- 1Collaborative Innovation Center for Cancer Medicine, State Key Laboratory of Oncology in South China, Guangzhou, China
- 2Department of Pediatric Oncology, Sun Yat-sen University Cancer Center, Guangzhou, China
Purpose: The combination of irinotecan, temozolomide and vincristine has been proposed as an effective salvage regimen for some pediatric malignancies. Thus, we sought to evaluate this combination for patients with relapsed and refractory neuroblastoma (NB).
Patients and Methods: In this retrospective study, forty-six patients with relapsed or refractory NB were treated with the combination of vincristine (1.5 mg/m2 i.v. day 1), irinotecan (50 mg/m2/day i.v. days 1–5) and temozolomide (100 mg/m2/day p.o. days 1–5) (VIT) during the period 2011–2019. All toxicities were documented.
Results: A total of 251 cycles (median 6 cycles/patient) were administered. A complete response (CR) was achieved in 5 patients, partial response (PR) in 27 patients, stable disease (SD) in 8 patients, and progression disease (PD) in 6 patients, with an overall objective response rate (CR+PR) of 69.6%. Eighteen patients developed diarrhea with Grade 3 or less. Grade 1-2 hematologic toxicity occurred in 10 patients. Grade 3-4 hematologic toxicity developed in 32 patients. VIT was an effective regimen for different metastatic sites. UGT1A*28 genotyping performed in 7 patients revealed wild type. Diarrhea occurred in 4 of them.
Conclusion: The shorter, 5-day VIT regimen is an active and well-tolerated salvage regimen in relapse/refractory NB.
Introduction
Neuroblastoma is the most common extracranial solid tumor in childhood. Although most of these patients were sensitive to chemotherapy, the long-term outcome of high-risk patients has remained dismal with 5-year overall survival (OS) rates less than 50%. Salvage therapy could prolong survival in multiply relapsed patients. Patients with relapsed or recurrent neuroblastoma at the Sun Yat-Sen University Cancer Center received chemotherapy comprising vincristine, irinotecan, and temozolomide (VIT therapy) from 2011 as salvage chemotherapy. In this study we retrospectively analyzed the therapeutic effects, and toxicity of VIT in patients with relapsed or refractory neuroblastoma.
Materials and Methods
Patients and Treatment
We retrospectively retrieved information from the medical charts of patients treated in Sun Yat-sen University Cancer Center between June 1, 2011 and March 31, 2019, who experienced relapse or progression after first-line therapy. All of them received a combination regimen of vincristine, irinotecan, and temozolomide (VIT) as the salvage therapy. VIT comprised irinotecan 50 mg/m2 intravenously (IV) on days 1–5 (250 mg/m2/course), temozolomide 100 mg/m2/dose orally on days 1–5 approximately 1 hour before irinotecan administration, and vincristine 0.05 mg/kg or 1.5 mg/m2 (maximum dose 2 mg) IV over one minute on day 1 for 21 days per cycle. For patients unable to swallow capsules, temozolomide was allowed to mix with apple sauce or juice in 100 ml. Every cycle was repeated at three-week interval. Patients received atropine 0.01mg/kg subcutaneously 30 minutes before irinotecan administration. Cefixime was administered for patients who experienced ≥grade 3 diarrhea before next chemotherapy cycle. Loperamide was started immediately after >grade 2 diarrhea occurred as follows: 2mg at initial dose followed by 1mg every 2 hours thereafter for patents younger than 6 years of age; 4mg at initial dose, followed by 2mg, every 2 hours thereafter for patents older than 6 years of age. Loperamide continued to be administered at 6 doses after diarrhea improved. Total courses didn’t exceed 48 hours. For each patient, we collated demographic, disease-related, treatment-related and outcome data, such as treatment course, acute and late toxicity, disease status, place of primary and metastatic tumor, regimen of chemotherapy, response, and clinical follow-up.
Assessment of Response
The response was evaluated using imaging technologies such as computed tomography or magnetic resonance imaging, bone marrow aspirates, serum neuron-specific enolase (NSE), the urine vanillic mandelic acid (VMA)-urine creatinine ratio and urinary homovanillic acid (HVA)- urine creatinine ratio every two courses and three weeks after completion of treatment. Recommendations of radiological assessment comprised computed tomography, magnetic resonance imaging, 18F-FDG PET/CT, 68Ga DOTATATE and SPECT. Bone marrow trephine was performed at initial diagnosis. If the examination was positive, we would perform bone marrow trephine after completion of VIT chemotherapy. 123I-MIBG scan was not available in our center during the period. Response Evaluation Criteria in Solid Tumors (RECIST) were used to evaluate tumor response at the end of cycles 2, 4, 6 and 8 according to World Health Organization (WHO) criteria. Response was defined as per COG criteria as follows: complete response (CR): the disappearance of all target lesions, partial response (PR): at least 50% decrease in all measurable lesions, progressive disease (PD): at least 25% increase in the size of any lesions or development of new lesions, stable disease (SD): 0 to <25% increase or decrease in lesions. Objective response rate (ORR) was defined as follows: number of (CR+PR)/total number of evaluable patients. Toxicity was graded according to NCI Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0.
