- Department of Neurosurgery, The First Affiliated Hospital of Nanchang University, Nanchang, China
Objective: Postoperative hypopituitarism associated with increased risks of premature mobility and mortality is often encountered in craniopharyngioma patients. The aim of our study is to construct nomograms related to injury types of the hypothalamus–pituitary axis (HPA) to predict hypopituitarism 1 year after surgery.
Methods: Craniopharyngioma patients undergoing initial endoscopic endonasal surgery between December 2012 and March 2021 in our center were retrospectively reviewed, and injury types of the HPA were categorized according to intraoperative endoscopic observation. Included patients were randomly divided into a training group and a validation group. Nomograms were established based on the results of multivariate logistic analysis. The predictive performance of the nomograms was evaluated in the training and validation groups.
Results: A total of 183 patients with craniopharyngioma were enrolled, and seven injury types of the HPA were summarized. Relative to intact HPA, exclusive hypothalamus injury significantly increased the risk of anterior (OR, 194.174; 95% CI, 21.311–1769.253; p < 0.001) and posterior pituitary dysfunction (OR, 31.393; 95% CI, 6.319–155.964; p < 0.001) 1 year after surgery, while exclusively sacrificing stalk infiltrated by tumors did not significantly increase the risk of anterior (OR, 5.633; 95% CI, 0.753–42.133; p = 0.092) and posterior pituitary dysfunction (OR, 1.580; 95% CI, 0.257–9.707; p = 0.621) 1 year after surgery. In the training group, the AUCs of nomograms predicting anterior and posterior pituitary dysfunction 1 year after surgery were 0.921 and 0.885, respectively, compared with 0.921 and 0.880 in the validation group.
Conclusions: Intact hypothalamus structure is critical in maintaining pituitary function. Moreover, our preliminary study suggests that the pituitary stalk infiltrated by craniopharyngioma could be sacrificed to achieve radical resection, without substantially rendering significantly worse endocrinological efficiency 1 year after surgery. The user-friendly nomograms can be used to predict hypopituitarism 1 year after surgery.
Introduction
Endocrinological deficiency associated with increased risks of premature mobility and mortality (1–3) is often encountered after surgery secondary to the injury of the hypothalamus–pituitary axis (HPA) in patients with craniopharyngioma (CP) (4–6). Meanwhile, it is well understood that the HPA plays a pivotal role in maintaining pituitary function. However, when evaluating endocrinological outcomes following surgery or addressing the issue that whether the pituitary stalk infiltrated by tumors can be sacrificed to achieve radical resection, previous studies only analyzed the effects of either the injury of the pituitary stalk (7–11) or the injury of the hypothalamus (12, 13) or the injury of the pituitary (14) on pituitary function, not mentioning any information about whether or not the other part of the HPA was injured. Given that the combined injury of the HPA is often encountered in patients with surgically treated CP due to different topographical characteristics of tumors, it is necessary to elucidate the impacts of combined injuries of the HPA on pituitary function, which is very helpful to comprehensively evaluate the impacts of the manipulation of the HPA on pituitary function and further tailor individual surgical strategies. Besides, risk factors associated with postoperative hypopituitarism in patients with CP are not well understood, which are pivotal to making informed decisions prior to and during surgery, aiming to improve endocrinological outcomes. Further, to date, there have been no prediction models to predict endocrinological deficiency for CP patients during follow-up, which is helpful to tailor individual endocrinological follow-up plans for patients with a high rate of postoperative endocrinological deficiency.
To address these gaps in the literature, we categorized the injury of the HPA into seven types based on intraoperative endoscopic observation and constructed nomograms incorporating independent risk factors to predict endocrinological deficiency 1 year after surgery.
Materials and Methods
Patient Population
After obtaining the board approval of the local ethics committee, the medical files and imaging data of consecutive craniopharyngioma patients who underwent fully endoscopic endonasal approach (EEA) between December 2012 and March 2021 in our center were retrospectively reviewed. Inclusion criteria were listed as follows: (1) the diagnosis of craniopharyngioma was histologically confirmed; (2) medical records were complete, including demographic data, pre- and postoperative imaging data, preoperative assessment, tumor characteristics, intraoperative videos, surgical results and complications, postoperative management, follow-up, and endocrinological outcomes; and (3) endocrinological evaluation with a minimum 1-year follow-up was required. Patients undergoing reoperations were excluded because the possible initial manipulation of the HPA could affect the identification of the injury type of the HPA and the endocrinological outcomes. In addition, patients undergoing tumor recurrence or regrowth within 1 year of follow-up were excluded, because of possible effects on endocrinological outcomes. The manuscript conforms to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.
Tumor size estimated as the maximal tumor diameter, tumor location, tumor consistency, calcification, and hydrocephalus were determined by MR images and computed tomography (CT) scans before surgery. Regarding the extent of resection (EOR), gross total resection (GTR) was defined as no residual tumor evaluated by postoperative contrast-enhanced MR images acquired within 72 h after surgery. In contrast, cases in which ≥80% of the tumor was resected were deemed as subtotal resection (STR) (9).
Regarding the injury of the HPA, seven injury categories, including intact HPA (HPA-intact type; Figure 1A), exclusive pituitary stalk sacrifice (PSS) type (Figure 1B), hypothalamic injury combined with pituitary stalk sacrifice (HI+PSS) type (Figure 1C), hypothalamic injury combined with pituitary stalk sacrifice and pituitary gland injury (HI+PSS+PGI) type (Figure 1D), exclusive hypothalamic injury (HI) type (Figure 1E), exclusive pituitary gland injury (PGI) type (Figure 1F), and pituitary stalk sacrifice combined with pituitary gland injury (PSS+PGI) type (Figure 1G), were defined based on intraoperative videos. For the purpose of our study, we defined HI as the lack of the integrity of the hypothalamus, including mild HI, unilateral HI, and bilateral HI, which we have previously described elsewhere (15). Given that the pituitary stalk partly anatomically preserved may show function, only patients subjected to complete transection of the PS were included into the PSS group. Patients with intrasellar CP or supradiaphragmatic CP extending into the intrasellar were deemed as having PGI because of inevitable intraoperative manipulation of the pituitary gland. The confirmation of injury types was conducted by two independent neurosurgeons who were blinded for clinical outcomes. If they failed to reach consensus, a third neurosurgeon was assigned to make the final decision.
