- Department of Oncology, The Second Affiliated Hospital of Dalian Medical University, Dalian, China
ANO1, a calcium-activated chloride channel (CACC), is also known as transmembrane protein 16A (TMEM16A). It plays a vital role in the occurrence, development, metastasis, proliferation, and apoptosis of various malignant tumors. This article reviews the mechanism of ANO1 involved in the replication, proliferation, invasion and apoptosis of various malignant tumors. Various molecules and Stimuli control the expression of ANO1, and the regulatory mechanism of ANO1 is different in tumor cells. To explore the mechanism of ANO1 overexpression and activation of tumor cells by studying the different effects of ANO1. Current studies have shown that ANO1 expression is controlled by 11q13 gene amplification and may also exert cell-specific effects through its interconnected protein network, phosphorylation of different kinases, and signaling pathways. At the same time, ANO1 also resists tumor apoptosis and promotes tumor immune escape. ANO1 can be used as a promising biomarker for detecting certain malignant tumors. Further studies on the channels and the mechanism of protein activity of ANO1 are needed. Finally, the latest inhibitors of ANO1 are summarized, which provides the research direction for the tumor-promoting mechanism of ANO1.
Introduction
ANO1, as a calcium-activated chloride channel (CaCC), also known as transmembrane protein 16A (TMEM16A), is a voltage-sensitive calcium-activated chloride channel with ten transmembrane fragments with amino and carboxyl-terminal structure (1). It is widely expressed in many cells, including epithelial cells, airways, smooth muscle cells, vascular endothelial cells, and myocardium. ANO1 also modulates several physiological functions, such as fluid and electrolyte secretion, intestinal motility, cardiac and neuronal excitability, vascular smooth muscle contraction, and heat pain (2, 3). Abnormal circulating ANO1 is associated with susceptibility and pathogenesis of several human diseases and pathological entities, including cystic fibrosis, various cancers, hypertension, and gastrointestinal motility disorders (4–6).
ANO1 also has a crucial role in tumor occurrence, development, metastasis, proliferation, anti-apoptosis, and epigenetic regulation is often overexpressed in tumor tissues and is an experimental target for antitumor therapies. However, the biological functions of malignant tumors remain a controversial issue. The present review aims to explore the role of ANO1 in cancer pathogenesis and therapeutics and to elucidate the mechanisms underlying the relation between ANO1 and malignancy.
Ano1 as A Potential Biomarker In Cancer
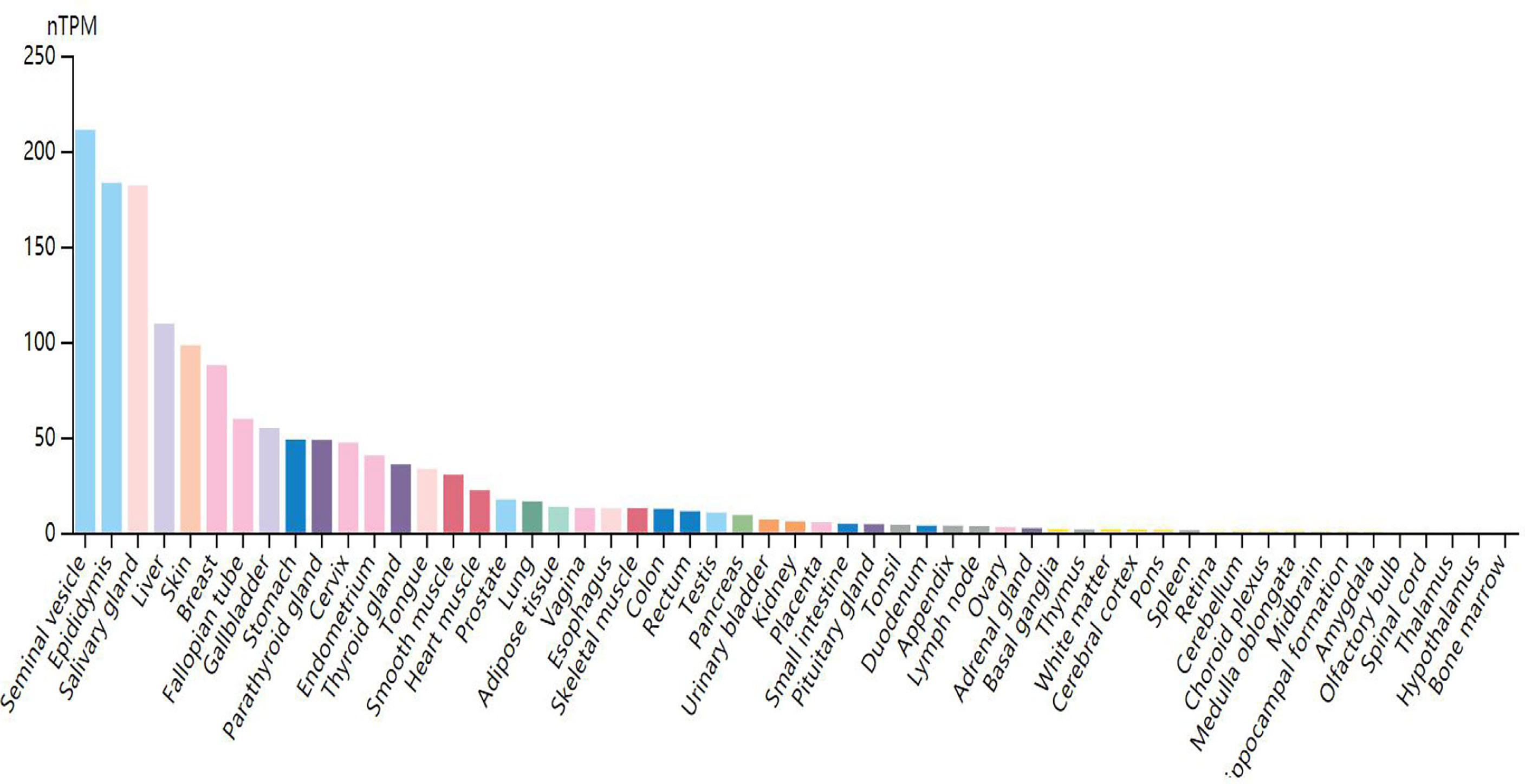
Through research on Human Protein Atlas and GEPIA database, ANO1 overexpression in various malignancies tissues (Figure 1). At the same time, up-regulation of ANO1 expression is associated with a worse prognosis in many malignant tumors (Figures 2, 3). ANO1 was reported as a promising biomarker of malignant potential, stage progression, and prognosis, aiding the monitoring of certain malignancies.
