- 1Department of Thoracic Surgery, Kanazawa Medical University, Kahoku-gun, Japan
- 2Department of Rehabilitation Medicine, Shimada Hospital, Fukui, Japan
- 3Center for Clinical Genomics, Kanazawa Medical University, Kahoku-gun, Japan
- 4Division of Genomic Medicine, Kanazawa Medical University, Kahoku-gun, Japan
- 5Department of Pathology and Laboratory Medicine, Kanazawa Medical University, Kahoku-gun, Japan
A female underwent a right middle lobectomy for a pulmonary adenocarcinoma (AD). She eventually died of a right malignant pleural mesothelioma (MPM; sarcomatoid type) 4 years and 7 months after the removal of the AD even though she did not have any history of asbestos exposure, smoking, or radiation exposure. Her chest CT revealed multiple pulmonary nodules and bilateral pleural effusion with a right pleural tumor directly invading into the abdominal cavity. The genomics of tumor origin and characteristics were examined for the AD and the MPM. As a result, 50 somatic variants were detected in the AD, and 29 somatic variants were detected in the MPM. The variants which were common in both the AD and the MPM were not present, which suggested that the AD and the MPM had occurred independently in different origins. The MPM had two driver oncogenes of TP53 and EP300, but the AD did not. Two driver oncogenes of TP53 and EP300 were hypothesized to make the MPM aggressive. The speed at which the MPM progressed without the patient having a history of asbestos exposure, smoking, or radiation exposure was alarming.
Introduction
Malignant mesothelioma (MM) is an aggressive malignancy of serosal membranes, including the pleura, peritoneum, pericardium, and the tunica vaginalis of the testes, predominantly caused by prior asbestos exposure (1). Malignant pleural mesothelioma (MPM) is the most common form of mesothelioma, accounting for approximately 80% of the disease, and is a lethal cancer with nearly 25,000 deaths worldwide in 2018 (2). Despite global efforts to reduce asbestos exposure through prohibition and mine closure in many countries, a decrease of mesothelioma incidences has not been achieved. It has been characterized by a long latency period between asbestos exposure and MPM presentation (13–70 years) and a lower survival rate (3). Some studies average the prognosis to be roughly 1 year after diagnosis (4). Genetic changes are required for the malignant transformation of mesothelial cells. Several oncogenes and tumor suppressors have been hypothesized to play a role in MPM carcinogenesis (5). MPM is histopathologically classified into three variants: epithelioid, sarcomatoid, and mixed/biphasic (6).
We experienced an impressive case in whom an aggressive MPM was diagnosed 4 years and 7 months after a curative pulmonary resection for pulmonary adenocarcinoma (AD). The origin and characteristics for the two kinds of tumors were examined from the point of genomics.
Background
A 77-year-old female patient did not have a history of asbestos exposure, smoking, or radiation exposure. She used to live in the country and was a housewife. She did not work in a factory and had no known contact with asbestos. She underwent a right middle lobectomy and nodal dissection for pulmonary AD which showed a ground glass opacity of 32 mm in size (Figure 1A). The diagnosis of AD was determined to be a minimally invasive AD (predominantly lepidic pattern) of pT2aN0M0 (pStage IB). For this diagnosis, we used not only morphological methods but also immunohistochemistry methods (Figures 2A–C). The AD was positive for TTF-1 and Napsin A and negative for calretinin, D2-40, or p40, which meant a pulmonary origin. Its epidermal growth factor receptor in real-time PCR was negative, and its anaplastic lymphoma kinase was negative. After the pulmonary resection, the patient had follow-up chest X-ray or chest CT every 6 months. At 2 years and 3 months after the pulmonary resection, the follow-up chest CT revealed a right pleural effusion (Figure 1B). The cytology of the right pleural effusion was negative for malignancy, and a recurrence of lung cancer was judged to be negative. At 4 years and 5 months after the pulmonary resection, there was not any symptom and any evidence of another tumor. At 4 years and 7 months after the pulmonary resection, the chest CT revealed multiple pulmonary nodules and bilateral pleural effusion with a right pleural tumor directly invading into the abdominal cavity (Figures 1C–F). The brain CT revealed a brain metastasis. The cytology of the right pleural effusion gave two negative results. The patient died of respiratory failure due to the malignant tumors within a month after the chest CT. An autopsy of the patient revealed that a right MPM (sarcomatoid type) invaded into the right adrenal gland and the liver with multiple pulmonary metastasis, multiple pleural metastasis, multiple metastasis to hilar and mediastinal lymph nodes, and oligometastasis into the heart. The MPM consisted of fusiform-shaped sarcomatoid mesothelial cells. The MPM was negative for TTF-1, Napsin A, or p40 and weakly positive for calretinin and positive for D2-40 (Figures 2D–F), which meant a mesothelium origin. The pathology was quite different from the adenocarcinoma.