Statistical Analysis
Overall survival after relapse/progression was defined from the date of the first cycle of VIT to death from any cause, or censored at the date of last follow-up for the patients who were alive. Progression-free survival (PFS) was defined as the interval between the beginning of VIT treatment until treatment failure, death, treatment discontinuation for any reason, or detection of second neoplasm. Relapse-free survival (RFS) was defined as the interval between the status of complete response achieved by VIT until treatment failure, death, treatment discontinuation for any reason, or detection of second neoplasm. The RFS and PFS were calculated with the Kaplan–Meier method by using SPSS Statistics, version 25.0 (IBM Corp., Armonk, NY, USA). All tests were two-tailed and P < 0.05 was considered statistically significant.
Ethical Approval
The present study was approved by the Ethics Board of the Sun Yat-sen University Cancer Center and conducted in accordance with the Helsinki Declaration. All original data were deposited on http://www.researchdata.org.cn (RDD number RDDA2019001318).
Results
Patient Characteristics
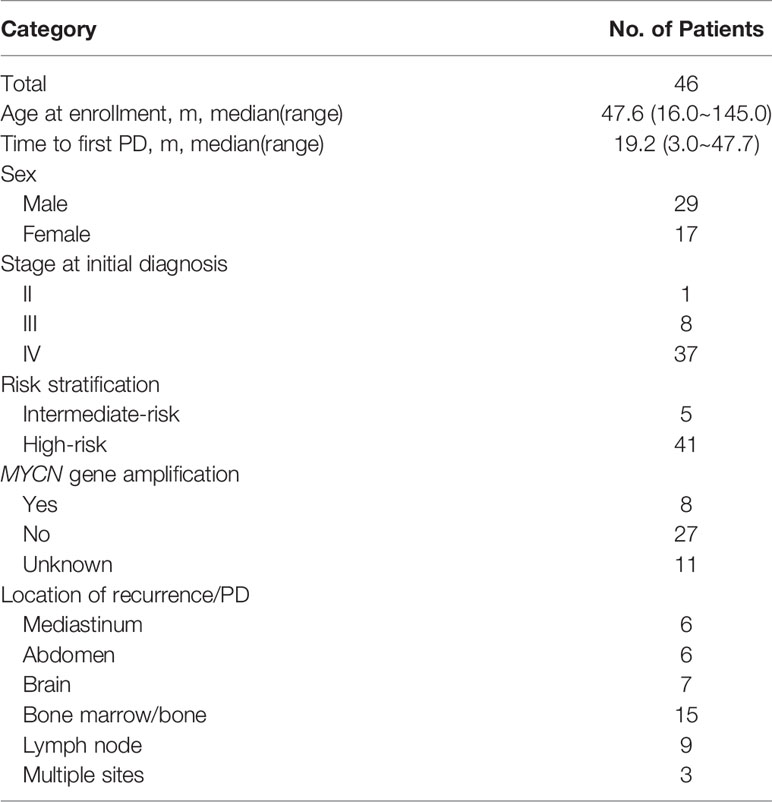
At SYSUCC, 46 children received VIT as salvage chemotherapy in refractory/relapsed (PD/Re) setting. There were 29 boys (63.0%) and 17 girls (37.0%) with a median age of 47.6 (range, 16.0 – 145.0) months. The International Neuroblastoma Staging System stage distribution was as follows: stage II: 1(2.2%) patient, stage III: 8 (17.4%) patients and stage IV: 37 (80.4%) patients. Five patients were stratified into intermediate-risk group and 41 were categorized into high-risk group at initial diagnosis on the basis of the following factors: stage, age, International Neuroblastoma Pathologic Classification, amplification of the MYCN oncogene within tumor tissue.
Thirty-five patients underwent MYCN amplification test on tumor specimens. MYCN amplification occurred in 8 patients and 27 patients were in the absence of MYCN amplification. Forty-six patients received first line therapy of vincristine/doxorubicin/cyclophosphamide (CAV) and ifosfamide/etoposide/cisplatin (VIP) at initial diagnosis. The median interval from the beginning date of initial diagnosis to the date of first relapse/progression was 19.2 (range, 3.0 – 47.7) months. Relapses or progression in the primary tumor occurred in 3 patients. Sites of metastases at relapse/progression were as follows: bone marrow/bone, lymph nodes, brain, mediastinum and abdomen. Patient characteristics are shown in Table 1.