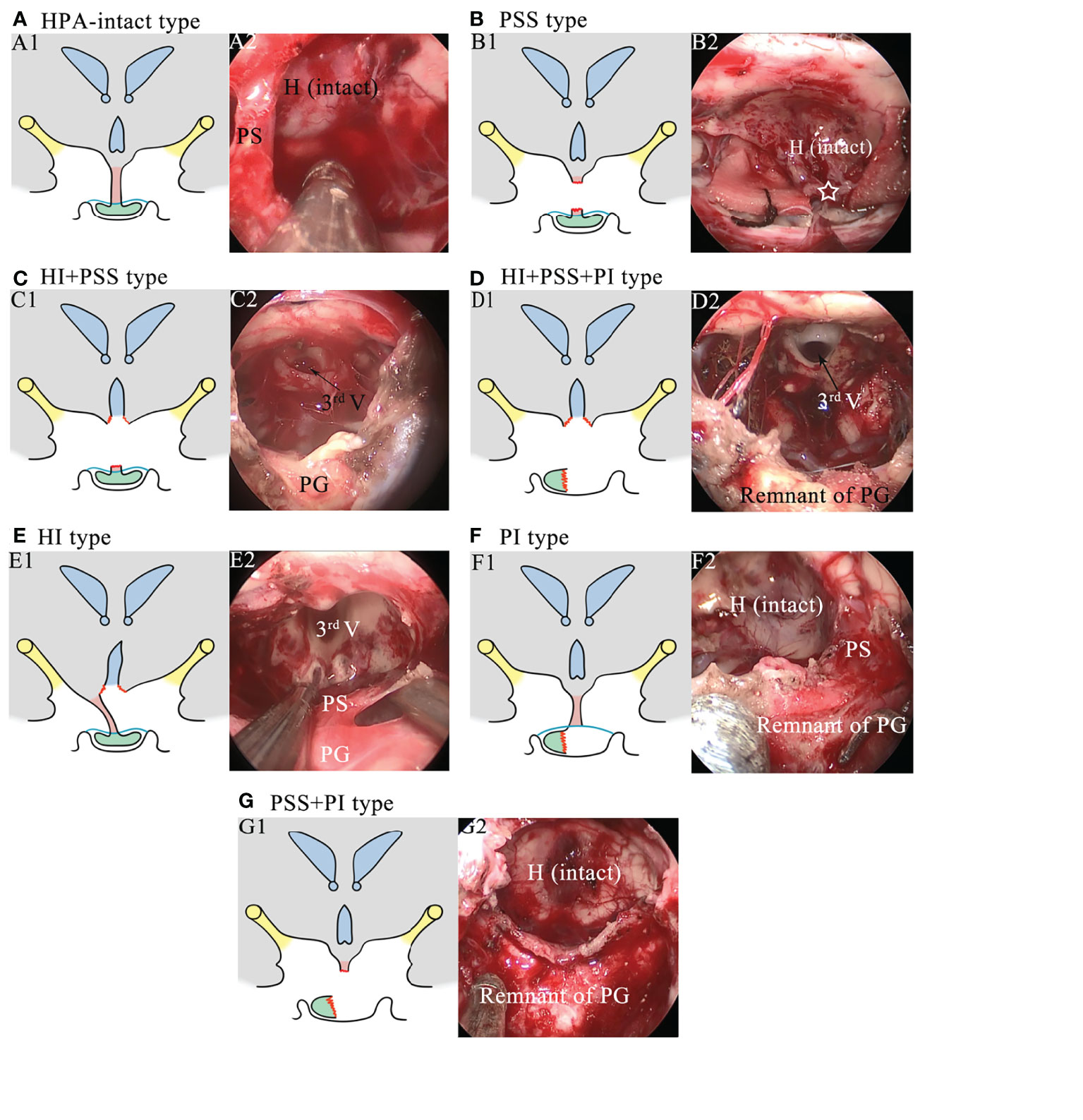
Figure 1 Schematics and intraoperative images showing the injury types of the hypothalamus–pituitary axis. (A) Schematic of the HPA-intact type (A1) and the corresponding intraoperative image (A2). The hypothalamus, the pituitary stalk, and the pituitary gland are intact. H, hypothalamus; PS, pituitary stalk. (B) Schematic of the PSS type (B1) and the corresponding intraoperative image (B2). The pituitary stalk is resected, while the hypothalamus and the pituitary gland are intact. The white star represents the stalk proximal stump. (C) Schematic of the HI+PSS type (C1) and the corresponding intraoperative image (C2). The third ventricle floor is open, and the pituitary stalk is resected, while the pituitary gland is intact. 3rd V, the third ventricle; PG, pituitary gland. (D) Schematic of the HI+PSS+PGI type (D1) and the corresponding intraoperative image (D2). The third ventricle floor is open, the pituitary stalk is resected, and the pituitary gland is injured, rendering a remnant of the pituitary gland. (E) Schematic of the HI type (E1) and the corresponding intraoperative image (E2). The third ventricle floor is open, while the pituitary stalk and the pituitary gland are preserved. (F) Schematic of the PGI type (F1) and the corresponding intraoperative image (F2). The pituitary gland is injured, while the hypothalamus and the pituitary stalk are preserved. (G) Schematic of the PSS+PGI type (G1) and the corresponding intraoperative image (G2). The hypothalamus is intact, while the pituitary stalk is resected and the pituitary gland is injured, rendering a remnant of the pituitary gland.
Endocrinological Evaluation
Presurgical and postsurgical endocrinological status was evaluated as described previously (16, 17). Partial hypopituitarism was defined as hormone deficiencies in one or two axes, and panhypopituitarism was defined as hormone deficiencies in three or more axes. Diabetes insipidus (DI) was evaluated preoperatively and postoperatively. Patients were diagnosed as having DI if they had polydipsia (excessive drinking; >3 l/day) and polyuria (excessive urination; >50 ml/kg body weight/24 h) combined with urine-specific gravity <1.005 and urine osmolality <300 mOsm/kg, as well as a positive response to desmopressin (15, 18). All the patients were recommended to receive imaging and clinical evaluation 3 months, 6 months, 12 months, and every 2 years subsequently after discharge. During follow-up, serum pituitary hormone levels were measured, and an endocrinologist analyzed the results. The protocol of hormone replacement was determined by the endocrinologist. Anterior pituitary dysfunction during follow-up was defined as replacement hormone required for at least one anterior pituitary hormone axis (14). For the purpose of our study, we focused on the endocrinological outcomes at 1 year after surgery and permanent DI representing posterior pituitary function was defined as at least 1 year of the need for desmopressin therapy after surgery (19).
Surgical Procedure, Surgical Principle, and Adjuvant Treatment
All procedures were performed using an EEA. An image-guided neuronavigation system was employed for all procedures. All surgeries were performed by the chief surgeon (TH) and assisted by attending surgeons (BT, SHX, and LY). The surgical nuances of CP resection did not differ significantly from those described in the literature (20). After tumor resection, the closure was performed with a multilayered reconstruction, as described in previous literatures (21). Of note, the following surgical principles were strictly followed during CP resections. First, all procedures, especially in the process of tumor dissection, must be performed under direct visualization, avoiding pulling blindly. Second, carefully identifying and sharply combined with bluntly dissecting the cleavage plane between tumors and the HPA were warranted to achieve radical resection of CP and preserve the anatomical integrity of the structure of the HPA involved in the attachment to the greatest extent. When the stalk was infiltrated by tumors and extremely thin, the stalk would be sacrificed to achieve a radical resection. In addition, less aggressive resection was performed for patients with the extensive involvement of the hypothalamus or with adherence of a thin CP cyst capsule to critical vessels to avoid the higher risk of hypothalamic damage and fatal complications. Radiotherapy was routinely performed in CP patients undergoing STR 3 months after surgery.
Statistical Analysis and Nomogram Construction
All patients were randomly divided into a training group and a validation group in a 7:3 ratio. Continuous variables were reported as mean (± standard deviation) or median with an interquartile range (IQR), which was determined by the Shapiro–Wilk test. Categorical data were reported as counts and proportions in each group. The baseline characteristics between the two groups were compared via the chi-square test (Fisher’s exact test where appropriate) for categorical variables and two-tailed Student (Mann–Whitney U-test where appropriate) for continuous variables. Interrater reliability for classifying the injury type of the HPA was performed using Cohen’s kappa, indicating a good level of agreement (κ = 0.781). Univariate and stepwise multivariate logistic regression was used to determine independent risk factors associated with the need for anterior pituitary hormone replacement 1 year after surgery and permanent DI in the training cohort. First, we performed univariate logistic regression analysis using all the clinical variables associated with endocrinological deficiency after CP surgery based on our subject matter knowledge and the established risk factors identified by previous literatures (7, 10, 14). Then, a stepwise multivariate logistic regression analysis was performed using variables with p < 0.1 in the univariate logistic regression analysis. A nomogram was drawn based on the results of the multivariate logistic regression analysis. To evaluate the discrimination efficiency and calibration power of the nomogram, the area under the receiver operating characteristic (ROC) curve (AUC) and calibration curve with 1,000 bootstrap samples were employed in the training cohort, respectively, and validated in the validation cohort. Regarding AUC, a value >0.8 was thought to have a good discrimination according to the grading guidelines (22). At last, for clinical use of the model, the total scores were calculated based on the nomogram for each patient. The procedures of conducting the nomograms followed the recommendations of “seven steps for development and an ABCD for validation” proposed by Steyerberg et al. (23).
SPSS (version 25.0; IBM Corp., Armonk, New York) was used to perform statistical analyses and plot ROC curves, while GraphPad Prism version 8 (GraphPad Software, La Jolla, CA) was used to plot histograms. R software (version 4.0.3; http://www.Rproject.org) was used to plot nomograms and calibration curves, with the “rms” package used. A two-sided level of p < 0.05 was considered statistically significant unless indicated otherwise.
Results
Clinical Characteristics
According to the inclusion and exclusion criteria, after excluding the patients undergoing reoperations, cases with perioperative mortality or loss to follow-up, and patients with endocrinological evaluation after surgery less than 1 year, 183 CP patients (104 men and 79 women) were enrolled into the final study cohort. The median follow-up duration of the entire cohort was 29.0 months (IQR 19–46 months). The included patients were randomly divided into the training (138 cases) and validation cohorts (45 cases).