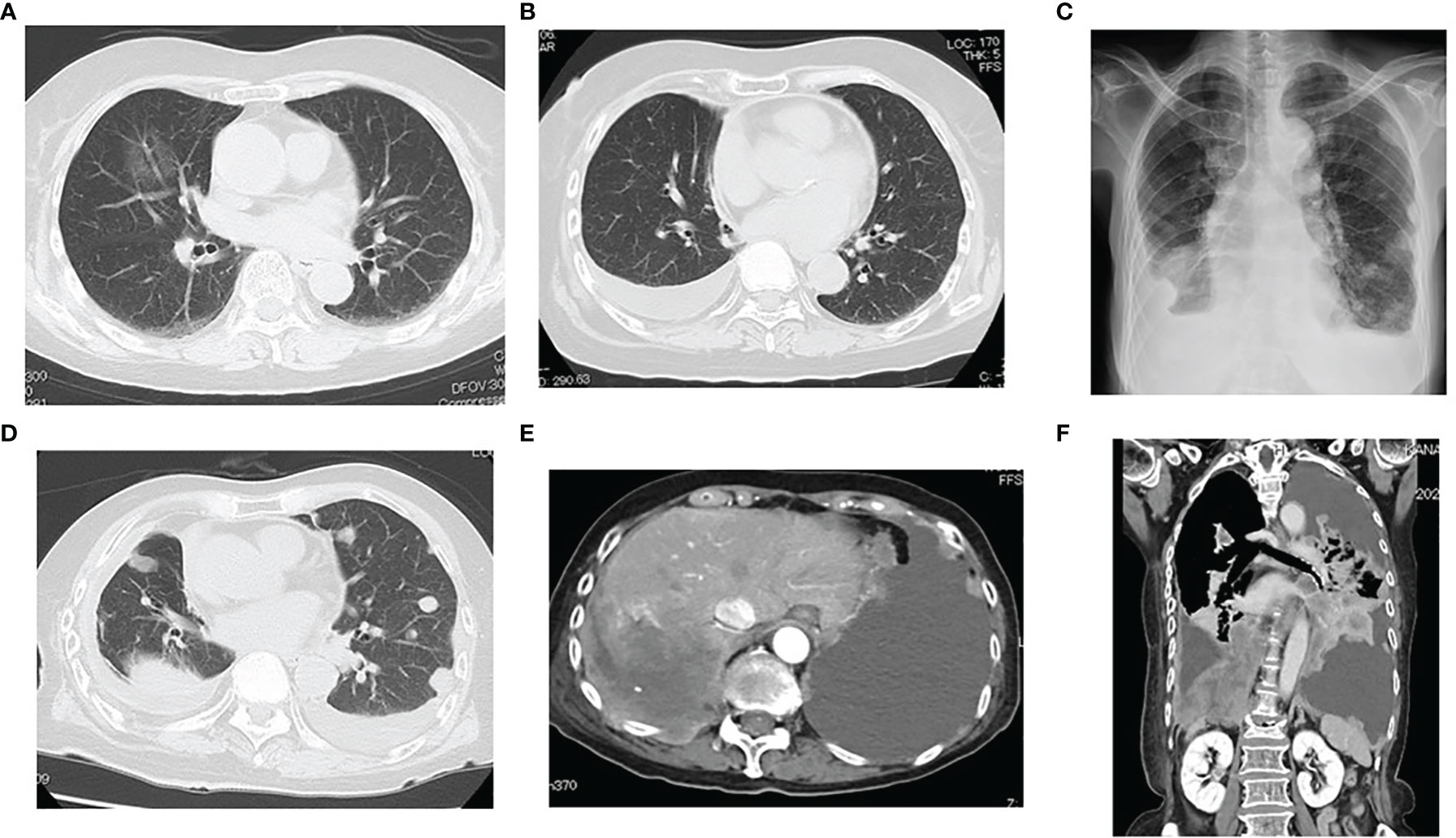
Figure 1 (A) Chest CT showing a ground glass opacity of 32 mm in size in her middle lobe of the right lung. (B) At 2 years and 3 months after pulmonary resection, the follow-up chest CT revealed right pleural effusion. (C–F) At 4 year and 7 months after pulmonary resection, the chest CT revealed multiple pulmonary nodules and bilateral pleural effusion with a right pleural tumor directly invading into the abdominal cavity. A right malignant pleural mesothelioma (sarcomatoid type) invaded to the right adrenal gland and the liver.