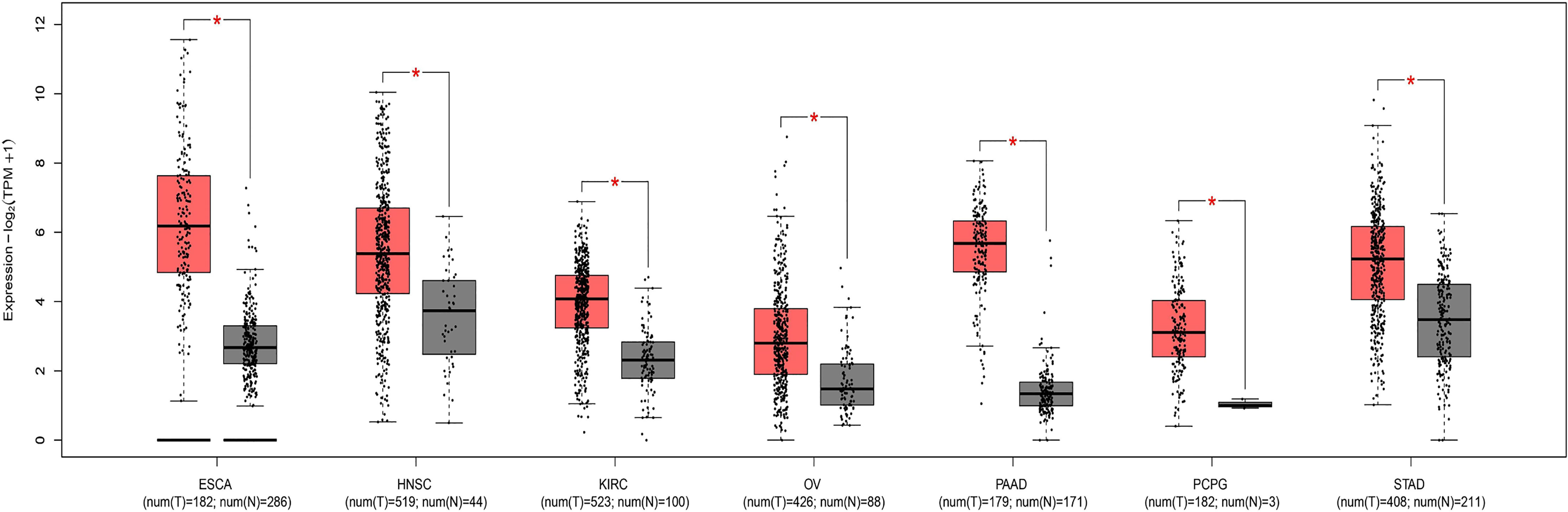
Figure 2 The expression of ANO1 was up-regulated in various malignant tumor ESCA Esophageal carcinoma; HNSC, Head and Neck squamous cell carcinoma; KIRC, Kidney renal clear cell carcinoma; OV, Ovarian serous cystadenocarcinoma; PAAD, Panecreatic adenocarcinoma; PCPG, Pheochromocytoma and Paraganglioma; STAD, Stomach adenocarcinoma. * indicates that the expression of ANO1 is statistically significant between groups.
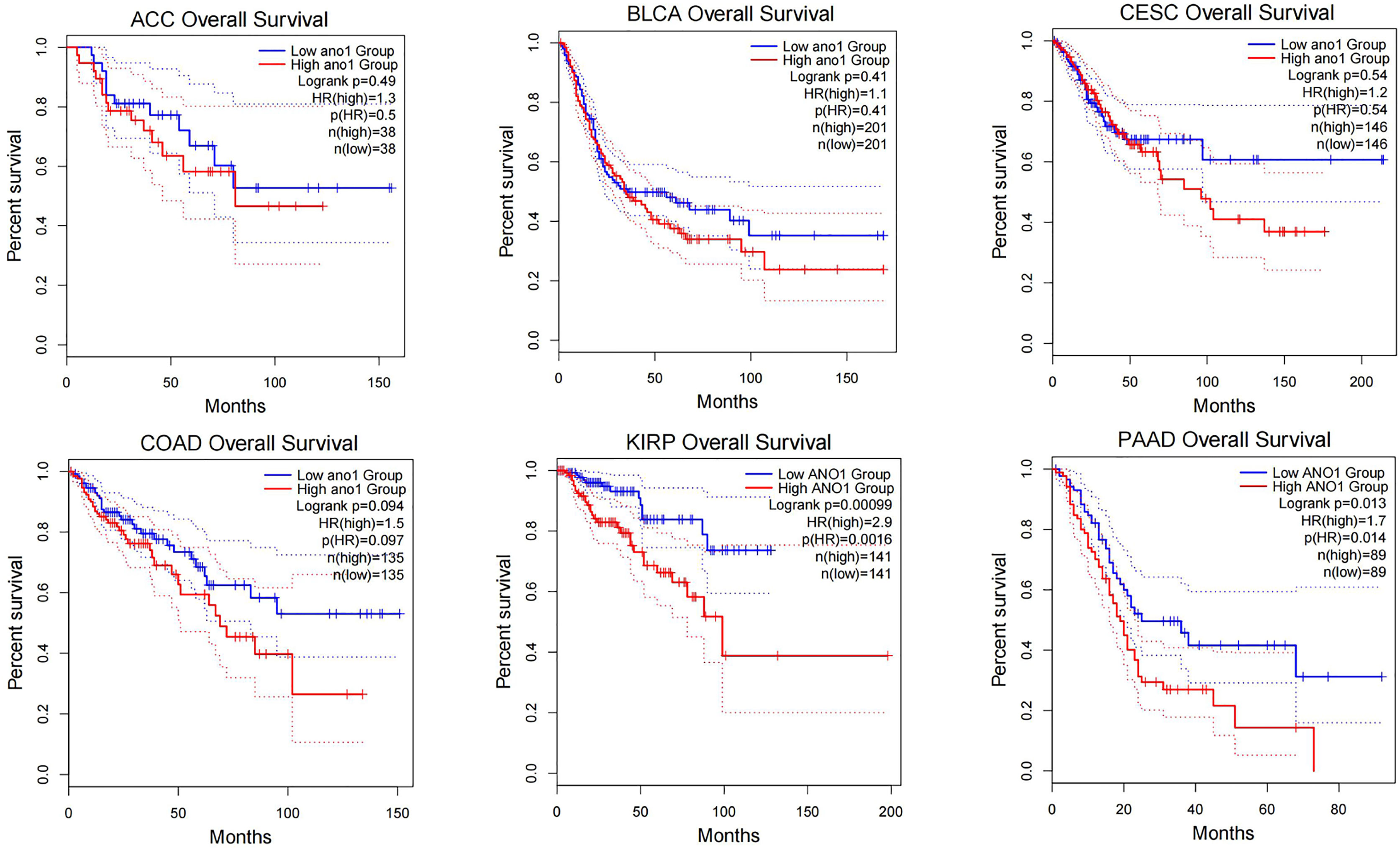
Figure 3 The increased expression of ANO1 is associated with poor prognosis in many malignat tumors ACC, Adrenocortical carcinoma; BLCA, Bladder Urothelial Carcinoma; CESC, Cervical squamous cell carcinoma and endocervical adenocarcinoma; COAD, Colon adenocarcinoma; KIRP, Kidney renal papillary cell carcinoma.
