- 1Department of Microbiology, Jagannath University, Dhaka, Bangladesh
- 2Department of Genetic Engineering and Biotechnology, Faculty of Biological Science and Technology, Jashore University of Science and Technology (JUST), Jashore, Bangladesh
- 3ABEx Bio-Research Center, Dhaka, Bangladesh
- 4Biochemistry and Molecular Biology department, Life Science faculty, Bangabandhu Sheikh Mujibur Rahman Science and Technology University, Gopalgonj, Bangladesh
- 5Biotechnology, University of Pécs, Medical School, Pécs, Hungary
- 6Department of Biotechnology and Genetic Engineering, Faculty of Life Science, Bangabandhu Sheikh Mujibur Rahman Science and Technology University, Gopalganj, Bangladesh
- 7Department of Microbiology, Chittagong University, Chittagong, Bangladesh
- 8Department of Pharmacy, Begum Gulchemonara (BGC) Trust University Bangladesh, Chittagong, Bangladesh
- 9Department of Pharmacy, Faculty of Allied Health Sciences, Daffodil International University, Dhaka, Bangladesh
- 10Laboratory of Pharmaceutical Biotechnology and Bioinformatics, Department of Genetic Engineering and Biotechnology, Jashore University of Science and Technology, Jashore, Bangladesh
- 11Korean Medicine (KM) Convergence Research Division, Korea Institute of Oriental Medicine, Daejeon, South Korea
- 12Plasma Bioscience Research Center, Kwangwoon University, Seoul, South Korea
- 13Global Biotechnology & Biomedical Research Network (GBBRN), Department of Biotechnology and Genetic Engineering, Faculty of Biological Sciences, Islamic University, Kushtia, Bangladesh
- 14Department of Pathology, College of Korean Medicine, Kyung Hee University, Seoul, South Korea
- 15Korean Medicine-Based Drug Repositioning Cancer Research Center, College of Korean Medicine, Kyung Hee University, Seoul, South Korea
Vaginal cancer is a rare and uncommon disease that is rarely discussed. Although vaginal cancer traditionally occurs in older postmenopausal women, the incidence of high-risk human papillomavirus (HPV)-induced cancers is increasing in younger women. Cervical cancer cells contain high-risk human papillomavirus (HPV) E6 and E7 proteins and inhibiting HPV gene expression leads the cells to stop proliferating and enter senescence. As E6, and E7 protein promoted the carcinogenesis mechanism, and here not only regulate the cellular degradation of P53, and pRb but also enhances the cell proliferation along with E6 protein targets the p53 for breakdown and subsequently promote the apoptotic cell death, and DNA repair inhibition, that is indispensable to the continue the lifecycle of the HPV. As a synchronous or metachronous tumor, vaginal cancer is frequently found in combination with cervical cancer. It is uncertain what causes invasive female vaginal organ cancer. HPV type 16 is the most often isolated HPV type in female vaginal organ cancers. Due to cancer’s rarity, case studies have provided the majority of etiologic findings. Many findings demonstrate that ring pessaries, chronic vaginitis, sexual behavior, birth trauma, obesity, vaginal chemical exposure, and viruses are all risk factors. Because of insufficient understanding and disease findings, we are trying to find the disease’s mechanism with the available data. We also address different risk factors, therapy at various stages, diagnosis, and management of vaginal cancer in this review.
Introduction
Cervical, endometrial, or vulvar cancer causes primary or recurring illness in the vaginal area (1, 2). The size and location of the original tumor in the vaginal canal, histologic type and grade, lymphatic vascular penetration, regional lymph node metastasis, and previous therapy are all critical factors in determining the prognosis (3–6). Because of the tumor’s closeness to the rectum, bladder, and urethra, vaginal cancers are seldom amenable to curative organ-sparing surgery (4, 7). Vaginal cancer is commonly encountered in conjunction with cervical cancer as a synchronous or metachronous tumor (8, 9). Furthermore, vaginal cancer has been reported to develop the following hysterectomy for cervical cancer (10), and newer reports have found condylomatous lesions in vaginal cancer tissue, as well as the presence of human papillomavirus antigens and/or DNA (11, 12). These findings have led to speculation that vaginal and cervical cancers may share etiologic characteristics (13).
Vaginal cancer is an uncommon gynecological cancer, accounting for just 1-2 percent of all gynecological cancers in developed countries (14, 15). Patients with canal cancer have a prognosis that is determined by their age, as well as the microscopic architecture and stage of the tumor (16). Only after the elimination of cervical, urethral, or vulvar origins should cases be categorized as vaginal carcinomas, according to the International Federation of Gynecology and Obstetrics (FIGO) (15). Because stage III studies have not been conducted because of the rarity of vaginal cancer. Based on retrospective or comparative research, current guidelines have been developed. This circumstance justifies the wide range of treatments available to women who have been impacted by those who suffer from this condition are subjected to it (17).
The genital organ is made up of the internal genitalia of a woman. Eighty-five percent to ninety percent of invasive female vaginal carcinomas are epithelial cell carcinomas. Glioma, basal cell malignant neoplastic illness, sarcoma, skin cancer, and undifferentiated malignant neoplastic disease are the remaining five hitters to fifteen (18). Primary duct carcinoma accounts for around 3% of all cancers of the female reproductive system. Squamous cell carcinoma in situ or invasive cancer of the vaginal canal affects around 1 in 100,000 women. Only two population-based case-control studies have been undertaken to establish the etiology of this disease due to its rarity (13). In the United States, about 3,000 cases are identified each year, with approximately 900 fatalities. Ductal carcinomas are becoming more common in young women, owing to precarious HPV infection (19, 20). The channel is a frequent website of pathologic process medicine cancer and its pathologic process or direct extension of non-gynecologic tumors to the channel also can occur from the bladder, urethra, rectum, and barely the breast, lung, or alternative sites (21, 22). A 35% risk of secondary malignancies spreading to other organs, including large intestine, anal, bladder, vulvar, and skin cancers, was discovered in one study of a woman who had girdle radiation. According to the findings of several research studies, Collins and Barclay identified seven vaginal malignancies during pregnancy that were accessible for extensive examination in 1963 (23). Likewise, additional five cases have also been reported in several research studies (24–28). Because vaginal squamous cell carcinoma in pregnancy is such an uncommon occurrence, discussing survival and prognosis is challenging. Only four individuals with vaginal squamous cell carcinoma during pregnancy survived, according to the literature. Eight of the patients showed a quick progression of symptoms. Three of the four survivors reacted well to an interstitial radium needle, while the other received selective pelvic lymph node dissection followed by concomitant cisplatin chemoradiation. Only three patients had a healthy baby as a result of their pregnancy (23).
Between 1968 and 1974, no cases of duct cancer were recorded in persons who were followed up on (29). After menopause or giving delivery, the majority of women with vaginal cancer report vaginal bleeding. Patients with prevaginal cancer are more prone to experience bladder discomfort and frequent contractions. Pelvic discomfort can also be caused by infections that extend outside the vaginal canal (20). The study gives an overview of epidemiological data on vulvar and vaginal malignancies in Germany. Morbidity, prevalence, therapeutic management, and survival data are seldom consistent. The New England Journal of Public Health published the study. The most frequent precancerous activity of the vulva is neoplasia in the epithelium. After doubling in the 1980s, this rate has been steadily growing since the mid-1970s (30). This rise can be attributed to several factors, including early intercourse and promiscuity. Squamous cell carcinoma (excluding 1.1% of sarcomas) accounts for 88% of vulvar malignancies, whereas melanoma accounts for 7.5%. The remaining 4.5% is made up of adenocarcinomas, multiple entities, and other malignant tumors (31, 32). Adenocarcinomas differ from squamous disease in that they commonly metastasis to the lung, supraclavicular, and pelvic nodes, and have a peak incidence between 17 and 21 years of age. Clear cell adenocarcinomas are uncommon and mostly affect people under the age of 30. They are often linked with adenosis. In women who have been exposed to DES, adenosis is the most prevalent histologic abnormality (33). Squamous cell vaginal carcinomas spread superficially at first, but many women develop metastatic illness as a result. The lungs and the liver are common sites of distant metastases (10). Squamous cell carcinoma is diagnosed at a rate of 1.35 to 1.5 per 100,000 people in the United States. Germany’s Robert Koch Institute (RKI) and the Registry of Cancer Epidemiology approximated data from 2004 reveal a new gynecological cancer (31, 34). The incidence of cancer of the female genitals will increase with age and the height incidence is seasoned between sixty and seventy-four years more matured (31). The remaining 5% to 15% are glioma, basal cell malignant neoplastic illness, sarcoma, malignant melanoma, and dedifferentiated malignant neoplastic disease. In 2011, 4340 women in the United States were diagnosed with invasive female genital organ cancer (18). The underlying cause of invasive female genital organ cancer is unknown. In female vaginal organ malignancies, HPV type 16 is the most isolated HPV type. Women under the age of 40 are more likely to be diagnosed with breast cancer (18, 35, 36). This is why most patients are over 60 years old (16). Although there is evidence that squamous cell carcinoma rates rise with age and are greater in blacks than whites (37, 38), few additional risk factors have been found. Because of the disease’s rarity, the majority of etiologic insights have come from case studies. These studies suggest that vaginal damage from ring pessaries, chronic vaginitis, sexual behavior, birthing trauma, obesity, exposure to chemicals in the vagina, and viruses are all risk factors (13).
The works on this topic are insufficient. There is no study where we found the mechanisms, therapy, risk factors, and so on all at once. We try to focus on the processes, kinds, risk factors, therapy at various stages, diagnosis, and management of vaginal cancer in this paper.
Diversifications of Vaginal Cancer
Generally, in the human body, there are two primary types of vaginal cancer. We have described these types in the following.
Squamous Cell Carcinoma
According to the research published in Annals of Internal Medicine, the age-adjusted incidence of invasive squamous cell carcinoma in black women was 72% higher in white women and 34% lower in Asian/Pacific Islander women. The most frequent histological malignancy in women was squamous cell cancer, which accounted for 71% of in situ and 66% of invasive cases. In situ cancer rates peaked around 70 years old and then began to fall, although they stayed steady or rose as people became older (39).
The most prevalent kind of vaginal cancer is squamous cell carcinoma. The thin, flat epithelial cells that line the vaginal surface give rise to these malignancies (40, 41). Squamous cell carcinomas account for around 80–90% of all primary vaginal malignancies (21, 42). Squamous cell carcinoma can be classified as differentiated [G1], moderately differentiated [G2], or poorly differentiated or undifferentiated [G3] on the basis of histological findings (43). They have a sluggish growth rate and may be caused by a precancerous disease called vaginal intraepithelial neoplasia (VAIN) (10).
Vaginal intraepithelial neoplasia [VAIN] can be a precursor to squamous cell carcinoma of the vaginal mucosa, although its real malignant potential is uncertain (21, 39, 44, 45). VAIN has been found in women who have had previous gynecological cancer radiation (46). When compared to VAIN that is not linked with radiation, this kind of VAIN appears to be more prone to return following surgical excision or ablation, and to develop to invasive malignancy (39).
Adenocarcinoma
Adenocarcinoma is the most common kind of vaginal cancer, accounting for around 15% of all cases (47). These malignancies start in the vaginal gland cells. They are more common in women over 50, but they can also happen in women whose mothers were exposed to diethylstilbestrol (DES) during pregnancy (48, 49). In 1971, a link was discovered between in-utero exposure to diethylstilbestrol during pregnancy and an increased risk of clear cell adenocarcinoma of the vaginal mucosa in young women. The typical age at diagnosis is 19 years, and 90% of patients are diagnosed with stage I–II illness. Neither the use of contraception nor pregnancy is linked to an increased risk of this malignancy. Endometriosis is the most common cause of vaginal endometrioid radiation (50, 51).