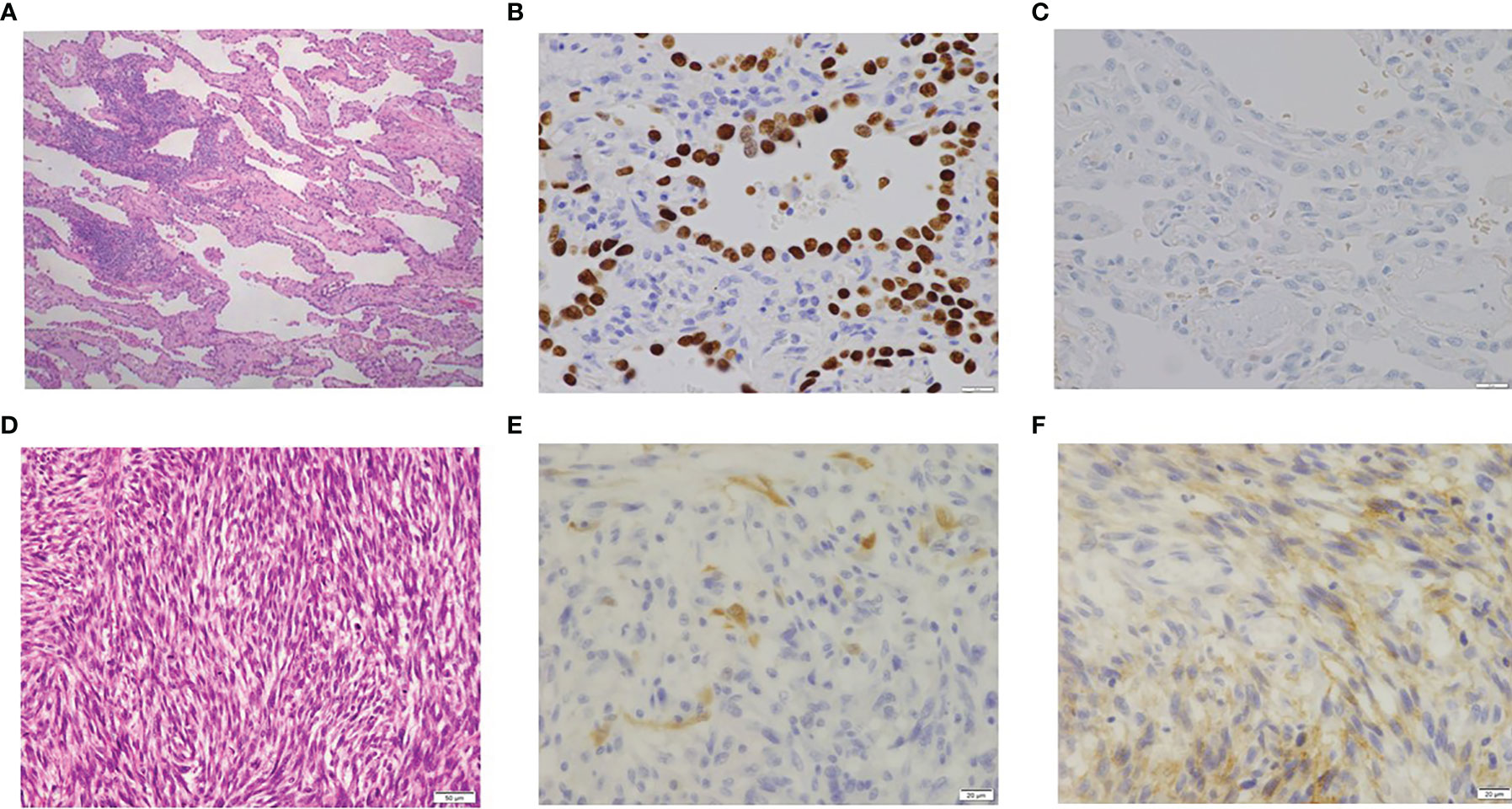
Figure 2 (A) Pathology of primary lung cancer (×100). (B) TTF-1 of lung cancer (positive; ×400). (C) Calretinin of lung cancer (negative; ×400). (D) Pathology of malignant pleural mesothelioma (×200). (E) Calretinin of malignant pleural mesothelioma (MPM; weakly positive; ×400). (F) D2-40 of MPM (positive; ×400).