Clinical data associated with risk of need for anterior pituitary hormone replacement 1 year after surgery and permanent DI are listed in Table 1. The baseline characteristics between these two cohorts were similar without significant difference. The number of permanent DI and needs for anterior pituitary hormone replacement 1 year after surgery was 71 (51.4%) and 94 (68.1%) in the training cohort, respectively, while 22 (48.9%) and 30 (66.7%) in the validation cohort, respectively.
Risk Factors for Need for Anterior Pituitary Hormone Replacement 1 Year After Surgery
Variables including presurgical, surgical, and postsurgical factors were selected to determine candidate risk factors via univariate logistic regression analysis. In this step, preop pituitary function (p = 0.001), tumor location (p = 0.012), and injury type (p < 0.001) were identified as potential risk factors and further included into stepwise multivariate logistic regression analysis to determine independent risk factors associated with the need for anterior pituitary hormone replacement 1 year after surgery (Table 2). On multivariate analysis, with results reported as odds ratio (OR, 95% CI), preop pituitary function (for partial hypopituitarism vs. normal, 4.184 [1.225–14.292]; for panhypopituitarism vs. normal, 13.742 [1.345–140.417]) and injury type (for HI vs. HPA-intact, 194.174 [21.311–1769.253]; for HI+PSS vs. HPA-intact, 89.443 [13.642–586.416]; for HI+PSS+PGI vs. HPA-intact, 68.111 [4.975–932.565]; for PSS vs. HPA-intact, 5.633 [0.753–42.133]; for PGI vs. HPA-intact, 159.790 [11.974–2132.357]; for PSS+PGI vs. HPA-intact, 31.921 [3.320–306.873]) were determined as independent risk factors and further used to form a nomogram (Table 3). Intriguingly, the difference in the risk of need for anterior pituitary hormone replacement 1 year after surgery between PSS and HPA-intact did not reach statistical significance, although stalk sacrifice had a higher risk (OR, 5.633; 95% CI, 0.753–42.133; p = 0.092).
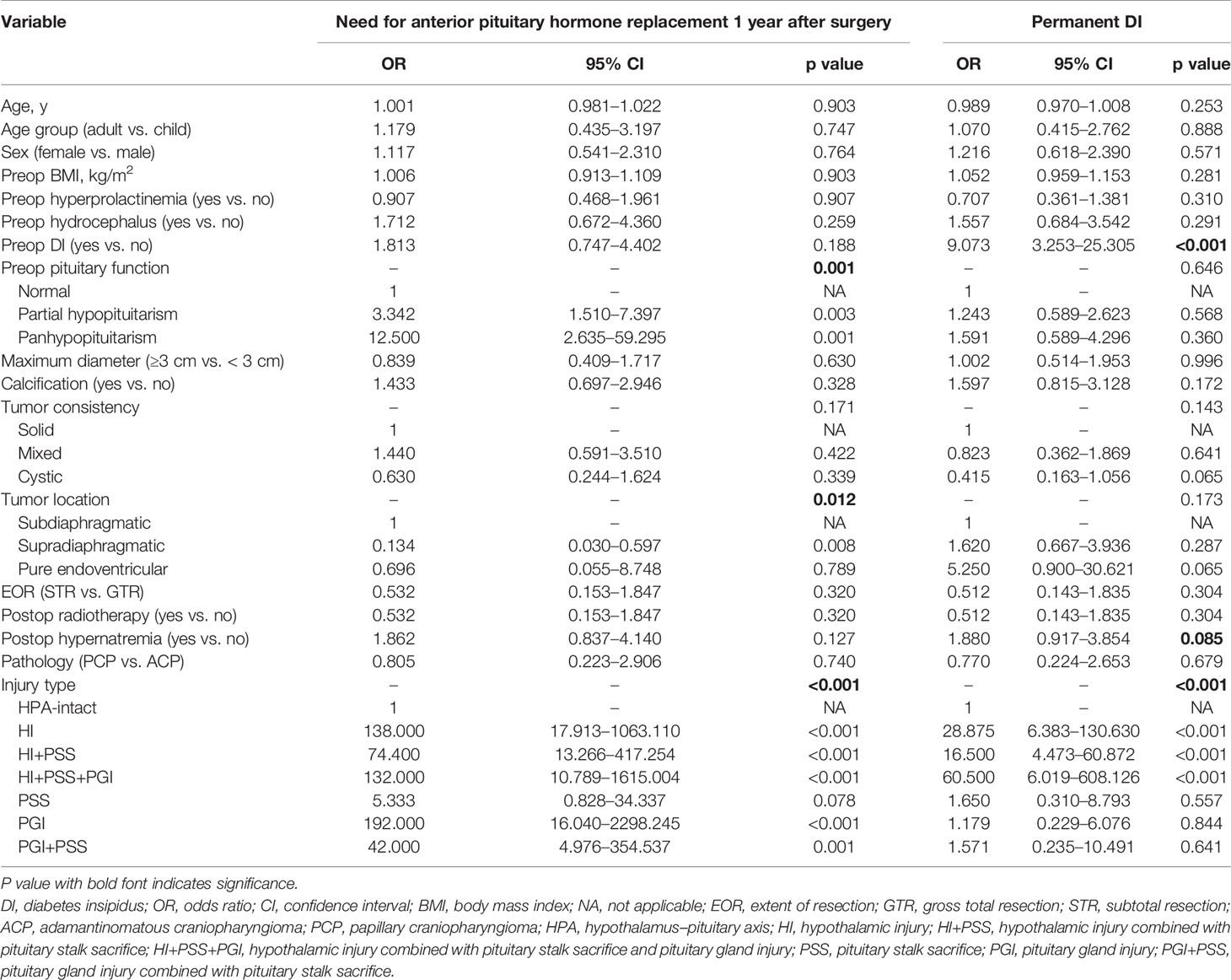
Table 2 Univariate logistic regression analysis of need for anterior pituitary hormone replacement 1 year after surgery and permanent diabetes insipidus (DI) based on pre-, intra-, and postoperative data in the training cohort.
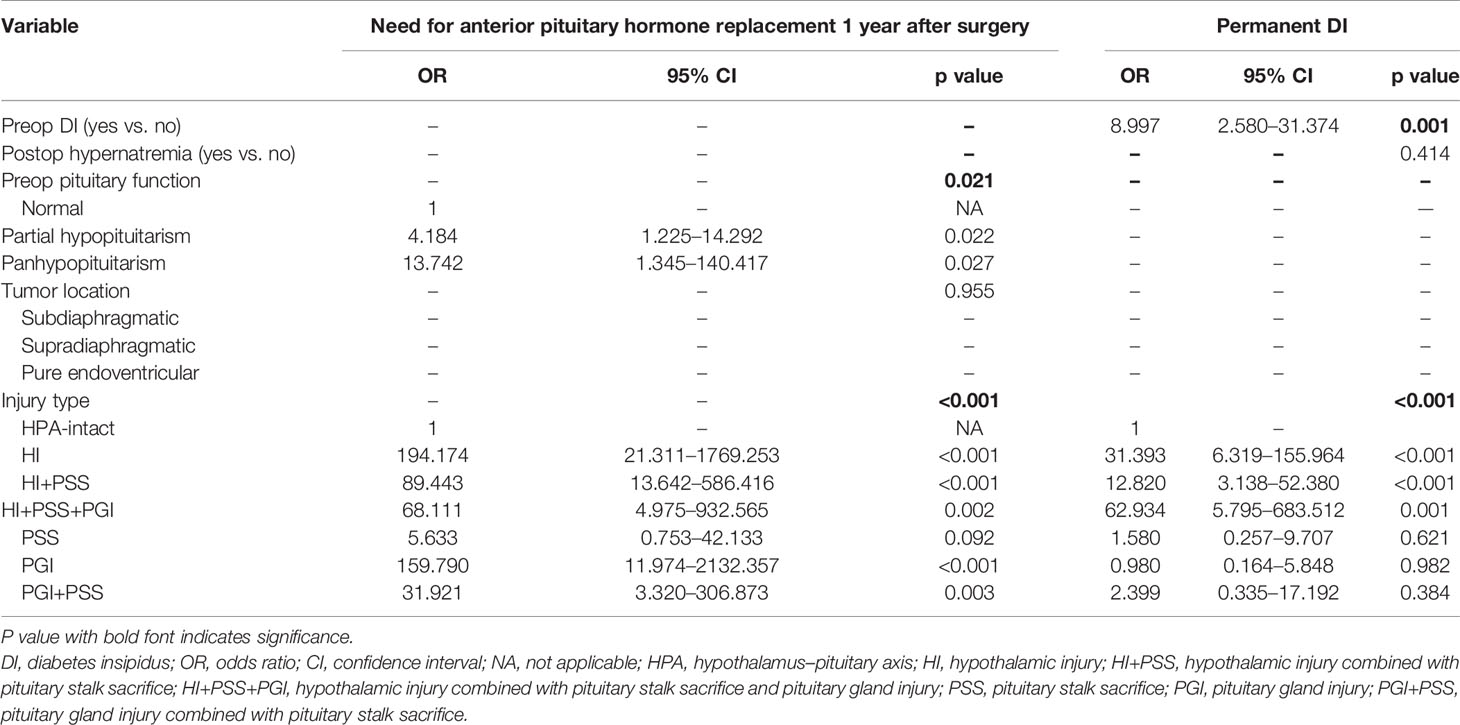
Table 3 Multivariate logistic regression analysis of need for anterior pituitary hormone replacement 1 year after surgery and permanent diabetes insipidus (DI) in the training cohort.
