, olverembatinib shows safety and efficacy in CP and BP-CML patients with at least two generations of TKI resistance or T315I mutations. We further recommend olverembatinib as the third-line treatment (37). In one study, the third-generation TKI ponatinib combined with fludarabine, cytarabine, granulocyte colony-stimulating factor, and idarubicin (FLAG-IDA) was used in Ph+ BP-CML patients, with 69% of patients in the second chronic phase after one cycle of treatment (46). Further studies are needed to investigate the efficacy and safety of olverembatinib in combination with other chemotherapies in AP-CML and BCR-ABL1T315I mutation patients.
Conclusion
Resistance to TKIs is a complex and multifactorial process that presents the selection of leukemia clones with the ability to evade treatment (47). Some specific mutations result in resistance to one particular TKI. Nilotinib is resistant to Y253H/F, E255K/V, F359V/C/I, and T315I mutations, whereas bosutinib works for all identified mutations with exceptions of V299L and T315I mutations (48, 49). The most common resistance mechanism is the ABL1 kinase domain point mutation. T315I mutation is the most aggressive point mutation identified in BCR-ABL1. Ponatinib was designed to overcome this mutation, while compound mutations, including T315I, cause resistance to ponatinib. Overall, olverembatinib shows a significant inhibitory effect in the presence of the T315I mutation or compound mutations because it does not form a hydrogen bond with the hydroxyl group at this residue (36); it has been demonstrated to be safe and efficacious in phase I/II clinical trials in CP-CML patients resistant to at least two TKIs. In China, olverembatinib is the only third-generation TKI approved for the treatment of CML patients with T315I mutation. Therefore, it is preferentially recommended in CML patients harboring the T315I mutation in China. Besides, olverembatinib as a third-line therapy might represent a viable option for patients failing first- or second-generation TKIs.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the major project of Wenzhou Municipal Science and Technology Bureau (No. ZY2021013).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Miranda-Filho A, Piñeros M, Ferlay J, Soerjomataram I, Monnereau A, Bray F. Epidemiological patterns of leukaemia in 184 countries: A population-based study. Lancet Haematol (2018) 5(1):e14–24. doi: 10.1016/S2352-3026(17)30232-6
2. Lin Q, Mao L, Shao L, Zhu L, Han Q, Zhu H, et al. Global, regional, and national burden of chronic myeloid leukemia, 1990-2017: A systematic analysis for the global burden of disease study 2017. Front Oncol (2020) 10:580759. doi: 10.3389/fonc.2020.580759
3. Thompson PA, Kantarjian HM, Cortes JE. Diagnosis and treatment of chronic myeloid leukemia in 2015. Mayo Clin Proc (2015) 90(10):1440–54. doi: 10.1016/j.mayocp.2015.08.010
4. Granatowicz A, Piatek CI, Moschiano E, El-Hemaidi I, Armitage JD, Akhtari M. An overview and update of chronic myeloid leukemia for primary care physicians. Korean J Fam Med (2015) 36(5):197–202. doi: 10.4082/kjfm.2015.36.5.197
5. Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med (2017) 376(10):917–27. doi: 10.1056/NEJMoa1609324
6. Saussele S, Pfirrmann M. Clinical trials in chronic myeloid leukemia. Curr Hematol Malig Rep (2012) 7(2):109–15. doi: 10.1007/s11899-012-0118-1
7. Mauro MJ, Baccarani M, Cervantes F, Lipton JH, Matloub Y, SinhaR R, et al. Dasatinib 2-year efficacy in patients with chronic-phase chronic myelogenous leukemia (CML-CP) with resistance or intolerance to imatinib (START-c). J Clin Oncol (2008) 26(15):7009. doi: 10.1200/jco.2008.26.15_suppl.7009