A dual deep sequence was performed to accurately compare the genomes of the two tumors by SureSelect NCC oncopanel (Agilent) (7). Two kinds of DNA polymerase (KAPA, Roche CustomBiotech; NEB, New England Biolabs) were employed for the first (pre-capture) library amplification, then two libraries were created for each of the AD and the MPM. Variant calls common to the KAPA and NEB libraries were selected in each tumor, and variant calls with variant allele frequency (VAF) less than 5% (<0.05) were eliminated as noise (7). As a result, 50 somatic variants were detected in the AD, and 29 somatic variants were detected in the MPM. The variants which were common both in the AD and the MPM were not present, which suggested that the origins of these tumors were different. The tumor content ratio was low (25 to 30%) in the adenocarcinoma, and all the detected mutations seemed to be heterozygous mutations (Table 1). Mutations of TP53 and EP300 were detected in MPM, not in AD. Although a specific driver mutation was not detected in AD, the mutations of PIK3R1 c.1915C>T p.(Arg639Ter) and FLT3 c.931C>T p.(Arg311Trp) were there, which were registered in the Catalogue of Somatic Mutations in Cancer (COSIMC). The tumor mutation burden (TMB) was 53.0 mut/Mbp in the AD and 30.7 mut/Mbp in the MPM, whose TMBs showed a higher hypermutation rate. There was no translocation of chromosome and no detection of fused genes in the AD and the MPM. As for the mutation of TP53 and EP300, VAF was high in MPM, the tumor content ratio was about 90%, and the mutations were interpreted as with accompanying homozygosity and loss of heterozygosity (LOH). The two tumors had different molecular pathologies and different origins. In the structural chromosomal aberration analysis by DNA microarray with OncoScan CNV (Affymetrix) (Figure 3), all chromosomes of the MPM showed abnormality, whereas the chromosomal aberration of AD showed relatively local abnormality. The commonality was not found in the pattern of the chromosomal aberration between the AD and the MPM. The entire chromosome 17 and EP300 locus of chromosome 22 of MPM exhibited a single copy, and LOH of TP53 and EP300 was confirmed to be due to chromosome deletion.
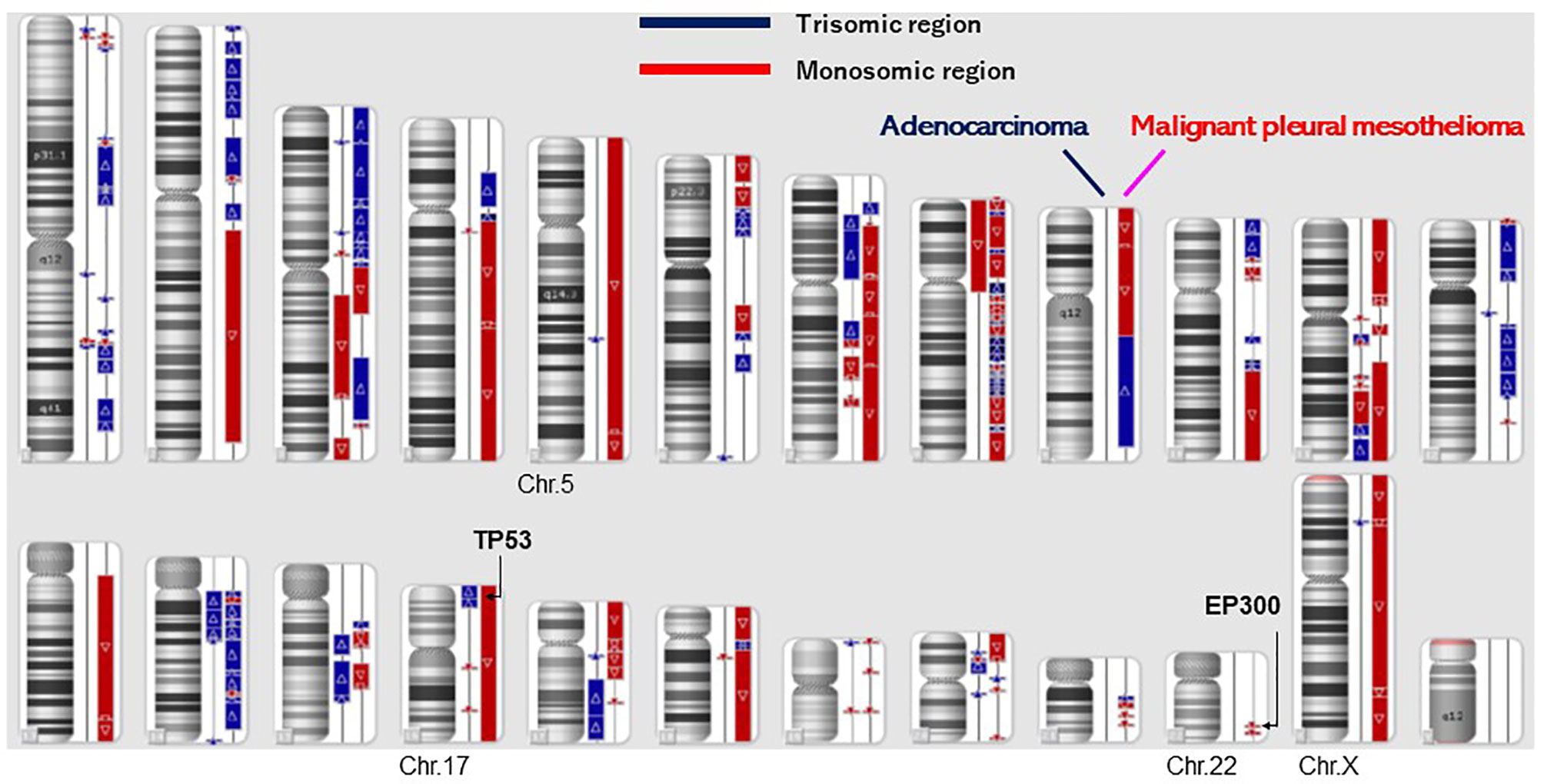
Figure 3 Structural chromosomal aberration analysis by DNA microarray (OncoScan CNV). All chromosomes showed abnormality in malignant pleural mesothelioma, whereas the chromosomal aberration of lung adenocarcinoma was relatively local. A commonality was not found in the patterns of the chromosomal aberration between the adenocarcinoma and the malignant pleural mesothelioma.
Discussion
