- 1Department of Internal Medicine, Zhongnan Hospital of Wuhan University, Wuhan, China
- 2Medical Research Institute of Wuhan University, Wuhan, China
Cardiac symptoms or signs as the first manifestations in acute lymphoblastic leukemia patients are sporadically reported which lead to misdiagnosis or delayed diagnosis due to lack of clinical experience and improper diagnosis procedures. Here, we documented the clinical features, procedures of diagnosis, treatments, and outcomes from the so-far reported 30 lymphoblastic leukemia cases that initially presented as cardiac problems and provided management recommendations based on the experiences and lessons learned from these patients to help physicians avoid misdiagnosis and improper treatment.
1 Introduction
Leukemic cardiac infiltration is frequently observed in postmortem autopsies (1) with incidence rate of 30%–44% (2–4). However, 99% of patients with cardiac infiltration were asymptomatic. Notably, a large sample of autopsy study (2) reported that cardiac infiltrations (51/116) were microscopic and occurred in the late stage of acute leukemia. As a result, the definite antemortem diagnosis of leukemic cardiac infiltration was rare (5, 6). In addition, the incidence of leukemic cardiac infiltration was significantly higher in acute myeloid leukemia than acute lymphoblastic leukemia (ALL) (1, 2). Therefore, macroscopic cardiac infiltration as the first manifestation of ALL is rather rare. In the real world, these patients usually first visit cardiologists, instead of oncologists. As a result, these patients usually missed the best treatment timeline due to the misdiagnosis or delayed diagnosis. Here, 30 scarce cases presented cardiac tamponade (7–14), cardiac mass (15–22), myocardium hypertrophy (23–30), or acute myocardial infarction (AMI) (31–36) as the first sign of ALL were carefully reviewed, and we provided recommendations of management procedures for the diagnosis and treatment in these atypical ALL patients.
2 Methods
A comprehensive literature search was performed using PubMed and the Cochrane database for English-language studies published from December 1985 through March 31, 2021. The following keywords were used: (“acute lymphoblastic leukemia” or “ALL”) and (“cardiac manifestation”, “cardiac disease”, “cardiac symptoms”, or “cardiac signs”). Additionally, the reference lists of all eligible cases were manually retrieved to obtain more literatures. Eventually, 30 cases that presented massive pericardial effusion or cardiac tamponade, cardiac mass, myocardium hypertrophy, or AMI as the first manifestation of ALL were included. Since this article is a review but not a clinical study, ethical approval is not applicable.
3 Results
In general, ALL patients with cardiac disorders as the first clinical manifestation showed a strong predominance in adolescent and middle-aged population. The most common cardiac abnormalities include persistent pericardial effusion or cardiac tamponade, severe cardiac hypertrophy, cardiac mass, and AMI-like manifestations.
3.1 Cardiac Tamponade as the First Manifestation of ALL
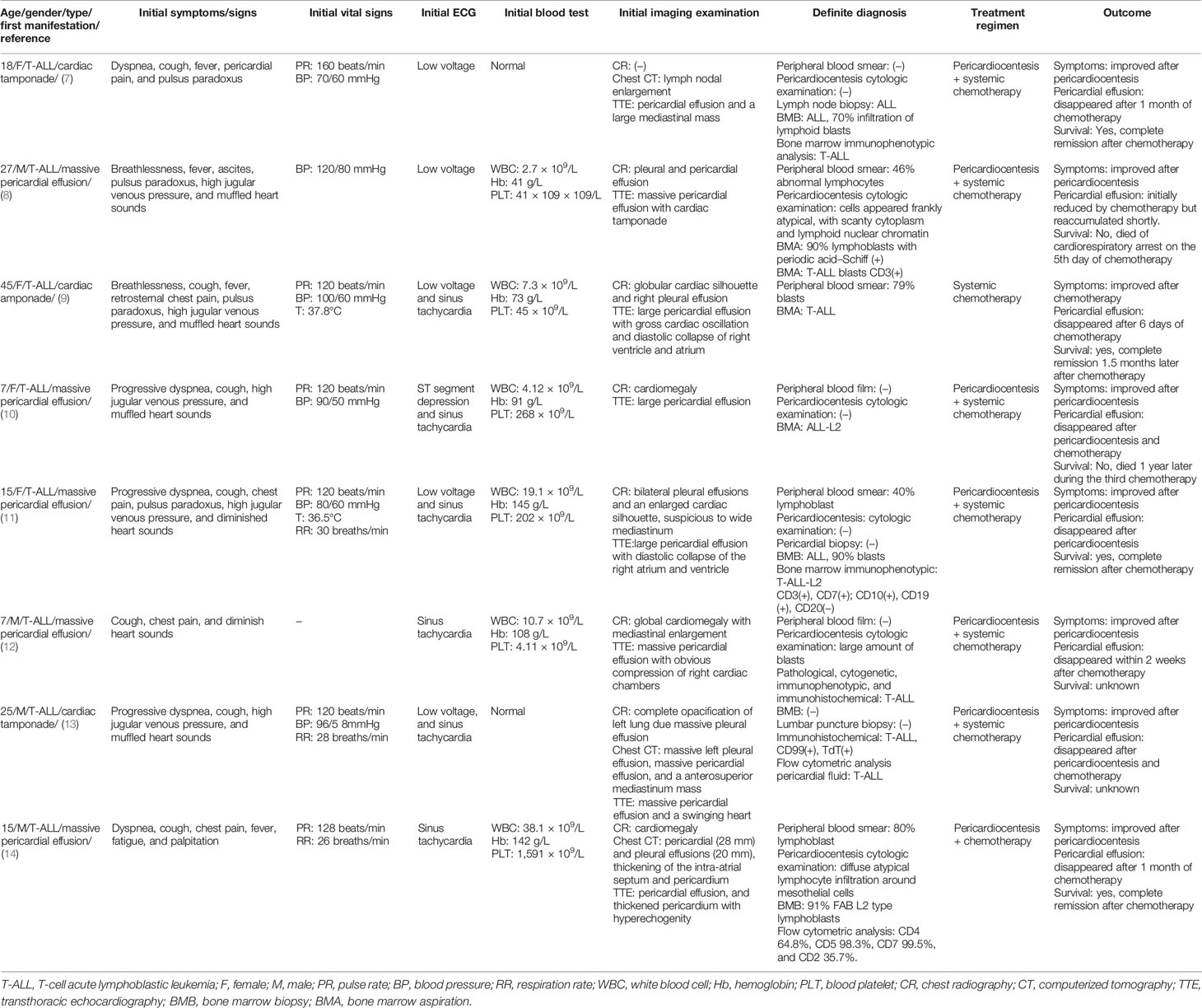
All of the eight reported T-cell acute lymphoblastic leukemia (T-ALL) cases presented massive pericardial effusion or cardiac tamponade as the first manifestation (Table 1). An adolescent predominance was observed with an average age of 19.8 years old (ranging from 7 to 45). Progressive dyspnea, cough, chest pain, and fever were recognized as the prominent symptoms, and typical signs include paradoxical pulse, elevated jugular venous pressure, and muffled heart sounds. In most cases, the resting heart rate was greater than 120 beats/min with a blood pressure usually lower than 100/60 mmHg. ECG was usually characterized as sinus tachycardia with low voltage. Notably, the initial blood test could be completely normal. The abnormal leukocyte associated with lymphoblast was observed at follow-up.
In imaging examinations (Table 1), chest radiography was used to screen pleural effusion, and chest computerized tomography (CT) was sensitive to lymph nodal or mediastinum mass detection. However, transthoracic echocardiography (TTE) was commonly used to detect pericardial effusion or cardiac tamponade, characterized by a swinging or oscillation heart with a diastolic collapse of the right ventricle and atrium. The present studies showed that chest CT scan had a positive rate of 25 (2/8) in detecting mediastinum involvement (Table 1).
