- Department of Hepatopancreatobiliary Surgery, Qingdao Municipal Hospital, Qingdao University, Qingdao, China
Background: Pancreatic neuroendocrine tumors (PanNETs) are a heterogeneous group of pancreatic malignancies. Surgical resection is the only curative treatment option for patients with localized PanNETs, yet the role of cancer-directed surgery (CDS) in the setting of oligometastatic liver metastasis remains a controversy.
Methods: All patients diagnosed with PanNETs and liver-only metastasis from 2010 to 2018 were identified from the SEER database. The biases of baseline characteristics between CDS and no-CDS cohorts were reduced by the propensity score-matching (PSM) method, and the prognostic role of CDS was estimated using the Kaplan–Meier method and Cox regression models. Logistic regression analysis was utilized to identify factors associated with patients who underwent CDS.
Results: A total of 1,270 PanNET patients with oligometastatic liver metastasis were included and analyzed. Of these patients, 283 (22.3%) patients underwent CDS of the primary tumor, while the remaining 987 (77.7%) did not. The OS and CSS were significantly better in the CDS cohort regardless of the propensity score analysis. Multivariate analysis revealed that age, tumor differentiation, tumor location, and lymph node status were significantly associated with patients who were more likely to receive CDS.
Conclusion: Our study demonstrated that CDS was associated with survival benefits in selected patients with PanNETs and liver-only metastasis based on a large population database.
Introduction
Pancreatic neuroendocrine tumors (PanNETs) are a heterogeneous group of pancreatic malignancies arising from the islet cells of the pancreas, accounting for only 1%–2% of all pancreatic tumors (1–3). However, the annual incidence of PanNETs has been increasing dramatically over the past 40 years, owing primarily to the widespread use of cross-sectional imaging (4, 5). Unlike pancreatic ductal adenocarcinoma (PDAC), these tumors are commonly regarded as characteristically slow-growing neoplasms associated with a favorable prognosis. Indolent behaviors may, to some extent, delay diagnosis, such as when metastases, predominantly to the liver, are present in patients at diagnosis. Also, compared to other gastroenteropancreatic NETs, PanNETs are more frequently diagnosed at advanced stages with the presence of distant metastases (6–8). Approximately 80% of metastatic patients have secondary liver lesions, and in nearly 50% of patients, the liver is the only metastatic site. Although surgical resection is the only curative treatment option for patients with localized PanNETs, the role of primary tumor resection in the setting of oligometastatic disease remains controversial (9–13). In consideration that PanNET patients with metastatic disease may even derive survival benefits from debulking operations according to several studies, there is an urgent need to gather evidence for the benefits of cancer-directed surgery and identify clinicopathological factors that assist in the selection of candidates for primary tumor removal.
Therefore, in the present study, we aimed to use the SEER database to determine whether primary tumor resection could confer survival benefits in patients with hepatic oligometastatic PanNETs and to establish clinical criteria for selecting patients most likely to benefit from cancer-directed surgery.
Materials and Methods
Records of patients with PanNETs between 2010 and 2018 were extracted and collected retrospectively from the SEER database, which covers nearly 30% of the population in the United States. The study was approved by the institutional review board (IRB) of Qingdao municipal hospital, and the informed consent was waived owing to the deidentified data source. The evaluated variables included patients’ demographics, tumor characteristics, treatment modalities, and survival outcomes. The inclusion criteria were as follows: (i) patients with histological confirmation of PanNET diagnosis, (ii) patients with liver-only metastases at the time of diagnosis, and (iii) patients with complete data on treatment and survival status. The exclusion criteria were set as: (i) patients with other metastatic sites such as bone, lung, and brain; and (ii) patients with missing information on treatment and oncological outcomes. The primary endpoint was overall survival (OS), which was defined as the interval from the initial treatment to death or the last follow-up time point.
Statistical Analysis
Categorical variables were expressed as numbers (percentage) and compared using the Chi-square test. Univariate and multivariate logistic regression analyses were performed to determine the association between clinical variables and receipt of primary tumor resection. The overall survival (OS) and cancer-specific survival (CSS) between the different groups were compared using Kaplan–Meier estimates with log-rank tests. Univariate and multivariate Cox proportional hazard regression models were applied to identify prognostic factors associated with OS in all PanNETs and in PanNET patients who received cancer-directed surgery, respectively. Propensity score-matching (PSM) analysis was conducted to reduce the selection biases and confounding variables between the study cohorts (14, 15). All statistical analyses were performed using R software (version 4.3.2). A p-value of <0.05 was considered statistically significant. In addition, patients with missing values on any of the analyzed predictors were not included in the regression model.
