- 1Endocrine Unit and Diabetes Center, Department of Clinical Therapeutics, Alexandra Hospital, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece
- 2Hematology and Oncology Unit, Department of Clinical Therapeutics, Alexandra Hospital, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece
Aim: The aim of this study was to investigate the association of endocrine complications after ICI immunotherapy with progression-free survival (PFS) and overall survival (OS) in a large single-center oncological cohort.
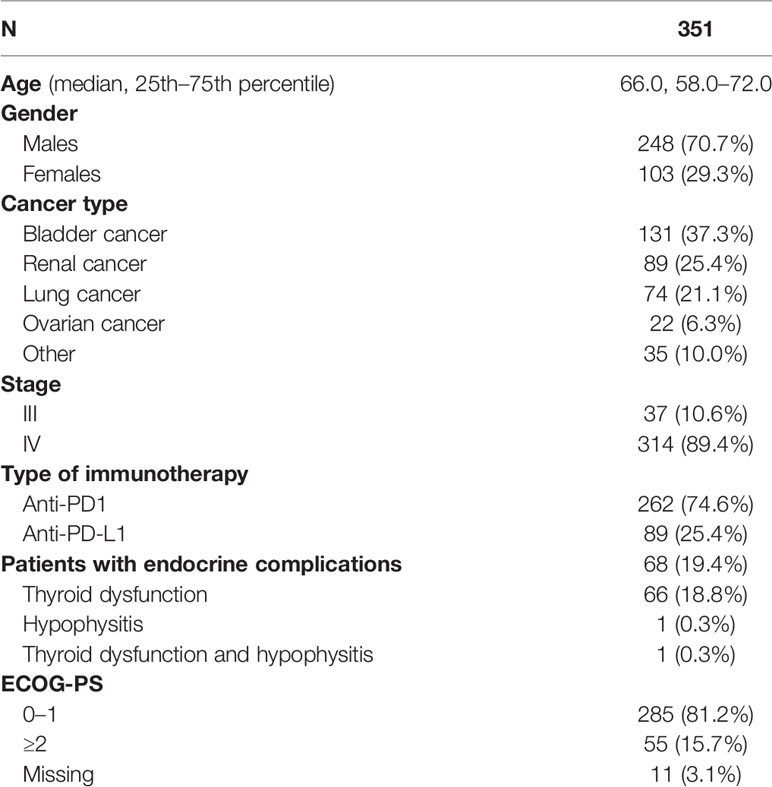
Patients and Methods: In total, 351 patients were included in the analysis, 248 men (70.7%) and 103 women (29.3%). The median age was 66 years. Patients had a variety of cancer types, namely, bladder cancer (131, 37.3%), renal cancer (89, 25.4%), lung cancer (74, 21.1%), ovarian cancer (22, 6.3%), and other types of cancer (35, 10%). The majority (314, 89.4%) were classified as stage IV, while 10.6% (37) were classified as stage III. Most of the patients received immunotherapy with anti-PD1 agents (262, 74.6%) and the rest with anti-PD-L1 agents (89, 25.4%). Kaplan–Meier estimates were used to describe and visualize the effect of categorical variables on OS and PFS. Survival analysis was performed by Kaplan–Meier curves, and survival differences between groups were estimated using the log-rank test. The estimation of the prognostic value of several variables with patients’ survival was made by Cox regression models.
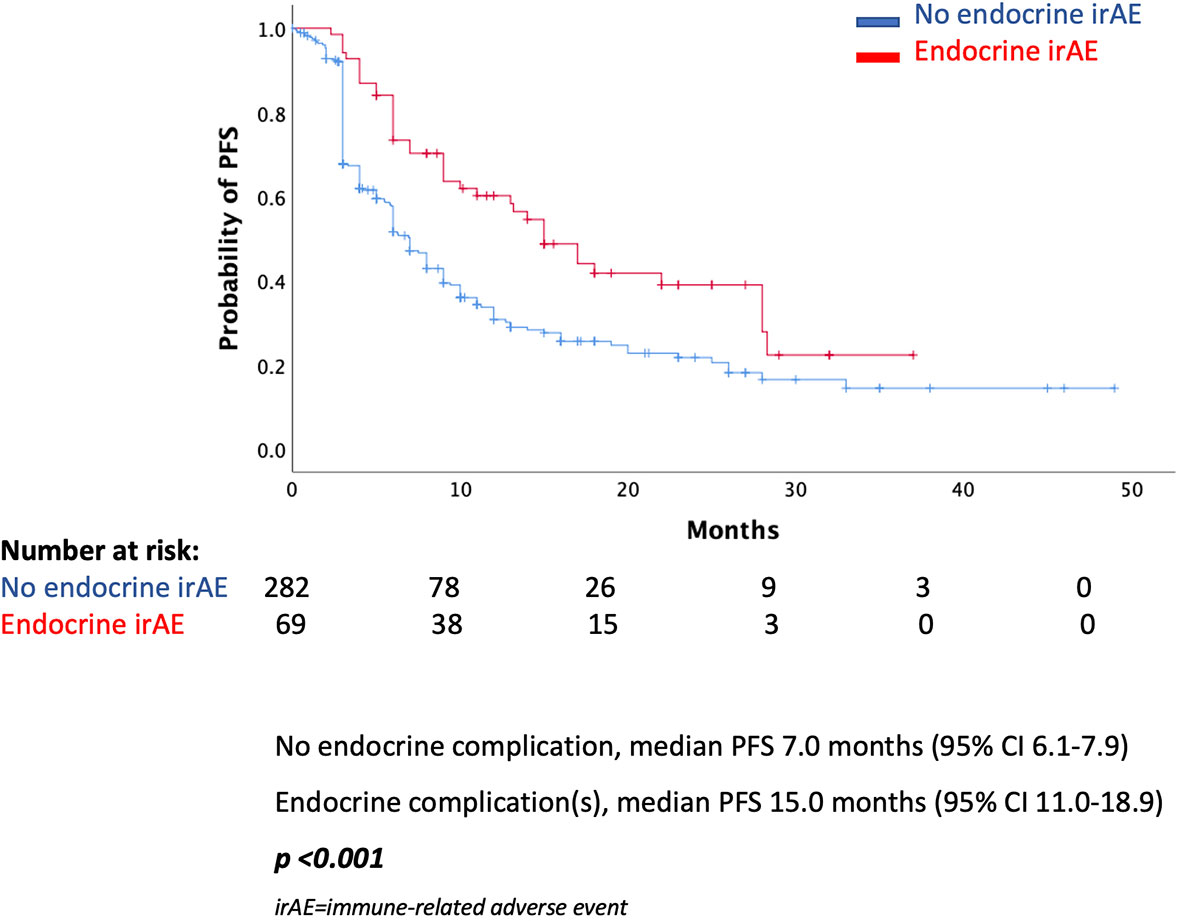
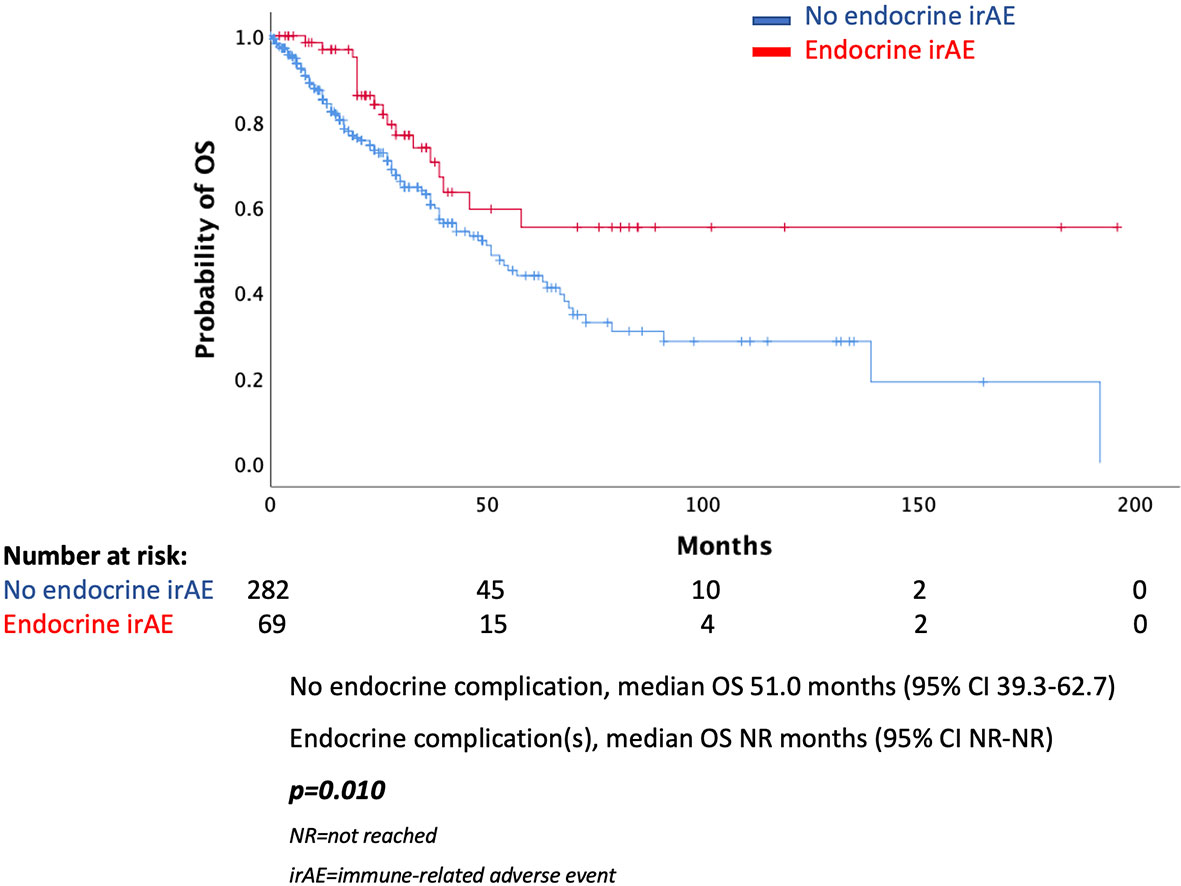
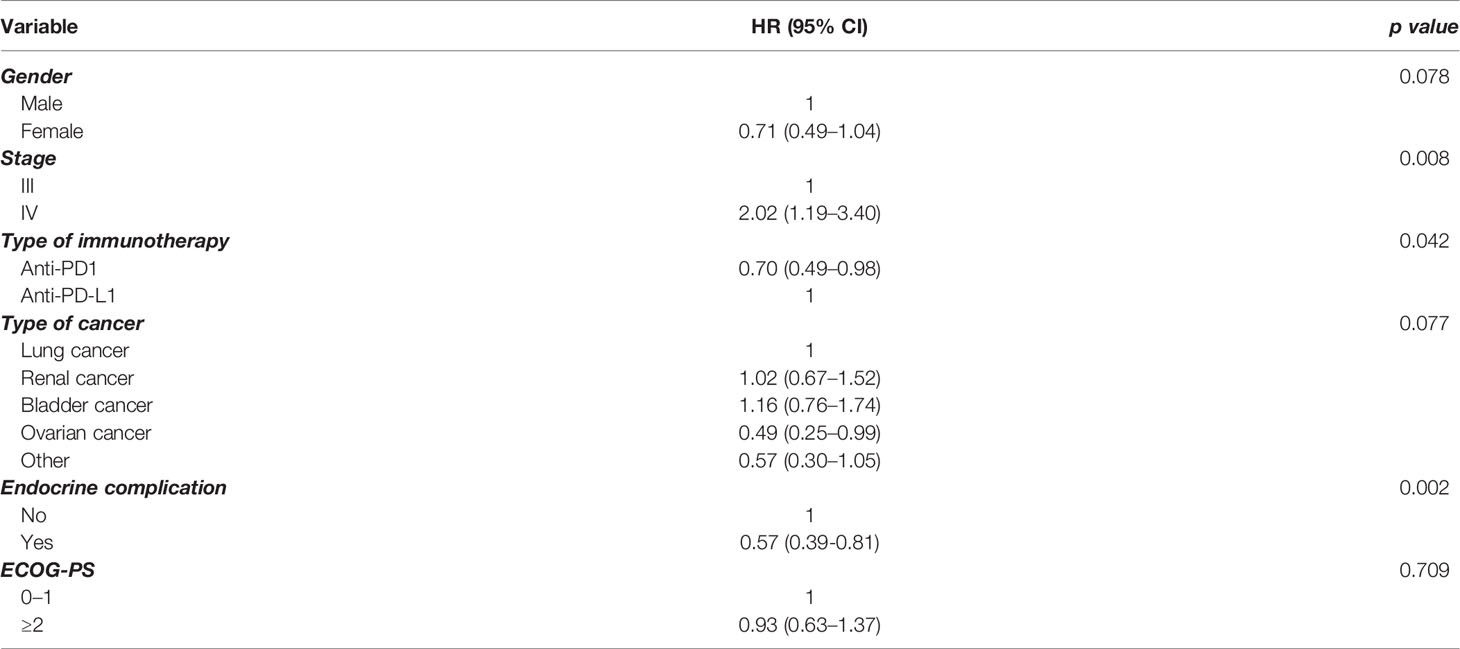
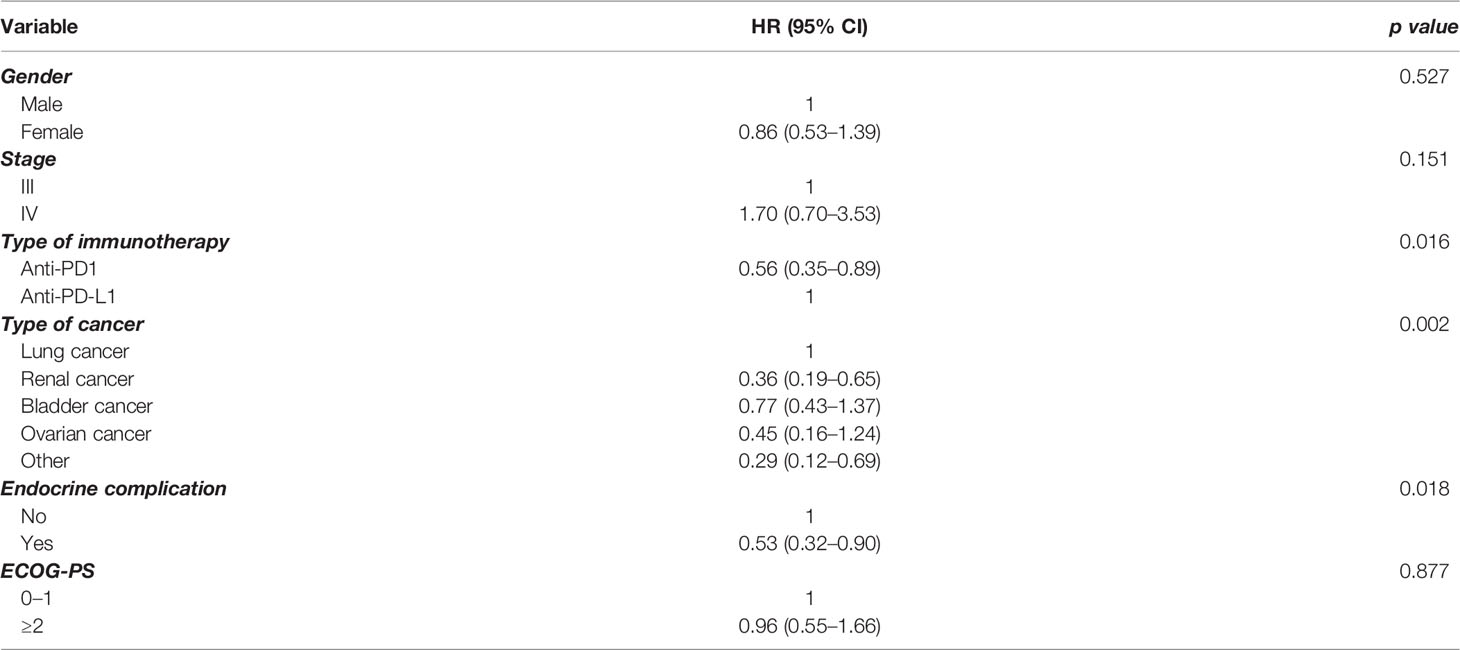