T-ALL diagnosis with atypical manifestations often requires comprehensive tests, such as pericardiocentesis cytologic examination, peripheral blood smear, bone marrow aspiration (BMA), bone marrow biopsy (BMB), immunophenotypes, immunohistochemistry, and flow cytometric analysis. While BMA or BMB were usually used to make a definite diagnosis, immunophenotypic, immunohistochemical, and flow cytometric analyses were usually used as a tool of qualitative assessment (T-cell markers). The present studies showed that BMA and BMB had a higher positive rate (6/7, 85.7%) than peripheral blood smear (4/6, 66.7%) and pericardiocentesis (4/7, 57.1%) test (Table 1).
Once diagnosed with T-ALL, pericardiocentesis and systemic chemotherapy were adopted (Table 1). The tamponade symptoms were released significantly after pericardiocentesis. Five patients achieved a resolution of pericardial effusion after 6 days to 4 weeks of chemotherapy. Three patients achieved complete remission from ALL after 4 to 6 weeks of chemotherapy. A particular case obtained complete remission after 3 years of intermittent chemotherapy due to recurrent supraventricular tachycardia caused by chemotherapy; one patient died of cardiorespiratory arrest on the 5th day of chemotherapy, and one patient died of chemotherapy resistance after 1-year treatment. The prognosis of the other two male patients was unknown due to lost follow-up.
3.2 Cardiac Mass as the First Manifestation of ALL
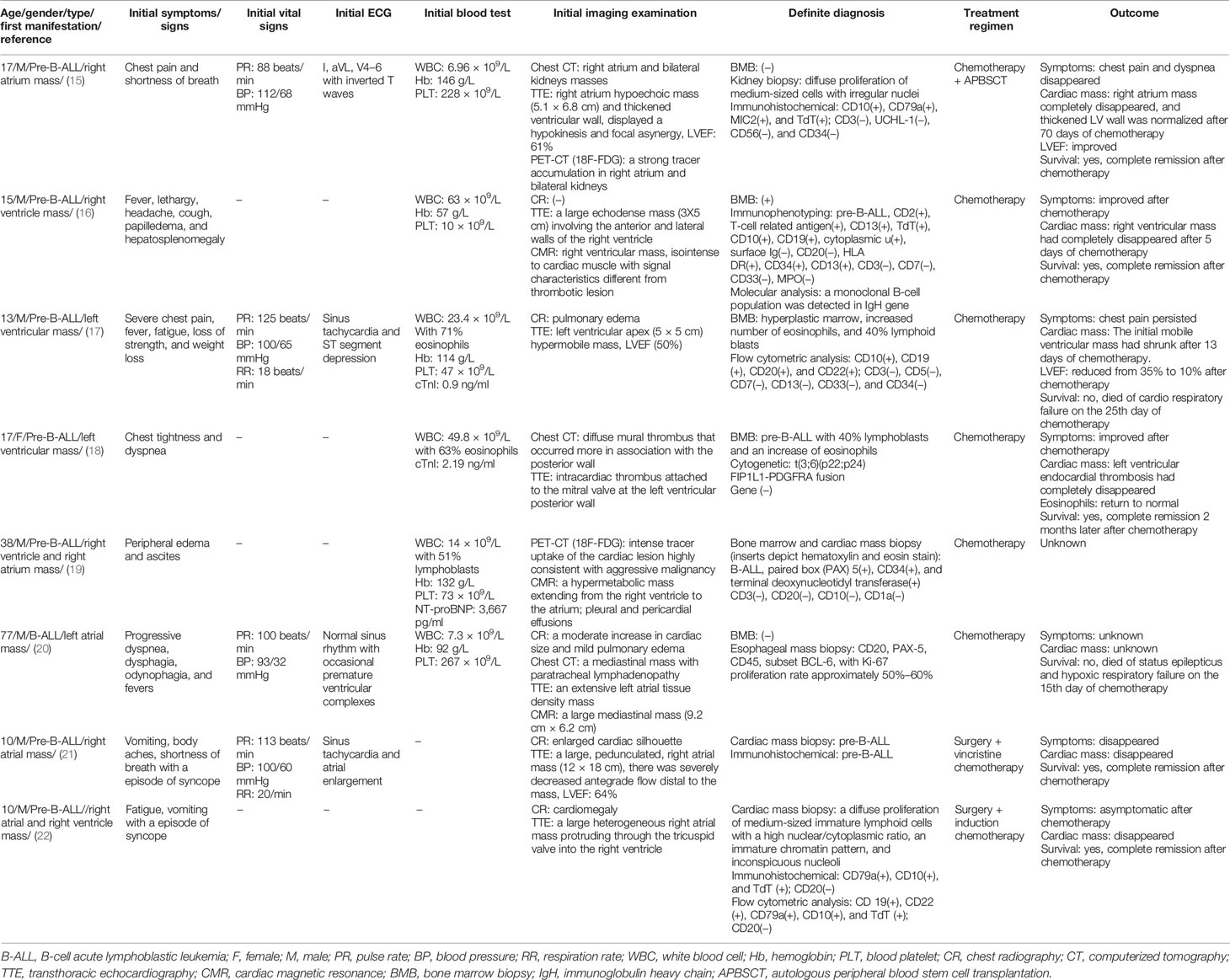
Eight B-cell acute lymphoblastic leukemia (B-ALL) patients presented cardiac mass as the first manifestation (Table 2). Similar to the T-ALL, an adolescent demographic feature was observed with an average age of 17 years old (ranging from 10 to 38). Cardiac mass was usually single and located in the cardiac cavity. The incidence of cardiac mass in the left and right heart chambers was 37.5% (3/8) and 62.5% (5/8), respectively. The most common symptoms include progressive dyspnea, chest pain, and syncope, usually companied with hypotension (<120/80 mmHg) and fast heart rate (>100 beats/min). In addition, the right heart mass usually presented peripheral edema, while the left heart mass usually presented severe chest pain with inverted T wave and/or depressed ST segment in the surface ECG and slightly elevated cardiac troponin I (cTnI) levels (0.9–2.19 ng/ml). In most cases, remarkably elevated peripheral blood leukocytes were observed.
Chest CT, TTE, cardiac magnetic resonance (CMR), and positron emission tomography-computed tomography (PET-CT) were commonly used for cardiac mass detection and pathological characteristic analysis (Table 2). However, the diagnosis of B-ALL largely depended on the biopsy of bone marrow and cardiac mass. The present studies showed that the positive rate for leukemic cardiac infiltration using mass biopsy and BMB was 100% (2/2) and 66.6% (4/6), respectively (Table 2). Notably, other than cardiac mass, kidney and esophageal invasion was occasionally observed.
After the diagnosis of B-ALL was made, the patients with severe hemodynamic disorder underwent cardiac surgery and chemotherapy, while others only adopted chemotherapy (Table 2). Five patients achieved cardiac mass resolution after 5 days to 2 months of chemotherapy. Five patients achieved complete remission from ALL after 2 months of chemotherapy. One patient died of respiratory failure 15 days after chemotherapy. One died of chemotherapy resistance after 25 days of treatment and one lost follow-up.