Risk Factors for Permanent DI
Similarly, through univariate (Table 2) and stepwise multivariate analyses, preop DI (yes vs. no, 8.997 [2.580–31.374]) and the injury type of HPA (for HI vs. HPA-intact, 31.393 [6.319–155.964]; for HI+PSS vs. HPA-intact, 12.820 [3.138–52.380]; for HI+PSS+PGI vs. HPA-intact, 62.934 [5.795–683.512]; for PSS vs. HPA-intact, 1.580 [0.257–9.707]; for PGI vs. HPA-intact, 0.980 [0.164–5.848]; for PSS+PGI vs. HPA-intact, 2.399 [0.335–17.192]) were determined as independent risk factors associated with permanent DI and were used to form a nomogram. Note that when we compared PSS vs. HPA-intact, PGI vs. HPA-intact, and PSS+PGI vs. HPA-intact, the significant difference in the risk of permanent DI was not found (Table 3).
Difference in Endocrinological Deficiency 1 Year After Surgery Between Injury Types
Further, we investigated the difference in endocrinological deficiency 1 year after surgery between the injury types of the HPA in the entire cohort (Figure 2). With regard to anterior pituitary dysfunction, the significant difference in the rate of need for anterior pituitary hormone replacement 1 year after surgery between injury types was found (p < 0.001). In addition, the rate of need for anterior pituitary hormone replacement 1 year after surgery was significantly lower in HPA-intact type and PSS type than HI type, HI+PSS type, HI+PSS+PGI type, PGI type, and PGI+PSS type (p < 0.05), whereas the difference between HPA-intact type and PSS type was not statistically significant (p > 0.05). Intriguingly, the difference in the rate of need for anterior pituitary hormone replacement 1 year after surgery between HI type, HI+PSS type, and HI+PSS+PGI type did not reach statistical significance (p > 0.05, Figure 2A). Regarding posterior pituitary dysfunction, there was a significant difference in the rate of permanent DI between injury types (p < 0.001). Moreover, the rate of permanent DI was significantly lower in HPA-intact type, PSS type, PGI type, and PGI+PSS type than HI type, HI+PSS type, and HI+PSS+PGI type (p < 0.05), whereas the difference between HPA-intact type, PSS type, PGI type, and PGI+PSS type was not statistically significant (p > 0.05). Likewise, the difference in the rate of permanent DI between HI type, HI+PSS type, and HI+PSS+PGI type did not reach statistical significance (p > 0.05, Figure 2B).
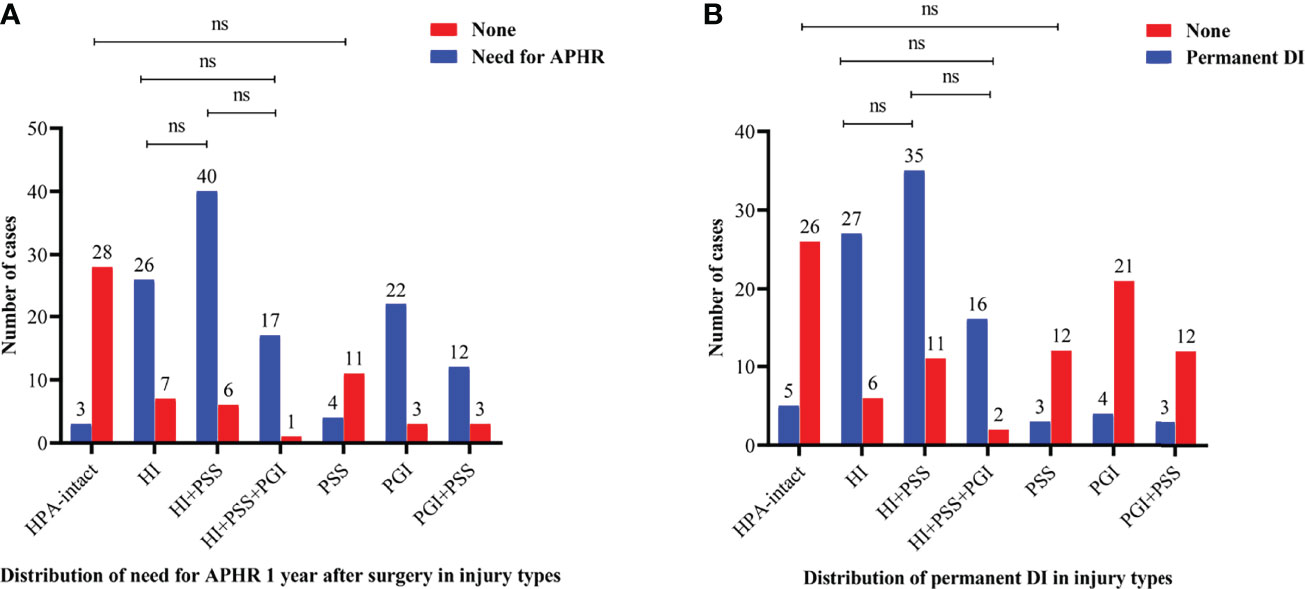
Figure 2 Bar charts showing the distributions of need for anterior pituitary hormone replacement 1 year after surgery (A) and permanent DI (B) between injury types in the entire cohort. HPA, hypothalamus–pituitary axis; PSS, pituitary stalk sacrifice; PGI+PSS, pituitary gland injury combined with pituitary stalk sacrifice; HI+PSS+PGI, hypothalamic injury combined with pituitary stalk sacrifice and pituitary gland injury; HI+PSS, hypothalamic injury combined with pituitary stalk sacrifice; PGI, pituitary gland injury; HI, hypothalamic injury; APHR, anterior pituitary hormone replacement; DI, diabetes insipidus; ns, not significant.
Nomograms and Model Performance
Nomograms based on the aforementioned independent risk factors to predict the probability of need for anterior pituitary hormone replacement 1 year after surgery and permanent DI are shown in Figure 3. The predicted probability of need for anterior pituitary hormone replacement 1 year after surgery and permanent DI for CP patients could be obtained, based on the sum of each variable score. Higher total scores calculated from the nomograms were associated with higher risk to suffer from endocrinological deficiency 1 year after surgery. For example, a CP patient with preoperative partial hypopituitarism, DI, and intraoperative pituitary stalk sacrifice would have a total of 110 scores (50 scores for partial hypopituitarism and 60 scores for pituitary stalk sacrifice) and a total of 92 scores (84 scores for preoperative DI and 18 scores for pituitary sacrifice), for a predicted need for anterior pituitary hormone replacement 1 year after surgery and permanent DI of 90.0% and 84.0%, respectively.