Results: In total, 68 (19.4%) of patients presented an endocrine complication after immunotherapy with ICIs. Specifically, 66 (18.8%) had thyroid dysfunction, 1 patient presented hypophysitis (0.3%), and 1 patient had a combination of thyroid dysfunction and hypophysitis (0.3%). Patients with an endocrine complication had mPFS of 15 months (95% CI 11.0–18.9 months), while in those without endocrine complication mPFS was 7 months (95% CI 6.1–7.9 months, p < 0.001). Similarly, median OS (mOS) was statistically significant lower in the patients’ group without endocrine complication. In fact, mOS was 51 months (95% CI 39.3–62.7 months) for these patients. The presence of endocrine complications after immunotherapy with ICIs retained its significance in terms of longer PFS (HR 0.57, 95% CI 0.39–0.81) and OS (HR 0.53, 95% CI 0.32–0.90) after multivariate analysis.
Conclusions: ICI endocrinopathies may be a positive predictor of immunotherapy response.
Introduction
Immune checkpoints (ICs) are molecules that modulate the duration and amplitude of physiological immune function, playing important roles in maintaining immune homeostasis (1). Immune checkpoint inhibitors (ICIs) are antibodies that target certain immune checkpoints (ICs), such as programmed death 1 (PD-1), its ligand (PD-L1), or cytotoxic T-lymphocyte antigen-4 (CTLA-4) (1, 2). ICIs have emerged as a powerful new tool for various types of cancers, namely, lymphoma, melanoma, lung cancer, renal cell carcinoma, urothelial carcinoma, etc. The indications of ICIs are currently increasing (1–5).
As these ICs are crucial for immunological self-tolerance, such therapies can trigger autoimmune adverse effects, affecting a number of organs (2, 6). Endocrinopathies are among the most common complications, including thyroid dysfunction, hypophysitis, and more rarely diabetes mellitus (DM) and primary adrenal insufficiency (6). The time of onset of endocrinopathies presents a great range (weeks to months after the initial dose) (7, 8). Furthermore, the severity of endocrinopathies presents a great range and grading systems are used in the clinical practice (1, 9). Combination of ICIs appears to increase the risk of endocrine complications (2, 3).