Response to VIT
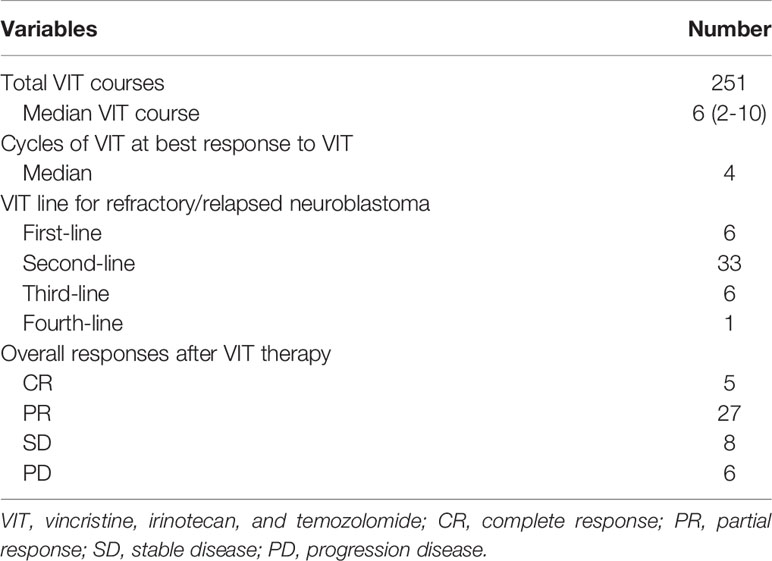
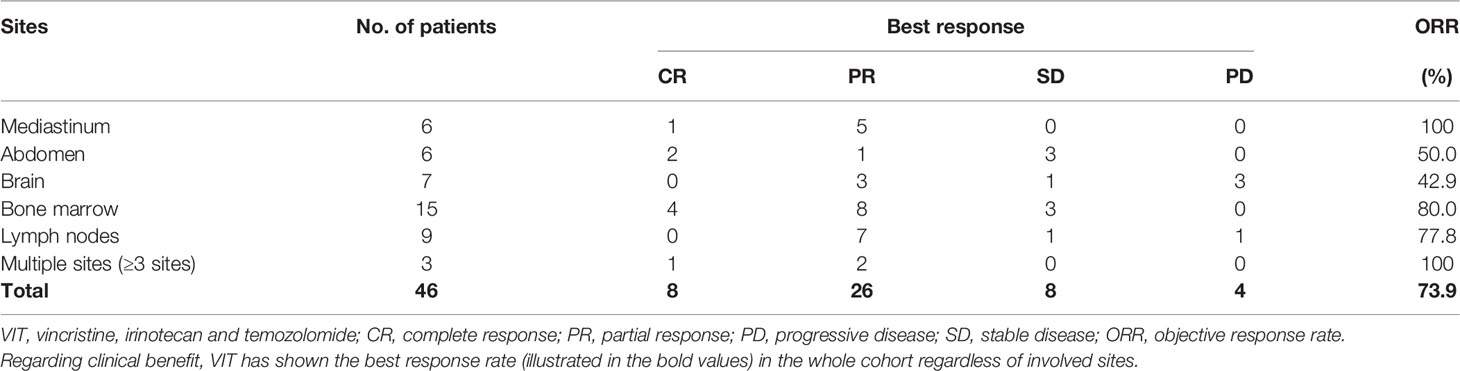
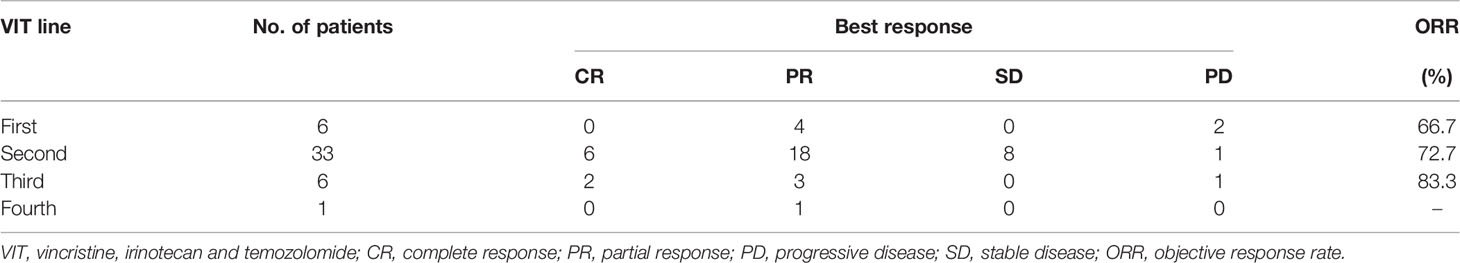
A total of 251 cycles were administered to 46 eligible patients (median of 6 cycles, range 2-10). VIT was used as first-line salvage therapy in 6 patients, second-line in 33 patients, third-line in 6 patients and fourth-line in 1 patient after progression or recurrence (Table 2). Overall response to VIT was assessed at the end of chemotherapy. Among all patients, 5 had complete response, 27 had partial response, 8 had SD, and 6 had progressive disease (Table 2). The objective response was 69.5% (complete response + partial response). The best response to VIT was achieved at the end of 4 courses for the median number of responders. During the treatment, one patient continued to have response in his lesions after 9 cycles. Further, among patients with different site involved, response rates to VIT regimen were as follows: mediastinum 100.0%, bone marrow/bone 80.0%, lymph node 77.8%, abdomen 50.0% and brain 42.9%, respectively. Thirty-four of the 46 patients (73.9%) had best responses (Table 3). In the patients who received VIT as first line, second line, third line and forth line salvage chemotherapy, the best response rates were 4/6, 24/33, 5/6 and 1/1, respectively (Table 4).
