- 1Cancer Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Department of Nutrition, Science and Research Branch, Islamic Azad University, Tehran, Iran
- 3Department of Medical Genetics, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
- 4Department of Medical Genetics, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 5Department of Clinical Nutrition and Dietetics, Faculty of Nutrition and Food Technology, National Nutrition and Food Technology Research Institute, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 6Research Center of Health and Enviroment, School of Health, Guilan University of Medical Sciences, Rasht, Iran
- 7Nursing and Midwifery School, Guilan University of Medical Sciences, Rasht, Iran
- 8Department of Biology and Biotechnology ”Charles Darwin”, Sapienza University of Rome, Rome, Italy
- 9Department of Pharmacognosy, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
- 10Reproductive Health Research Center, Department of Obstetrics and Gynecology, School of Medicine, Al-Zahra Hospital, Guilan University of Medical Sciences, Rasht, Iran
- 11Dept. of Nutrition Research, Faculty of Nutrition Sciences and Food Technology, National Nutrition and Food Technology Research Institute, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Objective: Genetics and dietary factors play important roles in the development of colorectal cancer (CRC). However, the underlying mechanisms of the interactions between CRC, gene polymorphisms, and dietary fat are unclear. This review study investigated the effects of polymorphisms of arachidonate lipoxygenase (ALOX) and cyclooxygenase (COX) genes in the association between CRC and dietary fat.
Methods: All the related papers published from 2000 to 2022 were collected from different databases such as PubMed, Science Direct, Scopus, and Cochran using related keywords such as colorectal cancer, ALOX, COX, polymorphism, and dietary fat. Non-English and unrelated documents were excluded.
Results: Some single-nucleotide polymorphisms (SNPs) in the ALOX and COX genes, such as rs2228065, rs6413416, and rs4986832 in the ALOX gene, and rs689465 in the COX gene may play significant roles in the association between the risk of CRC and dietary fats. SNPs of ALOX and COX genes may influence the effects of dietary fatty acids on the risk of CRC.
Conclusion: Some polymorphisms of the ALOX and COX genes may have important roles in the effects of dietary fat on the risk of CRC. If future studies confirm these results, dietary recommendations for preventing colorectal cancer may be personalized based on the genotype of the ALOX and COX genes.
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death in women and the third in men worldwide (1) and cause about 0.9 million deaths worldwide in 2020 (2). It has been reported that CRC originates from a combination of genetic, environmental, and behavioral risk factors. Some behavioral factors are associated with dietary intake, including higher intake of calories, red meat, and fats (3, 4).
Recently, various types of fatty acids have been reported as effective dietary factors in CRC development. Some fatty acids, such as saturated fatty acids, may have an adverse effect, whereas other fatty acids, such as omega-3 fatty acids, may have a beneficial effect on CRC prevention (5–7). One main mechanism through which dietary polyunsaturated fatty acids (PUFAs) may affect colonic carcinogenesis is the formation of specific eicosanoids (oxygenated metabolites of PUFAs) such as prostaglandins (PGs), thromboxanes (TXs), leukotrienes (LTs), and lipoxins (LXs) (7). Two enzymatic pathways related to the synthesis of these eicosanoids are the arachidonic lipoxygenase (ALOX) pathways and prostaglandin-endoperoxide synthase (PTGS), which are also known as cyclooxygenase (COX) pathways (8). The function of ALOX enzymes, such as ALOX5, ALOX12, and ALOX15, eventually leads to LT and LX formation, and the COX enzymes, like COX1 and COX2, result in the production of PGs and TXs (9, 10). Evidence has shown that changes in the sequence of COX and ALOX genes as single-nucleotide polymorphisms (SNPs) can influence the risk of CRC (8).
In terms of ALOX, previous research has established that ALOX15 expression and concentration of eicosanoic metabolites are reduced in polyps and colorectal tumors in humans (11). In contrast, increased ALOX5 expression has been reported in colorectal cancer cells (12). Moreover, it has been reported that some mutations in ALOX12 are associated with tumorigenesis in epithelial cancers (13). Recent studies have identified that the expression level of the COX2 gene and the levels of its metabolites, such as PGE2, PGD2, and PGF2α, are significantly increased in the colon of obese mice. Also, it has been shown that the administration of COX2 inhibitors can suppress inflammation, tumor growth, and tumor metastasis (14–16).
Notably, the effect of dietary fats on the risk of CRC may be influenced by gene polymorphisms (17–19). However, the interactions between CRC, dietary fat, and gene polymorphisms are still unknown. So, this review study investigated the effects of SNPs of the ALOX and COX genes on the association between dietary fats and CRC risk.
Materials and Methods
Search Strategy
The literature search was performed using the PubMed, Science Direct, Scopus, and Cochran databases, and all related papers published from 2000 to 2022 were collected using the following keywords: “dietary fat or fatty acid or fat or lipid” and “ALOX or COX or prostaglandin-endoperoxide synthase or PTGS or cyclo-oxygenase or COX or arachidonic lipoxygenase or lipoxygenase” and “colorectal cancer or colon cancer or rectal cancer” and “polymorphism or genetic variation or genotype or SNP.” All the collected papers and their references were reviewed.
Inclusion and Exclusion Criteria
All studies that examined the interaction of colorectal cancer with ALOX and COX genes, studies concerning the relationship between the ALOX and COX gene polymorphisms, and studies on the interactions between colorectal cancer, ALOX and COX genes, and dietary fat were included in this study. Unrelated and non-English papers, the review studies, studies on the relationship between ALOX and COX with other cancers, and animal studies were excluded.
Assessment of Methodological Rigor
In this review study, the quality of the collected studies was assessed by four researchers (MG, HS, SA, and SD). In the case of having opposing ideas, other researchers (MH and HS) would be involved in reaching an agreement. After collecting the papers, all unrelated studies were excluded from the review process according to their titles and abstracts. Then, the full texts of the relevant articles were studied precisely. The standard quality assessment method of the ‘EPOC Risk of Bias Tool’ was applied to assess the quality of the methodologies (20). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (21) was used to extract the required data from the included studies. Finally, the data about the participants, intended comparisons, obtained results, and study planning (PICOS) were collected.
Results
Description of the Identified Studies
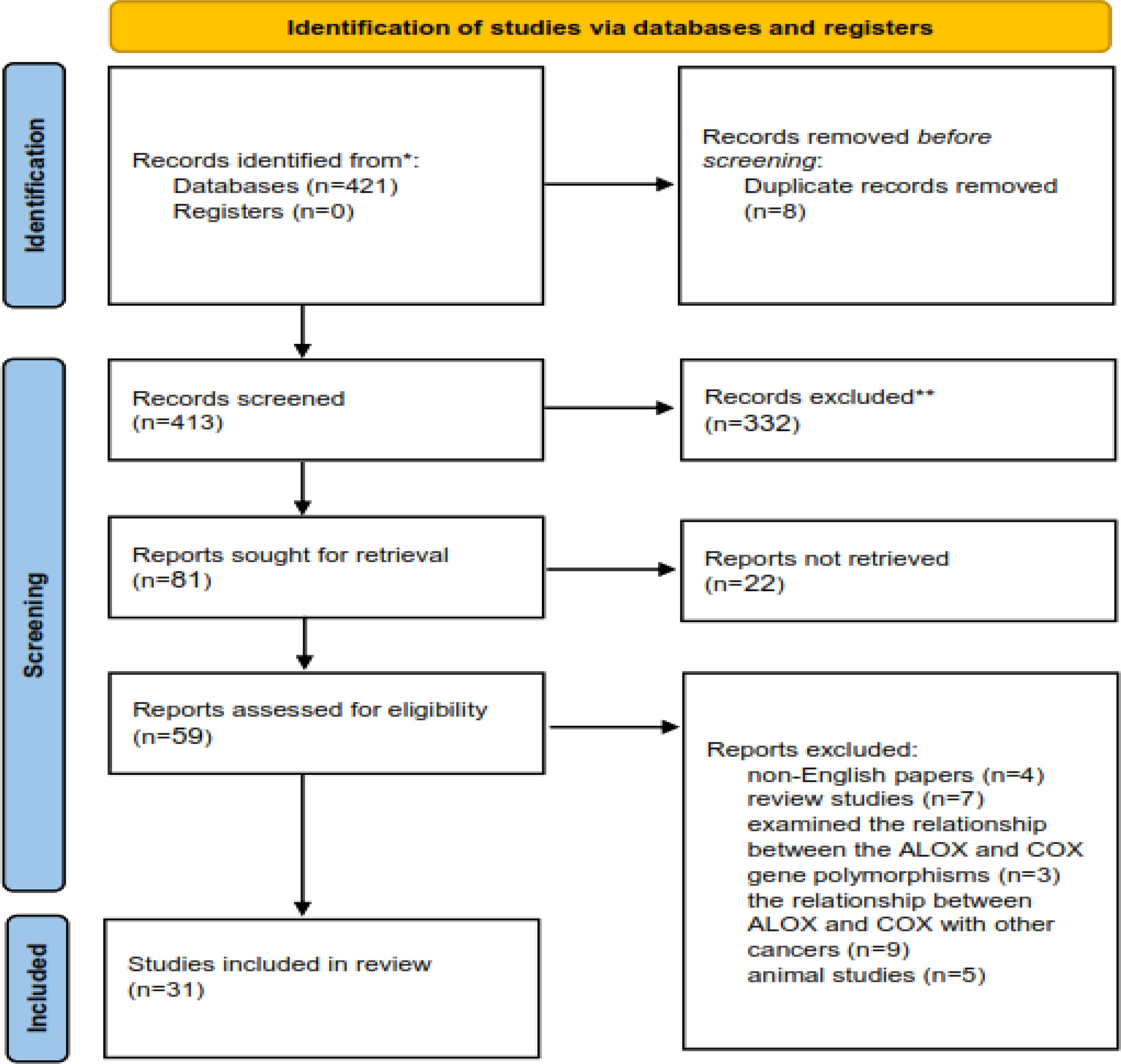
The process of including the appropriate studies is presented in Figure 1. A total of 413 articles were collected in the primary search, of which 354 articles were excluded after the screening of their titles and abstracts. Also, 28 articles were excluded after reading the full texts. Finally, 31 articles qualified to be included in the review process. All articles were published from 2000 to 2022 and were related to the interactions between CRC, ALOX, and COX genes and fat intake. The main characteristics of the studies are presented in Tables 1, 2.