3.3 Myocardium Hypertrophy as the First Manifestation of ALL
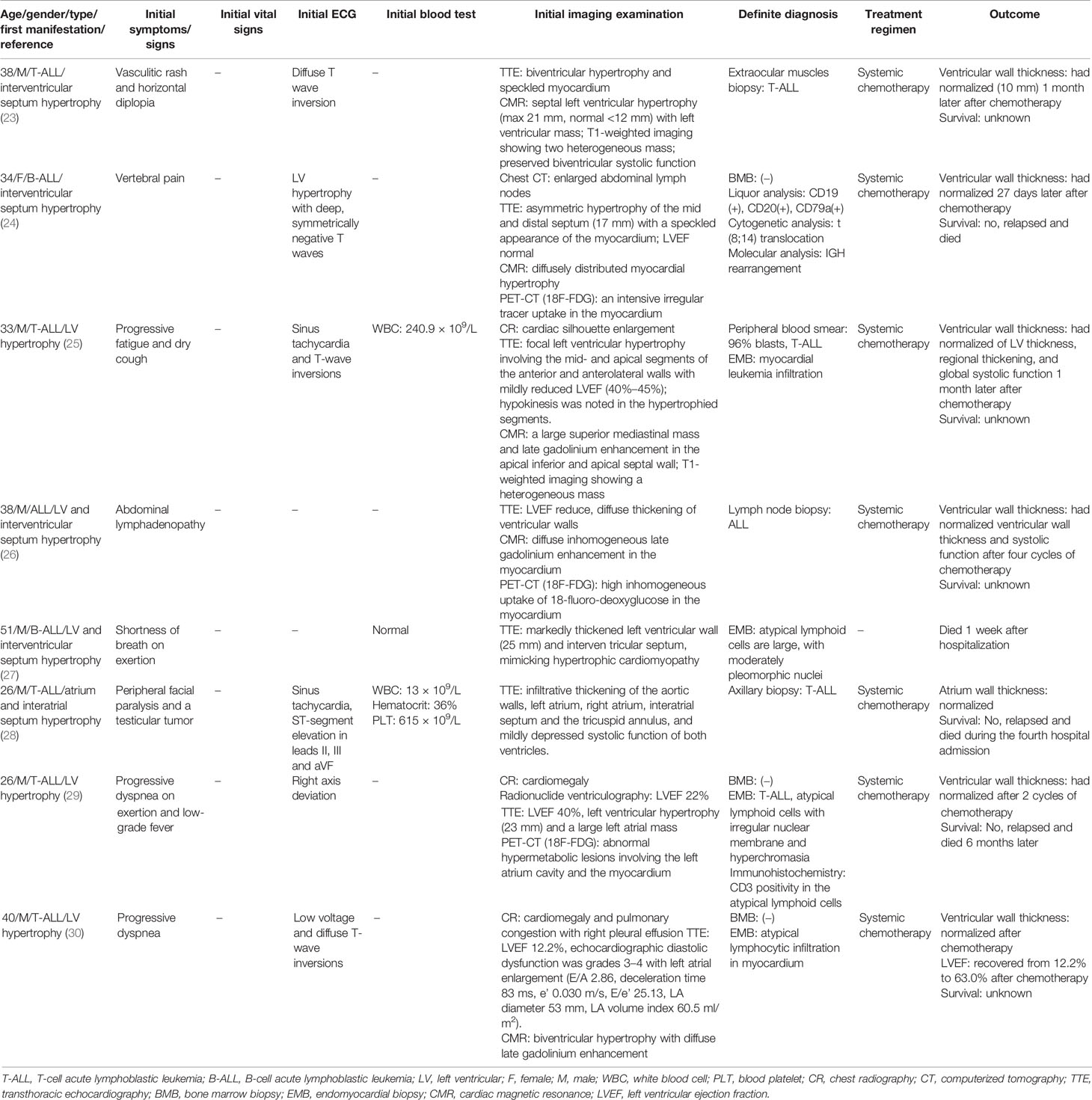
T-ALL-dominant pathological characteristics are presented in Table 3. A young to middle-aged preference was manifested with an average age of 35 years old (ranging from 26 to 51). The left ventricular (LV) wall was most frequently involved (5/8), followed by the ventricular septum (2/8) and atrium (1/8). Progressive dyspnea was the most common symptom. Unlike the hypertensive-hypertrophy, ECG usually displayed low voltage and diffuse T-wave inversion, occasionally with ST-segment elevation (II, III, aVF) and sinus tachycardia.
The leukemic infiltrated myocardium was characterized by speckle, hypokinesis, and/or multifocal regional thickening. TTE had an advantage in accessing the infiltration location, myocardial kinetics, and systolic function. CMR helped in recognizing the neoplasia myocardium infiltration by showing diffuse inhomogeneous late gadolinium enhancement, while PET-CT could monitor the relapse of ALL infiltration with manifestation of intensive irregular tracer uptake in the myocardium.
The diagnosis of ALL myocardial infiltration largely depended on the endomyocardial biopsy (EMB) rather than BMB. The present studies (Table 3) showed that the positive rate for EMB and BMB was 100% (4/4) and 0 (0/3), respectively. Occasionally, myocardium infiltration was accompanied by extraocular muscles and lymph node invasion.
After diagnosis of ALL with myocardium infiltration, all patients adopted systemic chemotherapy (Table 3) except for a 55-year-old male patient. Myocardium hypertrophy had gradually vanished in all patients who received chemotherapy. The one who did not accept chemotherapy died of deteriorated conditions 1 week after diagnosis. Three patients were relapsed and disseminated during follow-up and died after 6–14 months of treatment. However, the long-term outcomes of the other four patients were unknown.
3.4 AMI as the First Manifestation of ALL
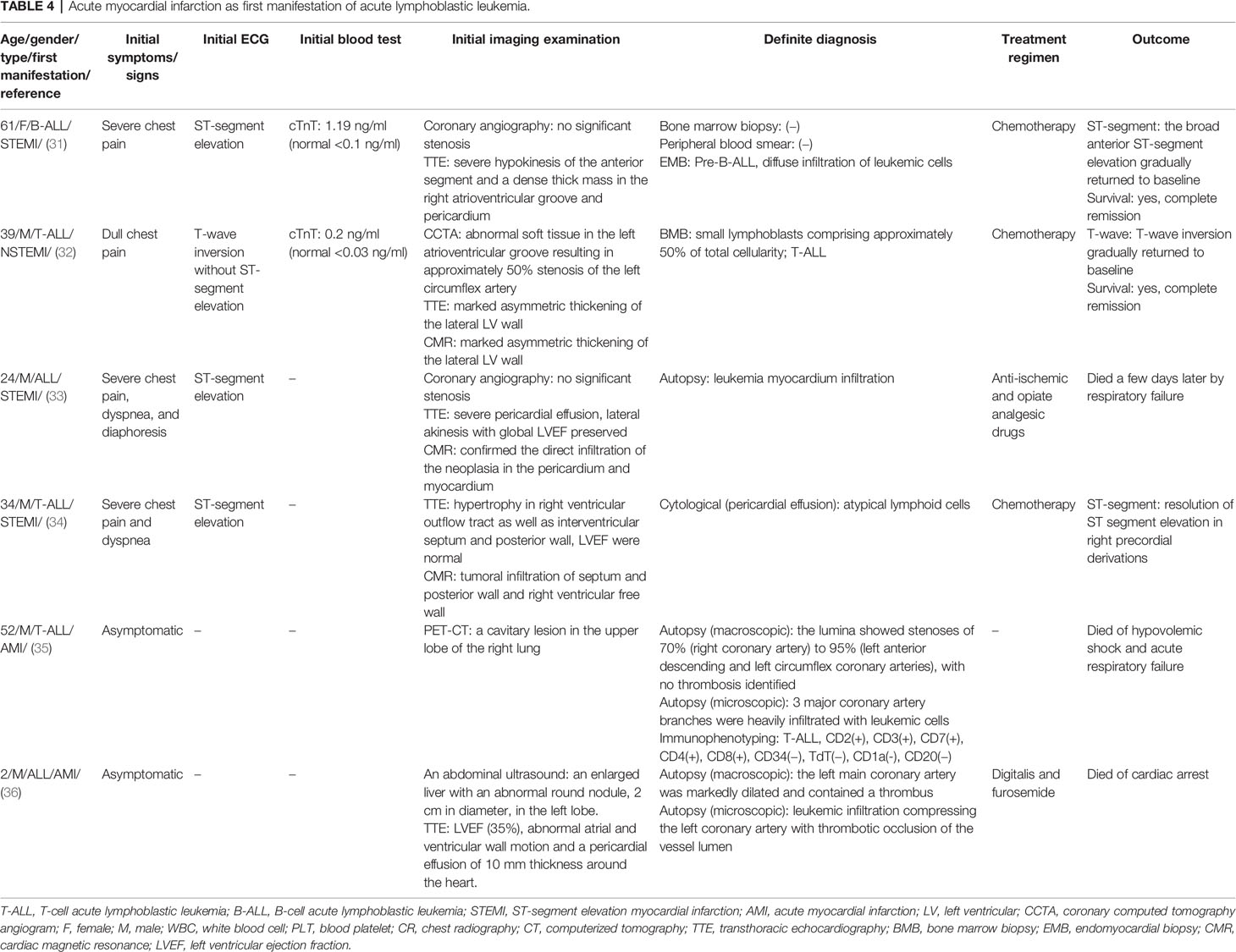
A total of six ALL cases reported AMI-like symptoms as the first clinical manifestation (Table 4), with an average age of 42 years old (ranging from 24 to 61) and exception of a 2-year-old boy. Surprisingly, 66.7% (4/6) of patients showed severe chest pain but without significant coronary artery obstruction, whereas 33.3% (2/6) of patients with severe coronary artery stenosis showed absence of chest pain. Initial ECG showed that 75% (3/4) of patients presented ST-segment elevation, and 25% (1/4) of patients presented T-wave inversion. Q wave had never been observed in any patient, although cTnI was significantly elevated in all patients with a range of 0.2–1.19 ng/ml (7–10-fold increase).