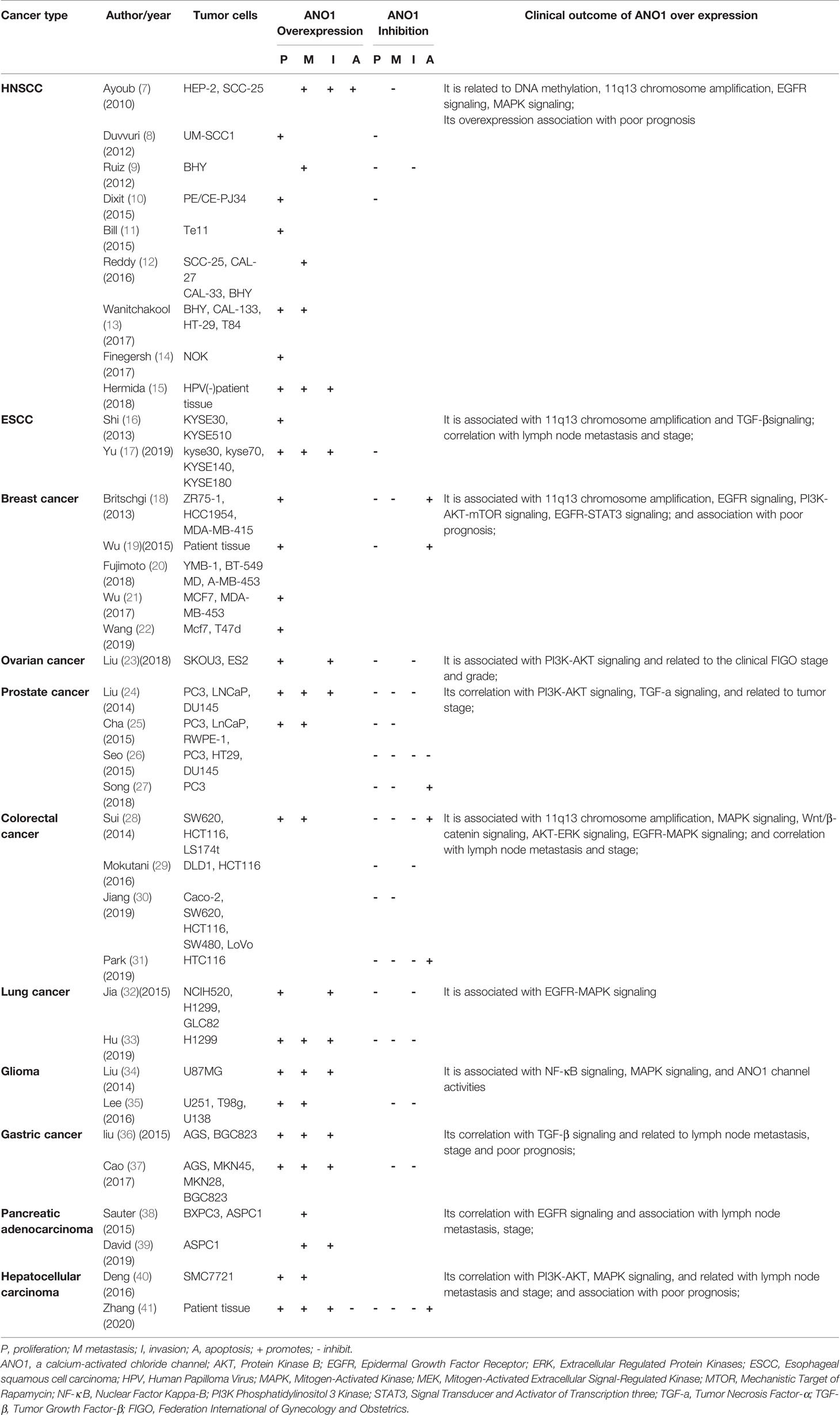
The tumor growth and metastasis were observed by transplantation of nude mice and tumor cell culture in vivo and vitro. Table 1 summarizes the role of ANO1 in different tumors. ANO1 was significantly upregulated in various malignant tumors and promotes the invasion of cancer cells. ANO1 overexpression is associated with lymph node metastasis, disease grading and poor prognosis in gastric cancer, liver cancer, and colon cancer (28, 36, 40). Overexpression of ANO1 promoted the growth and invasion of lung cancer, Head and Neck squamous cell carcinoma (HNSCC), breast cancer and pancreatic cancer through the epidermal growth factor receptor(EGFR)signal pathway (11, 22, 39). Especially in breast cancer cells, ANO1 and EGFR-signal transducer and activator of transcription 3(STAT3)signaling pathways form a positive feedback path to promote the proliferation and growth of breast cancer cells. At the same time, ANO1 also activates the Ras-Raf-MEK-ERK1/2 signaling pathway to promote HNSCC and colon cancer (22, 28). ANO1 can regulate the tumor growth factor-β(TGF-β) signaling pathway to promote Esophageal squamous cell carcinoma (ESCC) proliferation, migration, and invasion (17). Silencing or inhibition of ANO1 upregulates tumor necrosis factor-α (TNF-α) expression in prostate cancer cells (27). ANO1 can promote Hepatocellular carcinoma (HCC) and Ovarian cancer metastasis by activating the phosphatidylinositol three kinase-protein kinase B (PI3K-AKT) signaling pathway (23, 41). Meanwhile, ANO1 participates in immune escape in gastrointestinal stromal tumors (42). Finally, neutralization of ANO1 activity with gene knockdown or ANO1 inhibitors has shown anti-neoplastic actions both in vivo and in vitro models of cancer (Table 1).
In summary, it shows that ANO1 is related to cancer risk and progression in the following aspects: 1) its upregulation in a variety of tumor tissues; 2) its correlation with tumor proliferation, invasion depth, lymph node metastasis, distant metastasis, and apoptosis;3) the gene expression level of ANO1 is closely related to tumor size and differentiation; 4) its association with advanced stage and poor prognosis; 5) its interacts with various oncogenic signaling networks; 6) it regulate tumor microenvironmental tumorigenic signaling pathways.
