- 1Department of Radiation Oncology, China-Japan Union Hospital of Jilin University, Changchun, China
- 2Department of Gynecology, China-Japan Union Hospital of Jilin University, Changchun, China
Purpose: This study aims to evaluate clinical outcomes of MRI-guided adaptive brachytherapy (MR-IGABT) for each brachytherapy fraction in patients with locally advanced cervical cancer (LACC).
Methods and Materials: A retrospective analysis was performed on 97 consecutive patients with LACC treated with 44.0–50.4 Gy external beam radiotherapy (EBRT) ± concurrent platinum-containing chemotherapy followed by 4 × 7 Gy MR-IGABT between September 2014 and April 2019. Intracavitary (IC)/interstitial (IS)/hybrid intracavitary and interstitial (IC/IS) brachytherapy was used in MR-IGABT. Brachytherapy planning and dose reporting followed the GEC-ESTRO recommendations. Clinical outcomes including overall survival (OS), cancer-specific survival (CSS), progression-free survival (PFS), local control (LC), and treatment-related toxicity evaluated by the RTOG criteria were analyzed. Kaplan–Meier and univariable and multivariable Cox regression analyses were used to analyze the prognostic factor.
Results: Median follow-up was 21.1 months. Median dose to 90% (D90) of the high-risk clinical target volume (HR-CTV) was 91.7 Gy (range 76.7~107.2 Gy). Two-year OS, CSS, PFS, and LC were 83.5%, 84.1%, 71.1%, and 94.8%, respectively. Four patients (4.1%) suffered from grade 3 late gastrointestinal radiation toxicity, and no other grade 3 or greater radiation toxicity occurred. Initial HR-CTV was an independent factor of OS (p = 0.001, HR = 1.018/cm3), PFS (p = 0.012, HR = 1.012/cm3), and LC (p = 0.011, HR = 1.028/cm3). The HR-CTV D90 (p = 0.044, HR = 0.923/Gy) was an independent factor of PFS. Age was an independent factor of LC (p = 0.010, HR = 1.111/year).
Conclusion: For patients with LACC, MR-IGABT was effective and safe. It showed favorable LC, OS, and minimal toxicity. Moreover, initial HR-CTV, HR-CTV D90, and age were significant prognostic factors.
Introduction
In global cancer statistics, cervical cancer ranks fourth for both incidence and mortality in women (1). In China, cervical cancer had a significant upward trend in age-standardized incidence rates (2).
Stages IB2, IIA2, IIB, IIIA, IIIB, and IVA (FIGO 2009) cervical cancers are all locally advanced cervical cancer (LACC). To treat this type of cervical cancer, the National Comprehensive Cancer Network (NCCN) guidelines recommend the external beam radiotherapy (EBRT), concurrent platinum-containing chemotherapy, and brachytherapy (category 1) (3). As a critical component of the definitive radiation therapy, brachytherapy technology has been rapidly developing in recent years. In consideration of significant changes in the tumor regresses and the topography of the target and organs at risk during the course of treatment (4), image-guided adaptive brachytherapy (IGABT) became an individualized treatment method for patients with LACC. IGABT improves overall survival (OS) and generates a high rate of local tumor control (LC) with a moderate rate of treatment-related morbidity (5–8). IGABT has been developing particularly in Europe, North America, and Asia (9).
The preferred imaging technologies for IGABT for LACC are CT and MRI. Compared with CT, advantages of MRI lie in the soft tissue contrast and in discrimination of cervical cancer from normal uterine and adjacent tissue (10). This helps to define the tumor shrinkage and topography after EBRT (11). The NCCN guidelines recommend MRI as the best imaging modality to determine soft tissue and parametrial involvement in patients with advanced tumors (3). Even so, it is difficult for every institution to gain MRI access for each individual brachytherapy fraction (7, 12–17). Due to the limited MRI availability, some institutions use MRI only in some of the brachytherapy fractions (12, 16–18). The use of MRI-guided adaptive brachytherapy (MR-IGABT) in each fraction is still limited (18). The aim of this study was to evaluate the clinical outcomes of MR-IGABT in each fraction for Chinese patients with LACC.
Materials and Methods
Patients
Ninety-seven consecutive patients were included in this retrospective study, treated between September 2014 and April 2019. The following eligibility criteria were applied: patients with stages IB2 to IVA (FIGO 2009), who underwent the MR-IGABT (4 × 7 Gy) in our institution and did not have a previous history of malignancy. The present study was approved by the ethics committee of our institution.
Treatment
All patients received EBRT to the pelvis with and without concurrent platinum-containing chemotherapy as described below, followed by 4 × 7 Gy MR-IGABT. Each brachytherapy fraction was guided by T2-weighted (T2W) MRI.
Seventy-five (77.3%) patients underwent concurrent platinum-containing chemotherapy, 49 (50.5%) patients were administered platinum drugs as a single agent, and 26 (26.8%) patients were administered platinum combined with paclitaxel or docetaxel (Table 1 shows the patient information). EBRT (Synergy; Elekta AB, Stockholm, Sweden) used three-dimensional conformal radiotherapy (3D-CRT) or intensity modulated radiotherapy (IMRT), with a total prescribed dose of 44.0~50.4 Gy in 1.8~2.0 Gy fractions, with some patients receiving a pelvic nodal boost.
Each brachytherapy fraction utilizes ultrasound-assisted applicator/catheter insertion under general anesthesia (Figure 1). Applicator included Utrecht interstitial Fletcher CT/MRI Applicator Set (Elekta AB, Stockholm, Sweden), Interstitial Ring CT/MRI Applicator Set (Elekta AB, Stockholm, Sweden), Vaginal CT/MRI Multi Channel Applicator Set (Elekta AB, Stockholm, Sweden), self-made 3D-Printed applicator (This type of 3D-printed applicator consists of tandem, vaginal cylinder, and perineum template). Utrecht applicator and Ring applicator were appropriate for bulky disease. Multi Channel Applicator was appropriate for patients with vagina involvement. However, due to the invariable positions of the channel on the vaginal templates of the Utrecht applicator or Ring applicator, patients with bulky infiltrative extensive disease or narrow vagina cannot achieve the prescription dose. Self-made 3D-printing template and freehand interstitial technique provide the right and flexible position choices to make an adequate dose coverage (Figure 2).