Arachidonate Lipoxygenase (ALOX) Gene Polymorphisms and Risk of CRC
The primary function of ALOX is to convert arachidonic acid (AA) into hydroperoxyeicosatetraenoic acid (HPETE) and eventually leukotrienes, a class of paracrine hormones involved in the inflammatory response. For example, ALOX12 converts AA into 12-hydroperoxyeicosatetraenoic acid (12-HPETE), which is involved in the expression of pro-inflammatory cytokine genes such as tumor necrosis factor-α (TNF-α) (36). The role of the ALOX gene in inflammatory diseases and colorectal neoplasia has been frequently reported. For example, the arachidonate-5 lipoxygenase (ALOX5) and 12-lipoxygenase (ALOX12) played pro-carcinogenic roles in colorectal cancer (22). Additionally, overexpression of ALOX5 with its related downstream metabolites has been reported in other cancers such as breast, esophageal, pancreatic, and prostate cancers by stimulation of cell proliferation, tumor angiogenesis, and survival (37). Moreover, it has been reported that ALOX15 is associated with an increased risk of colorectal cancer, particularly in people with higher inflammatory factors (38). In another study, Ruan et al. examined the diagnostic and prognostic values of the ALOX gene family mRNA expression in 438 colon adenocarcinoma tumor samples and 41 adjacent tissue samples of Chinese patients by bioinformatics analysis. They showed that the expression level of ALOXE3, ALOX5, ALOX12, and ALOX12B was upregulated in colorectal tumor samples. Finally, they reported that ALOXE3 and ALOX12 might serve as potential independent prognostic indicators of colon adenocarcinoma (25). Thus, ALOX pathways in the AA metabolism process can be considered crucial pathways in the development of CRC (Figure 2).
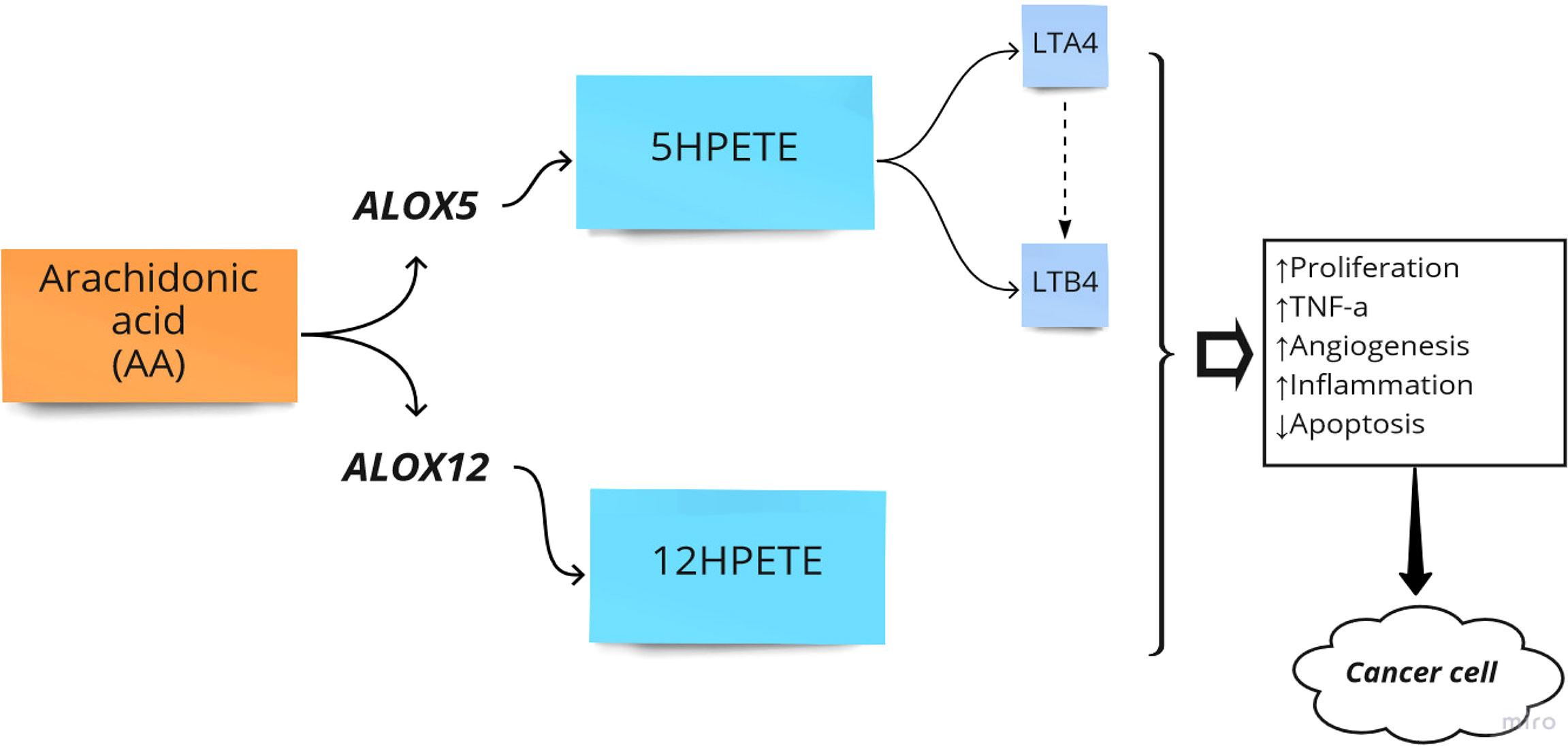
Figure 2 Arachidonate lipoxygenase (ALOX) in the metabolism of Arachidonic Acid (AA). HPETE, hydroperoxyeicosatetraenoic acid; LT, Leukotriene; ↑, Increase; ↓, Decrease.