Coronary imaging examinations, including coronary angiography and coronary computed tomography angiogram, showed that 3/6 of patients presented normal coronary artery, 1/6 of patients presented moderate stenosis (50%) in the left circumflex artery, and 2/6 of patients presented severe stenosis (70%–95%) in the right coronary artery, left anterior descending artery, left circumflex artery, or left main coronary artery. The stenosis of coronary artery caused by leukemia infiltrating was centripetal. Unlike conventional AMI, there were no plaques (i.e., atherosclerotic and calcified plaque) in the coronary artery. The lumen stenosis and occlusion were secondary to the vascular wall thickening caused by leukemia infiltration and the acute leukemia clots. Interestingly, ALL patients with AMI manifestation usually had asymmetric myocardial hypertrophy and/or cardiac mass, indicating the leukemic invasion in both coronary artery and myocardium (for details, see Table 4).
In these patients, 50% (3/6) did not show stenosis or occlusion of any coronary artery. Therefore, stent and thrombolytic therapy were not applied. Instead, two patients adopted chemotherapy and achieved a complete normalization of ECG (ST segment and T wave). However, the patient who only adopted anti-ischemic and opiate analgesic therapy died of respiratory failure. In patients who did not accept coronary angiography or CCTA (3/6), one patient adopted chemotherapy and achieved complete regression of ST segment and one patient only accepted symptomatic treatment and died of cardiac arrest. The other one without any medical treatment died of hypovolemic shock and acute respiratory failure. These outcomes indicate that chemotherapy is adequate for coronary leukemic invasion ALL patients.
4 Discussion
In some cases, cardiac manifestation could be the first clinical finding of ALL. These patients may first visit cardiologists instead of oncologists. Notably, the blood test could be completely normal at the onset of cardiac symptoms. This usually led to clinical tragedy due to misdiagnosis or delayed diagnosis. Although few cases were reported in the literature, many patients may have been misdiagnosed as heart disease and died. It is presumable that in the real world, the morbidity of ALL patients with heart disease as the primary manifestation may be much higher than we think. Therefore, it is helpful to document the clinical features of these patients and put forward feasible methods to avoid misdiagnosis and mistreatment.
4.1 Cardiac Manifestations Precede ALL Diagnosis
In the included 30 cases, 80% (24/30) of patients presented cardiac manifestations before ALL was diagnosed. In total, 33.3% (8/24) of cases (7–14) had detected a persistent pericardial effusion and/or cardiac tamponade, 33.3% (8/24) of cases (15–22) had detected a cardiac mass, and 33.3% (8/24) of cases (23–30) had detected severe cardiac hypertrophy by TTE (7–18, 20–22, 24–30) and/or CMR (19, 23) test. All of these 24 cases were diagnosed with ALL by further tests of BMB (7, 16–19), BMA (8–11, 14), EMB (25, 27, 29, 30), cytologic analysis (12, 13, 15, 24), or tissue biopsy (20–23, 26, 28).
However, 20% (6/30) of patients presented severe chest pain (31–34) or silent (asymptomatic) myocardium ischemia (35, 36) as the first manifestation of acute leukemia relapse, in which one patient (32) manifested an abnormal soft tissue in the left atrioventricular groove with a 50% stenosis in the left circumflex artery by CCTA; one patient (35) showed heavy leukemic infiltration in three major coronary artery branches at autopsy (microscopic); and one patient (36) showed leukemic infiltration with thrombotic occlusion of the vessel lumen at autopsy (microscopic). These results indicated that the AMI-like clinical manifestations (32, 35, 36) were possibly a result of coronary artery leukemic infiltration or leukemic thrombus.
4.2 Experience and Lessons Learned From Clinical Misdiagnosis
4.2.1 Experience
In ALL patients with massive pericardial effusion or cardiac tamponade, pericardiocentesis is an emergency measure to relieve cardiac tamponade symptoms and detect the malignant hematological cells (up to 50% positive rate). Therefore, it should be immediately conducted for diagnosis and treatment purposes once confirmed by echocardiography, especially for young people without previous cardiac problems or autoimmune diseases. In addition, the patients presented massive pericardial effusion or cardiac tamponade as the first sign of T-ALL usually manifested bleeding tendency due to the abnormal blood elements (8, 12). This needs to be considered before pericardiocentesis.
In ALL patients with intracardiac mass, the symptoms largely depend on the mass dimension and location. A large mass that occupied the entire cardiac chamber could cause severe hemodynamic disorder, such as syncope (21, 22). A right heart mass could cause systemic congestion, such as peripheral edema and ascites (19). In contrast, a LV medium-size mass could cause acute pulmonary congestion and present progressive dyspnea (18, 20). Moreover, a LV apex hypermobile mass could cause outflow tract obstruction and thus reduce cardiac output, leading to insufficient coronary perfusion and angina (17).
In ALL patients with infiltration-induced hypertrophy, the systolic function could be reduced depending on the region and severity of infiltration. The LV free wall infiltration could cause a reduced left ventricular ejection fraction (LVEF) (25–30) with progressive dyspnea, while the ventricular septum infiltration could have normal LVEF (23, 24) without cardiac symptoms.
In ALL patients with AMI, the severity of cardiac symptoms did not match the degrees of coronary stenosis. The patients who presented abnormal ECG (ST-segment elevation or T-wave inversion) and significantly elevated cTnI (0.2–1.19 ng/ml) experienced acute, severe chest pain with absence of coronary stenosis (31–34). The possible explanations include: (1) the ventricular infiltration of leukemic cells caused coronary artery compression; (2) vascular wall leukemic infiltration-induced centripetal thickening; (3) coronary microvessel occlusion; (4) coronary artery spasm and constriction; and (5) diffuse myocardial injury.
In contrast, two patients with severe coronary stenosis or occlusion were asymptomatic, including a case with occluded left main coronary artery (36) and a case with multiple severe coronary stenoses (35). The absence of symptoms in the conventional AMI was usually observed in elderly patients and patients with diabetics due to the insensitivity of the neve system. However, these two patients were young and without diabetics. Whether these patients had nerve injuries was unclear.
Taken together, ALL patients with severe chest pain and AMI-like ECG change and cTnI elevation may not be the actual onset of AMI caused by the rupture of coronary plaques. Instead, these changes could derive from the leukemic infiltration. Chemotherapy, other than coronary intervention, is usually effective. However, coronary angiography is required to identify the silent AMI. There was no knowledge about the pathologic feature of the coronary thrombus in the AMI-like ALL patients (e.g., the leukemic cluster or thrombus caused by the plaque rupture). However, this is critical for determining the proper coronary intervention strategy. Although currently not available, the autopsy data will address this issue.
4.2.2 Lessons
4.2.2.1 In ALL Patients With Cardiac Tamponade as the First Manifestation
A 7-year-old girl (10) presented cardiac tamponade as the first sign of T-ALL. She had a negative result in peripheral blood smear and pericardiocentesis. Therefore, she did not accomplish the BMB test due to these negative results. Her cardiac symptoms were improved after symptomatic treatment (broad-spectrum antibiotics, prednisone, lasix, digoxin). Unfortunately, she was diagnosed as ALL-L2 by BMB at her second hospital visit (1 month later) and died 1 year after the diagnosis.