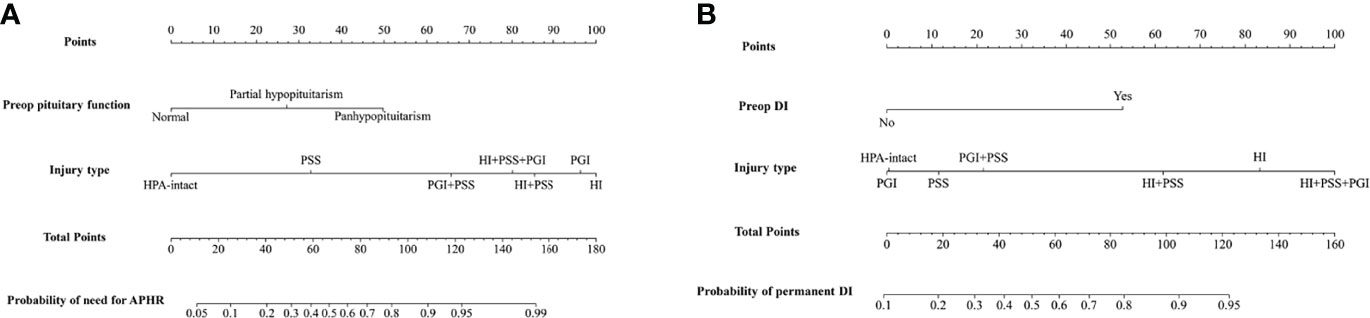
Figure 3 Nomograms to predict the rate of need for anterior pituitary hormone replacement 1 year after surgery (A) and permanent diabetes insipidus (B) in patients with craniopharyngioma. HPA, hypothalamus–pituitary axis; PSS, pituitary stalk sacrifice; PGI+PSS, pituitary gland injury combined with pituitary stalk sacrifice; HI+PSS+PGI, hypothalamic injury combined with pituitary stalk sacrifice and pituitary gland injury; HI+PSS, hypothalamic injury combined with pituitary stalk sacrifice; PGI, pituitary gland injury; HI, hypothalamic injury; APHR, anterior pituitary hormone replacement; DI, diabetes insipidus.
Further, ROC curves with AUCs and calibration curves were used to evaluate the performance of the nomograms. In the training cohort, for need for anterior pituitary hormone replacement 1 year after surgery and permanent DI predictions, the nomograms showed good discriminative abilities with AUCs of 0.921 and 0.885, respectively (Figures 4A, B). Calibration curves showed that the prediction curves were appropriately consistent with the ideal ones, which indicated that the nomograms had good calibration powers (Figures 4C, D). In addition, we validated the stability of the nomograms in the validation cohort. Similarly, good discriminative abilities with AUCs of 0.921 and 0.880 and moderate calibration powers of the nomograms were indicated by the ROC curves (Figures 5A, B) and calibration plots (Figures 5C, D), respectively.
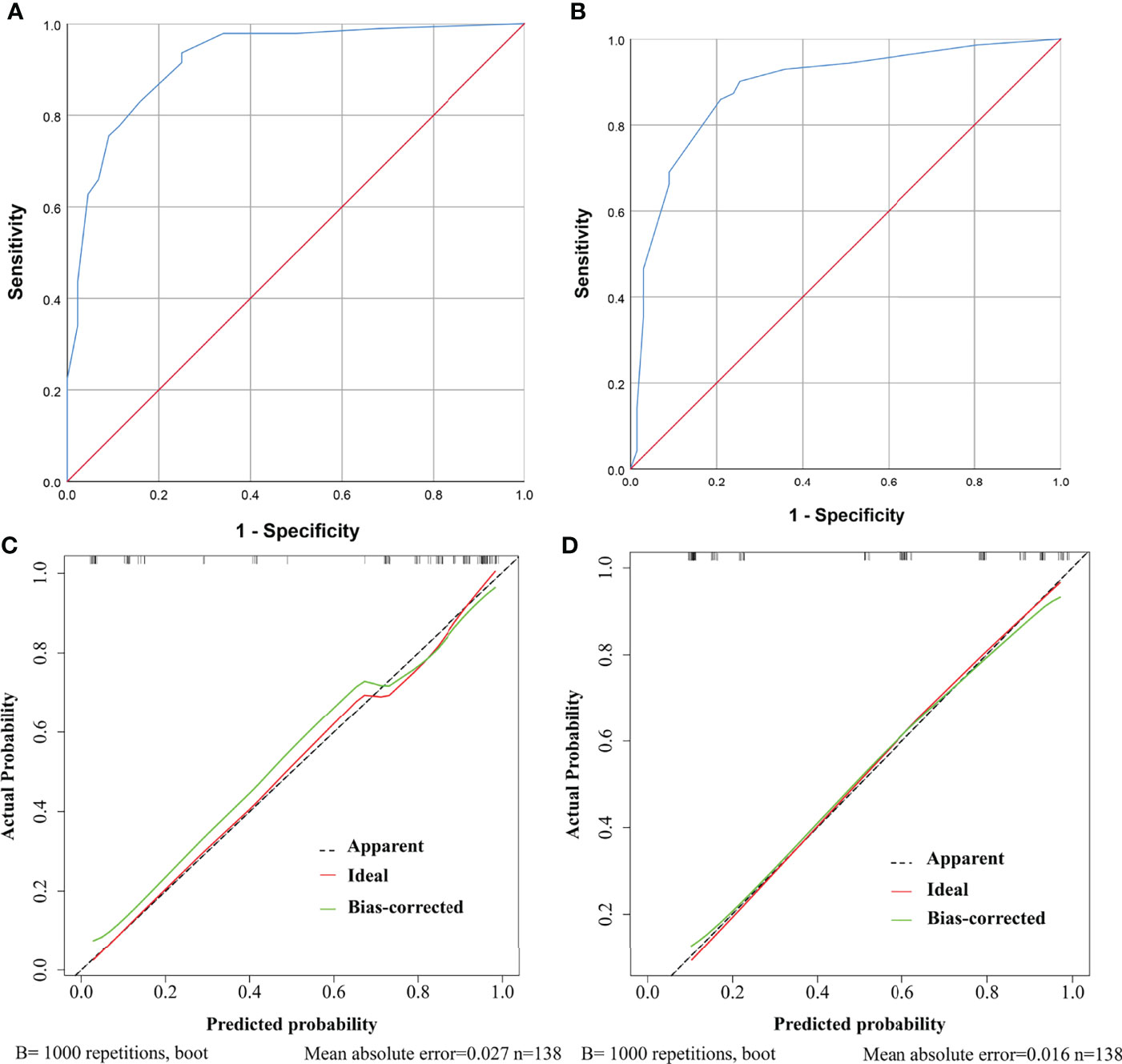
Figure 4 ROC curves and calibration curves of the models showing predictive performance for need for anterior pituitary hormone replacement 1 year after surgery and permanent diabetes insipidus (DI) in the training cohort. (A) The AUC of the model for need for anterior pituitary hormone replacement is 0.921, with 95% CI ranging from 0.871 to 0.970. (B) The AUC of the model for permanent DI is 0.885, with 95% CI ranging from 0.827 to 0.943. (C) Calibration curve of the prediction model for need for anterior pituitary hormone replacement. The grey dashed line represents the apparent curve (non-correction), the green line represents the bias-correction curve, and the red line represents the ideal curve. B = 1,000 repetitions; n = 138, mean absolute error = 0.027. (D) As in (C) but for permanent DI.