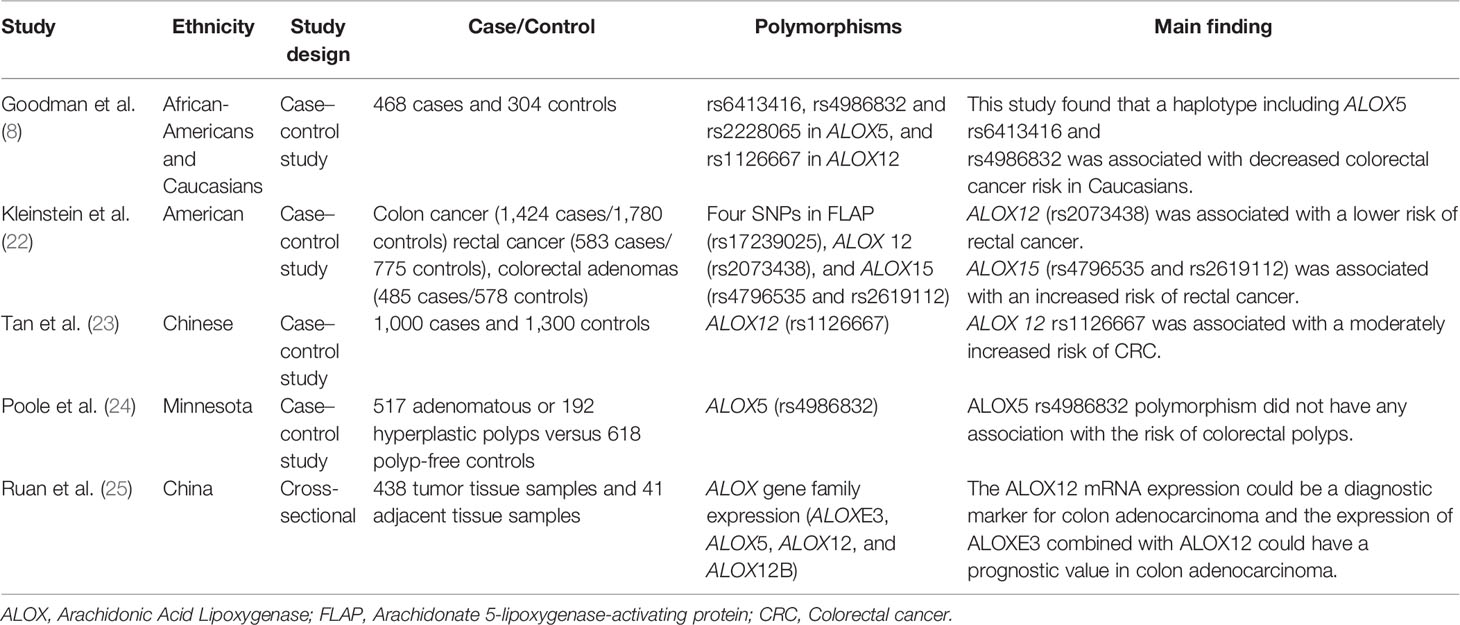
Notably, specific polymorphisms of the ALOX gene can affect the susceptibility to CRC. For instance, Goodman et al. assessed the effects of ALOX5 and ALOX12 gene polymorphisms on CRC in African-Americans and Caucasian patients. They found that the rs6413416 and rs4986832 polymorphisms of ALOX5 were associated with a decreased risk of CRC in Caucasians. They hypothesized that these polymorphisms could improve binding to the promoter region, leading to downregulation of ALOX5. In this way, they can lower the cancer risk by reducing enzymatic activity (8). However, the rs4986832 polymorphisms of ALOX5 had no association with the risk of colorectal polyps in Minnesota (24). Kleinstein et al. conducted a study on 2447 cases and 3133 controls regarding the effect of ALOX gene polymorphisms on the risk of CRC. The results showed that the rs2073438 polymorphism of ALOX 12 was related to a lower risk of rectal cancer (OR = 0.66, 95% CI: 0.42–1.04), while the rs4796535 and rs2619112 polymorphisms of ALOX15 were associated with an increased risk of rectal cancer (OR = 1.43, 95% CI: 1.03–1.97 and OR = 1.13, 95% CI: 0.85–1.55, respectively) (22). Moreover, a positive association was found between the ALOX12 rs1126667 polymorphism and a moderately increased risk of CRC (OR = 1.38, 95% CI: 1.09–1.74) (23). However, the association between rs1126667 polymorphism of ALOX12 and the risk of CRC has been reported in African-Americans and Caucasian patients (8). This discrepancy can be due to differences in ethnic or statistical power. A summary of studies on the association between ALOX polymorphisms and CRC is provided in Table 1.
Cyclooxygenase (COX) Gene Polymorphism and Risk of CRC
Prostaglandin H synthase, also known as cyclooxygenase and prostaglandin-endoperoxide synthase (PTGS), catalyzes the first step in the biosynthesis of all prostaglandins and prostacyclins by converting arachidonic acid to prostaglandin H (39). Two forms of human PTGS, PTGS1 and PTGS2 (COX1 and COX2), can be inhibited by non-steroidal anti-inflammatory drugs (NSAIDs). Also, the end products of COX are related to various biological pathways in stimulating tumor growth (26) (Figure 3).
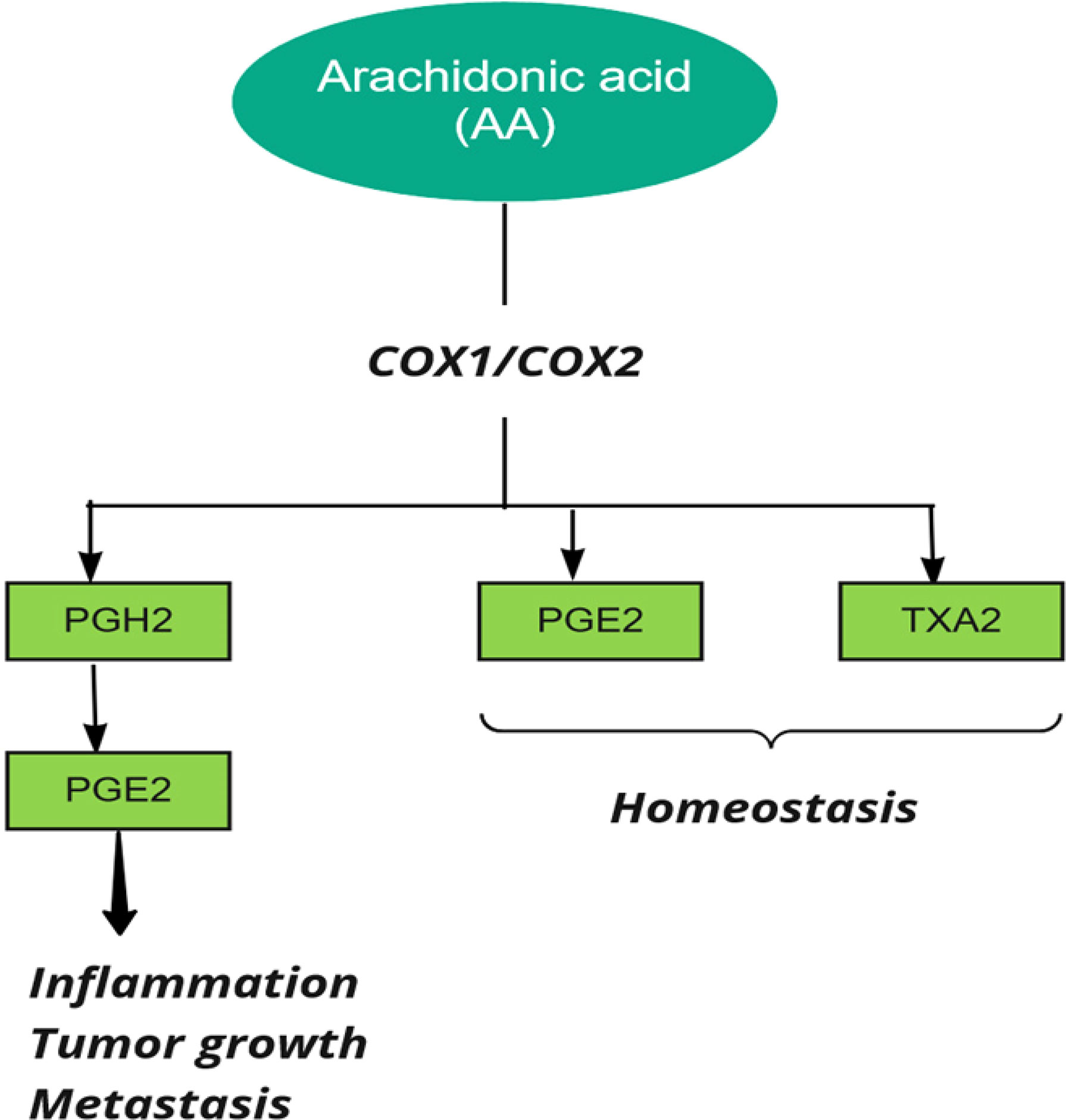
Figure 3 Cyclooxygenase (COX) in metabolism of Arachidonic Acid (AA). PG, Prostaglandin, TX, Thromboxane.
Prostaglandins are upregulated in colorectal cancer, and it was reported that genetic polymorphisms in both COX1 and COX2 are associated with CRC (27, 37). COX2 is involved in cell cycle control, and increased expression of COX-2 in CRC patients compared to normal controls indicates its possible role in the progression of CRC (40). COX2 influences cancer progression by increasing prostaglandin production, preventing tumor cell apoptosis, cell proliferation, and tumor angiogenesis (41). It was reported that aspirin plays a key role in preventing colon cancer by inhibiting COX (37). Ayiomamitis et al. examined the expression of COX1, COX2, prostaglandin-endoperoxide synthase 3 (PTGES3), and telomerase reverse transcriptase (TERT). They used bioinformatics analysis on the Cancer Genome Atlas Colon Adenocarcinoma (TCGA-COAD) and rectal adenocarcinoma (READ) datasets. The results showed an inverse relationship between COX2 expression and telomerase activity in CRC. In the end, they identified differentially methylated patterns within the promoter regions of COX2 and TERT (42). Joanna et al. observed COX2 overexpression in the early stages of colorectal cancer and higher COX2 gene expression in the advanced stages of the disease. The results also indicated that COX2 expression level could affect carcinogenicity by modulating local inflammation (43). In addition, a case–control study on Iraqi patients reported that the expression level of COX2 was upregulated at higher tumor grades (44). This result suggests that considering COX2 as an early marker of progression or initiation of colorectal carcinoma should be investigated by further studies. Moreover, Labda et al. found that COX2 expression was associated with tumor size and degree of differentiation in an observational study including 58 Indonesian CRC patients. However, there was no statistical correlation between COX2 expression and tumor location (45). Jin et al. conducted a case-control study involving 213 Chinese patients with colorectal cancer and 200 controls and reported that the expression level of IGF-IR and COX2 was directly related to the degree of progression and lymphatic metastasis and inversely related to the mean survival rate in CRC patients (46). The results of a Chinese study also indicated that PGE2 and COX2 expression were significantly associated with tumor invasion, tumor differentiation, lymph node metastasis, and TNM stage and were inversely related to patient survival (47).