Results
Baseline Characteristics
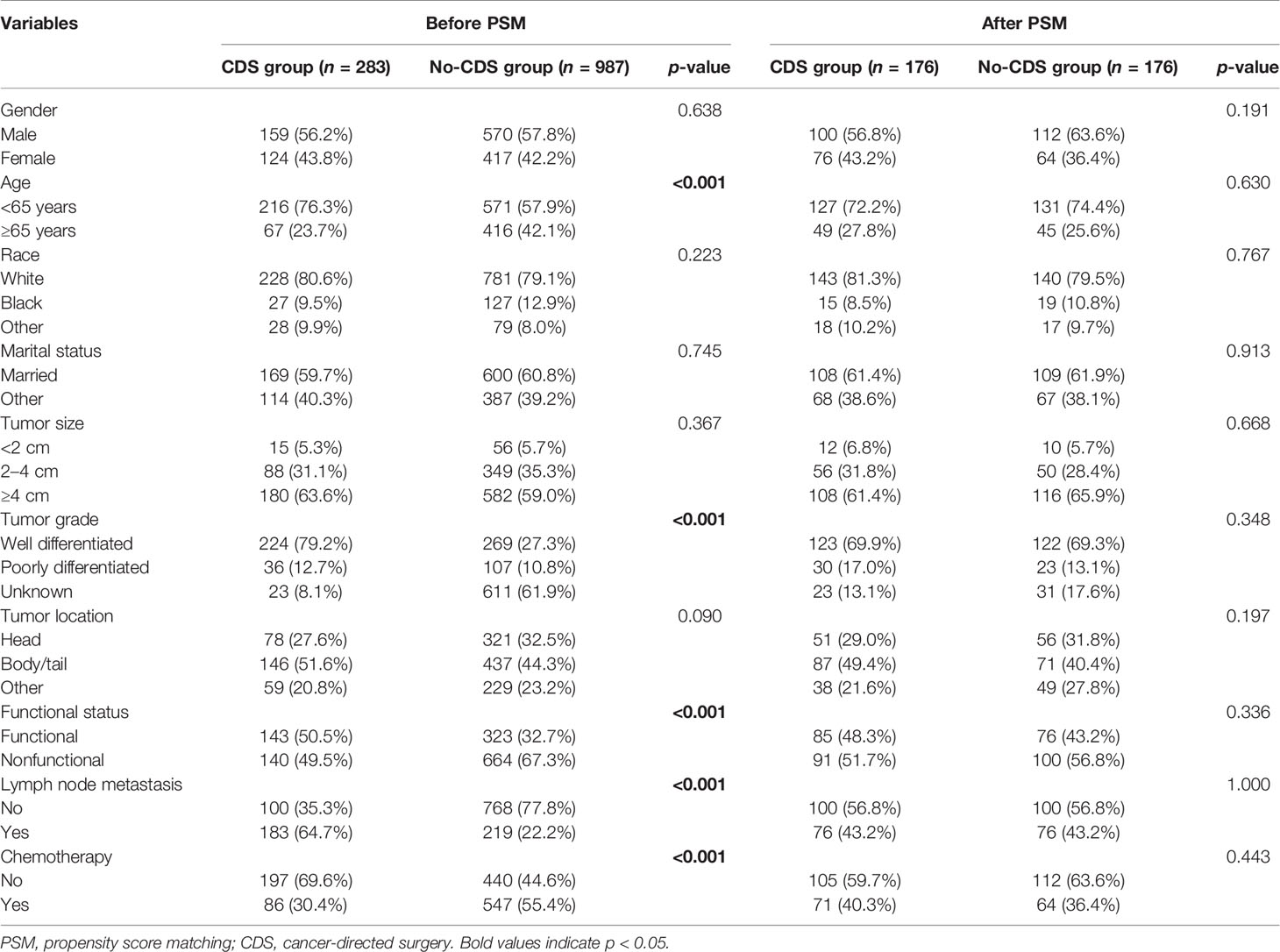
Between 2010 and 2018, a total of 1,270 patients with histologically confirmed PanNETs and hepatic metastasis at the time of diagnosis were identified and analyzed in our study. Of these patients, 283 (22.3%) underwent cancer-directed surgery (CDS) of the primary tumor, while the remaining 987 (77.7%) did not. Patient characteristics and clinicopathologic features are presented and compared in Table 1 before and after PSM. As shown in Table 1, before PSM, patients who underwent CDS were significantly younger and tended to be classified as having a higher proportion of lymph node metastasis compared to those who did not undergo CDS. In addition, patients in the CDS cohort were more likely to have well-differentiated and functional tumors. After PSM, 176 patients were matched in each group, and the comparisons between the two groups showed that baseline characteristics were well-balanced.
Survival Outcomes Before and After PSM
It is noteworthy that the overall survival (OS) and cancer-specific survival (CSS) were both significantly better in the CDS group than in the no-CDS group regardless of PSM or not. Before PSM, the median OS was 22.0 months in the no-CDS group and 95.0 months in the CDS group (p < 0.001). After PSM, the median OS was 95.0 and 31.0 months in the no-CDS and CDS cohorts, respectively (p < 0.001) (Figure 1).
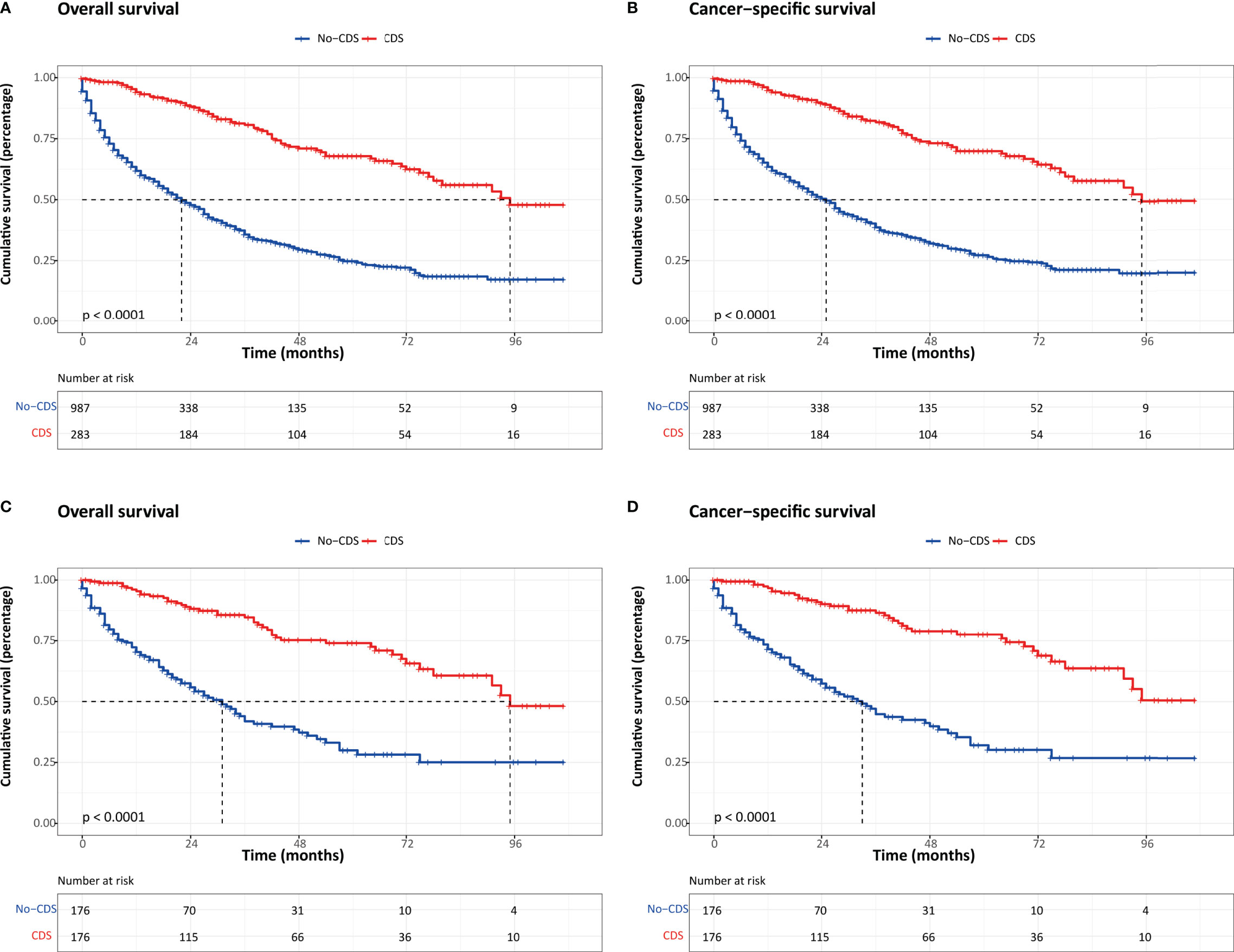
Figure 1 Kaplan–Meier survival estimates for patients in CDS and No-CDS groups: (A) overall survival before PSM. (B) Cancer-specific survival before PSM. (C) Overall survival after PSM. (D) Cancer-specific survival after PSM. CDS, cancer-directed surgery.