The expression of ANO1 seems to be controlled by various molecules and stimuli. Different cells have different regulatory mechanisms and signaling pathways. ANO1 can participate in various signal pathways not only with the interaction with various proteins but also with the ion pathway that regulates the ion homeostasis of tumor cells, thereby promoting the critical functions of tumor cells. Further studies to clarify the molecular mechanism are required, for up-regulated tumors, promoting proliferation, metastasis, invasion, and avoiding apoptosis. The mechanism of ANO1’s involvement in malignant tumors will be reviewed.
The Mechanism of Ano1 Involved in Cancer
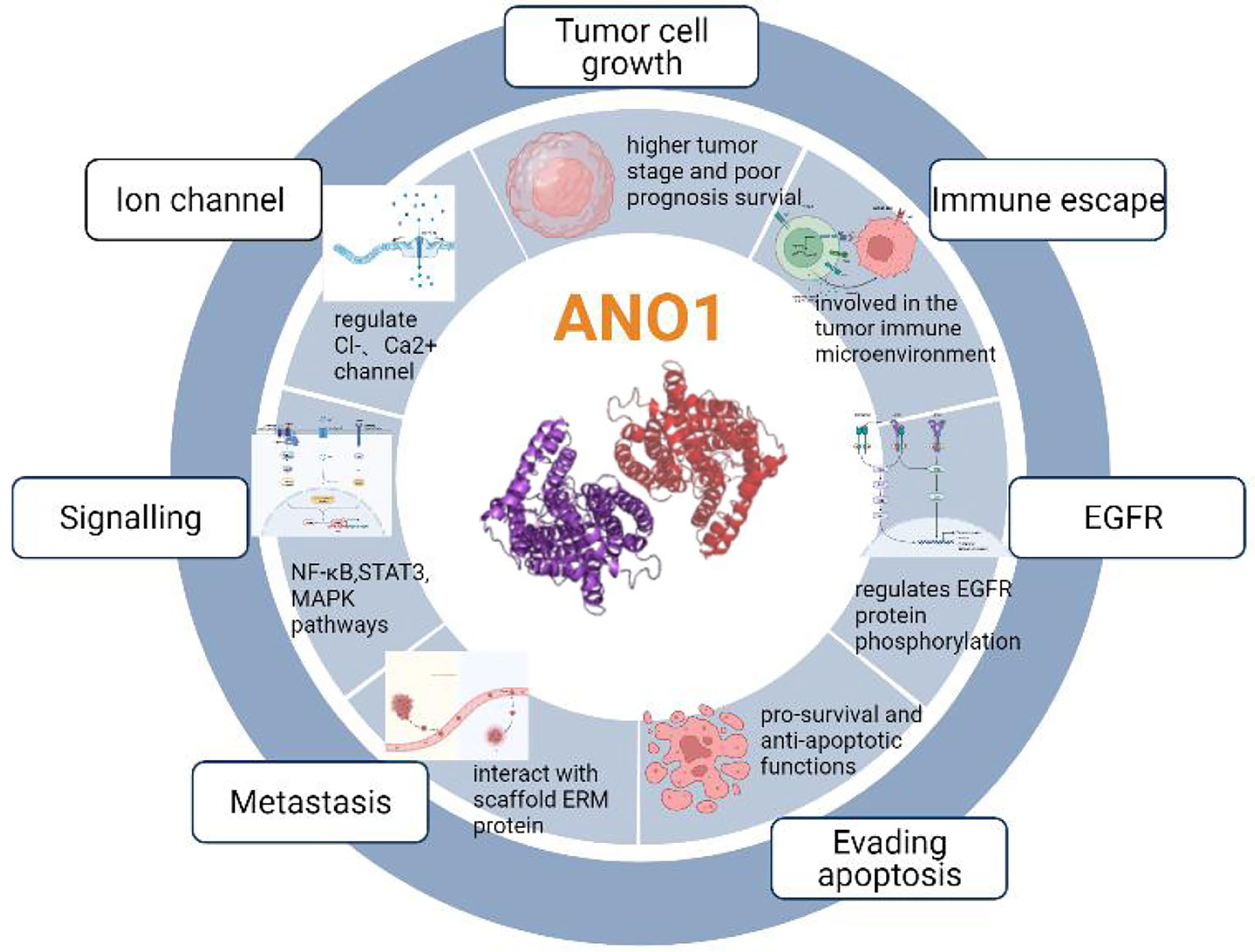
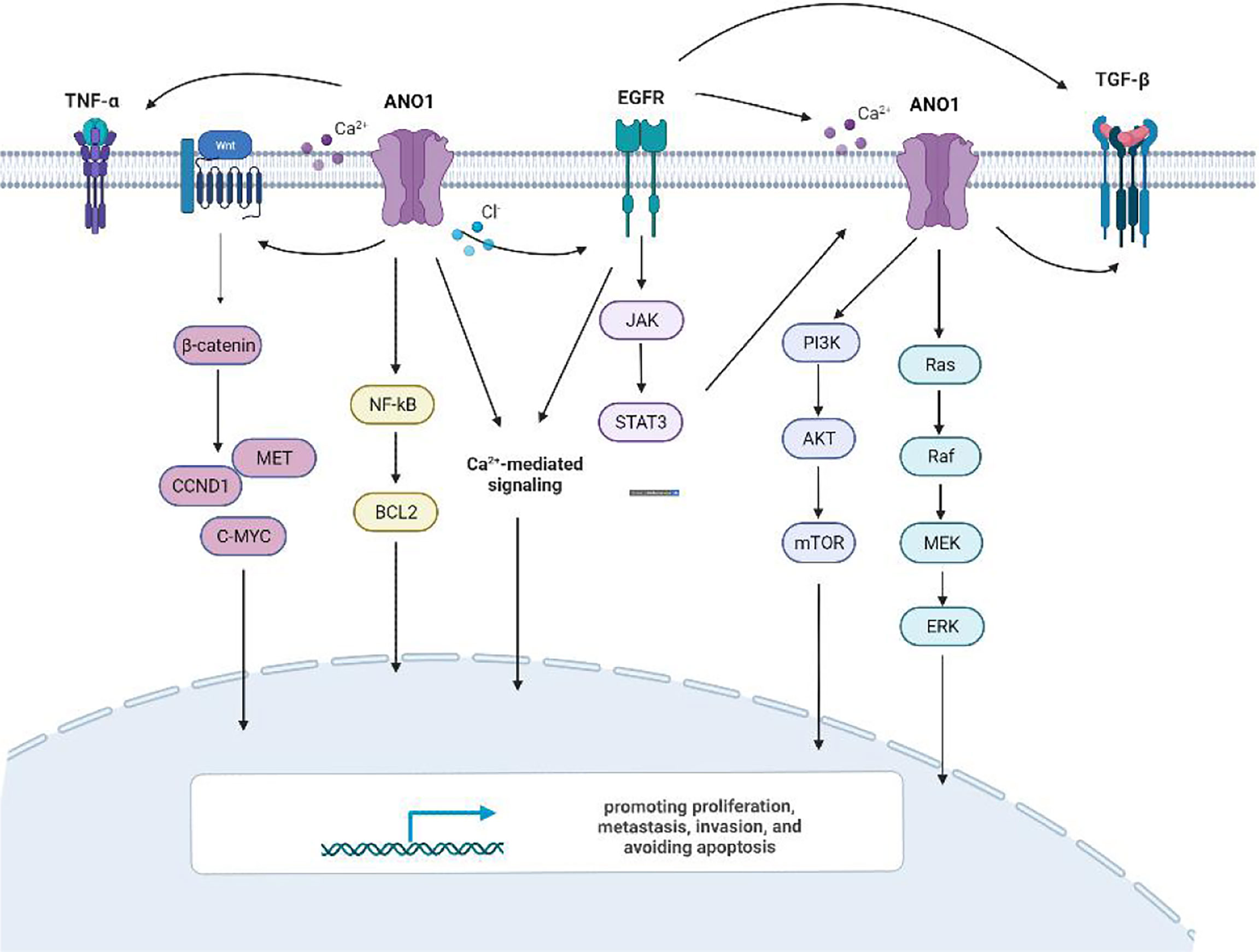
Previous studies have shown that ten biological functions formed during the multi-stage development of human tumors constitute the characteristics of cancer (43). These ten characteristics include self-sufficiency in growth signals, insensitivity to anti-growth signals, evading apoptosis, Limitless replicative potential, sustained angiogenesis, tissue invasion & metastasis, genome instability and mutation, tumor-Promoting inflammation, deregulating cellular energetics, avoiding immune destruction. The involvement of ANO1 in tumorigenesis is currently related to the following aspects (Figure 4).
ANO1 Overexpression Is Involved in the Unlimited Replication and Proliferation of Tumor Cells
ANO1 is upregulated in various tumor tissues and correlates with proliferation, invasion depth, lymph node metastasis, distant metastasis, and apoptosis. Multiple mechanisms can explain the overexpression of ANO1. ANO1 is located on human chromosome 11q13, commonly amplified in several cancers, and participates in tumorigenesis, invasion, and migration. Therefore, the most common mechanism of ANO1 overexpression in cancer amplifies the 11q13 locus (10, 44). This expansion has been described in some cancer types, including breast, gastric, esophageal, and lung carcinoma. However, it is essential to mention that 11q13 amplification resulted in a higher TMEM16A expression in human breast cancer and HNSCC than in non-11q13-amplified tumors. Meanwhile, ANO1 is sufficient to promote cell proliferation without 11q13 amplification, which shows that 11q13 gene amplification is not the only factor that promotes ANO1 overexpression (18).