It has been hypothesized that autoimmune complications or immune-related adverse events (irAEs) after ICIs may be a positive predictor of immunotherapy response (10). Particularly, several studies and a recent meta-analysis provided evidence that progression-free survival (PFS) and overall survival (OS) are longer in patients with development of autoimmune disorders in general and of endocrinopathies specifically (11–13). A recent study provided evidenced that the development of autoimmune complications was associated with improved survival in patients with advanced (stage III/IV) NSCLC treated with anti-PD-L1 agents (12). Such autoimmune complications, including endocrine ones, may represent bystander effects from activated T lymphocytes and despite undesirable symptoms can correlate with favorable disease outcomes. This phenomenon is known in the literature as “beneficial autoimmunity” (10, 14).
However, evidence so far is scarce and the exact mechanisms remain elusive. Moreover, not all studies have provided similar results and the topic remains quite controversial (13). As this hypothesis needs further investigation, we aimed to investigate the association of endocrine complications after ICI immunotherapy with PFS and OS in a large single-center oncological cohort.
Patients and Methods
Patients and Protocol
All patients who received immunotherapy with ICIs in the Unit of Oncology, Department of Clinical Therapeutics, Alexandra Hospital, School of Medicine, National and Kapodistrian University of Athens in Athens, Greece, from June 2016 to December 2020 were studied. In total, 351 patients were included in the analysis, 248 men (70.7%) and 103 women (29.3%). All patients have provided written consent for the use of their medical data. The study was granted approval by our Institutional Review Board and was conducted according to the declaration of Helsinki.