The patient did not have any history of asbestos exposure, smoking, occupational radiation exposure, or medical radiation therapy. Besides these, there was no evidence of mesothelioma in the right pleural cavity when she underwent a right middle lobectomy and nodal dissection for pulmonary AD. Although we should have had performed more detailed examinations to detect the new tumor at 4 years and 5 months after the pulmonary resection, we did not do so because we did not suspect the recurrence of the adenocarcinoma nor a newly developed tumor. The MPM was an extremely aggressive malignant tumor that progressed quickly, causing the patient to die within 2 months.
We evaluated the patient and analyzed the causes and the genomics of the AD and the MPM. The pulmonary AD was a minimally invasive AD (predominantly lepidic pattern) of pT2aN0M0 (pStage IB) and was removed by resection. In the literature, there were several case reports in which malignant pleural mesotheliomas invaded into the lung parenchyma with intrapulmonary lepidic spread (8, 9). In our case, the immunohistochemistry indicated that the adenocarcinoma originated from the right lung and was a typical minimally invasive AD with a predominantly lepidic pattern. Besides this, the immunohistochemistry indicated that the MPM originated from the mesothelium. Our case is quite different from such cases of a malignant pleural mesothelioma with intrapulmonary lepidic spread. An autopsy of the patient was performed to assess the cause of death. The patient died from the aggressive mesothelioma. The patient’s case was discussed by surgeons and pathologists. This research began postmortem, so we were not able to get her perspective.
Although asbestos was certainly the largest and most well-known cause of MM, roughly 20% of the patients did not have any known exposure to asbestos (4). A statistically significant increase of MM develops following radiation therapy for breast cancer, testicular cancer, Hodgkin’s lymphoma, and non-Hodgkin’s lymphoma (10–13). A couple of causal factors for MPM include occupational radiation exposure and medical radiation therapy (14). Our patient had no history of exposure to either of these causal factors. The rate of sarcomatoid and biphasic disease is higher in the pleura compared to the peritoneum. A pleural MM occurs much more commonly in men, while a peritoneal MM occurs in younger patients. Pleural MM has a relatively lower 5-year survival, even for the more favorable epithelioid histology. In contrast, it has been observed that a subset of epithelioid peritoneal MM patients had prolonged survival following an aggressive therapy. Collectively, these observations raise the possibility that pleural MM and peritoneal MM have a similar morphological representation but are biologically distinct from each other.