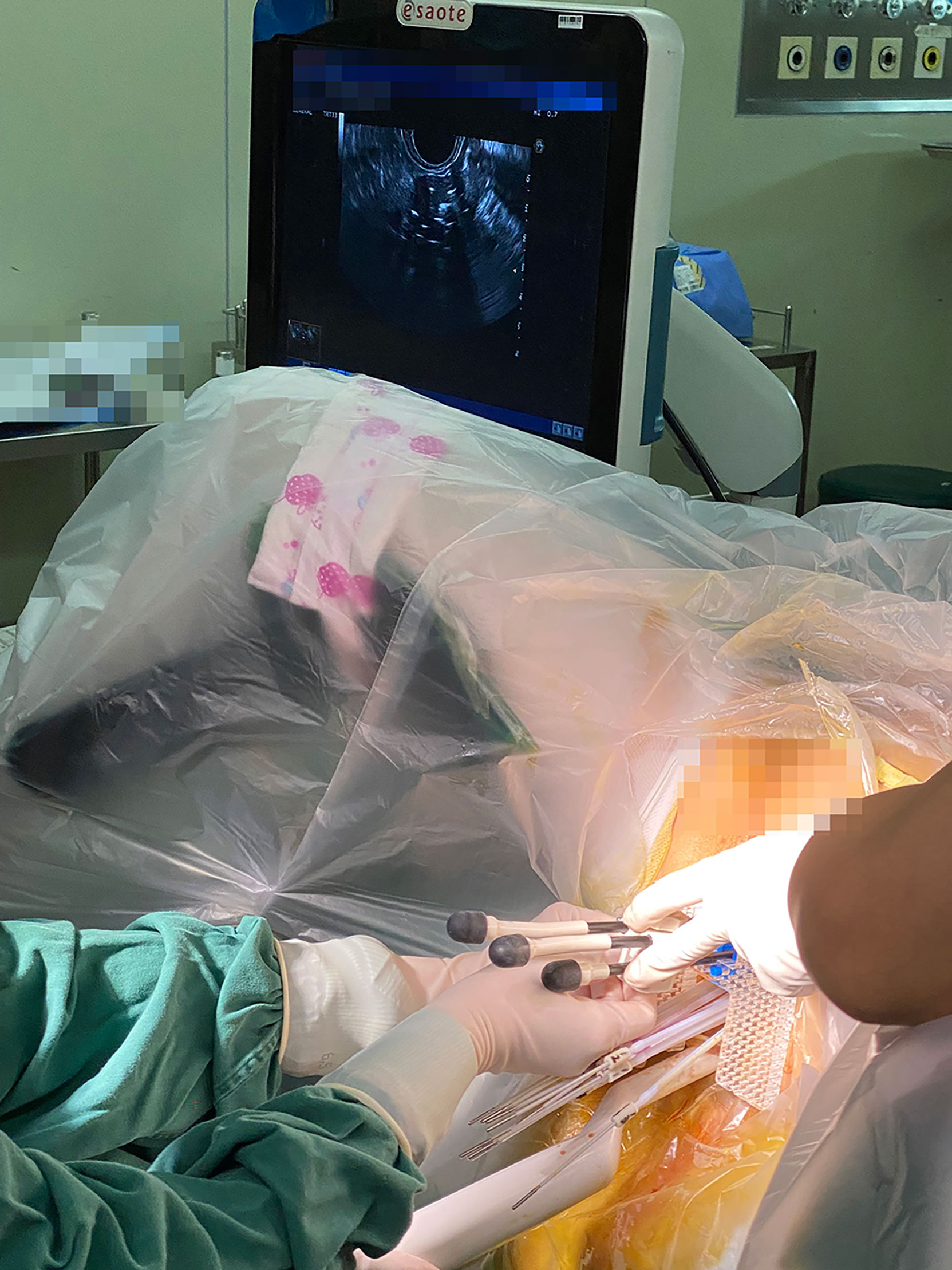
Figure 1 Use of hybrid intracavitary and interstitial (IC/IS) brachytherapy with assistant of real-time transrectal ultrasound. All the implants were MR compatible.
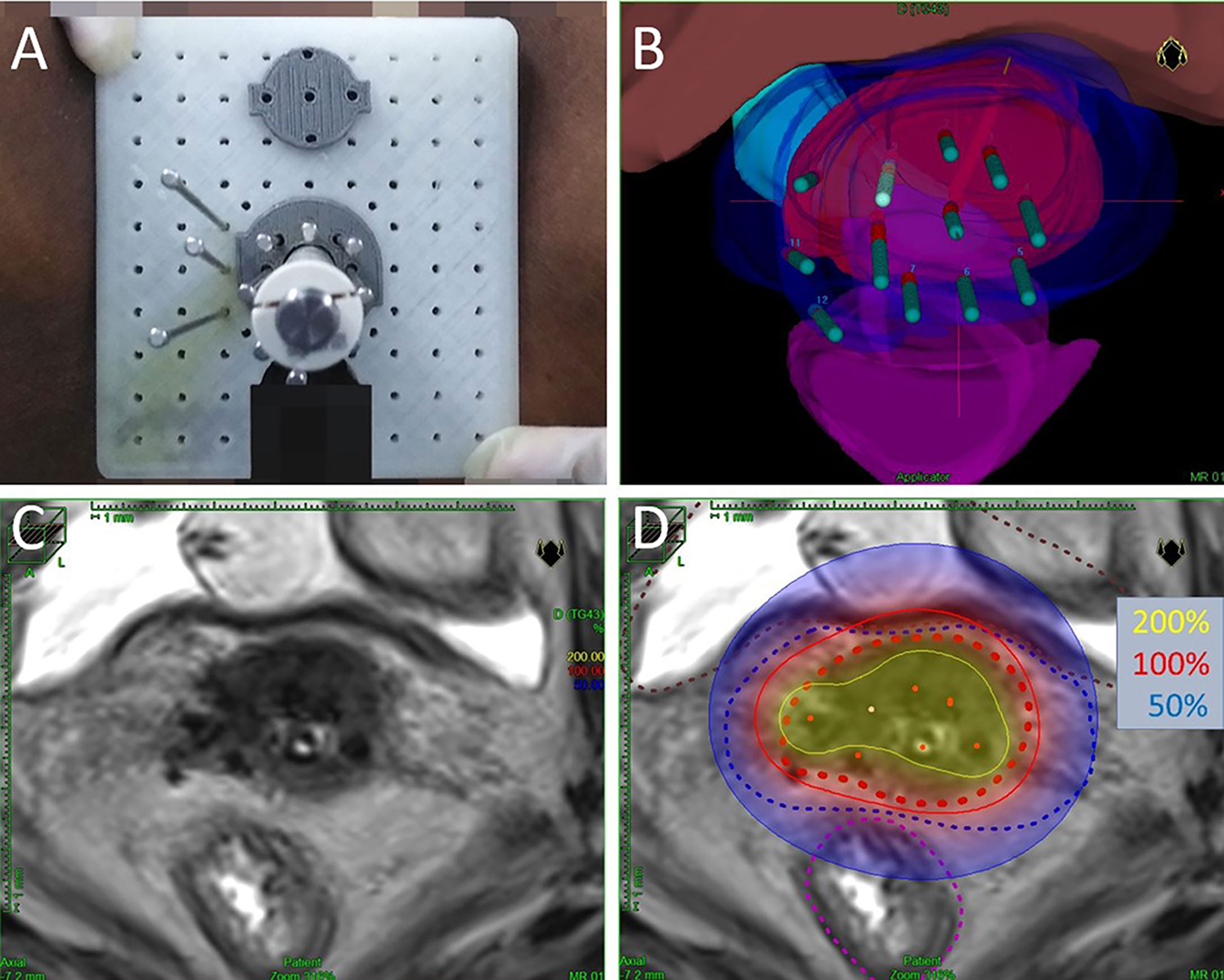
Figure 2 (A) Macroscopic view of self-made 3D-Printed applicator. (B) Three-dimensional view of the same implant planning data. The volumes represent HR-CTV (red), IR-CTV (blue), bladder (pink), rectum (purple), and sigmoid (cyan). (C) Axial view of T2-weighted magnetic resonance images (with implant in situ). (D) Axial view of brachytherapy dose distribution. Dotted red line is HR-CTV, dotted blue line is IR-CTV, dotted brown line represents the bladder, and dotted purple line represents the rectum. The isodose lines color code conventions are: solid yellow line = 200%; solid red line = 100%; solid blue line = 50% per treatment fraction.
A total of 388 brachytherapy fractions were included. Sixty-seven (17%) fractions used intracavitary (IC) brachytherapy. Three hundred and 21 (83%) fractions used hybrid intracavitary and interstitial (IC/IS) brachytherapy or interstitial (IS) brachytherapy alone. The IS brachytherapy alone was performed in a few brachytherapy fractions (6/388) with the obstruction of cervical canal orifice which were not appropriate to use tandem. In addition, 80 (20.6%) fractions used freehand interstitial brachytherapy, and for 90 patients (92.8%), the IC/IS or IS technique was used.
3.0-T MRI scans (Siemens Skyra, Erlangen, Germany) were performed after recovery from anesthesia (with implant in situ). T2W MRI of each brachytherapy fraction was used for the delineation of target volume and organs at risk (OARs), as referred to in the GEC-ESTRO recommendations (19, 20). High-dose rate (HDR) iridium-192 after-loading therapy (Microselectron V2 HDR; Nucletron, Veenendaal, The Netherlands. Treatment Planning System Oncentra V4.3; Nucletron, Veenendaal, The Netherlands) was applied to each brachytherapy fraction.
The equivalent dose based on linear-quadratic model in 2 Gy fraction (EQD2), with α/β of 10 Gy for tumor and 3 Gy for OARs, was used to calculate the cumulative doses from EBRT and MR-IGABT. Dosimetric parameters were evaluated after the GEC-ESTRO recommendations (19, 20).
Follow-Up and Endpoints
All patients were followed up by periodical check-up which consists of bimanual pelvic examination and imaging studies (pelvic MRI or CT scan) every 3 months in the first 2 years, at 6 months intervals for the next 3 years, and then annually.