Toxicity
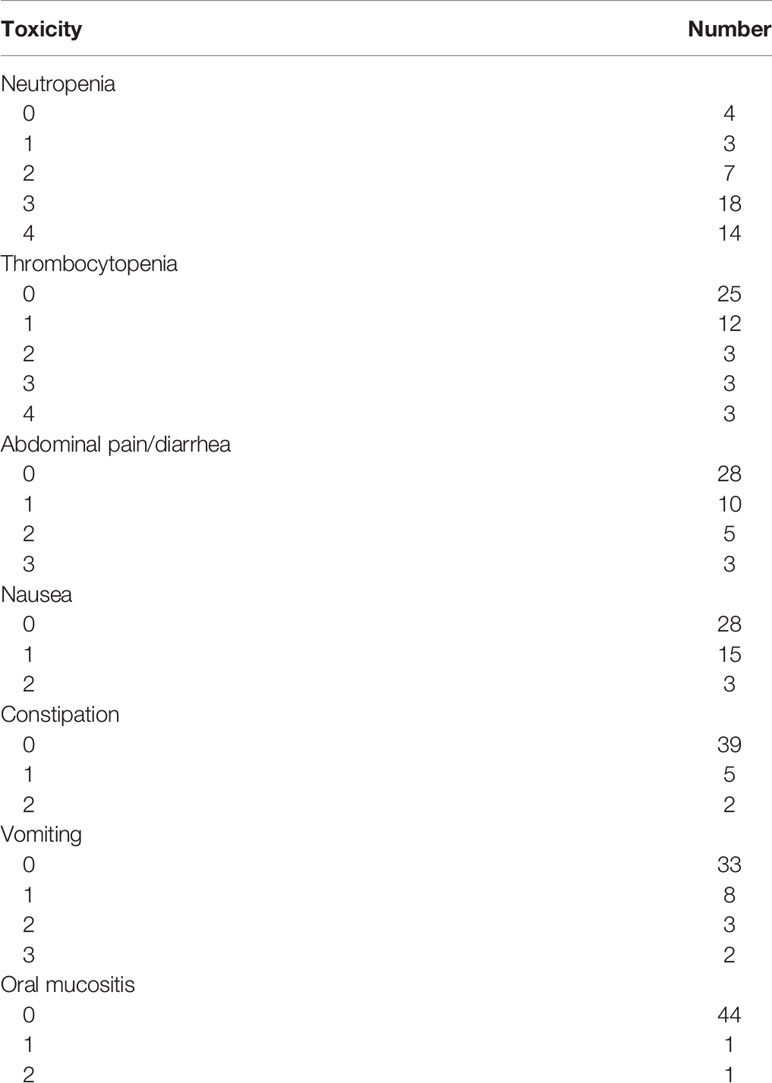
Toxicity was evaluated for the whole cohort. All toxicity results related to VIT chemotherapy were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version. Regarding neutropenia, toxicities included three patients with grade 1, seven with grade 2, eighteen with grade 3 and fourteen with grade 4, respectively. Grade 3–4 thrombocytopenia occurred in six patients (Table 5). Otherwise, non-haematologic toxicity observed included abdominal pain, diarrhea, nausea, vomiting, constipation and oral mucositis. Abdominal pain and diarrhea were the most common non-hematological adverse events. Toxicities are detailed in Table 5.
Association of UGT1A1(UDP-Glycosyltransferase 1 Polypeptide A1) Genotyping and Diarrhea
The UGT1A1*28 promoter polymorphism was genotyped by PCR amplification in seven patients. Analysis of the UGT1A1 gene revealed that the seven patients possessed a wild-type. Four patients experienced diarrheas. Two patients carried UGT1A1*6 heterozygous genotypes. One patient developed diarrhea.