Clinicopathological, treatment, and survival data were collected from patients’ records. More specifically, demographical data including patients’ date of birth, age at diagnosis, and date of first disease progression and/or death were collected. Type of surgery included primary or interval debulking, and surgery outcome, where available, was defined as optimal or suboptimal. Data regarding chemotherapy regimens and ICI therapy or combination were also collected. PFS and OS were calculated as part of the survival analysis. These were calculated as the number of months from the date of cancer diagnosis until disease progression or the date of death, respectively.
Evaluation of endocrine and other irAEs was performed according to CTCAE v4.03. Baseline evaluation for thyroid dysfunction is performed in all patients starting immunotherapy in our center and is periodically monitored during their treatment. Therefore, thyroid dysfunction could be detected as part of routine evaluation. Hypophysitis and other endocrine complications are suspected on the basis of relevant clinical symptoms, when additional testing is performed as appropriate (6).
Statistical Analysis
All data were coded and analyzed by a specifically designed database of the SPSS statistical package (SPSS Inc., Armonk, NY) version 24. The Kolmogorov–Smirnov test was used to assess the regularity of the data. Overall survival (OS) was defined as the time between the time of diagnosis and the date of death from any cause. Progression-free survival (PFS) was defined as the time between the time of diagnosis and the date of progression. Alive patients were censored at the date of last contact.
Kaplan–Meier estimates were used to describe and visualize the effect of categorical variables on OS and PFS. Survival analysis was performed by Kaplan–Meier curves, and survival differences between groups were estimated using the log-rank test. The estimation of the prognostic value of several variables with patients’ survival was made by Cox regression models. Multivariate Cox regression analysis was used to estimate the independent predictive value of the various factors in patients’ survival. All statistical correlations were considered significant in case of p < 0.05.
Results
Patients’ Characteristics
Characteristics of the patients are presented in Table 1. In total, 351 patients were included in the analysis, 248 men (70.7%) and 103 women (29.3%). The median age was 66 years. Patients had a variety of cancer types, namely, bladder cancer (131, 37.3%), renal cancer (89, 25.4%), lung cancer (74, 21.1%), ovarian cancer (22, 6.3%), and others (35, 10%). In the other types of category, pancreatic cancer (3 patients), breast cancer (2 patients), uterine cancer (7 patients), colon cancer (4 cases), hepatocellular cancer (1 patient), cervical cancer (6 patients), prostate cancer (3 patients), melanoma (2 patients), and multiple myeloma (7 patients) were included. The majority (314, 89.4%) were classified as stage IV, while 10.6% (37) were classified as stage III. Most of the patients received immunotherapy with anti-PD1 agents (262, 74.6%) and the rest with anti-PD-L1 agents (89, 25.4%).
In total, 68 (19.4%) of patients presented an endocrine complication after immunotherapy with ICIs. Specifically, 66 (18.8%) had thyroid dysfunction, 1 patient presented hypophysitis (0.3%), and 1 patient had a combination of thyroid dysfunction and hypophysitis (0.3%). Of note, endocrine irAEs were more common in renal carcinoma (25/89, 28.1%), urothelial cancer (28/131, 21.3%), and ovarian cancer (5/22, 22.7%) patients and less common in lung cancer (7/74, 9.5%) and other neoplasm (4/35, 11.4%) (p = 0.029) patients.
The median time of onset of endocrine complications was 10 weeks (range 14 days to 7.5 months). Most cases were treated with an appropriate hormonal supplementation. In the case with both hypophysitis and thyroid dysfunction, ICI immunotherapy was suspended. In two cases of thyroiditis, both levothyroxine and short-term corticosteroid therapy was used.
Survival
In the total population, there was a statistically significant difference in median PFS (mPFS) between those presented an endocrine complication and those who did not. More specifically, patients with an endocrine irAE had mPFS of 15 months (95% CI 11.0–18.9 months), while in those without endocrine complication mPFS was 7 months (95% CI 6.1–7.9 months, p < 0.001) (Figure 1). Similarly, median OS (mOS) was statistically significantly lower in the patients’ group without endocrine complication. In fact, mOS was 51 months (95% CI 39.3–62.7 months) for these patients, while mOS was not reached for patients with endocrine complications (Figure 2).
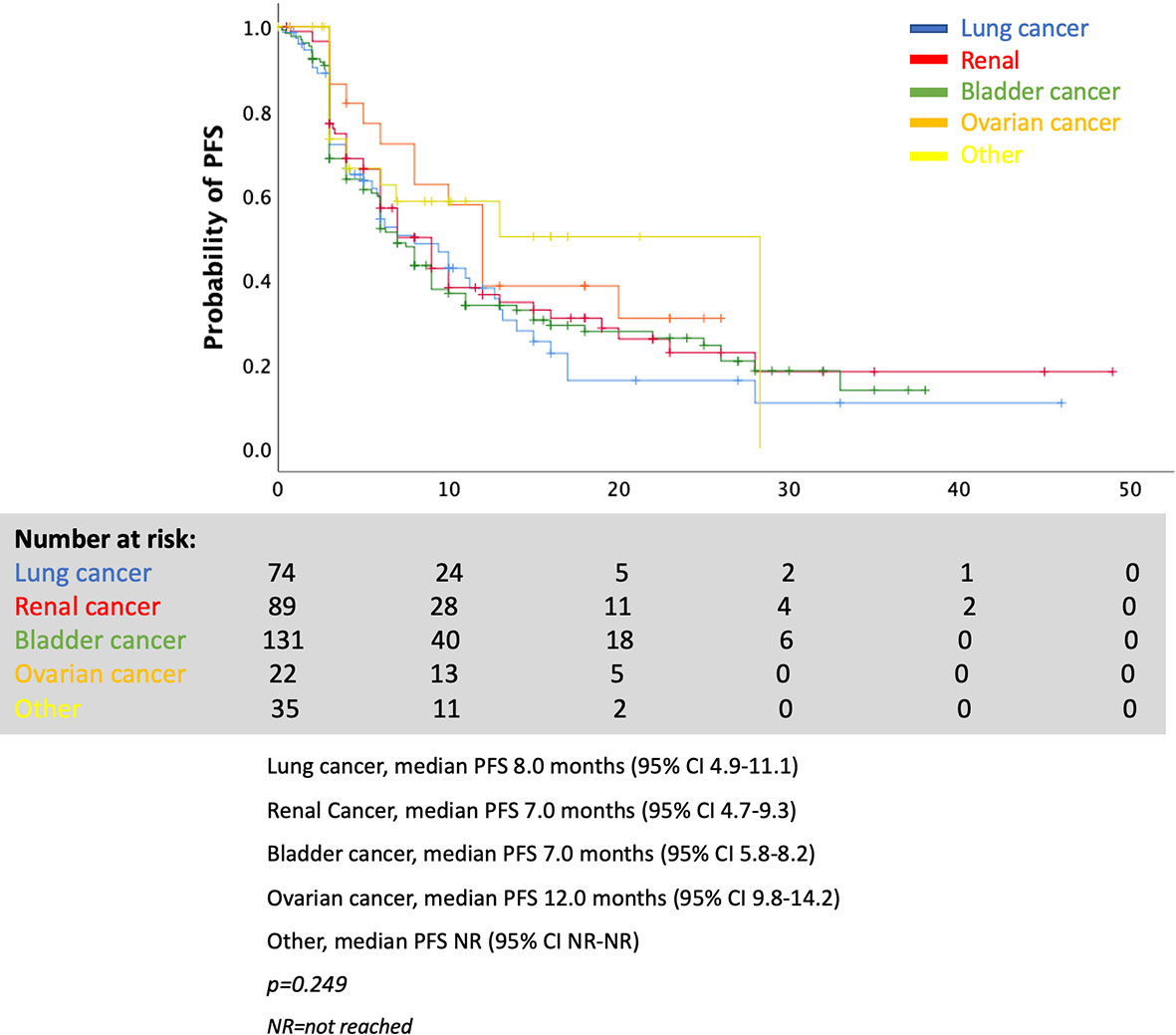
As shown in Figure 3, the various mPFSs in patients of different cancer types are presented. There are no statistically significant differences. When examining the effect of endocrine irAE presence of mPFS and mOS in patients with various cancers separately, we found that patients with renal cancer and bladder cancer who presented endocrine complications after ICIs had longer PFS. However, results were not statistically significant for patients with lung cancer, ovarian cancer, or other types of cancer (Figures 4A–E).