The RECIST guidelines (version 1.1) (21) were used to evaluate the initial tumor response. Overall survival (OS) and cancer-specific survival (CSS) were defined as the period from the date of diagnosis until the date of death and death by cervical cancer, respectively. Progression-free survival (PFS) was defined as the period from diagnosis to the date of first documented evidence of progressive or recurrent disease or death. Local control (LC) was defined as the period from the diagnosis to the date of local relapse. Acute radiation morbidity and late radiation morbidity were evaluated by the RTOG morbidity criteria (22). Severe toxicity was defined as grades 3–5 complications.
Statistical Analysis
Statistical analysis was performed using SPSS (version 24). Continuous variables and classification variables were described as medians (ranges) and counts (percentages), respectively. Continuous variables were compared using Student’s t-test or rank-sum test. The correlations were analyzed using Pearson’s or Spearman’s correlation. The survival curves were performed using the Kaplan–Meier method. Univariable factors were evaluated using log-rank tests and Cox regression analysis. Multivariable factors were evaluated with Cox regression analysis. p < 0.05 was considered statistically significant.
Results
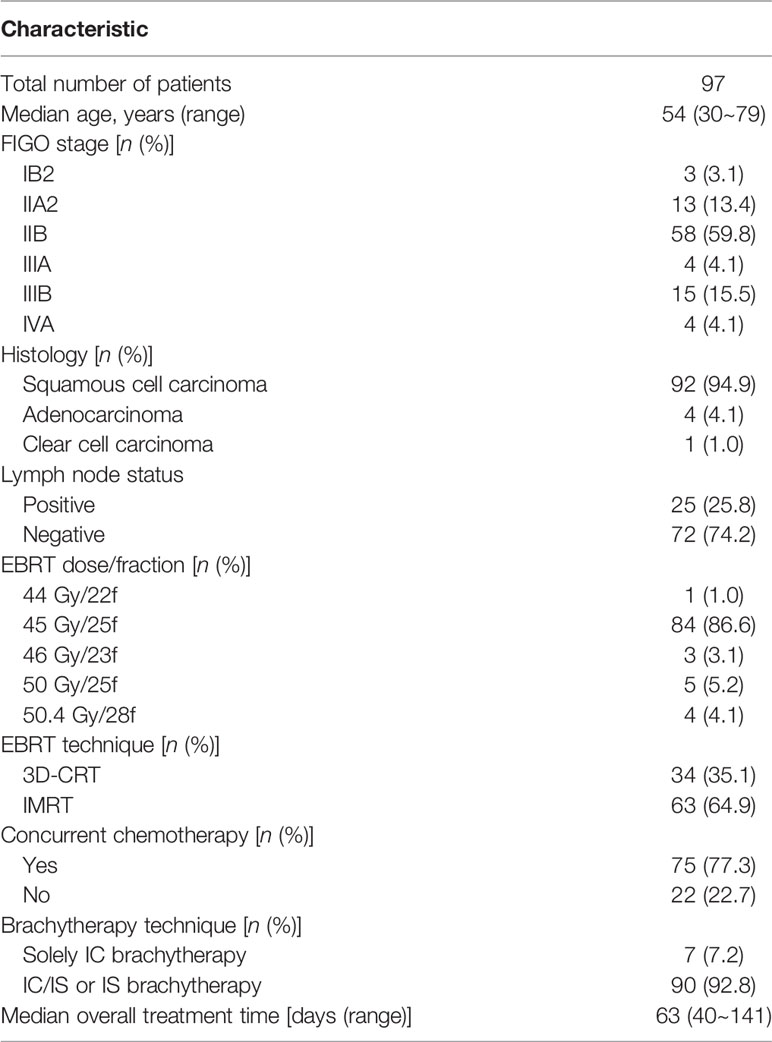
A total of 97 consecutive patients were included in this study, treated between September 2014 and April 2019. The median age at diagnosis was 54 (range 30~79) years. The median overall treatment time (OTT) was 63 days (range 40~141 days). Table 1 shows patients and treatment characteristics. For stages IIA2~IVA tumors, IC/IS and IS brachytherapy techniques were used in a higher proportion of 65%, 84%, 81%, 100%, and 94%, respectively.
Dose–Volume Parameters
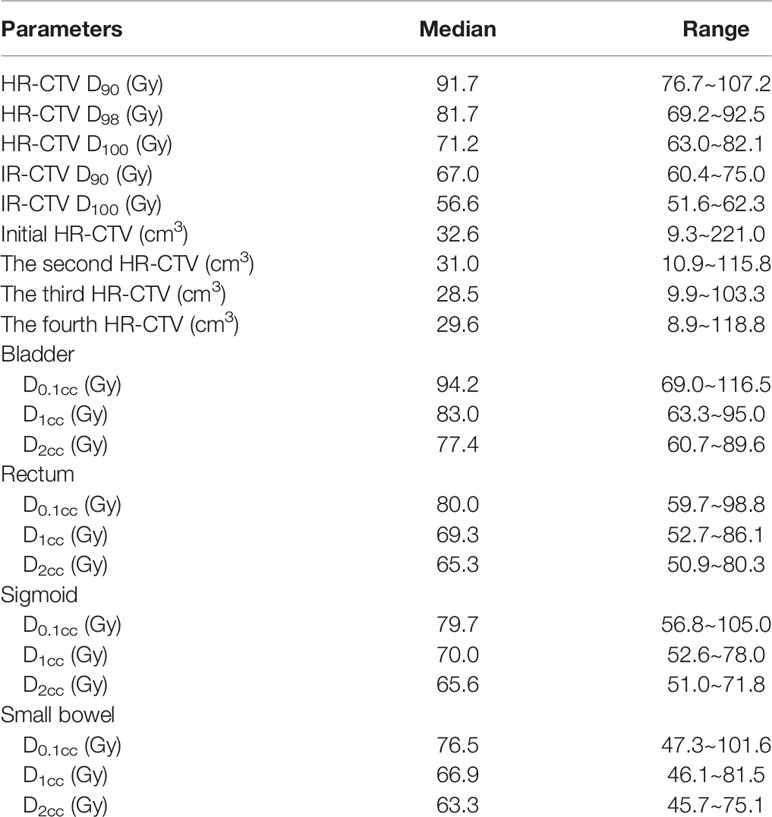
Dosimetric outcomes are presented in Table 2. The median HR-CTV D90, HR-CTV D98, HR-CTV D100, intermediate-risk CTV (IR-CTV) D90, and IR-CTV D100 were 91.7 Gy (76.7~107.2 Gy), 81.7 Gy (69.2~92.5 Gy), 71.2 Gy (63.0~82.1 Gy), 67.0 Gy (60.4~75.0 Gy), and 56.6 Gy (51.6~62.3 Gy), respectively. The initial, second, third, and fourth HR-CTV (range) were 32.6 cm3 (9.3~221.0 cm3), 31.0 cm3 (10.9~115.8 cm3), 28.5 cm3 (9.9~103.3 cm3), and 29.6 cm3 (8.9~118.8 cm3), respectively.
