- 1School of The Second Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, China
- 2Department of Hepatobiliary and Pancreatic Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 3Cancer Hospital of University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou, China
Background: For patients with colorectal cancer liver metastases (CRLMs), it is important to stratify patients according to the risk of recurrence. This study aimed to validate the predictive value of some clinical, imaging, and pathology biomarkers and develop an operational prognostic model for patients with CRLMs with neoadjuvant chemotherapy (NACT) before the liver resection.
Methods: Patients with CRLMs accompanied with primary lesion and liver metastases lesion resection were enrolled into this study. A nomogram based on independent risk factors was identified by Kaplan–Meier analysis and multivariate Cox proportional hazard analysis. The predictive ability was evaluated by receiver operating characteristic (ROC) curves and decision curve analysis (DCA). Calibration plot were also used to explore the consistency between prediction and reality.
Results: A total of 118 patients were enrolled into the study. Multivariable Cox analysis found that histopathological growth patterns (HGPs) [Hazard Rate (HR) = 2.130], radiology response (stable disease vs. partial response, HR = 2.207; progressive disease vs. partial response, HR = 3.824), lymph node status (HR = 1.442), and age (HR = 0.576) were independent risk factors for disease-free survival (DFS) (p < 0.05). Corresponding nomogram was constructed on the basis of the above factors, demonstrating that scores ranging from 5 to 11 presented better prognosis than the scores of 0–4 (median DFS = 14.3 vs. 4.9 months, p < 0.0001). The area under ROC curves of the model for 1-, 2-, and 3-year DFS were 0.754, 0.705, and 0.666, respectively, and DCA confirmed that the risk model showed more clinical benefits than clinical risk score. Calibration plot for the probability of DFS at 1 or 3 years verified an optimal agreement between prediction and actual observation. In the course of our research, compared with pure NACT, a higher proportion of desmoplastic HGP (dHGP) was detected in patients treated with NACT plus cetuximab (p = 0.030), and the use of cetuximab was an independent factor for decreased replacement HGP (rHGP) and increased dHGP (p = 0.049).
Conclusion: Our model is concise, comprehensive, and high efficient, which may contribute to better predicting the prognosis of patients with CRLMs with NACT before the liver resection. In addition, we observed an unbalanced distribution of HGPs as well.
Introduction
Colorectal cancer (CRC) is the third commonest malignancy, leading to about 0.9 million deaths in 2020 globally (1). The prognosis of colorectal cancer is closely relating to the stage; 5-year survival rate of stage I is more than 90%, whereas that of stage IV with distant metastasis is less than 14% (2). Accumulating evidence has revealed that liver is the most frequent site of CRC metastasis, 15%–25% of patients with CRC having liver metastasis at initial diagnosis and another 18%–25% developing liver metastasis within 5 years of early diagnosis (3, 4). The traditional sandwich approach of neoadjuvant therapy–surgical excision–adjuvant chemotherapy is considered the standard treatment for these patients, but the 5-year survival rate is less than 60% (5–7). Clinical risk score (CRS) is a mature biomarker that was used to predict the risk of recurrence in patients with colorectal cancer liver metastasis (CRLMs) after surgery and direct the preoperative treatment (8). However, CRS focuses on clinical traits of tumor and pays little attention to genetic, pathological, and imaging data of tumor. In addition, CRS assigns one point to all risk factors, without considering the weight of these factors. Furthermore, chemotherapy and follow-up after surgery are an important part of the treatment of CRLMs, but CRS does not seem to focus on this. Therefore, we urgently need a novel, comprehensive, and high-efficient recurrence risk prediction model in clinical practice to guide the postoperative treatment.
For patients receiving neoadjuvant chemotherapy (NACT) before liver resection, the response evaluation criteria in solid tumors (RECIST) are commonly used to estimate the curative effect of NACT and patients’ prognosis, which relies on magnetic resonance imaging (MRI) or computerized tomography (CT) or both (9–11). After hepatectomy, tumor regression grade (TRG), a pathological assessment approach, is utilized to assess the chemotherapy efficacy and outcome (12–14). Recently, histopathological growth patterns (HGPs), new pathology evaluation criteria, have been gradually introduced. Several studies have shown that CRLMs are present in one of three common HGPs referred to as desmoplastic HGP (dHGP), replacement HGP (rHGP), and pushing HGP (pHGP) (15, 16). dHGP presents as the metastatic cancer cells separated with hepatocytes by a margin of fibrous tissue; but in rHGP, cancer cells directly contact with hepatocytes and are in continuity with liver cell; finally, pHGP is the hepatocytes that surround the metastasis, which are pushed away and compressed (15, 17, 18). In terms of prognosis, dHGP is the best, followed by pHGP, and rHGP is the worst (15). Boris et al. further pointed out that patients with non-100% dHGP at liver–tumor interface had significantly lower survival than those with 100% dHGP (19).
Here, we investigated the value of some biomarkers to predict the prognosis of patients with CRLMs accepting NACT before liver resection. Then, we constructed an operational prognostic model for predicting recurrence and stratifying patients. In addition, we compared the purely predictive ability between our model and CRS.
Materials and Methods
Patients
Specimens were obtained from patients treated at the Zhejiang Cancer Hospital from May 2009 to December 2019 diagnosed with synchronous or metachronous CRLMs. All lesions presented in the liver before the first chemotherapy. Patients with insufficient basic information, without genetic test results, non-simultaneous radical liver metastases resection, and interventional treatment like radiofrequency ablation or selective hepatic arterial embolization before liver resection were excluded. Patients with extrahepatic metastases before liver resection were excluded from the study. Patients who died within 1 month of hepatectomy were also excluded. The characteristics like age, gender, NACT regimen, targeted drugs, number of metastases, diameter of maximum liver metastasis, time of liver metastasis, invasion depth and lymph node status, RAS and BRAF genes status (RAS gene includes KRAS and NRAS gene), and serum carcinoembryonic antigen (CEA) levels were recorded. Follow-up was done via outpatient and telephone visits. Regular liver and lung imaging and serum tumor marker levels were used to monitor tumor recurrence.
Pathological Assessment of Liver Metastases
All participants gave written informed consent. Liver resection specimens were fixed in formalin, embedded in paraffin, and stained with hematoxylin and eosin (H&E). The slices were independently and blindly evaluated by two experienced pathologists. In case of inconsistent conclusions, the HGPs and TRG results were reviewed by two experts. Positive surgical margins (R1/R2) were defined as residual tumor cells present at or within 1 cm of the resection margins, such patients were excluded. According to the National Comprehensive Cancer Network (NCCN) guideline–recommended evaluation criteria (20–22), TRGs 0 to 2 indicate major response, whereas TRG 3 indicates minor response. HGP analyses were done as the report quoted from the study by Boris et al. (19), with 100% dHGP at tumor–liver interface indicating dHGP and any proportion of rHGP or pHGP at tumor–liver interface indicating non-dHGP.
Evaluation Criterion of Other Biomarkers
Radiologic evaluation was done on the basis of RECIST version 1.1 (23). We evaluated all measurable lesions and pathological lymph nodes by CT or MRI. A sum of the diameters of longest tumor lesions and short axis for lymph nodes of all target lesions was calculated as baseline sum diameters. The response evaluation of NACT was implemented within 2 weeks after treatment. Next, the sum of shrunken or enlarged diameters of target lesions was determined. Finally, the therapeutic response of the target lesions was obtained by comparing the guideline, including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD).
CRS for each patient was determined the report from Fong et al. (8); each of the following criteria assigned 1 point: serum CEA level > 200 ng/ml, diameter of the largest hepatic tumor > 5 cm, number of metastases > 1, and positive lymph nodes and disease-free interval from primary lesion excision to liver metastases < 12 months. The first three clinical data were obtained from the first visit. The total score for each patient was then calculated; scores of 0 to 2 indicated low risk, whereas scores of 3 to 5 indicated high risk.