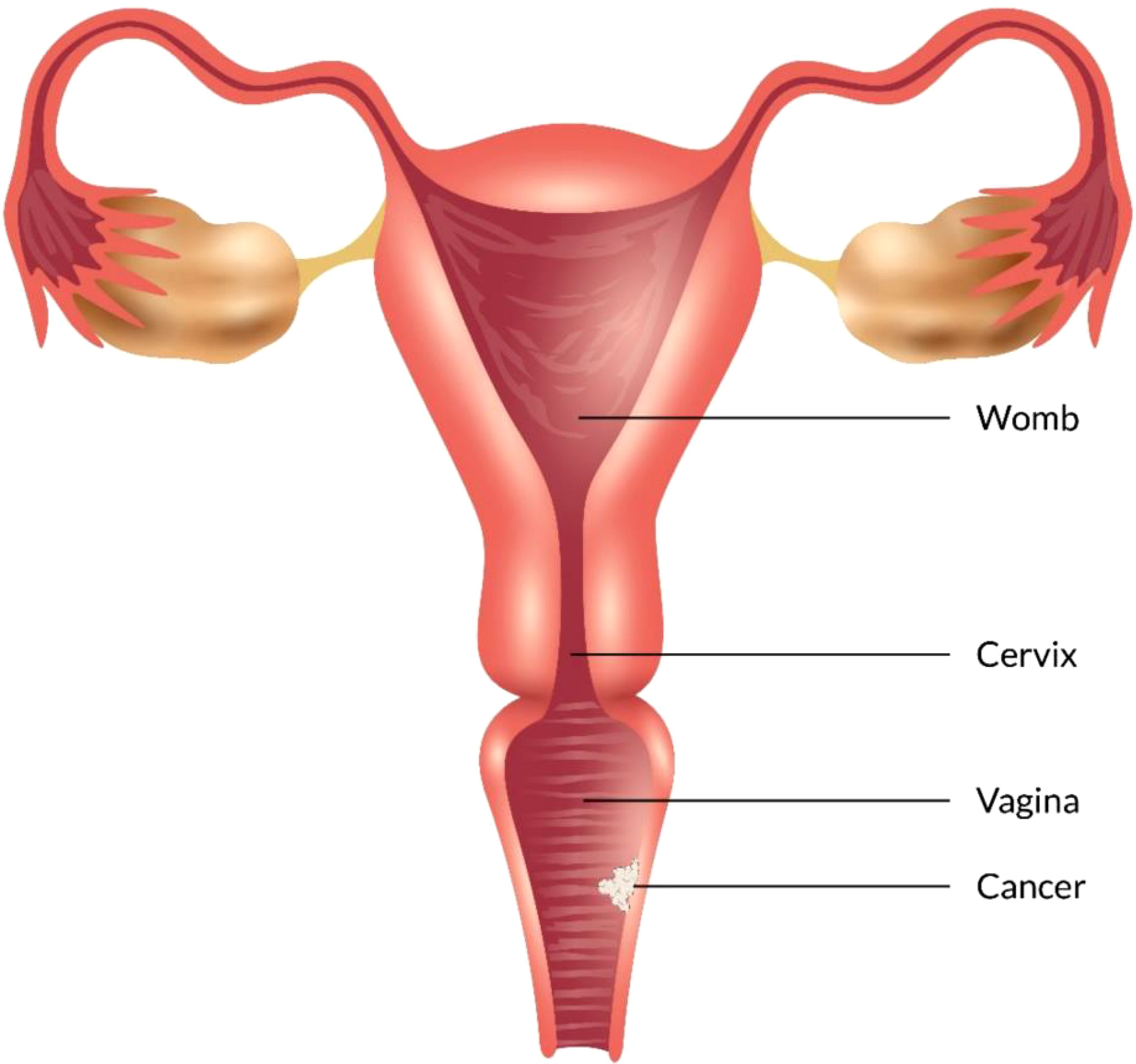
Some types of cancer, such as endometriosis and testicular cancer, can develop in the vagina. Certain cancers may spread to the vagina from their original site - such as uterine, cervical, rectal, or bladder cancers (Figure 1). These cancers are treated according to the primary cancer type (52, 53).
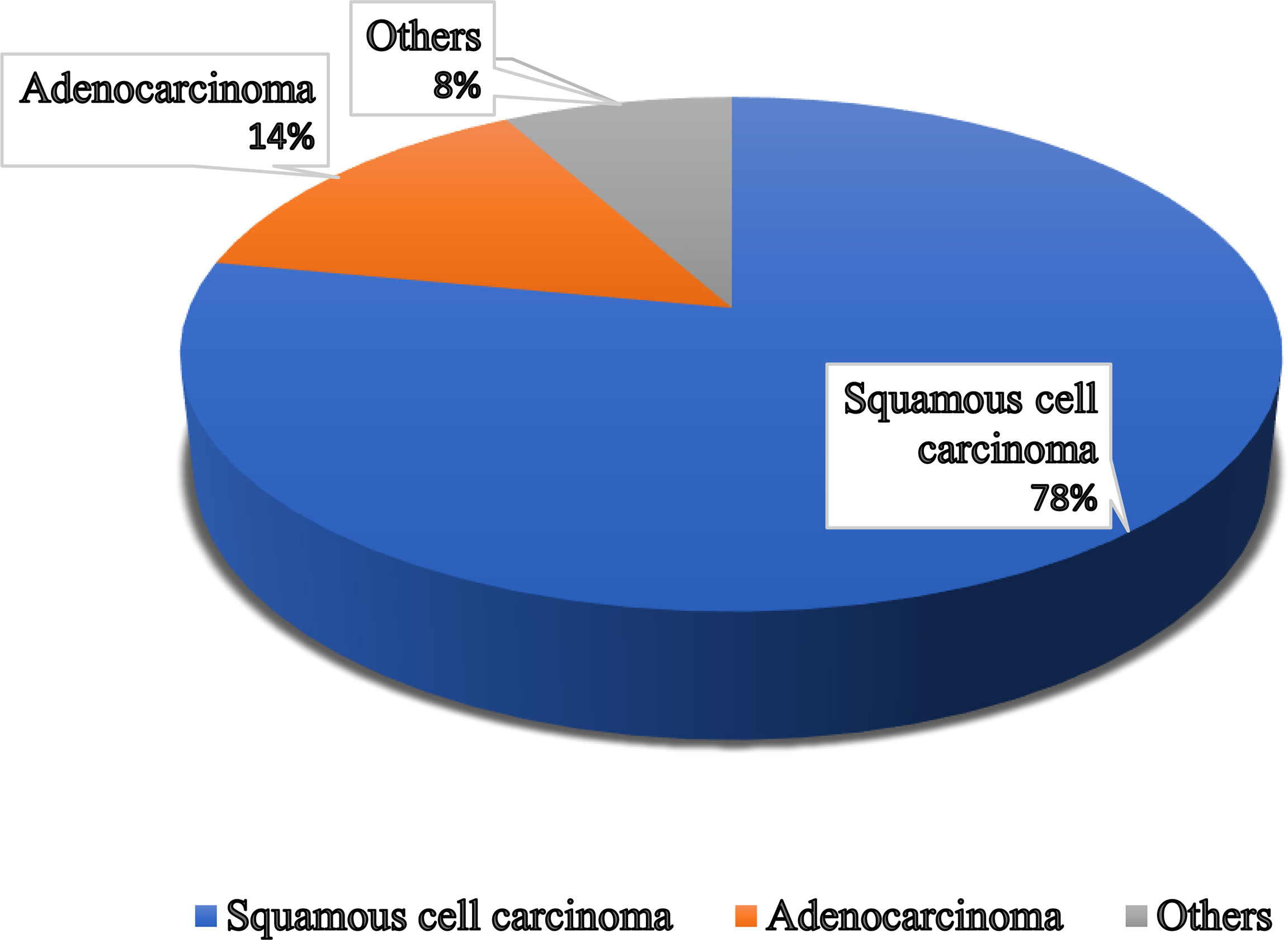
Figure 1 Different types of vaginal cancer and their infection rate. Various types of vaginal cancer are found in different parts of the vagina. Among them, squamous cell carcinoma, which is linked to the vaginal surface area and arises from epithelial cells, is the most prominent type of vaginal cancer. Adenocarcinoma is also found as a common type of vaginal cancer, which is responsible for 15% of all vaginal cancers. Besides, endrometriosis, testicular and some other types of cancer are noticed in the vaginal area.
Overview of Vaginal Cancer Development Stage
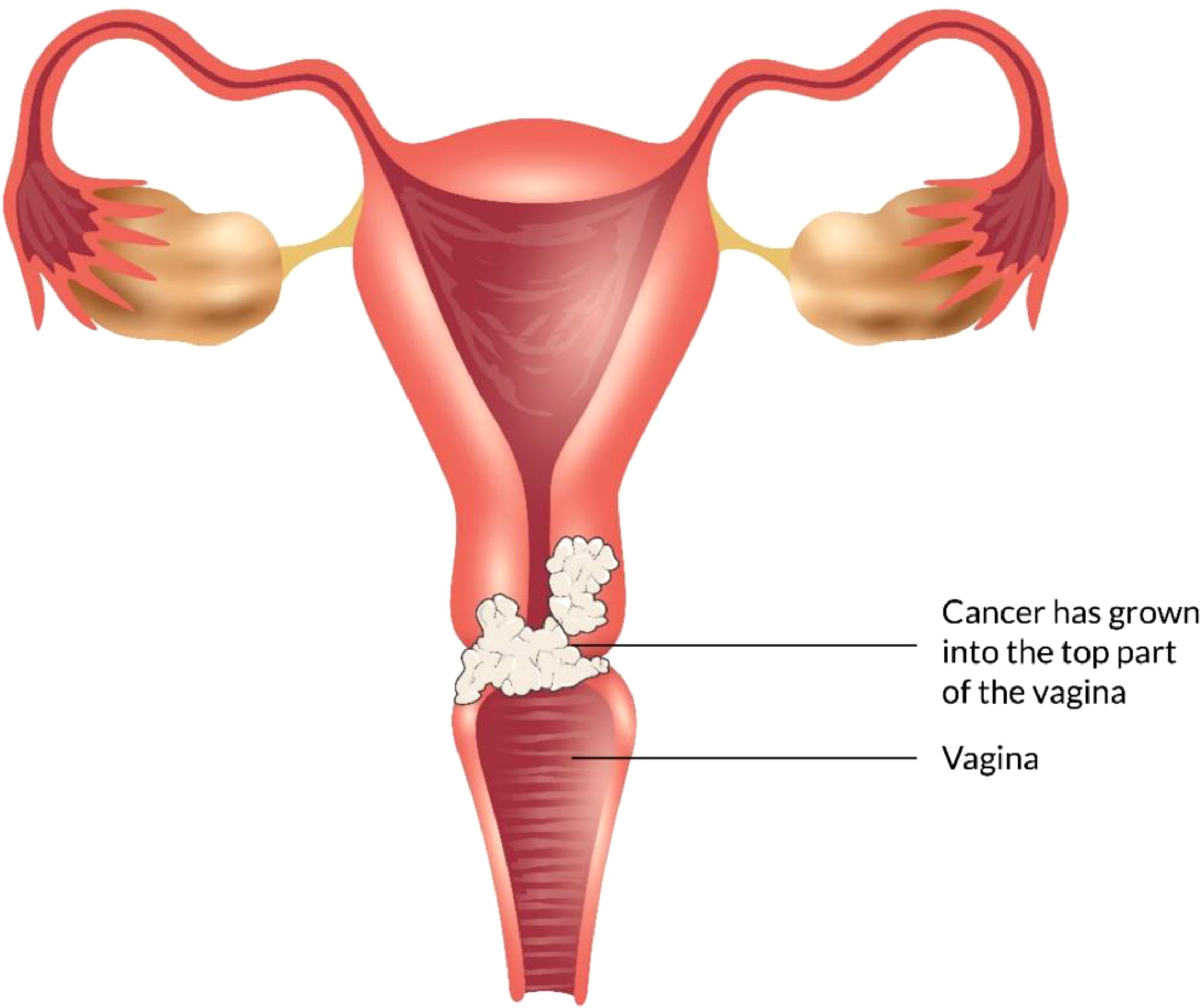
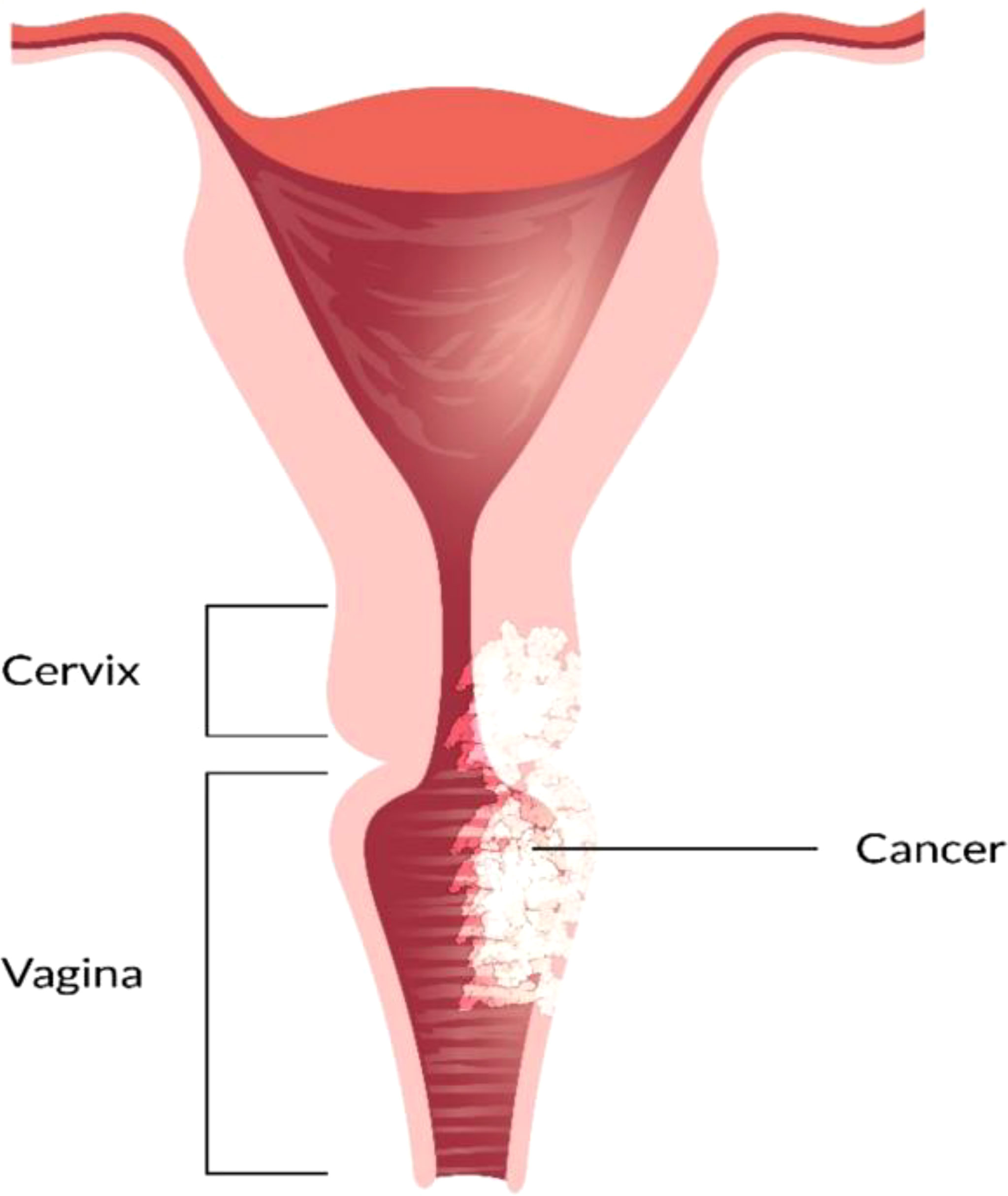
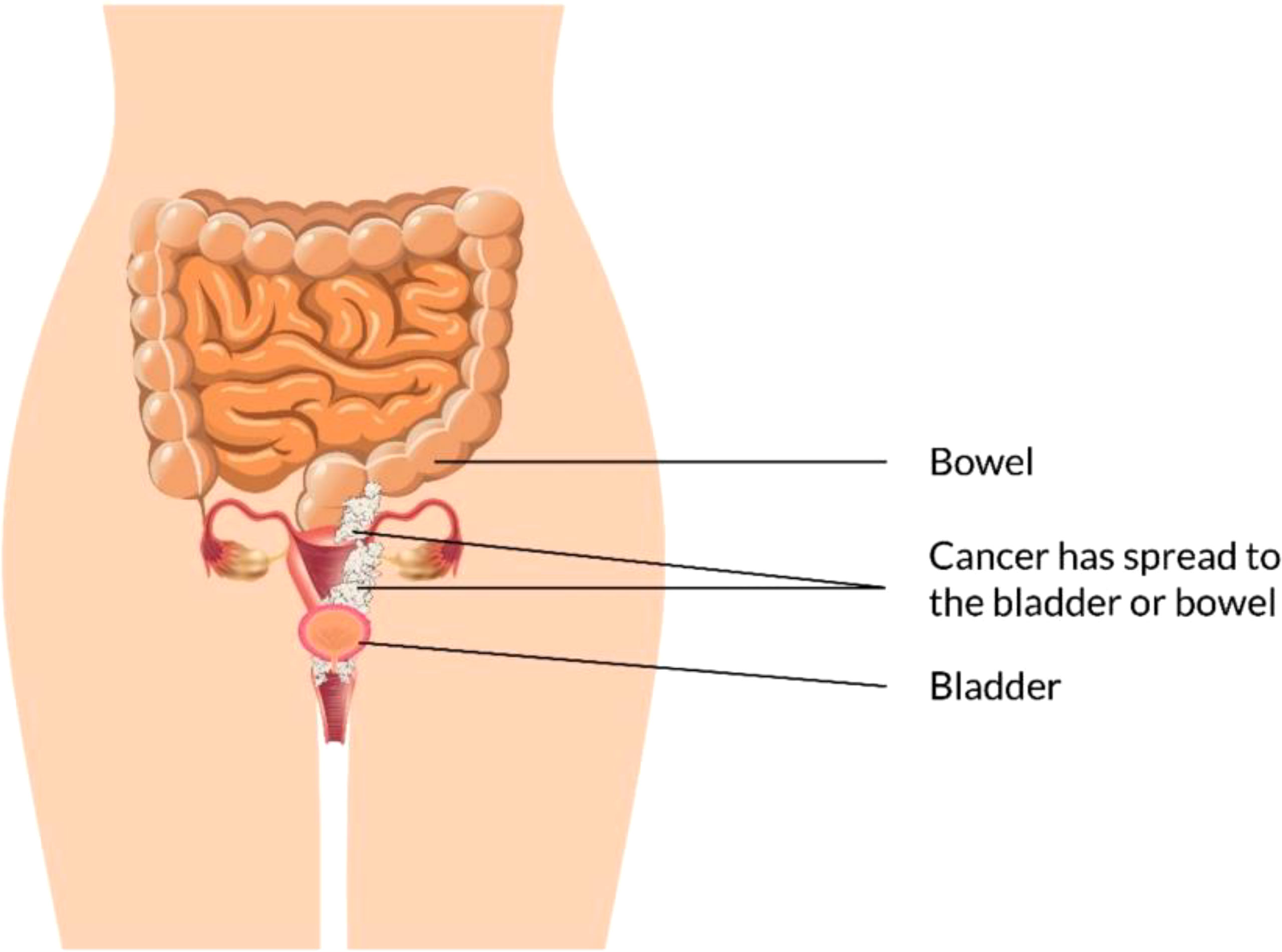
The vagina is a 3- to a 4-inch fibromuscular tube that runs from the lower side of the cervix to the vulva. It is useful for determining the location of tumors and lymphatic drainage (54). Lymph nodes are frequently involved in vaginal cancer, even in the early stages (55). The FIGO and the American Joint Committee on Cancer have developed recommendations for staging and classifying vaginal cancer (Figure 2) (56). Clinical parameters are used to determine the stage of vaginal cancer. Stage I cancer is restricted to the vaginal wall; Stage II cancer has spread to the sub-vaginal tissue but not to the pelvic wall; Stage III cancer has spread to the pelvic wall, and Stage IV cancer has spread beyond the true pelvis or has reached the bladder or rectum mucosa (15, 20).
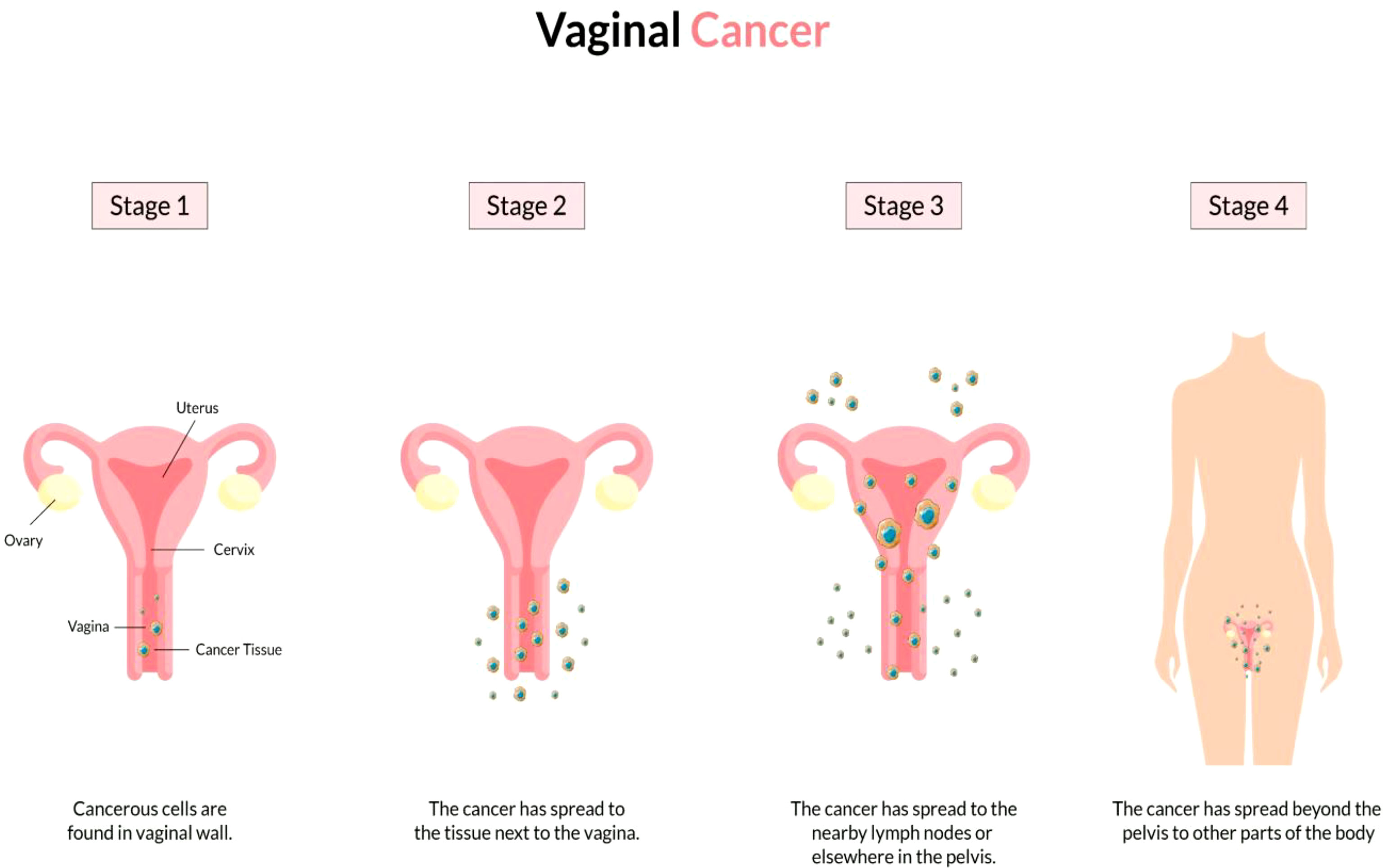
Figure 2 Overview of vaginal cancer and stages. Vaginal cancer is a rare kind of cancer that affects the vaginal area. The cells that line the vaginal surface are the most often affected by vaginal cancer. When found early on, vulvar cancer is generally treatable. Treatment for vaginal cancer that has progressed beyond the vagina is significantly