Treatment Outcomes
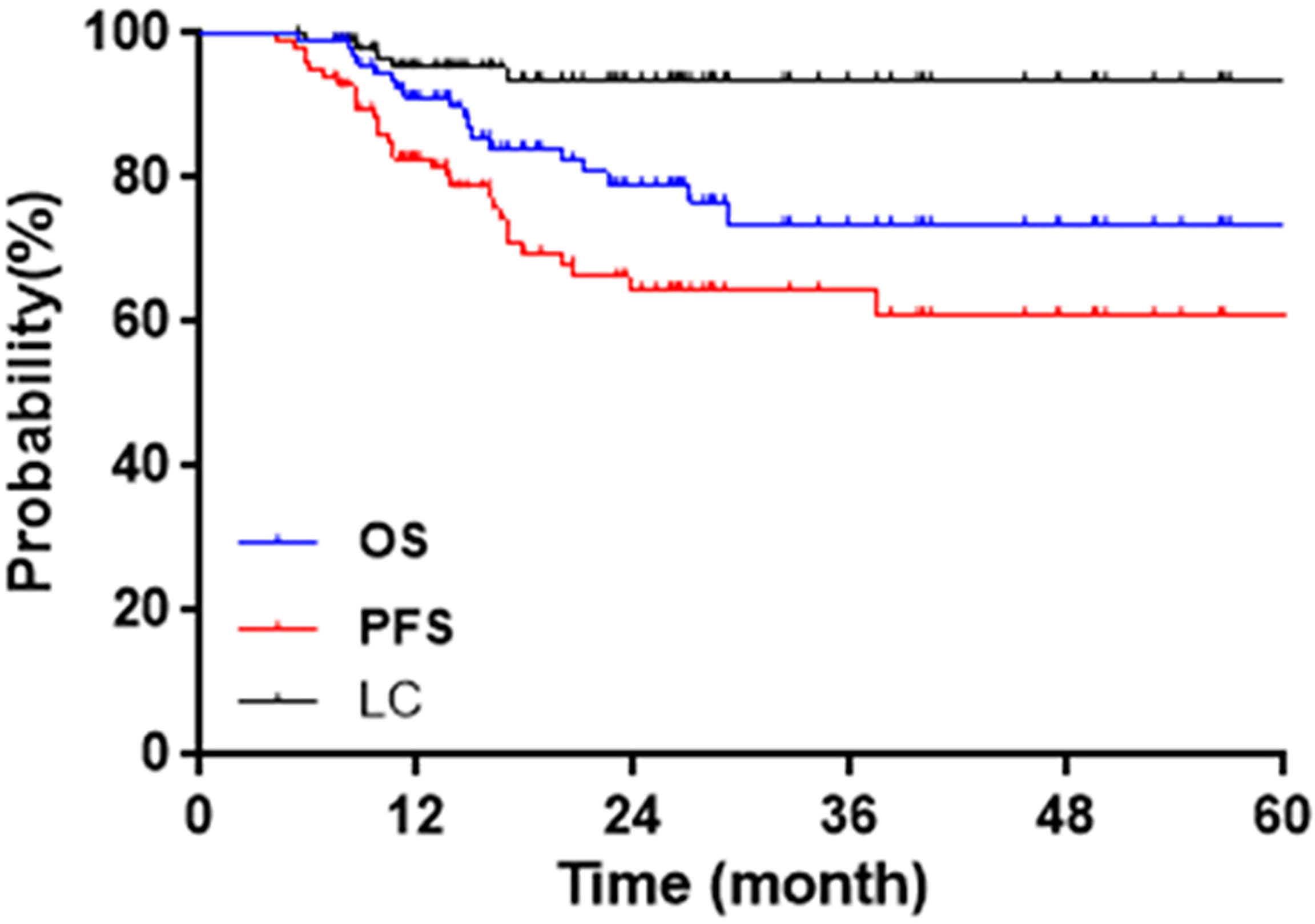
The median follow-up was 21.1 months (5.4~67.0 months). The initial tumor responses were 64 complete responses (CR) and 33 partial responses (PR). CR + PR were achieved in 97/97 (100%) patients. Two-year OS, CSS, PFS, and LC were 83.5%, 84.5%, 71.1%, and 94.8%, respectively. Figure 3 shows Kaplan–Meier curves for OS, PFS, and LC.
Eighteen patients have died, 17 from cervical cancer, 1 from a nontumor cause. The leading cause of the nontumor-caused death (OS = 16.0 months) was infection. This patient’s last physical examination showed positive hemoculture, without tumor recurrence, or digestive tract fistula. Therefore the death of this patient was not caused by cervical cancer or radiotherapy-related toxicity. Five patients suffered from local failures. Two-year LC was 100% for IB2, 92.3% for IIA2, 98.3% for IIB, 75% for IIIA, 93.3% for IIIB, and 75% for IVA. A total of 29 events occurred in PFS: 5 local failures (1 with pelvic metastasis), 2 pelvic metastasis, 20 distant metastasis (4 with pelvic metastasis), and 2 deaths.
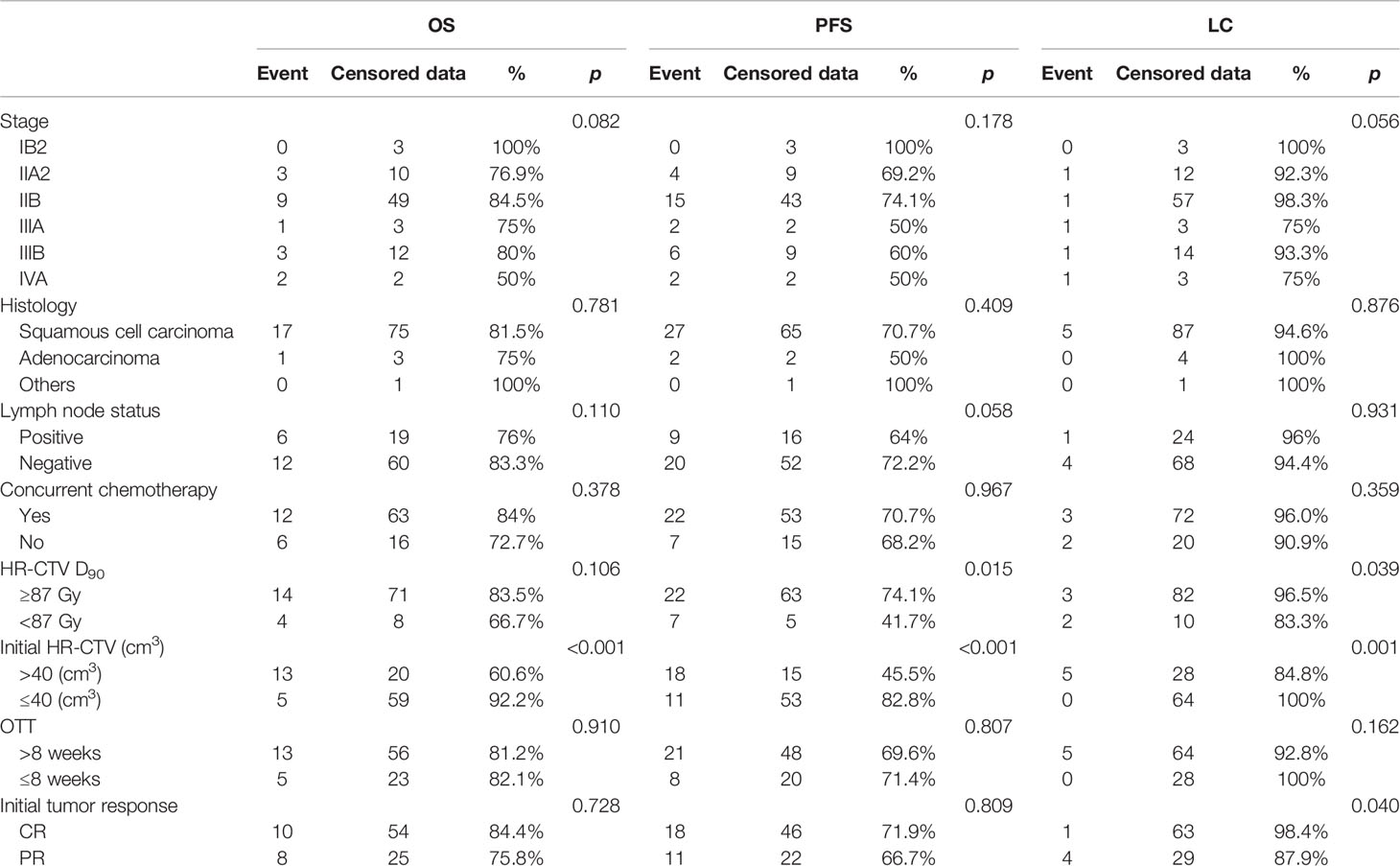
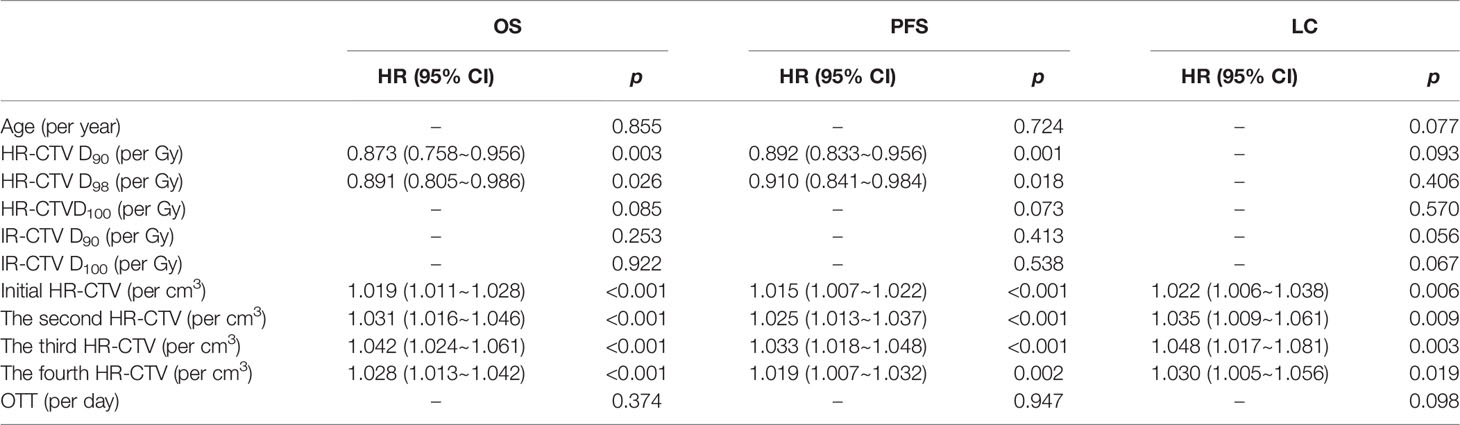
Outcomes of univariable and multivariable analyses are shown in Tables 3–5. Univariable analyses show: HR-CTV D90, HR-CTV D98, each fraction of HR-CTV, and initial HR-CTV >40 cm3 showed a statistical difference in OS. HR-CTV D90, HR-CTV D90 ≥87 Gy, HR-CTV D98, each fraction of HR-CTV, and initial HR-CTV >40 cm3 showed a statistical difference in PFS. HR-CTV D90 ≥87 Gy, each fraction of HR-CTV, and initial HR-CTV >40 cm3, the initial tumor response showed a statistical difference in LC.
