- 1Department of Oncology, Jinan Central Hospital, Jinan, China
- 2Department of Oncology, Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan, China
- 3Department of Thyroid Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
Lung cancer is the leading cause of cancer-related deaths worldwide. As the most prevalent molecular mutation subtypes in non-small cell lung cancer (NSCLC), EGFR-TKIs are currently a standard first-line therapy for targeting the mutated EGFR in advanced NSCLC patients. However, 20-30% of this subset of patients shows primary resistance to EGFR-TKIs. Patients with co-mutations of EGFR and several other genes have a poor response to EGFR-TKIs, whereas the prognostic and predictive significance of EGFR/TP53 co-mutation in NSCLC patients remains controversial. Meanwhile, little is known about how to choose an optimal therapeutic strategy for this subset of patients. Presently, no drugs targeting TP53 mutations are available on the market, and some p53 protein activators are in the early stage of clinical trials. A combination of EGFR-TKIs with antiangiogenic agents or chemotherapy or other agents might be a more appropriate strategy to tackle the problem. In this review, we describe the prognostic and predictive value of EGFR/TP53 co-mutation in NSCLC patients, investigate the mechanisms of this co-mutation affecting the response to EGFR-TKIs, and further explore optimal regimens effectively to prolong the survival time of the NSCLC patients harboring this co-mutation.
Introduction
Lung cancer is the second most commonly diagnosed malignancy worldwide and the leading cause of cancer-related death in 2020 (1). Non-small cell lung cancer(NSCLC) is the most common type of lung cancer, accounting for about 85-90% of lung cancer cases (2). The majority of patients present with advanced stages at initial diagnosis. Available treatments include chemotherapy, immunotherapy, and target therapy.
Epidermal growth factor receptor (EGFR) mutations are the most prevalent driver mutations in patients with NSCLC (3). An exon 19 short deletion (E746–A750) and an exon 21 point mutation, L858R, termed classical mutations, are the most common mutations accounting for about 85-90% (4). Atypical mutations include G719X, S768I, L861Q, and other point mutations (5), accounting for about 6-12% of EGFR mutations (6, 7). EGFR mutations occur most frequently in lung adenocarcinomas, women, never-smokers, and in Asian populations (6). Initially, first and second-generation EGFR-tyrosine kinase inhibitors (EGFR-TKIs) were approved as the standard for first-line treatment for patients with classical EGFR mutations (exon 19 deletions and L858R) whose median progression-free survival (mPFS) ranged from 9.2 to 12 months (8–11), and median overall survival (mOS) (22.1-28.2 months) (11–13). Subsequently, third-generation TKIs were confirmed as the first-line treatment with mPFS 18.9-19.3 months (14, 15), and mOS 33.1-38.6 months (15, 16).
However, 20-30% of patients with this subset of patients showed primary resistance to EGFR-TKIs (17). The understanding of the mechanisms underlying this primary resistance would be a prerequisite to overcoming the resistance and improving the therapeutic outcome of those patients. In recent years, accumulating research has focused on the molecular mechanisms of the acquired resistance to EGFR-TKIs and exploring alternative therapeutic strategies. Relatively few studies have been conducted on the mechanism of the primary EGFR-TKIs resistance. Uncommon EGFR mutations, such as de novo T790M mutation or exon 20 insertion mutation, could result in primary resistance to EGFR-TKIs (18). In addition, alternative pathway activation including BRAF mutation, HER2 mutation, KRAS mutation, and phosphatidylinositol3-kinase (PIK3CA) mutation, also caused primary resistance (19–22). With the development of next-generation sequencing (NGS), more and more co-mutations in advanced EGFR mutated-NSCLC patients were found, and these co-mutations might be one of the mechanisms of primary drug resistance, among which TP53 mutations were the most frequent co-mutations, accounting for about 17.3-72.5% (19, 23). Therefore, we will review the prognostic and predictive value of EGFR/TP53 co-mutation in NSCLC patients, investigate the mechanisms of this co-mutation affecting the response to EGFR-TKIs, and further explore optimal regimens effectively to prolong the survival time the NSCLC patients harboring this co-mutation.
Prognostic and Predictive Value of EGFR/TP53 Co-mutation in Advanced NSCLC Patients
TP53 gene, located on chromosome 17 short arm (17p13), is a tumor suppressor gene encoding tumor protein p53, which consists of 393 amino acids with four domains: an activation domain at the N-terminus (TAD), a central DNA-binding domain (DBD), a tetramerization domain (TD) in its C-terminal domain (CTD) and an extreme CTD regulatory domain. Protein p53 is involved in many biological processes, including DNA repair, metabolism, cell cycle arrest, apoptosis, and aging (24). TP53 mutations were associated with poor prognosis in a wide variety of cancers (25–31). In NSCLC, TP53 mutations were detected in approximately 40% of lung adenocarcinoma and 51% of squamous cell carcinoma (32, 33). Previous reports indicated that 17.3-72.5% of advanced EGFR-mutant lung cancers harbor TP53 mutations (19, 23).
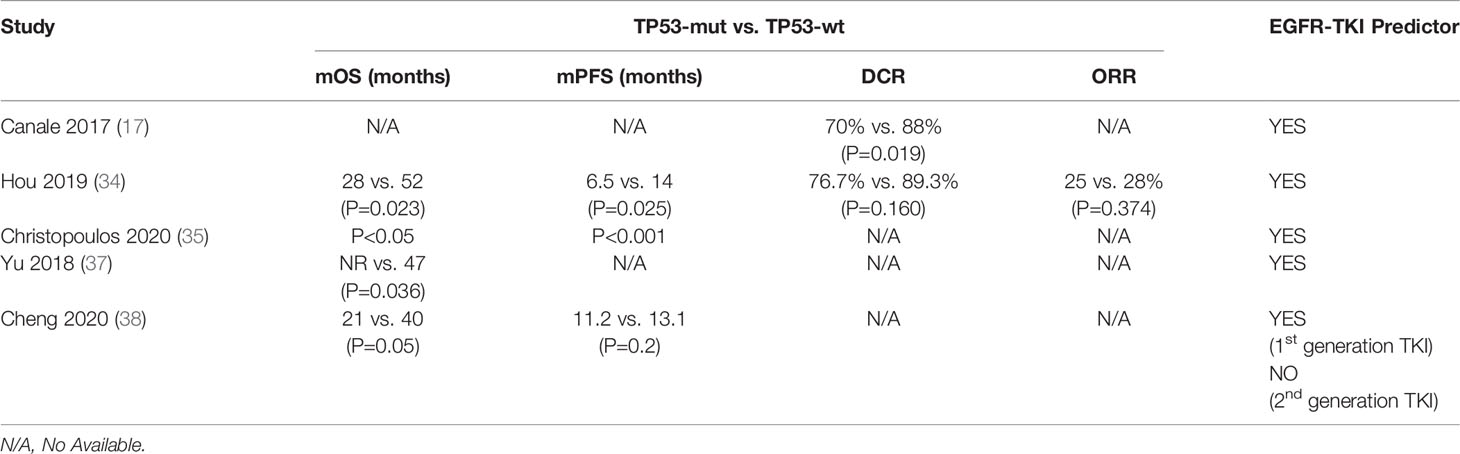
Canale et al. found that disease control rate (DCR) was 70% in TP53-mutated patients compared to 88% in TP53-wild type (TP53-wt) patients [relative risk, RR, of disease progression: 3.17 (95% CI 1.21-8.48), P=0.019] in 123 EGFR-mutated NSCLC patients receiving TKIs as first-line therapy (17). Subsequently, shorter mPFS (6.5 months VS 14.0 months, P=0.025) and mOS (28.0 months VS 52.0 months, P=0.023) were discovered in TP53 mutant patients treated with first-line TKIs compared with TP53 wild-type patients (34). In another study, TP53 co-mutation was discovered as a predictor for TKI efficacy and survival in EGFR+ NSCLC irrespective of other currently available parameters and it might be an important factor for risk stratification of newly diagnosed metastatic EGFR+ NSCLC (35). Several other researchers also obtained similar results (36, 37) (Table 1). In addition, Ying Cheng et al. integrated the genomic data and clinical outcomes in 179 patients with advanced EGFR-mutated NSCLC and found that EGFR-mutant patients harboring concomitant TP53 mutation were significantly associated with a poorer clinical prognosis (OS: 21 vs. 40 months, P = 0.05) after treated with 1st generation EGFR-TKI. In contrast, the presence of TP53 mutation did not affect the PFS or OS of patients treated with 2nd generation EGFR-TKI (38).