8. Kantarjian H, Pasquini R, Lévy V, Jootar S, Holowiecki J, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia resistant to imatinib at a dose of 400 to 600 milligrams daily: Two-year follow-up of a randomized phase 2 study (START-r). Cancer (2009) 115(18):4136–47. doi: 10.1002/cncr.24504
9. Shah NP, Rousselot P, Schiffer C, Rea D, Cortes JE, Milone J, et al. Dasatinib in imatinib-resistant or -intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034. Am J Hematol (2016) 91(9):869–74. doi: 10.1002/ajh.24423
10. Kantarjian HM, Giles FJ, Bhalla KN, Pinilla-Ibarz J, Larson RA, Gattermann N, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood (2011) 117(4):1141–5. doi: 10.1182/blood-2010-03-277152
11. Gambacorti-Passerini C, Brümmendorf TH, Kim DW, Turkina AG, Masszi T, Assouline S, et al. Bosutinib efficacy and safety in chronic phase chronic myeloid leukemia after imatinib resistance or intolerance: Minimum 24-month follow-up. Am J Hematol (2014) 89(7):732–42. doi: 10.1002/ajh.23728
12. Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood (2012) 119(5):1123–9. doi: 10.1182/blood-2011-08-376087
13. Cortes JE, Jiang Q, Wang J, Weng J, Zhu H, Liu X, et al. Dasatinib vs. imatinib in patients with chronic myeloid leukemia in chronic phase (CML-CP) who have not achieved an optimal response to 3 months of imatinib therapy: The DASCERN randomized study. Leukemia (2020) 34:2064–73. doi: 10.1038/s41375-020-0805-1
14. Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med (2010) 362(24):2251–9. doi: 10.1056/NEJMoa0912614
15. Pemmaraju N, Kantarjian H, Luthra R, O’Brien S, Jabbour E, Qunitas-Cardama A, et al. Results of a phase II trial of dasatinib as frontline therapy for chronic myeloid leukemia (CML) in chronic phase. Blood (2011) 118:1700. doi: 10.1182/blood.V118.21.1700.1700
16. Radich JP, Kopecky KJ, Appelbaum FR, Kamel-Reid S, Stock W, Malnassy G, et al. A randomized trial of dasatinib 100 mg versus imatinib 400 mg in newly diagnosed chronic-phase chronic myeloid leukemia. Blood (2012) 120(19):3898–905. doi: 10.1182/blood-2012-02-410688
17. Cortes JE, Gambacorti-Passerini C, Deininger MW, Mauro MJ, Chuah C, Kim DW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: Results from the randomized BFORE trial. J Clin Oncol (2018) 36(3):231–7. doi: 10.1200/JCO.2017.74.7162
18. Hochhaus A, Gambacorti-Passerini C, Abboud C, Gjertsen BT, Brümmendorf TH, Smith BD, et al. Bosutinib for pretreated patients with chronic phase chronic myeloid leukemia: Primary results of the phase 4 BYOND study. Leukemia (2020) 34(8):2125–37. doi: 10.1038/s41375-020-0915-9
19. Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med (2013) 369(19):1783–96. doi: 10.1056/NEJMoa1306494
20. Cortes J, Apperley J, Lomaia E, Moiraghi B, Undurraga Sutton M, Pavlovsky C, et al. Ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia: A randomized, open-label phase 2 clinical trial. Blood (2021) 138(21):2042–50. doi: 10.1182/blood.2021012082
21. Réa D, Mauro MJ, Boquimpani C, Minami Y, Lomaia E, Voloshin S, et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood (2021) 138(21):2031–41. doi: 10.1182/blood.2020009984
22. Cortes JE, Jones D, O'Brien S, Jabbour E, Ravandi F, Koller C, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol (2010) 28(3):398–404. doi: 10.1200/JCO.2009.25.4920
23. Rosti G, Palandri F, Castagnetti F, Breccia M, Levato L, Gugliotta G, et al. Nilotinib for the frontline treatment of ph(+) chronic myeloid leukemia. Blood (2009) 114(24):4933–8. doi: 10.1182/blood-2009-07-232595