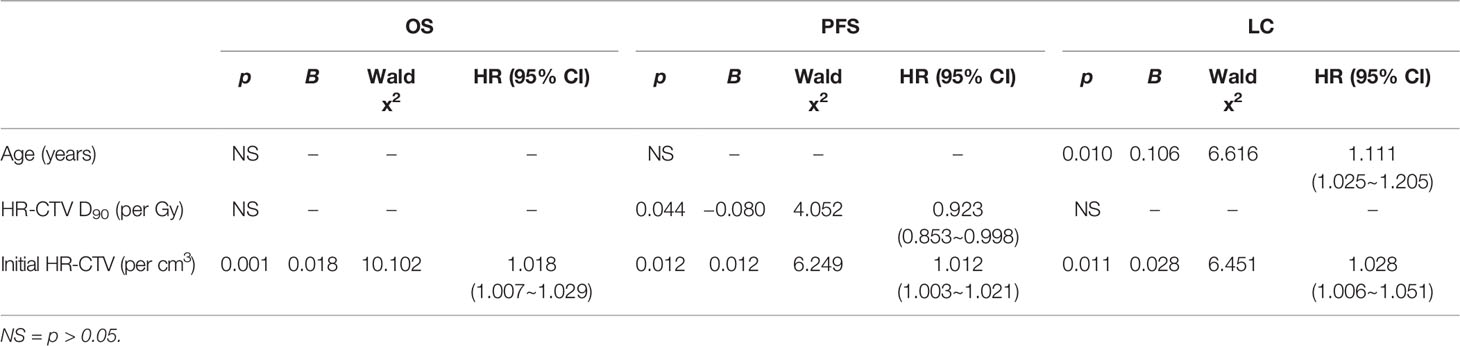
Multivariable analyses show the initial HR-CTV was an independent factor of OS (p = 0.001, HR = 1.018/cm3, 95% CI = 1.007~1.029), PFS (p = 0.012, HR = 1.012/cm3, 95% CI = 1.003~1.021), and LC (p = 0.011, HR = 1.028/cm3, 95% CI = 1.006~1.051). The HR-CTV D90 (p = 0.044, HR = 0.923/Gy, 95% CI = 0.853~0.998) was an independent factor of PFS. Age was an independent factor of LC (p = 0.010, HR = 1.111/year, 95% CI=1.025~1.205).
Toxicity
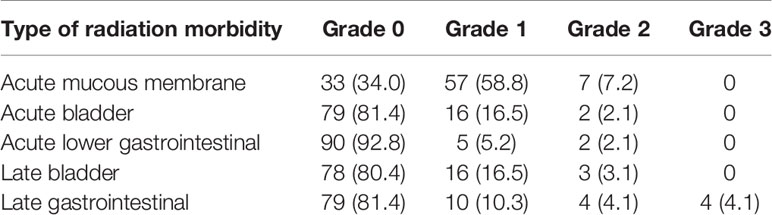
Four patients (4.1%) suffered from grade 3 late gastrointestinal radiation toxicity, and no other severe acute or late radiation toxicity occurred. Table 6 shows the distribution of different types of radiation toxicity. In addition, during the operation using interstitial technology, no serious bleeding or infections occurred.
Discussion
MRI has been recommended as gold standard imaging for cervical cancer contours, with some comparative studies previously published (14, 23, 24), and for MR-IGABT (repetitive MRI during complete brachytherapy treatment), several studies reported its clinical efficacy for LACC patients in Europe (7, 25) and North America (26). This study aimed to report the treatment outcomes of MR-IGABT for 97 Chinese LACC patients.
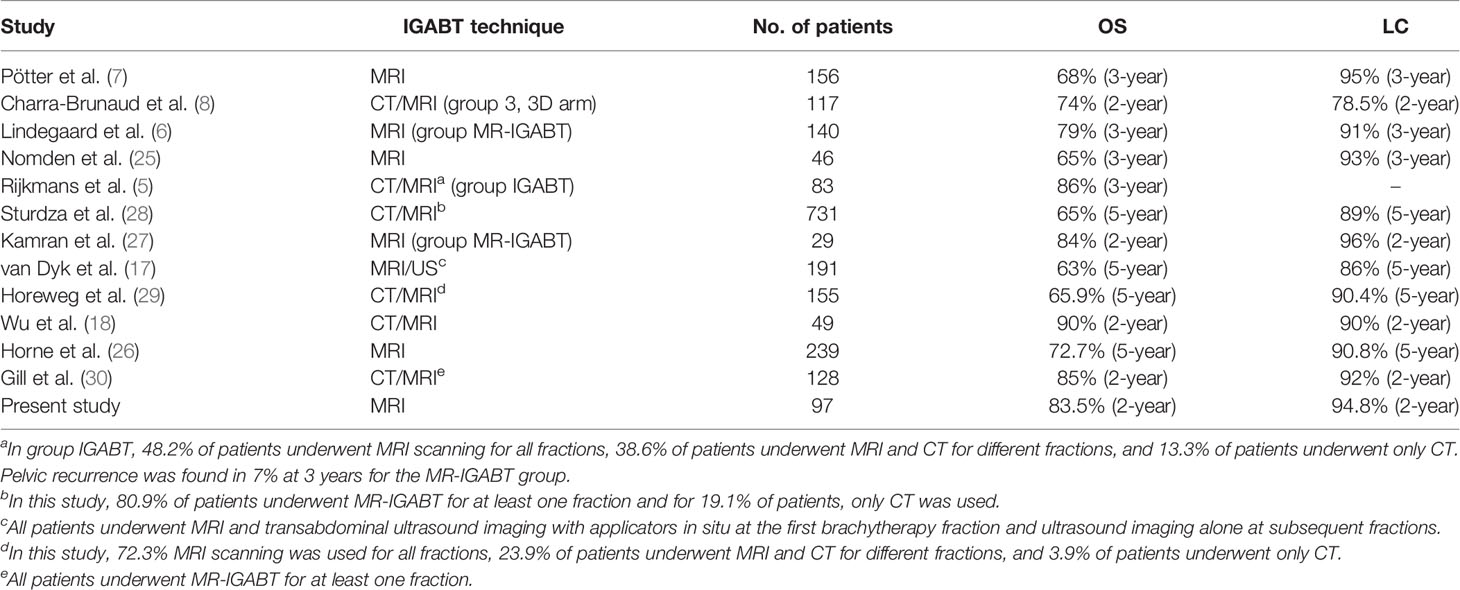
Whether MR-IGABT brings satisfactory clinical outcomes for cervical cancer, it has been a research priority of many radiotherapy centers in recent years. Lindegaard et al. (6) compared outcomes of LACC between 2D (X-ray)-guided brachytherapy and MR-IGABT. The 3-year OS of MR-IGABT showed a significant improvement (79% vs. 63%, p = 0.005), and 3-year LC of MR-IGABT was achieved in 91% of patients. Moreover, the moderate and severe late morbidity were both reduced by about 50% (p = 0.02). Kamran et al. (27) compared outcomes of LACC of MR-IGABT versus CT-guided brachytherapy. OS was significantly improved in MR-IGABT (84% vs. 56%, p = 0.036), and 2-year LC were 96% and 87% (p = 0.65), respectively. A large multicenter cohort study of Retro EMBRACE (28) included 731 LACC patients showed the efficacy and safety of MR-IGABT. Five hundred and ninety-two (80.9%) patients used MR-IGABT for at least one brachytherapy fraction, and 168 (23.0%) patients used IC/IS technique. The 3/5-year actuarial OS and LC were 74%/65% and 91%/89%, respectively. The 3/5-year grades 3–5 late morbidity was 4%/5% and 6%/7% for bladder and gastrointestinal tract, respectively. These excellent outcomes of MR-IGABT have been demonstrated in the western world. For Chinese patients with LACC, Wu et al. (18) recently evaluated the clinical outcomes of MR-IGABT where MRI was repeated at each implant (with implant in situ), in limited patient numbers (49 Chinese patients), with the first and the third brachytherapy fractions using MR-IGABT and other brachytherapy fractions were planned on CT imaging. Two-year OS and LC were both achieved in 90% of the patients with no severe late toxicity.