Statistical Analysis
Statistical analyses were performed using R and SPSS version 26.0. Ordinal and unordered categorical variables were compared using the Chi-square or Fisher exact test, as appropriate. Survival was evaluated by Kaplan–Meier (K-M) analysis and compared using the log-rank or Breslow test. Disease-free survival (DFS) is defined as the time from liver and primary tumor resection to tumor recurrence. Cox proportional hazard analysis was used to identify independent DFS prognostic factors. Multivariate analysis was completed for factors with p ≤ 0.200 after univariate analysis; p ≤ 0.05 was statistical significance. The Cox regression consequence was adjusted for confounding bias, checking for multicollinearity, and setting up disordered multi-category variable as dummy variable.
Results
Clinical Patient Characteristics
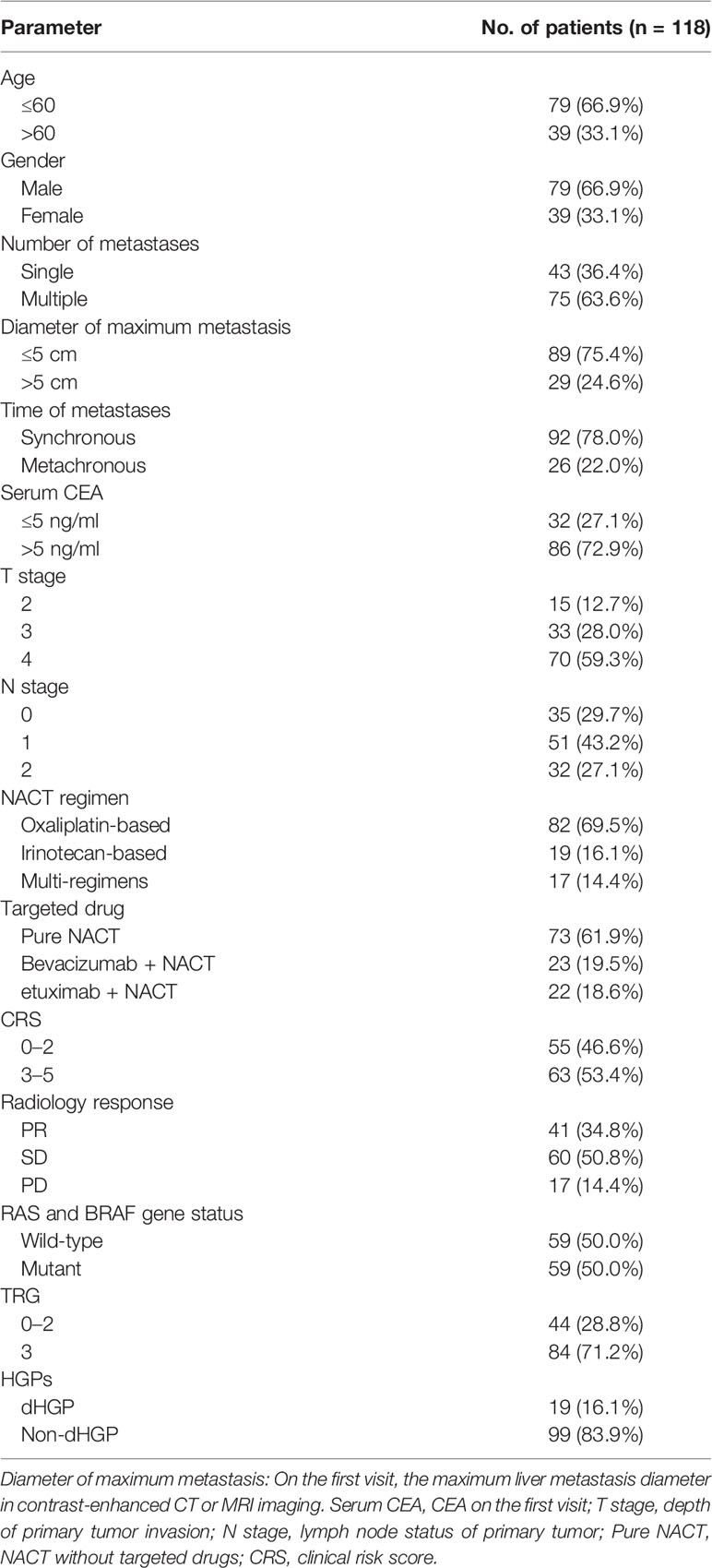
From May 2009 to December 2019, a total of 118 patients with CRLMs who underwent NACT before liver resection were recruited into the study after meeting inclusion criteria. The cohort’s characteristics were summarized on Table 1. Most patients were male (66.9%), and 66.9% were < 60 when they first came to the hospital due to colorectal cancer. In addition, 87.3% were in stage T3 or T4, 70.3% have positive lymph nodes, and 63.6% of patients had multiple metastases. Of these, 24.6% had resected lesions with maximum diameters of >5 cm, and 22% were metachronous metastases. Positive serum CEA levels of > 5ng/ml were detected in 72.9% of patients. RAS or BRAF mutations were observed in 50% of patients. With regard to NACT, 69.5% of patients received oxaliplatin-based regimens, 16.1% received irinotecan-based regimens, 17 patients received more than one regimen, and more than half of patients did not receive targeted drugs (61.9%).
According to radiology response, we found that none of patient achieved CR after several cycles of chemotherapy and more than half got SD (50.8%), whereas PD occurred in 14.4% of the patients. Patients with larger tumors tended to have a better regression. Only one (3.4%) patient with > 5-cm lesion progressed, whereas 16 (18.0%) patients with a < 5-cm metastasis progressed after chemotherapy (p = 0.001). Moreover, CRS low-risk and high-risk patients were approximately evenly distributed (46.6% vs. 53.4%).
In terms of pathological assessment, 19 (16.1%) patients had a dHGP. Considering the TRG score, patients with TRG 3 accounted for the vast majority (71.2%). Meanwhile, a high correlation between HGPs and TRG was observed, which found that non-dHGP accounted for 95.2% of TRG 3 (p < 0.001). Negative lymph node was more common in dHGP (52.6% vs. 25.3%, p = 0.017). Moreover, all patients with PD were non-dHGP (p = 0.009), and 91% of primary tumor had penetrated the muscularis propria in non-dHGP (p = 0.024).
Disease-Free Survival and Prognostic Factors
The median follow-up was 7.2 months, and the 1-, 2-, and 3-year DFS rates after liver resection were 38.0%, 18.5%, and 13.5%, respectively. A total of 39 (33.1%) patients had not recurrence at the last follow-up.