ANO1 Interacts With Various Proteins to Promote Tumor Proliferation and Invasion
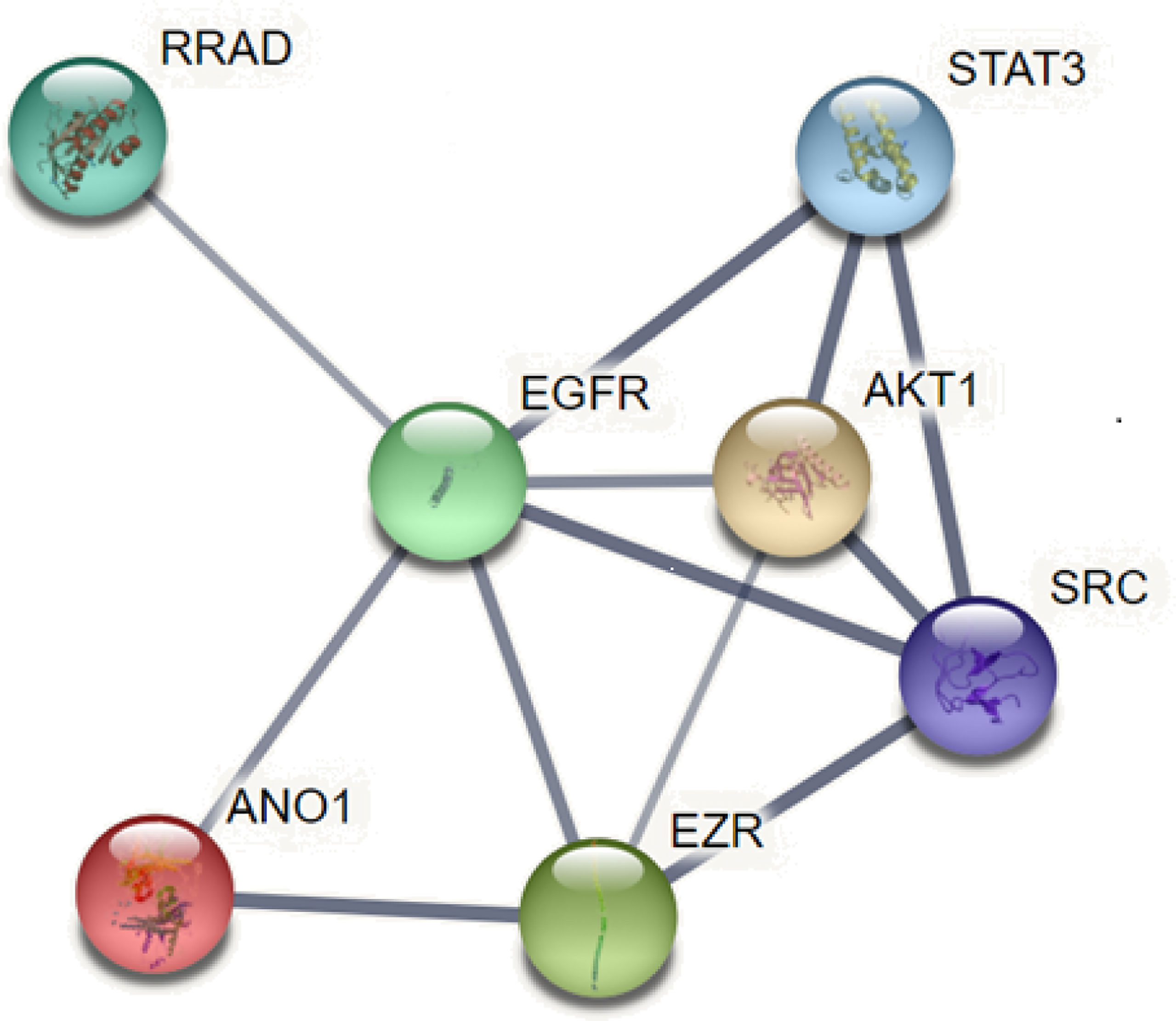
EGFR is overexpressed in many tumors such as HNSCC, breast cancer, and pancreatic cancer and is involved in tumorigenesis. ANO1 was also observed as a core protein in the STRING protein network pathway, which can regulate EGFR constitutive protein phosphorylation and related signal pathways, such as protein tyrosine kinase (SRC), protein kinase B (AKT), promote the proliferation of cancer cells (18, 45) (Figure 5). The interaction between ANO1 and EGFR can also increase ANO1 protein stability. At the same time, ANO1 also has a significant effect on the remodeling of EGF-induced protein phosphorylation. The study also found that the AKT and extracellular regulated protein kinases (ERK) signaling pathways are not affected by the expression of ANO1, which was further confirmed by the STRING pathway of ANO1. So, the relationship between the expression of ANO1 and EGFR in different signaling pathways is different (39). Therefore, it is crucial to understand how ANO1 regulates the EGFR signaling pathway.
Recent data in pancreatic cancer show that ANO1 is necessary to promote EGF-induced EGFR signal transduction, EGF-mediated Ca2+ storage depends on ANO1, and the ANO1-mediated Ca2+ signaling pathway regulates EGF-induced may be involved in the proliferation of pancreatic cancer cells, and may dramatically impact the migration (46, 47). It can be seen that ANO1 provides a new entry point for treating pancreatic cancer.
In the study of breast cancer cells, EGF initiates EGFR/STAT3 signal transduction, which promotes the overexpression of ANO1, while ANO1 overexpression further activates the EGFR-STAT3 signal transduction of breast cancer cells. The positive feedback loop between ANO1 and EGFR-STAT3 promotes the proliferation and migration of breast cancer cells. Knockout of ANO1 will reduce the phosphorylation of EGFR in breast cancer cells, further inhibit the activation of AKT, SRC, and ERK, and reduce the autocrine of EGFR ligands, EGF and TGF-β, suggesting that ANO1 may increase EGFR ligands The autocrine activates EGFR signal transduction (22). Therefore, we considered that ANO1 could activate the EGFR signaling pathway by increasing EGFR expression, phosphorylation, and EGFR ligand autocrine. More research is needed to confirm the connection between ANO1 and EGFR.
ANO1 also regulates cell morphology and volume and directly interacts with scaffold ERM protein (Ezrin/Radixin/Moesin) (48) (Figure 5). The ERM protein connects the plasma membrane with the actin cytoskeleton, affects the cell morphology of cancer cells, and regulates the movement, metastasis, and invasion of tumor cells. At the same time, ERM can be activated by many ligands as scaffold proteins to promote the signal transduction of membrane proteins, including EGF ligands, to promote the deformation and invasion of cancer cells. Studies have found that ANO1 may interact with EGFR to recruit ERM proteins, promoting EGFR signal transduction and activation of the MAPK/AKT pathway (11). The ANO1 inhibitor Des inhibited the activity and migration of non-small-cell lung carcinoma by decreasing the levels of Ano1, p-ERK1/2 and p-EGFR (49).
ANO1 Activates Multiple Signaling Pathways Involved in Tumor Proliferation, Migration and Invasion
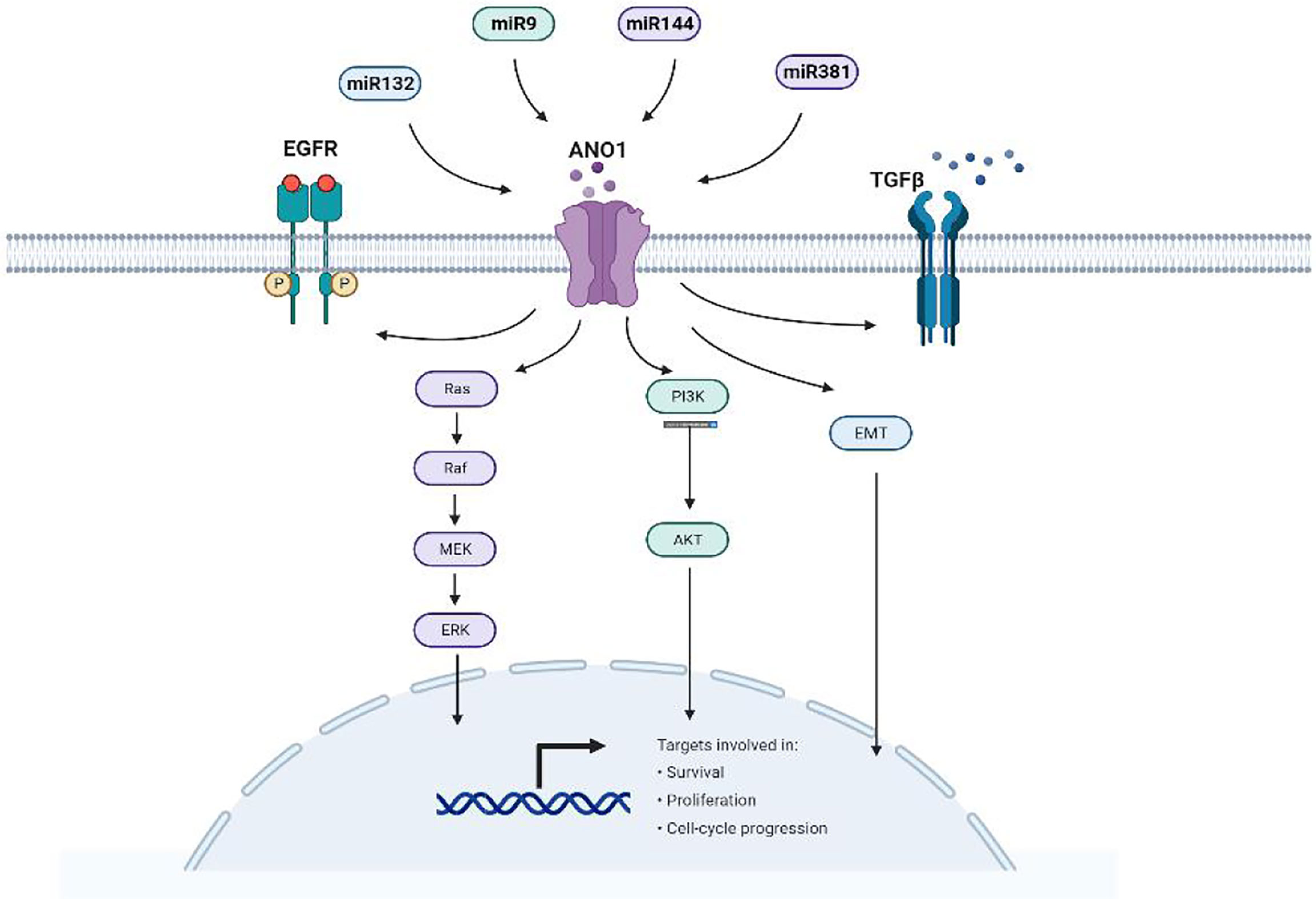
There is ample evidence that ANO1 triggers numerous signaling pathways and stimulates many biological effects in various cell types (Figure 6). Overexpression of ANO1 promoted the growth and invasion of lung cancer cells through the EGFR-MAPK signal pathway (33). ANO1 also activates the Ras-Raf-MEK-ERK1/2 signaling pathway to promote tumor cell growth of HNSCC (8). At the same time, ANO1 can regulate the TGF-β signaling pathway to promote ESCC cells proliferation, migration, and invasion (17). Silencing or inhibition of ANO1 inhibits cell growth, induces apoptosis, and upregulates TNF-α expression in prostate cancer cells (22). Calcium ions (SOCE) that activate ANO1 and ANO1-dependent storage can enter cells and regulate EGFR ligands to promote pancreatic cancer cell migration (39). The higher the expression level of ANO1 in HCC is accompanied by higher tumor grades, lesions, metastases, and a poor prognosis (40, 41). ANO1 can promote HCC metastasis by activating EGFR phosphorylation and subsequent PI3K-AKT signaling pathway (41). In breast cancer cells, ANO1 and EGFR-STAT3 signaling pathways form a positive feedback path to promote the proliferation of breast cancer cells (22). At the same time, ANO1 mediated the PI3K-AKT-mTOR and JAK-STAT3 signaling pathways of HER2-positive breast cancer cells by regulating intracellular Cl- to participate in HER2 transcription and promote tumor proliferation (21). Inhibit of ANO1 can inhibit PI3K-AKT signaling and inhibit the Ovarian cancer cell proliferation and invasion (23). ANO1 affects GBM cell proliferation by regulating NF-κB mediated genes. In the meantime, it is involved in cell proliferation cyclin D1, cyclin E and c-myc, and matrix metalloproteinase (MMP) 2 and MMP 9 induce GBM cell invasion and migration (34, 35). Higher expression of ANO1 was related to gastric and colorectal cancer lymph node metastasis, advanced tumor stage and poor prognosis. Knockout or pharmacological inhibition of ANO1 inhibits the proliferation and induces apoptosis of colorectal cancer cells via the Wnt/β-catenin signaling pathway (50).ANO1 is a direct target and negatively correlates with miR-9, miR-144 and miR-381 (30, 31, 37). Overexpression of miR-9 suppressed the expression of p-AKT, cyclin D1, and p-ERK. MiR-144 can inhibit colorectal cancer by inhibiting the EGFR-ERK signaling pathway targeting ANO1. Meanwhile, miR381 directly targets ANO1 to regulate the TGF-β pathway. ANO1 also has been considered an independent prognostic factor affecting the prognosis of gastric cancer patients.