more challenging. Stage I- Only the vulva or perineum (area between the anus and vulva) have cancerous cells. Stage II- Cancer has progressed to the urethra, anus, or vaginal region. Stage III- The malignancy has progressed to the lymph nodes in the surrounding area. Stage IV- Cancer has spread to other regions of the body from the lymph nodes.
Molecular Mechanisms of E6 and E7 Overexpression Due to Integration of Human Papilloma Virus in Vaginal cancer
Vaginal cancer is a multi-stage process that is developed by the accumulation of DNA changes of host cell genes. Oncogenes undergo epigenetic and genomic alterations as a result of these variations. The tumor suppressor genes that regulate cell cycle progression, chromosomal stability, telomeres, and apoptosis. However, the key step for carcinogenesis seems to be to integrate the viral genome into the human.
Human papillomavirus (HPV) enters the vagina and causes vaginal cancer. In HPV-mediated carcinogenesis, integrating HPV DNA into the host cell genome is crucial, resulting in abnormal proliferation and evil development (57, 58). This integration of viral DNA affects the host genome by promoting oncogenes and disrupting genes, leading to inter and intra chromosome rearrangements. Two tumor suppressor genes, such as p53 and pRb, maintain the cell’s normal proliferative function. Still, the problem is that when human papillomavirus enters the cell, E6 and E7 genes play a crucial role in interfering with the tumor suppressor gene’s function (59). The interaction between E7 and pRb protein leads to its breakdown and an abnormal start of the S-phase and the E2F transcriptional factor’s discharge that stimulates cyclones and other regulatory agents (60, 61). E6 and E7 induced carcinogenesis mechanism does not only regulate the cellular degradation of two tumor suppressor genes but also enhances the cell proliferation, and simultaneously. E6 protein targets p53 for breakdown and results in apoptotic cell death as well as DNA repair inhibition, which are essential to the entire lifecycle of the HPV (62). This is crucial for vaginal cancer development because it impedes the efficiency of oxidative stress to the DNA and enables secondary mutations to accumulate.