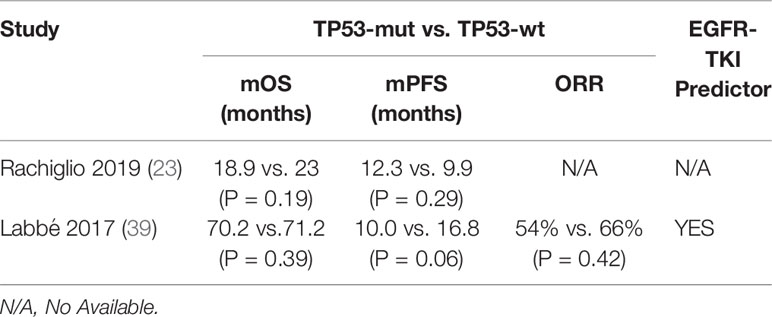
There were also several other studies failing to identify an adverse prognostic value of TP53 mutation (Table 2). Catherine et al. found that concomitant TP53 mutation status was not associated with relapse-free survival (RFS) or OS in patients with EGFR-mutant NSCLC at an early stage who underwent primary surgical resection and received adjuvant chemotherapy, suggesting that co-mutations were not a strong prognostic marker in early-stage patients without receiving EGFR-TKI therapy (39). Meanwhile, it was also found that objective response rate (ORR) was not significantly different (TP53-mut 54%, wt 66%, P=0.42) and there was a non-significant trend towards shorter mPFS on EGFR with TP53 mutation (HR 1.74, CI 0.98–3.10, P=0.06) in advanced NSCLC patients who received EGFR-TKI treatment (39). The work by Ying Jin et al. and Anna et al. supported this, showing that no significant difference in PFS was observed for TP53 co-mutation in advanced EGFR-mutated lung adenocarcinomas (19, 23). Therefore, the utility of EGFR/TP53 co-mutation as a prognostic and predictive biomarker for advanced EGFR-mutated NSCLC patients remains controversial.
A growing number of studies were conducted to determine whether the prognostic and predictive effect of TP53 mutations varied by type of gene mutation (Table 3). On basis of mutations subtypes, TP53 mutations showed a remarkable preference for missense mutations over nonsense and frameshift mutations that are commonly dominant in other tumor suppressor genes such as RB1, adenomatous polyposis coli (APC), and PTEN (44). One study showed that NSCLC patients with TP53 missense mutations had significantly shorter PFS treated with first-line EGFR-TKI therapy (HR 1.91, CI 1.01–3.60, P=0.04) (39). In another published study, TP53 non-missense mutations reduced responsiveness to first-generation TKIs and worsen the prognosis of EGFR-mutant advanced NSCLC patients (mPFS: 6.3 vs 14 months, P=0.041; HR 2.01, 95% CI: 1.00–4.05, P=0.046; mOS: 21.2 vs 52.5 months, P=0.001; HR 5.53, 95% CI: 1.79–16.95 P=0.001) (34). According to the functional effects on the p53 protein, TP53 mutations were divided into disruptive mutations and non-disruptive mutations (45). Disruptive mutations likely led to complete or almost complete loss of p53 protein activity, while non-disruptive mutations could retain some functional properties of wt-p53 (45). Molina-Vila’s study showed that non-disruptive mutations were predictive of shorter survival in the EGFR-mutated patients both in the training and in the validation cohorts (40). However, they found no significant association in erlotinib-treated patients carrying non-disruptive mutations (40). Multiple subsequent studies have demonstrated the prognostic roles of TP53 non-disruptive mutations. Meanwhile, it was found that these non-disruptive mutations could predict the response to first-line TKIs treatment in EGFR-mutated NSCLC patients (17, 25, 34). However, an analysis based on the cBioPortal database collected 1441 pieces of data from 1441 metastatic NSCLC patients and showed no prognostic value of disruptive or non-disruptive mutation of TP53 (41). Increasing research focused on the mutations based on the location of the mutation. The most common mutation site of TP53 is exon 4-8, accounting for about 44.8% (41), and exon 5-8 encodes DBD and recognizes consistent sequences in promoters of multiple genes involved in widely disparate biological effects (46, 47). Yang JJ et al. analyzed data obtained from a phase III randomized clinical trial (CTONG 0901) and found that mPFS in patients with mutations in exon 4 or 7 of TP53, other TP53 mutations, and wild-type TP53 were 9.4, 11.0, and 14.5 months (P=0.009), respectively. mOS were 15.8, 20.0, and 26.1 months (P=0.004), respectively. Mutations in exon 4 or 7 of TP53 served as independent prognostic factors for PFS (P=0.001) and OS (P=0.004) in advanced EGFR-mutant patients (42). Hou’s study consistently concluded that the mutation of exon 7 in exons 5-8 of TP53 greatly shortened the prognosis of patients compared with the wt-TP53 control group (mPFS: 5.0 vs 14.0 months, P=0.002, HR 3.98, 95% CI: 1.53-10.31, P=0.002, mOS: 14.0 vs 52.5 months, P=0.008, HR 5.29, 95% CI: 1.32-20.83, P=0.009) (34). However, another study showed that patients with exon 4, exon 6, mutation of the unknown site, and multiple mutations of TP53 demonstrated worse prognosis than with exon 5, exon 7, exon 8, and exon 9 mutation in EGFR exon 19/21 mutated patients (41). In Canale’s study, compared with patients with mutations in other TP53 exons and wt-TP53, patients with TP53 exon 8 mutation had shorter PFS (5.8 vs 12.4 vs 14.4 months), confirming the negative effect of exon 8 mutation on PFS (HR3.16,95%1.59-6.28, P=0.001) (43). In addition, it has also been shown that the T790M positive patients with TP53 R237C mutation failed to benefit from the subsequent osimertinib treatment and TP53 rs55863639 polymorphism was associated with the worse prognosis in TP53/EGFR co-mutation samples (48, 49). Thus, different classifications of TP53 mutations might result in different prognostic and predictive outcomes. How to identify the classification warrants further investigation in future studies.
Mechanisms of the EGFR/TP53 Co-mutation Affecting the Response to EGFR-TKIs in Advanced EGFR-Mutated NSCLC Patients
The negative prognostic effect of TP53 mutations might be attributed to their tumor-suppressive function loss, genomic instability function gain, and abilities of cancer cell transcriptome and phenotype regulation (40, 50, 51). However, the underlying mechanism of TP53 concurrent mutations as primary resistance to EGFR-TKIs in patients with advanced NSCLC remains poorly understood. A previous study investigated the role of p53 in growth-inhibitory and apoptotic effects of gefitinib in the human NSCLC cell lines NCI-H1299 and A549, which have no EGFR mutations and found that p53 enhanced gefitinib-induced growth inhibition and apoptosis by regulation of Fas (factor associated suicide) in NSCLC (52). However, whether the same mechanisms were shared in EGFR mutations was unknown. Another study showed that p53-knocked cells represented a significant reduction in sensitivity to EGFR inhibitors, compared to their parental cells. Conversely, restoration of functional p53 in EGFR inhibitor-resistant cells was sufficient to resensitize them to EGFR inhibitors or radiation in vitro and in vivo (53). The above findings demonstrated that tumor-suppressive functions loss of p53 determined EGFR inhibitors sensitivity.
Although these functions are traditionally thought of as the major functions of the p53 protein for tumor suppression, recent research has suggested that mutant p53 proteins showed oncogenic gain-of-function (GOF) properties, such as promoting tumor progression and acquiring drug resistance (40, 54, 55). Patricia et al. showed that mutant p53 expression can promote invasion, loss of directionality of migration, and metastatic behavior by constitutive activation of EGFR/integrin signaling (56). Molina-Vila’s confirmed that at least 11 non-disruptive mutations have induced GOF activity in cell models, and promoted tumor progression through the down-regulation of apoptosis and cell block genes, and the up-regulation of mitosis, angiogenesis, or drug-resistant tyrosine kinase receptor (Axl) genes (40, 57).
A bioinformatics study showed that in dual mutation samples, differential expression genes (DEGs) were strikingly enriched in regulating the metabolism of important amino acids, cell division, cell cycle regulation, cell adhesion, and extracellular matrix composition which were mainly enriched in signaling pathways such as PI3K-Akt, cytokine–cytokine receptor interaction, focal adhesions, and extracellular matrix receptor interaction, and PPI network suggested that GPC3, CCL28, GPR37, and NPY genes were up-regulated (58). Another study analyzed the data of 491 patients from The Cancer Genome Atlas (TCGA) and demonstrated that co-mutation of EGFRL858R/TP53 increased the expression of COMP and ITGB8, which are involved in an extracellular matrix organization and cell surface receptor signaling pathways, thus contributing to poor prognosis in lung adenocarcinoma. Validation was performed using three GEO profiles and similar results were obtained. CCK-8 and cell colony formation assays indicated that comutant EGFRL858R/TP53MUT promoted cell proliferation ability compared to the cowild A549 NC and TP53MUT H1299 NC. Additionally, the results revealed that EGFRL858R/TP53MUT resulted in increasing migration abilities compared with A549 and H1299 cells in the NC group (49). Although the exact mechanism of EGFR/TP53 co-mutation on prognosis is not specified, the aforementioned studies indicate that they may depend, at least partially, on the cellular functions and pathways.