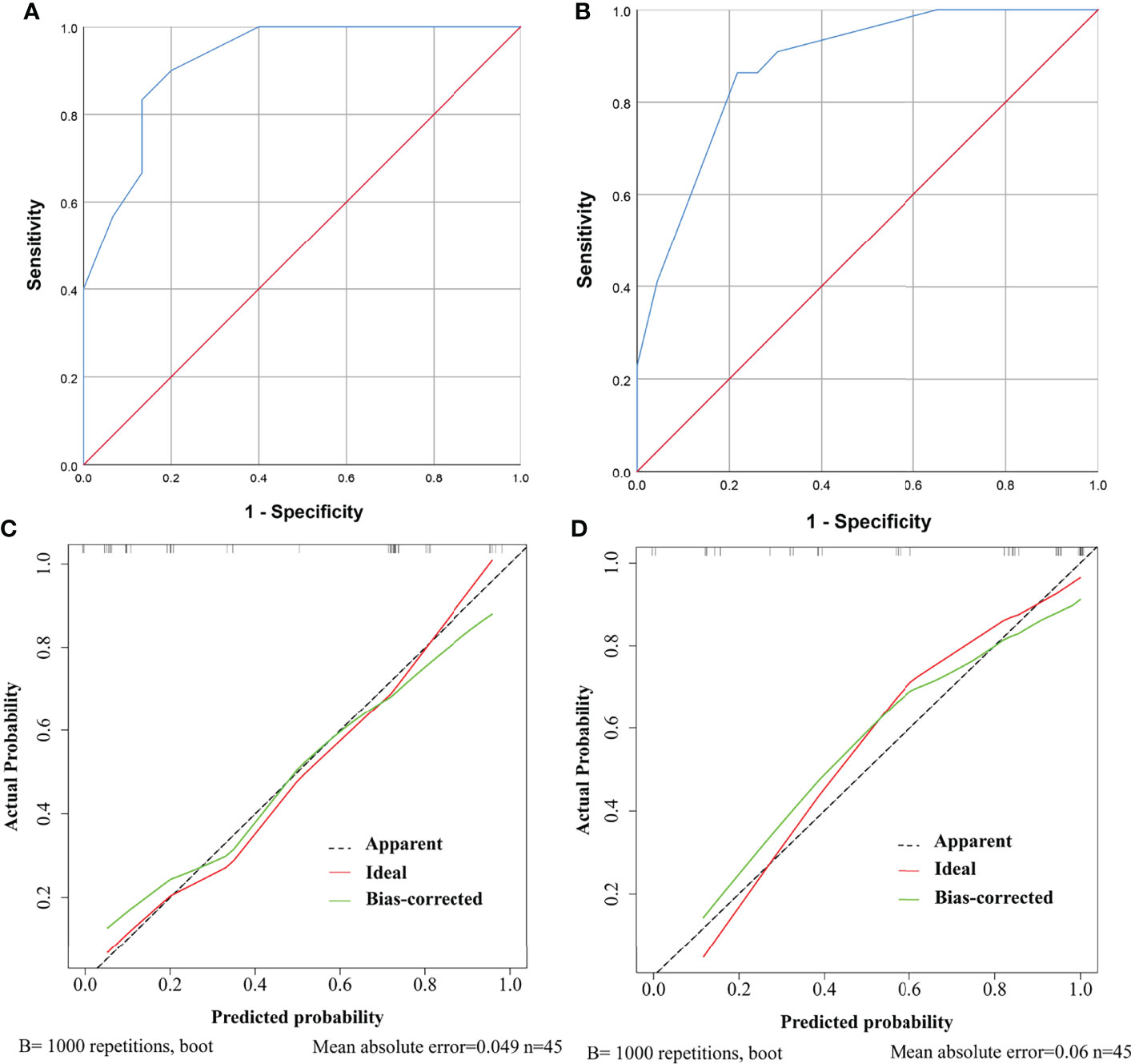
Figure 5 ROC curves and calibration curves of the models showing predictive performance for need for anterior pituitary hormone replacement 1 year after surgery and permanent diabetes insipidus (DI) in the validation cohort. (A) The AUC of the model for need for anterior pituitary hormone replacement is 0.921, with 95% CI ranging from 0.834 to 1.000. (B) The AUC of the model for permanent DI is 0.880, with 95% CI ranging from 0.782 to 0.979. (C) Calibration curve of the prediction model for need for anterior pituitary hormone replacement. The grey dashed line represents the apparent curve (non-correction), the green line represents the bias-correction curve, and the red line represents the ideal curve. B = 1,000 repetitions; n = 45, mean absolute error = 0.049. (D) As in (C) but for permanent DI.
Discussion
Via categorizing the injury of the HPA into seven types, our study shows that although the stalk and the pituitary were anatomically preserved, relative to the HPA-intact type, the exclusive injury of the hypothalamus could significantly increase the risk of endocrinological deficiency 1 year after surgery. In addition, when the hypothalamus and the pituitary were intact, relative to the HPA-intact type, sacrificing the stalk infiltrated by tumors did not significantly increase the risk of anterior pituitary dysfunction 1 year after CP surgery. Meanwhile, when the hypothalamus was intact, relative to HPA intact, sacrificing the PS infiltrated by CPs did not significantly increase the risk of permanent DI. By contrast, when the hypothalamus was injured, sacrificing the stalk infiltrated by CPs did not have a significantly worse pituitary function 1 year after surgery than stalk preservation. Based on aforementioned results, we conclude that the role of the hypothalamus in maintaining pituitary function is critical and the stalk infiltrated by CPs could be sacrificed to achieve GTR, not substantially resulting in significantly worse endocrinological efficiency 1 year after surgery. Additionally, via multivariate analysis, we found that preop pituitary function and the injury type of the HPA were independent risk factors for need for anterior pituitary hormone replacement 1 year after surgery, while preop DI and the injury type of the HPA were independent risk factors for permanent DI. At last, for the first time, we developed nomograms harboring good discriminations and calibration powers to predict endocrinological deficiency 1 year after CP resection.
As published clinical literature evaluating the ramifications of the hypothalamus damage after CP surgery mainly focused on hypothalamic obesity, cognitive dysfunction, sleep disorders, and impaired temperature regulation, knowledge about the effects of exclusive hypothalamus injury on pituitary function is limited (12, 24, 25). Via focusing on the effects of exclusive injury of the hypothalamus on anterior pituitary function after surgery, we found that patients suffering from exclusive hypothalamus injury had a significantly increased rate of need for anterior pituitary hormone replacement 1 year after surgery, which shows a critical role of the intact hypothalamus in maintaining anterior pituitary function. Moreover, we also found that exclusively sacrificing the stalk infiltrated by tumors would not significantly increase the rate of need for anterior pituitary hormone replacement 1 year after surgery. Not completely consistent with our results, previous studies reported that sacrificing the stalk infiltrated by tumors could result in worse anterior pituitary function after CP surgery (7, 10, 11). The following reasons may account for this discrepancy. First, the results in our study are based on the premise that the hypothalamus and the pituitary are intact, which is not mentioned in aforementioned studies. Second, some previous studies do not report whether or not the difference in anterior pituitary function after surgery between stalk sacrifice and stalk preservation has reached statistical significance (10, 11). At last, the different duration of follow-up is another reason. The following underlying mechanisms are thought to account for our results. First, hypothalamic releasing factors (HRFs) produced in the hypothalamus are transported along neurons to the median eminence, where they are secreted into the portal vein and sequentially stimulate the anterior pituitary to synthesize and release anterior pituitary hormones (26–30). Second, previous studies have reported that when the stalk is resected, portal vein recanalization could occur over a long period of time demonstrated by MR images (31, 32). Thus, based on aforementioned evidence, we speculate that the maintaining of anterior pituitary function 1 year after stalk sacrifice is attributed to the fact that through the preserved median eminence, the HRFs are secreted into the reestablished portal vein and sequentially act on the preserved anterior pituitary lobe (32). At last, it must be highlighted that according to our surgical strategies, the stalk sacrificed has been infiltrated or destroyed by CPs before surgery, so some degree of compensation may have existed. Nevertheless, our results require more multicenter clinical studies with a large sample size to validate.
Likewise, regarding posterior pituitary dysfunction after CP surgery, we found that the role of the intact hypothalamus in maintaining posterior pituitary function is critical and resecting the stalk below the level of the median eminence could not significantly increase the rate of permanent DI. In accordance with our results, many studies have reported that low-level stalk transection usually could not result in permanent DI (18, 32–36). The maintaining of posterior pituitary function after sacrificing stalk infiltrated by tumors may attribute to being hypersensitive to plasma-intrinsic antidiuretic hormone (ADH), occurrence of ectopic posterior lobe, and portal vein recanalization (31, 32, 35, 37). As to the underlying compensatory mechanism for DI, based on a rat experiment, Feng et al. (38) speculated that the functional ectopic posterior lobe increased the GAP-43 expression via PI3K/AKT pathways to alleviate DI after stalk sacrifice. Likewise, more clinical and laboratory studies are needed to elucidate the accurate compensatory mechanism for DI after stalk sacrifice.
In accordance with our results, preoperative pituitary dysfunction as a risk factor associated with endocrinological deficiency during follow-up has been widely reported, as preexisting endocrinological deficiency of CP patients undergoing surgery is usually only mildly improved during follow-up (9, 39, 40). There is an agreement in the literature that radiotherapy can worsen pituitary function (5, 7, 41, 42). Intriguingly, our statistical analysis excluded the role of radiotherapy as an independent risk factor associated with endocrinological deficiency 1 year after surgery. Limited sample size and relatively short time interval from receiving radiotherapy to the endpoint of our study may account for our results. In addition, some studies (43, 44) have reported that the EOR could affect endocrinological outcomes, which is not confirmed by our study. One possible explanation is that in the context that the goal of our surgery is to resect as much tumors as possible, the factors determining GTR or STR, such as adherence of CP to critical vessels, do not lead to a difference in the manipulation of HPA between GTR and STR. Thus, EOR (STR vs. GTR) has no significant difference in endocrinological outcomes, which is in accordance with previous studies (7, 10).