The intermolecular interaction between E6 and E7, known as the HPV interactome, regulates the gene expression profile and intracellular signaling pathways and changes the structure of epithelial cells (63). E6 also binds to and degrades the FAS-associated death domain protein (FADD), inhibiting Fas-mediated apoptosis (64). All these molecular changes facilitate the resistance to programmed cell death. Caspases are the main molecular participants in the programmed cell death regulatory network. Therefore, the continued action of proteins E6 and E7 causes abnormal cell proliferation, mutation of the oncogene, and eventually vaginal cancer (65, 66).
Analysis of the molecular mechanism of HPV integration and overview of E6/E7 overexpression Integration usually leads to greater expression and consistency of viral oncogenic transcripts (E6 and E7) that are recognized to inactivate or speed up the degradation of diverse cellular proteins, such as the retinoblastoma protein (E7) and p53 (E6). The integration site is dispersed over the entire genome as chromosomally fragile areas where DNA cracks in double strands are not repaired (67). Integration begins with DNA strand breaks caused by oxidative stress or HPV protein, followed by DNA damage responses. HPV DNA breaks are initiated when the virus is replicated, and these breaks cannot be remedied. This injury response channel is used by HPV to generate a sufficient number of episomal HPVs, which enhance the presence of more HPV DNA for inclusion into the host DNA. At the time of DNA breaking, some DNA damage reaction (DDR) stimulates the deposition of replication factors in the replication focal area and acts as an incentive for viral replication (68). The virus uses DDR machinery to boost viral amplification, while the oncoprotein of the virus makes the cells cope with harmful downstream effects. When CDKs inhibitors (P21, P27) and P53 protein are inhibited, E6 and E7 oncoproteins damage the checkpoint of the cell cycle. The oncoprotein PV-16 E7 mitigates the reaction of the DNA damage control point by boosting up the claspin proteolytic revenue during ATR/CHK1 signaling and DNA damage control point retrieval in the cell cycle at phase G2 (Figure 3) (69).
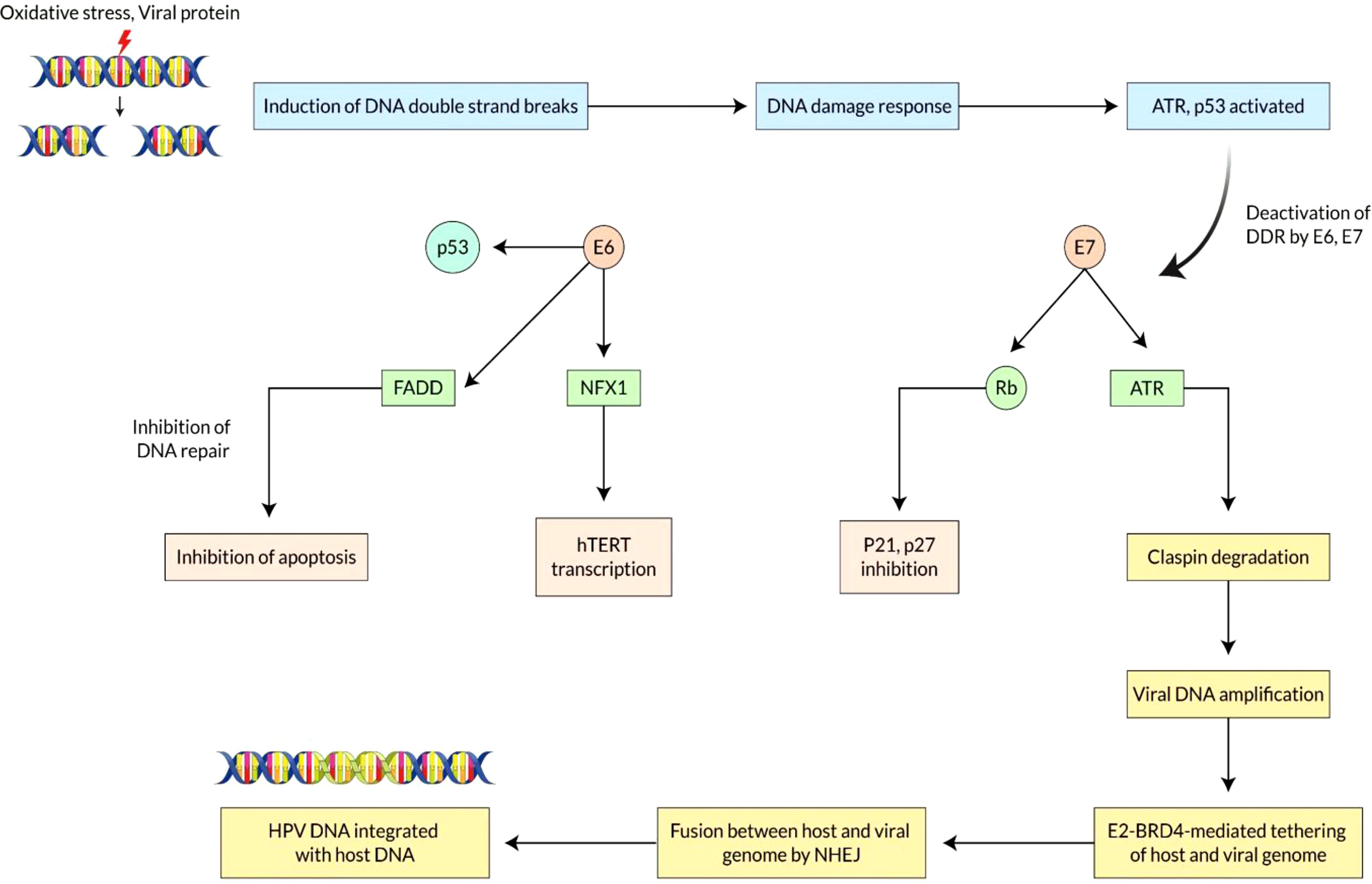
Figure 3 Mechanisms of E6 and E7 overexpression due to integration of Human Papilloma Virus in Vaginal cancer. Vaginal cancer is a multi-stage process that is developed by the accumulation of DNA changes of host cell genes. Integrating HPV DNA into the host cell genome is crucial, resulting in abnormal proliferation and development. E6 and E7 genes play a crucial role in interfering with the tumor suppressor gene’s function. The continued action of proteins E6 and E7 causes abnormal cell proliferation, mutation of the oncogene, and eventually vaginal cancer. The integration site is dispersed over the entire genome as chromosomally fragile areas where DNA cracks in double strands are not repaired. HPV DNA breaks are initiated when the virus is replicated, and these breaks cannot be remedied. When CDKs inhibitors (P21, P27) and P53 protein are inhibited, E6 and E7 oncoproteins damage the checkpoint of the cell cycle. DNA damaging reaction pathways regulate fusion between the two genomes by homologous or heterologous recombination. HPV genome directly binds to the host chromosome via HPVE2-BRD4 complex for dividing genomes into daughter cells.
Moreover, E2-BRD4-mediated therapies could enhance the viability of viral inclusion into the host genomes. DNA damaging reaction pathways such as ATM/ATR and DNA PK pathway regulate fusion between the two genomes by homologous or heterologous recombination (70). The combination of tandem arrangement in the vaginal cancer cell’s chromosome shows that a linear concentric HPV genome is produced by a rolling-cycle replication mechanism integrated into the host genome (71). It has already been revealed that only crumbled replication fork during the replication of viral genomes is created by homologous recombination machines recruited to the territories of dual strand breaks. HPV genome directly binds to the host chromosome via HPVE2-BRD4 (Bromodomain protein 4) complex for dividing genomes into daughter cells (72). The link between BRD4 and the fragile chromosomal area indicates that BRD4 can significantly enhance mechanistic assimilation viability. At last, the destruction of E2 ORF throughout inclusion led to increased expression of E6 and E7 viral oncogenes, leading to disruption of the critical cell genome and causing cellular death.
Risk Factors
The most frequently detected types of HPV can be found in most vaginal tumors and HPV 16 (73, 74). Nearly 60% of the intrusive cell carcinomas and 80% to 90% of in situ, constitute HPV-DNA. The threat of in-situ tumors will double with HPV18. Invasive carcinoma probability is approximately six times increasing with the existence of HPV 16 antibodies. The health issues for vaginal intraepithelial neoplasia (VAIN) or vaginal cancer, particularly HPV-16 infection, were associated in a community retrospective analysis with 156 patients (75). The probability of in situ vaginal cancer has been reported to enhance almost six times by genital warts, but the connections with infective tumors are less clear (76). There has also been an improved risk for persons with an earlier SCC, which may be associated with HPV infection (77). About 30% of patients identified with preliminary stages have suffered before five years from invasive vaginal cancer (78). It has been reported that radiation treatment could occupy a part in the development of intermediate vaginal cancer in youth patients. There have also been higher chances for vaginal cancer for female patients with cancers in plenty of different anogenital locations or with an anogenital familial history (79). Lower educated communities and individuals living under economic conditions are associated with the risk of higher vaginal cancer (36, 80, 81).
Updated Vaginal Cancer Identification Approaches
Bleeding, discharge, and urine retention are the most significant signs of vaginal cancer. Rectal symptoms such as constipation or blood in the stool might be caused by tumors that grow on the posterior vaginal wall. The most essential technique for determining the local extent of vaginal cancer is still a pelvic examination (15, 82, 83).
Treatment of Vaginal Cancer Based on its Different Stages
Vaginal cancer is so uncommon as a result, there are lots of debate and disagreement over the perfect treatment. Radiation and resection can be applied in vaginal cancer if the diagnosis can be done in the early stage (Table 1) (84). According to FIGO, treatment should be tailored based on the stage of diseases and the location of vaginal attachment.
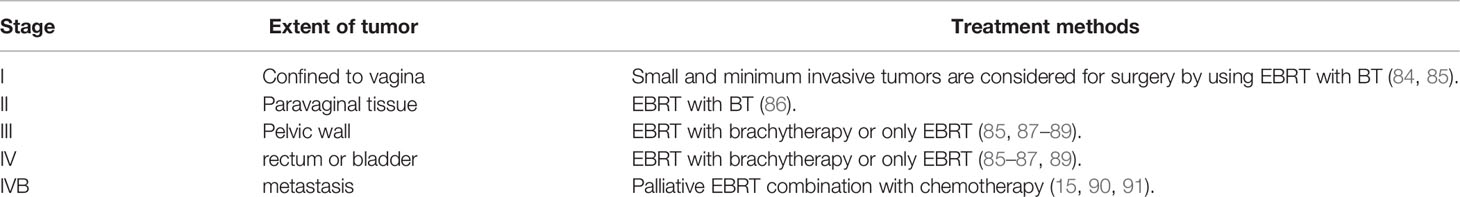
Table 1 Treatment of vaginal cancer by International Federation of Gynecology and Obstetrics’ stage.
Stage I
In this stage, tumors are restricted to the vaginal mucosa which is classified as FIGO stage I. Radiotherapy combination with surgery or only radiotherapy is an effective treatment for FIGO stage I vaginal cancer. In FIGO stage I, the lymph nodal involvement rate is minimal which ranges from 6 to 16% (90, 92). As the surgical method is needed to be thoroughly standardized, a variety of techniques have been documented. For the selected patients who possess tiny neoplasms mass excision has been performed (Figure 4) (84, 92–94). There are also several adopted procedures such as entire simple vaginectomy (84, 93, 94), partial vaginectomy (92), radical vaginectomy (82, 84, 92, 93). The affected vaginal tissue is removed to the pelvic sidewall in radical vaginectomy (93). In order to perform negative margins, the lower portion of the vagina may require vulva vaginectomy (82, 92, 93). Several studies (82, 84, 92, 93, 95–97) show that 174 patients with a cumulative 5 years survival 56-90% were treated only surgery in FIGO stage I. Some writers also argued that adjuvant radiotherapy should be used for the patients who are at high risk of recurrence, with 5 years survival rate of 79-100% (55, 82, 92, 95, 96, 98–100). Combining of intracavitary and interstitial therapy (55, 82, 86, 94–96, 99, 101, 102) with EBRT in patients with increasing risk prognostic factor (6, 55, 95, 96, 99, 100, 103) was the most after used radiotherapeutic technique. The EBRT is often recommended for poor differentiated or large tumors (6, 88). In most cancer patients with advanced stages, radiation which consists of brachytherapy and bean radiation is important to treat vaginal cancer treatment (85). Radiation is beneficial because of its preservation on the vagina (85, 104). CT simulation is used for 3D conformal treatment planning which results in tumor dosage with better efficacy.