ANO1 participates in various signaling pathways to promote proliferation, metastasis, and invasion. The signal pathway involved in each tumor is not unique, and one signal pathway can correspond to multiple tumor types. To this end, it would be significant to determine the ANO1 receptor and clarify its signaling pathway in tumor promotion.
Meanwhile, ion channels also participate in ANO1 regulated signaling pathways. Some scholars believe that ANO1 may regulate EGF through Ca2+ signaling to promote pancreatic cancer cell migration (39). Some scholars have also found that silencing the expression of ANO1 reduces the intracellular Cl-, thereby reducing the secretion of TGF-β and inhibiting the metastasis and invasion of gastric cancer cells (36). At the same time, in breast cancer cells, a decrease in the activity of the Cl- channel can down-regulate the EGFR signaling pathway (18). The mechanism of ANO1 in each tumor is different, so that it may constitute the heterogeneity of ANO1 overexpressing cancer cells. However, further studies are needed to clarify whether specific cancer types have unique ionic states.
Alteration of ANO1 Protein Level and Ion Channel Activity Promotes Cancer Cell Proliferation and Migration
Many studies are researching how the increase in ANO1 protein level and channel activity is involved in the proliferation and migration of malignant tumor cells. The gating characteristics of ANO1 are complex because it involves the increase in intracellular calcium ion concentration, membrane depolarization, and the interaction between extracellular chloride or transition anions and intracellular protons (11).
ANO1 contains ten putative transmembrane domains, intracellular NH2 and COOH end, and two calmodulin-binding domains (51). E702 and E705 are presumed to be Ca2+ binding sites, and ANO1 expresses multiple splice variants with variable sensitivity to cytoplasmic Ca2+. Studies have found an X-ray crystal structure (52) (a fungus with Ca2+ activated lipid interfering enzyme activity nhTMEM16) ANO1 subtype has 39-42% homology with mammalian ANO1 (53, 54). nhTMEM16 contains ten transmembrane subunit fragments and six residues (including glutamic acid and aspartic acid). Exploring the fungal model of ANO1 will help determine the Ca2+ binding site. However, the exact Ca2+ binding mechanism of ANO1 still needs to be further explored, even identifying the Ca2+ binding site and ionic conductive hole of ANO1.
For the structural study of ANO1, the current research shows two main ion phenomena: A) ANO1 is activated by voltage when Ca2+ is absent in tumor cells. B) After reducing the concentration of extracellular Cl-, the conductivity of Cl- in ANO1 decreases (55). The intracellular Ca2+ signaling is an essential regulator of cell proliferation, and ANO1 can control intracellular Ca2+ levels by regulating Ras-Raf-MEK-ERK1/2, PIK3-AKT, and DAG-IP3 receptor signals (11, 51). In many systems, the transient increase of intracellular Ca2+ and the continuous activation of the Ras-Raf-MEK-ERK1/2 pathway are the central links in cell proliferation (51, 56, 57). The steady-state regulation of Ca2+ and chloride ion conductance in ANO1 channel activity is also involved in the EGF-induced EGFR pathway. In addition, Cepharanthine significantly inhibits cell proliferation, migration and induces apoptosis in lung adenocarcinoma cells via endogenous ANO1 currents (58). Change and regulation of Ca2+ concentration play an essential role in the ANO1 function of cancer cells.
At the same time, the Cl- channel is the key to maintaining cell volume, so it is crucial in tumor cell migration and invasion (44, 59, 60). In cancer, related changes in intracellular water content can regulate cell volume, and the resulting morphological changes are critical to cell division, migration, invasion, and prevention of cell death (61, 62). The osmotic adjustment represents a mechanism that links ion flux to different cancer cell functions. Some reports have observed that inhibiting ANO1 can affect the size or morphology of cancer cells (7, 60, 63). Furthermore, the activation of Cl- concentration in ANO1 can regulate cell proliferation, and the overexpression of cell ANO1 protein and the increase of channel activity may lead to changes in Cl- concentration. Intracellular Cl- can function as a second messenger, regulating various proteins in many signaling pathways (48, 64). So far, it is still unclear whether the overexpression of ANO1 will increase or decrease the concentration of Cl-. Some scholars have put forward the hypothesis: If ANO1 overexpression can reduce the concentration of Cl-, it may be by increasing the outflow of Cl- in the cell. Experiments have shown that the Cl- channel associated with glioblastoma can be transported to the plasma membrane and act as an ion channel, explicitly participating in tumorigenesis (65).