24. Gambacorti-Passerini C, le Coutre P, Piazza R. The role of bosutinib in the treatment of chronic myeloid leukemia. Future Oncol (2020) 16(2):4395–408. doi: 10.2217/fon-2019-0555
25. Gurion R, Gafter-Gvili A, Vidal L, Leader A, Ram R, Shacham-Abulafia A, et al. Has the time for first-line treatment with second generation tyrosine kinase inhibitors in patients with chronic myelogenous leukemia already come? systematic review and meta-analysis. Haematologica (2013) 98(1):95–102. doi: 10.3324/haematol.2012.063172
26. Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCRABL gene mutation or amplification. Science (2001) 293(5531):876–80. doi: 10.1126/science.1062538
27. Parker WT, Ho M, Scott HS, Hughes TP, Branford S. Poor response to second-line kinase inhibitors in chronic myeloid leukemia patients with multiple low-level mutations, irrespective of their resistance profile. Blood (2012) 119(10):2234–8. doi: 10.1182/blood-2011-08-375535
28. Liu J, Yang H, Xu X, Yi S, Meng L. Mutations in the BCR-ABL1 kinase domain in patients with chronic myeloid leukaemia treated with TKIs or at diagnosis. Oncol Lett (2020) 20(2):1071–6. doi: 10.3892/ol.2020.11650
29. Deininger MW, Hodgson JG, Shah NP, Cortes JE, Kim DW, Nicolini FE, et al. Compound mutations in BCR-ABL1 are not major drivers of primary or secondary resistance to ponatinib in CP-CML patients. Blood (2016) 127(6):703–12. doi: 10.1182/blood-2015-08-660977
30. Zabriskie MS, Eide CA, Tantravahi SK, Vellore NA, Estrada J, Nicolini FE, et al. BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in ph chromosome-positive leukemia. Cancer Cell (2014) 26(3):428–42. doi: 10.1016/j.ccr.2014.07.006
31. Dhillon S. Olverembatinib: First approval. Drugs (2022) 82(4):469–75. doi: 10.1007/s40265-022-01680-9
32. Ren X, Pan X, Zhang Z, Wang D, Lu X, Li Y, et al. Identification of GZD824 as an orally bioavailable inhibitor that targets phosphorylated and nonphosphorylated breakpoint cluster region-Abelson (Bcr-abl) kinase and overcomes clinically acquired mutation-induced resistance against imatinib. J Med Chem (2013) 56(3):879–94. doi: 10.1021/jm301581y
33. Wang Y, Zhang L, Tang X, Luo J, Tu Z, Jiang K, et al. GZD824 as a FLT3, FGFR1 and PDGFRα inhibitor against leukemia in vitro and in vivo. Transl Oncol (2020) 13(4):100766. doi: 10.1016/j.tranon.2020.100766
34. Fang DD, Zhu H, Tang Q, Wang G, Min P, Wang Q, et al. FLT3 inhibition by olverembatinib (HQP1351) downregulates MCL-1 and synergizes with BCL-2 inhibitor lisaftoclax (APG-2575) in preclinical models of FLT3-ITD mutant acute myeloid leukemia. Transl Oncol (2021) 15(1):101244. doi: 10.1016/j.tranon.2021.101244
35. Ye W, Jiang Z, Lu X, Ren X, Deng M, Lin S, et al. GZD824 suppresses the growth of human b cell precursor acute lymphoblastic leukemia cells by inhibiting the SRC kinase and PI3K/AKT pathways. Oncotarget (2017) 8(50):87002–15. doi: 10.18632/oncotarget.10881
36. Liu X, Wang G, Yan X, Qiu H, Min P, Wu M, et al. Preclinical development of HQP1351, a multi-kinase inhibitor targeting a broad spectrum of mutant KIT kinases, for the treatment of imatinib-resistant gastrointestinal stromal tumors. Cell Biosci (2019) 9:88. doi: 10.1186/s13578-019-0351-6
37. Jiang Q, Li Z, Qin Y, Li W, Xu N, Liu B, et al. Olverembatinib (HQP1351), a well-tolerated and effective tyrosine kinase inhibitor for patients with T315I-mutated chronic myeloid leukemia: Results of an open-label, multicenter phase 1/2 trial. J Hematol Oncol (2022) 15(1):113. doi: 10.1186/s13045-022-01334-z
38. Lu M, Deng C, Xiong Y, Wang H, Xu P, Men L, et al. Exposure-response (E-r) analysis of olverembatinib (HQP1351) in Chinese patients with chronic myeloid leukemia (CML). Blood (2020) 136(Suppl 1):5–6. doi: 10.1182/blood-2020-141268