Progression Free Survival After First VIT Therapy
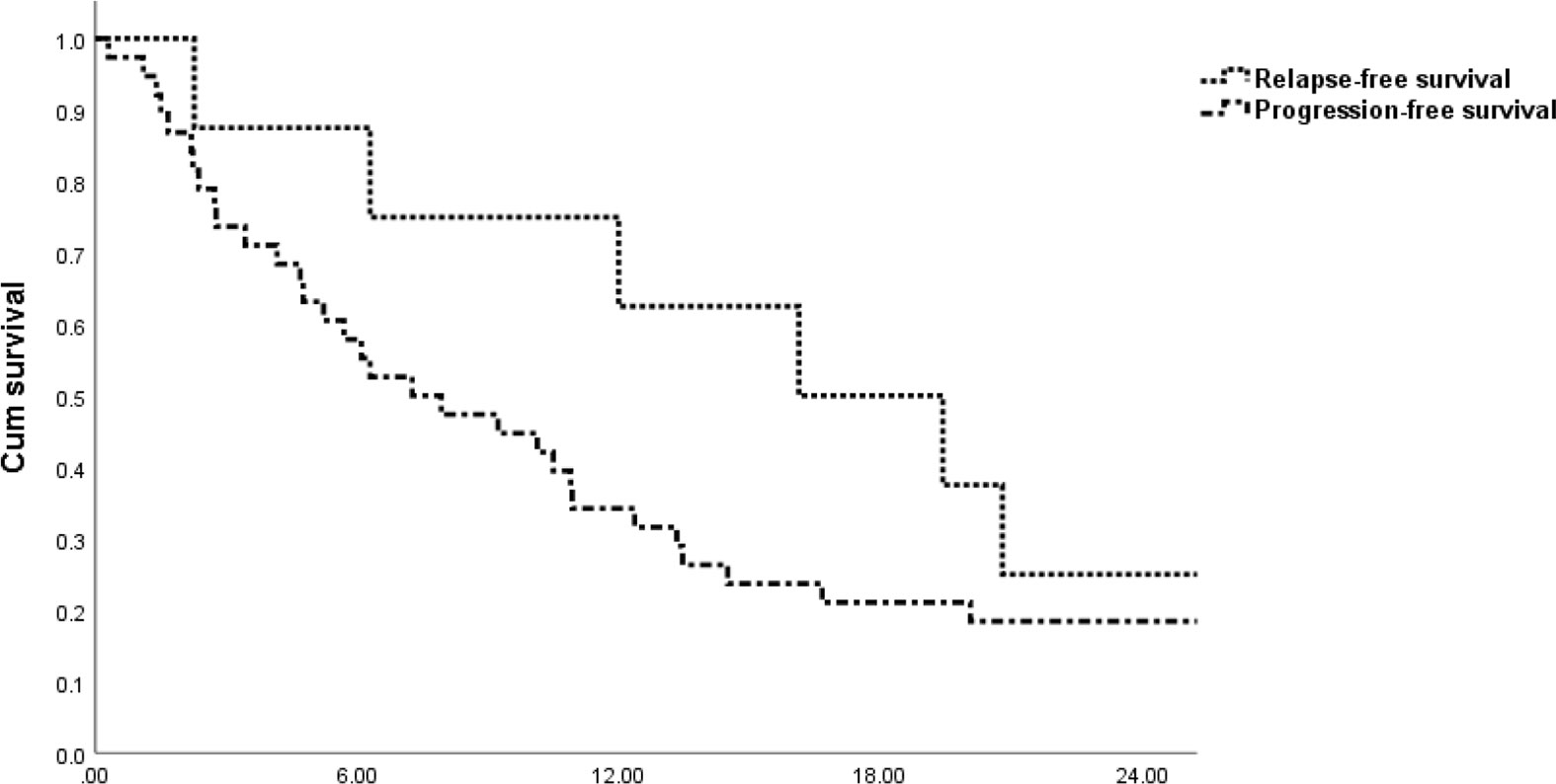
The median follow-up time was 43.9 months (range: 17.8–133.6 months) for all patients. The median RFS time and PFS time from the beginning of VIT treatment for the whole cohort were 16.1 (range, 2.3–32.0) months and 7.3 (range, 0.3-50.7) months, respectively (Figure 1).
Discussion
In our center, treatment for our patients with neuroblastoma is based on the Children’s Oncology Group protocol. Such patients received risk-stratified therapy. First-line adjuvant chemotherapy with cycles of vincristine, doxorubicin, cyclophosphamide alternating with cycles of ifosfamide, cisplatin and etoposide was administered to high-risk patients. First-line treatment included only standard dose chemotherapy courses. Each cycle was detailed as follows: vincristine 1.5 mg/m2 (2 mg maximum total dose) intravenously on days 1, doxorubicin 50 mg/m2 intravenously on day 1, cyclophosphamide 1 g/m2 intravenously on days 1-2; ifosfamide 1.5 g/m2 intravenously on days 1-4, cisplatin 25 mg/m2 intravenously on days 1-4, etoposide 100 mg/m2 intravenously on days 1-4. Every cycle was repeated at the 3-week interval. Total cycles of chemotherapy were 8 courses. Although the event-free survival of high-risk patients is still dismal, they could still achieve CR after salvage therapy. In the absence of anti-disialoganglioside immunotherapy, overall survival rates have improved owing to progress in multimodality treatment. The combination of irinotecan, and temozolomide has shown activity against relapsed or refractory neuroblastoma (1).In 19 refractory NB patients treated with VIT, 2 had CR and 7 had object responses with an objective response (CR+PR) of 47.4%. In 17 patients with progressive disease, one had PR and two had objective responses.