In contrast, an 18-year-old female patient (7) presented cardiac tamponade as the first sign of T-ALL also showed negative results in peripheral blood smear and pericardiocentesis. However, she had BMB test due to a large mediastinal mass indicated by TTE and swollen mediastinal lymph nodes indicated by chest CT. She was diagnosed with T-ALL by BMB and achieved complete remission after chemotherapy. Moreover, a 15-year-old male patient (16) presented cardiac mass as the first sign. He had accomplished a BMB test due to fever, hepatosplenomegaly, and abnormal blood test (WBC: 63 × 109/L, Hb: 57 g/L, PLT: 10 × 109/L). He obtained a definite diagnosis of B-ALL by BMB and achieved complete remission after chemotherapy.
These cases indicated that the BMB is critical for the diagnosis of ALL. It should be routinely conducted in patients with cardiac mass or massive pericardial effusion or cardiac tamponade for unknown reason, especially for young people, even the peripheral blood test and pericardiocentesis results are normal.
Although BMB was important for ALL diagnoses, BMB was sometimes negligently conducted. For instance, a 13-year-old male patient (17) presented a left ventricular mass as the first sign of B-ALL was diagnosed as hypereosinophilic syndrome due to the careless examination of bone marrow. In fact, there was at least 40% lymphoblast in his bone marrow section, which was found 3 months later by retrograde reviewing the bone marrow section. Unfortunately, this misdiagnosis largely delayed his treatment and caused his death. A similar misdiagnosis was made in another 13-year-old male patient (37). Therefore, BMB needs to be routinely and carefully reviewed in the highly suspected ALL patients.
4.3 In ALL Patients With Myocardium Hypertrophy as the First Manifestation
A 26-year-old male patient (29) had accomplished EMB due to left ventricular hypertrophy and large left atrial mass. He obtained a definite diagnosis of T-ALL by EMB and had a complete resolution of hypertrophic myocardium and left atrial mass after chemotherapy. By contrast, a 40-year-old male patient (30) had accomplished EMB due to low voltage in the ECG with remarkable left ventricular hypertrophy. He obtained a definite diagnosis of T-ALL by EMB and had a complete resolution of myocardium hypertrophy after chemotherapy. In addition, a 33-year-old male patient (25) had accomplished EMB due to delayed gadolinium enhancement in the apical inferior and apical septal wall and a large superior mediastinal mass. He was diagnosed with T-ALL by EMB and reached a complete resolution for the myocardium hypertrophy and superior mediastinal mass after chemotherapy.
4.4 In ALL Patients With Clinical Manifestations of AMI
A 39-year-old male (32) presented severe chest pain with T wave inversion. CCTA showed extensive abnormal soft tissue in the left atrioventricular groove, right atrium and lateral pericardium, resulting in about 50% stenosis of the left circumflex artery. It indicated that the stenosis was likely caused by neoplasia myocardium infiltration. Notably, his cTnI level was slightly increased (0.2 ng/ml, reference range <0.03). Considering only a 50% stenosis in the coronary artery, the elevation of troponin I was possibly caused by a neoplasia myocardium infiltration rather than coronary stenosis. He had accomplished BMB due to asymmetric left ventricular hypertrophy and delayed gadolinium enhancement in the left ventricular lateral wall. He obtained a definite diagnosis by BMB and achieved a remarkable resolution of left ventricular hypertrophy with normalized T wave after five months of chemotherapy.
In contrast, the coronary angiography of a 61-year-old female patient (31) showed no stenosis. Interestingly, the cTnI levels (1.19 ng/ml) were significantly elevated, and the imaging examinations (TTE, enhanced CT, and gallium scintigraphy) revealed an abnormal neoplasia infiltration of the myocardium and pericardium. Therefore, the chest pain was likely caused by neoplasia cardiac infiltration rather than coronary stenosis. She had accomplished EMB examination, although the result of BMB test was negative. She obtained a definite diagnosis of B-ALL by EMB and had a remarked resolution of cardiac infiltration after two weeks of chemotherapy.
The pattern of changes in high-sensitivity troponin I (hs-TnI) could be helpful to distinguish AMI-like leukemia from the conventional AMI. For instance, in the conventional AMI, the TnI curve contains ascending and descending phases (with a peak) due to acute death of a large numbers of cardiomyocytes. Owing to the one-time cardiac death, the curve will manifest a clear peak 10–24 h after the onset. In addition, conventional AMI patients have no evidence of neoplasia myocardium infiltration. In contrast, the AMI-like ALL presented neoplasia myocardium infiltration (31–34) and had no evidence of severe coronary stenosis (31, 33, 34). The TnI curve could show an ascending trend and then stabilization at a higher level without a typical peak due to the continuous cardiomyocyte injury caused by leukemic cell infiltration. EMB needs to be conducted for ALL diagnose, but it should not be conducted in conventional AMI patients due to the risk of ventricular rupture. Unfortunately, all of the AMI-like ALL patients (31–36) in the present study did not monitor the dynamic changes of cTnI levels and thus missed the opportunity to understand the underlying pathology.
Notably, two B-ALL patients (17, 18) presented cardiac mass as the first sign with remarkably elevated eosinophils in peripheral blood. In fact, in some cases, eosinophilia might be the initial presentation of B-ALL (38–40). As a large number of eosinophils might mask underlying or coexisting leukemia due to the absence of lymphoblasts in peripheral blood (37), the BMB is strongly recommended in patients presented cardiac mass with eosinophilia. Furthermore, Sumners et al. (2) compared 1-week and 1-month antemortem peripheral leukocyte count and found that the numbers of the peripheral leukocytes were significantly higher in ALL patients with cardiac infiltration than those without cardiac infiltration. Thus, the increased levels of eosinophil (17, 18, 37), leukocyte (8, 11, 16–19, 25, 28), and lymphoblast (8, 9, 11, 19, 25) might be a clue of ALL, and the blood test and peripheral blood smear should be routinely conducted in patients with massive pericardial effusion or cardiac tamponade, cardiac mass, or suspected myocardium infiltration. In rare cases, the laboratory examinations, including pericardial effusion cytologic examination, routine blood test, peripheral blood smear, BMB, and EMB showed negative results. If highly suspected, the flow cytometric measurement, immunophenotypic, immunohistochemical, cytogenetic, and genome-wide single nucleotide polymorphism study should be performed to search T/B cell markers.
For instance, Fournier et al. (41) claimed a specific L3-IgH rearrangement in B-ALL with eosinophilia. Consistent with this, the rearrangement of L3-IgH was observed in a patient (37) with eosinophilia who was finally diagnosed as B-ALL. Therefore, the L3-IgH rearrangement test is helpful for screening ALL patients who presented cardiac mass with eosinophilia.
Importantly, cardiac symptoms or signs could be absent in the AMI-like ALL patients. For example, a 52-year-old male patient (35) with a history of untreated T-ALL, the PET-CT indicated a pulmonary lesion, he did not conduct ECG and cTnI test due to the absence of cardiac symptoms, a few days later, he died of hypovolemic shock and acute respiratory failure. His autopsy revealed multiple, severe coronary stenosis (70% in right coronary artery, 95% in left anterior descending and 95% in left circumflex artery), indicating that he likely experienced a silent (asymptomatic) myocardium ischemia before death (the cTnI level has not been documented). Similarly, a 2-year-old boy (36) had a complete remission of ALL after chemotherapy. However, during the follow-up, the auxiliary examinations (WBC: 0.6 × 109/L, liver nodule, splenomegaly, pericardial effusion) indicated a relapse of ALL. He did not accomplish ECG and cTnI examination due to the absence of cardiac symptoms. Unfortunately, he died of cardiac arrest 17 days later. His autopsy revealed massive leukemic cell infiltration in the left main coronary artery with a thrombus occlusion of the vessel lumen. This indicates that in the patients with a deteriorated or disseminated ALL, regardless of cardiac symptoms, ECG and cTnI should be routinely examined. Once the abnormality was observed, coronary angiography or CCTA should be conducted.
