- 1Department of Surgery, Division of Breast Surgery, Istituti di Ricovero e Cura a Carattere Scientifico (IRCCS) Regina Elena National Cancer Institute, Rome, Italy
- 2Hematology Unit, Department of Research and Clinical Oncology, Istituti di Ricovero e Cura a Carattere Scientifico (IRCCS) Regina Elena National Cancer Institute, Rome, Italy
- 3Department of Applied Clinical Sciences and Biotechnology, University of L’Aquila, L’Aquila, Italy
- 4Pathology Department, Istituti di Ricovero e Cura a Carattere Scientifico (IRCCS) Regina Elena National Cancer Institute, Rome, Italy
- 5Department of Surgery, Division of Plastic and Reconstructive Surgery, Istituti di Ricovero e Cura a Carattere Scientifico (IRCCS) Regina Elena National Cancer Institute, Rome, Italy
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a rare T-cell lymphoma associated with textured breast implants. The most common presentation is a periprosthetic seroma that occurs at least 1 year after an aesthetic or reconstructive implantation, and in these cases, the surgical treatment seems to be successful. More rarely, BIA-ALCL presents with locally advanced mass-formed disease and a related regional lymph node involvement. In all these cases with worse prognosis, a multidisciplinary approach is required, including adjuvant chemotherapy, radiation therapy, and surgery. We present a clinical case of a 49-year-old woman who developed on the left side of the breast a mass-formed stage 3 BIA-ALCL 15 years after a bilateral breast augmentation with textured silicone implant. Our multidisciplinary team (MDT) scheduled the patient for a “reverse-strategy” sequential approach consisting of induction chemotherapy, hematopoietic stem cell mobilization, and harvest followed by autologous stem cell transplant (ASCT). After 100 days from the stem cell transplant, the patient showed a complete pathologic response and was a candidate for radical surgery. She underwent removal of both implants with total en bloc capsulectomy. On the left site, the periprosthetic mass was also en bloc removed. We did not perform any axillary dissection. Our surgical and hemato-oncological teams followed the patient every 3 months, and no local or systemic recurrences were observed 24 months after surgery. This case report has demonstrated the effectiveness of neoadjuvant chemotherapy as part of a “reverse strategy” in selected cases of advanced-stage BIA-ALCL in which it was not possible to perform an immediate radical surgery. Furthermore, in our case, the de-escalation strategy adopted permitted a less demolitic surgery with good functional and aesthetic results.
Introduction
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a recently recognized non-Hodgkin lymphoma of T-cell origin. Despite its low incidence, the increasing use of breast implants for aesthetic reconstruction or post-mastectomy purposes poses BIA-ALCL as an emerging medical challenge (1). All clinical case reports have demonstrated a strong relationship between BIA-ALCL and textured breast implants.
Although most patients with BI-ALCL have a relatively indolent clinical course presenting as a delayed effusion or persistent seroma around the implants, one-third of the cases develop a peri-capsular mass and a small number of lymph-node involvement or distant disease (2, 3).
The National Comprehensive Cancer Network (NCCN) guidelines and the UK guidelines on the Diagnosis and Treatment of BIA-ALCL suggest that during the early stage, surgical removal of the implant with the surrounding capsule intact usually has a curative effect. For mass-forming cases, surgery was also indicated with a total en bloc capsulectomy with removal of any associated mass and abnormal regional lymph nodes followed by adjuvant chemotherapy and/or radiotherapy (4, 5). In advanced-stage patients, the role of neoadjuvant therapy has not been investigated yet.
We report a case of a woman with locally advanced BIA-ALCL treated with an unconventional combined approach in which a sequential chemotherapeutic program was applied in a neoadjuvant setting (reverse strategy).