Table 7 (5–8, 17, 18, 25–30) summarizes the clinical outcomes of IGABT mentioned in this study and/or other studies recently published. The clinical outcomes of MR-IGABT, including the present study, show favorable OS, LC, and limited severe morbidity. The 2-year rates for OS and LC were achieved in 83.5% and 94.8% in the present study. Studies (8, 31) showed most local failures occurring less than 2 years after treatment. Tan et al. (32) summarized the distribution of local failure by time: 44.9% (year 1), 29.0% (year 2), 8.7% (year 3), 8.7% (year 4), 2.9% (year 5), 1.4% (years 6–10), and 4.3% (>10 years).
PFS is another important clinical outcome. Two-year PFS in the present study was 71.1%. A total of 20 distant metastasis occurred, which was the largest share of PFS (20/29). This was similar to other studies (7, 32, 33). Potter et al. (7) reported that IGABT technology significantly reduces local failure, which will further make distant metastases the predominant failure pattern. In order to reduce the risk of distant metastases and improve PFS, intensified chemoradiotherapy (34) or other therapy (such as molecular targeted therapy or immunotherapy) should be taken into account.
The most common radiation toxicity that occurred was grade 1 acute mucous membrane radiation toxicity (58.1%). Other incidences of radiation toxicity (acute bladder, acute lower gastrointestinal, late bladder, and late gastrointestinal) were all less than 20% (19.4%, 9.2%, 17.3%, and 19.4%, respectively). Only 4 patients (4.1%) showed grade 3 late gastrointestinal radiation toxicity. After symptomatic treatment, 2 patients fell to grade 1 and 2 patients fell to grade 0. ABS guideline (35) recommended the D2cc to the bladder, rectum, and sigmoid are ≤90, ≤75, and ≤75 Gy, respectively. In this study, only 2 (2%) patients had a rectum D2cc higher than 75 Gy. The interstitial-related side effect (such as pain, bleeding, infection) was settled with symptomatic treatment. MR-IGABT with interstitial technique can fully conform to dose limits for OARs, which will further lead to a well- tolerated treatment.
In the present study, the larger initial HR-CTV (per cm3) was an independent factor for worse OS, PFS, and LC. We investigated the correlations between initial HR-CTV and other dose–volume parameters. The initial HR-CTV was negatively correlated with HR-CTV D90 (p = 0.002), HR-CTV D98 (p = 0.016), and HR-CTVD100 (p = 0.006) and positively correlated with bladder D0.1cc (p = 0.047), bladder D1cc (p < 0.001), bladder D2cc (p < 0.001), rectum D0.1cc (p = 0.027), rectum D1cc (p < 0.001), rectum D2cc (p < 0.001). The initial HR-CTV showed no correlation between the dose to sigmoid and small bowel. The probable cause is the geometrical uncertainties in OARs (36) (e.g., filling status and motion in relation to the radiation sources). These uncertainties are highly related to the motility of the organs, such as, the sigmoid and small bowel. The high dose of HR-CTV D90 was an independent prognostic factor for improved PFS. Furthermore, we found that age was an independent factor for LC. A large national cohort analysis (37), which included 24,126 patients found that age was an independent predictor for the receipt of complete treatment (concurrent chemotherapy with combination external beam radiation and brachytherapy to total dose ≥70 Gy) for cervical cancer. Its multivariable analysis showed age groups of women older than 61 (group 61–70, group 71–80, group 80+) were less likely to be treated with complete treatment. In the present study, age was significantly different (p = 0.016) between patients with and without concurrent chemotherapy [median age was 53 (range 30–76) vs. 61.5 (range 33–79)]. The older age resulted in incomplete treatment, which may influence the LC.
The independent prognostic factors for LC in previous studies (26, 38, 39) where patients received MR-IGABT have reported stage, histology, HR-CTV D90, and initial HR-CTV, OTT. Dimopoulos et al. (39) found that LC was clearly greater if the HR-CTV D90 ≥87 Gy. Horne et al. (26) found that LC was affected by HR-CTV >40 cm3. In the present study, 2-year LC was 96.5% for HR-CTV D90 ≥87 Gy versus 83.3% for HR-CTV D90 <87 Gy (log-rank, p = 0.039) and 100% for initial HR-CTV ≤40 cm3 versus 84.8% for initial HR-CTV >40 cm3 (log-rank, p = 0.001). In addition, we found that 2-year LC was significantly different between CR and PR (98.4% for CR vs. 87.9% for PR, log-rank, p = 0.040), indicating the initial tumor response may influence LC.
Interstitial techniques including IC/IS and IS techniques were used as a dose-escalation method in 90 of 97 patients in this study, with 85 patients (87.6%) HR-CTV D90 ≥87 Gy and 96 patients (99.0%) HR-CTV D90 ≥80 Gy. The median HR-CTV D90 was 91.7 Gy, which meet the ABS (35) and NCCN guidelines (3). Only 1 patient (1%) showed HR-CTV D90 lower than 80 Gy, due to the large volume of initial HR-CTV (93.08 cm3). Compared with traditional IC brachytherapy alone, interstitial technique is more feasible with adequate coverage of disease in the vagina and parametrium (40).
In the present study, 69 patients (71.1%) had OTT longer than 8 weeks, which was suggested within 8 weeks (3). The main reason was patients did not receive timely brachytherapy treatment after the end of EBRT, which can be further optimized. The reduction of OTT to avoid repopulation in cervical cancer is known to be one of the ways to improve LC. Compared with the difficulty to deliver a higher dose of radiation to cervical cancer, OTT can be more easily kept within certain limits. Mazeron et al. (41) reported that the inverse correlation (probit model) between overall treatment time and local control and excessive OTT was an independent factor of LC for LACC treated by IGABT, with a cutoff of 55 days (Log-rank, p = 0.004, and Cox model, p = 0.047). Although, for OTT, there was no statistical significance in OS, PFS, and LC in the present study, we found OTT was significantly different (p = 0.035) between CR and PR [median OTT was 62 days (range 40–125 days) vs. 64 days (range 54–141 days)]. In consideration of the significant difference of 2-year LC between CR and PR in this study, the longer OTT results in a poorer initial tumor response, which further impacts LC. However, there is still insufficient high-level evidence with regard to OTT effects because OTT is closely related to many factors such as tumor response, dose, fractionation, and treatment-related side effects.