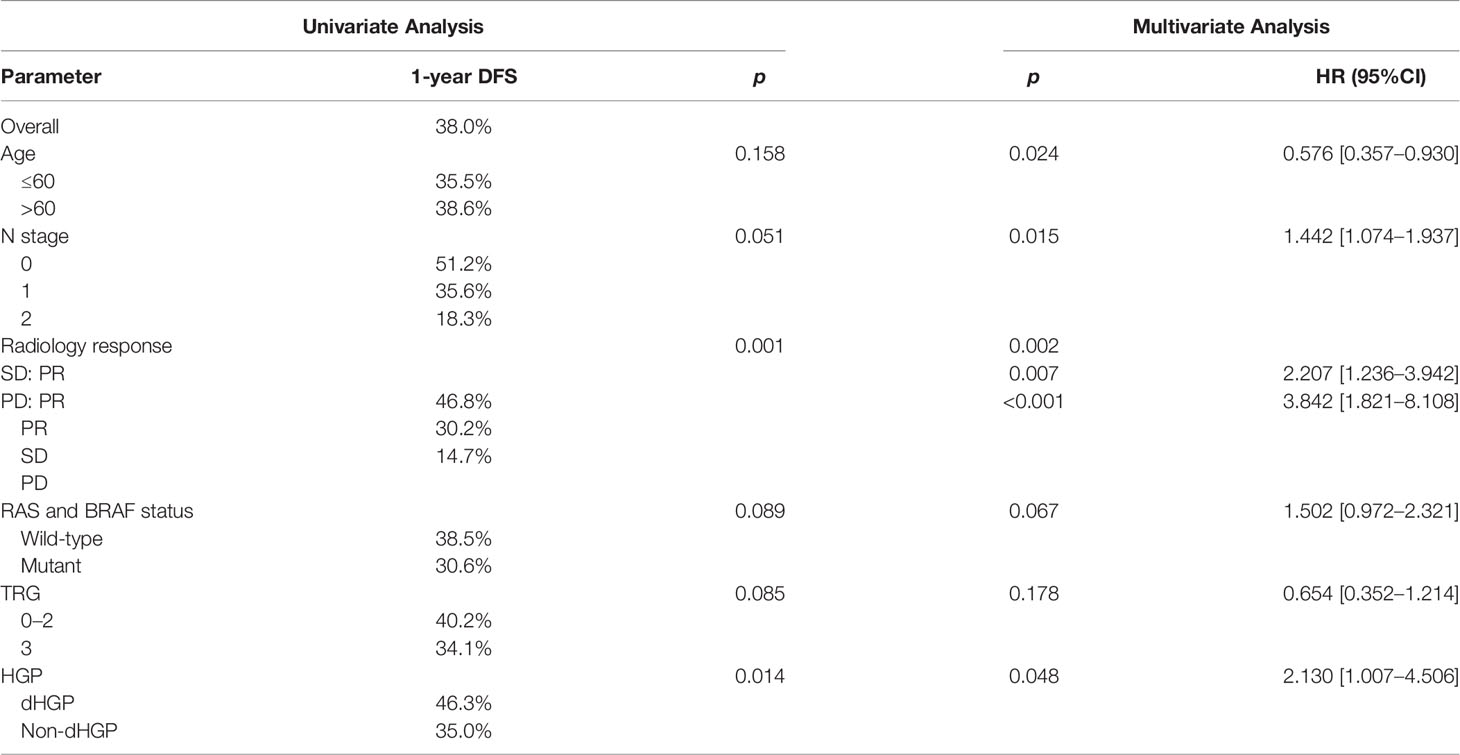
Results of univariate and multivariate analysis of DFS prognostic factors are shown in Table 2. Univariate analysis showed that HGPs (p = 0.014, Figure 1A) and radiology response (p = 0.001, Figure 1B) correlated with prognosis. In the multivariate one, HGPs was confirmed as an independent prognostic factor for DFS (HR = 2.130, p = 0.048). The median DFS of patients with dHGP was 14 months [interquartile range (IQR): 3–26 months] compared with 7 months (IQR: 6–8 months) in patients with non-dHGP. Additional independent prognostic factors were age (HR = 0.576, p = 0.024), lymph node status (HR = 1.442, p = 0.015), and radiology response (SD vs. PR, HR = 2.207, p = 0.007; PD vs. PR, HR = 3.842, p < 0.001). Radiology response was set as a dummy variable in data processing. No multicollinearity was found.
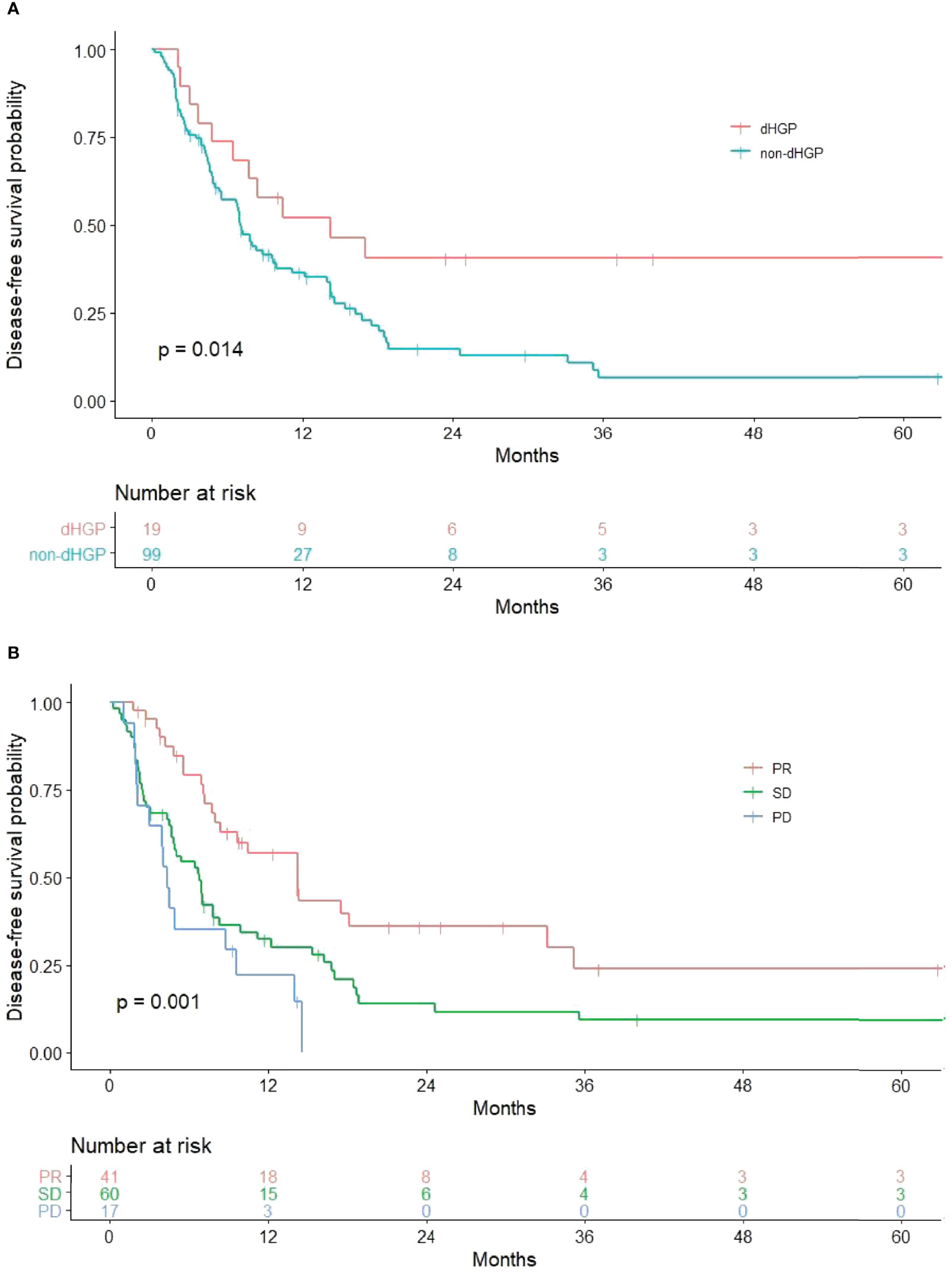
Figure 1 Significant results in univariate analysis. (A) Relationship between HGPs and DFS. The non-dHGP is associated with shorter DFS than the dHGP (median DFS = 7.0 vs. 14.2 months, p = 0.014). (B) Relationship between radiology response and DFS (median DFS for PR vs. SD vs. PD = 14.2 vs. 6.8 vs. 4.3 months, p = 0.001).