Case report
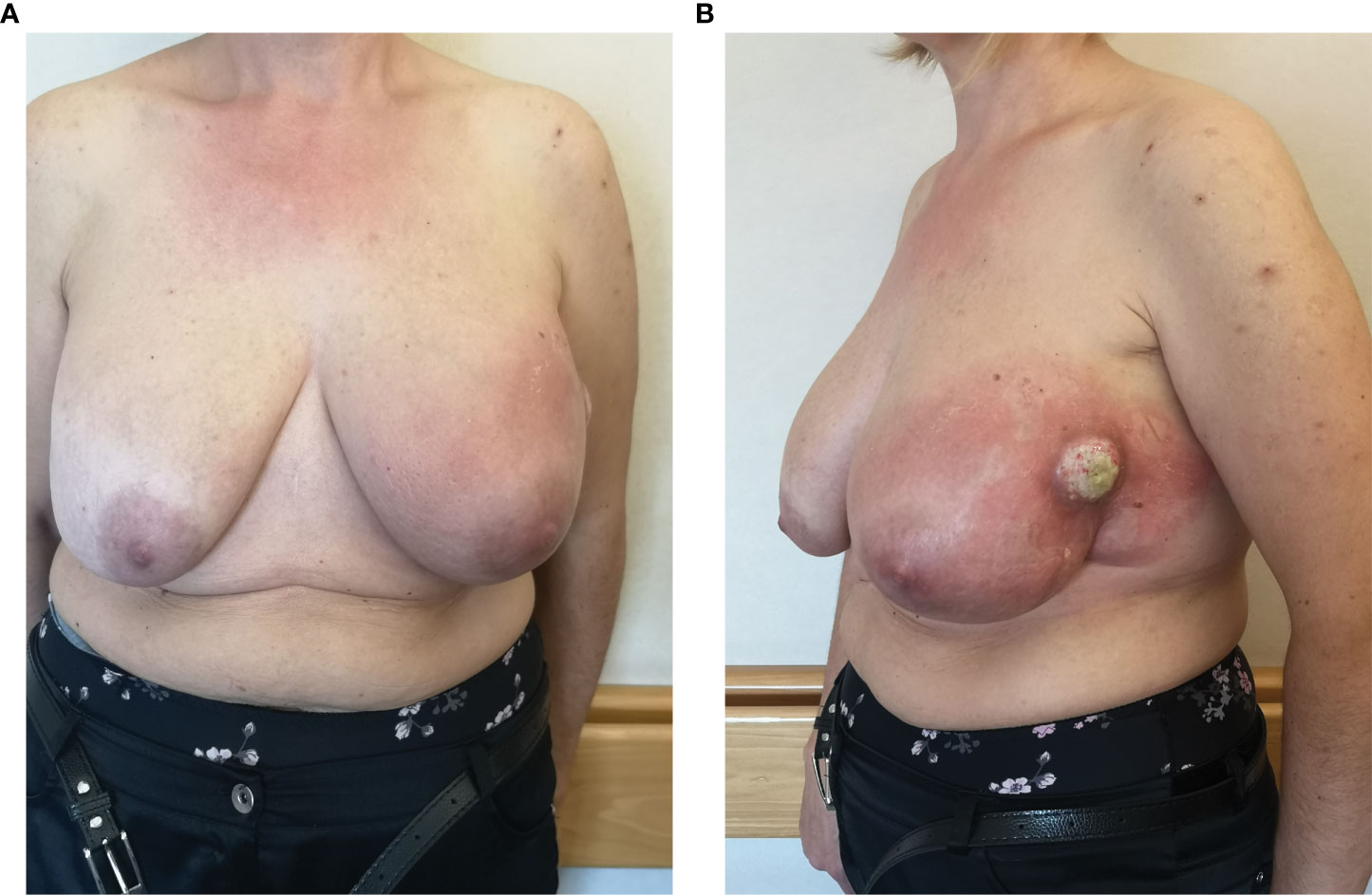
A 49-year-old woman with a history of bilateral breast augmentation with silicone implant macro textured 15 years earlier presented with the appearance of a palpable large mass associated with a volumetric increase of the left breast. She had no postoperative complications or previous trauma to the breast, nor did she undergo any additional breast surgery before presentation to the clinic. The patient did not show any significant previous clinical history or relevant comorbidities. Clinical examination revealed swelling, erythema, palpable mass, and peau d’orange on the left breast (Figures 1A, B). The patient underwent breast ultrasound and magnetic resonance imaging. The latter showed signs of intracapsular ruptures.