Stage II
In stage II vaginal cancer, the neoplasia affects the area of sub vaginal tissue but does not reach the pelvic wall (Figure 5). Radiotherapy is considered the most prevalent treatment method for stage II cancer disease. A mix of branchy therapy and EBRT is commonly used in standard radiation treatment (86). Only radical surgery or radical surgery combination with radiotherapy are generally preferred (55, 92). For the treatment of stage II-IVA vaginal cancer, chemotherapy is commonly recommended as a radiosensitizer. Based on randomized data Perez et al, reported that 30% of patients are in stage II and 50% are in stage III. Several strategies can be utilized to increase the radiation dosage to the main tumor location. The size and location of the tumor dictate the treatment strategy chosen. By using 5-FU and mitomycin with radiotherapy and concurrent chemotherapy, it is reported to show promising results in vaginal cancer treatment (105). To assess the therapeutic efficiency of chemotherapeutic or chemo radio therapeutic regimen, more research is needed.
Stage III/IV
EBRT with branchy therapy or only EBRT is used for the treatment of stage III cancer disease. In females with stage III (Figure 6) and stage IV (advanced) (Figure 7) vaginal cancer, combined chemoradiation is found to perform higher metabolic and clinical response (106). EBRT combination with branchy therapy or only EBRT is used for the treatment of IVA cancer disease which is similar to the treatment of stage III cancer disease (55, 87, 89).
Palliative radiotherapy combined with chemotherapy is usually suggested for stage IVB vaginal cancer disease treatment (15, 25–27, 90, 91).
Other Possible Strategies for The Prevention of Vaginal Cancer
The vagina is a muscular, tube-like structure that stretches from the cervix where vaginal cancer is generally developed and a very uncommon type of cancer. Vaccination against HPV (the Human Papillomavirus) giving up smoking and safer sex are all recommended by NYU Langone specialists (107).
HPV Vaccination:
HPV has been associated with at least five other mucosal cancers: vaginal, vulvar, anal, penile, and oropharyngeal cancers. HPV is the most prevalent sexually transmitted infection in the world, with 50-80% of sexually active people developing it at some point in their life (108–110).. Vaginal cancer is responsible for 95% of all cervical cancer cases, as well as the majority of HPV-related morbidity and death. The Human Papillomavirus vaccine Gardasil has been licensed by U.S. FDA (Food and Drug Administration) to prevent precancer and cancer disease in the vagina. Gardasil also assists to prevent infections from the most common types of the strain of HPV (111, 112). Because they attack the skin, anogenital, and oral mucosal epithelial cells (108).
Regular Gynecologic Examinations
Women who possess risk factors for vaginal cancer, need to assume early-stage gynecologic examinations regularly which will assist to prevent precancerous and cancer in the vaginal area (113). The doctor will examine the vagina, uterus, cervix and other reproductive organs also to check for any unexpected abnormalities in these parts of a women’s body (114). The Pap test, which examines the epithelial lesion to detect vaginal cancer, Cervicography, which captures a vaginal picture and analyzes the vaginal size to detect vaginal cancer, and others are among the diagnostics available for detecting vaginal cancer (115).. Various types of cancer are developed by various factors. Scientists are still looking to find out the cause for genital vaginal cancer development and solutions to prevent it (116).
Discussion
Female vaginal cancer appears to be a malignancy that affects women in their older years of life, with the highest prevalence happening between the sixth and seventh decades of their lives. It is estimated that 15 percent of patients are diagnosed before the age of 50 and that 10 percent of tumor cells emerge in people under the age of 40 (95, 98, 117, 118). Squamous cell carcinomas account for more than 90% of vaginal cancer cases, while adenocarcinomas account for approximately 5% (95, 117, 118). Although squamous cell vaginal carcinomas immediately expanded superficially, several women were eventually diagnosed with metastatic cancer stage (10). Adenocarcinomas are distinct from squamous cancer in that they occur most frequently between the ages of 17 and 21 and often metastasize to the supraclavicular, pelvic nodes, or lung. It is rare to develop clear cell adenocarcinoma, and it usually affects people under the age of thirty. They are often linked with adenosis. Adenosis is the most prevalent histological abnormality in DES-exposed pregnant mothers (33). p53 mutations are common in HPV-negative malignancies in older women, and they are linked to an increased risk of mortality. The following are risk factors for SCC vaginal cancer: first intercourse before the age of seventeen, five or more sexual partners (83), low socioeconomic status, smoking, prior abnormal cytology, a history of genital warts, and prior hysterectomy (if the patient has had a hysterectomy) (13, 75, 119). Chronic irritating vaginitis, particularly because of constant exposure to foreign bodies like vaginal pessary and HPV infections, was associated with invasive vaginal carcinoma (120, 121). Patients who have previously developed cervical carcinoma are at increased risk of developing vaginal carcinoma, which is thought to be due to the fact that these sites are exposed to or susceptible to the same carcinogenic stimuli, whether endogenous or exogenous (82, 104).
When human papillomavirus enters the cell, E6 and E7 genes play a crucial role in interfering with the tumor suppressor gene’s function. The interaction between E7 and pRb protein leads to its breakdown and an abnormal start of the S-phase and the E2F transcriptional factor’s discharge. This is crucial for vaginal cancer development because it impedes the efficiency of oxidative stress to the DNA and enables secondary mutations to accumulate (57–59). The protein E6 regulates the gene expression profile and intracellular signaling pathways and changes the structure of epithelial cells. E6 binds and breaks down the FAS-associated death domain protein (FADD), inhibiting apoptosis-inducing communication capability through the Fas pathway. The continued action of proteins E6 and E7 causes abnormal cell proliferation, mutation of the oncogene, and eventually vaginal cancer (61, 65, 66). Viral oncogenic transcripts E6 and E7 are recognized to inactivate or speed up the degradation of diverse cellular proteins, such as retinoblastoma protein (E7) and p53 (E6). HPV DNA breaks are initiated when the virus is replicated, and these breaks cannot be remedied. The HPV genome directly binds to the host chromosome via the HPVE2-BRD4 (Bromodomain protein 4) complex for dividing genomes into daughter cells. BRD4 can significantly enhance the mechanistic assimilation viability of viral genomes into the host (69–71).
The stage of this cancer heavily influences prognosis. Indeed, the tumor stage strongly correlates with survival, distant metastasis, and local control (15). Additionally, the size of the tumor is used as a predictor of survival and regional or distant control (88, 122). Chyle and colleagues found that lesions with maximum diameters of less than 5 cm had a 10-year local recurrence rate of 20%. In contrast, lesions with maximum diameters greater than 5 cm had a recurrence rate of 40% (88). In particular, it is claimed in the series of Perez et al. that the lesion size is a valid predictor of pelvic tumor control and disease-free life, but only in Stage 2, when paravaginal submucosal extension is not involved with parametrial (6, 18). It has been shown that the tumor size is not a significant prognostic factor in Stages 1, II with parametrial involvement, or Stage III.
Another significant consideration while evaluating a vaginal lesion is its location. However, the effect of the position of the lesion on prognosis is controversial. According to Tarraza et al., upper-third lesions have more local recurrences, whereas lower-third lesions have a higher number of sidewall and distant recurrences (123). However, a larger series could not detect any site difference based on the location of the primary lesion (86). Many researchers have demonstrated that patients with cancers affecting the proximal half of the vagina have better overall survival and fewer recurrences than those with cancers affecting the distal half of the vagina or those affecting the entire length of the vagina, according to their findings (88, 103, 124, 125). Aside from that, lesions involving the posterior wall have a poorer prognosis than lesions involving the other vaginal walls (88, 98).
Additionally, the tumor’s location is significant as a predictor of lymph nodal status. In accordance with one theory, the efferent lymphatics from the upper section of the vaginal canal drain largely into the internal and external iliac lymph nodes, with the interiliac lymph nodes receiving the greatest amount of outflow. The lower part of the vagina lymph is drained directly into the inguino-femoral lymph nodes. Depending on the individual, other routes may also lead to the presacral or common iliac nodes (126). A higher incidence of lymph node metastasis (65%) was observed in lesions involving the posterior wall as opposed to the anterior wall or vaginal apex when the lesions were first discovered (16%) (92). The presence of nodes in vaginal cancer is thought to be a prognostic factor (6, 92, 103). Finally, the significance of age as a prognostic factor remains debatable. In some studies, the age of the patients is considered a prognostic factor; however, in other studies, this is not the case (6, 98, 125, 127). In terms of histologic type and grade, several studies have demonstrated that grading is a reliable and significant predictor of survival in the absence of other risk factors (103, 122, 125). Additionally, Chyle et al. (88) demonstrated that individuals with adenocarcinoma experienced a significantly higher rate of local recurrence than patients with squamous cell carcinoma.
In most situations, the prognosis is given too late, by which time the disease has spread to the submucosal tissue and, sometimes in cases, the pelvic sidewall of the patient, resulting in death. Even more challenging, the syndrome is most frequent in older persons, making treatment even more difficult to obtain. At the moment, radiation is used to treat the great majority of malignant tumors. Brachytherapy can be used on the primary tumor, whereas external beam radiation can be used on metastasized lymph nodes.
In the initial stages of vulvar, only surgery is considered as the backbone of treatment, whereas in advanced stages, surgery is combined with chemotherapy or radiation (128–130). Surgical intervention ensures adequate local control, but it comes with anatomical or functional limitations that compromise quality of life and sexual function. In recent times, a more comprehensive surgical treatment has been performed for the selected persons which permit anatomic reconstruction (131–133). By using tension-free skin closure, reconstructive surgery should be able to replace the lost tissue with a healthy donor region. Despite the fact that several reconstructive surgical treatments have been offered throughout the years, the prevalence of postoperative problems continues to be significant (134, 135). Furthermore, a significant complication rate affects surgical consequences for malignant gynecological pathologist (136). Wound dehiscences, low genital tract infections, vaginal and anovaginal shrinkage, urethral damage, and anti-aesthetic reconstructions, in particular, might exacerbate the patient’s quality of life and necessitate reintervention (137). The fasciocutaneous advancement flaps are the most commonly utilized by oncologists and gynecologists because of their ease of usage and good surgical results (137). With V-Y fasciocutaneous flap repair, Hand et al. found 33 and 15% surgical dehiscence and infection, respectively (138). The vitality of the restored tissue following its displacement is an important factor in vulvar flap success (128). Some points about reconstructive surgery. V-Y advancement gluteal fold flap (VYGF), Medial thigh fascio-cutaneous flap (MTF), Lotus petal flap (LPF) etc (131). The fascio-cutaneous flasps are distinguished from other type’s flasps by their simple feasibility, lower donor site morbidity and thus, they are frequently performed by oncologist (139). The operation has traditionally been reserved for the very beginning stages of this cancer. Improved anesthesia has made it possible to subject a larger number of patients to surgery, including those who are elderly or have comorbidities, and the discovery that tumors of the lower genital tract are chemo-sensitive, as demonstrated by several neoadjuvant chemotherapy trials, the field of urology has seen a resurgence in recent years (140–142). As time progresses, it is expected that surgery will play an increasingly important role. When comparing surgery to radiotherapy alone in stage I, investigations conducted on retrospective series have consistently demonstrated that surgery is either equal to or superior. The use of concurrent chemoradiotherapy, which has become the standard of care in cervical cancer, is another treatment method that warrants additional exploration. It is consequently important to adapt the treatment by a gynecologist based on the features of each individual. It is hoped that the adoption of widespread HPV vaccination programs would result in an even greater reduction in the incidence of this disease in the future. Furthermore, given the fact that most women fall prey to local illness, it is hoped that better techniques such as NACT+ radiation and chemotherapy in cervical cancer with similar survival and without compromising the quality of life can be relevant to this condition.