Identifying independent risk factors is of great importance to comprehensively understand the prognosis of a given disease. Further, a nomogram based on independent risk factors can provide more individual and accurate prognosis data for treating clinicians and patients or their relatives. In the last two decades, neurosurgical clinical prediction models have increased exponentially, for a variety of clinical outcomes (23, 45). This is the first study to construct models to predict postoperative endocrinological prognosis in patients with CP. Our nomograms were constructed following standard procedures and harbored good discriminations and calibration powers, which merits application in clinical works.
Limitations
First, due to the retrospective nature of the study, selection bias inevitably exists. Second, the present findings were based on limited data from a single center and on patients only undergoing EEA, it is necessary to validate them in multiple centers with a large sample size. Particularly, attention must be paid when interpreting our results that pituitary stalk infiltrated by tumors could be sacrificed, as only 15 patients underwent exclusive stalk sacrifice, warranting further investigation. Third, we defined endocrinological deficiency as the need for pituitary hormone replacement rather than objective measurement of pituitary hormone levels, which may underestimate the rate of pituitary deficiency, although it arguably gives a more clinically meaningful result (14). At last, the injury type of the HPA cannot be quantitatively defined, which may lead to bias when determining injury types between different neurosurgeons.
Conclusion
Collectively, our preliminary study suggests that the role of the intact hypothalamus in maintaining pituitary function is critical and the pituitary stalk infiltrated by CPs could be sacrificed to achieve GTR, not substantially resulting in significantly worse endocrinological efficiency 1 year after surgery. Moreover, our study highlights that more clinical and laboratory studies are required to elucidate the accurate mechanism and validate our results. The established nomograms with good predictive performance can provide a user-friendly tool to predict the rate of hypopituitarism 1 year after surgery, which is helpful for clinicians to better counsel patients and provide anticipatory guidance on postoperative expectations and management.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of the First Affiliated Hospital of Nanchang University. For this retrospective study, formal consent was not required.
Author Contributions
TH and JW contributed to the study’s conception and design. Analysis of the data was performed by TH, JW, XW, and LY. Material preparation and data collection were performed by JW, XW, LY, SHX, BT, ZGT, BWW, HD, YYB, LZ, and YQY. The first draft of the manuscript was written by JW, and all authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant nos. 82060246 and 81460381), Ganpo555 engineering excellence of the Jiangxi Science and Technology Department (2013), and the Key Research and Invention Plan of Jiangxi Science and Technology Department (20192BBG70026).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jasim S, Alahdab F, Ahmed A, Tamhane S, Prokop L, Nippoldt T, et al. Mortality in Adults With Hypopituitarism: A Systematic Review and Meta-Analysis. Endocrine (2017) 56(1):33–42. doi: 10.1007/s12020-016-1159-3
2. Pappachan J, Raskauskiene D, Kutty V, Clayton R. Excess Mortality Associated With Hypopituitarism in Adults: A Meta-Analysis of Observational Studies. J Clin Endocrinol Metab (2015) 100(4):1405–11. doi: 10.1210/jc.2014-3787
3. Tomlinson J, Holden N, Hills R, Wheatley K, Clayton R, Bates A, et al. Association Between Premature Mortality and Hypopituitarism. West Midlands Prospective Hypopituitary Study Group. Lancet (London England) (2001) 357(9254):425–31. doi: 10.1016/s0140-6736(00)04006-x
4. Karavitaki N, Cudlip S, Adams CB, Wass JA. Craniopharyngiomas. Endocr Rev (2006) 27(4):371–97. doi: 10.1210/er.2006-0002
5. DeVile CJ, Grant DB, Hayward RD, Stanhope R. Growth and Endocrine Sequelae of Craniopharyngioma. Arch Dis Child (1996) 75(2):108–14. doi: 10.1136/adc.75.2.108
6. Muller HL, Merchant TE, Warmuth-Metz M, Martinez-Barbera JP, Puget S. Craniopharyngioma. Nat Rev Dis Primers (2019) 5(1):75. doi: 10.1038/s41572-019-0125-9
7. Ordóñez-Rubiano EG, Forbes JA, Morgenstern PF, Arko L, Dobri GA, Greenfield JP, et al. Preserve or Sacrifice the Stalk? Endocrinological Outcomes, Extent of Resection, and Recurrence Rates Following Endoscopic Endonasal Resection of Craniopharyngiomas. J Neurosurg (2018), 1–9. doi: 10.3171/2018.6.Jns18901
8. Li K, Lu X, Yang N, Zheng J, Huang B, Li L. Association of Pituitary Stalk Management With Endocrine Outcomes and Recurrence in Microsurgery of Craniopharyngiomas: A Meta-Analysis. Clin Neurol Neurosurg (2015) 136:20–4. doi: 10.1016/j.clineuro.2015.05.019
9. Dho Y, Kim Y, Se Y, Han D, Kim J, Park C, et al. Endoscopic Endonasal Approach for Craniopharyngioma: The Importance of the Relationship Between Pituitary Stalk and Tumor. J Neurosurg (2018) 129(3):611–9. doi: 10.3171/2017.4.Jns162143
10. Honegger J, Buchfelder M, Fahlbusch R. Surgical Treatment of Craniopharyngiomas: Endocrinological Results. J Neurosurg (1999) 90(2):251–7. doi: 10.3171/jns.1999.90.2.0251
11. Jung T, Jung S, Choi J, Moon K, Kim I, Kang S. Adult Craniopharyngiomas: Surgical Results With a Special Focus on Endocrinological Outcomes and Recurrence According to Pituitary Stalk Preservation. J Neurosurg (2009) 111(3):572–7. doi: 10.3171/2008.10.Jns0880
12. Elowe-Gruau E, Beltrand J, Brauner R, Pinto G, Samara-Boustani D, Thalassinos C, et al. Childhood Craniopharyngioma: Hypothalamus-Sparing Surgery Decreases the Risk of Obesity. J Clin Endocrinol Metab (2013) 98(6):2376–82. doi: 10.1210/jc.2012-3928
13. Hayashi Y, Sasagawa Y, Oishi M, Misaki K, Kozaka K, Tachibana O, et al. Radiological and Endocrinological Evaluations With Grading of Hypothalamic Perifocal Edema Caused by Craniopharyngiomas. Pituitary (2019) 22(2):146–55. doi: 10.1007/s11102-019-00945-z
14. Waqar M, Rampersad S, Bennett D, Kearney T, Gnanalingham K. Pre- and Postoperative Need for Pituitary Hormone Replacement in non-Adenomatous Sellar and Parasellar Lesions: Importance of the Sellar Encroachment Score. Acta Neurochirurgica (2020) 162(10):2371–9. doi: 10.1007/s00701-020-04440-4
15. Yang L, Xie S, Tang B, Wu X, Tong Z, Fang C, et al. Hypothalamic Injury Patterns After Resection of Craniopharyngiomas and Correlation to Tumor Origin: A Study Based on Endoscopic Observation. Cancer Med (2020) 9(23):8950–61. doi: 10.1002/cam4.3589
16. Tang B, Xie S, Xiao L, Huang G, Wang Z, Yang L, et al. A Novel Endoscopic Classification for Craniopharyngioma Based on its Origin. Sci Rep (2018) 8(1):10215. doi: 10.1038/s41598-018-28282-4
17. Tang B, Xie S, Huang G, Wang Z, Yang L, Yang X, et al. Clinical Features and Operative Technique of Transinfundibular Craniopharyngioma. J Neurosurg (2020) 133(1):119–28. doi: 10.3171/2019.3.JNS181953
18. Christ-Crain M, Bichet DG, Fenske WK, Goldman MB, Rittig S, Verbalis JG, et al. Diabetes Insipidus. Nat Rev Dis Primers (2019) 5(1):54. doi: 10.1038/s41572-019-0103-2
19. Burke WT, Cote DJ, Penn DL, Iuliano S, McMillen K, Laws ER. Diabetes Insipidus After Endoscopic Transsphenoidal Surgery. Neurosurgery (2020) 87(5):949–55. doi: 10.1093/neuros/nyaa148
20. Kassam AB, Gardner PA, Snyderman CH, Carrau RL, Mintz AH, Prevedello DM. Expanded Endonasal Approach, A Fully Endoscopic Transnasal Approach for the Resection of Midline Suprasellar Craniopharyngiomas: A New Classification Based on the Infundibulum. J Neurosurg (2008) 108(4):715–28. doi: 10.3171/JNS/2008/108/4/0715
21. Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH, et al. A Novel Reconstructive Technique After Endoscopic Expanded Endonasal Approaches: Vascular Pedicle Nasoseptal Flap. Laryngoscope (2006) 116(10):1882–6. doi: 10.1097/01.mlg.0000234933.37779.e4