Subgroup Analysis of OS
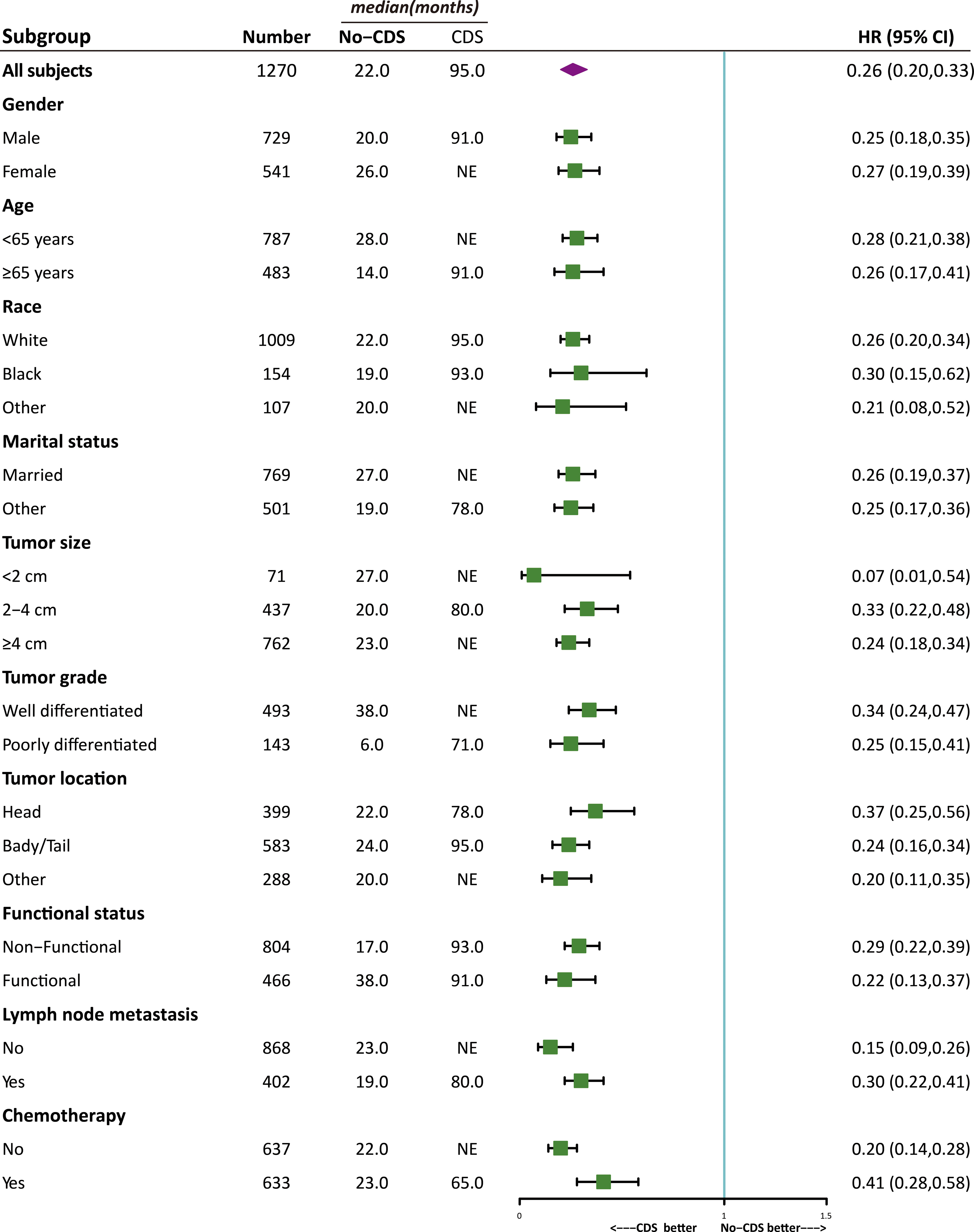
As shown in Figures 2, 3, the effect of CDS on survival outcomes in all prespecified subgroups was then examined. After CDS, patients in the CDS cohort were associated with prolonged OS among all subgroups compared to those in the no-CDS cohort.