4.5 Experience and Lessons Learned From Clinical Therapy
In ALL patients with massive pericardial effusion or cardiac tamponade presented, mild clinical condition usually achieved complete remission with a resolution of pericardial effusion after chemotherapy (7, 9, 12, 14). However, a 27-year-old male patient (8) presented severe condition with developed bilateral parietal lobe hemorrhage with reaccumulated pericardial effusion after 4 days of chemotherapy and died of cardiorespiratory arrest 5 days later. Also, a 7-year-old girl (10) who presented severe clinical condition did not obtain remission after 4 cycles of chemotherapy and finally developed systemic and cerebral diffusion and died after 1 year of treatment. These indicate that the outcome of chemotherapy for the ALL patients presented massive pericardial effusion or cardiac tamponade largely depends on the severity of the basic condition of ALL itself. The cardiac effusion would usually be absorbed after chemotherapy, and cardiac dysfunction has not been observed.
Interestingly, a 15-year-old female patient (11) with persistent pericardial effusion developed a mediastinal mass and two large right atrium masses with severe chest pain after 1 week of chemotherapy. She was lucky since the histologic sections revealed necrotic thrombus for the right atrial masses and chronic mediastinitis for the mediastinal mass. Thus, her chemotherapy continued and achieved complete remission with a resolution of pericardial effusion 1.5 years later. Whether the necrotic thrombus and mediastinitis were associated with the chemotherapy remains unclear, although very likely.
The ALL patients with myocardium hypertrophy or cardiac mass, if manifested with normal or preserved LVEF, usually tolerate chemotherapy well. Most of them could achieve a complete resolution of myocardium hypertrophy and mass. However, a 26-year-old male patient (29) presented significantly decreased LV systolic function (LVEF 22%) and developed chemotherapy resistance on the fourth treatment cycle. He died of ALL relapse after 6 cycles of chemotherapy. In contrast, a 40-year-old male patient (30) presented remarkably reduced LV systolic function (LVEF 12.2%) with no evidence of chemotherapy resistance completed the chemotherapy and achieved a resolution of LV hypertrophy with a complete recovery of LV systolic function (LVEF 63.0%) at the end of chemotherapy. These indicated that no matter the patients with normal or reduced LVEF, chemotherapy needs to be conducted, and the outcome largely depends on the sensitivity of the patients to the chemotherapy.
ALL patients could present severe chest pain, ischemic ECG changes (ST-segment elevation and/or T-wave inversion) and significant elevation of cTnI (7–10-fold increase), a similar manifestation of AMI (31–34). However, coronary angiography or CCTA did not show significant coronary stenosis (31–33). Therefore, the ECG changes and elevated cTnI levels were likely caused by neoplasia myocardium infiltration, other than the coronary ischemic myocardial injury. This was proved by the success of chemotherapy, with achievement of symptom relief and normalization of ECG, although the curvilinear trajectory of cTnI had not been documented.
4.6 General Perspectives of Anticancer Therapy
The tolerance of ALL patients with cardiac infiltration is likely a concern for anticancer therapy, especially in consideration of cardiac toxicity. However, the cardiac manifestation in ALL patients is primarily caused by neoplasia infiltration rather than the primary cardiac problem. Therefore, anticancer therapy can generally relieve and gradually vanish cardiac symptoms. In fact, in patients who accepted chemotherapy, a complete resolution was achieved in 62.5% (5/8) of patients with pericardial effusion or cardiac mass (Tables 1, 2), and 100% of patients with myocardium hypertrophy (7/7) and AMI-like cardiac injury (3/3) (Tables 3, 4). Notably, deterioration of clinical condition with chemotherapy was also detected in 25% (2/8) of patients with cardiac tamponade or cardiac mass (Tables 1, 2). Considering that these patients had a severe basic condition before chemotherapy, it indicates that the patients with severe clinical condition may not tolerate chemotherapy well. Therefore, chemotherapy needs to be evaluated cautiously and adjusted individually before conducting, such as the time point, course of treatment and drug selection (avoiding drugs with severe cardiac toxicity). Moreover, 42.9% (3/7) of ALL patients with myocardium hypertrophy relapsed after chemotherapy (Table 3), although the myocardial infiltration itself was highly sensitive to chemotherapy, indicating a poor prognosis for these patients.
Considering leukemia prone to recurrence, TTE, routine blood test, peripheral blood smear, cTnI, and ECG should be conducted to monitor the relapse during the follow-up phase.
5 Management Recommendations
We put forward suggestive management procedures for the diagnosis and treatment of the ALL patients who initially presented as cardiac disease:
1. In young patients who present triad of pericardial effusion, systemic congestion and/or hypotension, TTE should be performed to detect pericardial effusion or intracardiac mass. Pericardiocentesis is recommended in patients with cardiac tamponade for symptom relief and etiological judgment. However, digitalis therapy is not recommended in patients with cardiac tamponade due to nonoptimal response. BMB is strongly recommended to rule out ALL in patients with persistent pericardial effusion or tamponade without basic heart disease.
2. For patients with myocardium hypertrophy but ECG showed low voltage and diffuse T-wave inversion, CMR and/or PET-CT are strongly recommended to identify the possible myocardium infiltration, especially for patients with asymmetric ventricular hypertrophy without hypertension. If suspected with myocardium infiltration, EMB should be conducted.
3. For young patients with AMI-like clinical manifestations, CCTA and/or coronary angiography need to be performed to identify the coronary stenosis, and the dynamic changes of TnI levels and ECG also need to be monitored. The conventional AMI usually has a TnI peak and typical ST and T-wave regression with or without Q-wave formation. However, neoplasia myocardium infiltration-induced AMI-like manifestations usually come with a stabilized TnI at a high level and consistent ECG changes without Q-wave formation. Once AMI is excluded, BMB and EMB need to be conducted to rule out or confirm the diagnosis of ALL.
4. Tissue biopsy is recommended in patients with cardiac mass or suspected neoplasia infiltration. If BMB and EMB showed negative results, the flow cytometric measurement should be performed to search T/B cell markers if highly suspected. L3-IgH rearrangement examination by sequencing is recommended in patients with eosinophilia since it could be a B-ALL marker.
5. Once the definite diagnosis is made, systemic chemotherapy should be carried out as soon as possible in ALL patients who presented massive pericardial effusion or cardiac tamponade, cardiac mass, myocardium hypertrophy and/or AMI-like myocardium injury. LV dysfunction is not an absolute contraindication to chemotherapy. In most cases, the cardiac function could be significantly improved after chemotherapy because the cardiac dysfunction is caused by neoplasia infiltration rather than the primary heart disease. However, chemotherapy should be adopted with careful evaluation and individualized in patients with severe basic conditions.
6. For patients with complete remission, TTE, cTnI, ECG, routine blood test and peripheral blood smear should be routinely conducted to monitor the relapse during follow-up.
These recommendations are illustrated in Figure 1.