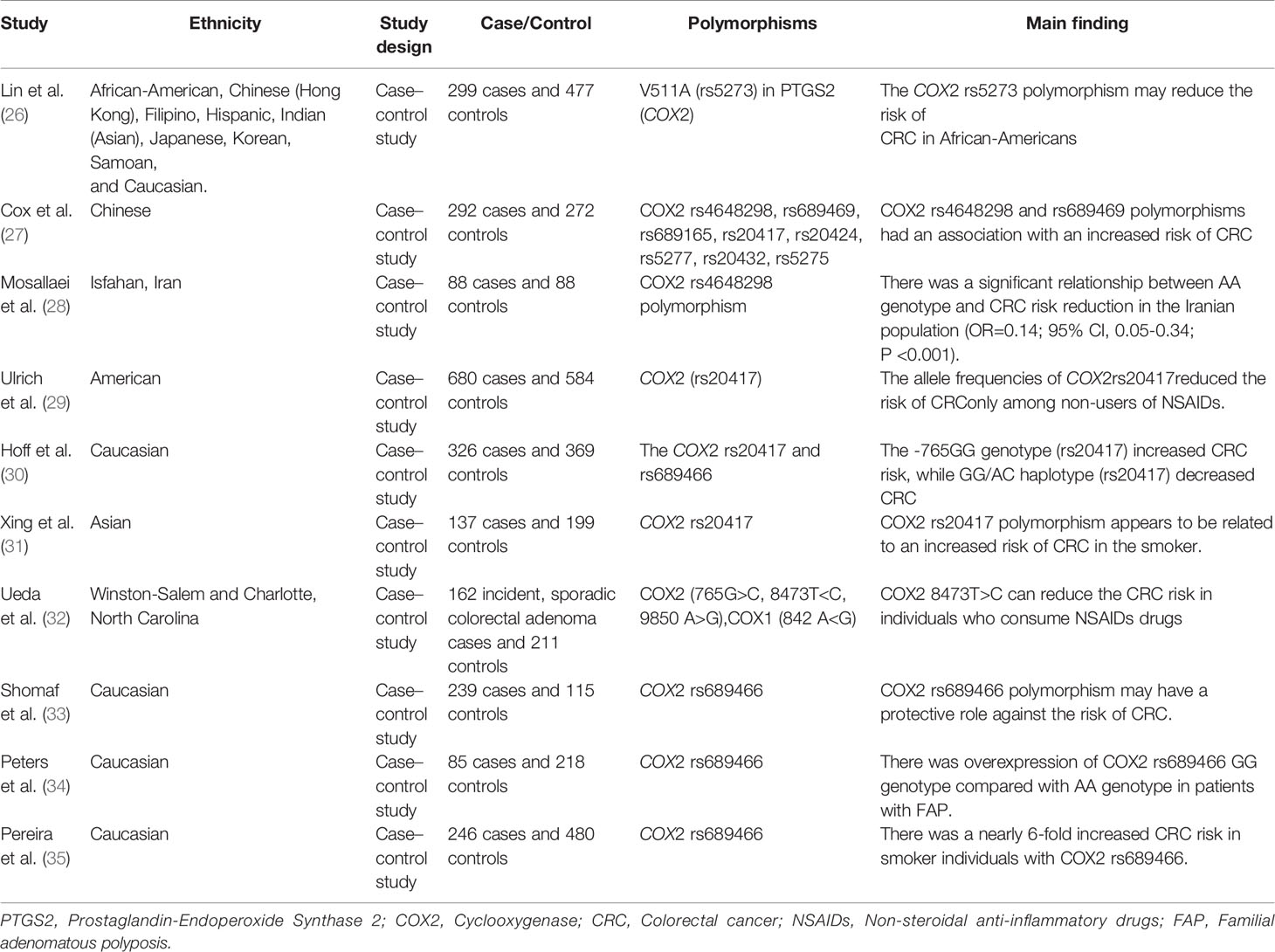
Polymorphisms of the COX gene can affect the risk of CRC. In this regard, Lin et al. found that the COX2 rs5273 polymorphism, in about 5% of African Americans, was associated with a lowered risk of CRC (OR = 0.78, 95% CI: 0.49–1.23) (26). The COX2 rs4648298 and rs689469 polymorphisms were reported to be associated with an increased risk of CRC. Analysis of haplotypes confirmed that people with these variants were at an increased risk of colorectal cancer (OR = 2.17, 95% CI: 0.97–4.84, P = 0.06) (27). In contrast with these results, Mosallaei et al. observed a significant relationship between COX2 rs4648298 polymorphism (AA genotype) and a reduced risk of CRC in the Iranian population (OR = 0.14; 95% CI: 0.05–0.34; P <0.001). Interestingly, they found this significant association only in non-smokers (28). This finding suggests that environmental factors may influence the association between the COX gene polymorphism and CRC. Another study showed that the GG genotype of COX2 rs20417 was associated with an increased risk of developing CRC in the Dutch population (OR, 1.45; 95% CI, 1.03–2.04) (30). Interestingly, Xing et al. observed the positive association between the GG genotype of the COX2 rs20417 polymorphism and increased CRC risk in China, especially in smokers and in people with a high Body Mass Index (BMI) (OR: 1.107, 95% CI: 1.107–3.726; P = 0.022) (31). In this line, the Minnesota-based case-control study discovered that COX2 gene expression or COX2 enzyme activity is suppressed and the risk of colorectal polyps is reduced by NSAIDs in individuals with the GG genotype of COX2 rs20417 (OR: 0.66; 95% CI: 0.48–0.92) (29). Ueda et al. investigated the association between the COX2 position 765 G<C, 8473 T>C, and 9850 A>G and CRC risk and reported that among the studied polymorphisms, COX2 8473T>C may reduce the CRC risk in people who consume NSAIDs (OR: 1.57, 95% CI: 1.04–2.38) (32). Another previous study on 104 cases of adenomatous polyps and 115 matched control samples found that COX2 rs689466 polymorphism may have a protective effect on the risk of development of CRC (33). In contrast, Peters et al. identified that overrepresentation of COX2 was associated with a high risk for CRC development in patients with familial adenomatous polyposis (FAP) who had the rs689466 polymorphism GG genotype compared with AA genotype carriers (OR = 2.81; 95% CI = 1.00–7.91, P= 0.042) (34). In addition, Pereira et al. suggested that smoker people with COX2 rs689466 polymorphism had a nearly 6-fold increased CRC risk compared with people without rs689466 risk allele (95% CI: 1.49–22.42, P = 0.011) (35). Some reasons for these conflicting results on the association between CRC and COX gene can be due to effects of different environmental factors such as lifestyle on this association. Table 2 presents the summary of studies regarding COX polymorphisms and CRC risk.