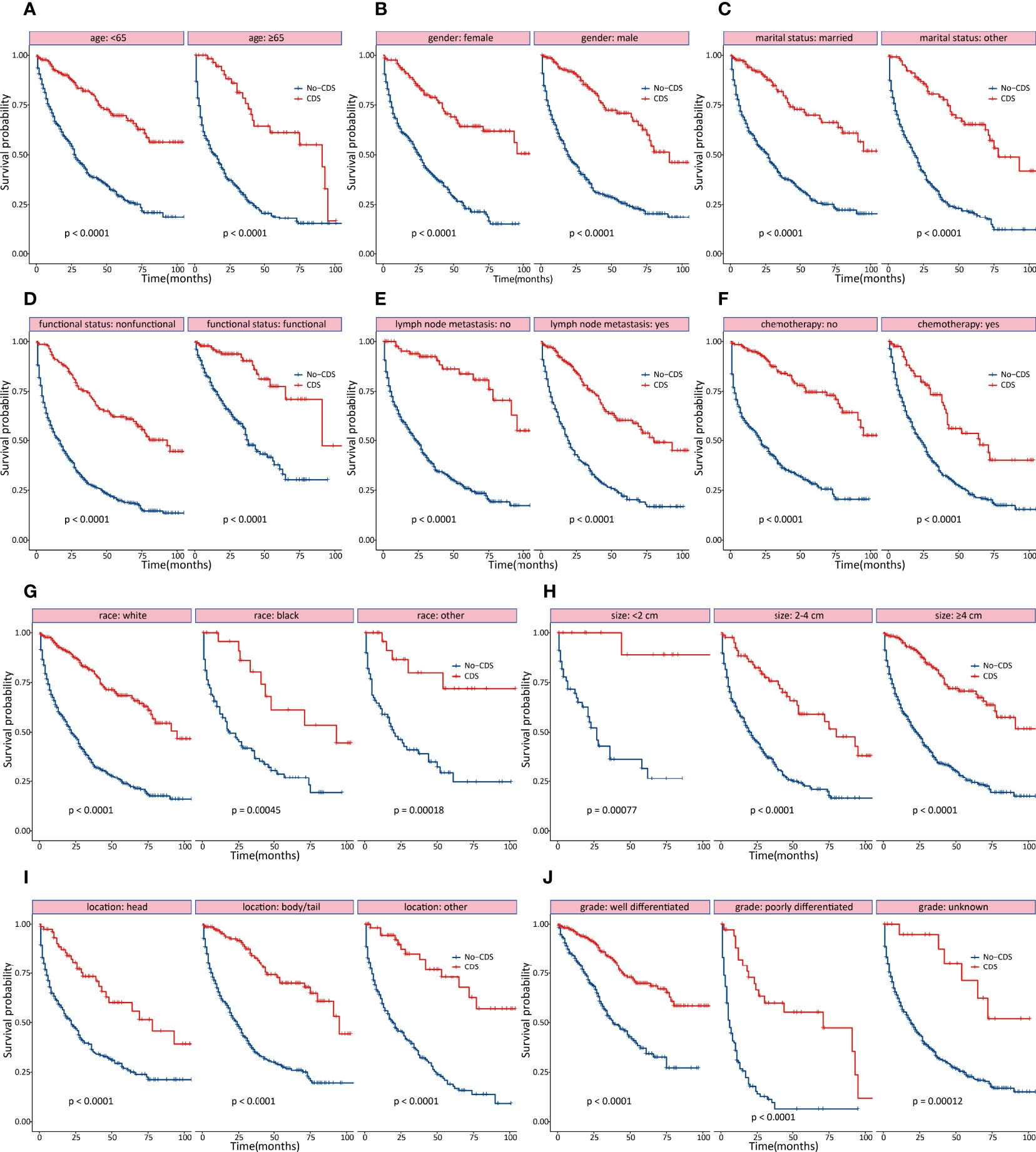
Figure 2 Subgroup analyses of OS stratified by clinicopathological characteristics. (A) age (B) gender (C) marital status (D) functional status (E) lymph node status (F) chemotherapy (G) race (H) tumor size (I) tumor location (J) tumor differentiation.
Predictors of Survival
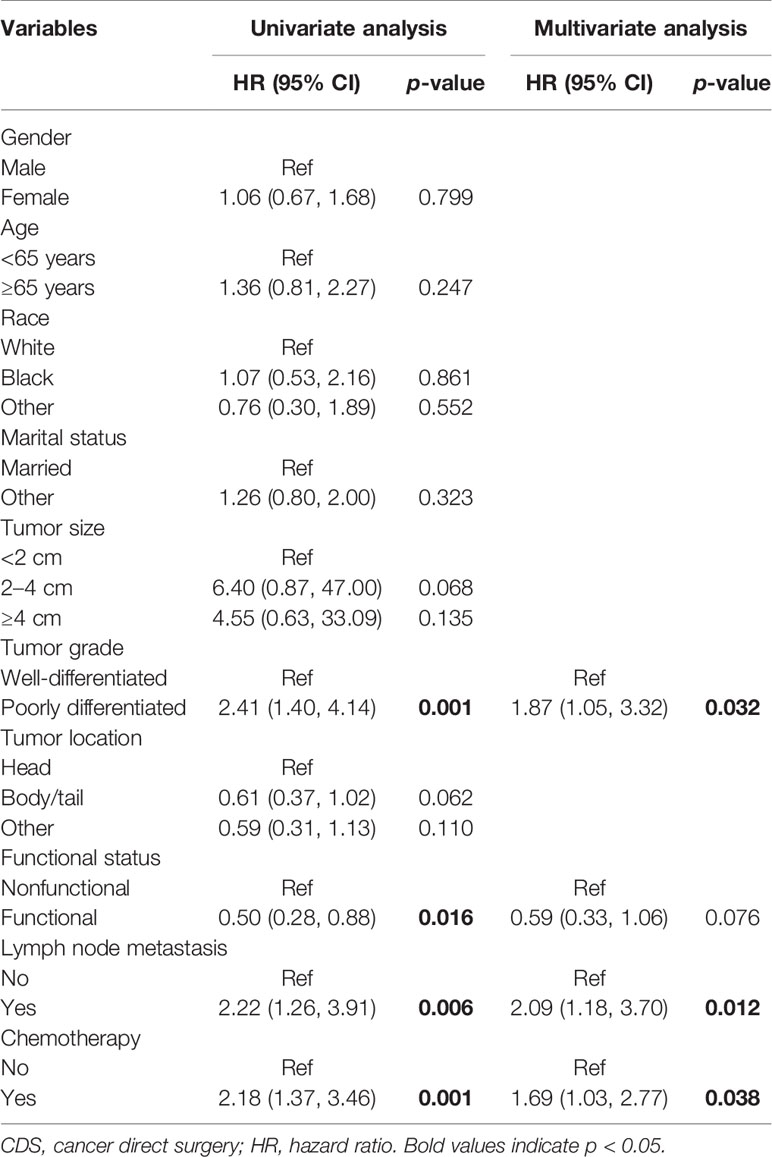
In multivariate COX regression analysis, when considering the whole population, age at diagnosis, marital status, tumor grade, functional status, and surgery were independently associated with OS (Table 2). As for patients who underwent CDS, multivariate analysis found that tumor grade, lymph node metastasis, and chemotherapy were independent prognostic factors for OS (Table 3).