Concluding Remarks and Future Directions
In summary, Vaginal cancer in females appears to be a malignancy in their older years of life, specifically the sixth and seventh decades of their lives possess the highest prevalence. It is now clear that approximately 15% of vaginal cancer diagnosed at the age of 50 years, whereas before the age of 40 determine the only 10% vaginal cancer prevalence. However, Squamous cell carcinomas (SCC), as well as adenocarcinomas are two fundamental types of Vaginal Cancer; therefore, Squamous cell carcinomas account for nearly 90% of vaginal cancer cases, while adenocarcinomas account for approximately more than 5%. But most importantly SCC immediately expanded superficially. To detect the etiology of Vaginal cancer, identify the HPV infection, where it enters the host vaginal cells, E6 and E7 genes of the virus play a crucial role in interfering with the tumor suppressor genes (mainly P53, and pRb) function. As E6, and E7 protein promoted the carcinogenesis mechanism, and here not only regulate the cellular degradation of P53, and pRb but also enhances the cell proliferation along with E6 protein targets the p53 for breakdown and subsequently promote the apoptotic cell death, and DNA repair inhibition, that is indispensable to the continue the lifecycle of the HPV. During the SCC mediated vaginal cancer exhibited the following risk factors notably-first intercourse before the age of seventeen, five or more sexual partners, smoking, prior abnormal cytology, low socioeconomic status, a history of genital warts, and so forth. But to date adenocarcinomas, risk factors are not detected precisely. The most common signs and symptoms of vaginal cancer are bleeding, discharge, and urine retention; in some cases, identify rectal symptoms like constipation or blood in the stool. Pelvic examination is the primary technique to distinctively detect vaginal cancer. If the diagnosis can be done in an early stage of vaginal cancer, then radiation therapy, and surgery to cure this disease. only EBRT, EBRT with brachytherapy, Palliative EBRT combination with chemotherapy are mandatory to address the late stage of this severe disease. At present, several preventive rules are out in diverse scientific reports and added here like HPV vaccination, regular gynecologic examinations, and there are lots of tests are available like Pap test, Cervicography where examine the epithelial lesion of the vagina, and analyzing the vaginal size. More research is required to perfectly meet the molecular mechanism, risk factors, diagnosis, and prevention of Vaginal cancer.
Author Contributions
Conceptualization by SB, PB, DD, and MI; Writing and main draft preparation by SB, PB, MK, MI, DD, MS, and AM; Figures drawing by TR; Review and editing by TEB, MH, IH, and M-KJ; Visualization and supervision by MR, and BK. The Project Funded by BK. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Korea Institute of Oriental Medicine (grant number KSN2021240), Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1I1A2066868), the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A5A2019413), a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HF20C0116), and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HF20C0038).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Aalders J, Abeler V. Recurrent Adenocarcinoma of the Endometrium: A Clinical and Histopathological Study of 379 Patients. Oncology (1984). doi: 10.1016/0090-8258(84)90063-5
3. Curran J, Whittington R. Vaginal Recurrences of Endometrial Carcinoma: The Prognostic Value of Staging by a Primary Vaginal Carcinoma System. (1988). doi: 10.1016/0360-3016(88)90110-1
4. Dancuart F, Delclos L, Wharton J. Primary Squamous Cell Carcinoma of the Vagina Treated by Radiotherapy: A Failures Analysis—the MD Anderson Hospital Experience 1955–1982. (1988). doi: 10.1016/0090-8258(84)90063-5
5. Viswanathan AN, Erickson BE, Rownd J. Image-Based Approaches to Interstitial Brachytherapy. Berlin, Heidelberg: Gynecol. Radiat. Ther. Springer Berlin Heidelberg (2011) p. pp 247–59.
6. Perez CA, Grigsby PW, Garipagaoglu M, Mutch DG, Lockett MA. Factors Affecting Long-Term Outcome of Irradiation in Carcinoma of the Vagina. Int J Radiat Oncol (1999) 44:37–45. doi: 10.1016/s0360-3016(98)00530-6
7. Beriwal S, Demanes DJ, Erickson B, Jones E, De Los Santos JF, Cormack RA, et al. American Brachytherapy Society Consensus Guidelines for Interstitial Brachytherapy for Vaginal Cancer. Brachytherapy (2012) 11:68–75.
8. Rose PG, Herterick EE, Boutselis JG, Moeshberger M, Sachs L. Multiple Primary Gynecologic Neoplasms. Am J Obstet Gynecol (1987) 157:261–7. doi: 10.1016/s0002-9378(87)80148-5
9. Choo Y. Neoplasms of the Vagina Following Cervical Carcinoma. Oncology (1982) 14:125–32. doi: 10.1016/0090-8258(82)90059-2
10. Gallup DG, Talledo OE, Shah KJ, Hayes C. Invasive Squamous Cell Carcinoma of the Vagina: A 14-Year Study. Obstet Gynecol (1987) 69:782–5.
11. Campion MJ. Clinical Manifestations and Natural History of Genital Human Papillomavirus Infection. America MC-O Gynecol Clinics N (1987).
12. Okagaki T. Female Genital Tumors Associated With Human Papillomavirus Infection, and the Concept of Genital Neoplasm-Papilloma Syndrome (GENPS). Annu TO-P (1984).
13. Brinton LA, Nasca PC, Mallin K, Schairer C, Rosenthal J, Rothenberg R, et al. Case-Control Study of in Situ and Invasive Carcinoma of the Vagina. Gynecol Oncol (1990) 38:49–54. doi: 10.1016/0090-8258(90)90010-I
14. Parkin DM, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA Cancer J Clin (2005) 55:74–108. doi: 10.3322/canjclin.55.2.74
15. Di Donato V, Bellati F, Fischetti M, Plotti F, Perniola G, Benedetti Panici P. Vaginal Cancer. Crit Rev Oncol Hematol (2012) 81:286–95. doi: 10.1016/j.critrevonc.2011.04.004
16. Grigsby PW. Vaginal Cancer. Curr Treat Options Oncol (2002) 3:125–30. doi: 10.1007/s11864-002-0058-4
17. Bacalbasa N. Therapeutic Strategies in Primary Malignancies of the Vagina–a Literature Review. Rom J Med Pract (2015).
18. Carter JS, Downs LS. Vulvar and Vaginal Cancer. Obstet Gynecol Clin North Am (2012) 39:213–31. doi: 10.1016/j.ogc.2012.04.002
19. Siegel R, Ma J, Zou Z, Jemal A. Cancer Statistics, 2014. CA Cancer J Clin (2014) 64:9–29. doi: 10.3322/caac.21208
20. Rajaram S, Maheshwari A, Srivastava A. Staging for Vaginal Cancer. Best Pract Res Clin Obstet Gynaecol (2015) 29:822–32. doi: 10.1016/j.bpobgyn.2015.01.006
21. Hacker NF, Eifel PJ, van der Velden J. Cancer of the Vagina. Int J Gynecol Obstet (2012) 119:S97–9. doi: 10.1016/S0020-7292(12)60022-8
22. Abdel-Hafez SM, Hathout RM, Sammour OA. Attempts to Enhance the Anti-Cancer Activity of Curcumin as a Magical Oncological Agent Using Transdermal Delivery. Adv Tradit Med (2021) 21:15–29. doi: 10.1007/s13596-020-00439-5
23. Fujita K, Aoki Y, Tanaka K. Stage I Squamous Cell Carcinoma of Vagina Complicating Pregnancy: Successful Conservative Treatment. Gynecol Oncol (2005) 98:513–5. doi: 10.1016/j.ygyno.2005.05.007
24. Palumbo L, Shingleton HM, Fishburne JI, Pepper FD, Koch GG. Primary Carcinoma of the Vagina. South Med J (1969) 62:1048–53. doi: 10.1097/00007611-196909000-00004
25. Baruah N, Sangupta S. Primary Carcinoma of Vagina Complicating Pregnancy. J Indian Med Assoc (1973) 60:469–70.
26. Lutz MH, Underwood PB, Rozier JC, Putney FW. Genital Malignancy in Pregnancy. Am J Obstet Gynecol (1977) 129:533–5. doi: 10.1016/0002-9378(77)90093-X
27. Beck I, CLAYTON JK. Vaginal Carcinoma Arising in Vaginal Condylomata. Case Report. BJOG Int J Obstet Gynaecol (1984) 91:503–5. doi: 10.1111/j.1471-0528.1984.tb04792.x
28. Steed H. Invasive Squamous Cell Carcinoma of the Vagina During Pregnancy1. Obstet Gynecol (2002) 100:1105–8. doi: 10.1016/S0029-7844(02)02107-5
30. Sturgeon SR, Curtis RE, Johnson K, Ries L, Brinton LA. Second Primary Cancers After Vulvar and Vaginal Cancers. Am J Obstet Gynecol (1996) 174:929–33. doi: 10.1016/S0002-9378(96)70328-9
31. Dittmer C, Katalinic A, Mundhenke C, Thill M, Fischer D. Epidemiology of Vulvar and Vaginal Cancer in Germany. Arch Gynecol Obstet (2011) 284:169–74. doi: 10.1007/s00404-011-1850-9
32. Munich Tumor Registry at the Munich Tumor Center. Available at: https://www.tumorregister-muenchen.de/ (Accessed 4 Aug 2021).
33. Herbst AL, Robboy SJ, Scully RE, Poskanzer DC. Clear-Cell Adenocarcinoma of the Vagina and Cervix in Girls: Analysis of 170 Registry Cases. Am J Obstet Gynecol (1974) 119:712. doi: 10.1016/0002-9378(74)90138-0
34. Santos M, Montagut C, Mellado B, García Á, Cajal S, Cardesa A, et al. Immunohistochemical Staining for P16 and P53 in Premalignant and Malignant Epithelial Lesions of the Vulva. Int J Gynecol Pathol (2004) 23:206–14. doi: 10.1097/01.pgp.0000130108.03231.89
35. Zaino: Carcinoma of the Vulva, Urethra, and Bartholin’s. - Google Scholar. Available at: https://scholar.google.com/scholar?cluster=3250917822265087738&hl=en&as_sdt=2005&sciodt=0,5 (Accessed 4 Aug 2021).
36. Trimble CL, Hildesheim A, Briton LA, Shah KV, Kurman RJ. Heterogeneous Etiology of Squamous Carcinoma of the Vulva. Obstet Gynecol (1996) 87:59–64. doi: 10.1016/0029-7844(95)00351-7
37. Peters R, Mack T. 2016 Parallels in the Epidemiology of Selected Anogenital Carcinomas. J National Cancer Institute (1984) 72:609–615.
38. Young J, Percy C, Asire A, US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute. Surveillance, Epidemiology, and End Results: Incidence and Mortality Data, 1973-77. (1981).
39. Gadducci A, Fabrini MG, Lanfredini N, Sergiampietri C. Squamous Cell Carcinoma of the Vagina: Natural History, Treatment Modalities and Prognostic Factors. Crit Rev Oncol Hematol (2015) 93:211–24. doi: 10.1016/j.critrevonc.2014.09.002
40. Sugarbaker PH. Observations Concerning Cancer Spread Within the Peritoneal Cavity and Concepts Supporting an Ordered Pathophysiology. Cancer Treat Res (1996) 82:79–100. doi: 10.1007/978-1-4613-1247-5_6
41. Okamura H, Cytol HK-IR. Pathophysiological Dynamics of Human Ovarian Surface Epithelial Cells in Epithelial Ovarian Carcinogenesis. Int Rev Cytol (2005) 242:1–54. doi: 10.1016/S0074-7696(04)42001-4
42. Frank SJ, Deavers MT, Jhingran A, Bodurka DC, Eifel PJ. Primary Adenocarcinoma of the Vagina Not Associated With Diethylstilbestrol (DES) Exposure. Gynecol Oncol (2007) 105:470–4. doi: 10.1016/j.ygyno.2007.01.005
43. Beller U, Benedet J, Creasman W, Ngan H, Quinn M, Maisonneuve Pecorelli S, et al. Carcinoma of the Vagina. Int J Gynecol Obstet (2006) 95:S29–42. doi: 10.1016/S0020-7292(06)60029-5
44. Ratnavelu N, Patel A, Fisher A, Galaal K, Cross P, Naik R. High-Grade Vaginal Intraepithelial Neoplasia: Can We be Selective About Who We Treat? BJOG Int J Obstet Gynaecol (2013) 120:887–93. doi: 10.1111/1471-0528.12223
45. Gunderson CC, Nugent EK, Elfrink SH, Gold MA, Moore KN. A Contemporary Analysis of Epidemiology and Management of Vaginal Intraepithelial Neoplasia. Am J Obstet Gynecol (2013) 208:410.e1–6. doi: 10.1016/j.ajog.2013.01.047
46. Liao JB, Jean S, Wilkinson-Ryan I, Ford AE, Tanyi JL, Hagemann AR, et al. Vaginal Intraepithelial Neoplasia (VAIN) After Radiation Therapy for Gynecologic Malignancies: A Clinically Recalcitrant Entity. Gynecol Oncol (2011) 120:108–12. doi: 10.1016/j.ygyno.2010.09.005
47. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer Statistics, 2012. CA Cancer J Clin (2015) 65:87–108. doi: 10.3322/caac.21262
48. Duong TH, Flowers LC. Vulvo-Vaginal Cancers: Risks, Evaluation, Prevention and Early Detection. Obstet Gynecol Clin North Am (2007) 34:783–802. doi: 10.1016/j.ogc.2007.10.003
49. Mittendorf R. Teratogen Update: Carcinogenesis and Teratogenesis Associated With Exposure to Diethylstilbestrol (DES) In Utero. Teratology (1995) 51:435–45. doi: 10.1002/tera.1420510609
51. Palmer J, Anderson D, Helmrich S. Risk Factors for Diethylstilbestrol-Associated Clear Cell Adenocarcinoma. Gynecology (2000). doi: 10.1097/00006250-200006000-00007
52. Types of Vaginal Cancer: Common, Rare and More . CTCA. Available at: https://www.cancercenter.com/cancer-types/vaginal-cancer/types (Accessed 14 Oct 2021).