A New Recurrence Risk Prediction Model
The DFS nomogram showed in Figure 2A based on biomarkers including age, lymph node status, radiology response, and HGPs. Then, we queried patients score from each item and rounded to the nearest whole number before calculating the total score. We then performed a K-M analysis and found that patients’ outcome was improved significantly in score ≥5. Therefore, we classified patients with a score of 5 to 11 as low risk and those with a score of 0 to 4 as high risk. We found DFS to be markedly higher in low-risk relative to high-risk patients (median DFS = 14.3 vs. 4.9 months, p < 0.0001, Figure 2B). The calibration plot for the probability of 1- or 3-year DFS after surgery demonstrated satisfactory consensus between the prediction via nomogram and actual observation (Figures 3A, B). Receiver operating characteristic (ROC) curve to predict short- and long-term prognosis based on this risk model revealed area under the curve (AUC) of the risk model 1-, 2-, and 3-year DFS to be 0.754, 0.705, and 0.666, respectively, and the new model has a better AUC than CRS (Figures 4A, B). On the basis of Figure 4C, the survival decision curve analysis (DCA), which confirmed that the risk model displayed more clinical benefits than CRS.
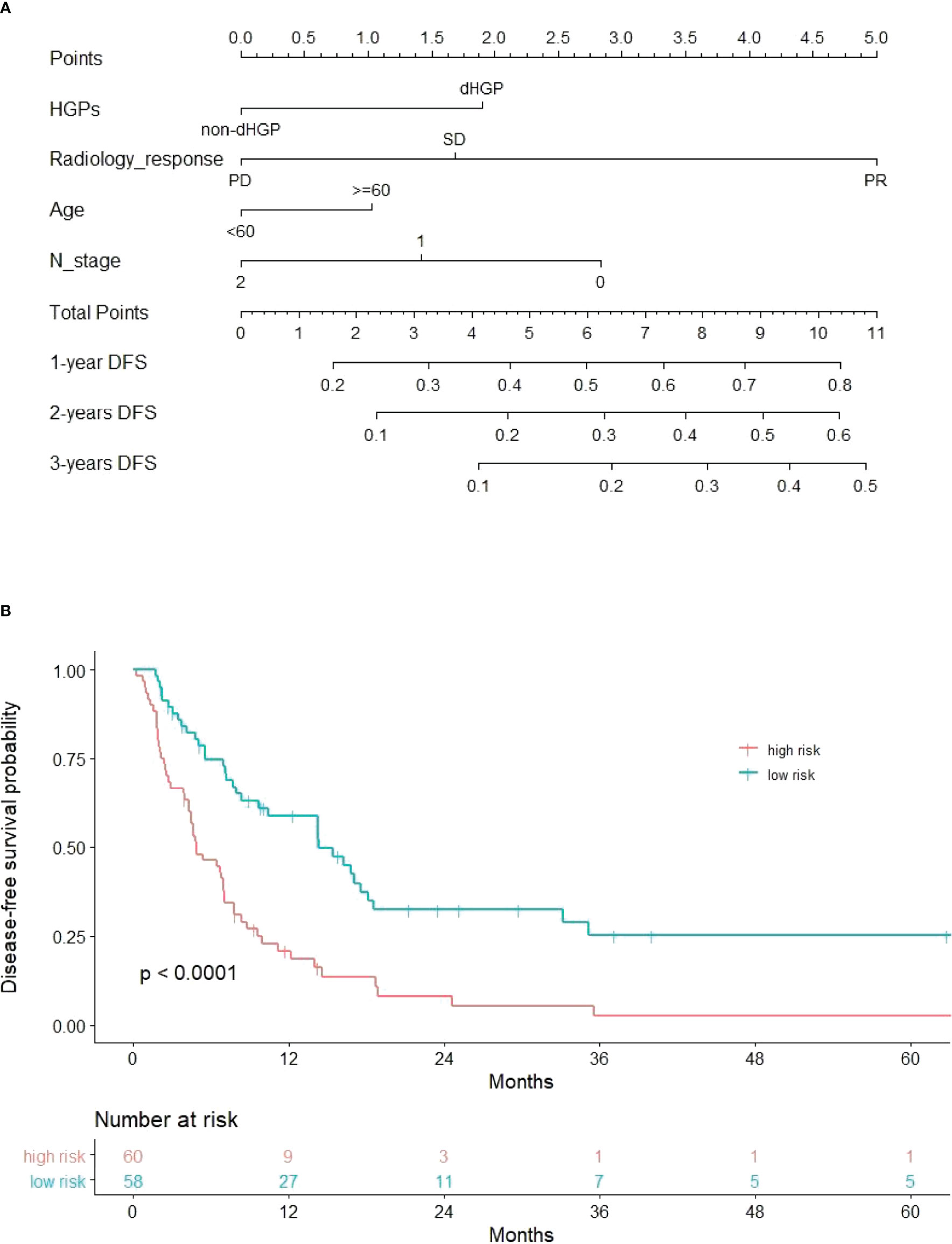
Figure 2 Nomogram and recurrence curve. (A) Nomogram incorporating HGPs, radiology response, lymph node status of primary tumor, and age for predicting the DFS of patients with CRLMs. Total points were obtained by summing up individual points from the respective variables, and lower points indicate poorer survival. (B) Differences in DFS between high risk and low risk patients (median DFS = 4.9 vs. 14.3 months, p < 0.0001).
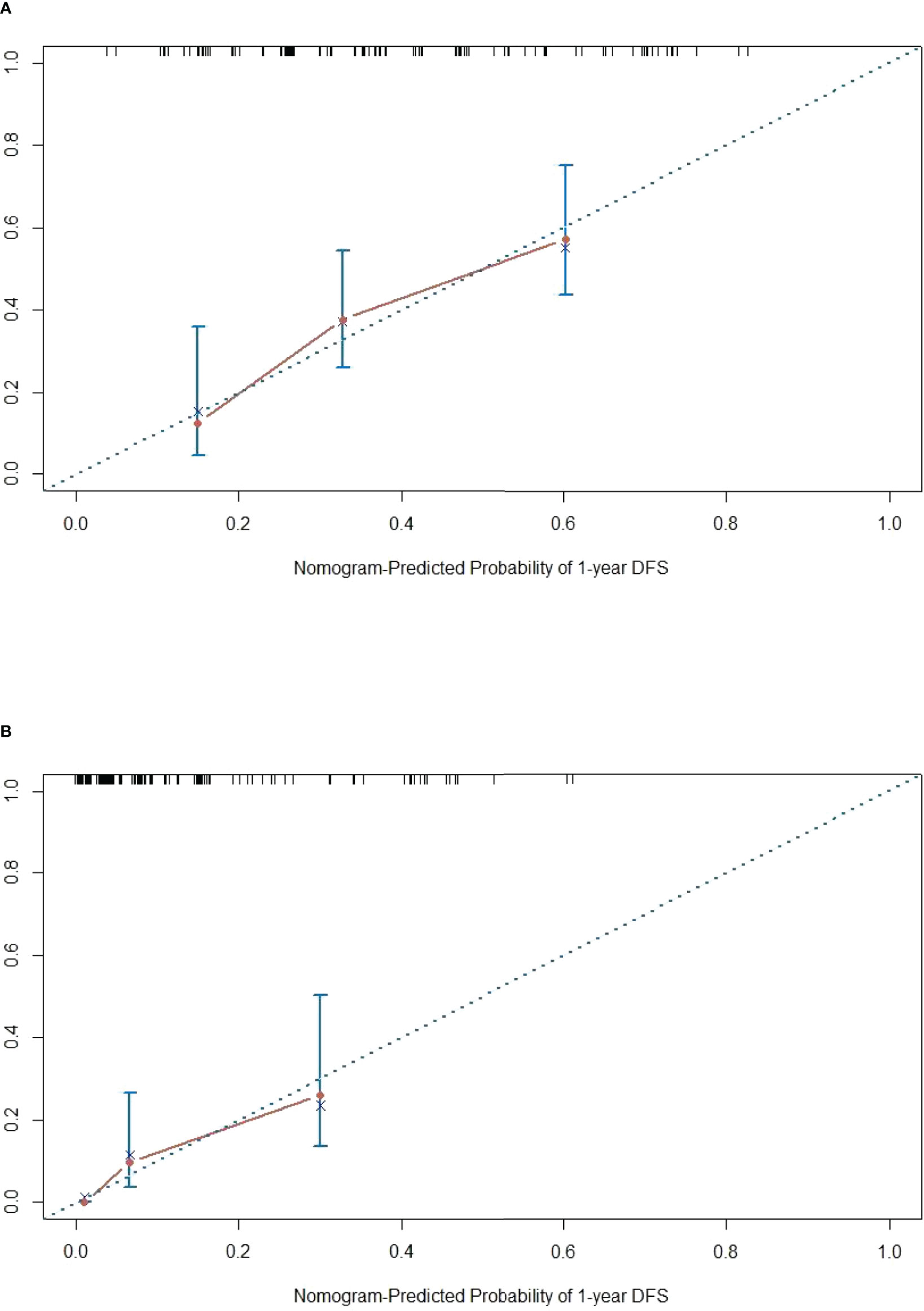
Figure 3 Calibration diagram. (A, B) One-year calibration and 3-year calibration diagram for assessment of the nomogram. The nearer distance of red dots to the diagonal line, the more accurate is the prediction of the nomogram.