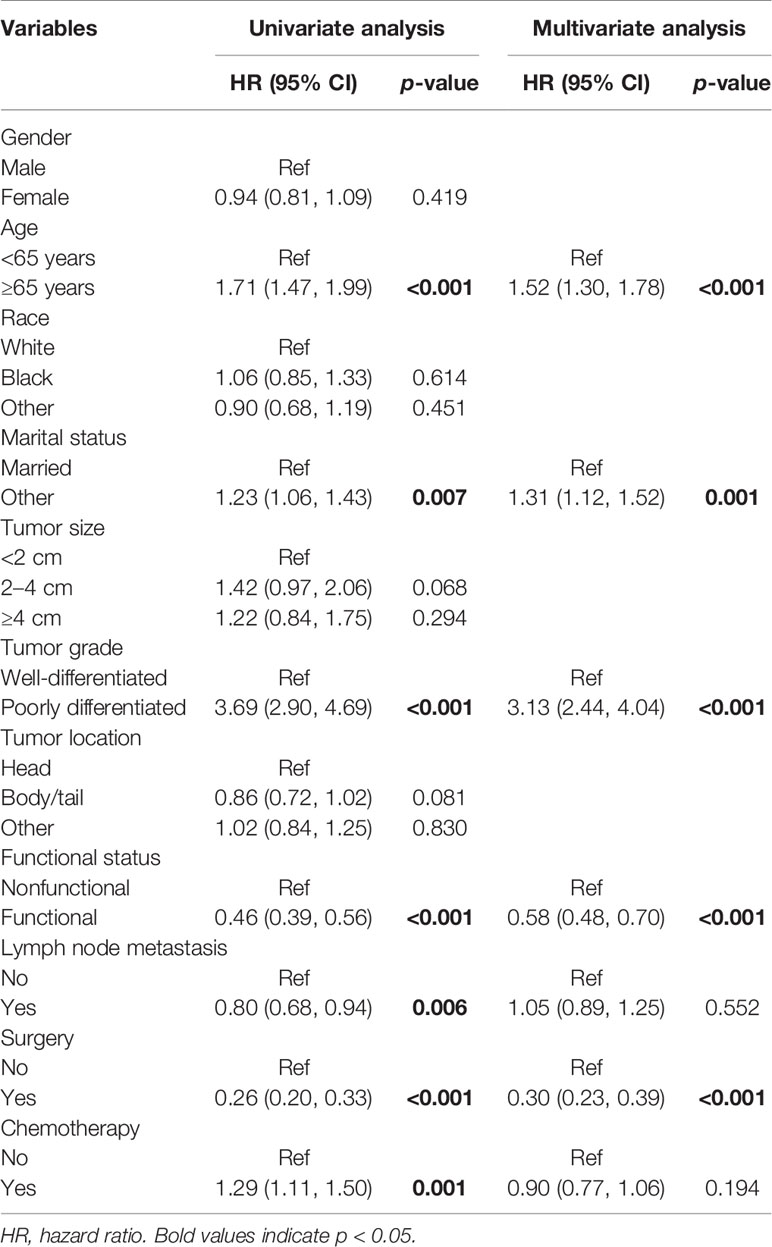
Table 2 Association of clinicopathological factors with OS in oligometastatic liver metastasis in PNET patients.
Factors Associated With CDS
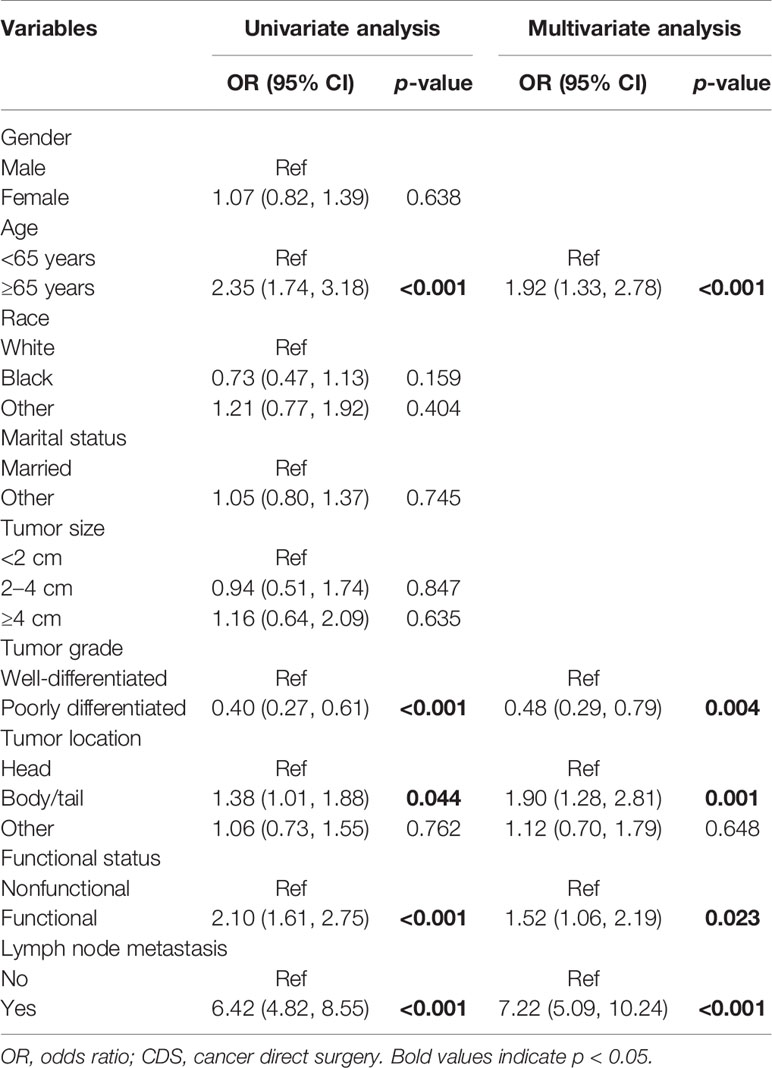
To gain insight on patient selection, the logistic regression model was used to analyze the factors correlated with CDS (Table 4). On univariable analysis, age at diagnosis, tumor grade, tumor location, functional status, and lymph node status were found to be associated with patients, whether they received CDS or not. The significant factors in univariate analysis were then incorporated into the multivariate logistic regression model. Multivariate analysis demonstrated that those who were older than 65, with well-differentiated tumors, with tumors located in the pancreatic body or tail, and had lymph node metastasis were more frequently to undergo CDS. Among all the identified variables, lymph node status showed the most powerful association with CDS.