Interaction Between CRC, ALOX and COX Polymorphisms, and Dietary Fatty Acids
COX enzymes (COX1, COX2) are important factors in the biosynthetic pathway of PGs from AA. ALOX enzymes (ALOX5, ALOX12, and ALOX15) convert PUFA to fatty acid hydroperoxides, which results in the production of LTs. Recent studies reported an association between the CRC risk with the amount of fatty acids intake and COX and ALOX polymorphisms (Figure 4). Habermann et al. identified an association between COX1 rs10306110 polymorphism and low intake of docosahexaenoic acid (DHA), a fatty acid with anti-inflammatory properties, with an increased risk of colon cancer (OR = 1.6, 95% CI: 1.1–2.3, adjusted P = 0.06) (38). Notably, supplementation with some fatty acids such as ω-3 fatty acids plays a protective role in colon cancer by attenuating the pro-inflammatory state and decreasing the production of PGE2 (48). These results conform to studies that indicated that omega-3 long-chain polyunsaturated fatty acids (n−3 LC-PUFA) may lower cancer risk by suppressing oxidative stress, tumor apoptosis, and inflammatory pathways by modulation of COX activity and inhibition of arachidonic acid-derived eicosanoids (49–51). However, a case–control study on 310 patients with colorectal cancer and 1,177 controls provided epidemiological evidence for the possible link between PGs production from n−6 PUFAs through the enzymatic activity of COX2 and increased risk of colon cancer. They reported an association between COX2 rs20417 polymorphism and an increased risk of colon cancer in individuals with high n−6 PUFA intake (OR = 2.38, 95% CI = 1.23–4.59, P = 0.07). However, there was no association between this polymorphism and the risk of rectal cancer regardless of the dietary n−6 PUFA intake levels (52). These results emphasize the importance of lifestyle modification in the carriers of the high-risk allele of the COX gene. Moreover, Siezen et al. demonstrated that colorectal adenoma risk could be modified by the interaction between polymorphisms in AA pathway genes and fish consumption. They showed that the COX2 rs5277 polymorphism in people with high fish consumption played a protective role against CRC compared with people with low fish intake (53). In another work, Siezen et al. confirmed the inverse association between high fish consumption and CRC risk. However, they could not find any significant interaction between CRC and SNPs in the genes involved in the AA pathway (54). Interestingly, another study indicated that the effects of n−3 PUFA intake and NSAIDs on CRC may differ in people with COX1 polymorphisms. Among the wild-type homozygous individuals (PP genotype) with COX1 rs3842787 polymorphism, high fish consumption and regular use of NSAIDs were associated with a decreased risk of CRC. In comparison, an inverse association was observed in individuals with at least one risk allele (PL, LL genotypes) in the COX-1 rs3842787 polymorphism (55). Furthermore, dietary supplementation with n−3 PUFA, particularly DHA and EPA, was reported to have antineoplastic effects on CRC by modifying the epigenetic modification like DNA methylation (56). Table 3 summarizes studies regarding the association between ALOX, COX polymorphism, dietary fatty acid, and CRC risk.
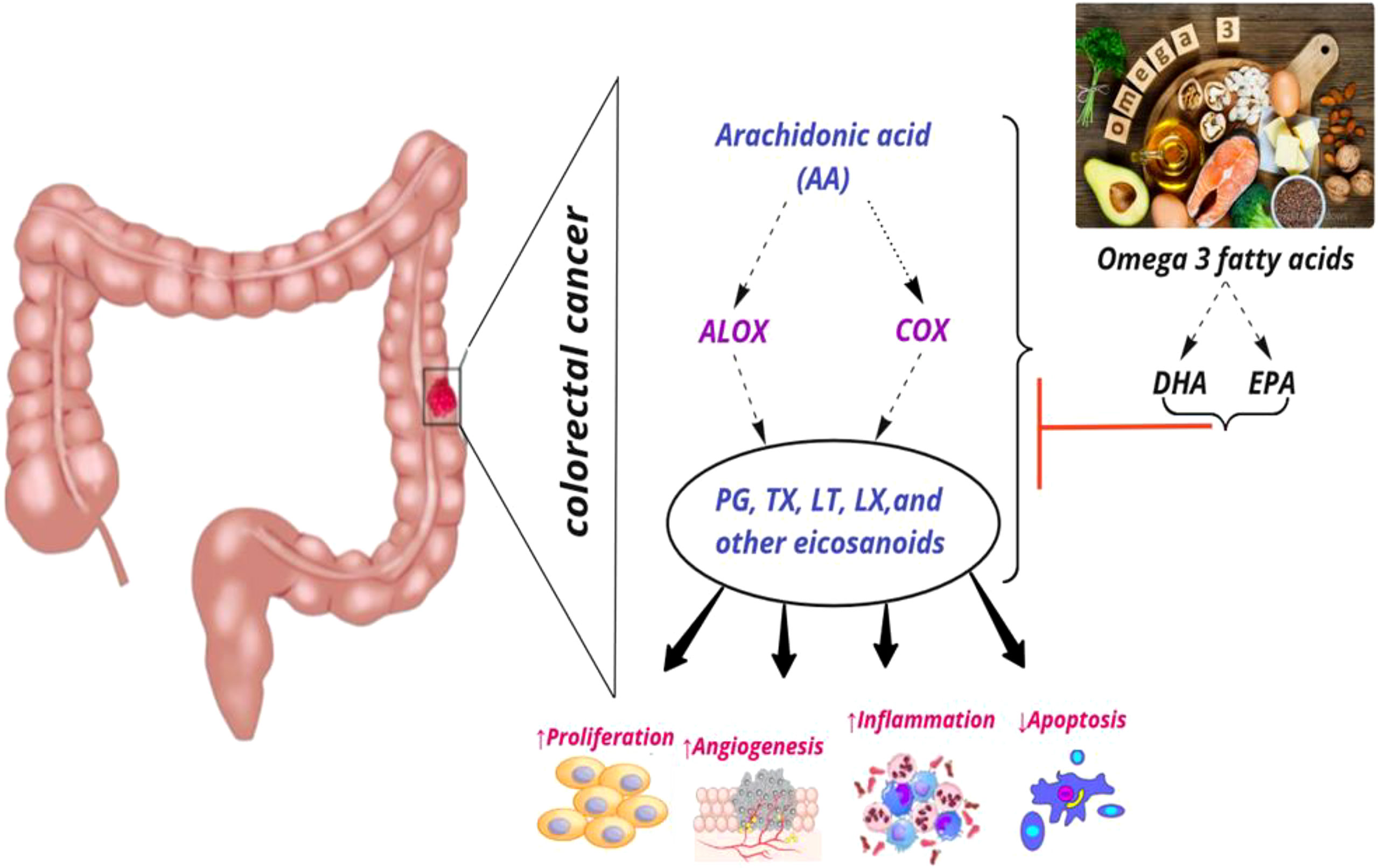
Figure 4 Interaction among dietary fatty acids, ALOX (Arachidonate lipoxygenase) and COX (Cyclooxygenase) in metabolic pathway of AA (Arachidonic Acid), and CRC (colorectal cancer) risk. PG, Prostaglandin; TX, Thromboxane; LT, Leukotriene; LX, Lipoxin; DHA, Docosahexaenoic acid; EPA, Eicosapentaenoic acid; ↑, increase; ↓, decrease.
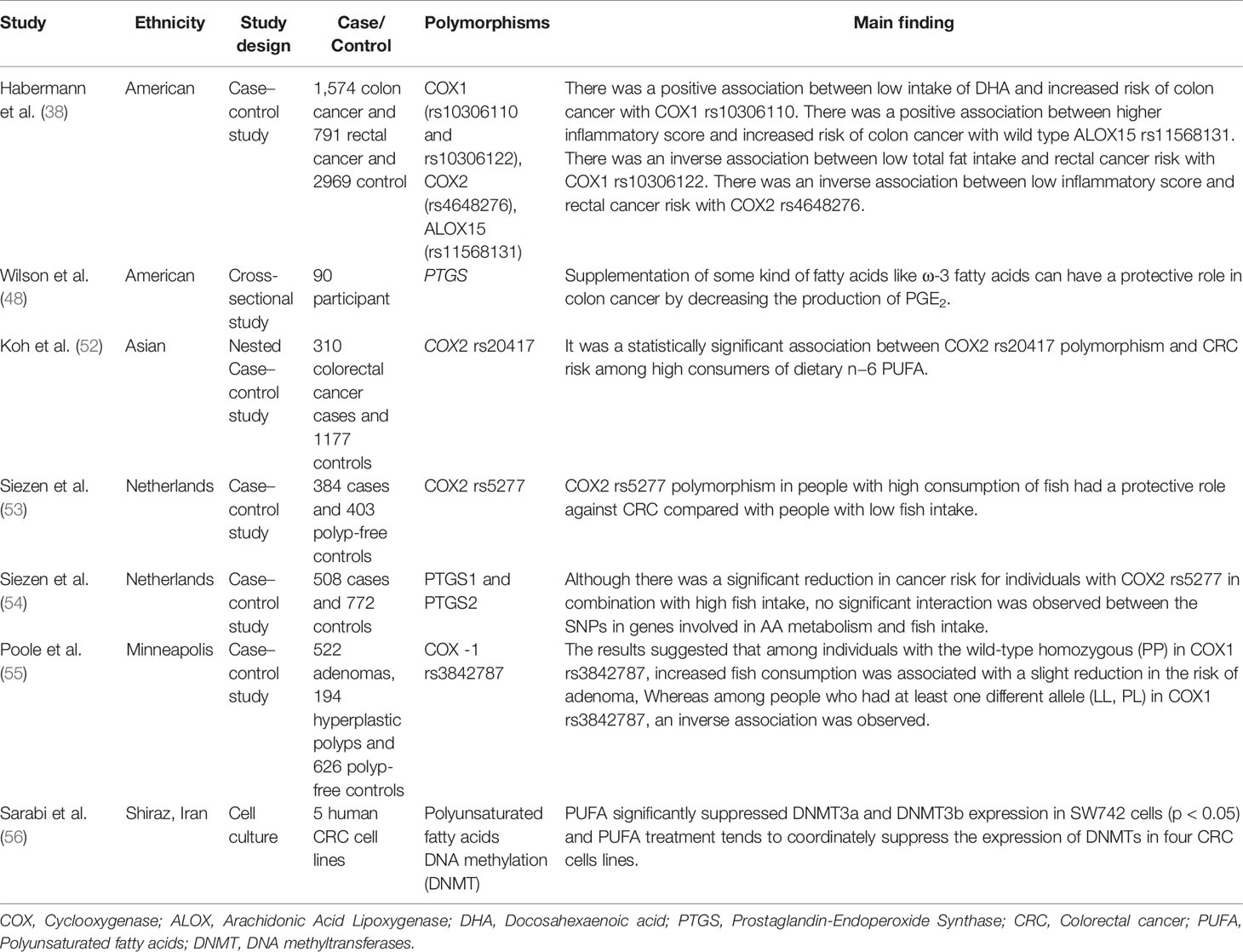
Table 3 Summary of studies regarding interactions between ALOX, COX polymorphism, dietary fatty acid, and CRC risk.