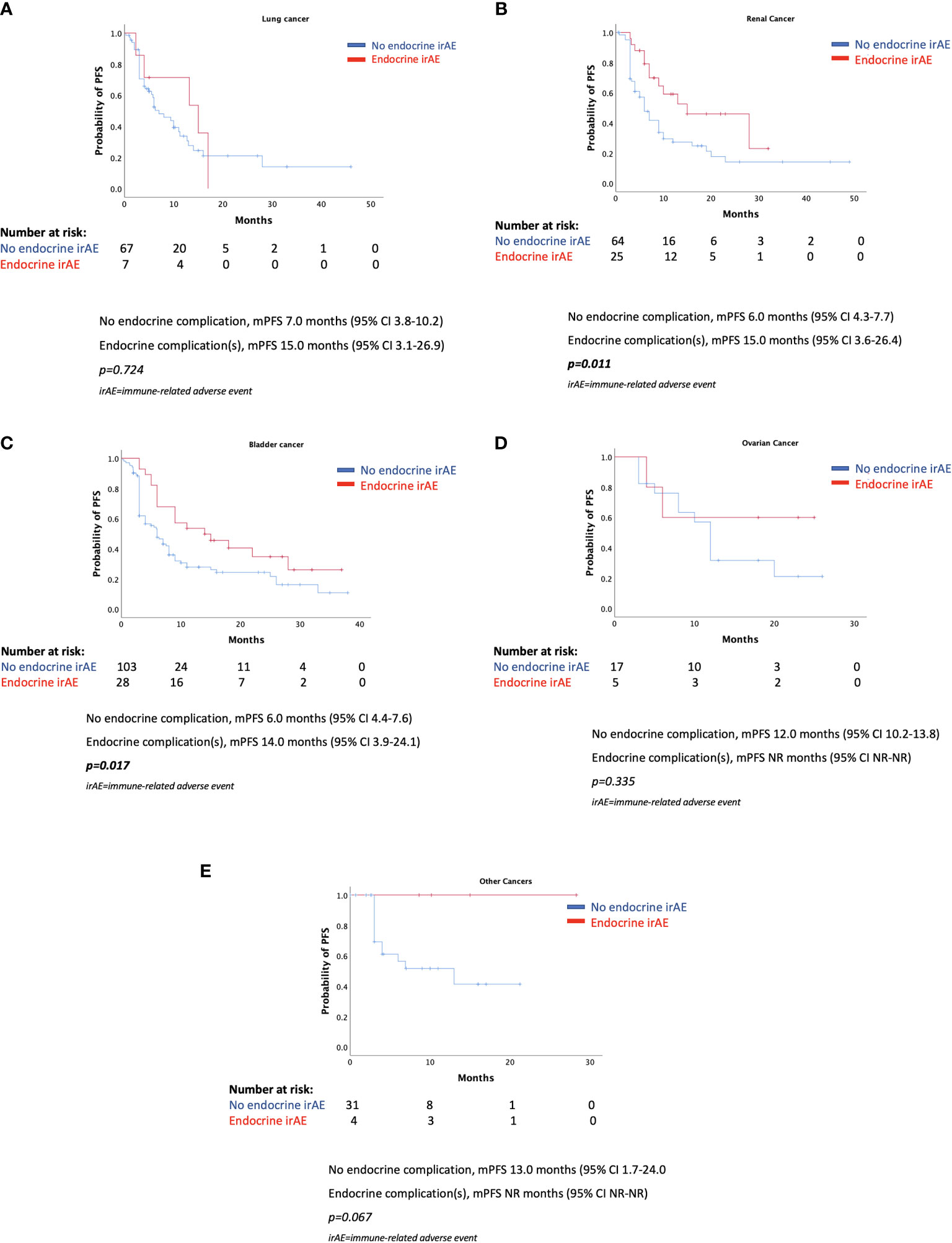
Figure 4 (A–E) Progression-free survival according to the presence of endocrine complications by cancer type.
In order to further elucidate the findings in the whole group, we performed multivariate analysis for PFS (Table 2) and OS (Table 3), including a number of parameters, such as gender, stage, type of immunotherapy, type of cancer, ECOG performance status scale, and endocrine complication. Interestingly, the presence of endocrine complications after immunotherapy with ICIs retained its significance in terms of longer PFS (HR 0.57, 95% CI 0.39–0.81) (Table 2) and OS (HR 0.53, 95% CI 0.32–0.90) (Table 3).
Discussion
The aim of this study was to investigate the association of endocrine complications after ICI immunotherapy with PFS and OS in a large cohort of oncological patients. We observed that 19.4% of participants presented endocrinopathies in total. Oncological patients with an endocrine complication after ICIs had longer mPFS compared with those without endocrine complication. Similarly, median OS was statistically significantly lower in the patients’ group without endocrine complication. These results retained significance even after multivariate analysis, including gender, stage of cancer, type of immunotherapy, and type of cancer.