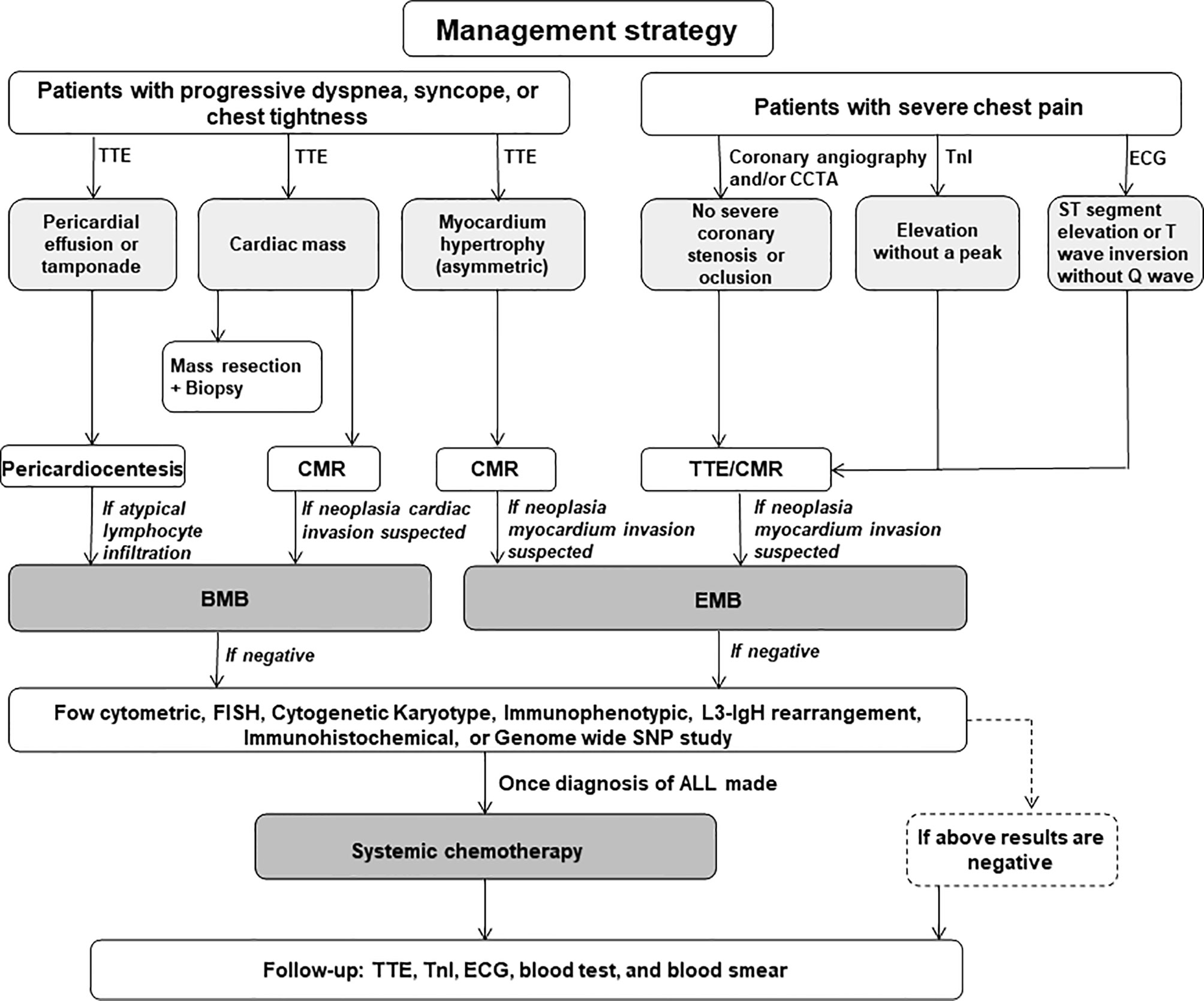
Figure 1 The management recommendations for the acute lymphoblastic leukemia patients with cardiac problems as the first manifestation. TTE, transthoracic echocardiography; CCTA, coronary computed tomography angiogram; TnI, troponin I; CMR, cardiac magnetic resonance; EMB, endomyocardium biopsy; FISH, fluorescence in situ hybridization; SNP, single nucleotide polymorphism; ALL, acute lymphoblastic leukemia.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
YW and ZL conceived and designed this study as well as drafted the manuscript. ZL and JC carried out the literature search and collected and organized data. YW was responsible for data interpretation, revision, and approval of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants awarded to YW from the National Natural Science Foundation of China (NSFC, Grant Nos. 81270304, 81873507, and 81420108004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Roberts WC, Bodey GP, Wertlake PT. The Heart in Acute Leukemia. A Study of 420 Autopsy Cases. Am J Cardiol (1968) 21:388–412. doi: 10.1016/0002-9149(68)90143-4
2. Sumners JE, Johnson WW, Ainger LE. Childhood Leukemic Heart Disease. A Study of 116 Hearts of Children Dying of Leukemia. Circulation (1969) 40:575–81. doi: 10.1161/01.CIR.40.4.575
3. Kirshbaum JD, Preus FS. Leukemia: Clinical and Pathologic Study of One Hundred and Twenty-Three Fatal Cases in a Series of 14,400 Necropsies. Arch Intem Med (1943) 71:777. doi: 10.1001/archinte.1943.00210060038003
4. Javier BV, Yount WJ, Crosby DJ, Hall TC. Cardiac Metastasis in Lymphoma and Leukemia. Dis Chest (1967) 52:481–4. doi: 10.1378/chest.52.4.481
5. Hori T, Suzuki N, Mizue N, Hatakeyama N, Takamuro M, Tsutsumi H. Relapse of T-Cell All After Stem Cell Transplant Presenting as Hypertrophic Cardiomyopathy: The Value of Non-Invasive Diagnostic Imaging in Detecting Cardiac Leukemia. Pediatr Blood Cancer (2006) 46:108–11. doi: 10.1002/pbc.20409
6. Chang K, Kim DY, Lee KH, Huh J, Kang JW, Shin DY, et al. An Isolated Cardiac Relapse After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Lymphoblastic Leukemia. Korean J Intern Med (2017) 32:753–7. doi: 10.3904/kjim.2015.115
7. Mancuso L, Marchi S, Giuliano P, Pitrolo F. Cardiac Tamponade as First Manifestation of Acute Lymphoblastic Leukemia in a Patient With Echographic Evidence of Mediastinal Lymph Nodal Enlargement. Am Heart J (1985) 110:1303–4. doi: 10.1016/0002-8703(85)90032-8
8. Basu D, Siddaraju N, Murugan P, Badhe BA, Akkarappatty C, Dutta TK. Cytologic Aspects of T-Cell Acute Lymphoblastic Leukemia Presenting as a Massive Pericardial Effusion: A Case Report. Acta Cytol (2009) 53:337–40. doi: 10.1159/000325321
9. Leung WH, Tai YT, Lau CP, Wong CK, Cheng CH, Chan TK. Cardiac Tamponade Complicating Leukaemia: Immediate Chemotherapy or Pericardiocentesis? Postgrad Med J (1989) 65:773–5. doi: 10.1136/pgmj.65.768.773
10. Mutai L, Abdallah F, Kaiser F. An Unusual Presentation Of Acute Lymphoblastic Leukaemia With Peri-Cardial Effusion Causing Cardiac Tamponade. East Afr Med J (2012) 89:142–3.
11. Zareifar S, Cheriki S, Namdari M, Farahmandfar M, Jannati A. Pericardial Effusion and Atrial Thrombosis: A Rare Complication of Childhood Leukemia. Iran J Pediatr (2012) 22:117–20.
12. Ozdemir R, Meşe T, Karadeniz C, Doksöz Ö. Unusual Presentation of Pre-T Cell Acute Lymphoblastic Leukemia: Massive Pericardial Effusion Only. Pediatr Emerg Care (2015) 31:711–2. doi: 10.1097/PEC.0000000000000451
13. Kapur S, Levin MB. Precursor T-Cell Lymphoblastic Lymphoma Presenting as Cardiac Tamponade in a 25-Year-Old Male: A Case Report and Review of Literature. World J Oncol (2014) 5:129–34. doi: 10.14740/wjon785w
14. Malbora B, Ozyurek E, Yildirim SV, Avci Z, Ozbek N. Cardiac Involvement in an Adolescent With Acute Lymphoblastic Leukemia. Pediatr Hematol Oncol (2010) 27:476–81. doi: 10.3109/08880018.2010.493572
15. Manabe M, Yoshii Y, Mukai S, Sakamoto E, Kanashima H, Nakao T, et al. Precursor B-Lymphoblastic Lymphoma Involving an Intracardiac Mass and Myocardial Infiltration: A Case Report. Intern Med (2012) 51:315–9. doi: 10.2169/internalmedicine.51.6075
16. Barbaric D, Holley D, Lau KC, McCowage G. It Is ALL in the Heart: A Patient With Acute Lymphoblastic Leukemia and Cardiac Infiltration at Time of Diagnosis. Leuk Lymphoma (2002) 43:2417–9. doi: 10.1080/1042819021000040161
17. Gharabaghi MA, Aghajanzadeh P, Zahedi G, Borji R, Derakhskan L, Sattarzadeh R, et al. Cardiac Disease in a Case of Precursor B Acute Lymphoblastic Leukaemia With Eosinophilia(ALL/Eo). BMJ Case Rep (2012) 2012:bcr0420114128. doi: 10.1136/bcr.04.2011.4128
18. Nie YL, Jan SL, Fu LS, Chang TK, Wang JD. Congestive Heart Failure as Presentation of Acute Lymphoblastic Leukaemia With Eosinophilia. Br J Haematol (2010) 149:633. doi: 10.1111/j.1365-2141.2010.08103.x
19. Werner RA, Rudelius M, Thurner A, Higuchi T, Lapa C. Cardiac Manifestation of Acute Lymphoblastic Leukemia. Clin Nucl Med (2016) 41:570–1. doi: 10.1097/RLU.0000000000001235
20. Ban-Hoefen M, Zeglin MA, Bisognano JD. Diffuse Large B Cell Lymphoma Presenting as a Cardiac Mass and Odynophagia. Cardiol J (2008) 15:471–4.