Regarding the interactions between ALOX and COX gene polymorphisms, Siezen et al. reported that these SNPs are associated with colorectal adenoma risk and that these associations are modified by fish consumption. No association was found between SNP rs5277 in the COX2 gene and rs743646 in the ALOX15 gene (53).
Discussion
The results of this study indicated that some SNPs of the ALOX and COX genes can be associated with the interaction between dietary fats and the risk of CRC. The metabolizing effects of ALOX and COX enzymes on AA were reported to be associated with the production of carcinogenic factors in the colon (57). The association between ALOX12 and colorectal neoplasia has been reported (22). However, Goodman et al. found that the ALOX5 gene haplotype, including the rs6413416 and rs4986832 polymorphisms, was associated with a reduced risk of CRC in Caucasians. They assumed that these polymorphisms could augment the binding to the regulatory region of the promoter. Thus, attenuating the enzymatic activity could lead to a lower cancer risk. While this association was not observed in the African-American population, this inconsistency can be related to the existence of effective genetic or environmental factors in the African-American population (8).
Concerning the role of the COX enzyme in CRC risk, it has been reported that COX2 is involved in the early stages of colon cancer development (42). Low COX2 expression is observed in the early stages of colon cancer and COX2 overexpression is more common in the advanced stages of the disease (43). COX2 expression was significantly associated with CRC tumor invasion, tumor location, tumor size, degree of differentiation, and metastasis (45, 58). However, there was no significant relationship between COX2 expression and the histological type of CRC (45). On the other hand, an inverse association was reported between CRC with COX2 expression as well as methylation patterns within the promoter regions of COX2 (42). Regarding the association between CRC and COX2 genotype, some studies found no association between rs20420 and rs5273 polymorphisms of the COX2 gene and CRC risk (8, 59). In contrast, Lin and Schumaf reported the protective effect of COX2 rs689466 and rs5273 polymorphisms against colorectal neoplasms (26, 33), and some other studies reported an increased risk of CRC carriers of some COX2 polymorphisms such as rs689466 and rs20417 (31, 32, 35, 43). These conflicting results of the studies can be due to differences in ethnicity, environmental factors, and tumor type.
The role of polyunsaturated fatty acids (PUFAs) in the prevention of various types of malignancy, such as CRC, has been frequently reported (56). Recent studies found that dietary fatty acids may influence the association between CRC with ALOX and COX genes. For example, Habermann et al. indicated the effects of different fatty acid intake patterns on the association between colon cancer risk and COX1 rs10306110 and ALOX15 rs11568131 polymorphisms and also on the association between rectal cancer risk and COX1 rs10306122 and ALOX12 rs11571339 polymorphisms. They reported a possible increase in CRC risk among those with low intake of the marine sources of n−3 PUFAs such as EPA and DHA in people with a risk allele of COX1 rs10306110 polymorphism (38). The evidence indicates that n−3 LC-PUFA may decrease cancer risk by suppressing oxidative stress, tumor apoptosis, and inflammatory pathways. They can decrease inflammation via the modulation of COX activity and inhibition of arachidonic acid-derived eicosanoids (49–51). It has also been reported that consuming higher n−3 fatty acids may reduce the production of pro-inflammatory eicosanoids, which may be involved in colon cancer (17). In this regard, Wilson et al. reported that ω-3 fatty acid supplementation upregulated COX1 expression and reduced the pro-inflammatory state. Individuals with higher mRNA expression of COX2 after ω-3 fatty acid supplementation had reduced colonic PGE2 (48). The ALOX and COX gene expression level in CRC patients is supposed to be dependent on dietary fats. Koh et al. provided epidemiological evidence for a possible association between the production of prostaglandin n−6 PUFAs through COX2 enzymatic activity and an increased risk of colon cancer. They also showed a significant association between the COX2 rs20417 and colorectal cancer risk among people with a higher intake of n−6 PUFA (52). The ratio of omega-6 fatty acids (as precursors of inflammatory eicosanoids) to omega-3 fatty acids (as precursors of anti-inflammatory eicosanoids) may affect the extent to which the ALOX and COX genes affect colorectal cancer risk. These results highlight the importance of the intake of different types of dietary fats in carriers of the risk alleles of the ALOX and COX genes. However, few studies directly assess this interaction. Also, different factors, such as ethnic and racial differences, may influence the obtained results on the interactions of CRC risk with dietary fats and ALOX and COX genes. Further studies are needed to understand the interaction between dietary fat, genetics, and colorectal cancer. Moreover, other genes involved in enzymatic pathways for synthesizing eicosanoids from dietary fats and possible mechanisms for their relationship with CRC risk should be investigated. If the findings of this review study are confirmed in future longitudinal studies, it could be an important step in providing a specific diet to prevent colorectal cancer, especially in COX and ALOX risk allele carriers.
Conclusion
In conclusion, COX and ALOX genes may play a significant role in CRC risk. Additionally, dietary fats may play an essential role in the effects of the ALOX and COX genes on the risk of CRC. Generally, enhancing the knowledge of nutritional genomics can lead to finding new methods to prevent, treat, and manage CRC. The results of this review article emphasize that environmental factors, such as dietary fat intake, may influence the association between colorectal cancer and the genotype of an individual. If the results are confirmed in future longitudinal studies, the importance of personalized medicine and the recommendation of personalized diets according to the genotype of individuals to prevent colorectal cancer will be further highlighted. Further longitudinal studies in this field of nutritional genomics can lead to the discovery of personalized dietary recommendations for CRC prevention.