We found the main driver mutations of TP53 (p53) and EP300 in the MPM and hypothesized that these driver mutations made the MPM aggressive. TP53 was reported in Li-Fraumeni syndrome, which was an autosomal dominant inheritance disease of pathologic mutation of the TP53 gene in the germline on ClinVar. TP53 is the most frequently mutated gene (>50%) in human cancer, indicating that the TP53 gene plays a crucial role in preventing cancer formation. The TP53 gene is located on the short arm of chromosome 17 (17p13.1). MPMs with TP53 mutations were reported to have a more aggressive phenotype (15). The mutation of the TP53 gene predicted shorter survival (16). A univariate regression analysis revealed that the overexpression of TP53 and B-cell lymphoma-2-associated X protein (BAX) in colorectal cancer tissues was associated with poor patient outcome (17). TP53 is associated with important cell functions, such as the termination on the border, apoptosis instruction, DNA repair promotion, and neovascularization suppression. EP300, also known as histone acetyltransferase p300, E1A-associated protein p300, or p300, is an enzyme that is encoded by the EP300 gene. EP300 mutations contribute to an unfavorable phenotype in a number of solid tumors and hematological malignancies, and therefore EP300 is often considered as a tumor suppressor (18). This enzyme plays an essential role in regulating cell growth and division, prompting cells to mature and preventing the growth of cancerous tumors. The downregulation of EP300 gene expression was associated with higher anti-tumor immunity in most solid malignancies (19). The EP300 gene is located on the long arm of chromosome 22 (22p13.2). EP300 and BAX contribute to the regulation of the cell cycle and apoptosis, cellular processes that are often impaired in cancer cells. Dysregulations of the expression of EP300, TP53, and BAX genes were found to contribute to colorectal cancer pathogenesis (17).
MPM is characterized by a low mutation load (20). MPM does not appear to be involved in the aberrant expression of many well-studied growth control genes, such as HRAS, KRAS, TP53 (p53), and RB1 (21–23), although the SV40 T antigen has been proposed to inactivate p53 function in some MPM tumors (24). MPM was reported to be characterized by the frequent inactivation of tumor suppressor genes, e.g., the homozygous deletion of the cyclin-dependent kinase inhibitor 2A/2B, various genetic alterations that inactivate BRCA1-associated protein-1 (BAP1), neurofibromin 2, large tumor-suppressor kinase 2, and tumor protein p53 (TP53) (4, 25–27). In our case study, the mesothelioma did not have either the BAP1 or CDKN2A mutation. The impact that the BAP1 and CDKN2A mutations have on MPM is unclear. TP53 and RB1 tumor suppressor genes were important in maintaining genetic homeostasis in MPM (28).
Although a specific driver mutation was not detected in the adenocarcinoma, there were mutations with registration in COSIMC. PIK3R1 c.1915C>T p.(Arg639Ter) is a mutation with registration in COSIMC, and pathologic significance is confirmed (COSV57126125). The mutation was observed in colorectal cancers and prostate cancers (https://cancer.sanger.ac.uk/cosmic/search?q=PIK3R1+c.1915C). FLT3 c.931C>T p.(Arg311Trp) is also a mutation with registration in COSIMC, and pathologic significance is confirmed (COSV54057282). The mutation was observed in colorectal cancers and prostate cancers (https://cancer.sanger.ac.uk/cosmic/search?q=FLT3+c.931C). As a result, PIK3R1 and FLT3 were recognized to be mutations that were not correlated to metastasis and the recurrence of a malignant tumor.
The patterns of chromosomal aberration of the AD and the MPM were quite different. The AD and the MPM showed different patterns in the chromosome structure analysis by OncoScan; specifically, the MPM had structural abnormalities in all chromosomes. The NCC Oncopanel showed no pathologic fusion gene in the AD and the MPM. The two kinds of tumor had different molecular pathologies and occurred in different origins. The TMB was 53.0 mut/Mbp in the AD and 30.7 mut/Mbp in the MPM. The TMBs showed higher hypermutation rates, and immune checkpoint inhibitors could be effective for patient therapy.
Concluding Remarks
AD and MPM occurred independently and had different origins. The MPM had the two oncogenes of TP53 and EP300, but the AD did not. The two driver oncogenes of TP53 and EP300 were hypothesized to make the MPM more aggressive. The MPM progressed quickly without a history of asbestos exposure, smoking, or radiation exposure.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
KU performed the research and wrote the paper. MI, SI, AY, YI, and NM performed therapy on a patient. YN contributed to the analysis of the genetic status of this patient. SY performed a pathological examination of lung cancers. HU contributed to the supervision of this study and revision of the manuscript. Dustin Keeling, whose native language is English, revised the paper. All authors contributed to the article and approved the submitted version.