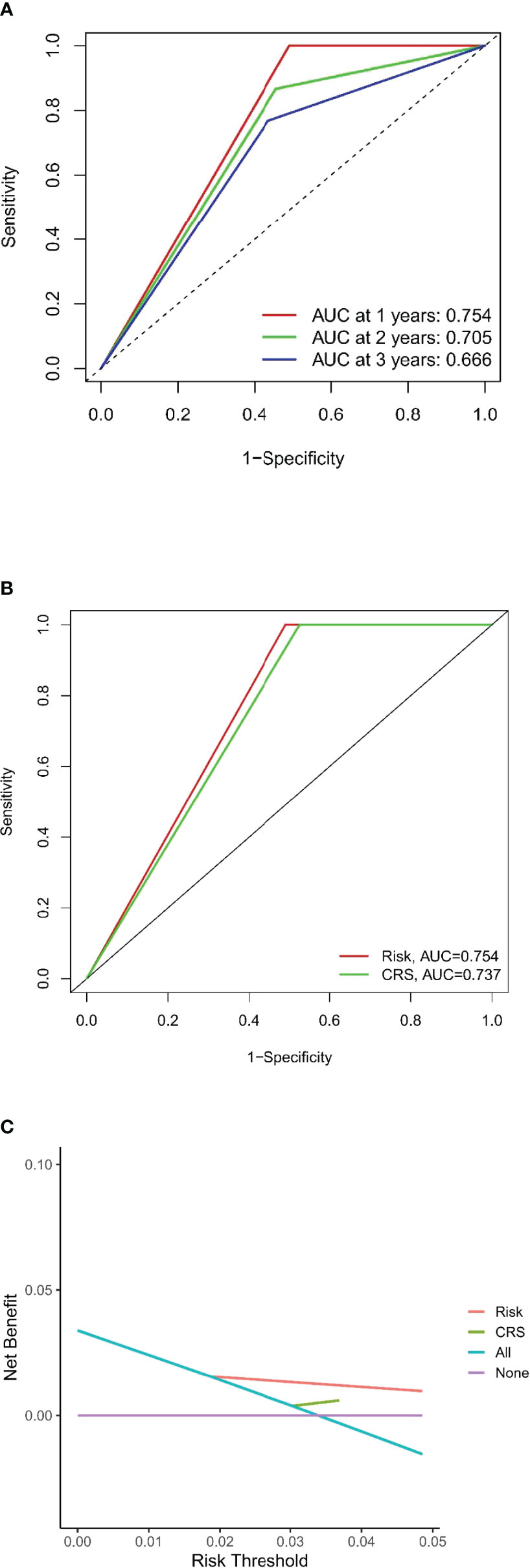
Figure 4 Evaluation of the predictive model and DCA analysis. (A, B) ROC curves of the predictive model. (C) DCA analysis for new model and CRS.
Cetuximab Led to a Low rHGP Proportion
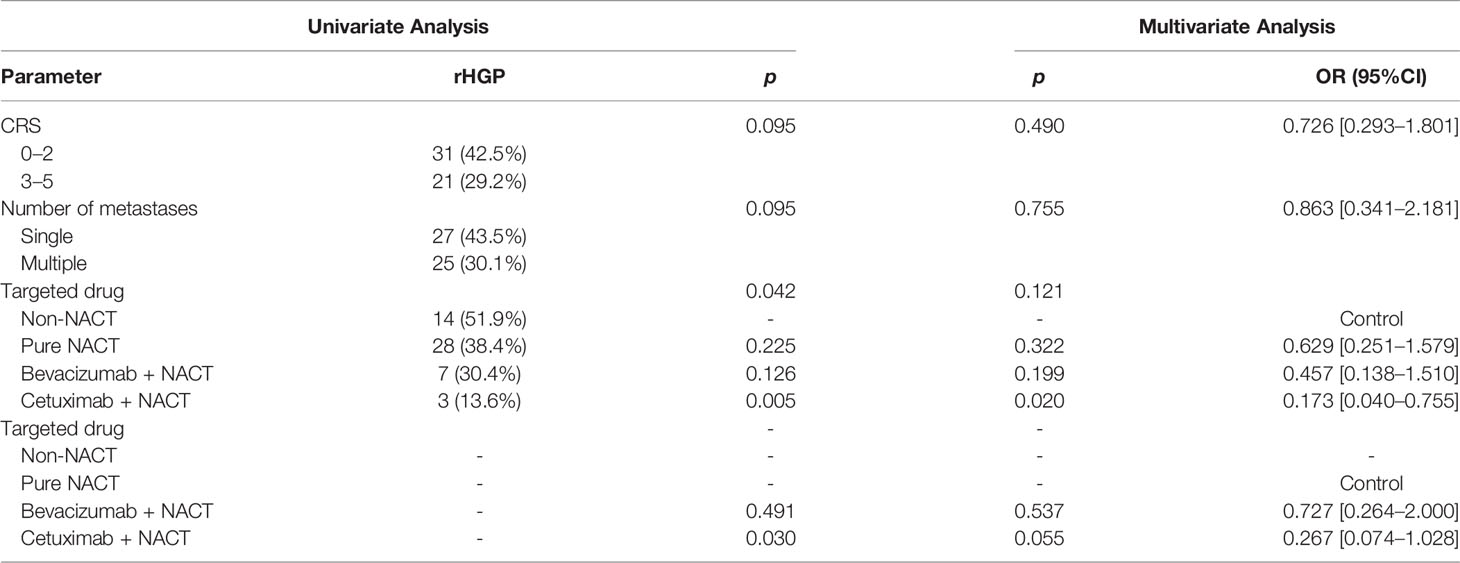
In our study, we found a strong correlation between HGPs and cetuximab. rHGP was rare in patients who used cetuximab in NACT before lesion excision. We added a cohort (27 patients) that did not accept NACT before liver resection (control group); thereby, the study cohort was extended to 145 patients. Then, HGP was obtained from every patient in light of the latest international consensus guidelines (15): Taking 50% as the cutoff value, rHGP < 50% rim of liver–tumor interface is non-rHGP and rHGP ≥ 50% rim of liver–tumor interface is rHGP. In univariate analysis, compared with control group, patients who accepted pure NACT without targeted drugs had a lower proportion of rHGP (51.9% vs. 38.4%, p = 0.225, Table 3). The addition of bevacizumab or cetuximab plus chemotherapy attenuated the rHGP occurrence (51.9% vs. 30.4%, p = 0.126; 51.9% vs. 13.6%, p = 0.005). Furthermore, cetuximab plus chemotherapy as the one and only independent factor influencing HGPs (HR = 0.173, p = 0.020), no similar effect was observed with bevacizumab (38.4% vs. 30.4%, p = 0.491). Transforming patients who treated with pure NACT as control group, cetuximab plus chemotherapy still significantly reduced rHGP incidence (38.4% vs. 13.6%, p = 0.030). In addition, cetuximab plus chemotherapy was not an independent factor (HR = 0.267, p =0.055). After ruled out patients who had a more than 50% pHGP or mixture HGP in non-rHGP subgroup, a separate multivariate analysis was calculated. In this further analysis, we also discovered low rHGPs level in cetuximab plus chemotherapy subgroup [HR = 0.176, p = 0.025 (vs. non-NACT patients); HR = 0.256, p = 0.049 (vs. pure NACT patients)].