Author Contributions
MG, SMD, and AH designed the study, and were involved in the data collection, analysis, and drafting of the manuscript. NM, SA, SA, SP, MNJ, SD, HS, MH, MA, and AA were involved in the design of the study, analysis of the data, and critically reviewed the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
Funding for this study was provided by School of Nutrition and Food Sciences, National Nutrition and Food Technology Research Institute, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code 27846).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Becker N. Epidemiology of Colorectal Cancer. Der Radiologe (2003) 43(2):98–104. doi: 10.1007/s00117-003-0868-9
2. Xi Y, Xu P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Trans Oncol (2021) 14(10):101174. doi: 10.1016/j.tranon.2021.101174
3. Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary Long-Chain N– 3 Fatty Acids for the Prevention of Cancer: A Review of Potential Mechanisms. Am J Clin Nutr (2004) 79(6):935–45. doi: 10.1093/ajcn/79.6.935
5. Vinikoor LC, Millikan RC, Satia JA, Schroeder JC, Martin CF, Ibrahim JG, et al. Trans-Fatty Acid Consumption and Its Association With Distal Colorectal Cancer in the North Carolina Colon Cancer Study Ii. Cancer Causes Control (2010) 21(1):171–80. doi: 10.1007/s10552-009-9447-3
6. Whelan J, McEntee MF. Dietary (N-6) PUFA and Intestinal Tumorigenesis. J Nutr (2004) 134(12):3421S–6S. doi: 10.1093/jn/134.12.3421S
7. Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, et al. Dietary-Fat-Induced Taurocholic Acid Promotes Pathobiont Expansion and Colitis in Il10–/– Mice. Nature (2012) 487(7405):104–8. doi: 10.1038/nature11225
8. Goodman JE, Bowman ED, Chanock SJ, Alberg AJ, Harris CC. Arachidonate Lipoxygenase (ALOX) and Cyclooxygenase (COX) Polymorphisms and Colon Cancer Risk. Carcinogenesis (2004) 25(12):2467–72. doi: 10.1093/carcin/bgh260
10. Doaei S, Kalantari N, Izadi P, Salonurmi T, Mosavi Jarrahi A, Rafieifar S, et al. Changes in FTO and IRX3 Gene Expression in Obese and Overweight Male Adolescents Undergoing an Intensive Lifestyle Intervention and the Role of FTO Genotype in This Interaction. J Transl Med (2019) 17(1):1–8. doi: 10.1186/s12967-019-1921-4
11. Lee SI, Zuo X, Shureiqi I. 15-Lipoxygenase-1 as a Tumor Suppressor Gene in Colon Cancer: Is the Verdict in? Cancer Metastasis Rev (2011) 30(3):481–91. doi: 10.1007/s10555-011-9321-0
12. Melstrom LG, Bentrem DJ, Salabat MR, Kennedy TJ, Ding X-Z, Strouch M, et al. Overexpression of 5-Lipoxygenase in Colon Polyps and Cancer and the Effect of 5-LOX Inhibitors In Vitro and in a Murine Model. Clin Cancer Res (2008) 14(20):6525–30. doi: 10.1158/1078-0432.CCR-07-4631
13. Mashima R, Okuyama T. The Role of Lipoxygenases in Pathophysiology; New Insights and Future Perspectives. Redox Biol (2015) 6:297–310. doi: 10.1016/j.redox.2015.08.006
14. Wang W, Yang J, Zhang J, Wang Y, Hwang SH, Qi W, et al. Lipidomic Profiling Reveals Soluble Epoxide Hydrolase as a Therapeutic Target of Obesity-Induced Colonic Inflammation. Proc Natl Acad Sci (2018) 115(20):5283–8. doi: 10.1073/pnas.1721711115
15. Tanaka K-I, Suemasu S, Ishihara T, Tasaka Y, Arai Y, Mizushima T. Inhibition of Both COX-1 and COX-2 and Resulting Decrease in the Level of Prostaglandins E2 is Responsible for non-Steroidal Anti-Inflammatory Drug (NSAID)-Dependent Exacerbation of Colitis. Eur J Pharmacol (2009) 603(1-3):120–32. doi: 10.1016/j.ejphar.2008.11.058
16. Doaei S, Jarrahi SM, Moghadam AS, Akbari M, Kooshesh SJ, Badeli M, et al. The Effect of Rs9930506 FTO Gene Polymorphism on Obesity Risk: A Meta-Analysis. Biomol Concepts (2019) 10(1):237–42. doi: 10.1515/bmc-2019-0025
17. Hall MN, Chavarro JE, Lee I-M, Willett WC, Ma J. A 22-Year Prospective Study of Fish, N-3 Fatty Acid Intake, and Colorectal Cancer Risk in Men. Cancer Epidemiol Prev Biomarkers (2008) 17(5):1136–43. doi: 10.1158/1055-9965.EPI-07-2803
18. Busstra M, Siezen C, Grubben M, Van Kranen H, Nagengast F, van't Veer P. Tissue Levels of Fish Fatty Acids and Risk of Colorectal Adenomas: A Case–Control Study (Netherlands). Cancer Causes Control (2003) 14(3):269–76. doi: 10.1023/A:1023650201730
19. Tanaka T, Hosokawa M, Yasui Y, Ishigamori R, Miyashita K. Cancer Chemopreventive Ability of Conjugated Linolenic Acids. Int J Mol Sci (2011) 12(11):7495–509. doi: 10.3390/ijms12117495
20. AL-Taie A, Warka’a T, Shabil FYM. Correlation Study of Retinol Binding Protein (-4), Nesfatin and Thyroid Hormones in Colorectal Cancer Iraqi Male Patients. Plant Archives (2020) 20(1):2841–6.
21. Fan W-J, Ding H, Chen X-X, Yang L. All-Trans Retinoic Acid Potentiates Antitumor Efficacy of Cisplatin by Increasing Differentiation of Cancer Stem-Like Cells in Cervical Cancer. Ann Clin Lab Sci (2021) 51(1):22–9.
22. Kleinstein SE, Heath L, Makar KW, Poole EM, Seufert BL, Slattery ML, et al. Genetic Variation in the Lipoxygenase Pathway and Risk of Colorectal Neoplasia. Genes Chromosomes Cancer (2013) 52(5):437–49. doi: 10.1002/gcc.22042
23. Tan W, Wu J, Zhang X, Guo Y, Liu J, Sun T, et al. Associations of Functional Polymorphisms in Cyclooxygenase-2 and Platelet 12-Lipoxygenase With Risk of Occurrence and Advanced Disease Status of Colorectal Cancer. Carcinogenesis (2007) 28(6):1197–201. doi: 10.1093/carcin/bgl242
24. Poole EM, Bigler J, Whitton J, Sibert JG, Potter JD, Ulrich CM. Prostacyclin Synthase and Arachidonate 5-Lipoxygenase Polymorphisms and Risk of Colorectal Polyps. Cancer Epidemiol Prev Biomarkers (2006) 15(3):502–8. doi: 10.1158/1055-9965.EPI-05-0804
25. Ruan G-T, Gong Y-Z, Zhu L-C, Gao F, Liao X-W, Wang X-K, et al. The Perspective of Diagnostic and Prognostic Values of Lipoxygenases mRNA Expression in Colon Adenocarcinoma. OncoTargets Ther (2020) 13:9389. doi: 10.2147/OTT.S251965
26. Lin HJ, Lakkides KM, Keku TO, Reddy ST, Louie AD, Kau IH, et al. Prostaglandin H Synthase 2 Variant (Val511Ala) in African Americans may Reduce the Risk for Colorectal Neoplasia. Cancer Epidemiol Prev Biomarkers (2002) 11(11):1305–15.
27. Cox D, Pontes C, Guinó E, Navarro M, Osorio A, Canzian F, et al. Polymorphisms in Prostaglandin Synthase 2/Cyclooxygenase 2 (PTGS2/COX2) and Risk of Colorectal Cancer. Br J Cancer (2004) 91(2):339–43. doi: 10.1038/sj.bjc.6601906
28. Mosallaei M, Simonian M, Ahangari F, Miraghajani M, Mortazavi D, Salehi AR, et al. Single Nucleotide Polymorphism Rs4648298 in miRNAs Hsa-Mir21 and Hsa-Mir590 Binding Site of COX Gene Is a Strong Colorectal Cancer Determinant. J Gastrointestinal Oncol (2018) 9(3):448. doi: 10.21037/jgo.2017.11.01
29. Ulrich CM, Whitton J, Yu J-H, Sibert J, Sparks R, Potter JD, et al. PTGS2 (COX-2)– 765g> C Promoter Variant Reduces Risk of Colorectal Adenoma Among Nonusers of Nonsteroidal Anti-Inflammatory Drugs. Cancer Epidemiol Prev Biomarkers (2005) 14(3):616–9. doi: 10.1158/1055-9965.EPI-04-0510
30. Hoff JH, Te Morsche RH, Roelofs HM, van der Logt EM, Nagengast FM, Peters WH. COX-2 Polymorphisms-765G→ C and-1195A→ G and Colorectal Cancer Risk. World J Gastroenterol: WJG (2009) 15(36):4561. doi: 10.3748/wjg.15.4561
31. Xing L-L, Wang Z-N, Jiang L, Zhang Y, Xu Y-Y, Li J, et al. Cyclooxygenase 2 Polymorphism and Colorectal Cancer:-765G> C Variant Modifies Risk Associated With Smoking and Body Mass Index. World J Gastroenterol: WJG (2008) 14(11):1785. doi: 10.3748/wjg.14.1785
32. Ueda N, Maehara Y, Tajima O, Tabata S, Wakabayashi K, Kono S. Genetic Polymorphisms of Cyclooxygenase-2 and Colorectal Adenoma Risk: The Self Defense Forces Health Study. Cancer Sci (2008) 99(3):576–81. doi: 10.1111/j.1349-7006.2007.00711.x