In addition, Michael Offin et al. found that patients with EGFR/RB1/TP53-mutant lung cancers had a shorter time to discontinuation than EGFR/TP53- and EGFR-mutant-only cancers(9.5 versus 12.3 versus 36.6 months, respectively, P=2×10-9). It was demonstrated that TP53 inactivation and RB1 loss might be early events of small cell transformation which would generate resistance to TKIs (59).
Clinical Implications in Advanced EGFR-Mutated NSCLC Patients With TP53 Co-mutation
Recent studies suggested that the current routine testing of EGFR for selecting NSCLC patients treatable with first-line targeted therapy was not enough to predict the response to the TKIs (32). As mentioned above, patients with TP53 co-mutation in advanced EGFR-mutated NSCLC showed poor prognosis and insensitivity to EGFR-TKIs. However, little was known about how to choose the best treatment modality in the aforementioned populations. At present, no drugs targeting TP53 mutations are available on the market, and some p53 protein activators, such as ARP-246, are in the early stage of clinical trials (60). A combination of EGFR-TKI therapy with chemotherapy or antiangiogenic agents or other agents might be a more appropriate strategy to tackle the problem. Thus, this paper will explore the combination treatment options in patients with TP53 mutations to provide therapeutic strategies for clinicians.
Combination of EGFR-TKIs and Chemotherapy
Before 2011, platinum-based chemotherapy was the cornerstone of treatment of advanced NSCLC. As the driver oncogenes were identified, therapeutic strategies for NSCLC, especially for those with driver mutations have been revolutionized in recent years. EGFR TKIs have become a standard of first-line treatment for patients with EGFR-mutated NSCLC. Recently, a randomized phase III study (NEJ009) showed that compared with gefitinib alone, gefitinib combined with carboplatin plus pemetrexed improved PFS in patients with untreated advanced NSCLC with EGFR mutations (20.9 vs 11.2 months, P < 0.001) (9). The results from a phase II study (JMIT) supported this observation as well, showing that pemetrexed + gefitinib improved PFS (15.8 vs 10.9 months, P=0.028) compared with gefitinib alone in East Asian patients with advanced NSCLC and activating EGFR mutations (61).
Could patients with TP53 co-mutation benefit from combined treatment with EGFR-TKIs and chemotherapy? Here we first reviewed the effects of the sensitivity of tumor cells to chemotherapy by the status of p53. In vitro experiments showed that NCI-H1299 (p53-null) cells were insensitive to cisplatin (CDDP) (62, 63). Similar results were observed in other studies. Cisplatin (CDDP) significantly induced apoptosis in A549 (p53-wt) cells, but not in H1299 cells, and p53-deficient tumor cells show chemoresistance to drugs, suggesting that a functional p53 might affect the chemosensitivity of NSCLC (64, 65). One study was analyzed for the p53 genotype in patients with advanced NSCLC who had received neoadjuvant chemotherapy and found that the presence of a mutant p53 genotype was highly indicative of resistance to induction chemotherapy (P<0.002) (66). Another study showed that in advanced NSCLC the response rate of the p53 positive group was 26% versus 57% of the p53 negative group (P=0.004), and in multivariate analyses, positive p53 was identified as an independent predictive factor for resistance to cisplatin-based chemotherapy(P=0.006) (67). However, some studies were inconsistent with the above observation, which demonstrated that TP53 mutations were not significant predictive of response to cisplatin-based chemotherapy (68–70). To date, no studies have been published for EGFR-TKIs plus chemotherapy in advanced EGFR/TP53 co-mutation NSCLC. In ClinicalTrials.gov, one registered phase III clinical study comparing osimertinib monotherapy to combination therapy with osimertinib, carboplatin, and pemetrexed for untreated patients with advanced non-squamous NSCLC with concurrent EGFR and TP53 mutations is currently undergone. We can look forward to the final results of this study in the coming years.
Combination of EGFR-TKIs and Antiangiogenic Drugs
Since the Eastern Cooperative Oncology Group (ECOG) 4599 study showed a significant survival benefit with the use of bevacizumab in combination with carboplatin and paclitaxel in comparison with CP chemotherapy alone in patients with previously untreated advanced, metastatic or recurrent NSCLC (71), antiangiogenic drugs were increasingly being used in clinical practice (72).
Antiangiogenic drugs mainly targeted vascular endothelial growth factor (VEGF)/vascular endothelial growth factor receptor (VEGFR) signal pathway in tumor angiogenesis. A previous study showed that TP53 mutation was significantly associated with higher VEGF expression level (P=0.006) (73), and mutant p53 binding to the VEGFR2 promoter transcription start site, regulated angiogenesis by promoting VEGFR2 up-regulation (74).
So far, several studies have suggested TP53 mutation might be predictive of clinical sensitivity to antiangiogenic therapies in different tumor types. One prospective study analyzed outcomes based on VEGF/VEGFR inhibitor treatment and the presence of TP53 mutations in various tumor types. The results showed that treatment with VEGF/VEGFR inhibitors was independently associated with improvement in all outcome parameters [rate of SD≥6 months/PR/CR, length of TTF and OS (all P≤ 0.01)] for the patients harboring TP53-mutant cancers, but the improvement was not seen in any of these parameters for the group of patients with TP53 wild-type neoplasms (75). Another study retrospectively reviewed 19 cases of patients with advanced sarcoma treated with VEGFR inhibition and discovered that the PFS of patients with TP53 mutations was significantly greater than TP53 wild-type tumors with the median PFS of 208 versus 136 days, respectively [P=0.036, hazards ratio 0.38 (95% confidence interval 0.09-0.83)] (76). Similar findings were reported in non-small cell lung cancer. Said with his colleagues retrospectively analyzed the response to standard systemic therapy of 145 patients with documented tumor p53 mutational status (mutant-type [mtp53] vs. wild-type [wtp53]), and the results showed that PFS was significantly longer with bevacizumab-containing regimens as compared to non-bevacizumab containing regimen in patients with mtp53 (median 11.0 [95% CI 5.9-16.0], n=22 vs. 4.0 months [95% CI: 3.6-5.7], n=35, P<0.0001), but not those with wtp53 (median 5.0 [95% CI: 2.0-7.6] vs. 6.0 [95% CI 4.0-7.5] months, P=0.318) (77). Anlotinib, a novel oral multi-target antiangiogenic TKI directed against VEGFR-1/2/3, fibroblast growth factor receptor (FGFR), was approved as a third-line treatment for advanced NSCLC in China (78). Fang discussed three NSCLC patients with TP53 mutation treated with anlotinib as a second or third- line regimen, all three patients achieved PR and PFS of 8 months, 6.5 months, and 5 months respectively (79).
However, the above-mentioned studies were conducted without knowing EGFR mutation status. For patients with EGFR mutation, multiple studies had demonstrated that antiangiogenic agents plus EGFR-TKIs significantly improved PFS compared with EGFR-TKIs alone (80–84). Were similar results obtained in EGFR-mutated patients with TP53 concurrent mutations? Results from RELAY showed that a combination of the antiangiogenic agent ramucirumab with EGFR-TKI targeted therapy provided significant and similar clinical benefit for both EGFR ex19del and ex21L858R NSCLC, and its subgroup analyses showed that in patients with co-mutations of EGFR and TP53, ramuzumab plus erlotinib showed better PFS treatment advantage than erlotinib alone (P<0.001) (85). A single-center retrospective analysis by Cheng Y et al. recruited 179 patients with advanced EGFR-mutated NSCLC, and the results showed that bevacizumab combined with EGFR-TKI regimen could significantly improve PFS (14 vs 9.7months, P=0.034) in patients with TP53 co-mutation compared with EGFR-TKI single drug (38). At present, for this clinical condition, combination therapies of EGFR-TKIs and antiangiogenic agents might provide a better benefit for patients with TP53 co-mutation.
Combination of EGFR-TKIs and Immunotherapy
Immune checkpoint inhibitors, including monoclonal antibodies against programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1), either as monotherapy or combination therapy, have made a breakthrough in clinical treatment for patients with advanced NSCLC (86). However, subgroup analyses from several clinical trials demonstrated that no PFS and/or OS benefit was achieved from anti-PD-1/PD-L1 therapy in the patients harboring EGFR mutations (87–89). Based on the above trials, immunotherapy is not recommended as the preferred treatment of patients with NSCLC harboring EGFR mutation by the NCCN guidelines at present. Meanwhile, safety concerns caused by the combination of EGFR-TKIs and anti-PD-1/PD-L1 therapy resulted in the termination of some clinical trials (90, 91). Thus, the combination of EGFR-TKI and immunotherapy could not achieve the expected therapeutic value.