Discussion
The novel recurrence risk prediction model has shown great effectiveness for patients who received NACT before radical primary tumor and liver metastases resection. All the indicators of this model are from the routine preoperative and postoperative examinations of patients, which does not require additional unnecessary medical examinations and will not increase the economic burden of patients. Patient who underwent chemotherapy would evaluate the suitability for surgery using imaging tests before lesions resection, so it is very convenient to acquire every patient’s radiology response. Lymph node status and HGPs assessment are available to perform via pathological evaluations on surgical specimens. Compared with other examinations, postoperative pathological examination can more directly reflect the characteristics and the response to preoperative chemotherapy of the tumor and may provide guidance for the selection of postoperative chemotherapy regimens.
CRS system, a basis for the formulation of treatment strategies, provides reliable stratification of patients and is now widely used in clinical practice (24–26). Previous studies have shown no difference in DFS between patients in the low-CRS group who underwent direct surgery and those who received NACT before surgery, but patients in the high-CRS group who received NACT pre-operation had significantly better DFS than those who underwent direct surgery (27). Furthermore, the NCCN guidelines have recommended CRS as the standard stratification for the patients with CRLMs to determine whether patients receive preoperative chemotherapy (20, 21). In clinical practice, there are more patients with high CRS. However, a part of low-CRS patients who have some high-risk factors such as local progression of the primary lesion, the large metastases, or hepatic vascular invasion will also receive NACT. Our model was designed for patients with high CRS and some low CRS who received NACT before surgery. Five criteria of CRS are from the preoperative, focusing on the preoperative personalized treatment of patients with CRLMs. Our model includes both preoperative and postoperative indicators, which can more accurately predict patients’ DFS and provide a reference for clinicians to formulate personalized follow-up and treatment plans for patients after surgery. Nomogram has the inherent advantage of providing different coefficient for different risk factors. Our model and CRS can be used together to demonstrate the precision treatment of cancer, which is in line with the direction of modern cancer treatment. Our model outperforms CRS if it only predicts the prognosis of patients receiving NACT before surgery.
The prognostic value of HGPs was demonstrated again in our study; this is an emerging biomarker and is also the focus of this study. It is also being studied by more and more oncologist and pathologist (28–30). However, the mechanism of different HGPs influencing prognosis is still not clear at present, and it is well known that the angiogenesis of rHGP and dHGP is different. In dHGP, angiogenenic pattern is characterized by a sprouting angiogenesis. As for rHGP, cancer cells replace hepatocytes in the liver cell plates, allowing metastases to integrate in the sinusoidal blood vessels at the tumor–liver interface, without inducing sprouting angiogenesis, which is a process termed “vessel co-option” (15, 17, 18). What is known is that bevacizumab is only effective against sprouting angiogenesis; it may contribute to better outcomes for patients with dHGP (18). Therefore, the underlying molecular mechanisms require further investigation.
Radiology response is the one of the strongest prognostic factors in our study, and the prognosis has a prominent difference between different stratifications. RECIST has been revealed strongly predictive potential in other studies (31, 32). Although TRG did not representing prognostic value in multivariate Cox regression, we detected that almost all (95.2%) TRG 3 were non-dHGP. Compared with dHGP, metastatic tumors with non-dHGP tend to have worse prognostic characteristics in other aspects, such as positive lymph node, tumor progression after chemotherapy, and less tumor regression.
Some researchers have pointed out that liver metastases with a more than one growth pattern represented tumors transitioning from one growth pattern to another in some cases (15, 19). Frentzas et al. discovered that chemotherapy plus bevacizumab might increase the proportion of rHGP in patients with recurrence or progression (18, 33). We speculated that cetuximab might convert rHGP to dHGP, resulting in a lower proportion of rHGP in the cetuximab plus chemotherapy subgroup. Tumors with rHGP exhibit reduced immune cells infiltration; in addition, in dHGP, we found dense that lymphocytes infiltration appears in the liver–tumor interface (Figures 5A, B). Several studies found that rHGP exhibited reduced CD8+ immune cells infiltration (34, 35), and high levels of peri-tumor infiltration by CD4+, CD45RO+, and CD8+ cells would appear in dHGP (15, 34). Höppener et al. reported peritumoral and intratumoral enrichment of cytotoxic CD8+ T cells in dHGP as well as a higher CD8+/CD4+ ratio (36). An immunoglobulin G1 isotype monoclonal antibody (IgG1 mAb), cetuximab, elicits immune reactions like antibody-dependent cell-mediated cytotoxicity (ADCC) involving natural killer cells and T-cell recruitment to the tumor (37). In addition, tumor regression by cetuximab is ADCC-dependent and is mediated by tumor infiltrating CD8+ T effector cells. This novel mechanism of ADCC mediated by CD8+T effector cells was restricted to IgG1 anti-EGFR mAb (38). Possibly through CD8+ T-cell recruitment and improved natural killer cell-mediated ADCC efficiency, cetuximab alters the tumor microenvironment, thereby mediating conversion from rHGP to dHGP.
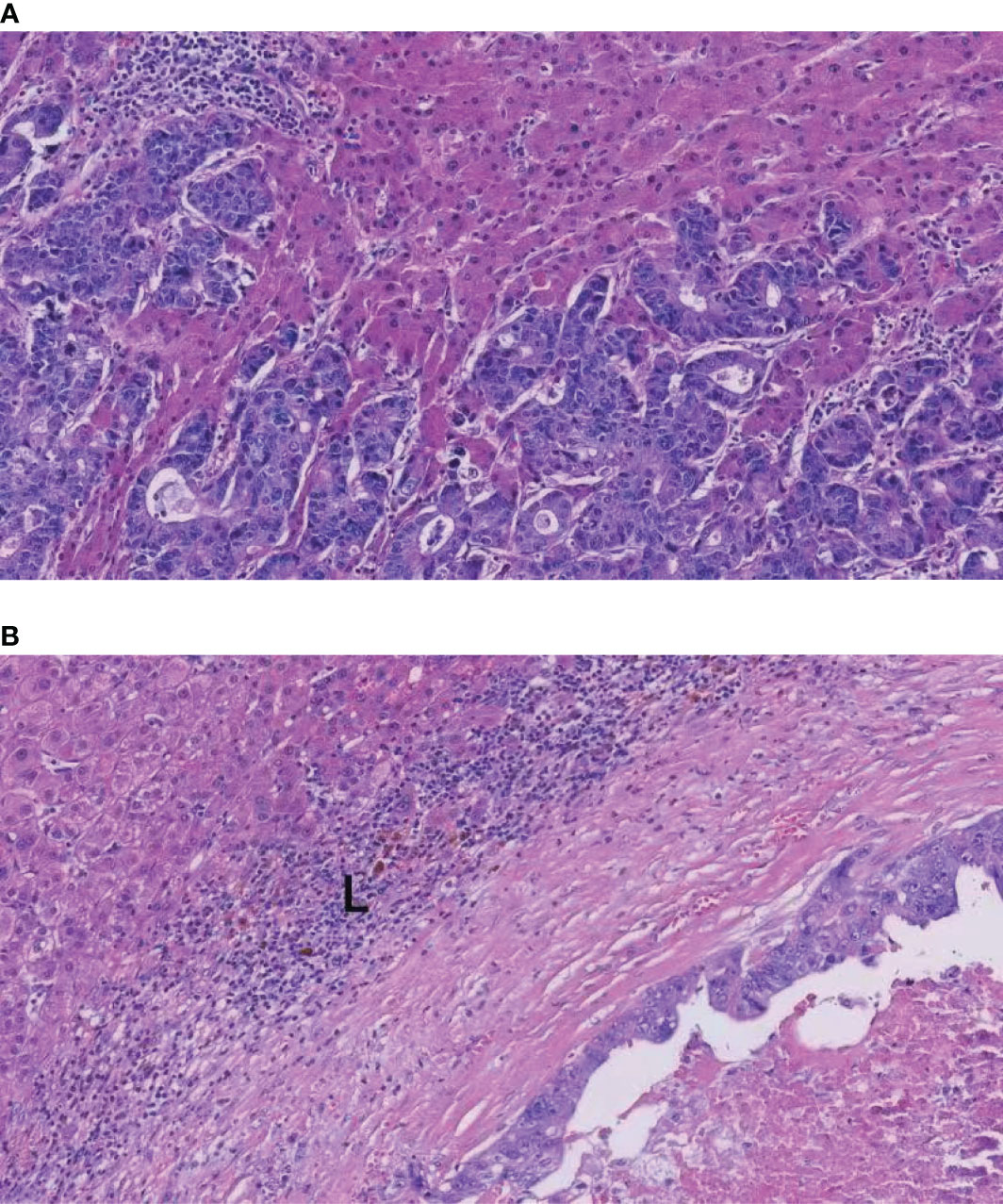
Figure 5 H&E images of the rHGP and dHGP. (A) High magnification image of the rHGP. (B) High magnification image of the dHGP. L: lymphocyte infiltrate.