As a second generation alkylating agent, temozolomide is prodrug of MTIC (3-methyl-(triazen-1-yl)imidazole-4-carboxamide). Oral temozolomide has demonstrated activity for pediatric rhabdomyosarcoma (2–4). Irinotecan is a camptothecin prodrug which is converted by endogenous carboxylesterases to SN-38, a topoisomerase-I inhibitor causing inhibition of both DNA replication and transcription. The combination of temozolomide and irinotecan has shown synergistic activity against PD/Re pediatric solid tumors (5). The synergy can be explained due to DNA methylation by temozolomide and recruitment of topoisomerase I cleavage complexes, potentiating irinotecan to stabilize the DNA–enzyme complex that caused cytotoxicity of the tumor cells (6). Irinotecan is administered intravenously on days 1-5 approximately one hour after temozolomide intake in order to maximize the synergistic effect (7). Temozolomide was obtained commercially in the form of capsules. Patients were allowed to open them and mix with apple sauce or juice because of steady state in acidic environment and oral mucositis. Irinotecan was administered intravenously over 90 minutes at the dose of 50mg/m2.
The combination of irinotecan and temozolomide(IT) has demonstrated activity in various types of PD/Re solid tumors. Based on preclinical data, irinotecan was administered as protracted regimen as follows: 20mg/m2, 5 days per week for 2 consecutive weeks (daily×5×2). VIT was administered in shortened regimen every 21 days as follows: vincristine, 1.5 mg/m2 intravenously (IV) on day 1; irinotecan, 50mg/m2 IV, days 1–5; temozolomide 100mg/m2 orally, days1–5. Vincristine (1.5mg/m2, max 2mg). Study exploring irinotecan administration have shown that shorter course of 50mg/m2/day×5 days on a 3-week schedule is equivalent to the protracted schedule (6). Although several studies have shown that there was difference in efficacy between protracted regimen and shortened regimen, more data evaluating the efficacy and safety of the two regimens shows no significant difference in response rates or toxicity (7). We utilized shortened regimen on 3-week interval for patient convenience. All patients were heavily pretreated with multiagent regimen that includes doxorubicin. Irinotecan was less influenced by P-glycoprotein multi-drug resistance compared with topoisomerase II inhibitors such as doxorubicin and etoposide (8). Irinotecan has shown antitumour activity in preclinical models of topotecan-resistant xenografts (8). Previous study with small sample size demonstrated that the number of previous treatment regimens received did not affect the outcome of those who received VIT as salvage therapy (6). An optimal responses were seen in patients who used VIT as first, second or third line of therapy after relapse or progression (Table 4). VIT did not show significant difference in response rates among different metastatic sites (Table 5). Treatment directed against pediatric medulloblastoma has been shown activity in preclinical mouse models because of its blood brain barrier penetration. One patient with brain metastasis localized to the parenchyma in our cohort responded well to chemotherapy with VIT, resulting in CR in the bone marrow and disease-free survival of 8 years after multidisciplinary treatment. There is synergistic activity, efficient penetration through the blood–brain barrier as well as no cross-resistance, and hence therapy of VIT could benefit PD/Re patients with neuroblastoma.
Both preclinical and clinical trials showed activity of VIT in a variety of pediatric cancers. VIT regimen was used as third-line chemotherapy in 34 patients with PD/Re solid tumors including 8 patients with neuroblastoma (9). Salvage chemotherapy prior to VIT comprised ifosfamide/etoposide and ifosfamide/carboplatin/etoposide. Among the 8 patients, 1 had complete response, 1 had partial response, 3 had stable disease, and 3 had progressive disease. Median progression free time was three months in all NB patients (9). The overall objective response (complete response + partial response) in our cohort was 69.6% (34/46) with 7.3 months median progression free time which may have contributed to raise the opportunity for local control. The combination of irinotecan and temozolomide (IT) have been used for relapsed/refractory neuroblastoma in previous study. The combination of IT produced objective responses of 33% and 15%, respectively (1, 10). Vincristine in combination with IT have shown synergistic activity in patients with rhabdomyosarcoma (11). Our study has demonstrated better ORR than results in previous study likely due to the addition of vincristine to the IT backbone.
The shortened schedule of irinotecan resulted in less grade 3–4 toxicity. A trial comparing protracted versus short schedule of irinotecan combined with vincristine and temozolomide showed higher frequency of dose limiting toxicity in the protracted regimen (12). Temozolomide has relatively minor myelosuppressive side effect and its dose limiting toxicity is mainly neutropenia and thrombocytopenia. Dose-limiting toxicities of irinotecan are primarily diarrhea and abdominal pain, accounting for 20% of patients (9). Overall the toxicities observed in this study were diarrhea and abdominal pain, accounting for 39.1%. Irinotecan could induce cholinergic syndrome, characterized by diarrhea, vomiting and abdominal pain. Irinotecan-related cholinergic syndrome is managed with atropine in prophylaxis. Irinotecan is metabolized by a specific route into SN38. Enteric bacteria cleave the drug-glucuronide bond in SN38. The resultant free SN38 metabolite causes diarrhea through its direct effect on the intestinal mucosa. Cefixime was used to eliminate intestinal bacteria and reduce irinotecan associated diarrhea. Cefixime 8mg/kg daily (maximum 400mg) was administered starting 2 days before each cycle for patients who experienced >grade 2 diarrhea in last cycles (13). Loperamide and hydration support were administered to patients with diarrhea. Eighteen patients developed diarrhea among 46 patients. No >grade 3 diarrhea occurred in this cohort. Patients treated on our study did not experience severe intestinal obstruction and neuropathy. Vincristine was omitted for patients with >grade 3 neuropathy (14). Myelosuppression associated with VIT regimen was mild. Myelosuppression was manageable, with eighteen patients experiencing grade 3 and fourteen developing grade 4 myelosuppression. These heavily pretreated patients tolerated VIT regimen after first line of intense therapy. VIT regimens were readily administered in the outpatient setting and hence had the benefits of shorter duration of total hospital days. A few clinical trials have explored IT regimen in combination with other cytotoxic therapies to improve outcomes. For example, the addition of vincristine or bevacizumab to IT exhibited better anti-tumor activity and associated with more durable response than irinotecan alone (15). In addition, activity is further improved when synergistic combinations of IT regimen and radiotherapy were used.