Are all advanced NSCLC patients with EGFR mutation not suitable for immunotherapy? In the phase II ATLANTIC study, the investigators assessed the effect of durvalumab (anti-PD-L1) treatment as third-line or later treatment in three cohorts of patients with NSCLC defined by EGFR/ALK status and tumor expression of PD-L1. The proportion of patients with EGFR-/ALK- NSCLC achieving a response was higher than that with EGFR+/ALK+NSCLC. The ORR among the patients with EGFR+ NSCLC with ≥ 25% of tumor cells expressing PD-L1 remained encouraging (12.2%) relative to that (4%) among patients with < 25% PD-L1 expression (92). Therefore, how to choose the proper patients suitable for durvalumab in the EGFR+NSCLC patients warrants further investigation.
Patients with EGFR/TP53 co-mutation might gain little benefit from EGFR-TKIs treatment, could they benefit from ICIs? So far no definitive biomarker for immunotherapy has been established. Currently, PD-L1 expression status in tumor tissue is now considered a predictive biomarker for selecting patients who could benefit the most (93). A retrospective study examined tumor PD-L1 expression and eleven gene mutations in 247 surgically resected primary and 26 advanced NSCLC patients and revealed that PD-L1 expression was significantly associated with TP53 mutation (P=0.014) using multivariate logistic regression (94). Similar results were obtained by Yoon JC et al. (95). Moreover, Hao Sun et al. demonstrated that the TP53-missense-mutation group showed increased PD-L1 (CD274) level and enriched IFN-γ signatures compared with the TP53-wild-type subgroup, but no differences were noted in patients with nonsense-mutant vs wild-type p53 (96). Furthermore, a real-world study of a large Chinese cohort suggested that mutations in TP53 were significantly associated with high PD-L1 expression (both P < 0.001). High PD-L1 expression was significantly associated with EGFR co-mutation with tumor suppressor genes such as TP53, while EGFR mutation alone was not associated with high PD-L1 expression, and these results might explain why advanced NSCLC patients with EGFR mutation alone showed poor response to immunotherapy and patients with EGFR/TP53 co-mutation were likely to benefit from anti-PD-1/PD-L1 treatment (97). The tumor mutation burden (TMB) is another important biomarker used to predict the response to cancer immunotherapy in NSCLC patients (98). One study investigated the relationship between TMB and the imaging, histologic, and genetic features in NSCLC and found that tumors with high TMB were more prevalent in those with a TP53 mutation (P<0.0001) (99).
Emerging evidence from a few recent clinical studies suggested that advanced TP53-mutated NSCLC patients could benefit from immunotherapy. Sandra et al. revealed that in advanced NSCLC patients treated with nivolumab, with or without CTLA-4 blocker ipilimumab, or pembrolizumab, the median OS in the TP53-mutated group was 18.1 months (95% CI 6.6-not reached) vs 8.1 months (95% CI 2.2–14.5, hazard ratio [HR] = 0.48; 95% CI 0.25-0.95, P=0.04) in the TP53-wild-type group and the mPFS was significantly longer in TP53-mutated patients (4.5 months, 95% CI 2.8–18.1 versus 1.4, 95% CI 1.1–3.5; P=0.03). In multivariate analysis, TP53 mutations were independently associated with longer OS (HR = 0.35, 95% CI 0.16-0.77, P=0.009), whereas TP53 mutation status failed to significantly influence PFS (P=0.32) (100). Another study showed that TP53-mutated lung adenocarcinoma treated with ICIs showed significantly prolonged PFS (P=0.017, HR=0.69 [95%CI: 0.50-0.94]) (101). Dong et al. retrospectively showed in 30 NSCLC patients treated with pembrolizumab that median PFS was significantly longer in the TP53-mutated group than in the TP53-wild-type group (14.5 versus 3.5 months, P=0.042) (102). Jun Lu et al. also indicated that the potential predictors of immunotherapy were significantly different, especially between patients with TP53 (+) and TP53 (−) (103). Alternatively, it has been shown that TP53 missense but not nonsense mutants were associated with better clinical benefits taking antiPD-1/L1. However, all such TP53 subgroups responded well to nivolumab (antiPD-L1) plus ipilimumab (antiCTLA-4) therapy (96). An increasing number of recent studies have demonstrated that TP53 and other gene co-mutations might serve as a predictive biomarker for ICI responses in NSCLC. Dong ZY et al. observed that the TP53/KRAS co-mutated subgroup manifested exclusive increased expression of PD-L1 and the highest proportion of PD-L1+/CD8A+, increased mutation burden, and specifically enriched in the transversion-high (TH) cohort. And it was also found that TP53 or KRAS mutation patients, especially those with co-occurring TP53/KRAS mutations, showed remarkable clinical benefit to PD-1 inhibitors (102). Another subsequent research suggested that the presence of KRAS/STK11 co-mutation and KRAS/STK11/TP53 co-mutation affected OS only in patients treated with ICIs (HR=10.936, 95% CI: 2.337-51.164, P=0.002; HR=17.609, 95% CI: 3.777-82.089, P<0.001, respectively) (104). One multiple-cohort study included patients with NSCLC from the Gene plus Institute, the Cancer Genome Atlas (TCGA), and the Memorial Sloan Kettering Cancer Center (MSKCC) databases and from the POPLAR and OAK randomized controlled trials and found that TP53 and ataxia-telangiectasia mutated (ATM) co-mutation was associated with a significantly higher TMB compared with the sole mutation and with no mutation. Among patients treated with ICIs in the POPLAR and OAK cohort, TP53 and ATM co-mutation was associated with better PFS and OS than a single mutation and no mutation groups (mPFS: TP53 and ATM co-mutation, 10.4 months; TP53 mutation, 1.6 months; ATM mutation, 3.5 months; no mutation, 2.8 months; P=0.01; mOS: TP53 and ATM co-mutation, 22.1 months; TP53 mutation, 8.3 months; ATM mutation, 15.8 months; no mutation, 15.3 months; P=0.002) (105). In addition, TP53/KMT2C co-mutation was also considered a potential predictive factor in guiding anti-PD-1/PD-L1 immunotherapy (106). Based on these previous results, immunotherapy could be a valuable option for advanced NSCLC patients with EGFR/TP53 co-mutation.
However, how could we improve the clinical outcome of ICIs, monotherapy, or in combination with other agents, such as another ICI, conventional chemotherapy, and antiangiogenic drugs? The IMpower150 trial could provide some implications for us. In this phase III study participants with chemotherapy-naive metastatic non-small-cell lung cancer were randomly assigned (1:1:1) to receive atezolizumab plus bevacizumab plus carboplatin plus paclitaxel (ABCP), atezolizumab plus carboplatin plus paclitaxel, or the standard-of-care bevacizumab plus carboplatin plus paclitaxel (BCP) every three weeks. Efficacy was assessed in a key subgroup with EGFR mutations previously treated with one or more tyrosine kinase inhibitors within the intention-to-treat population. It was found that improved OS with ABCP versus BCP was observed in patients with EGFR mutations in the intention-to-treat population (19.8 months vs 14.9 months; HR 0.76, 95% CI: 0.63-0.93) (107). Despite the limited sample size of EGFR-mutated patients, this result could indicate that patients with EGFR‐TKI resistance might benefit from the combination of ICIs, antiangiogenic drugs, and conventional chemotherapy. In summary, further investigations will be warranted within current molecular stratification for appropriate therapeutic regimens.
Conclusion
So far, most studies suggested that TP53 mutations were an important marker of poor prognosis and predictor in advanced EGFR-mutated NSCLC. However, the classification of TP53 mutations was very complicated, and which mutation contributed to prognosis and prediction, had not been consistently concluded. The answers to these inconsistencies will be further investigated in future studies. Although the exact mechanism of EGFR/TP53 co-mutation on prognosis is not specified, current studies indicate that they may depend, at least partially, on the cellular functions and pathways and small cell transformation generating resistance to TKIs. At present, to this clinical condition, combinations with immunotherapy or combination therapies of EGFR-TKIs and antiangiogenic agents may be valuable options for the advanced NSCLC patients with EGFR/TP53 co-mutation. However, all of these studies were retrospective, and most with small patient numbers. Thus, further verification using a large sample size and stratifying patients based on TP53 mutation status in studies, especially prospective studies are needed in the future.
Author Contributions
All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Natural Science Foundation of China (81501989), the Natural Science Foundation of Shandong Province (ZR2020MH210, ZR2020QH179), and the Jinan Medical and Health Science and Technology Development Project (grant no. 201907118).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks to the support of the National Natural Science Foundation of China (81501989), the Natural Science Foundation of Shandong Province (ZR2020MH210, ZR2020QH179), and the Jinan Medical and Health Science and Technology Development Project (grant no. 201907118).