Exploratory Analyses
To compare the efficacy of chemotherapy, the survival outcomes were evaluated between patients who underwent CDS only and those who received CDS plus chemotherapy using the PSM method. Before PSM, we found that elderly patients (≥65 years), patients with well-differentiated, and functional tumors were more likely to undergo CDS alone (Table 5). Also, the OS and CSS were significantly better compared to those who underwent a combination of CDS and chemotherapy. After PSM, the baseline characteristics did not significantly differ between groups, and the survival outcomes were comparable (Figure 4).
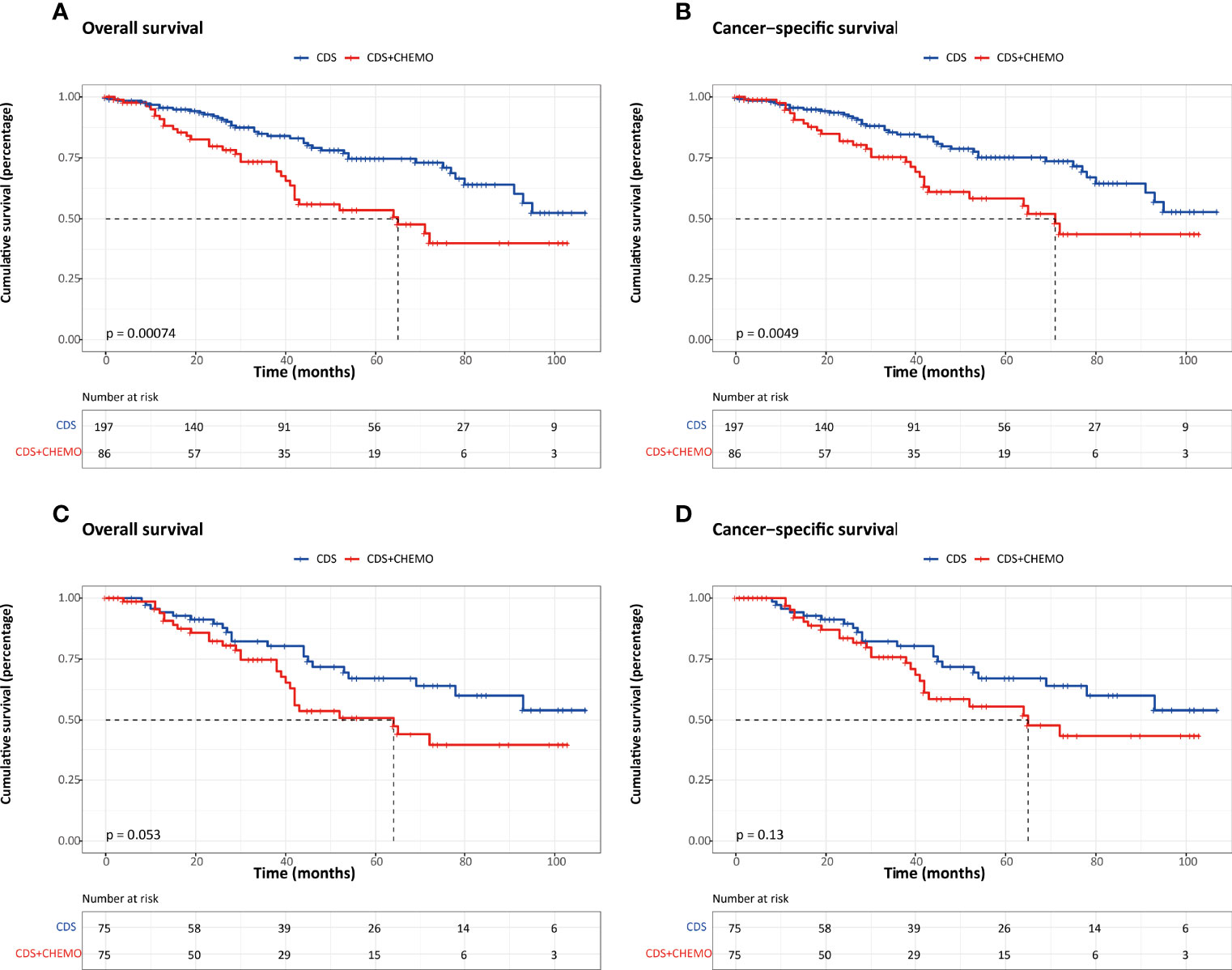
Figure 4 Kaplan–Meier survival estimates between patients who underwent CDS and those who underwent CDS and chemotherapy. (A) Overall survival before PSM. (B) Cancer-specific survival before PSM. (C) Overall survival after PSM. (D) Cancer-specific survival after PSM. CDS, cancer-directed surgery.
Discussion
A comparison of matched cohorts from the SEER database indicated that PanNET patients with liver-only metastasis who underwent CDS had significantly better survival outcomes compared to those without CDS. Our study also revealed tumor grade, lymph node metastasis, and chemotherapy were independent prognostic factors for OS in those who received CDS. In addition, we found that the combination of CDS and chemotherapy did not show survival advantages in comparison to CDS alone among the patients who performed CDS. To the best of our knowledge, it is the first study to verify the survival benefit of primary tumor resection focusing on PanNETs with oligometastatic liver metastasis based on a large population database from the United States.