21. Hahn B, Rao S, Shah B. Case Report of Precursor B-Cell Lymphoblastic Lymphoma Presenting as Syncope and Cardiac Mass in a Nonimmunocompromised Child. Pediatr Emerg Care (2007) 23:576–9. doi: 10.1097/PEC.0b013e31812eef6b
22. Bassi D, Lentzner BJ, Mosca RS, Alobeid B. Primary Cardiac Precursor B Lymphoblastic Lymphoma in a Child: A Case Report and Review of the Literature. Cardiovasc Pathol (2004) 13:116–9. doi: 10.1016/S1054-8807(03)00131-5
23. Baritussio A, Gately A, Pawade J, Marks DI, Bucciarelli-Ducci C. Extensive Cardiac Infiltration in Acute T-Cell Lymphoblastic Leukemia: Occult Extra-Medullary Relapse and Remission After Salvage Chemotherapy. Eur Heart J (2017) 38:1933. doi: 10.1093/eurheartj/ehw393
24. Bergler-Klein J, Knoebl P, Kos T, Streubel B, Becherer A, Schwarzinger I, et al. Myocardial Involvement in a Patient With Burkitt’s Lymphoma Mimicking Hypertrophic Cardiomyopathy. J Am Soc Echocardiogr (2003) 16:1326–30. doi: 10.1067/j.echo.2003.08.013
25. Prenner SB, Franken AA, Murphy IG, Mikati IA. Rapid Reversal of Focal Left Ventricular Hypertrophy and Systolic Dysfunction Resulting From Myocardial Infiltration by Acute Lymphoblastic Leukemia. Circulation (2016) 133:678–9. doi: 10.1161/CIRCULATIONAHA.115.020035
26. Kuchynka P, Palecek T, Lambert L, Masek M, Knotkova V. Cardiac Involvement in Lymphoma Mimicking Hypertrophic Cardiomyopathy. Kardiol Pol (2018) 76:1278. doi: 10.5603/KP.2018.0167
27. Lee PW, Woo KS, Chow LT, Ng HK, Chan WW, Yu CM, et al. Images in Cardiovascular Medicine. Diffuse Infiltration of Lymphoma of the Myocardium Mimicking Clinical Hypertrophic Cardiomyopathy. Circulation (2006) 113:662–4. doi: 10.1161/CIRCULATIONAHA.105.576306
28. Vinicki JP, Cianciulli TF, Farace GA, Saccheri MC, Lax JA, Kazelian LR, et al. Complete Regression of Myocardial Involvement Associated With Lymphoma Following Chemotherapy. World J Cardiol (2013) 5:364–8. doi: 10.4330/wjc.v5.i9.364
29. Santhosh S, Bahl A, Saikia UN, Lad D, Mittal BR, Malhotra P, et al. FDG PET/CT in the Staging and Follow-Up of Primary Cardiac ‘T’ Cell Lymphoma Presenting as Hypertrophic Cardiomyopathy. J Nucl Cardiol (2016) 23:581–4. doi: 10.1007/s12350-015-0238-9
30. Lee GY, Kim WS, Ko YH, Choi JO, Jeon ES. Primary Cardiac Lymphoma Mimicking Infiltrative Cardiomyopathy. Eur J Heart Fail (2013) 15:589–91. doi: 10.1093/eurjhf/hfs193
31. Kakefuda Y, Sato A, Hoshi T, Ishizu T, Tada H, Satomi K, et al. Isolated Cardiac Involvement of B-Cell Acute Lymphoblastic Leukemia Mimicking Acute Myocardial Infarction With Persistent Broad ST-Segment Elevation. Circulation (2012) 125:979–82. doi: 10.1161/CIRCULATIONAHA.111.050930
32. Fursevich D, Zuchowski C, Limback J, Kendall M, Ramirez A, Fanaian N, et al. Leukemic Ischemia: A Case of Myocardial Infarction Secondary to Leukemic Cardiac Involvement. Case Rep Cardiol (2017) 2017:7298347. doi: 10.1155/2017/7298347
33. Vivas D, Ruiz-Mateos B, Franco E. Acute Myocardial Infarction Secondary to Direct Myocardial Infiltration by a Malignant Neoplasia. Eur Heart J (2010) 31:2261. doi: 10.1093/eurheartj/ehq177
34. Geçmen Ç, Geçmen G, Kahyaoğlu M, Önal Ç, İzgi İA. Case Images: Cardiac Infiltration of Leukemia With Persistent ST Segment Elevation. Turk Kardiyol Dern Ars (2016) 44:618. doi: 10.5543/tkda.2016.65737
35. Cheng H, Feldman T, Butt Y, Chow KF, Yang XY, Bhattacharyya PK, et al. T-Cell Prolymphocytic Leukemia With Extensive Cardiovascular Infiltrate Leading to Multiple Myocardial Infarctions and Cardiac Death. Tex Heart Inst J (2014) 41:626–30. doi: 10.14503/THIJ-13-3581
36. Perkkiö M, Tikanoja T, Marin S. Leukemic Coronary Artery Occlusion in a Child With Acute Lymphoblastic Leukemia. Pediatr Cardiol (1997) 18:64–5. doi: 10.1007/s002469900113
37. Yu H, Wertheim G, Shankar S, Paessler M, Aplenc R, Pillai V. Marked Eosinophilia Masking B Lymphoblastic Leukemia. Am J Hematol (2016) 91:543–4. doi: 10.1002/ajh.24266
38. Ferruzzi V, Santi E, Gurdo G, Arcioni F, Caniglia M, Esposito S. Acute Lymphoblastic Leukemia With Hypereosinophilia in a Child: Case Report and Literature Review. Int J Environ Res Public Health (2018) 15:1169. doi: 10.3390/ijerph15061169
39. Song G, Liu H, Sun F, Gu L, Wang S. Acute Lymphocytic Leukemia With Eosinophilia: A Case Report and Review of the Literature. Aging Clin Exp Res (2012) 24:555–8. doi: 10.3275/8337
40. Wilson F, Tefferi A. Acute Lymphocytic Leukemia With Eosinophilia: Two Case Reports and a Literature Review. Leuk Lymphoma (2005) 46:1045–50. doi: 10.1080/10428190500085537
Keywords: acute lymphoblastic leukemia, cardiac problems, management, recommendations, misdiagnosis and mistreatment
Citation: Luo Z, Cheng J and Wang Y (2022) Cardiac Infiltration as the First Manifestation of Acute Lymphoblastic Leukemia: A Systematic Review. Front. Oncol. 12:805981. doi: 10.3389/fonc.2022.805981
Received: 31 October 2021; Accepted: 03 January 2022;
Published: 27 January 2022.
Edited by:
Charles De Bock, Children’s Cancer Institute, AustraliaReviewed by:
Michael Diamantidis, University Hospital of Larissa, GreeceSabrina Spoto, ARNAS Ospedali Civico Di Cristina Benfratelli, Italy
Copyright © 2022 Luo, Cheng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanggan Wang, d2IwMDA4MTNAd2h1LmVkdS5jbg==
 Zhi Luo
Zhi Luo Jun Cheng2
Jun Cheng2 Yanggan Wang
Yanggan Wang