39. Vener C, Banzi R, Ambrogi F, Ferrero A, Saglio G, Pravettoni G, et al. First-line imatinib vs second- and third generation TKIs for chronic-phase CML: A systematic review and meta-analysis. Blood Adv (2020) 4(12):2723–35. doi: 10.1182/bloodadvances.2019001329
40. Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L, et al. Nilorinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, philadlephia chromosome-positive, chronic myeloid leukemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol (2011) 12:841–51. doi: 10.1016/S1470-2045(11)70201-7
41. Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med (2010) 362:2260–70. doi: 10.1056/NEJMoa1002315
42. Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. (2020) 34(4):966–84. doi: 10.1038/s41375-020-0776-2
43. Monestime S, Al Sagheer T, Tadros M. Asciminib (Scemblix): A third-line treatment option for chronic myeloid leukemia in chronic phase with or without T315I mutation. Am J Health Syst Pharm (2022), zxac286. doi: 10.1093/ajhp/zxac286
44. Baccarani M, Castagnetti F, Gugliotta G, Palandri F, Rosti G. Treatment recommendations for chronic myeloid leukemia. Mediterr J Hematol Infect Dis (2014) 6(1):e2014005. doi: 10.4084/MJHID.2014.005
45. Anagnostou T, Litzow MR. Spotlight on ponatinib in the treatment of chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia: Patient selection and perspectives. Blood Lymphat Cancer (2017) 8:1–9. doi: 10.2147/BLCTT.S130197
46. Copland M, Slade D, McIlroy G, Horne G, Byrne JL, Rothwell K, et al. Ponatinib with fludarabine, cytarabine, idarubicin, and granulocyte colony-stimulating factor chemotherapy for patients with blast-phase chronic myeloid leukaemia (MATCHPOINT): A single-arm, multicenter, phase 1/2 trial. Lancet Haematol (2022) 9(2):e121–32. doi: 10.1016/S2352-3026(21)00370-7
47. Soverini S, Branford S, Nicolini FE, Talpaz M, Deininger MW, Martinelli G, et al. Implications of BCR-ABL1 kinase domain-mediated resistance in chronic myeloid leukemia. Leuk Res (2014) 38(1):10–20. doi: 10.1016/j.leukres.2013.09.011
48. Redaelli S, Mologni L, Rostagno R, Piazza R, Magistroni V, Ceccon M, et al. Three novel patient derived BCR/ABL mutants show different sensitivity to second and third generation tyrosine kinase inhibitors. Am J Hematol (2012) 87(11):E125–8. doi: 10.1002/ajh.23338
49. Soverini S, Hochhaus A, Nicolini FE, Gruber F, Lange T, Saglio G, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: Recommendations from an expert panel on behalf of European LeukemiaNet. Blood (2011) 118(5):1208–15. doi: 10.1182/blood-2010-12-326405
Keywords: olverembatinib, chronic myeloid leukemia, tyrosine kinase inhibitors, breakpoint cluster region protein and abelson tyrosine-protein kinase 1, T315I mutation
Citation: Qian H, Gang D, He X and Jiang S (2022) A review of the therapeutic role of the new third-generation TKI olverembatinib in chronic myeloid leukemia. Front. Oncol. 12:1036437. doi: 10.3389/fonc.2022.1036437
Received: 04 September 2022; Accepted: 16 November 2022;
Published: 08 December 2022.
Edited by:
Shimin Hu, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Mengxing Xue, The First Affiliated Hospital of Soochow University, ChinaGianni Binotto, University of Padua, Italy
Daniele Cattaneo, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, Italy
Copyright © 2022 Qian, Gang, He and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Songfu Jiang, amlhbmdzb25nZnVGRkBob3RtYWlsLmNvbQ==
 Honglan Qian
Honglan Qian Dongxu Gang
Dongxu Gang Songfu Jiang
Songfu Jiang