According to the clinical presentation, PanNETs were classified as functional and nonfunctional tumors; of these, nonfunctional PanNETs accounted for nearly 80% (16, 17). Owing to a lack of specific symptoms and the indolent clinical course, a relatively large proportion of PanNETs were found to present with metastases at the time of diagnosis. Surgical resection is the mainstay treatment for patients with localized disease. However, issues regarding the management of PanNETs with metastases remain a subject of investigation, including the efficacy of primary tumor removal as well as the established criteria for selecting patients most likely to benefit from surgical resection (18, 19). Both the ENETS and NANETS guidelines currently do not recommend routine surgical resection in patients with metastatic PanNETs (20, 21). Other literature argued that neuroendocrine tumors have been regarded as one of few tumor types in which debulking operation could still yield survival benefits in metastatic disease (22, 23). Several previous studies have demonstrated the feasibility and safety of cancer-directed surgery in metastatic PanNET patients (24, 25). However, these publications only included small sample sizes of patients or analyzed patients with various metastatic sites. Our current study, on the other hand, included a large cohort of the population who were diagnosed with PanNETs and liver-only metastasis. A retrospective study including 882 patients with metastatic nonfunctioning pancreatic neuroendocrine tumors showed that removal of the primary tumor was associated with improved survival compared to those without resection (26). Feng et al. identified 350 patients with metastatic pancreatic neuroendocrine carcinoma and confirmed the survival benefits of primary tumor resection (27). Givi et al. investigated whether primary tumor resection in patients with gastrointestinal carcinoid neoplasm and hepatic metastases provides improved survival outcomes. A total of 84 patients were enrolled, 60 of whom received primary tumor resection, and survival analysis displayed that the resected group had a significantly longer median survival compared with the nonresected group (22). Compared with previous reports focusing on broader samples of metastatic PanNETs, our study differed in the respect that we concentrated specifically on patients with oligometastatic liver metastasis at diagnosis. Therefore, some differences may be attributed to the study population under analysis. In our study, we performed propensity score analysis to reduce the selection biases between groups and demonstrated the survival benefits of CDS even in the setting of liver-only metastatic disease. Furthermore, our results indicated that age at diagnosis, marital status, tumor grade, functional status, and surgery were independently associated with OS in all study populations.
In particular, multivariate logistic regression found that elderly patients, good tumor differentiation, tumors located in the body or tail of the pancreas, and lymph node metastasis were significantly correlated to patients who underwent CDS. In other words, these clinical variables might be used in patient selection when considering surgical treatment on PanNETs with liver-only metastasis. Subgroup survival analysis suggested that CDS was associated with survival advantages in all stratified groups, even in those with the presence of lymph node involvement. Owing to the indolent biological behavior, more aggressive surgical resection should be considered even in advanced stage patients.
Although there are various treatment options for PanNETs, surgery represents the cornerstone of the management because of the potential symptomatic and survival benefits (28–30). However, the role of chemotherapy in patients with liver metastasis remains unclear. In our study, we investigated the efficacy of chemotherapy in selected patients using the PSM method. In the matched cohorts, the survival results were comparable between patients who performed CDS alone and those who received CDS and chemotherapy.
Our study has some limitations. Firstly, the selection biases in this retrospective study cannot be fully avoided even though we used the PSM method. Secondly, the SEER database did not give us information on the extent of liver metastasis, such as the number and size of metastases. Thirdly, some important factors were not recorded in the SEER database, including the Ki-67 index, comorbidities, preoperative treatments, chemotherapy regimens, targeted therapy, and surgery-related data, which may influence our analysis. For example, patients with serious comorbidities were less likely to undergo surgery and were associated with poor survival results. Lastly, the definition of the functional status of PanNETs may not be quite accurate. Despite these limitations, our study sheds light on the role of CDS in oligometastatic liver metastatic PanNET patients.
Conclusion
In conclusion, our study based on a large population database revealed that CDS was associated with survival benefits in selected patients with PanNETs and liver-only metastasis. It is imperative to keep in mind that the treatment option should be guided based on patient characteristics and interdisciplinary consultation.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://seer.cancer.gov/.
Author Contributions
GS and KL contributed to the conception and designed the study. ZY and JL drafted the manuscript and conducted the statistical analysis. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Perri G, Prakash LR, Katz MHG. Pancreatic Neuroendocrine Tumors. Curr Opin Gastroenterol (2019) 35(5):468–77. doi: 10.1097/mog.0000000000000571
2. Kotagal M, von Allmen D. Gastroenteropancreatic Neuroendocrine Tumors. Semin Pediatr Surg (2020) 29(3):150928. doi: 10.1016/j.sempedsurg.2020.150928
3. Ma ZY, Gong YF, Zhuang HK, Zhou ZX, Huang SZ, Zou YP, et al. Pancreatic Neuroendocrine Tumors: A Review of Serum Biomarkers, Staging, and Management. World J Gastroenterol (2020) 26(19):2305–22. doi: 10.3748/wjg.v26.i19.2305
4. Cives M, Strosberg JR. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J Clin (2018) 68(6):471–87. doi: 10.3322/caac.21493
5. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol (2017) 3(10):1335–42. doi: 10.1001/jamaoncol.2017.0589
6. Xu Z, Wang L, Dai S, Chen M, Li F, Sun J, et al. Epidemiologic Trends of and Factors Associated With Overall Survival for Patients With Gastroenteropancreatic Neuroendocrine Tumors in the United States. JAMA Netw Open (2021) 4(9):e2124750. doi: 10.1001/jamanetworkopen.2021.24750
7. Das S, Dasari A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr Oncol Rep (2021) 23(4):43. doi: 10.1007/s11912-021-01029-7
8. Clift AK, Kidd M, Bodei L, Toumpanakis C, Baum RP, Oberg K, et al. Neuroendocrine Neoplasms of the Small Bowel and Pancreas. Neuroendocrinology (2020) 110(6):444–76. doi: 10.1159/000503721
9. Raphael MJ, Chan DL, Law C, Singh S. Principles of Diagnosis and Management of Neuroendocrine Tumours. Cmaj (2017) 189(10):E398–e404. doi: 10.1503/cmaj.160771
10. Mpilla GB, Philip PA, El-Rayes B, Azmi AS. Pancreatic Neuroendocrine Tumors: Therapeutic Challenges and Research Limitations. World J Gastroenterol (2020) 26(28):4036–54. doi: 10.3748/wjg.v26.i28.4036
11. Vaghaiwalla T, Keutgen XM. Surgical Management of Pancreatic Neuroendocrine Tumors. Surg Oncol Clin N Am (2020) 29(2):243–52. doi: 10.1016/j.soc.2019.11.008
12. Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, et al. Gastroenteropancreatic Neuroendocrine Neoplasms: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2020) 31(7):844–60. doi: 10.1016/j.annonc.2020.03.304
13. Jeune F, Taibi A, Gaujoux S. Update on the Surgical Treatment of Pancreatic Neuroendocrine Tumors. Scand J Surg (2020) 109(1):42–52. doi: 10.1177/1457496919900417
14. Kane LT, Fang T, Galetta MS, Goyal DKC, Nicholson KJ, Kepler CK, et al. Propensity Score Matching: A Statistical Method. Clin Spine Surg (2020) 33(3):120–2. doi: 10.1097/bsd.0000000000000932
15. Schober P, Vetter TR. Propensity Score Matching in Observational Research. Anesth Analg (2020) 130(6):1616–7. doi: 10.1213/ane.0000000000004770
16. Andreasi V, Partelli S, Muffatti F, Manzoni MF, Capurso G, Falconi M. Update on Gastroenteropancreatic Neuroendocrine Tumors. Dig Liver Dis (2021) 53(2):171–82. doi: 10.1016/j.dld.2020.08.031
17. Wu J, Sun C, Li E, Wang J, He X, Yuan R, et al. Non-Functional Pancreatic Neuroendocrine Tumours: Emerging Trends in Incidence and Mortality. BMC Cancer (2019) 19(1):334. doi: 10.1186/s12885-019-5543-2
18. Andreasi V, Muffatti F, Guarneri G, Falconi M, Partelli S. Surgical Principles in the Management of Pancreatic Neuroendocrine Neoplasms. Curr Treat Options Oncol (2020) 21(6):48. doi: 10.1007/s11864-020-00736-w
19. Finkelstein P, Sharma R, Picado O, Gadde R, Stuart H, Ripat C, et al. Pancreatic Neuroendocrine Tumors (panNETs): Analysis of Overall Survival of Nonsurgical Management Versus Surgical Resection. J Gastrointest Surg (2017) 21(5):855–66. doi: 10.1007/s11605-017-3365-6
20. Kulke MH, Anthony LB, Bushnell DL, de Herder WW, Goldsmith SJ, Klimstra DS, et al. NANETS Treatment Guidelines: Well-Differentiated Neuroendocrine Tumors of the Stomach and Pancreas. Pancreas (2010) 39(6):735–52. doi: 10.1097/MPA.0b013e3181ebb168
21. Pavel M, Baudin E, Couvelard A, Krenning E, Öberg K, Steinmüller T, et al. ENETS Consensus Guidelines for the Management of Patients With Liver and Other Distant Metastases From Neuroendocrine Neoplasms of Foregut, Midgut, Hindgut, and Unknown Primary. Neuroendocrinology (2012) 95(2):157–76. doi: 10.1159/000335597
22. Givi B, Pommier SJ, Thompson AK, Diggs BS, Pommier RF. Operative Resection of Primary Carcinoid Neoplasms in Patients With Liver Metastases Yields Significantly Better Survival. Surgery (2006) 140(6):891–7. doi: 10.1016/j.surg.2006.07.033
23. Fahmy JN, Varsanik MA, Hubbs D, Eguia E, Abood G, Knab LM, et al. Pancreatic Neuroendocrine Tumors: Surgical Outcomes and Survival Analysis. Am J Surg (2021) 221(3):529–33. doi: 10.1016/j.amjsurg.2020.12.037
24. Jiang J, Park J, Kim S, Daan A, Donahue T, Girgis MD. Surgical Resection of High-Grade Nonfunctional Pancreatic Neuroendocrine Carcinoma is Associated With Improved Survival. J Surg Oncol (2021) 124(8):1373–80. doi: 10.1002/jso.26644
25. Hill JS, McPhee JT, McDade TP, Zhou Z, Sullivan ME, Whalen GF, et al. Pancreatic Neuroendocrine Tumors: The Impact of Surgical Resection on Survival. Cancer (2009) 115(4):741–51. doi: 10.1002/cncr.24065
26. Keutgen XM, Nilubol N, Glanville J, Sadowski SM, Liewehr DJ, Venzon DJ, et al. Resection of Primary Tumor Site is Associated With Prolonged Survival in Metastatic Nonfunctioning Pancreatic Neuroendocrine Tumors. Surgery (2016) 159(1):311–8. doi: 10.1016/j.surg.2015.05.042
27. Feng T, Lv W, Yuan M, Shi Z, Zhong H, Ling S, et al. Surgical Resection of the Primary Tumor Leads to Prolonged Survival in Metastatic Pancreatic Neuroendocrine Carcinoma. World J Surg Oncol (2019) 17(1):54. doi: 10.1186/s12957-019-1597-5
28. Goretzki PE, Mogl MT, Akca A, Pratschke J. Curative and Palliative Surgery in Patients With Neuroendocrine Tumors of the Gastro-Entero-Pancreatic (GEP) Tract. Rev Endocr Metab Disord (2018) 19(2):169–78. doi: 10.1007/s11154-018-9469-9
29. Knigge U, Hansen CP. Surgery for GEP-NETs. Best Pract Res Clin Gastroenterol (2012) 26(6):819–31. doi: 10.1016/j.bpg.2012.12.005
Keywords: pancreatic neuroendocrine tumors, liver metastasis, surgery, survival, PSM
Citation: Yang Z, Liang J, Leng K and Shi G (2022) Survival Benefit of Surgical Resection for Pancreatic Neuroendocrine Tumors With Oligometastatic Liver Metastasis: A Retrospective and Propensity Score-Matching Analysis. Front. Oncol. 12:903560. doi: 10.3389/fonc.2022.903560
Received: 24 March 2022; Accepted: 18 May 2022;
Published: 30 June 2022.
Edited by:
Ravindra Deshpande, Wake Forest School of Medicine, United StatesReviewed by:
Shravan Girada, University of California, San Diego, United StatesJagadeeswara Rao Bommi, Case Western Reserve University, United States
Copyright © 2022 Yang, Liang, Leng and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangjun Shi, c2dqenBAaG90bWFpbC5jb20=; Kaiming Leng, bGVuZ2thaW1pbmcxMTI4QGhvdG1haWwuY29t
 Zhen Yang
Zhen Yang Jie Liang
Jie Liang