54. Gupta S. A Comprehensive Textbook of Obstetrics and Gynecology. Gynecol Oncol (2011) 92:873–80. doi: 10.5005/jp/books/11278
55. Gardner CS, Sunil J, Klopp AH, Devine CE, Sagebiel T, Viswanathan C, et al. Primary Vaginal Cancer: Role of MRI in Diagnosis, Staging and Treatment. Br J Radiol (2015) 88:20150033. doi: 10.1259/bjr.20150033
56. FIGO Committee on Gynecologic Oncology. Current FIGO Staging for Cancer of the Vagina, Fallopian Tube, Ovary, and Gestational Trophoblastic Neoplasia. Int J Gynecol Obstet (2009) 105:3–4. doi: 10.1016/j.ijgo.2008.12.015
57. Daniel B, Rangarajan A. The Link Between Integration and Expression of Human Papillomavirus Type 16 Genomes and Cellular Changes in the Evolution of Cervical Intraepithelial Neoplastic. J General Virol (1997) 78:1095–101. doi: 10.1099/0022-1317-78-5-1095
58. Hudelist G, Manavi M. Physical State and Expression of HPV DNA in Benign and Dysplastic Cervical Tissue: Different Levels of Viral Integration are Correlated With Lesion Grade. (2004). doi: 10.1016/j.ygyno.2003.11.035
59. Ojesina A, Lichtenstein L, Freeman S, Nature C--. Landscape of Genomic Alterations in Cervical Carcinomas. Nature (2014) 506:371–75. doi: 10.1038/nature12881
60. Antinore MJ, Birrer MJ, Patel D, Nader L, McCance DJ. The Human Papillomavirus Type 16 E7 Gene Product Interacts With and Trans-Activates the AP1 Family of Transcription Factors. EMBO J (1996) 15:1950–60.
61. Funk J, Waga S, Harry J. Inhibition of CDK Activity and PCNA-Dependent DNA Replication by P21 is Blocked by Interaction With the HPV-16 E7 Oncoprotein. Genes & development (1997) 11:2090–100. doi: 10.1101/gad.11.16.2090
62. Doorbar J. Molecular Biology of Human Papillomavirus Infection and Cervical Cancer. science (2006).
63. Halim TA, Farooqi AA, Zaman F. Nip the HPV Encoded Evil in the Cancer Bud: HPV Reshapes TRAILs and Signaling Landscapes. Cancer Cell Int (2013). doi: 10.1186/1475-2867-13-61
64. Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, et al. The Biology and Life-Cycle of Human Papillomaviruses. Elsevier (2012). doi: 10.1016/j.vaccine.2012.06.083
65. Filippova M. The Human Papillomavirus 16 E6 Protein Binds to Fas-Associated Death Domain and Protects Cells From Fas-Triggered Apoptosis. Journal of Biological Chemistry (2004) 279:25729–25744. doi: 10.1074/jbc.M401172200
66. Hongmei Z. Extrinsic and Intrinsic Apoptosis Signal Pathway Review. Apoptosis and medicine. InTechOpen, UK. (2012).
67. Münger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M, et al. Mechanisms of Human Papillomavirus-Induced Oncogenesis. J Virol (2004) 78:11451–60. doi: 10.1128/JVI.78.21.11451-11460.2004
68. Fradet-Turcotte A. Nuclear Accumulation of the Papillomavirus E1 Helicase Blocks S-Phase Progression and Triggers an ATM-Dependent DNA Damage Response. Am Soc Microbiol (2011) 85:8996–9012. doi: 10.1128/JVI.00542-11
69. Spardy N, Covella K, Cha E, Hoskins E. Human Papillomavirus 16 E7 Oncoprotein Attenuates DNA Damage Checkpoint Control by Increasing the Proteolytic Turnover of Claspin. Cancer Res (2009) 69:7022–7029. doi: 10.1158/0008-5472.CAN-09-0925
70. Jackson S, Nature J--. The DNA-Damage Response in Human Biology and Disease. Nature (2009) 461:1071–1078. doi: 10.1038/nature08467
71. Kusumoto-Matsuo R, Kanda T. Rolling Circle Replication of Human Papillomavirus Type 16 DNA in Epithelial Cell Extracts. Cell (2011) 16:23–33. doi: 10.1111/j.1365-2443.2010.01458.x
72. Jang MK, Anderson DE, van Doorslaer K, Mcbride AA. A Proteomic Approach to Discover and Compare Interacting Partners of Papillomavirus E2 Proteins From Diverse Phylogenetic Groups. Proteomics (2015) 15:2038–50. doi: 10.1002/pmic.201400613
73. Bj T, Dillner J, Anttila T, Engeland A, Bmj T--. Prospective Seroepidemiological Study of Role of Human Papillomavirus in non-Cervical Anogenital Cancers. Bmj (1997) 7109:646–9. doi: 10.1136/bmj.315.7109.646
74. Carter J, Madeleine M, Shera K. Human Papillomavirus 16 and 18 L1 Serology Compared Across Anogenital Cancer Sites. Research (2001) 61:1934–1940.
75. Daling J, Madeleine M, Schwartz S. A Population-Based Study of Squamous Cell Vaginal Cancer: HPV and Cofactors. Gynecologic Oncol (2002) 84:263–70. doi: 10.1006/gyno.2001.6502
76. Madsen BS, Jensen HL, Van Den Brule AJC, Wohlfahrt J, Frisch M. Risk Factors for Invasive Squamous Cell Carcinoma of the Vulva and Vagina - Population-Based Case-Control Study in Denmark. Int J Cancer (2008) 122:2827–34. doi: 10.1002/ijc.23446
77. Wassberg C, Thörn M. Second Primary Cancers in Patients With Squamous Cell Carcinoma of the Skin: A Population-Based Study in Sweden. Cancer (1999). doi: 10.1002/(SICI)1097-0215(19990209)80:4<511::AID-IJC5>3.0.CO;2-P
78. Pride GL, Schultz AE, Chuprevich TW, Bucler DA. Primary Invasive Squamous Carcinoma of the Vagina. Obstetrics and Gynecology (1979) 53:218–225.
79. Hussain SK, Sundquist J, Hemminki K. Familial Clustering of Cancer at Human Papillomavirus-Associated Sites According to the Swedish Family-Cancer Database. Int J Cancer (2008) 122:1873–8. doi: 10.1002/ijc.23265
80. Parazzini F, Moroni S, Negri E. Selected Food Intake and Risk of Vulvar Cancer. Cancer (1995). doi: 10.1002/1097-0142(19951201)76:11<2291::aid-cncr2820761117>3.0.co;2-w
81. Basta A, Adamek K. Intraepithelial Neoplasia and Early Stage Vulvar Cancer. Epidemiological, Clinical and Virological Observations. Gynaecological (1999).
82. Rubin SC, Young J, Mikuta JJ. Squamous Carcinoma of the Vagina: Treatment, Complications and Long-Term Follow-Up. Gynecol Oncol (1985) 20:346–53. doi: 10.1016/0090-8258(85)90216-1
83. Herman JM, Homesley HD, Dignan MB. Is Hysterectomy a Risk Factor for Vaginal Cancer? JAMA (1986) 256:601–3.
85. Frank SJ, Jhingran A, Levenback C, Eifel PJ. Definitive Radiation Therapy for Squamous Cell Carcinoma of the Vagina. Int J Radiat Oncol (2005) 62:138–47. doi: 10.1016/j.ijrobp.2004.09.032
86. Perez CA, Camel HM, Galakatos AE, Grigsby PW, Kuske RR, Buchsbaum G, et al. Definitive Irradiation in Carcinoma of the Vagina: Long-Term Evaluation of Results. Int J Radiat Oncol (1988) 15:1283–90. doi: 10.1016/0360-3016(88)90222-2
87. Tran PT, Su Z, Lee P, Lavori P, Husain A, Teng N, et al. Prognostic Factors for Outcomes and Complications for Primary Squamous Cell Carcinoma of the Vagina Treated With Radiation. Gynecol Oncol (2007) 105:641–9. doi: 10.1016/j.ygyno.2007.01.033
88. Chyle V, Zagars GK, Wheeler JA, Wharton JT, Delclos L. Definitive Radiotherapy for Carcinoma of the Vagina: Outcome and Prognostic Factors. Int J Radiat Oncol (1996) 35:891–905. doi: 10.1016/0360-3016(95)02394-1
89. Lian J, Dundas G, Carlone M, Ghosh S, Pearcey R. Twenty-Year Review of Radiotherapy for Vaginal Cancer: An Institutional Experience. Gynecol Oncol (2008) 111:298–306. doi: 10.1016/j.ygyno.2008.07.007
90. Dalrymple JL, Russell AH, Lee SW, Scudder SA, Leiserowitz GS, Kinney WK, et al. Chemoradiation for Primary Invasive Squamous Carcinoma of the Vagina. Int J Gynecol Cancer (2004) 14:110–7. doi: 10.1136/ijgc-00009577-200401000-00015
91. Samant R, Lau B EC, Le T, Tam T. Primary Vaginal Cancer Treated With Concurrent Chemoradiation Using Cis-Platinum. Int J Radiat Oncol (2007) 69:746–50. doi: 10.1016/j.ijrobp.2007.04.015
92. Stock RG, Chen ASJ, Seski J. A 30-Year Experience in the Management of Primary Carcinoma of the Vagina: Analysis of Prognostic Factors and Treatment Modalities. Gynecol Oncol (1995) 56:45–52. doi: 10.1006/gyno.1995.1008
93. Ball HG, Berman ML. Management of Primary Vaginal Carcinoma. Gynecol Oncol (1982) 14:154–63. doi: 10.1016/0090-8258(82)90085-3
94. Tjalma WAA, Monaghan JM, de Barros Lopes A, Naik R, Nordin AJ, Weyler JJ. The Role of Surgery in Invasive Squamous Carcinoma of the Vagina. Gynecol Oncol (2001) 81:360–5. doi: 10.1006/gyno.2001.6171
95. Davis KP, Stanhope CR, Garton GR, Atkinson EJ, O’Brien PC. Invasive Vaginal Carcinoma: Analysis of Early-Stage Disease. Gynecol Oncol (1991) 42:131–6. doi: 10.1016/0090-8258(91)90332-Y
96. Creasman W. The National Cancer Data Base Report on Cancer of the Vagina. Cancer: Interdisciplinary International Journal of the American Cancer Society (1998) 83:1033–1040. doi: 10.1002/(SICI)1097-0142(19980901)83:5<1033::AID-CNCR30>3.0.CO;2-6
97. Chu AM, Beechinor R. Survival and Recurrence Patterns in the Radiation Treatment of Carcinoma of the Vagina. Gynecol Oncol (1984) 19:298–307. doi: 10.1016/0090-8258(84)90196-3
98. Dixit S, Singhal S, Baboo HA. Squamous Cell Carcinoma of the Vagina: A Review of 70 Cases. Gynecol Oncol (1993) 48:80–7. doi: 10.1006/gyno.1993.1013
99. Leung S, Sexton M. Radical Radiation Therapy for Carcinoma of the Vagina—Impact of Treatment Modalities on Outcome: Peter MacCallum Cancer Institute Experience 1970–1990. Int J Radiat Oncol (1993) 25:413–8. doi: 10.1016/0360-3016(93)90061-Y
100. Dunn LJ, Napier JG. Primary Carcinoma of the Vagina. Am J Obstet Gynecol (1966) 96:1112–6. doi: 10.1016/0002-9378(66)90519-9
101. Lee WR, Marcus RB, Sombeck MD, Mendenhall WM, Morgan LS, Freeman DE, et al. Radiotherapy Alone for Carcinoma of the Vagina: The Importance of Overall Treatment Time. Int J Radiat Oncol (1994) 29:983–8. doi: 10.1016/0360-3016(94)90392-1
102. Tewari KS, Cappuccini F, Puthawala AA, Kuo JV, Burger RA, Monk BJ, et al. Primary Invasive Carcinoma of the Vagina. Cancer (2001) 91:758–70. doi: 10.1002/1097-0142(20010215)91:4<758::AID-CNCR1062>3.0.CO;2-U
103. Kucera H, Vavra N. Radiation Management of Primary Carcinoma of the Vagina: Clinical and Histopathological Variables Associated With Survival. Gynecol Oncol (1991) 40:12–6. doi: 10.1016/0090-8258(91)90076-H
105. Kersh C, Constable W, Spaulding C, Cancer S--. A Phase I-II Trial of Multimodality Management of Bulky Gynecologic Malignancy: Combined Chemoradiosensitization and Radiotherapy. Cancer (1990) 66:30–34. doi: 10.1002/1097-0142(19900701)66:1<30::AID-CNCR2820660107>3.0.CO;2-C
106. Kunos CA, Radivoyevitch T, Waggoner S, et al. Radiochemotherapy Plus 3-Aminopyridine-2-Carboxaldehyde Thiosemicarbazone (3-AP, NSC #663249) in Advanced-Stage Cervical and Vaginal Cancers. Gynecol Oncol (2013) 130:75–80. doi: 10.1016/j.ygyno.2013.04.019