However, evidence that cetuximab promotes rHGP-to-dHGP conversion is insufficient because we could not prove that the distribution of various HGPs was uniform among the four subgroups before treatment. The best way to demonstrate HGPs conversion is comparing HGPs before and after treatment with cetuximab in the same lesion. There are still many limitations in our study. First of all, this is a retrospective study with weakness related to its study type. Second, although the novel prognostic model has a satisfactory predictive capacity, there is no external validation using data from other experienced centers. Third, because of the low incidence of dHGP, the patients with no dHGP were significantly more than dHGP in our study.
Our model is concise, comprehensive, and high efficient, which may contribute to better predicting the prognosis of patients with CRLMs with NACT before liver resection and provide reference for postoperative treatment of patients. Imaging and pathology data make a fundamental contribution to predicting patient prognosis. We observed an unbalanced distribution of HGPs, and the mechanism of this phenomenon needs further investigation.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The study was reviewed and approved by the ethics committee of the Cancer Hospital of University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou, China. The study was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants for their participation in this study.
Author Contributions
HJ and ZZ contributed to conception and design of the study. ZZ, WW, and DS organized the database. ZZ and XH performed the statistical analysis. ZZ and XH wrote the first draft of the manuscript. ZL wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal Cancer Statistics, 2020. CA: Cancer J Clin (2020) 70(3):145–64. doi: 10.3322/caac.21601
3. van der Geest LG, Lam-Boer J, Koopman M, Verhoef C, Elferink MA, de Wilt JH. Nationwide Trends in Incidence, Treatment and Survival of Colorectal Cancer Patients With Synchronous Metastases. Clin Exp metastasis. (2015) 32(5):457–65. doi: 10.1007/s10585-015-9719-0
4. van Gestel YR, de Hingh IH, van Herk-Sukel MP, van Erning FN, Beerepoot LV, Wijsman JH, et al. Patterns of Metachronous Metastases After Curative Treatment of Colorectal Cancer. Cancer Epidemiol (2014) 38(4):448–54. doi: 10.1016/j.canep.2014.04.004
5. House MG, Ito H, Gönen M, Fong Y, Allen PJ, DeMatteo RP, et al. Survival After Hepatic Resection for Metastatic Colorectal Cancer: Trends in Outcomes for 1,600 Patients During Two Decades at a Single Institution. J Am Coll Surgeons. (2010) 210(5):744–52, 52-5. doi: 10.1016/j.jamcollsurg.2009.12.040
6. Viganò L, Capussotti L, Lapointe R, Barroso E, Hubert C, Giuliante F, et al. Early Recurrence After Liver Resection for Colorectal Metastases: Risk Factors, Prognosis, and Treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol (2014) 21(4):1276–86. doi: 10.1245/s10434-013-3421-8
7. Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S. Survival After Hepatic Resection for Colorectal Metastases: A 10-Year Experience. Ann Surg Oncol (2006) 13(5):668–76. doi: 10.1245/ASO.2006.05.039
8. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical Score for Predicting Recurrence After Hepatic Resection for Metastatic Colorectal Cancer: Analysis of 1001 Consecutive Cases. Ann surgery. (1999) 230(3):309–18. doi: 10.1097/00000658-199909000-00004
9. Lucidarme O, Wagner M, Gillard P, Kim S, Bachet JB, Rousseau B, et al. RECIST and CHOI Criteria in the Evaluation of Tumor Response in Patients With Metastatic Colorectal Cancer Treated With Regorafenib, a Prospective Multicenter Study. Cancer Imaging Off Publ Int Cancer Imaging Society. (2019) 19(1):85. doi: 10.1186/s40644-019-0271-z
10. Sager S, Akgün E, Uslu-Beşli L, Asa S, Akovali B, Sahin O, et al. Comparison of PERCIST and RECIST Criteria for Evaluation of Therapy Response After Yttrium-90 Microsphere Therapy in Patients With Hepatocellular Carcinoma and Those With Metastatic Colorectal Carcinoma. Nucl Med Commun (2019) 40(5):461–8. doi: 10.1097/MNM.0000000000001014
11. Vera R, Gómez ML, Ayuso JR, Figueras J, García-Alfonso P, Martínez V, et al. Correlation of RECIST, Computed Tomography Morphological Response, and Pathological Regression in Hepatic Metastasis Secondary to Colorectal Cancer: The AVAMET Study. Cancers (2020) 12(8):2259. doi: 10.3390/cancers12082259
12. Viganò L, Capussotti L, De Rosa G, De Saussure WO, Mentha G, Rubbia-Brandt L. Liver Resection for Colorectal Metastases After Chemotherapy: Impact of Chemotherapy-Related Liver Injuries, Pathological Tumor Response, and Micrometastases on Long-Term Survival. Ann surgery. (2013) 258(5):731–40. doi: 10.1097/SLA.0b013e3182a6183e
13. Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, et al. Importance of Histological Tumor Response Assessment in Predicting the Outcome in Patients With Colorectal Liver Metastases Treated With Neo-Adjuvant Chemotherapy Followed by Liver Surgery. Ann Oncol Off J Eur Soc Med Oncol (2007) 18(2):299–304. doi: 10.1093/annonc/mdl386
14. Cai Y, Lu X, Zhu X, Ju H, Sun W, Wu W. Histological Tumor Response Assessment in Colorectal Liver Metastases After Neoadjuvant Chemotherapy: Impact of the Variation in Tumor Regression Grading and Peritumoral Lymphocytic Infiltration. J Cancer. (2019) 10(23):5852–61. doi: 10.7150/jca.31493
15. van Dam PJ, van der Stok EP, Teuwen LA, Van den Eynden GG, Illemann M, Frentzas S, et al. International Consensus Guidelines for Scoring the Histopathological Growth Patterns of Liver Metastasis. Br J cancer. (2017) 117(10):1427–41. doi: 10.1038/bjc.2017.334
16. Nierop PMH, Galjart B, Höppener DJ, van der Stok EP, van den Braak C, Vermeulen PB, et al. Salvage Treatment for Recurrences After First Resection of Colorectal Liver Metastases: The Impact of Histopathological Growth Patterns. Clin Exp metastasis. (2019) 36(2):109–18. doi: 10.1007/s10585-019-09960-7