The results of our study are in accordance with other recent findings, proposing that autoimmune complications after ICIs may be a positive predictor of therapy response. A recent large meta-analysis (13) included 30 (15–44) studies with 4,971 individuals and provided evidence that patients with autoimmune complications after ICIs experienced both an OS benefit and a PFS benefit compared with patients who did not develop such complications [OS: hazard ratio (HR) 0.54, 95% CI 0.45–0.65; p < 0.001; PFS: HR 0.52, 95% CI 0.44–0.61, p < 0.001). Specific analysis regarding endocrine complications yielded similar results (OS: HR 0.52, 95% CI, 0.44–0.62, p < 0.001). The same was found for dermatological (OS: HR 0.45, 95% CI 0.35–0.59, p < 0.001) and low-grade irAEs (OS: HR 0.57, 95% CI 0.43–0.75; p < 0.001) (13). Another later multicenter cohort study confirmed that the development of multisystem immune-related adverse events was associated with improved survival in patients with advanced (stage III/IV) NSCLC treated with anti-PD-L1 agents (12).
We need to note that a definite conclusion on the topic has not been drawn yet, as several single studies have provided negative or contradictory results (34–46). However, recent and increasing evidence, including our findings, suggests that such complications can be a positive predictor of immunotherapy response, despite the undesirable symptoms. The exact mechanisms of the association between autoimmune complications and survival benefits are not completely clear. Such adverse events are thought to represent bystander effects from activated T lymphocytes. An immune response against both the tumor and healthy cells or tissues seems to take place, probably through antigen mimicry procedures. The humoral immunity dysregulation has been additively proposed as a possible explanation for the phenomenon. For example, the PD-1 signaling pathway modulates B cell activation in both T-cell-dependent and -independent pathways (10, 13).
There is a term in the literature known as “beneficial autoimmunity.” Importantly, the nature of “beneficial autoimmunity” is substantially influenced by the type of cancer treated. For example, the induction of vitiligo in the case of melanoma is a good prognostic sign, while the repigmentation is associated with progression. In contrast, the presentation of pneumonitis in a patient with lung cancer would indicate poor prognosis. The difference reflects the fact that autoimmune responses that lead to the eradication of non-essential cells, such as melanocytes, are affordable to the body whereas damage of essential tissues, such as respiratory cells in the lung, is not (10). This is in accordance with the data from the recent meta-analysis that confirmed the association of endocrine, dermatological, and low-grade autoimmune complications with favorable outcomes (13). Of note, thyroid dysfunction is considered the most frequent ICI complication, irrespective of the location of the primary tumor (2, 6, 10, 13). Similarly, in our study most patients presented with thyroid dysfunction. Specifically, 66 (18.8%) had thyroid dysfunction, 1 patient presented hypophysitis (0.3%), and 1 patient had a combination of thyroid dysfunction and hypophysitis (0.3%).
In our study, most of the patients received immunotherapy with anti-PD1 agents (262, 74.6%) and the rest with anti-PD-L1 agents (89, 25.4%). Interestingly, the recent meta-analysis (13) showed that the association between autoimmune complication development and a favorable benefit on survival was significant in those patients undergoing treatment with anti-PD1 agents (OS: HR 0.51, 95% CI, 0.42–0.62, p < 0.001), but not with CTLA-4 inhibitors (OS: HR 0.89, 95% CI, 0.49–1.61, p = 0.706). In the meta-analysis, 26 studies adopted anti-PD-1 agents, 3 anti-CTLA-4 agents, and 1 anti-PD-1/PD-L1 agents (13). We need to take into consideration the lower numbers regarding CTLA-4 inhibitors; however, there may be a pathophysiological explanation too. Indeed, CTLA-4 inhibition activates T cells at an earlier stage of their development. Therefore, such an inhibition may directly disrupt central tolerance without affecting tumor immune response at the same time (1, 6, 13).
We found no statistically significant differences in mPFS of different cancer types, while the presence of endocrine complications was a positive predictor for longer mPFS in patients with renal cancer and bladder cancer, but not in patients with lung cancer, ovarian cancer, or other types of cancer. We need to note that numbers of the latter patients’ subgroups may be low to prove such an outcome for each different type of cancer. Indeed, several previous studies have shown favorable outcomes for patients with melanoma and lung cancer who developed various irAEs after immunotherapy with ICIs (12, 13, 15–17, 19–23, 25, 26).
The main strength of the study is the large number of participants. This is one of the largest cohorts in the literature. All patients come from the same academic unit, which implies another strength—that all participants were followed up by the same multidisciplinary team and with the same protocol. Numbers may be low for separate types of cancers, and this can be considered as a limitation. Moreover, data are retrospectively analyzed, however deriving from a prospectively collected cohort. Last but not least, a potential survivorship bias may exist for the development of irAEs.
In conclusion, this study provided evidence that oncological patients who present endocrine complications after ICI immunotherapy had longer mPFS and mOS compared with those without endocrine complication. These results retained significance even after multivariate analysis, including gender, stage of cancer, type of immunotherapy, and type of cancer. Therefore, physicians dealing with such patients should be aware that endocrine complications after ICIs may be a positive predictor of immunotherapy response and a prognostic factor of longer PFS and OS.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Alexandra Hospital Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contribution
SP designed the protocol and wrote the manuscript. ML participated in the collection of the data and performed the statistical analysis. EE-P participated in the collection of the data. KS participated in the collection of the data. CM participated in the collection of the data. KK participated in the collection of the data. FZ participated in the collection of the data and revised the manuscript. TP participated in the collection of the data and revised the manuscript. M-AD designed the protocol and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pardoll DM. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat Rev Cancer (2012) 12:252–64. doi: 10.1038/nrc3239
2. Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L. Endocrine Toxicity of Cancer Immunotherapy Targeting Immune Checkpoints. Endocr Rev (2019) 40:17–65. doi: 10.1210/er.2018-00006
3. Massari F, Mollica V, Rizzo A, Cosmai L, Rizzo M, Porta C. Safety Evaluation of Immune-Based Combinations in Patients With Advanced Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Expert Opin Drug Saf (2020) 19(10):1329–38. doi: 10.1080/14740338.2020.1811226
4. Massari F, Rizzo A, Mollica V, Rosellini M, Marchetti A, Ardizzoni A, et al. Immune-Based Combinations for the Treatment of Metastatic Renal Cell Carcinoma: A Meta-Analysis of Randomised Clinical Trials. Eur J Cancer (2021) 154:120–7. doi: 10.1016/j.ejca.2021.06.015
5. Rizzo A, Brandi G. Biochemical Predictors of Response to Immune Checkpoint Inhibitors in Unresectable Hepatocellular Carcinoma. Cancer Treat Res Commun (2021) 27:100328. doi: 10.1016/j.ctarc.2021.100328
6. Paschou SA, Stefanaki K, Psaltopoulou T, Liontos M, Koutsoukos K, Zagouri F, et al. How We Treat Endocrine Complications of Immune Checkpoint Inhibitors. ESMO Open (2021) 6(1):100011. doi: 10.1016/j.esmoop.2020.100011
7. Okura N, Asano M, Uchino J, Morimoto Y, Iwasaku M, Kaneko Y, et al. Endocrinopathies Associated With Immune Checkpoint Inhibitor Cancer Treatment: A Review. J Clin Med (2020) 9:2033. doi: 10.3390/jcm9072033
8. Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, et al. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-Analysis. JAMA Oncol (2018) 4:173–82. doi: 10.1001/jamaoncol.2017.3064
9. United States National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) (2017). Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#c (Accessed 5 February 2022).