On the same side, it had morbid tissue with a necrotic component localized in the external quadrant extending from the skin to the muscle layer (4 × 8 cm); the local swelling and the increase in skin thickness affect all the breast tissue. In addition, suspicious axillary lymph nodes have been reported in the same side. Given the complexity of the case, the patient was sent to our Breast Surgery Department of the “Regina Elena National Cancer Institute” in Rome, considered as a reference point in central-southern Italy for breast cancer (6, 7). We subjected the patient to 18-FDG positron emission tomography/computed tomography scan (PET/TC), which showed a focal increase of the left breast tissue intake (SUV max 39.4) going from the skin to the muscular layer of the outer quadrants and of the axillary and retro-pectoral lymph nodes (SUV max 7.8; dmt max 26 mm). Clinical and radiographic evaluations of the right breast did not show any abnormalities. The patient was subjected to fine needle aspiration of mass; cytology exam showed round, large cells with pleomorphic and cerebroid nuclei (CD45+++, Vimentin++, Keratin−). The following percutaneous biopsy of the mass demonstrated a proliferation of phenotypically aberrant population of large cells that expressed CD30 and CD45, negative for CD3, CD20, and ALK1, diagnostic for anaplastic large cell lymphoma, ALK-negative (ALK-ALCL) (Figures 2A, B) (8).
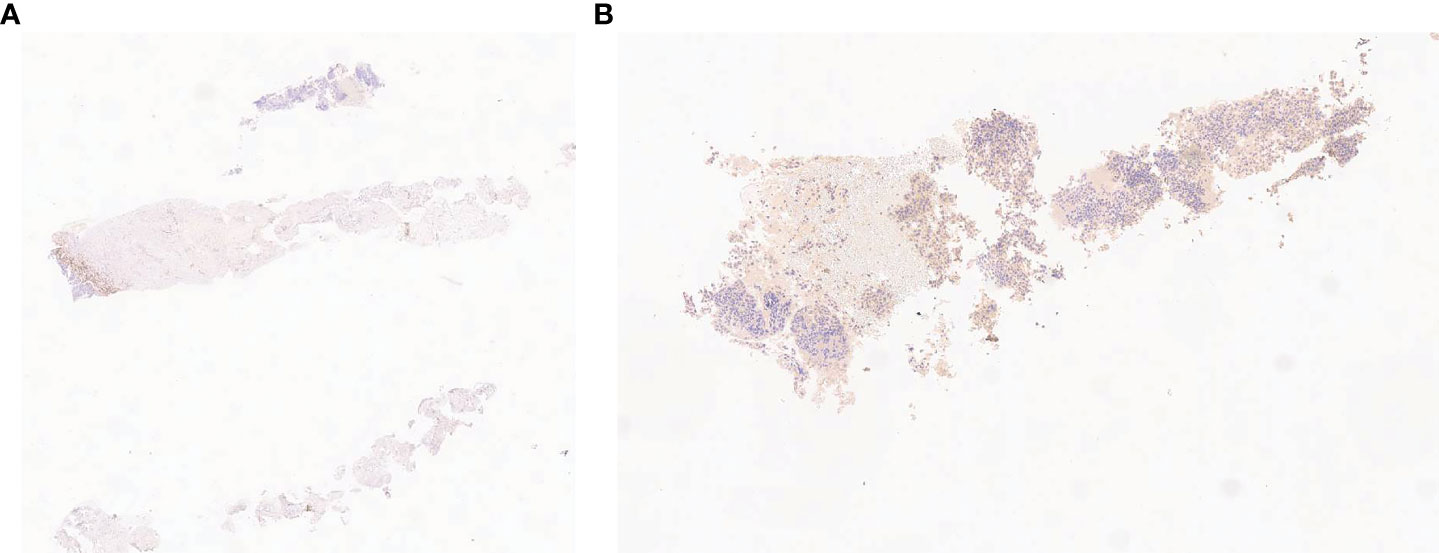
Figure 2 Fine needle cytology: neoplastic proliferation of large round cells with pleomorphic nuclei, negative for epithelial marker CK (A) and positive for linfoid marker and vimentin (B).