17. Donnem T, Reynolds AR, Kuczynski EA, Gatter K, Vermeulen PB, Kerbel RS, et al. Non-Angiogenic Tumours and Their Influence on Cancer Biology. Nat Rev Cancer. (2018) 18(5):323–36. doi: 10.1038/nrc.2018.14
18. Frentzas S, Simoneau E, Bridgeman VL, Vermeulen PB, Foo S, Kostaras E, et al. Vessel Co-Option Mediates Resistance to Anti-Angiogenic Therapy in Liver Metastases. Nat Med (2016) 22(11):1294–302. doi: 10.1038/nm.4197
19. Galjart B, Nierop PMH, van der Stok EP, van den Braak R, Höppener DJ, Daelemans S, et al. Angiogenic Desmoplastic Histopathological Growth Pattern as a Prognostic Marker of Good Outcome in Patients With Colorectal Liver Metastases. Angiogenesis (2019) 22(2):355–68. doi: 10.1007/s10456-019-09661-5
20. Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Network JNCCN (2018) 16(7):874–901. doi: 10.6004/jnccn.2018.0061
21. Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J Natl Compr Cancer Network JNCCN (2018) 16(4):359–69. doi: 10.6004/jnccn.2018.0021
22. Ryan R, Gibbons D, Hyland JM, Treanor D, White A, Mulcahy HE, et al. Pathological Response Following Long-Course Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Histopathology (2005) 47(2):141–6. doi: 10.1111/j.1365-2559.2005.02176.x
23. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
24. Brudvik KW, Jones RP, Giuliante F, Shindoh J, Passot G, Chung MH, et al. RAS Mutation Clinical Risk Score to Predict Survival After Resection of Colorectal Liver Metastases. Ann surgery. (2019) 269(1):120–6. doi: 10.1097/SLA.0000000000002319
25. Maithel SK, Gönen M, Ito H, Dematteo RP, Allen PJ, Fong Y, et al. Improving the Clinical Risk Score: An Analysis of Molecular Biomarkers in the Era of Modern Chemotherapy for Resectable Hepatic Colorectal Cancer Metastases. Surgery (2012) 151(2):162–70. doi: 10.1016/j.surg.2011.07.020
26. Shin SJ, Ahn JB, Choi JS, Choi GH, Lee KY, Baik SH, et al. Implications of Clinical Risk Score to Predict Outcomes of Liver-Confined Metastasis of Colorectal Cancer. Surg Oncol (2012) 21(3):e125–30. doi: 10.1016/j.suronc.2012.04.002
27. Ayez N, van der Stok EP, Grünhagen DJ, Rothbarth J, van Meerten E, Eggermont AM, et al. The Use of Neo-Adjuvant Chemotherapy in Patients With Resectable Colorectal Liver Metastases: Clinical Risk Score as Possible Discriminator. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol (2015) 41(7):859–67. doi: 10.1016/j.ejso.2015.04.012
28. Fonseca GM, Herman P, Faraj SF, Kruger JAP, Coelho FF, Jeismann VB, et al. Pathological Factors and Prognosis of Resected Liver Metastases of Colorectal Carcinoma: Implications and Proposal for a Pathological Reporting Protocol. Histopathology (2018) 72(3):377–90. doi: 10.1111/his.13378
29. Latacz E, van Dam PJ, Vanhove C, Llado L, Descamps B, Ruiz N, et al. Can Medical Imaging Identify the Histopathological Growth Patterns of Liver Metastases? Semin Cancer Biol (2020) 71:33–41. doi: 10.1016/j.semcancer.2020.07.002
30. Palmieri V, Lazaris A, Mayer TZ, Petrillo SK, Alamri H, Rada M, et al. Neutrophils Expressing Lysyl Oxidase-Like 4 Protein are Present in Colorectal Cancer Liver Metastases Resistant to Anti-Angiogenic Therapy. J Pathol (2020) 251(2):213–23. doi: 10.1002/path.5449
31. Beer L, Hochmair M, Haug AR, Schwabel B, Kifjak D, Wadsak W, et al. Comparison of RECIST, iRECIST, and PERCIST for the Evaluation of Response to PD-1/PD-L1 Blockade Therapy in Patients With Non-Small Cell Lung Cancer. Clin Nucl Med (2019) 44(7):535–43. doi: 10.1097/RLU.0000000000002603
32. Lim TS, Rhee H, Kim GM, Kim SU, Kim BK, Park JY, et al. Alpha-Fetoprotein, Des-Gamma-Carboxy Prothrombin, and Modified RECIST Response as Predictors of Survival After Transarterial Radioembolization for Hepatocellular Carcinoma. J Vasc interventional Radiol JVIR. (2019) 30(8):1194–200.e1. doi: 10.1016/j.jvir.2019.03.016
33. Mentha G, Terraz S, Morel P, Andres A, Giostra E, Roth A, et al. Dangerous Halo After Neoadjuvant Chemotherapy and Two-Step Hepatectomy for Colorectal Liver Metastases. Br J Surg (2009) 96(1):95–103. doi: 10.1002/bjs.6436
34. Brunner SM, Kesselring R, Rubner C, Martin M, Jeiter T, Boerner T, et al. Prognosis According to Histochemical Analysis of Liver Metastases Removed at Liver Resection. Br J Surg (2014) 101(13):1681–91. doi: 10.1002/bjs.9627
35. Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, et al. Histopathologic-Based Prognostic Factors of Colorectal Cancers are Associated With the State of the Local Immune Reaction. J Clin Oncol Off J Am Soc Clin Oncol (2011) 29(6):610–8. doi: 10.1200/JCO.2010.30.5425
36. Höppener DJ, Nierop PMH, Hof J, Sideras K, Zhou G, Visser L, et al. Enrichment of the Tumour Immune Microenvironment in Patients With Desmoplastic Colorectal Liver Metastasis. Br J cancer. (2020) 123(2):196–206. doi: 10.1038/s41416-020-0881-z
37. García-Foncillas J, Sunakawa Y, Aderka D, Wainberg Z, Ronga P, Witzler P, et al. Distinguishing Features of Cetuximab and Panitumumab in Colorectal Cancer and Other Solid Tumors. Front Oncol (2019) 9:849. doi: 10.3389/fonc.2019.00849
Keywords: colorectal cancer liver metastases, neoadjuvant chemotherapy, histopathological growth patterns, recurrence risk prediction model, nomogram
Citation: Zhou Z, Han X, Sun D, Liang Z, Wu W and Ju H (2022) A Comprehensive Prognostic Model for Colorectal Cancer Liver Metastasis Recurrence After Neoadjuvant Chemotherapy. Front. Oncol. 12:855915. doi: 10.3389/fonc.2022.855915
Received: 16 January 2022; Accepted: 17 May 2022;
Published: 16 June 2022.
Edited by:
Kun Wang, Peking University, ChinaReviewed by:
Satvinder Singh Mudan, Imperial College London, United KingdomTommaso Stecca, ULSS2 Marca Trevigiana, Italy
Copyright © 2022 Zhou, Han, Sun, Liang, Wu and Ju. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haixing Ju, anVoYWl4aW5nQDEyNi5jb20=; Wei Wu, d3V3ZWlAempjYy5vcmcuY24=
†These authors have contributed equally to this work and share first authorship
 Zhenyuan Zhou
Zhenyuan Zhou Xin Han
Xin Han Diandian Sun
Diandian Sun Zhiying Liang1
Zhiying Liang1