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2019. CA Cancer J Clin (2019) 69(1):7–34. doi: 10.3322/caac.21551
3. Greenhalgh J, Boland A, Bates V, Vecchio F, Dundar Y, Chaplin M, et al. First-Line Treatment of Advanced Epidermal Growth Factor Receptor (EGFR) Mutation Positive Non-Squamous Non-Small Cell Lung Cancer. Cochrane Database Syst Rev (2021) 3:CD010383. doi: 10.1002/14651858.CD010383.pub3
4. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR Mutations and Lung Cancer. Annu Rev Pathol (2011) 6:49–69. doi: 10.1146/annurev-pathol-011110-130206
5. Chiu CH, Yang CT, Shih JY, Huang MS, Su WC, Lai RS, et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Treatment Response in Advanced Lung Adenocarcinomas With G719X/L861Q/S768I Mutations. J Thorac Oncol (2015) 10(5):793–9. doi: 10.1097/JTO.0000000000000504
6. Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A Prospective, Molecular Epidemiology Study of EGFR Mutations in Asian Patients With Advanced Non-Small-Cell Lung Cancer of Adenocarcinoma Histology (PIONEER). J Thorac Oncol (2014) 9(2):154–62. doi: 10.1097/JTO.0000000000000033
7. Kobayashi Y, Mitsudomi T. Not All Epidermal Growth Factor Receptor Mutations in Lung Cancer Are Created Equal: Perspectives for Individualized Treatment Strategy. Cancer Sci (2016) 107(9):1179–86. doi: 10.1111/cas.12996
8. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib Versus Cisplatin Plus Docetaxel in Patients With Non-Small-Cell Lung Cancer Harbouring Mutations of the Epidermal Growth Factor Receptor (WJTOG3405): An Open Label, Randomised Phase 3 Trial. Lancet Oncol (2010) 11(2):121–8. doi: 10.1016/S1470-2045(09)70364-X
9. Hosomi Y, Morita S, Sugawara S, Kato T, Fukuhara T, Gemma A, et al. Gefitinib Alone Versus Gefitinib Plus Chemotherapy for Non-Small-Cell Lung Cancer With Mutated Epidermal Growth Factor Receptor: NEJ009 Study. J Clin Oncol (2020) 38(2):115–23. doi: 10.1200/JCO.19.01488
10. Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients With Metastatic Lung Adenocarcinoma With EGFR Mutations. J Clin Oncol (2013) 31(27):3327–34. doi: 10.1200/JCO.2012.44.2806
11. Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib Versus Cisplatin Plus Gemcitabine for First-Line Treatment of Asian Patients With Advanced Non-Small-Cell Lung Cancer Harbouring EGFR Mutations (LUX-Lung 6): An Open-Label, Randomised Phase 3 Trial. Lancet Oncol (2014) 15(2):213–22. doi: 10.1016/S1470-2045(13)70604-1
12. Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib Versus Cisplatin-Based Chemotherapy for EGFR Mutation-Positive Lung Adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of Overall Survival Data From Two Randomised, Phase 3 Trials. Lancet Oncol (2015) 16(2):141–51. doi: 10.1016/S1470-2045(14)71173-8
13. Zhong WZ, Wang Q, Mao WM, Xu ST, Wu L, Shen Y, et al. Gefitinib Versus Vinorelbine Plus Cisplatin as Adjuvant Treatment for Stage II-IIIA (N1-N2) EGFR-Mutant NSCLC (ADJUVANT/CTONG1104): A Randomised, Open-Label, Phase 3 Study. Lancet Oncol (2018) 19(1):139–48. doi: 10.1016/S1470-2045(17)30729-5
14. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med (2018) 378(2):113–25. doi: 10.1056/NEJMoa1713137
15. Lu S, Dong X, Jian H, Chen J, Chen G, Sun Y, et al. Randomized Phase III Trial of Aumolertinib (HS-10296, Au) Versus Gefitinib (G) as First-Line Treatment of Patients With Locally Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC) and EGFR Exon 19 Del or L858R Mutations (EGFRm). Wolters Kluwer Health (2021) 39(15):9013. doi: 10.1200/JCO.2021.39.15_suppl.9013
16. Ramalingam S, Gray J, Ohe Y, Cho B, Vansteenkiste J, Zhou C, et al. Osimertinib vs Comparator EGFR-TKI as First-Line Treatment for EGFRm Advanced NSCLC (FLAURA): Final Overall Survival Analysis. Ann Oncol (2019) 30:v914–5. doi: 10.1093/annonc/mdz394.076
17. Canale M, Petracci E, Delmonte A, Chiadini E, Dazzi C, Papi M, et al. Impact of TP53 Mutations on Outcome in EGFR-Mutated Patients Treated With First-Line Tyrosine Kinase Inhibitors. Clin Cancer Res (2017) 23(9):2195–202. doi: 10.1158/1078-0432.CCR-16-0966
18. Pao W, Chmielecki J. Rational, Biologically Based Treatment of EGFR-Mutant Non-Small-Cell Lung Cancer. Nat Rev Cancer (2010) 10(11):760–74. doi: 10.1038/nrc2947
19. Jin Y, Shi X, Zhao J, He Q, Chen M, Yan J, et al. Mechanisms of Primary Resistance to EGFR Targeted Therapy in Advanced Lung Adenocarcinomas. Lung Cancer (2018) 124:110–6. doi: 10.1016/j.lungcan.2018.07.039
20. Wang J, Wang B, Chu H, Yao Y. Intrinsic Resistance to EGFR Tyrosine Kinase Inhibitors in Advanced Non-Small-Cell Lung Cancer With Activating EGFR Mutations. Onco Targets Ther (2016) 9:3711–26. doi: 10.2147/OTT.S106399
21. Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, et al. KRAS Mutations and Primary Resistance of Lung Adenocarcinomas to Gefitinib or Erlotinib. PloS Med (2005) 2(1):e17. doi: 10.1371/journal.pmed.0020017
22. Brose MS, Volpe P, Feldman M, Kumar M, Rishi I, Gerrero R, et al. BRAF and RAS Mutations in Human Lung Cancer and Melanoma. Cancer Res (2002) 62(23):6997–7000.
23. Rachiglio AM, Fenizia F, Piccirillo MC, Galetta D, Crino L, Vincenzi B, et al. The Presence of Concomitant Mutations Affects the Activity of EGFR Tyrosine Kinase Inhibitors in EGFR-Mutant Non-Small Cell Lung Cancer (NSCLC) Patients. Cancers (Basel) (2019) 11(3):341. doi: 10.3390/cancers11030341
24. Lane D, Levine A. P53 Research: The Past Thirty Years and the Next Thirty Years. Cold Spring Harb Perspect Biol (2010) 2(12):a000893. doi: 10.1101/cshperspect.a000893
25. Yu R, Bai H, Li T, Gao B, Han J, Chang G, et al. TP53 Mutations in Circulating Tumor DNA in Advanced Epidermal Growth Factor Receptor-Mutant Lung Adenocarcinoma Patients Treated With Gefitinib. Transl Oncol (2021) 14(9):101163. doi: 10.1016/j.tranon.2021.101163
26. Zhang Z, Hao R, Guo Q, Zhang S, Wang X. TP53 Mutation Infers a Poor Prognosis and Is Correlated to Immunocytes Infiltration in Breast Cancer. Front Cell Dev Biol (2021) 9:759154. doi: 10.3389/fcell.2021.759154
27. Moon S, Balch C, Park S, Lee J, Sung J, Nam S. Systematic Inspection of the Clinical Relevance of TP53 Missense Mutations in Gastric Cancer. IEEE/ACM Trans Comput Biol Bioinform (2019) 16(5):1693–701. doi: 10.1109/TCBB.2018.2814049
28. Li H, Zhang J, Tong JHM, Chan AWH, Yu J, Kang W, et al. Targeting the Oncogenic P53 Mutants in Colorectal Cancer and Other Solid Tumors. Int J Mol Sci (2019) 20(23):5999. doi: 10.3390/ijms20235999
29. Chen X, Lin X, Shen Q, Qian X. Combined Spiral Transformation and Model-Driven Multi-Modal Deep Learning Scheme for Automatic Prediction of TP53 Mutation in Pancreatic Cancer. IEEE Trans Med Imaging (2021) 40(2):735–47. doi: 10.1109/TMI.2020.3035789
30. Xu Y, Zhang Q, Miao C, Dongol S, Li Y, Jin C, et al. CCNG1 (Cyclin G1) Regulation by Mutant-P53 via Induction of Notch3 Expression Promotes High-Grade Serous Ovarian Cancer (HGSOC) Tumorigenesis and Progression. Cancer Med (2019) 8(1):351–62. doi: 10.1002/cam4.1812
31. Wen XM, Xu ZJ, Jin Y, Xia PH, Ma JC, Qian W, et al. Association Analyses of TP53 Mutation With Prognosis, Tumor Mutational Burden, and Immunological Features in Acute Myeloid Leukemia. Front Immunol (2021) 12:717527. doi: 10.3389/fimmu.2021.717527
32. Guo Y, Song J, Wang Y, Huang L, Sun L, Zhao J, et al. Concurrent Genetic Alterations and Other Biomarkers Predict Treatment Efficacy of EGFR-TKIs in EGFR-Mutant Non-Small Cell Lung Cancer: A Review. Front Oncol (2020) 10:610923. doi: 10.3389/fonc.2020.610923
33. Mitsudomi T, Hamajima N, Ogawa M, Takahashi T. Prognostic Significance of P53 Alterations in Patients With Non-Small Cell Lung Cancer: A Meta-Analysis. Clin Cancer Res (2000) 6(10):4055–63.