The major limitation of this study is its retrospective nature, with 23 patients receiving EBRT in other institutions before the MR-IGABT in our center. This results in a lack of unity in the EBRT dosimetric data and treatment characteristics. In addition, a longer follow-up may be required to obtain more meaningful results.
Conclusions
This retrospective study including 97 consecutive Chinese patients with LACC has shown that MR-IGABT was effective and safe. It showed favorable LC, OS, toxicity, and morbidity. Moreover, initial HR-CTV, HR-CTV D90, and age were significant prognostic factors. Future investigations and systemic treatment need to be emphasized.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of China-Japan Union Hospital of Jilin University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YC: manuscript writing, data collection, and data analysis. YP: protocol development. NZ: manuscript writing. DH: data management. XG: data analysis. ZM: data analysis. GC: protocol development and manuscript editing. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was partially supported by grants from the National Natural Science Foundation of China (grant numbers 82073331, 81201737, 31600679, 81703034, 82003208), Project of Science and Technology Department of Jilin Province (grant number 20190303151SF, 20210401138YY), and Horizontal Project of Jilin University (grant numbers 2019YX435, 2019155).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer Statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
3. Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2019) 17(1):64–84. doi: 10.6004/jnccn.2019.0001
4. Tanderup K, Georg D, Pötter R, Kirisits C, Grau C, Lindegaard JC. Adaptive Management of Cervical Cancer Radiotherapy. Semin Radiat Oncol (2010) 20(2):121–9. doi: 10.1016/j.semradonc.2009.11.006
5. Rijkmans EC, Nout RA, Rutten IH, Ketelaars M, Neelis KJ, Laman MS, et al. Improved Survival of Patients With Cervical Cancer Treated With Image-Guided Brachytherapy Compared With Conventional Brachytherapy. Gynecol Oncol (2014) 135(2):231–8. doi: 10.1016/j.ygyno.2014.08.027
6. Lindegaard JC, Fokdal LU, Nielsen SK, Juul-Christensen J, Tanderup K. MRI-Guided Adaptive Radiotherapy in Locally Advanced Cervical Cancer From a Nordic Perspective. Acta Oncol (2013) 52(7):1510–9. doi: 10.3109/0284186x.2013.818253
7. Pötter R, Georg P, Dimopoulos JC, Grimm M, Berger D, Nesvacil N, et al. Clinical Outcome of Protocol Based Image (MRI) Guided Adaptive Brachytherapy Combined With 3D Conformal Radiotherapy With or Without Chemotherapy in Patients With Locally Advanced Cervical Cancer. Radiother Oncol (2011) 100(1):116–23. doi: 10.1016/j.radonc.2011.07.012
8. Charra-Brunaud C, Harter V, Delannes M, Haie-Meder C, Quetin P, Kerr C, et al. Impact of 3D Image-Based PDR Brachytherapy on Outcome of Patients Treated for Cervix Carcinoma in France: Results of the French STIC Prospective Study. Radiother Oncol (2012) 103(3):305–13. doi: 10.1016/j.radonc.2012.04.007
9. Pötter R, Tanderup K, Kirisits C, de Leeuw A, Kirchheiner K, Nout R, et al. The EMBRACE II Study: The Outcome and Prospect of Two Decades of Evolution Within the GEC-ESTRO GYN Working Group and the EMBRACE Studies. Clin Transl Radiat Oncol (2018) 9:48–60. doi: 10.1016/j.ctro.2018.01.001
10. Pötter R, Fidarova E, Kirisits C, Lang S, Reinthaller A, Dimopoulos J. 3d MRI-Based Brachytherapy for Cervical Cancer. Expert Rev Obstetrics Gynecol (2008) 3(3):351–8. doi: 10.1586/17474108.3.3.351
11. Pötter R, Federico M, Sturdza A, Fotina I, Hegazy N, Schmid M, et al. Value of Magnetic Resonance Imaging Without or With Applicator in Place for Target Definition in Cervix Cancer Brachytherapy. Int J Radiat Oncol Biol Phys (2016) 94(3):588–97. doi: 10.1016/j.ijrobp.2015.09.023
12. Nesvacil N, Pötter R, Sturdza A, Hegazy N, Federico M, Kirisits C. Adaptive Image Guided Brachytherapy for Cervical Cancer: A Combined MRI-/CT-Planning Technique With MRI Only at First Fraction. Radiother Oncol (2013) 107(1):75–81. doi: 10.1016/j.radonc.2012.09.005
13. Ohno T, Wakatsuki M, Toita T, Kaneyasu Y, Yoshida K, Kato S, et al. Recommendations for High-Risk Clinical Target Volume Definition With Computed Tomography for Three-Dimensional Image-Guided Brachytherapy in Cervical Cancer Patients. J Radiat Res (2017) 58(3):341–50. doi: 10.1093/jrr/rrw109
14. Viswanathan AN, Dimopoulos J, Kirisits C, Berger D, Pötter R. Computed Tomography Versus Magnetic Resonance Imaging-Based Contouring in Cervical Cancer Brachytherapy: Results of a Prospective Trial and Preliminary Guidelines for Standardized Contours. Int J Radiat Oncol Biol Phys (2007) 68(2):491–8. doi: 10.1016/j.ijrobp.2006.12.021
15. Grover S, Harkenrider MM, Cho LP, Erickson B, Small C, Small W Jr, et al. Image Guided Cervical Brachytherapy: 2014 Survey of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys (2016) 94(3):598–604. doi: 10.1016/j.ijrobp.2015.11.024
16. Tharavichitkul E, Tippanya D, Jayavasti R, Chakrabandhu S, Klunklin P, Onchan W, et al. Two-Year Results of Transabdominal Ultrasound-Guided Brachytherapy for Cervical Cancer. Brachytherapy (2015) 14(2):238–44. doi: 10.1016/j.brachy.2014.11.001
17. van Dyk S, Narayan K, Bernshaw D, Kondalsamy-Chennakesavan S, Khaw P, Lin MY, et al. Clinical Outcomes From an Innovative Protocol Using Serial Ultrasound Imaging and a Single MR Image to Guide Brachytherapy for Locally Advanced Cervix Cancer. Brachytherapy (2016) 15(6):817–24. doi: 10.1016/j.brachy.2016.07.008
18. Wu PY, Wong TPW, Yip YYC, Chang TYA, Chan LKL, Lee MCH, et al. MRI-Guided Adaptive Brachytherapy for Locally Advanced Cervix Cancer: Treatment Outcomes From a Single Institution in Hong Kong. Brachytherapy (2019) 18(2):171–9. doi: 10.1016/j.brachy.2018.11.007
19. Haie-Meder C, Pötter R, Van Limbergen E, Briot E, De Brabandere M, Dimopoulos J, et al. Recommendations From Gynaecological (GYN) GEC-ESTRO Working Group (I): Concepts and Terms in 3D Image Based 3D Treatment Planning in Cervix Cancer Brachytherapy With Emphasis on MRI Assessment of GTV and CTV. Radiother Oncol (2005) 74(3):235–45. doi: 10.1016/j.radonc.2004.12.015
20. Pötter R, Haie-Meder C, Van Limbergen E, Barillot I, De Brabandere M, Dimopoulos J, et al. Recommendations From Gynaecological (GYN) GEC ESTRO Working Group (II): Concepts and Terms in 3D Image-Based Treatment Planning in Cervix Cancer Brachytherapy-3D Dose Volume Parameters and Aspects of 3D Image-Based Anatomy, Radiation Physics, Radiobiology. Radiother Oncol (2006) 78(1):67–77. doi: 10.1016/j.radonc.2005.11.014
21. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
22. Cox JD, Stetz J, Pajak TF. Toxicity Criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys (1995) 31(5):1341–6. doi: 10.1016/0360-3016(95)00060-c