10. Zitvogel L, Perreault C, OJ F, Kroemer G. Beneficial Autoimmunity Improves Cancer Prognosis. Nat Rev Clin Oncol (2021) 18(9):591–602.1. doi: 10.1038/s41571-021-00508-x
11. Matsuoka H, Hayashi T, Takigami K, Imaizumi K, Shiroki R, Ohmiya N, et al. Correlation Between Immune-Related Adverse Events and Prognosis in Patients With Various Cancers Treated With Anti PD-1 Antibody. BMC Cancer (2020) 20:656. doi: 10.1186/s12885-020-07142-3
12. Shankar B, Zhang J, Naqash AR, Forde PM, Feliciano JL, Marrone KA, et al. Multisystem Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors for Treatment of Non-Small Cell Lung Cancer. JAMA Oncol (2020) 6(12):1952–6. doi: 10.1001/jamaoncol.2020.5012
13. Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are Immune-Related Adverse Events Associated With the Efficacy of Immune Checkpoint Inhibitors in Patients With Cancer? A Systematic Review and Meta-Analysis. BMC Med (2020) 18(1):87. doi: 10.1186/s12916-020-01549-2
14. Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on Tumor Cells in the Escape From Host Immune System and Tumor Immunotherapy by PD-L1 Blockade. Proc Natl Acad Sci USA (2002) 99:12293–7. doi: 10.1073/pnas.192461099
15. Indini A, Di Guardo L, Cimminiello C, Prisciandaro M, Randon G, De Braud F, et al. Immune-Related Adverse Events Correlate With Improved Survival in Patients Undergoing Anti-PD1 Immunotherapy for Metastatic Melanoma. J Cancer Res Clin Oncol (2019) 145(2):511–21. doi: 10.1007/s00432-018-2819-x
16. Owen DH, Wei L, Bertino EM, Edd T, Villalona-Calero MA, He K, et al. Incidence, Risk Factors, and Effect on Survival of Immune Related Adverse Events in Patients With Non–Small-Cell Lung Cancer. Clin Lung Cancer (2018) 19(6):e893–900. doi: 10.1016/j.cllc.2018.08.008
17. Okada N, Kawazoe H, Takechi K, Matsudate Y, Utsunomiya R, Zamami Y, et al. Association Between Immune-Related Adverse Events and Clinical Efficacy in Patients With Melanoma Treated With Nivolumab: A Multicenter Retrospective Study. Clin Ther (2019) 41(1):59–67. doi: 10.1016/j.clinthera.2018.11.004
18. Verzoni E, Carteni G, Cortesi E, Giannarelli D, De Giglio A, Sabbatini R, et al. Real-World Efficacy and Safety of Nivolumab in Previously-Treated Metastatic Renal Cell Carcinoma, and Association Between Immune-Related Adverse Events and Survival: The Italian Expanded Access Program. Cancer Sci (2019) 7(1):99. doi: 10.1186/s40425-019-0579-z
19. Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, et al. High-Dose Glucocorticoids for the Treatment of Ipilimumabinduced Hypophysitis Is Associated With Reduced Survival in Patients With Melanoma. Cancer (2018) 124(18):3706–14. doi: 10.1002/cncr.31629
20. Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association With Outcomes. Clin Cancer Res (2016) 22(4):886–94. doi: 10.1158/1078-0432.CCR-15-1136
21. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small Cell Lung Cancer. JAMA Oncol (2018) 4(3):374–8. doi: 10.1001/jamaoncol.2017.2925
22. Kim HI, Kim M, Lee SH, Park SY, Kim YN, Kim H, et al. Development of Thyroid Dysfunction is Associated With Clinical Response to PD-1 Blockade Treatment in Patients With Advanced Non-Small Cell Lung Cancer. Oncoimmunology (2017) 7(1):e1375642. doi: 10.1080/2162402X.2017.1375642
23. Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, et al. Antibody-Mediated Thyroid Dysfunction During T-Cell Checkpoint Blockade in Patients With Non-Smallcell Lung Cancer. Ann Oncol (2017) 28(3):583–9. doi: 10.1093/annonc/mdw640
24. Yamauchi I. Incidence, Features, and Prognosis of Immune-Related Adverse Events Involving the Thyroid Gland Induced by Nivolumab. Immunotherapy (2019) 14(5):e0216954. doi: 10.1371/journal.pone.0216954
25. Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet-Bressand M, Khobta N, et al. Association Between Immune-Related Adverse Events and Efficacy of Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer. Clin Lung Cancer (2018) 20(3):201–7. doi: 10.1016/j.cllc.2018.10.002
26. Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, et al. Impact of Immune-Related Adverse Events on Survival in Patients With Advanced Non-Small Cell Lung Cancer Treated With Nivolumab: Long-Term Outcomes From a Multi-Institutional Analysis. J Cancer Res Clin Oncol (2019) 145(2):479–85. doi: 10.1007/s00432-018-2805-3
27. Lei M, Michael A, Patel S, Wang D. Evaluation of the Impact of Thyroiditis Development in Patients Receiving Immunotherapy With Programmed Cell Death-1 Inhibitors. J Oncol Pharm Pract (2019) 25(6):1402–11. doi: 10.1177/1078155219829813
28. Ishihara H, Takagi T, Kondo T, Homma C, Tachibana H, Fukuda H, et al. Association Between Immune-Related Adverse Events and Prognosis in Patients With Metastatic Renal Cell Carcinoma Treated With Nivolumab. Urol Oncol (2019) 37(6):355.e21–.e29. doi: 10.1016/j.urolonc.2019.03.003
29. Toi Y, Sugawara S, Sugisaka J, Ono H, Kawashima Y, Aiba T, et al. Profiling Preexisting Antibodies in Patients Treated With Anti-PD-1 Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol (2018) 5(3):376–83. doi: 10.1001/jamaoncol.2018.5860
30. Cortellini A, Chiari R, Ricciuti B, Metro G, Perrone F, Tiseo M, et al. Correlations Between the Immune-Related Adverse Events Spectrum and Efficacy of Anti-PD1 Immunotherapy in NSCLC Patients. Eurasian J Med (2019) 20(4):237–47. doi: 10.1016/j.cllc.2019.02.006