22. Kleinbaum DG, Klein M. Logistic Regression, A Self-Learning Text. 3rd ed. New York: Springer (2010).
23. Steyerberg EW, Vergouwe Y. Towards Better Clinical Prediction Models: Seven Steps for Development and an ABCD for Validation. Eur Heart J (2014) 35(29):1925–31. doi: 10.1093/eurheartj/ehu207
24. Roth CL, Eslamy H, Werny D, Elfers C, Shaffer ML, Pihoker C, et al. Semiquantitative Analysis of Hypothalamic Damage on MRI Predicts Risk for Hypothalamic Obesity. Obes (Silver Spring) (2015) 23(6):1226–33. doi: 10.1002/oby.21067
25. Van Gompel JJ, Nippoldt TB, Higgins DM, Meyer FB. Magnetic Resonance Imaging-Graded Hypothalamic Compression in Surgically Treated Adult Craniopharyngiomas Determining Postoperative Obesity. Neurosurg Focus (2010) 28(4):E3. doi: 10.3171/2010.1.FOCUS09303
26. Huo L, Munzberg H, Nillni EA, Bjorbaek C. Role of Signal Transducer and Activator of Transcription 3 in Regulation of Hypothalamic Trh Gene Expression by Leptin. Endocrinology (2004) 145(5):2516–23. doi: 10.1210/en.2003-1242
27. van Swieten MM, Pandit R, Adan RA, van der Plasse G. The Neuroanatomical Function of Leptin in the Hypothalamus. J Chem Neuroanat (2014) 61-62:207–20. doi: 10.1016/j.jchemneu.2014.05.004
28. Stamatiades GA, Kaiser UB. Gonadotropin Regulation by Pulsatile GnRH: Signaling and Gene Expression. Mol Cell Endocrinol (2018) 463:131–41. doi: 10.1016/j.mce.2017.10.015
29. Marshall JC, Kelch RP. Gonadotropin-Releasing Hormone: Role of Pulsatile Secretion in the Regulation of Reproduction. N Engl J Med (1986) 315(23):1459–68. doi: 10.1056/nejm198612043152306
30. Bluet-Pajot M, Epelbaum J, Gourdji D, Hammond C, Kordon C. Hypothalamic and Hypophyseal Regulation of Growth Hormone Secretion. Cell Mol Neurobiol (1998) 18(1):101–23. doi: 10.1023/a:1022579327647
31. Fujisawa I. Magnetic Resonance Imaging of the Hypothalamic-Neurohypophyseal System. J Neuroendocrinol (2004) 16(4):297–302. doi: 10.1111/j.0953-8194.2004.01183.x
32. Kikuchi K, Fujisawa I, Momoi T, Yamanaka C, Kaji M, Nakano Y, et al. Hypothalamic-Pituitary Function in Growth Hormone-Deficient Patients With Pituitary Stalk Transection. J Clin Endocrinol Metab (1988) 67(4):817–23. doi: 10.1210/jcem-67-4-817
33. Lipsett MB, Maclean JP, West CD, Li MC, Pearson OH. An Analysis of the Polyuria Induced by Hypophysectomy in Man. J Clin Endocrinol Metab (1956) 16(2):183–95. doi: 10.1210/jcem-16-2-183
34. Verbalis JG. Acquired Forms of Central Diabetes Insipidus: Mechanisms of Disease. Best Pract Res Clin Endocrinol Metab (2020) 34(5):101449. doi: 10.1016/j.beem.2020.101449
35. Nishizawa S, Ohta S, Oki Y. Spontaneous Resolution of Diabetes Insipidus After Pituitary Stalk Sectioning During Surgery for Large Craniopharyngioma. Endocrinological Evaluation and Clinical Implications for Surgical Strategy. Neurologia Medico Chirurgica (2006) 46(3):126–34. doi: 10.2176/nmc.46.126
36. Ikeda H, Gotoh H, Watanabe K. Outcome of Endoscopy-Assisted Microscopic Extended Transsphenoidal Surgery for Suprasellar Adult Craniopharyngiomas. Front Endocrinol (2012) 3:25. doi: 10.3389/fendo.2012.00025
37. Fujisawa I, Kikuchi K, Nishimura K, Togashi K, Itoh K, Noma S, et al. Transection of the Pituitary Stalk: Development of an Ectopic Posterior Lobe Assessed With MR Imaging. Radiology (1987) 165(2):487–9. doi: 10.1148/radiology.165.2.3659371
38. Feng Z, Ou Y, Zhou M, Wu G, Ma L, Zhang Y, et al. Functional Ectopic Neural Lobe Increases GAP-43 Expression via PI3K/AKT Pathways to Alleviate Central Diabetes Insipidus After Pituitary Stalk Lesion in Rats. Neurosci Lett (2018) 673:1–6. doi: 10.1016/j.neulet.2018.02.038
39. Koutourousiou M, Gardner P, Fernandez-Miranda J, Tyler-Kabara E, Wang E, Snyderman C. Endoscopic Endonasal Surgery for Craniopharyngiomas: Surgical Outcome in 64 Patients. J Neurosurg (2013) 119(5):1194–207. doi: 10.3171/2013.6.Jns122259
40. Cavallo L, Frank G, Cappabianca P, Solari D, Mazzatenta D, Villa A, et al. The Endoscopic Endonasal Approach for the Management of Craniopharyngiomas: A Series of 103 Patients. J Neurosurg (2014) 121(1):100–13. doi: 10.3171/2014.3.Jns131521
41. Combs S, Thilmann C, Huber P, Hoess A, Debus J, Schulz-Ertner D. Achievement of Long-Term Local Control in Patients With Craniopharyngiomas Using High Precision Stereotactic Radiotherapy. Cancer (2007) 109(11):2308–14. doi: 10.1002/cncr.22703
42. Minniti G, Saran F, Traish D, Soomal R, Sardell S, Gonsalves A, et al. Fractionated Stereotactic Conformal Radiotherapy Following Conservative Surgery in the Control of Craniopharyngiomas. Radiother Oncol (2007) 82(1):90–5. doi: 10.1016/j.radonc.2006.11.005
43. Ravindra VM, Okcu MF, Ruggieri L, Frank TS, Paulino AC, McGovern SL, et al. Comparison of Multimodal Surgical and Radiation Treatment Methods for Pediatric Craniopharyngioma: Long-Term Analysis of Progression-Free Survival and Morbidity. J Neurosurg Pediatr (2021), 1–8. doi: 10.3171/2020.11.Peds20803
44. Clark A, Cage T, Aranda D, Parsa A, Auguste K, Gupta N. Treatment-Related Morbidity and the Management of Pediatric Craniopharyngioma: A Systematic Review. J Neurosurg Pediatr (2012) 10(4):293–301. doi: 10.3171/2012.7.Peds11436
Keywords: craniopharyngioma, nomogram, endocrinological deficiency, hypothalamus–pituitary axis, pituitary stalk
Citation: Wu J, Wu X, Yang L, Xie S, Tang B, Tong Z, Wu B, Yang Y, Ding H, Bao Y, Zhou L and Hong T (2022) Nomograms to Predict Endocrinological Deficiency in Patients With Surgically Treated Craniopharyngioma. Front. Oncol. 12:840572. doi: 10.3389/fonc.2022.840572
Received: 21 December 2021; Accepted: 15 April 2022;
Published: 19 May 2022.
Edited by:
Songbai Gui, Capital Medical University, ChinaReviewed by:
Emanuele La Corte, University of Bologna, ItalyLuiz Eduardo Armondi Wildemberg, Instituto Estadual do Cérebro Paulo Niemeyer (IECPN), Brazil
Copyright © 2022 Wu, Wu, Yang, Xie, Tang, Tong, Wu, Yang, Ding, Bao, Zhou and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Hong, aHQyMDAwQHZpcC5zaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jie Wu†
Jie Wu† Le Yang
Le Yang YouQing Yang
YouQing Yang Tao Hong
Tao Hong