34. Hou H, Qin K, Liang Y, Zhang C, Liu D, Jiang H, et al. Concurrent TP53 Mutations Predict Poor Outcomes of EGFR-TKI Treatments in Chinese Patients With Advanced NSCLC. Cancer Manag Res (2019) 11:5665–75. doi: 10.2147/CMAR.S201513
35. Christopoulos P, Kirchner M, Roeper J, Saalfeld F, Janning M, Bozorgmehr F, et al. Risk Stratification of EGFR(+) Lung Cancer Diagnosed With Panel-Based Next-Generation Sequencing. Lung Cancer (2020) 148:105–12. doi: 10.1016/j.lungcan.2020.08.007
36. Hong S, Gao F, Fu S, Wang Y, Fang W, Huang Y, et al. Concomitant Genetic Alterations With Response to Treatment and Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With EGFR-Mutant Advanced Non-Small Cell Lung Cancer. JAMA Oncol (2018) 4(5):739–42. doi: 10.1001/jamaoncol.2018.0049
37. Yu HA, Suzawa K, Jordan E, Zehir A, Ni A, Kim R, et al. Concurrent Alterations in EGFR-Mutant Lung Cancers Associated With Resistance to EGFR Kinase Inhibitors and Characterization of MTOR as a Mediator of Resistance. Clin Cancer Res (2018) 24(13):3108–18. doi: 10.1158/1078-0432.CCR-17-2961
38. Cheng Y, Ma L, Liu Y, Zhu J, Xin Y, Liu X, et al. Comprehensive Characterization and Clinical Impact of Concomitant Genomic Alterations in EGFR-Mutant NSCLCs Treated With EGFR Kinase Inhibitors. Lung Cancer (2020) 145:63–70. doi: 10.1016/j.lungcan.2020.04.004
39. Labbe C, Cabanero M, Korpanty GJ, Tomasini P, Doherty MK, Mascaux C, et al. Prognostic and Predictive Effects of TP53 Co-Mutation in Patients With EGFR-Mutated Non-Small Cell Lung Cancer (NSCLC). Lung Cancer (2017) 111:23–9. doi: 10.1016/j.lungcan.2017.06.014
40. Molina-Vila MA, Bertran-Alamillo J, Gasco A, Mayo-de-las-Casas C, Sanchez-Ronco M, Pujantell-Pastor L, et al. Nondisruptive P53 Mutations Are Associated With Shorter Survival in Patients With Advanced Non-Small Cell Lung Cancer. Clin Cancer Res (2014) 20(17):4647–59. doi: 10.1158/1078-0432.CCR-13-2391
41. Jiao XD, Qin BD, You P, Cai J, Zang YS. The Prognostic Value of TP53 and its Correlation With EGFR Mutation in Advanced Non-Small Cell Lung Cancer, an Analysis Based on Cbioportal Data Base. Lung Cancer (2018) 123:70–5. doi: 10.1016/j.lungcan.2018.07.003
42. Li XM, Li WF, Lin JT, Yan HH, Tu HY, Chen HJ, et al. Predictive and Prognostic Potential of TP53 in Patients With Advanced Non-Small-Cell Lung Cancer Treated With EGFR-TKI: Analysis of a Phase III Randomized Clinical Trial (CTONG 0901). Clin Lung Cancer (2021) 22(2):100–9.e3. doi: 10.1016/j.cllc.2020.11.001
43. Canale M, Petracci E, Delmonte A, Bronte G, Chiadini E, Ludovini V, et al. Concomitant TP53 Mutation Confers Worse Prognosis in EGFR-Mutated Non-Small Cell Lung Cancer Patients Treated With TKIs. J Clin Med (2020) 9(4):1047. doi: 10.3390/jcm9041047
44. Bykov VJN, Eriksson SE, Bianchi J, Wiman KG. Targeting Mutant P53 for Efficient Cancer Therapy. Nat Rev Cancer (2018) 18(2):89–102. doi: 10.1038/nrc.2017.109
45. Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, et al. TP53 Mutations and Survival in Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med (2007) 357(25):2552–61. doi: 10.1056/NEJMoa073770
46. Savli H, Sertdemir N, Aydin D, Dursun B, Kurtas O, Reka S, et al. TP53, EGFR and PIK3CA Gene Variations Observed as Prominent Biomarkers in Breast and Lung Cancer by Plasma Cell-Free DNA Genomic Testing. J Biotechnol (2019) 300:87–93. doi: 10.1016/j.jbiotec.2019.05.005
47. Monti P, Menichini P, Speciale A, Cutrona G, Fais F, Taiana E, et al. Heterogeneity of TP53 Mutations and P53 Protein Residual Function in Cancer: Does It Matter? Front Oncol (2020) 10:593383. doi: 10.3389/fonc.2020.593383
48. Fu Y, Wang A, Zhou J, Feng W, Shi M, Xu X, et al. Advanced NSCLC Patients With EGFR T790M Harboring TP53 R273C or KRAS G12V Cannot Benefit From Osimertinib Based on a Clinical Multicentre Study by Tissue and Liquid Biopsy. Front Oncol (2021) 11:621992. doi: 10.3389/fonc.2021.621992
49. Zheng C, Li X, Ren Y, Yin Z, Zhou B. Coexisting EGFR and TP53 Mutations in Lung Adenocarcinoma Patients Are Associated With COMP and ITGB8 Upregulation and Poor Prognosis. Front Mol Biosci (2020) 7:30. doi: 10.3389/fmolb.2020.00030
50. Talos F, Nemajerova A, Flores ER, Petrenko O, Moll UM. P73 Suppresses Polyploidy and Aneuploidy in the Absence of Functional P53. Mol Cell (2007) 27(4):647–59. doi: 10.1016/j.molcel.2007.06.036
51. Oren M, Rotter V. Mutant P53 Gain-of-Function in Cancer. Cold Spring Harb Perspect Biol (2010) 2(2):a001107. doi: 10.1101/cshperspect.a001107
52. Rho JK, Choi YJ, Ryoo BY, Na II, Yang SH, Kim CH, et al. P53 Enhances Gefitinib-Induced Growth Inhibition and Apoptosis by Regulation of Fas in Non-Small Cell Lung Cancer. Cancer Res (2007) 67(3):1163–9. doi: 10.1158/0008-5472.CAN-06-2037
53. Huang S, Benavente S, Armstrong EA, Li C, Wheeler DL, Harari PM. P53 Modulates Acquired Resistance to EGFR Inhibitors and Radiation. Cancer Res (2011) 71(22):7071–9. doi: 10.1158/0008-5472.CAN-11-0128
54. Hanel W, Marchenko N, Xu S, Yu SX, Weng W, Moll U. Two Hot Spot Mutant P53 Mouse Models Display Differential Gain of Function in Tumorigenesis. Cell Death Differ (2013) 20(7):898–909. doi: 10.1038/cdd.2013.17
55. Xu J, Reumers J, Couceiro JR, De Smet F, Gallardo R, Rudyak S, et al. Gain of Function of Mutant P53 by Coaggregation With Multiple Tumor Suppressors. Nat Chem Biol (2011) 7(5):285–95. doi: 10.1038/nchembio.546
56. Muller PA, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S, et al. Mutant P53 Drives Invasion by Promoting Integrin Recycling. Cell (2009) 139(7):1327–41. doi: 10.1016/j.cell.2009.11.026
57. Vaughan CA, Singh S, Windle B, Yeudall WA, Frum R, Grossman SR, et al. Gain-Of-Function Activity of Mutant P53 in Lung Cancer Through Up-Regulation of Receptor Protein Tyrosine Kinase Axl. Genes Cancer (2012) 3(7-8):491–502. doi: 10.1177/1947601912462719
58. Wang F, Zhao N, Gao G, Deng HB, Wang ZH, Deng LL, et al. Prognostic Value of TP53 Co-Mutation Status Combined With EGFR Mutation in Patients With Lung Adenocarcinoma. J Cancer Res Clin Oncol (2020) 146(11):2851–9. doi: 10.1007/s00432-020-03340-5
59. Offin M, Chan JM, Tenet M, Rizvi HA, Shen R, Riely GJ, et al. Concurrent RB1 and TP53 Alterations Define a Subset of EGFR-Mutant Lung Cancers at Risk for Histologic Transformation and Inferior Clinical Outcomes. J Thorac Oncol (2019) 14(10):1784–93. doi: 10.1016/j.jtho.2019.06.002
60. Bykov VJ, Zhang Q, Zhang M, Ceder S, Abrahmsen L, Wiman KG. Targeting of Mutant P53 and the Cellular Redox Balance by APR-246 as a Strategy for Efficient Cancer Therapy. Front Oncol (2016) 6:21. doi: 10.3389/fonc.2016.00021