33. Shomaf M, Yousef A, Ababna N, Bobali Y. Cyclooxygenase-2 (COX2) Gene Polymorphisms and the Risk of Sporadic Colorectal Cancer and Polyps Among Jordanian Population. Turk J Gastroenterol (2015) 26(2):154–8. doi: 10.5152/tjg.2015.6174
34. Peters WH, te Morsche RH, Roelofs HM, Mathus-Vliegen EM, Berkhout M, Nagengast FM. COX-2 Polymorphisms in Patients With Familial Adenomatous Polyposis. Oncol Res (2009) 17(8):347–51. doi: 10.3727/096504009788428451
35. Pereira C, Queiros S, Galaghar A, Sousa H, Pimentel-Nunes P, Brandao C, et al. Genetic Variability in Key Genes in Prostaglandin E2 Pathway (COX-2, HPGD, ABCC4 and SLCO2A1) and Their Involvement in Colorectal Cancer Development. PloS One (2014) 9(4):e92000. doi: 10.1371/journal.pone.0092000
36. Chakrabarti SK, Cole BK, Wen Y, Keller SR, Nadler JL. 12/15-Lipoxygenase Products Induce Inflammation and Impair Insulin Signaling in 3T3-L1 Adipocytes. Obesity (2009) 17(9):1657–63. doi: 10.1038/oby.2009.192
37. Silverman ES, Drazen JM. Genetic Variations in the 5-Lipoxygenase Core Promoter: Description and Functional Implications. Am J Respir Crit Care Med (2000) 161(supplement_1):S77–80. doi: 10.1164/ajrccm.161.supplement_1.ltta-16
38. Habermann N, Ulrich CM, Lundgreen A, Makar KW, Poole EM, Caan B, et al. PTGS1, PTGS2, ALOX5, ALOX12, ALOX15, and FLAP SNPs: Interaction With Fatty Acids in Colon Cancer and Rectal Cancer. Genes Nutr (2013) 8(1):115–26. doi: 10.1007/s12263-012-0302-x
39. Brenna JT, Salem N, Sinclair AJ, Cunnane SC. α-Linolenic Acid Supplementation and Conversion to N-3 Long-Chain Polyunsaturated Fatty Acids in Humans. Prostaglandins Leukotrienes Essential Fatty Acids (2009) 80(2-3):85–91. doi: 10.1016/j.plefa.2009.01.004
40. Salem AM, Elfeky MA, Nawar N, Alattar AZ, Elekiabi OA, Elaidy MM. Prognostic Value of Combined; Cox-2, Cyclin D1 and P21 Expression in Colorectal Cancer (CRC) Patients: An Immunohistochemical Study. Open J Pathol (2018) 8(3):106–21. doi: 10.4236/ojpathology.2018.83013
41. Wasti H, Shawana S. Comparision Of BRAF V600E, COX–2 and P53 As Biomarkers For The Early Detection Of Colorectal Cancer. J Bahria Univ Med Dental Coll (2019) 9(2):147–50. doi: 10.51985/JBUMDC2019017
42. Ayiomamitis GD, Notas G, Vasilakaki T, Tsavari A, Vederaki S, Theodosopoulos T, et al. Understanding the Interplay Between COX-2 and hTERT in Colorectal Cancer Using a Multi-Omics Analysis. Cancers (2019) 11(10):1536. doi: 10.3390/cancers11101536
43. Joanna B, Jolanta B, Agnieszka G, Diana H-Z, Krystyna S, et al. Linoleic Acid, Arachidonic Acid and COX-2 in Colorectal Cancer Patients in Relation to Disease Stage, Tumour Localisation and Disease Progression. Arab J Gastroenterol (2019) 20(3):121–6. doi: 10.1016/j.ajg.2019.05.007
44. Madhloom ZS, Melconian AK, Mahmood AS. Comparison Of The Expression Levels Of Cox-2 In Colorectal Cancer Tissue And Adjacent Non-Cancerous Tissues (2020) 20(1):3437–43.
45. Labeda I, Uwuratuw JA, Nelwan B, Prihantono P. The Relationship of Cyclooxygenase-2 (COX-2) Expression With Clinical Presentation, Staging, and Degree of Differentiation in Colorectal Cancer. Int J Sci Basic Appl Res (2017) 35(1):64–75.
46. Jin M, Long Z-W, Yang J, Lin X. Correlations of IGF-1R and COX-2 Expressions With Ras and BRAF Genetic Mutations, Clinicopathological Features and Prognosis of Colorectal Cancer Patients. Pathol Oncol Res (2018) 24(1):45–57. doi: 10.1007/s12253-017-0195-5
47. Jiachi M, Zhao X, Jianbo Q, Guo Q, Gong Z, Jianbo D, et al. Expression of PGE2 and COX-2 in Colorectal Cancer and the Clinical Significance. Chin J Gen Surgery (2018) 33(4):322–5.
48. Wilson MJ, Sen A, Bridges D, Turgeon DK, Brenner DE, Smith WL, et al. Higher Baseline Expression of the PTGS2 Gene and Greater Decreases in Total Colonic Fatty Acid Content Predict Greater Decreases in Colonic Prostaglandin-E2 Concentrations After Dietary Supplementation With ω-3 Fatty Acids. Prostaglandins Leukotrienes Essential Fatty Acids (2018) 139:14–9. doi: 10.1016/j.plefa.2018.11.001
49. Yurko-Mauro K, Van Elswyk M, Teo L. A Scoping Review of Interactions Between Omega-3 Long-Chain Polyunsaturated Fatty Acids and Genetic Variation in Relation to Cancer Risk. Nutrients (2020) 12(6):1647. doi: 10.3390/nu12061647
50. Cockbain AJ, Volpato M, Race AD, Munarini A, Fazio C, Belluzzi A, et al. Anticolorectal Cancer Activity of the Omega-3 Polyunsaturated Fatty Acid Eicosapentaenoic Acid. Gut (2014) 63(11):1760–8. doi: 10.1136/gutjnl-2013-306445
51. Mauermann J, Pouliot F, Fradet V. Dietary Omega-3 Fatty Acids, Genetic Variation and Risk of Breast and Prostate Cancers. Healthy Agriculture Healthy Nutrition Healthy People (2011) 102:156–71. doi: 10.1159/000327805
52. Koh W, Yuan J, Van Den Berg D, Lee H, Yu M. Interaction Between Cyclooxygenase-2 Gene Polymorphism and Dietary N-6 Polyunsaturated Fatty Acids on Colon Cancer Risk: The Singapore Chinese Health Study. Br J Cancer (2004) 90(9):1760–4. doi: 10.1038/sj.bjc.6601797
53. Siezen CL, van Leeuwen AI, Kram NR, Luken ME, van Kranen HJ, Kampman E. Colorectal Adenoma Risk Is Modified by the Interplay Between Polymorphisms in Arachidonic Acid Pathway Genes and Fish Consumption. Carcinogenesis (2005) 26(2):449–57. doi: 10.1093/carcin/bgh336
54. Siezen CL, Bueno-de-Mesquita HB, Peeters PH, Kram NR, van Doeselaar M, van Kranen HJ. Polymorphisms in the Genes Involved in the Arachidonic Acid-Pathway, Fish Consumption and the Risk of Colorectal Cancer. Int J Cancer (2006) 119(2):297–303. doi: 10.1002/ijc.21858
55. Poole EM, Bigler J, Whitton J, Sibert JG, Kulmacz RJ, Potter JD, et al. Genetic Variability in Prostaglandin Synthesis, Fish Intake and Risk of Colorectal Polyps. Carcinogenesis (2007) 28(6):1259–63. doi: 10.1093/carcin/bgm026
56. Sarabi MM, Naghibalhossaini F. The Impact of Polyunsaturated Fatty Acids on DNA Methylation and Expression of DNMTs in Human Colorectal Cancer Cells. Biomed Pharmacother (2018) 101:94–9. doi: 10.1016/j.biopha.2018.02.077
57. Austin Pickens C, Yin Z, Sordillo LM, Fenton JI. Arachidonic Acid-Derived Hydroxyeicosatetraenoic Acids Are Positively Associated With Colon Polyps in Adult Males: A Cross-Sectional Study. Sci Rep (2019) 9(1):1–10. doi: 10.1038/s41598-019-48381-0
58. MA J, Zhao X QIJ, Guo Q, Gong Z, DU J, et al. Expression of PGE2 and COX-2 in Colorectal Cancer and the Clinical Significance. Chin J Gen Surg (2018), 322–5.
Keywords: colorectal cancer, polymorphism, dietary fat, lipoxygenase, cyclooxygenase
Citation: Gholamalizadeh M, Majidi N, Tajaddod S, Abdollahi S, Poorhosseini SM, Ahmadzadeh M, Naimi Joubani M, Dahka SM, Shafaei H, Hajiesmaeil M, Alizadeh A, Doaei S and Houshiar-Rad A (2022) Interactions of Colorectal Cancer, Dietary Fats, and Polymorphisms of Arachidonate Lipoxygenase and Cyclooxygenase Genes: A Literature Review. Front. Oncol. 12:865208. doi: 10.3389/fonc.2022.865208
Received: 29 January 2022; Accepted: 26 May 2022;
Published: 19 July 2022.
Edited by:
Lisardo Bosca, Autonomous University of Madrid, SpainReviewed by:
Rocío Brea Contreras, Autonomous University of Madrid, SpainZaki A. Sherif, Howard University, United States
Copyright © 2022 Gholamalizadeh, Majidi, Tajaddod, Abdollahi, Poorhosseini, Ahmadzadeh, Naimi Joubani, Mirzaei Dahka, Shafaei, Hajiesmaeil, Alizadeh, Doaei and Houshiar-Rad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saeid Doaei, RG9hZWlAZ3Vtcy5hYy5pcg==; Anahita Houshiar-Rad, c2RvYWVpQHNibXUuYWMuaXI=
 Maryam Gholamalizadeh1
Maryam Gholamalizadeh1 Saeid Doaei
Saeid Doaei