Funding
This research was supported partly by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (grant number: 20K09172).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Robinson BM. Malignant Pleural Mesothelioma: An Epidemiological Perspective. Ann Cardiothorac Surg (2012) 1:491–6. doi: 10.3978/j.issn.2225-319X.2012.11.04
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
3. Lanphear BP, Buncher CR. Latent Period for Malignant Mesothelioma of Occupational Origin. J Occup Med (1992) 34:718–21.
4. Abbott DM, Bortolotto C, Benvenuti S, Lancia A, Filippi AR, Stella GM. Malignant Pleural Mesothelioma: Genetic and Microenviromental Heterogeneity as an Unexpected Reading Frame and Therapeutic Challenge. Cancers (2020) 12(5):1186. doi: 10.3390/cancers12051186
5. Lehman TA, Reddel R, Peiifer AM, Spillare E, Kaighn ME, Weston A, et al. Oncogenes and Tumor-Suppressor Genes. Environ Health Perspect (1991) 93:133–4. doi: 10.1289/ehp.9193133
6. Galateau-Salle F, Churg A, Roggli V, Travis WD. World Health Organization Committee for Tumors of the Pleura. The 2015 World Health Organization Classification of Tumors of the Pleura: Advances Since the 2004 Classification. J Thorac Oncol (2016) 11:142–54. doi: 10.1016/j.jtho.2015.11.005
7. Hiroki U, Sumihito T, Yo N. Dual Deep Sequencing Improves the Accuracy of Low-Frequency Somatic Mutation Detection in Cancer Gene Panel Testing. Int J Mol Sci (2020) 21:3530. doi: 10.3390/ijms21103530
8. Rossi G, Cavazza A, Turrini E, Costantini M, Casali C, Morandi, et al. Exclusive Intrapulmonary Lepidic Growth of a Malignant Pleural Mesothelioma Presenting With Pneumothorax and Involving the Peritoneum. Int J Surg Pathol (2006) 14(3):234–7. doi: 10.1177/1066896906290360
9. Jimbo N, Kawahara K, Tsukamoto, Minami K, Tanaka Y, Maniwa Y, et al. Malignant Pleural Mesothelioma Showing Intrapulmonary Lepidic Spread. Pathol Int (2020) 70(6):373–5. doi: 10.1111/pin.12927
10. Tward JD, Wendland MM, Shrieve DC, Szabo A, Gaffney DK. The Risk of Secondary Malignancies Over 30 Years After the Treatment of Non-Hodgkin Lymphoma. Cancer (2006) 107:108–15. doi: 10.1002/cncr.21971
11. Teta MJ, Lau E, Sceurman BK, Wagner ME. Therapeutic Radiation for Lymphoma: Risk of Malignant Mesothelioma. Cancer (2007) 109:1432–8. doi: 10.1002/cncr.22526
12. Travis LB, Fossa SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H, et al. Second Cancers Among 40,576 Testicular Cancer Patients: Focus on Long-Term Survivors. J Natl Cancer Inst (2005) 97:1354–65. doi: 10.1093/jnci/dji278
13. Brown LM, Chen BE, Pfeiffer RM, Schairer C, Hall P, Storm H, et al. Risk of Second Non-Hematological Malignancies Among 376,825 Breast Cancer Survivors. Breast Cancer Res Treat (2007) 106:439–51. doi: 10.1007/s10549-007-9509-8
14. Cavazza A, Travis LB, Travis WD, Wolfe JT, Foo ML, Gillespie DJ, et al. Post-Irradiation Malignant Mesothelioma. Cancer (1996) 77:1379–85. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1379::AID-CNCR24>3.0.CO;2-Y
15. Lo Iacono M, Monica V, Righi L, Grosso F, Libener R, Vatrano S, et al. Targeted Next-Generation Sequencing of Cancer Genes in Advanced Stage Malignant Pleural Mesothelioma: A Retrospective Study. J Thorac Oncol (2015) 10:492–9. doi: 10.1097/JTO.0000000000000436
16. May P, May E. Twenty Years of P53 Research: Structural and Functional Aspects of the P53 Protein. Oncogene (1999) 18:7621–36. doi: 10.1038/sj.onc.1203285