Following a multi-disciplinary tumor board discussion, involving hematologists, surgeons, pathologists, and radiotherapists, an immediate surgical treatment was considered not indicated, as a consequence of the extremely advanced disease stage not allowing a minimally invasive surgical intervention. After the administration of informed consent, we decided first to start a sequential chemotherapeutic approach consisting of induction chemotherapy, hematopoietic stem cell mobilization, and harvest followed by autologous stem cell transplant (ASCT), according to our institutional policy and international guidelines about treatment of advanced-stage ALK-negative peripheral T-cell lymphoid malignancies (9–12). In particular, induction chemotherapy consisted of six courses of alternating CHOEP (cyclophosphamide, vincristine, doxorubicin, etoposide, and prednisone; three cycles) and DHAP (cisplatin, cytarabin, and dexamethasone; three cycles). Hematopoietic stem cells were collected after the last DHAP cycle (CD34+ × 106/kg collected: 7.024). At the end of the induction phase, a disease assessment performed by a CT/PET scan showed a complete remission. Afterwards, a consolidation treatment with ASCT after BEAM conditioning regimen (BCNU, etoposide, cytarabine, and melphalan) was finally performed (Figures 3A, B).
One hundred days after the stem cell transplant, she underwent removal of both implants with total en bloc capsulectomy. On the left, the site of the periprosthetic mass was also removed (Figures 4A, B).

Figure 4 Surgical specimen: implants with total en bloc capsulectomy (A) and periprosthetic tissue previously involved by BIA-ALCL (B).
We did not perform any axillary dissection. The histology, performed as reported by Lyapichev et al. (13), revealed a complete pathologic response with CD30 immunohistochemistry negative (Figures 5A, B).
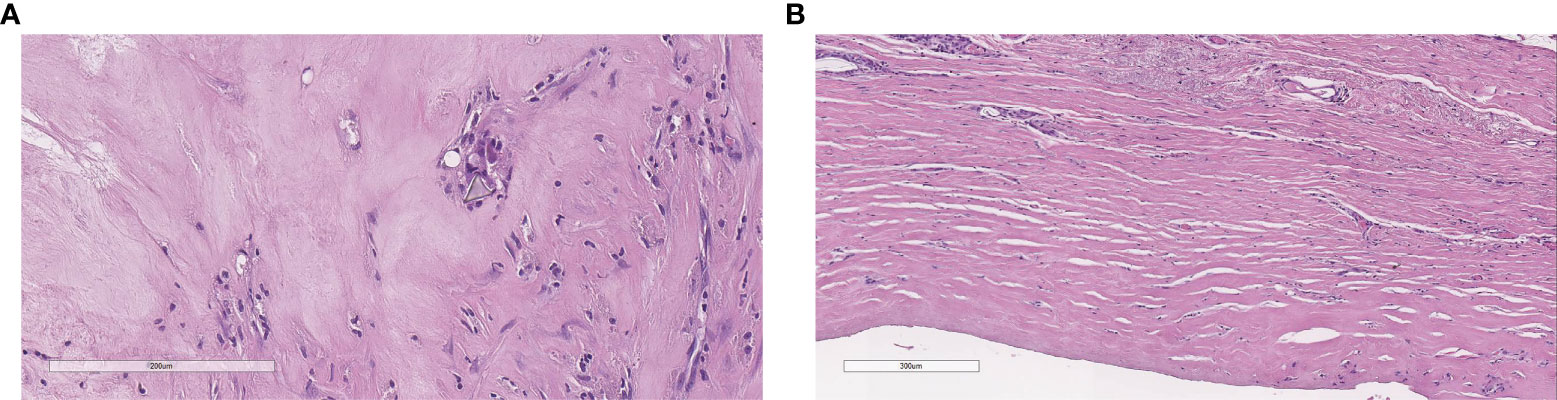
Figure 5 Complete pathological response: histology does not show neoplastic cells with a polyurethane crystal and rare inflammatory cells (A), flat synovial metaplasia, and foreign body giant cell granulomas (B).
Given his complete pathological response, multidisciplinary consensus did not consider adjuvant radiotherapy necessary. The patient was followed by our surgical and hemato-oncological teams every 3 months and no local or systemic recurrences were observed 24 months after surgery.
Discussion
BIA-ALCL has been identified as an emerging disease entity and represents a novel variant of the clinic pathologic subtypes of anaplastic large cell lymphoma (ALCL) associated with breast implants with a textured outer. Since its first description in 1997 by Keech and Creech (1, 14), an increasing number of cases have been reported in literature (15), and recently, the World Health Organization has listed BIA-ALCL as a unique pathological entity (16).