61. Cheng Y, Murakami H, Yang PC, He J, Nakagawa K, Kang JH, et al. Randomized Phase II Trial of Gefitinib With and Without Pemetrexed as First-Line Therapy in Patients With Advanced Nonsquamous Non-Small-Cell Lung Cancer With Activating Epidermal Growth Factor Receptor Mutations. J Clin Oncol (2016) 34(27):3258–66. doi: 10.1200/JCO.2016.66.9218
62. Deng X, Li Y, Gu S, Chen Y, Yu B, Su J, et al. P53 Affects PGC1alpha Stability Through AKT/GSK-3beta to Enhance Cisplatin Sensitivity in Non-Small Cell Lung Cancer. Front Oncol (2020) 10:1252. doi: 10.3389/fonc.2020.01252
63. Hu C, Zhang M, Moses N, Hu CL, Polin L, Chen W, et al. The USP10-HDAC6 Axis Confers Cisplatin Resistance in Non-Small Cell Lung Cancer Lacking Wild-Type P53. Cell Death Dis (2020) 11(5):328. doi: 10.1038/s41419-020-2519-8
64. Zhang Y, Han CY, Duan FG, Fan XX, Yao XJ, Parks RJ, et al. P53 Sensitizes Chemoresistant Non-Small Cell Lung Cancer via Elevation of Reactive Oxygen Species and Suppression of EGFR/PI3K/AKT Signaling. Cancer Cell Int (2019) 19:188. doi: 10.1186/s12935-019-0910-2
65. Jia L, Lu XA, Liu G, Wang S, Xu M, Tian Y, et al. Endostatin Sensitizes P53-Deficient Non-Small-Cell Lung Cancer to Genotoxic Chemotherapy by Targeting DNA-Dependent Protein Kinase Catalytic Subunit. J Pathol (2017) 243(2):255–66. doi: 10.1002/path.4952
66. Kandioler D, Stamatis G, Eberhardt W, Kappel S, Zochbauer-Muller S, Kuhrer I, et al. Growing Clinical Evidence for the Interaction of the P53 Genotype and Response to Induction Chemotherapy in Advanced Non-Small Cell Lung Cancer. J Thorac Cardiovasc Surg (2008) 135(5):1036–41. doi: 10.1016/j.jtcvs.2007.10.072
67. Gregorc V, Ludovini V, Pistola L, Darwish S, Floriani I, Bellezza G, et al. Relevance of P53, Bcl-2 and Rb Expression on Resistance to Cisplatin-Based Chemotherapy in Advanced Non-Small Cell Lung Cancer. Lung Cancer (2003) 39(1):41–8. doi: 10.1016/s0169-5002(02)00391-4
68. Ma X, Rousseau V, Sun H, Lantuejoul S, Filipits M, Pirker R, et al. Significance of TP53 Mutations as Predictive Markers of Adjuvant Cisplatin-Based Chemotherapy in Completely Resected Non-Small-Cell Lung Cancer. Mol Oncol (2014) 8(3):555–64. doi: 10.1016/j.molonc.2013.12.015
69. Graziano SL, Tatum A, Herndon JE, Box J, Memoli V, Green MR, et al. Use of Neuroendocrine Markers, P53, and HER2 to Predict Response to Chemotherapy in Patients With Stage III Non-Small Cell Lung Cancer: A Cancer and Leukemia Group B Study. Lung Cancer (2001) 33(2-3):115–23. doi: 10.1016/s0169-5002(01)00183-0
70. Gajra A, Tatum AH, Newman N, Gamble GP, Lichtenstein S, Rooney MT, et al. The Predictive Value of Neuroendocrine Markers and P53 for Response to Chemotherapy and Survival in Patients With Advanced Non-Small Cell Lung Cancer. Lung Cancer (2002) 36(2):159–65. doi: 10.1016/s0169-5002(01)00463-9
71. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-Carboplatin Alone or With Bevacizumab for Non-Small-Cell Lung Cancer. N Engl J Med (2006) 355(24):2542–50. doi: 10.1056/NEJMoa061884
72. Tian W, Cao C, Shu L, Wu F. Anti-Angiogenic Therapy in the Treatment of Non-Small Cell Lung Cancer. Onco Targets Ther (2020) 13:12113–29. doi: 10.2147/OTT.S276150
73. Schwaederle M, Lazar V, Validire P, Hansson J, Lacroix L, Soria JC, et al. VEGF-A Expression Correlates With TP53 Mutations in Non-Small Cell Lung Cancer: Implications for Antiangiogenesis Therapy. Cancer Res (2015) 75(7):1187–90. doi: 10.1158/0008-5472.CAN-14-2305
74. Pfister NT, Fomin V, Regunath K, Zhou JY, Zhou W, Silwal-Pandit L, et al. Mutant P53 Cooperates With the SWI/SNF Chromatin Remodeling Complex to Regulate VEGFR2 in Breast Cancer Cells. Genes Dev (2015) 29(12):1298–315. doi: 10.1101/gad.263202.115
75. Wheler JJ, Janku F, Naing A, Li Y, Stephen B, Zinner R, et al. TP53 Alterations Correlate With Response to VEGF/VEGFR Inhibitors: Implications for Targeted Therapeutics. Mol Cancer Ther (2016) 15(10):2475–85. doi: 10.1158/1535-7163.MCT-16-0196
76. Koehler K, Liebner D, Chen JL. TP53 Mutational Status Is Predictive of Pazopanib Response in Advanced Sarcomas. Ann Oncol (2016) 27(3):539–43. doi: 10.1093/annonc/mdv598
77. Said R, Hong DS, Warneke CL, Lee JJ, Wheler JJ, Janku F, et al. P53 Mutations in Advanced Cancers: Clinical Characteristics, Outcomes, and Correlation Between Progression-Free Survival and Bevacizumab-Containing Therapy. Oncotarget (2013) 4(5):705–14. doi: 10.18632/oncotarget.974
78. Wang P, Fang X, Yin T, Tian H, Yu J, Teng F. Efficacy and Safety of Anti-PD-1 Plus Anlotinib in Patients With Advanced Non-Small-Cell Lung Cancer After Previous Systemic Treatment Failure-A Retrospective Study. Front Oncol (2021) 11:628124. doi: 10.3389/fonc.2021.628124
79. Fang S, Cheng W, Zhang M, Yang R. Association of TP53 Mutations With Response to Anlotinib Treatment in Advanced Non-Small Cell Lung Cancer. OncoTargets Ther (2020) 13:6645–50. doi: 10.2147/OTT.S257052
80. Zhao H, Yao W, Min X, Gu K, Yu G, Zhang Z, et al. Apatinib Plus Gefitinib as First-Line Treatment in Advanced EGFR-Mutant NSCLC: The Phase III ACTIVE Study (Ctong1706). J Thorac Oncol (2021) 16(9):1533–46. doi: 10.1016/j.jtho.2021.05.006
81. Scagliotti GV, Krzakowski M, Szczesna A, Strausz J, Makhson A, Reck M, et al. Sunitinib Plus Erlotinib Versus Placebo Plus Erlotinib in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer: A Phase III Trial. J Clin Oncol (2012) 30(17):2070–8. doi: 10.1200/JCO.2011.39.2993
82. Zhou Q, Xu CR, Cheng Y, Liu YP, Chen GY, Cui JW, et al. Bevacizumab Plus Erlotinib in Chinese Patients With Untreated, EGFR-Mutated, Advanced NSCLC (ARTEMIS-CTONG1509): A Multicenter Phase 3 Study. Cancer Cell (2021) 39(9):1279–91 e3. doi: 10.1016/j.ccell.2021.07.005
83. Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, et al. Erlotinib Plus Bevacizumab Versus Erlotinib Alone in Patients With EGFR-Positive Advanced Non-Squamous Non-Small-Cell Lung Cancer (NEJ026): Interim Analysis of an Open-Label, Randomised, Multicentre, Phase 3 Trial. Lancet Oncol (2019) 20(5):625–35. doi: 10.1016/S1470-2045(19)30035-X
84. Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, et al. Erlotinib Alone or With Bevacizumab as First-Line Therapy in Patients With Advanced Non-Squamous Non-Small-Cell Lung Cancer Harbouring EGFR Mutations (JO25567): An Open-Label, Randomised, Multicentre, Phase 2 Study. Lancet Oncol (2014) 15(11):1236–44. doi: 10.1016/S1470-2045(14)70381-X