Next, we determined that the homeostasis regulation of Ca2+ and Cl- currents also has a more significant effect on ANO1 in cancer cells. Some scholars established a 12-state Markov chain model by studying the mechanism of membrane potential, Ca2+, and Cl- on ANO1 ion channel gating (54). This model interprets the activation of ANO1 as 2 Ca2+ dependent on the membrane voltage, directly and continuously coupled with the external Cl- dependent on the membrane potential, and transition into a voltage-dependent state. This model assumes that further experiments prove no significant change in extracellular Cl- affinity for ANO1 Ca2+. However, experiments have also found that extracellular Cl- can promote voltage-dependent activation by stabilizing the open configuration induced by Ca2+. The establishment of the model helped to understand the ion channel mechanism of ANO1. However, the related mechanism of ANO1 channel activity and tumor also needs to be confirmed in other models.
While most reports have confirmed the role of ANO1 overexpression in cancer cell proliferation and migration, there is insufficient information on the role of ANO1 in cell proliferation and migration, mainly due to the increase in ANO1 protein level or the increase in channel activity. Studies have found that reducing the expression level of ANO1 protein may be more critical than blocking the activity of ANO1 channels (66).
ANO1 Induces Tumor Cells to Escape Apoptosis and Regulate the Cell Cycle
Apoptosis is a highly regulated process of cells, essential for cell growth and tissue development (67, 68). Exogenous pathways can trigger apoptosis, including extracellular, pro-apoptotic, ligand-receptor interaction, and endogenous (69). In breast cells, the expression of ANO1 increased anti-apoptotic proteins BCL2 and MCL-1, indicating that ANO1 has pro-survival and anti-apoptotic functions (18). TNF-α expression was negatively correlated with ANO1 expression in prostate cancer cells. Inhibition of ANO1 can activate the TNF-α signaling and induce apoptosis via caspase activation (27). ANO1 also may regulate HCC cell apoptosis through the control AKT/MAPK signaling pathway (41). ANO1 expression correlates with increased ERK 1/2 activity and less apoptotic activity in HNSCC (70). ANO1 inhibitors can cause the overexpression of miR9 to inhibit the proliferation of colorectal cancer cells and induce cell cycle arrest at the GO/G1 phase leading to cell apoptosis (31). At the same time, inhibiting ANO1 can reduce the chloride channel activity and the expression of EGFR and calmodulin dependent kinase (CAMKII), thus promoting breast cancer cell apoptosis (18). Therefore, Suppression of ANO1 overexpression induces apoptosis and offers a promising new modality for the future treatment of malignant tumors.
The G1 phase arrest of the cell cycle can slow cell proliferation. Studies have found that inhibiting ANO1 can induce cell cycle G2/M phase arrest. Nevertheless, it does not affect cell cycle distribution (71). Meanwhile, changes in ANO1 expression affect proteins used explicitly for transcription or cell division. Such as cyclin A2, cyclin D1, and cyclin E. Up-regulation of cyclin-dependent kinases 1 and 2 (CDK1 and CDK2) in cancer cells can cause an increase in the expression of ANO1 (34, 52). The above data indicate that ANO1 may be involved in the cell cycle process, but it remains to be clarified how ANO1 induces tumor cell cycle arrest. Existing studies have found that the ANO1 inhibitor Caccinh-A01 may reduce the chloride current in gastric cancer cells and cause the G1 phase of the cell cycle block (72). At the same time, research on colorectal cancer found that cyclin D1 protein expression was positively correlated with ERK1/2 and ANO1.ANO1 inhibits G1 to S phase progression by down-regulating the expression of the ANO1, which is consistent with the decreased expression of cyclin D1 (28). Suppressing ANO1 expression also had significantly lower cyclin D1, leading to changes in p27Kip1 distribution and correlated with a cell cycle arrest phenotype in HNSCC (8, 73).
ANO1 Is Involved in Tumor Immune Evasion
Tumor immune escape signifies that tumor cells escape recognition and attack by the immune system through various mechanisms to survive and proliferate (74). Future development of cancer immunotherapy depends on the crosstalk between cytokines produced by the tumor microenvironment and biology. In the study of gastrointestinal stromal tumors, ANO1 gene expression was significantly negatively correlated with plasma cells and activated memory CD4+ T cells, implying that ANO1 may be involved in the functional inhibition of helper T cells (42). We consider that ANO1 may be related to the immune microenvironment of malignant tumors. These specific mechanisms also remain to be systematically investigated.
Perspectives and Future Directions of Ano1
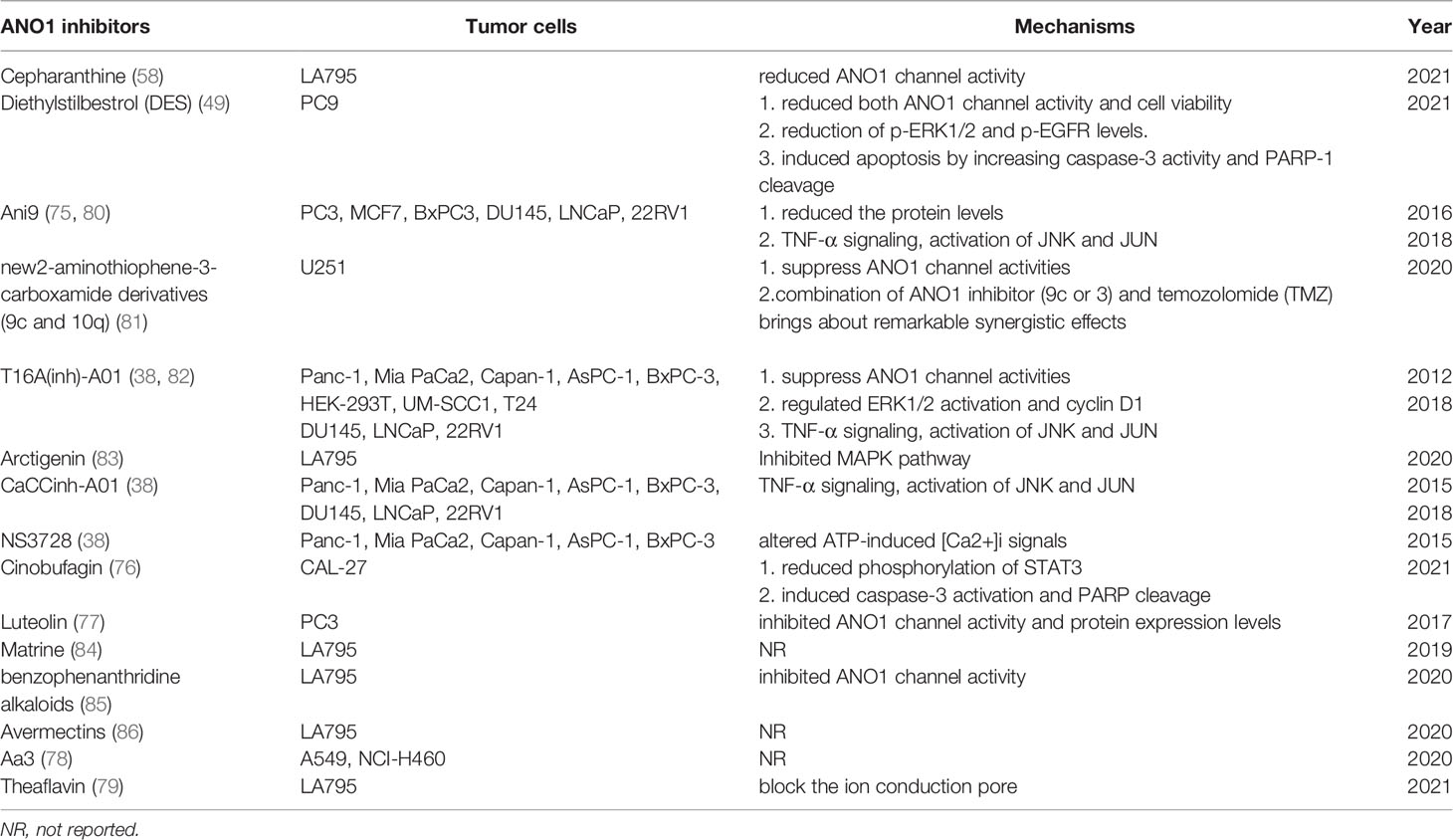
As a calcium-activated chloride channel, ANO1’s expression is affected by various molecular mechanisms. Current research shows that ANO1 may be involved in malignant tumours’ occurrence, development, and metastasis. Finally, novel, more effective, and safer ANO1-centered therapeutic interventions may open new horizons in oncotherapy and provide a new cancer treatment strategy. Some compounds such as cepharanthine (58), Ani9 (36, 75), cinobufagin (76), luteolin (77), Aa3 (78), theaflavin (79), 2-aminothiophene-3-carboxamidederivatives (80), matrine (76), have been identified as inhibitors of ANO1 Agent. It has been validated in multiple in vitro and vivo tumor models (Table 2).