The most common presentation of BIA-ALCL is a large spontaneous periprosthetic fluid collection occurring at least 1 year and on the average from 7 to 10 years following the implantation with a textured surface breast implant. In addition to large fluid collections and delayed seromas, 8% to 24% of patients present an associated palpable mass and 4% to 12% present with lymphadenopathy (4). Less than 5% of the cases are unfrequently described and manifest local and systemic signs, such as rash, fever, and capsular contracture (17).
Surgical excision is the established standard of care to treat the localized disease, but the BIA-ALCL treatment algorithm suggested by international guidelines is limited in case of unusual, progressive, or complex patient scenarios (18, 19). This smaller subset of patients that present with a tumor mass associated with the fibrous capsule or lymph node involvement are more likely to have worse prognosis. Miranda et al. found that only 72% of patients with tumor masses achieved remission, as opposed to 93% when disease was confined to the fibrous capsule (20). Ferrufino-Schmidt et al. found out a significant reduction in 5-year overall survival in patients with lymph node involvement compared to patients who are not clinically or radiologically suspected to have lymph node disease (75% vs. 97.8%) (21).
Our clinical case falls into one of those advanced BIA-ALCL stages (stage III) (13, 22) with worse prognosis. The patient had a large mass forming on the left side extending from the skin to the muscle layer and suspected ipsilateral axillary lymph nodes.
Our multidisciplinary oncology board, which involved hematologists, surgeons, pathologists, and radiotherapists, did not consider the patient as a candidate for immediate surgery due to the advanced stage of the disease. Such an intervention would have been extremely destructive and not safe. Keeping in mind the importance of obtaining safe negative surgical margins and conservative surgery with oncoplastic techniques, the patient was scheduled to administer chemotherapy and ASCT before surgery, reversing the commonly recommended strategy (reverse strategy).
In accordance with our institutional policy and international guidelines about treatment of advanced-stage ALK-negative peripheral T-cell lymphoid malignancies (9–12), patients were submitted to induction chemotherapy by six courses of alternating CHOEP and DHAP. Finally, a consolidation treatment with ASCT after BEAM conditioning regimen was performed. The chemotherapeutic regimen used in our patients is in line with the regimen proposed in literature to treat advanced BIA-ALCL in an adjuvant setting (4, 5). At the end of the induction phase, a clinical and radiological restaging manifested a complete remission of the disease.
Keeping in mind the pivotal role of “free” surgical margins to reduce local recurrences (22), the surgical procedure performed on treated tissue allows for a less destructive surgery without compromising its radical nature. In our case, a radiological complete remission was obtained, 100 days from the stem cell transplant; the patient underwent bilateral capsulectomy with, on the left side, a local excision of previous pericapsular mass to ensure that no tumor was present at the margins.
Contrary to guideline recommendations, we did not perform any axillary dissection, despite the initial suspicion of multi-lymphonodal involvement. A complete response was obtained for the concomitant axillary lymph node, as expected after the medical management of common ALCL so that we avoided needless axillary dissection. Our patient was not interested in maintaining an enlarged breast and asked to avoid an immediate reconstruction with smooth implants. At follow-up, a device-free reconstruction was planned, leaving the breast in a natural-looking shape. Two other cases, in which neoadjuvant therapy has been administered to treat advanced BIA-ALCL, have been reported in the literature (18, 19).
In both cases, a complete pathological response was achieved after neoadjuvant chemotherapy, and the adjuvant radiotherapy was deemed unnecessary, following a multidisciplinary consensus.
Our case has shown that the use of neoadjuvant chemotherapy is effective in cases of advanced BIA-ALCL, when immediate surgical treatment is not indicated for the extension of the disease. In these scenarios, the reverse strategy, which involves the use of neoadjuvant chemotherapy, may obtain near-clinical complete response allowing for conservative surgery.
The incidence of BIA-ALCL has continued to rise, but consistent data regarding recommendations for patients with a more aggressive presentation of BIA-ALCL are still lacking in the literature. In this optic, the role of unconventional therapeutic strategy should be investigated. Our report underlines the important role of neoadjuvant chemotherapy in BIA-ALCL to control disease burden before tumor resection in selected cases in which radical surgery is not possible or precluded due to the general condition of the patient.