107. Sahoo C, Satyanarayana K, Nayak P. AN OVERVIEW ON CERVICAL CANCER. Sahoo World J Pharm Res (2014) 3:323.
108. St. Laurent J, Luckett R, Feldman S. HPV Vaccination and the Effects on Rates of HPV-Related Cancers. Curr Probl Cancer (2018) 42:493–506. doi: 10.1016/j.currproblcancer.2018.06.004
109. Zur Hausen H, de Villiers E-M. Reprint of: Cancer “Causation” by Infections–Individual Contributions and Synergistic Networks. Semin Oncol (2015) 42:207–22. doi: 10.1053/j.seminoncol.2015.02.019
110. Winer RL, Koutsky LA. The Epidemiology of Human Papillomavirus Infections. Cancer Prevention — Cancer Causes book series (CPCC, volume 2) (2004) 143–87. doi: 10.1007/1-4020-2016-3_6
111. Herrero R, González P. Present Status of Human Papillomavirus Vaccine Development and Implementation. Oncology (2015) 16:e206–16. doi: 10.1016/S1470-2045(14)70481-4
112. Lowy D, Herrero R. Primary Endpoints for Future Prophylactic Human Papillomavirus Vaccine Trials: Towards Infection and Immunobridging. Oncology (2015). doi: 10.1016/S1470-2045(15)70075-6
113. Herzog T. The Impact of Cervical Cancer on Quality of Life—the Components and Means for Management. oncology (2007). doi: 10.1016/j.ygyno.2007.09.019
114. Edwards JNT, Morris HB. Langerhans’ Cells and Lymphocyte Subsets in the Female Genita1 Tract. BJOG Int J Obstet Gynaecol (1985) 92:974–82. doi: 10.1111/j.1471-0528.1985.tb03080.x
115. Averette HE, Steren A, Nguyen HN. Screening in Gynecologic Cancers. Cancer (1993) 72:1043–9. doi: 10.1002/1097-0142(19930801)72:3
116. Duong TH, Flowers LC. Vulvo-vaginal cancers: risks, evaluation, prevention and early detection. Obstetrics and Gynecology Clinics of North Am (2007) 34(4):783–802. doi: 10.1016/j.ogc.2007.10.003
117. Merino MJ. Vaginal Cancer: The Role of Infectious and Environmental Factors. Am J Obstet Gynecol (1991) 165:1255–62. doi: 10.1016/S0002-9378(12)90738-3
118. Macnaught R, Symonds RP, Hole D, Watson ER. Improved Control of Primary Vaginal Tumours by Combined External-Beam and Interstitial Radiotherapy. Clin Radiol (1986) 37:29–32. doi: 10.1016/S0009-9260(86)80160-X
119. Okagaki T, Twiggs LB, Zachow KR, Clark BA, Ostrow RS, Faras AJ. Identification of Human Papillomavirus DNA in Cervical and Vaginal Intraepithelial Neoplasia With Molecularly Cloned Virus-Specific DNA Probes. Int J Gynecol Pathol (1983) 2:153–9. doi: 10.1097/00004347-198302000-00006
120. Weed JrJC, Lozier C. Human Papilloma Virus in Multifocal, Invasive Female Genital Tract Malignancy. Gynecology.
121. Bouma J, Burger MPM, Krans M, Hollema H, Pras E. Squamous Cell Carcinoma of the Vagina: A Report of 32 Cases. Int J Gynecol Cancer (1994) 4:389–94. doi: 10.1046/j.1525-1438.1994.04060389.x
122. Kirkbride P, Fyles A, Rawlings GA, Manchul L, Levin W, Murphy KJ, et al. Carcinoma of the Vagina—Experience at the Princess Margaret Hospital (1974-1989). Gynecol Oncol (1995) 56:435–43. doi: 10.1006/gyno.1995.1077
123. Hiniker SM, Roux A, Murphy JD, Harris JP, Tran PT, Kapp DS, et al. Primary squamous cell carcinoma of the vagina: prognostic factors, treatment patterns, and outcomes. Gynecologic Oncology (2013) 131(2):380–385. doi: 10.1016/j.ygyno.2013.08.012"10.1016/j.ygyno.2013.08.012
124. Ali M, Huang D. Radiation Alone for Carcinoma of the Vagina: Variation in Response Related to the Location of the Primary Tumor. Cancer: Interdisciplinary International Journal of the American Cancer Society (1996) 77:1934–1939. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1934::AID-CNCR25>3.0.CO;2-#
125. Urbański K, Kojs Z, Reinfuss M, Fabisiak W. Primary Invasive Vaginal Carcinoma Treated With Radiotherapy: Analysis of Prognostic Factors. Gynecol Oncol (1996) 60:16–21. doi: 10.1006/gyno.1996.0004
126. Reiffenstuhl G. The Lymphatics of the Female Genital. - Google Scholar. Available at: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Reiffenstuhl%2C+G.+The+lymphatics+of+the+female+genital+organs%3B+Lippincott%3A+Annals+of+Surgery%2C+1964%3B+Volume+162.6.&btnG (Accessed 14 Sep 2021).
127. Eddy GL, Marks RD, Miller MC, Underwood Jr.PB. Primary Invasive Vaginal Carcinoma. Am J Obstet Gynecol (1991) 165:292–8. doi: 10.1016/0002-9378(91)90081-2
128. Capozzi VA, Monfardini L, Sozzi G, Armano G, Rosati A, Gueli Alletti S, et al. Subcutaneous Vulvar Flap Viability Evaluation With Near-Infrared Probe and Indocyanine Green for Vulvar Cancer Reconstructive Surgery: A Feasible Technique. Front Surg (2021) 8. doi: 10.3389/fsurg.2021.721770
129. Tans L, Ansink AC, van Rooij PH, Kleijnen C, Mens JW. The Role of Chemo-Radiotherapy in the Management of Locally Advanced Carcinoma of the Vulva. Am J Clin Oncol (2011) 34:22–6. doi: 10.1097/COC.0b013e3181cae6a1
130. Moore DH, Ali S, Koh W-J, Michael H, Barnes MN, McCourt CK, et al. A Phase II Trial of Radiation Therapy and Weekly Cisplatin Chemotherapy for the Treatment of Locally-Advanced Squamous Cell Carcinoma of the Vulva: A Gynecologic Oncology Group Study. Gynecol Oncol (2012) 124:529–33. doi: 10.1016/j.ygyno.2011.11.003
131. Di Donato V, Bracchi C, Cigna E, Domenici L, Musella A, Giannini A, et al. Vulvo-Vaginal Reconstruction After Radical Excision for Treatment of Vulvar Cancer: Evaluation of Feasibility and Morbidity of Different Surgical Techniques. Surg Oncol (2017) 26:511–21. doi: 10.1016/j.suronc.2017.10.002
132. Windhofer C, Papp C, Staudach A, Michlits W. Local Fasciocutaneous Infragluteal (FCI) Flap for Vulvar and Vaginal Reconstruction: A New Technique in Cancer Surgery. Int J Gynecol Cancer (2012) 22:132–8. doi: 10.1097/IGC.0b013e318234fa0a
133. Tan B-K, Kang GC-W, Tay EH, Por YC. Subunit Principle of Vulvar Reconstruction: Algorithm and Outcomes. Arch Plast Surg (2014) 41:379. doi: 10.5999/aps.2014.41.4.379
134. Salgarello M, Farallo E, Barone-Adesi L, Cervelli D, Scambia G, Salerno G, et al. Flap Algorithm in Vulvar Reconstruction After Radical, Extensive Vulvectomy. Ann Plast Surg (2005) 54:184–90. doi: 10.1097/01.sap.0000141381.77762.07
135. Wills A, Obermair A. A Review of Complications Associated With the Surgical Treatment of Vulvar Cancer. Gynecol Oncol (2013) 131:467–79. doi: 10.1016/j.ygyno.2013.07.082
136. Bizzarri N, du Bois A, Fruscio R, De Felice F, De Iaco P, Casarin J, et al. Is There Any Therapeutic Role of Pelvic and Para-Aortic Lymphadenectomy in Apparent Early Stage Epithelial Ovarian Cancer? Gynecol Oncol (2021) 160:56–63. doi: 10.1016/j.ygyno.2020.10.028
137. Hand LC, Maas TM, Baka N, Mercier RJ, Greaney PJ, Rosenblum NG, et al. Utilizing V Y Fasciocutaneous Advancement Flaps for Vulvar Reconstruction. Gynecol Oncol Rep (2018) 26:24–8. doi: 10.1016/j.gore.2018.08.007
138. Kim SI, Lee M, Kim HS, Chung HH, Kim J-W, Park NH, et al. Effect of BRCA Mutational Status on Survival Outcome in Advanced-Stage High-Grade Serous Ovarian Cancer. J Ovarian Res (2019) 12:40. doi: 10.1186/s13048-019-0511-7
139. Langstein HN, Robb GL. Reconstructive Approaches in Soft Tissue Sarcoma. Semin Surg Oncol (1999) 17:52–65. doi: 10.1002/(SICI)1098-2388(199907/08)17:1<52::AID-SSU7>3.0.CO;2-I
140. Benedetti Panici P, Bellati F, Plotti F, Di Donato V, Antonilli M, Perniola G, et al. Neoadjuvant Chemotherapy Followed by Radical Surgery in Patients Affected by Vaginal Carcinoma. Gynecol Oncol (2008) 111:307–11. doi: 10.1016/j.ygyno.2008.07.005
141. Benedetti-Panici P, Greggi S, Colombo A, Amoroso M, Smaniotto D, Giannarelli D, et al. Neoadjuvant Chemotherapy and Radical Surgery Versus Exclusive Radiotherapy in Locally Advanced Squamous Cell Cervical Cancer: Results From the Italian Multicenter Randomized Study. J Clin Oncol (2002) 20:179–88. doi: 10.1200/JCO.2002.20.1.179
Keywords: vaginal cancer, postmenopausal, human papillomavirus (HPV) E6 and E7 proteins, synchronous or metachronous, carcinogenesis mechanisms, HPV type 16
Citation: Baral SK, Biswas P, Kaium MA, Islam MA, Dey D, Saber MA, Rahaman TI, M A, Emran TB, Hasan MN, Jeong M-K, Han I, Rahman MA and Kim B (2022) A Comprehensive Discussion in Vaginal Cancer Based on Mechanisms, Treatments, Risk Factors and Prevention. Front. Oncol. 12:883805. doi: 10.3389/fonc.2022.883805
Received: 25 February 2022; Accepted: 23 June 2022;
Published: 18 July 2022.
Edited by:
Saikat Mitra, University of Dhaka, BangladeshReviewed by:
Vito Andrea Capozzi, University Hospital of Parma, ItalyChy Mohammad Monirul Hasan, National University of Malaysia, Malaysia
Abu Montakim Tareq, University of Houston, United States
Copyright © 2022 Baral, Biswas, Kaium, Islam, Dey, Saber, Rahaman, M, Emran, Hasan, Jeong, Han, Rahman and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Md. Ataur Rahman, YXRhdXIxOTgxcmFobWFuQGhvdG1haWwuY29t; Bonglee Kim, Ym9uZ2xlZWtpbUBraHUuYWMua3I=
†These authors have contributed equally to this work
 Sumit Kumar Baral
Sumit Kumar Baral Partha Biswas
Partha Biswas Md. Abu Kaium2
Md. Abu Kaium2 Dipta Dey
Dipta Dey Tanjim Ishraq Rahaman
Tanjim Ishraq Rahaman Talha Bin Emran
Talha Bin Emran Md. Nazmul Hasan
Md. Nazmul Hasan Mi-Kyung Jeong
Mi-Kyung Jeong Ihn Han
Ihn Han Md. Ataur Rahman
Md. Ataur Rahman Bonglee Kim
Bonglee Kim