23. Tuntipumiamorn L, Lohasammakul S, Dankulchai P, Nakkrasae P. Comparison of Impact of Target Delineation of Computed Tomography- and Magnetic Resonance Imaging-Guided Brachytherapy on Dose Distribution in Cervical Cancer. J Contemp Brachyther (2018) 10(5):418–24. doi: 10.5114/jcb.2018.78993
24. Federico M, Hernandez-Socorro CR, Ribeiro I, Martin JG, Oramas MDR, Saez-Bravo ML, et al. Prospective Intra/Inter-Observer Evaluation of Pre-Brachytherapy Cervical Cancer Tumor Width Measured in TRUS and MR Imaging. Radiat Oncol (2019) 14(1):173. doi: 10.1186/s13014-019-1352-7
25. Nomden CN, de Leeuw AA, Roesink JM, Tersteeg RJ, Moerland MA, Witteveen PO, et al. Clinical Outcome and Dosimetric Parameters of Chemo-Radiation Including MRI Guided Adaptive Brachytherapy With Tandem-Ovoid Applicators for Cervical Cancer Patients: A Single Institution Experience. Radiother Oncol (2013) 107(1):69–74. doi: 10.1016/j.radonc.2013.04.006
26. Horne ZD, Karukonda P, Kalash R, Edwards RP, Kelley JL, Comerci JT, et al. Single-Institution Experience in 3D MRI-Based Brachytherapy for Cervical Cancer for 239 Women: Can Dose Overcome Poor Response? Int J Radiat Oncol Biol Phys (2019) 104(1):157–64. doi: 10.1016/j.ijrobp.2018.12.042
27. Kamran SC, Manuel MM, Cho LP, Damato AL, Schmidt EJ, Tempany C, et al. Comparison of Outcomes for MR-Guided Versus CT-Guided High-Dose-Rate Interstitial Brachytherapy in Women With Locally Advanced Carcinoma of the Cervix. Gynecol Oncol (2017) 145(2):284–90. doi: 10.1016/j.ygyno.2017.03.004
28. Sturdza A, Pötter R, Fokdal LU, Haie-Meder C, Tan LT, Mazeron R, et al. Image Guided Brachytherapy in Locally Advanced Cervical Cancer: Improved Pelvic Control and Survival in RetroEMBRACE, a Multicenter Cohort Study. Radiother Oncol (2016) 120(3):428–33. doi: 10.1016/j.radonc.2016.03.011
29. Horeweg N, Creutzberg CL, Rijkmans EC, Laman MS, Velema LA, Coen V, et al. Efficacy and Toxicity of Chemoradiation With Image-Guided Adaptive Brachytherapy for Locally Advanced Cervical Cancer. Int J Gynecol Cancer (2019) 29(2):257–65. doi: 10.1136/ijgc-2018-000057
30. Gill BS, Kim H, Houser CJ, Kelley JL, Sukumvanich P, Edwards RP, et al. MRI-Guided High-Dose-Rate Intracavitary Brachytherapy for Treatment of Cervical Cancer: The University of Pittsburgh Experience. Int J Radiat Oncol Biol Phys (2015) 91(3):540–7. doi: 10.1016/j.ijrobp.2014.10.053
31. van Nagell JR Jr, Rayburn W, Donaldson ES, Hanson M, Gay EC, Yoneda J, et al. Therapeutic Implications of Patterns of Recurrence in Cancer of the Uterine Cervix. Cancer (1979) 44(6):2354–61. doi: 10.1002/1097-0142(197912)44:6<2354::aid-cncr2820440653>3.0.co;2-j
32. Tan LT, Pötter R, Sturdza A, Fokdal L, Haie-Meder C, Schmid M, et al. Change in Patterns of Failure After Image-Guided Brachytherapy for Cervical Cancer: Analysis From the RetroEMBRACE Study. Int J Radiat Oncol Biol Phys (2019) 104(4):895–902. doi: 10.1016/j.ijrobp.2019.03.038
33. Mazeron R, Gilmore J, Dumas I, Champoudry J, Goulart J, Vanneste B, et al. Adaptive 3D Image-Guided Brachytherapy: A Strong Argument in the Debate on Systematic Radical Hysterectomy for Locally Advanced Cervical Cancer. Oncologist (2013) 18(4):415–22. doi: 10.1634/theoncologist.2012-0367
34. Dueñas-González A, Zarbá JJ, Patel F, Alcedo JC, Beslija S, Casanova L, et al. Phase III, Open-Label, Randomized Study Comparing Concurrent Gemcitabine Plus Cisplatin and Radiation Followed by Adjuvant Gemcitabine and Cisplatin Versus Concurrent Cisplatin and Radiation in Patients With Stage IIB to IVA Carcinoma of the Cervix. J Clin Oncol (2011) 29(13):1678–85. doi: 10.1200/jco.2009.25.9663
35. Viswanathan AN, Beriwal S, De Los Santos JF, Demanes DJ, Gaffney D, Hansen J, et al. American Brachytherapy Society Consensus Guidelines for Locally Advanced Carcinoma of the Cervix. Part II: High-Dose-Rate Brachytherapy. Brachytherapy (2012) 11(1):47–52. doi: 10.1016/j.brachy.2011.07.002
36. Pötter R, Kirisits C, Erikson B, Haie-Meder C, Van Limbergen E, Lindegaard JC, et al. Prescribing, Recording, and Reporting Brachytherapy for Cancer of the Cervix. J icru (2013) 13(1-2). doi: 10.1093/jicru/ndw027
37. Albert A, Lee A, Allbright R, Vijayakumar S. Impact of Age on Receipt of Curative Treatment for Cervical Cancer: An Analysis of Patterns of Care and Survival in a Large, National Cohort. J Geriatr Oncol (2019) 10(3):465–74. doi: 10.1016/j.jgo.2018.10.005
38. Tanderup K, Fokdal LU, Sturdza A, Haie-Meder C, Mazeron R, van Limbergen E, et al. Effect of Tumor Dose, Volume and Overall Treatment Time on Local Control After Radiochemotherapy Including MRI Guided Brachytherapy of Locally Advanced Cervical Cancer. Radiother Oncol (2016) 120(3):441–6. doi: 10.1016/j.radonc.2016.05.014
39. Dimopoulos JC, Lang S, Kirisits C, Fidarova EF, Berger D, Georg P, et al. Dose-Volume Histogram Parameters and Local Tumor Control in Magnetic Resonance Image-Guided Cervical Cancer Brachytherapy. Int J Radiat Oncol Biol Phys (2009) 75(1):56–63. doi: 10.1016/j.ijrobp.2008.10.033
40. Keller A, Rodríguez-López JL, Patel AK, Vargo JA, Kim H, Houser CJ, et al. Early Outcomes After Definitive Chemoradiation Therapy With Vienna/Venezia Hybrid High-Dose Rate Brachytherapy Applicators for Cervical Cancer: A Single-Institution Experience. Brachytherapy (2021) 20(1):104–11. doi: 10.1016/j.brachy.2020.08.006
41. Mazeron R, Castelnau-Marchand P, Dumas I, del Campo ER, Kom LK, Martinetti F, et al. Impact of Treatment Time and Dose Escalation on Local Control in Locally Advanced Cervical Cancer Treated by Chemoradiation and Image-Guided Pulsed-Dose Rate Adaptive Brachytherapy. Radiother Oncol (2015) 114(2):257–63. doi: 10.1016/j.radonc.2014.11.045
Keywords: locally advanced cervical cancer, magnetic resonance imaging guided adaptive brachytherapy, intracavitary brachytherapy, interstitial brachytherapy, hybrid intracavitary/interstitial brachytherapy, clinical outcome
Citation: Chi Y, Pan Y, Zhang N, Han D, Guo X, Mao Z and Cheng G (2022) Clinical Outcomes of MRI-Guided Adaptive Brachytherapy for Each Fraction in Locally Advanced Cervical Cancer: A Single Institution Experience. Front. Oncol. 12:841980. doi: 10.3389/fonc.2022.841980
Received: 23 December 2021; Accepted: 17 February 2022;
Published: 17 March 2022.
Edited by:
Jing Cai, Hong Kong Polytechnic University, Hong Kong, SAR ChinaReviewed by:
Yingming Sun, Fujian Medical University, ChinaKevin Albuquerque, University of Texas Southwestern Medical Center, United States
Copyright © 2022 Chi, Pan, Zhang, Han, Guo, Mao and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanghui Cheng, Y2hlbmdnaEBqbHUuZWR1LmNu
†These authors have contributed equally to this work
 Yunbo Chi1†
Yunbo Chi1† Guanghui Cheng
Guanghui Cheng