Irinotecan is converted by endogenous carboxylesterases to metabolite SN-38. UGT1A1 is responsible for detoxification of SN-38 through the process of glucuronidation. Expression of UGT1A1 is, in part, under the control of a polymorphic dinucleotide repeat sequence within the UGT1A1 promoter TATA box, varying from five to eight copies of a TA repeat([TA]nTAA) (16). The (TA) 6 TAA allele is considered wild-type. The UGT1A1∗ 28 allele consisting of 7 TA repeats is the most frequently variant type. Homozygous UGT1A1 ∗ 28 polymorphism is associated with a reduction of about 80% of UGT1A1 transcription. Reduced UGT1A1 gene expression leads to a decrease of inactive SN-38G and hence increases irinotecan-related toxicity. There was no clear relationship observed between UGT1A1 genotype and irinotecan-induced toxicity in adult. Blood samples for UGT1A1*28 genotyping were collected from 7 children in our study. All patients carried the UGT1A1*28 wild (6/6) genotype. We are unable to draw a definite conclusion of association between UGT1A1*28 genotype and irinotecan-related diarrhea in the limited number of patients. Factors other than UGT1A1*28 genotype may be responsible for irinotecan-induced diarrhea (13).
In conclusion, the VIT regimen had an objective response of 69.6% in heavily pretreated refractory/relapsed neuroblastoma. Among the 46 evaluable patients, the best response to VIT was achieved at the median of 4 cycles. The 5-day VIT regimen is an active and well-tolerated salvage regimen in relapse/refractory NB with manageable toxicities. Vomiting induced by oral temozolomide is another concern. The study can also serve as a template for intravenous temozolomide to the vincristine, irinotecan chemotherapy backbone in the future.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Board of the Sun Yat-sen University Cancer Center. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
JZ, XS, and YZ conceived and designed the analysis. TC and JW collected the data. JH, SL, and FS contributed data and analysis tools. ZZ performed the analysis. JZ, TC, and ZZ wrote the paper. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Chunmei Chen for developing the study database.
References
1. Kushner BH, Kramer K, Modak S, Cheung NK. Irinotecan Plus Temozolomide for Relapsed or Refractory Neuroblastoma. J Clin Oncol: Off J Am Soc Clin Oncol (2006) 24:5271–6. doi: 10.1200/JCO.2006.06.7272
2. Nicholson HS, Krailo M, Ames MM, Seibel NL, Reid JM, Liu-Mares W, et al. Phase I Study of Temozolomide in Children and Adolescents With Recurrent Solid Tumors: A Report From the Children’s Cancer Group. J Clin Oncol: Off J Am Soc Clin Oncol (1998) 16:3037–43. doi: 10.1200/JCO.1998.16.9.3037
3. Estlin EJ, Lashford L, Ablett S, Price L, Gowing R, Gholkar A, et al. Phase I Study of Temozolomide in Paediatric Patients With Advanced Cancer. United Kingdom Children’s Cancer Study Group. Br J Cancer (1998) 78:652–61. doi: 10.1038/bjc.1998.555
4. De Sio L, Milano GM, Castellano A, Jenkner A, Fidani P, Dominici C, et al. Temozolomide in Resistant or Relapsed Pediatric Solid Tumors. Pediatr Blood Cancer (2006) 47:30–6. doi: 10.1002/pbc.20516
5. Mixon BA, Eckrich MJ, Lowas S, Engel ME. Vincristine, Irinotecan, and Temozolomide for Treatment of Relapsed Alveolar Rhabdomyosarcoma. J Pediatr Hematol/Oncol (2013) 35:e163–6. doi: 10.1097/MPH.0b013e31825802c2
6. Setty BA, Stanek JR, Mascarenhas L, Miller A, Bagatell R, Okcu F, et al. VIncristine, Irinotecan, and Temozolomide in Children and Adolescents With Relapsed Rhabdomyosarcoma. Pediatr Blood Cancer (2018) 65:e26728-33. doi: 10.1002/pbc.26728