85. Nakagawa K, Nadal E, Garon EB, Nishio M, Seto T, Yamamoto N, et al. RELAY Subgroup Analyses by EGFR Ex19del and Ex21L858R Mutations for Ramucirumab Plus Erlotinib in Metastatic Non-Small Cell Lung Cancer. Clin Cancer Res (2021) 27(19):5258–71. doi: 10.1158/1078-0432.CCR-21-0273
86. Xiong A, Wang J, Zhou C. Immunotherapy in the First-Line Treatment of NSCLC: Current Status and Future Directions in China. Front Oncol (2021) 11:757993. doi: 10.3389/fonc.2021.757993
87. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab Versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
88. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab Versus Docetaxel in Patients With Previously Treated Non-Small-Cell Lung Cancer (OAK): A Phase 3, Open-Label, Multicentre Randomised Controlled Trial. Lancet (2017) 389(10066):255–65. doi: 10.1016/S0140-6736(16)32517-X
89. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab Versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7
90. Oxnard GR, Yang JC, Yu H, Kim SW, Saka H, Horn L, et al. TATTON: A Multi-Arm, Phase Ib Trial of Osimertinib Combined With Selumetinib, Savolitinib, or Durvalumab in EGFR-Mutant Lung Cancer. Ann Oncol (2020) 31(4):507–16. doi: 10.1016/j.annonc.2020.01.013
91. Yang JC, Shepherd FA, Kim DW, Lee GW, Lee JS, Chang GC, et al. Osimertinib Plus Durvalumab Versus Osimertinib Monotherapy in EGFR T790M-Positive NSCLC Following Previous EGFR TKI Therapy: CAURAL Brief Report. J Thorac Oncol (2019) 14(5):933–9. doi: 10.1016/j.jtho.2019.02.001
92. Garassino MC, Cho BC, Kim JH, Mazieres J, Vansteenkiste J, Lena H, et al. Durvalumab as Third-Line or Later Treatment for Advanced Non-Small-Cell Lung Cancer (ATLANTIC): An Open-Label, Single-Arm, Phase 2 Study. Lancet Oncol (2018) 19(4):521–36. doi: 10.1016/S1470-2045(18)30144-X
93. Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther (2015) 14(4):847–56. doi: 10.1158/1535-7163.MCT-14-0983
94. Liu Y, Wu A, Li X, Wang S, Fang S, Mo Y. A Retrospective Analysis of Eleven Gene Mutations, PD-L1 Expression and Clinicopathological Characteristics in Non-Small Cell Lung Cancer Patients. Asian J Surg (2022) 45(1):367–75. doi: 10.1016/j.asjsur.2021.06.030
95. Cha YJ, Kim HR, Lee CY, Cho BC, Shim HS. Clinicopathological and Prognostic Significance of Programmed Cell Death Ligand-1 Expression in Lung Adenocarcinoma and Its Relationship With P53 Status. Lung Cancer (2016) 97:73–80. doi: 10.1016/j.lungcan.2016.05.001
96. Sun H, Liu SY, Zhou JY, Xu JT, Zhang HK, Yan HH, et al. Specific TP53 Subtype as Biomarker for Immune Checkpoint Inhibitors in Lung Adenocarcinoma. EBioMedicine (2020) 60:102990. doi: 10.1016/j.ebiom.2020.102990
97. Jin Y, Xue Q, Shen X, Zheng Q, Chen H, Zhou X, et al. PD-L1 Expression and Comprehensive Molecular Profiling Predict Survival in Nonsmall Cell Lung Cancer: A Real-World Study of a Large Chinese Cohort. Clin Lung Cancer (2022) 23(1):43–51. doi: 10.1016/j.cllc.2021.08.009
98. Hellmann MD, Nathanson T, Rizvi H, Creelan BC, Sanchez-Vega F, Ahuja A, et al. Genomic Features of Response to Combination Immunotherapy in Patients With Advanced Non-Small-Cell Lung Cancer. Cancer Cell (2018) 33(5):843–52 e4. doi: 10.1016/j.ccell.2018.03.018
99. Zhang N, Wu J, Yu J, Zhu H, Yang M, Li R. Integrating Imaging, Histologic, and Genetic Features to Predict Tumor Mutation Burden of Non-Small-Cell Lung Cancer. Clin Lung Cancer (2020) 21(3):e151–e63. doi: 10.1016/j.cllc.2019.10.016
100. Assoun S, Theou-Anton N, Nguenang M, Cazes A, Danel C, Abbar B, et al. Association of TP53 Mutations With Response and Longer Survival Under Immune Checkpoint Inhibitors in Advanced Non-Small-Cell Lung Cancer. Lung Cancer (2019) 132:65–71. doi: 10.1016/j.lungcan.2019.04.005
101. Lin X, Wang L, Xie X, Qin Y, Xie Z, Ouyang M, et al. Prognostic Biomarker TP53 Mutations for Immune Checkpoint Blockade Therapy and Its Association With Tumor Microenvironment of Lung Adenocarcinoma. Front Mol Biosci (2020) 7:602328. doi: 10.3389/fmolb.2020.602328
102. Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z, Liu SY, et al. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin Cancer Res (2017) 23(12):3012–24. doi: 10.1158/1078-0432.CCR-16-2554
103. Lu J, Zhong R, Lou Y, Hu M, Yang Z, Wang Y, et al. TP53 Mutation Status and Biopsy Lesion Type Determine the Immunotherapeutic Stratification in Non-Small-Cell Lung Cancer. Front Immunol (2021) 12:732125. doi: 10.3389/fimmu.2021.732125
104. Pavan A, Bragadin AB, Calvetti L, Ferro A, Zulato E, Attili I, et al. Role of Next Generation Sequencing-Based Liquid Biopsy in Advanced Non-Small Cell Lung Cancer Patients Treated With Immune Checkpoint Inhibitors: Impact of STK11, KRAS and TP53 Mutations and Co-Mutations on Outcome. Trans Lung Cancer Res (2021) 10(1):202–20. doi: 10.21037/tlcr-20-674
105. Chen Y, Chen G, Li J, Huang YY, Li Y, Lin J, et al. Association of Tumor Protein P53 and Ataxia-Telangiectasia Mutated Co-Mutation With Response to Immune Checkpoint Inhibitors and Mortality in Patients With Non-Small Cell Lung Cancer. JAMA network Open (2019) 2(9):e1911895. doi: 10.1001/jamanetworkopen.2019.11895
106. Shi Y, Lei Y, Liu L, Zhang S, Wang W, Zhao J, et al. Integration of Comprehensive Genomic Profiling, Tumor Mutational Burden, and PD-L1 Expression to Identify Novel Biomarkers of Immunotherapy in Non-Small Cell Lung Cancer. Cancer Med (2021) 10(7):2216–31. doi: 10.1002/cam4.3649
107. Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab Plus Bevacizumab and Chemotherapy in Non-Small-Cell Lung Cancer (IMpower150): Key Subgroup Analyses of Patients With EGFR Mutations or Baseline Liver Metastases in a Randomised, Open-Label Phase 3 Trial. Lancet Respir Med (2019) 7(5):387–401. doi: 10.1016/S2213-2600(19)30084-0
Keywords: non-small cell lung cancer (NSCLC), EGFR, TP53, comutation, primary resistance, treatment
Citation: Liu S, Yu J, Zhang H and Liu J (2022) TP53 Co-Mutations in Advanced EGFR-Mutated Non–Small Cell Lung Cancer: Prognosis and Therapeutic Strategy for Cancer Therapy. Front. Oncol. 12:860563. doi: 10.3389/fonc.2022.860563
Received: 23 January 2022; Accepted: 16 March 2022;
Published: 04 April 2022.
Edited by:
Lin Zhang, University of Pittsburgh, United StatesReviewed by:
Francesco Pepe, University of Naples Federico II, ItalyChengzhi Zhou, Clinical Management Department of National Respiratory Medical Center, China
Copyright © 2022 Liu, Yu, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Liu, c2RqbmxqamllQDEyNi5jb20=
 Surui Liu
Surui Liu Jin Yu1
Jin Yu1 Jie Liu
Jie Liu