31. Berner F, Bomze D, Diem S, Ali OH, Fassler M, Ring S, et al. Association of Checkpoint Inhibitorinduced Toxic Effects With Shared Cancer and Tissue Antigens in Non-Small Cell Lung Cancer. JAMA Oncol (2019) 5(7):1043–47. doi: 10.1001/jamaoncol.2019.0402
32. Ahn BC, Pyo KH, Xin CF, Jung D, Shim HS, Lee CY, et al. Comprehensive Analysis of the Characteristics and Treatment Outcomes of Patients With Non-Small Cell Lung Cancer Treated With Anti-PD-1 Therapy in Real-World Practice. J Cancer Res Clin Oncol (2019) 145(6):1613–23. doi: 10.1007/s00432-019-02899-y
33. Nakamura Y, Tanaka R, Asami Y, Teramoto Y, Imamura T, Sato S, et al. Correlation Between Vitiligo Occurrence and Clinical Benefit in Advanced Melanoma Patients Treated With Nivolumab: A Multi-Institutional Retrospective Study. J Dermatol (2017) 44(2):117–22. doi: 10.1111/1346-8138.13520
34. Judd J, Zibelman M, Handorf E, O’Neill J, Ramamurthy C, Bentota S, et al. Immune-Related Adverse Events as a Biomarker in Non-Melanoma Patients Treated With Programmed Cell Death 1 Inhibitors. Oncologist (2017) 22(10):1232–7. doi: 10.1634/theoncologist.2017-0133
35. Ksienski D, Wai ES, Croteau N, Fiorino L, Brooks E, Poonja Z, et al. Efficacy of Nivolumab and Pembrolizumab in Patients With Advanced Non–Small-Cell Lung Cancer Needing Treatment Interruption Because of Adverse Events: A Retrospective Multicenter Analysis. Clin Lung Cancer (2019) 20(1):e97–e106. doi: 10.1016/j.cllc.2018.09.005
36. Rogado J, Sánchez-Torres JM, Romero-Laorden N, Ballesteros AI, Pacheco- Barcia V, Ramos-Leví A, et al. Immune-Related Adverse Events Predict the Therapeutic Efficacy of Anti–PD-1 Antibodies in Cancer Patients. Eur J Cancer (2019) 109:21–7. doi: 10.1016/j.ejca.2018.10.014
37. Lesueur P, Escande A, Thariat J, Vauleon E, Monnet I, Cortot A, et al. Safety of Combined PD-1 Pathway Inhibition and Radiation Therapy for Non-Small-Cell Lung Cancer: A Multicentric Retrospective Study From the GFPC. Cancer Med (2018) 7(11):5505–13. doi: 10.1002/cam4.1825
38. Lisberg A, Tucker DA, Goldman JW, Wolf B, Carroll J, Hardy A, et al. Treatment-Related Adverse Events Predict Improved Clinical Outcome in NSCLC Patients on KEYNOTE-001 at a Single Center. Cancer Immunol Res (2018) 6(3):288–94. doi: 10.1158/2326-6066.CIR-17-0063
39. Bjørnhart B, Hansen KH, Jørgensen TL, Herrstedt J, Schytte T. Efficacy and Safety of Immune Checkpoint Inhibitors in a Danish Real Life Non-Small Cell Lung Cancer Population: A Retrospective Cohort Study. Acta Oncol (2019) 58(7):953–61. doi: 10.1080/0284186X.2019.1615636
40. Lang N, Dick J, Slynko A, Schulz C, Dimitrakopoulou-Strauss A, Sachpekidis C, et al. Clinical Significance of Signs of Autoimmune Colitis in (18)F-Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography of 100 Stage-IV Melanoma Patients. Immunotherapy (2019) 11(8):667–76. doi: 10.2217/imt-2018-0146
41. Fujimoto D, Yoshioka H, Kataoka Y, Morimoto T, Kim YH, Tomii K, et al. Efficacy and Safety of Nivolumab in Previously Treated Patients With Non-Small Cell Lung Cancer: A Multicenter Retrospective Cohort Study. Lung Cancer (2018) 119:14–20. doi: 10.1016/j.lungcan.2018.02.017
42. Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, et al. Correlation Between Immune-Related Adverse Events and Efficacy in Non-Small Cell Lung Cancer Treated With Nivolumab. Lung Cancer (2018) 115:71–4. doi: 10.1016/j.lungcan.2017.11.019
43. Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, et al. Pembrolizumab Cutaneous Adverse Events and Their Association With Disease Progression. JAMA Dermatol (2015) 151(11):1206–12. doi: 10.1001/jamadermatol.2015.1916
44. de Moel EC, Rozeman EA, Kapiteijn EH, Verdegaal EME, Grummels A, Bakker JA. Autoantibody Development Under Treatment With Immune-Checkpoint Inhibitors. Cancer Immunol Res (2019) 7(1):6–11. doi: 10.1158/2326-6066.CIR-18-0245
45. Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol (2017) 35(7):785–92. doi: 10.1200/JCO.2015.66.1389
46. Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol (2015) 33(28):3193–8. doi: 10.1200/JCO.2015.60.8448
Keywords: immunotherapy, endocrine, thyroiditis, diabetes, hypophysitis, cancer
Citation: Paschou SA, Liontos M, Eleftherakis-Papaiakovou E, Stefanaki K, Markellos C, Koutsoukos K, Zagouri F, Psaltopoulou T and Dimopoulos M-A (2022) Oncological Patients With Endocrine Complications After Immunotherapy With Checkpoint Inhibitors Present Longer Progression-Free and Overall Survival. Front. Oncol. 12:847917. doi: 10.3389/fonc.2022.847917
Received: 03 January 2022; Accepted: 22 February 2022;
Published: 24 March 2022.
Edited by:
Rodabe N. Amaria, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Arun Khattri, Indian Institute of Technology (BHU), IndiaAlessandro Rizzo, National Cancer Institute Foundation (IRCCS), Italy
Chengzhi Zhou, Clinical Management Department of National Respiratory Medical Center, China
Copyright © 2022 Paschou, Liontos, Eleftherakis-Papaiakovou, Stefanaki, Markellos, Koutsoukos, Zagouri, Psaltopoulou and Dimopoulos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meletios-Athanasios Dimopoulos, bWRpbW9wQG1lZC51b2EuZ3I=
 Stavroula A. Paschou
Stavroula A. Paschou Michael Liontos
Michael Liontos Evangelos Eleftherakis-Papaiakovou2
Evangelos Eleftherakis-Papaiakovou2 Flora Zagouri
Flora Zagouri Theodora Psaltopoulou
Theodora Psaltopoulou Meletios-Athanasios Dimopoulos
Meletios-Athanasios Dimopoulos