7. Kurucu N, Sari N, Ilhan IE. Irinotecan and Temozolamide Treatment for Relapsed Ewing Sarcoma: A Single-Center Experience and Review of the Literature. Pediatr Hematol Oncol (2015) 32:50–9. doi: 10.3109/08880018.2014.954070
8. Kushner BH, Kramer K, Modak S, Yataghene K, Cheung NK. High-Dose Cyclophosphamide-Irinotecan-Vincristine for Primary Refractory Neuroblastoma. Eur J Cancer (2011) 47:84–9. doi: 10.1016/j.ejca.2010.09.014
9. Buyukkapu Bay S, Kebudi R, Gorgun O, Zulfikar B, Darendeliler E, Cakir FB. Vincristine, Irinotecan, and Temozolomide Treatment for Refractory/Relapsed Pediatric Solid Tumors: A Single Center Experience. J Oncol Pharm Practice: Off Publ Int Soc Oncol Pharm Practitioners (2019) 25:1343–48. doi: 10.1177/1078155218790798
10. Bagatell R, London WB, Wagner LM, Voss SD, Stewart CF, Maris JM, et al. Phase II Study of Irinotecan and Temozolomide in Children With Relapsed or Refractory Neuroblastoma: A Children’s Oncology Group Study. J Clin Oncol: Off J Am Soc Clin Oncol (2011) 29:208–13. doi: 10.1200/JCO.2010.31.7107
11. Mascarenhas L, Lyden ER, Breitfeld PP, Walterhouse DO, Donaldson SS, Paidas CN, et al. Randomized Phase II Window Trial of Two Schedules of Irinotecan With Vincristine in Patients With First Relapse or Progression of Rhabdomyosarcoma: A Report From the Children’s Oncology Group. J Clin Oncol: Off J Am Soc Clin Oncol (2010) 28:4658–63. doi: 10.1200/JCO.2010.29.7390
12. Venkatramani R, Malogolowkin M, Davidson TB, May W, Sposto R, Mascarenhas L. A Phase I Study of Vincristine, Irinotecan, Temozolomide and Bevacizumab (Vitb) in Pediatric Patients With Relapsed Solid Tumors. PloS One (2013) 8:e68416. doi: 10.1371/journal.pone.0068416
13. Wagner LM, Perentesis JP, Reid JM, Ames MM, Safgren SL, Nelson MD Jr., et al. Phase I Trial of Two Schedules of Vincristine, Oral Irinotecan, and Temozolomide (VOIT) for Children With Relapsed or Refractory Solid Tumors: A Children’s Oncology Group Phase I Consortium Study. Pediatr Blood Cancer (2010) 54:538–45. doi: 10.1002/pbc.22407
14. Wagner L, Turpin B, Nagarajan R, Weiss B, Cripe T, Geller J. Pilot Study of Vincristine, Oral Irinotecan, and Temozolomide (VOIT Regimen) Combined With Bevacizumab in Pediatric Patients With Recurrent Solid Tumors or Brain Tumors. Pediatr Blood Cancer (2013) 60:1447–51. doi: 10.1002/pbc.24547
15. McNall-Knapp RY, Williams CN, Reeves EN, Heideman RL, Meyer WH. Extended Phase I Evaluation of Vincristine, Irinotecan, Temozolomide, and Antibiotic in Children With Refractory Solid Tumors. Pediatr Blood Cancer (2010) 54:909–15. doi: 10.1002/pbc.22460
Keywords: neuroblastoma, relapse, refractory, vincristine, irinotecan, temozolomide, efficacy, toxicity
Citation: Zhu J, Wang J, Sun F, Zhen Z, Chen T, Lu S, Huang J, Zhang Y and Sun X (2022) Vincristine, Irinotecan, and Temozolomide in Patients With Relapsed/Refractory Neuroblastoma. Front. Oncol. 12:804310. doi: 10.3389/fonc.2022.804310
Received: 29 October 2021; Accepted: 03 February 2022;
Published: 09 March 2022.
Edited by:
Jaume Mora, Hospital Sant Joan de Déu Barcelona, SpainReviewed by:
Stefano Mastrangelo, Catholic University of the Sacred Heart, Rome, ItalyDinesh Babu Somasundaram, University of Oklahoma Health Sciences Center, United States
Copyright © 2022 Zhu, Wang, Sun, Zhen, Chen, Lu, Huang, Zhang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaofei Sun, c3VueGZAc3lzdWNjLm9yZy5jbg==; Yizhuo Zhang, emhhbmd5emhAc3lzdWNjLm9yZy5jbg==
†These authors have contributed equally to this work and share first authorship
 Jia Zhu
Jia Zhu Juan Wang1,2†
Juan Wang1,2† Feifei Sun
Feifei Sun Zijun Zhen
Zijun Zhen Suying Lu
Suying Lu Junting Huang
Junting Huang Yizhuo Zhang
Yizhuo Zhang Xiaofei Sun
Xiaofei Sun