Consent to participate
Informed consent was obtained from the participant for the publication of this case report including all data and images.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Central Ethics Committee IRCCS Lazio – IRCCS I.F.O. with number RS1719/22. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SC and FM wrote the main manuscript, its acquisition data, and the interpretation. LP, FPa, and FPe contributed to data acquisition. MCI made a critical review of the article. CB and MCo were in charge of the final approval of the version to be published. The final manuscript has been read and approved by all the authors.
Funding
This work was financially supported through funding from the institutional “Ricerca Corrente” granted by the Italian Ministry of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Keech JA, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline- filled breast implant. Plast Reconstr Surg (1997) 100:554–5. doi: 10.1097/00006534-199708000-00065
2. Sheena Y, Smith S, Dua S, Morgan M, Ramakrishnan V. Current risk estimate of breast implant-associated anaplastic Large cell lymphoma in textured breast implants. Plast Reconstr Surg (2020) 145(2):446e. doi: 10.1097/PRS.0000000000006506
3. Marra A, Viale G, Pileri SA, Pravettoni G, Viale G, De Lorenzi F, et al. Breast implant-associated anaplastic large cell lymphoma: A comprehensive review. Cancer Treat Rev (2020) 84:101963. doi: 10.1016/j.ctrv.2020.101963
4. Clemens MW, Jacobsen ED, Horwitz SM. NCCN consensus guidelines on the diagnosis and treatment of breast implant-associated anaplastic Large cell lymphoma (BIA-ALCL). Aesthet Surg J (2019) 39(Suppl_1):S3–S13. doi: 10.1093/asj/sjy331
5. Turton P, El-Sharkawi D, Lyburn I, Sharma B, Mahalingam P, Turner SD, et al. UK Guidelines on the diagnosis and treatment of breast implant-associated anaplastic Large cell lymphoma on behalf of the medicines and healthcare products regulatory agency plastic, reconstructive and aesthetic surgery expert advisory group. Br J Haematol (2021) 192(3):444–58. doi: 10.1111/bjh.17194
6. Pelle F, Cappelli S, Graziano F, Piarulli L, Cavicchi F, Magagnano D, et al. Breast cancer surgery during the covid-19 pandemic: a monocentre experience from the Regina Elena national cancer institute of Rome. J Exp Clin Cancer Res (2020) 39(1):171. doi: 10.1186/s13046-020-01683-y
7. Cappelli S, Corallino D, Clementi M, Guadagni S, Pelle F, Puccica I, et al. Surgical site infections in patients undergoing breast oncological surgery during the lockdown: An unexpected lesson from the COVID-19 pandemic. G Chir (2022) 42(2):e02. doi: 10.1097/IA9.0000000000000003
8. WHO Classification of Tumours Editorial Board. Haematolymphoid tumours. In: Lyon (France): International agency for research on cancer; forthcoming, 5th ed (2002) vol. 11 WHO Classificationof tumours series. Available at: https://publications.iarc.fr.