17. Kowalczyk AE, Krazinski BE, Godlewski J, Kiewisz J, Kwiatkowski P, Sliwinska-Jewsiewicka A, et al. Expression of the EP300, TP53 and BAX Genes in Colorectal Cancer: Correlations With Clinicopathological Parameters and Survival. Oncol Rep (2017) 38:201–10. doi: 10.3892/or.2017.5687
18. Godlewski J, Krazinski BE, Kowalczyk AE, Kiewisz J, Kiezun J, Kwiatkowski P, et al. Expression and Prognostic Significance of EP300, TP53 and BAX in Clear Cell Renal Cell Carcinoma. Anticancer Res (2017) 37:2927–37. doi: 10.21873/anticanres.11646
19. Krupar R, Watermann C, Idel C, Ribbat-Idel J, Offermann A, Pasternack H, et al. In Silico Analysis Reveals EP300 as a Pancancer Inhibitor of Anti-Tumor Immune Response via Metabolic Modulation. Sci Rep (2020) 10:9389. doi: 10.1038/s41598-020-66329-7
20. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 Human Cancer Genomes Reveals the Landscape of Tumor Mutational Burden. Genome Med (2017) 9(1):34. doi: 10.1186/s13073-017-0424-2
21. Metcalf RA, Welsh JA, Bennet WP, Seddon MB, Lehman TA, Pelin K, et al. P53 and Kirsten-Ras Mutations in Human Mesothelioma Cell Lines. Cancer Res (1992) 52:2610 –5.
22. Mor O, Yaron P, Huszar M, Yellin A, Kakobovitz O, Brok-Simoni F, et al. Absence of P53 Mutations in Malignant Mesotheliomas. Am J Respir Cell Mol Biol (1997) 16:9 –13. doi: 10.1165/ajrcmb.16.1.8998073
23. Gavin J, Gordon, Graham N, Rockwell, Roderick V, Jensen, et al. Identification of Novel Candidate Oncogenes and Tumor Suppressors in Malignant Pleural Mesothelioma Using Large-Scale Transcriptional Profiling. Am J Pathol (2005) 166:1827–40. doi: 10.1016/S0002-9440(10)62492-3
24. Carbone M, Rizzo P, Grimley PM, Procopio A, Mew DJY, Shridhar V, et al. Simian Virus-40 Large-T Antigen Binds P53 in Human Mesothelioma. Nat Med (1997) 3:908 –12. doi: 10.1038/nm0897-908
25. Yang H, Xu D, RA S, Peng RW. Biomarker-Guided Targeted and Immunotherapies in Malignant Pleural Mesothelioma. Ther Adv Med Oncol (2020) 12:1758835920971421. doi: 10.1177/1758835920971421
26. Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L, et al. The Nucleardeubiquitinase BAP1 Is Commonly Inactivated by Somatic Mutations and 3p21.1 Losses in Malignant Pleural Mesothelioma. Nat Genet (2011) 43(7):668–72. doi: 10.1038/ng.855
27. Cokiroglu E, Senturk S. Genomics and Functional Genomics of Malignant Pleural Mesothelioma. Int J Mol Sci (2020) 21(17):6342. doi: 10.3390/ijms21176342
Keywords: lung cancer, genomics, oncogene, adenocarcinoma, malignant pleural mesothelioma
Citation: Usuda K, Niida Y, Ishikawa M, Iwai S, Yamagata A, Iijima Y, Motono N, Yamada S and Uramoto H (2022) Genomics of Tumor Origin and Characteristics for Adenocarcinoma and Malignant Pleural Mesothelioma: A Case Report. Front. Oncol. 12:858094. doi: 10.3389/fonc.2022.858094
Received: 19 January 2022; Accepted: 30 March 2022;
Published: 19 May 2022.
Edited by:
Nobukazu Fujimoto, Okayama Rosai Hospital, JapanReviewed by:
Federica Pezzuto, University of Padua, ItalyDragana Jovanovic, University of Belgrade, Serbia
Copyright © 2022 Usuda, Niida, Ishikawa, Iwai, Yamagata, Iijima, Motono, Yamada and Uramoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katsuo Usuda, dXN1ZGFAa2FuYXphd2EtbWVkLmFjLmpw
 Katsuo Usuda
Katsuo Usuda Yo Niida
Yo Niida Masahito Ishikawa1
Masahito Ishikawa1 Nozomu Motono
Nozomu Motono Sohsuke Yamada
Sohsuke Yamada Hidetaka Uramoto
Hidetaka Uramoto