9. El-Asmar J, Reljic T, Ayala E, Hamadani M, Nishihori T, Kumar A, et al. Efficacy of high-dose therapy and autologous hematopoietic cell transplantation in peripheral T cell lymphomas as front-line consolidation or in the Relapsed/Refractory setting: A systematic Review/Meta-analysis. Biol Blood Marrow Transplant (2016) 22(5):802–14. doi: 10.1016/j.bbmt.2015.12.004
10. Fossard G, Broussais F, Coelho I, Bailly S, Nicolas-Virelizier E, Toussaint E, et al. Role of up-front autologous stem-cell transplantation in peripheral T-cell lymphoma for patients in response after induction: an analysis of patients from LYSA centers. Ann Oncol (2018) 29(3):715–23. doi: 10.1093/annonc/mdx787
11. Park SI, Horwitz SM, Foss FM, Pinter-Brown LC, Carson KR, Rosen ST, et al. The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: Report from COMPLETE, a prospective, multicenter cohort study. Cancer (2019) 125(9):1507–17. doi: 10.1002/cncr.31861
12. D'Amore F, Gaulard P, Trümper L, Corradini P, Kim WS, Specht L, et al. Peripheral T-cell lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2015) 26(Suppl 5):v108–15. doi: 10.1093/annonc/mdv201
13. Lyapichev KA, Piña-Oviedo S, Medeiros LJ, Evans MG, Liu H, Miranda AR, et al. A proposal for pathologic processing of breast implant capsules in patients with suspected breast implant anaplastic large cell lymphoma. Modern Pathol (2020) 33(3):367–79. doi: 10.1038/s41379-019-0337-2
14. Lyapichev KA, Medeiros LJ, Clemens MW, Ferrufino-Schmidt MC, Marques-Piubelli ML, Chai SM, et al. Reconsideration of the first recognition of breast implant-associated anaplastic large cell lymphoma: A critical review of the literature. Ann Diagn Pathol (2020) 45:151474. doi: 10.1016/j.anndiagpath.2020.151474
15. Wang Y, Zhang Q, Tan Y, Lv W, Zhao C, Xiong M, et al. Current progress in breast implant-associated anaplastic Large cell lymphoma. Front Oncol (2022) 11:785887. doi: 10.3389/fonc.2021.785887
16. WHO Classification of Tumours Editorial Board. Breast tumours. In: Lyon (France): International agency for research on cancer, 5th ed, vol. 2. (2019) WHO Classificationof tumours series. Available at: https://publications.iarc.fr.
17. Clemens MW, Brody GS, Mahabir RC, Miranda RN. How to diagnose and treat breast implant-associated anaplastic Large cell lymphoma. Plast Reconstr Surg (2018) 141(4):586e–99e. doi: 10.1097/PRS.0000000000004262
18. Caputo GG, Alban A, D'Alì L, Mariuzzi L, Galvano F, Parodi PC. Locally advanced breast implant-associated anaplastic large-cell lymphoma: a combined medical-surgical approach. Eur Rev Med Pharmacol Sci (2021) 25(9):3483–8. doi: 10.26355/eurrev_202105_25830
19. Thibodeau R, Fan KL, Wehner PB. Stage IV breast implant-associated anaplastic Large-cell lymphoma with complete pathologic response to neoadjuvant chemotherapy. Plast Reconstr Surg Glob Open (2019) 7(9):e2446. doi: 10.1097/GOX.0000000000002446
20. Miranda RN, Aladily TN, Prince HM, Kanagal-Shamanna R, De Jong D, Fayad LE, et al. Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol (2014) 32(2):114–20. doi: 10.1200/JCO.2013.52.7911
21. Ferrufino-Schmidt MC, Medeiros LJ, Liu H, Clemens MW, Hunt KK, Laurent C, et al. Clinicopathologic features and prognostic impact of lymph node involvement in patients with breast implant-associated anaplastic Large cell lymphoma. Am J Surg Pathol (2018) 42(3):293–305. doi: 10.1097/PAS.0000000000000985
Keywords: breast implant-associated anaplastic large cell lymphoma (BIA-ALCL), reverse-strategy, neoadjuvant chemotherapy, stem cell transplant, conservative surgery
Citation: Cappelli S, Marchesi F, Clementi M, Perracchio L, Palombi F, Pelle F, Botti C and Costantini M (2023) Reverse strategy to locally advanced breast implant-associated anaplastic large cell lymphoma: A case report. Front. Oncol. 12:1062389. doi: 10.3389/fonc.2022.1062389
Received: 05 October 2022; Accepted: 14 December 2022;
Published: 10 January 2023.
Edited by:
Riccardo Bertolo, Hospital San Carlo di Nancy, ItalyReviewed by:
Kirill Lyapichev, University of Texas Medical Branch at Galveston, United StatesMatteo Vittori, Hospital San Carlo di Nancy, Italy
Copyright © 2023 Cappelli, Marchesi, Clementi, Perracchio, Palombi, Pelle, Botti and Costantini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Clementi, bWFyY28uY2xlbWVudGlAdW5pdmFxLml0
†ORCID: Marco Clementi, orcid.org/0000-0002-9652-5148
 Sonia Cappelli1
Sonia Cappelli1 Francesco Marchesi
Francesco Marchesi Marco Clementi
Marco Clementi Claudio Botti
Claudio Botti Maurizio Costantini
Maurizio Costantini