Simultaneously, microRNAs (miRNAs) can also directly target and inhibit ANO1 expression suppressing tumor cells. MiRNA is small non-coding RNAs with about 22 nucleotides. miRNAs play an essential regulatory role in various cancers, including gastric cancer, pancreatic cancer, and hepatocellular carcinoma. They bind to the 3’-untranslated region (3’-UTR) of target genes, resulting in irreversible inhibition of transcription or translation. Because abnormally expressed miRNA also contributes to cancer cells’ proliferation, apoptosis, and metastasis (87–89). Moreover, miRNA expression is considered a promising biomarker of cancer diagnosis, prognosis, and therapy (90).
Existing studies have found that luciferase detection shows that ANO1 is a direct target and is negatively correlated with miR132, miR144, miR381, and miR9 (Figure 7). High miR144 and miR9 directly target ANO1 to inhibit colorectal cell proliferation and migration and promote apoptosis (30, 31). miR9 overexpression inhibits ANO1 miRNA and protein expression and inhibits the expression of P-AKT, CD1, and P-ERK proteins in colorectal cancer cells (31). miR381 expression is related to gastric cancer proliferation, lymph node metastasis, advanced stage, and poor prognosis by directly targeting ANO1 to regulate the TGF-β pathway (37). MiR144 also down-regulates the expression of PTEN to activate the Ras-Raf-MEK-ERK1/2 pathway to inhibit breast cell proliferation and survival (30). Exploring the relationship between ANO1 and microRNA has broad prospects for the occurrence and development of tumors.
Summary
ANO1 has recently attracted considerable attention because of its potential role in cancer chemoprevention and chemotherapy. ANO1 can participate in various signal pathways by interacting with a variety of proteins or by regulating the ion conduction of malignant tumor cell ion homeostasis, thereby affecting the biological behavior of malignant tumors. Moreover, ANO1 expression can constitute a vital diagnosis and therapy monitoring marker. Advances in ANO1 biology and ANO1 inhibitors may hold promise for ANO1 use as a potential cancer biomarker and therapeutic target. The development of ANO1 inhibitors is currently desirable and validated in multiple in vitro and vivo tumor models and used in clinical trials. Simultaneously, many questions remain to be answered, yet other fields need additional exploration.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was partially supported by China’s National Natural Science Foundation(81900198), the Dalian Science and Technology Innovation Program, and the Natural Science Foundation of Liaoning Province(2020-MS-257).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Paulino C, Neldner Y, Lam AK, Kalienkova V, Brunner JD, Schenck S, et al. Structural Basis for Anion Conduction in the Calcium-Activated Chloride Channel TMEM16A. Elife (2017) 6:1–23. doi: 10.7554/eLife.26232
2. Schroeder BC, Cheng T, Jan YN, Jan LY. Expression Cloning of TMEM16A as a Calcium-Activated Chloride Channel Subunit. Cell (2008) 134:1019–29. doi: 10.1016/j.cell.2008.09.003
3. Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, et al. TMEM16A, a Membrane Protein Associated With Calcium-Dependent Chloride Channel Activity. Science (2008) 322:590–4. doi: 10.1126/science.1163518
4. Pedemonte N, Galietta LJV. Structure and Function of TMEM16 Proteins (Anoctamins). Physiol Rev (2014) 94:419–59. doi: 10.1152/physrev.00039.2011
5. Oh U, Jung J. Cellular Functions of TMEM16/anoctamin. Pflugers Archiv (2016) 468:443–53. doi: 10.1007/s00424-016-1790-0
6. Qu ZQ, Yao WC, Yao RY, Liu XP, Yu K, Hartzell C. The Ca(2+) -Activated Cl(-) Channel, ANO1 (TMEM16A), Is a Double-Edged Sword in Cell Proliferation and Tumorigenesis. Cancer Med (2014) 3:453–61. doi: 10.1002/cam4.232
7. Ayoub C, Wasylyk C, Li Y, Thomas E, Marisa L, Robe A, et al. ANO1 Amplification and Expression in HNSCC With a High Propensity for Future Distant Metastasis and Its Functions in HNSCC Cell Lines. Br J Cancer (2010) 103:715–26. doi: 10.1038/sj.bjc.6605823
8. Duvvuri U, Shiwarski DJ, Xiao D, Bertrand C, Huang X, Edinger RS, et al. TMEM16A Induces MAPK and Contributes Directly to Tumorigenesis and Cancer Progression. Cancer Res (2012) 72:3270–81. doi: 10.1158/0008-5472.CAN-12-0475-T
9. Ruiz C, Martins JR, Rudin F, Schneider S, Dietsche T, Fischer CA, et al. Enhanced Expression of ANO1 in Head and Neck Squamous Cell Carcinoma Causes Cell Migration and Correlates With Poor Prognosis. PloS One (2012) 7:e43265. doi: 10.1371/journal.pone.0043265
10. Dixit R, Kemp C, Kulich S, Seethala R, Chiosea S, Ling S, et al. TMEM16A/ANO1 is Differentially Expressed in HPV-Negative Versus HPV-Positive Head and Neck Squamous Cell Carcinoma Through Promoter Methylation. Sci Rep (2015) 5:16657. doi: 10.1038/srep16657
11. Bill A, Gaither LA. The Mechanistic Role of the Calcium-Activated Chloride Channel ANO1 in Tumor Growth and Signaling. Adv Exp Med Biol (2017) 966:1–14. doi: 10.1007/5584_2016_201
12. Reddy RB, Bhat AR, James BL, Govindan SV, Mathew R, Ravindra DR, et al. Meta-Analyses of Microarray Datasets Identifies ANO1 and FADD as Prognostic Markers of Head and Neck Cancer. PloS One (2016) 11:e0147409. doi: 10.1371/journal.pone.0147409
13. Wanitchakool P, Wolf L, Koehl GE, Sirianant L, Schreiber R, Kulkarni S, et al. Role of Anoctamins in Cancer and Apoptosis. Philos Trans R Soc Lond B Biol Sci (2014) 369:20130096. doi: 10.1098/rstb.2013.0096
14. Finegersh A, Kulich S, Guo T, Favorov AV, Fertig EJ, Danilova LV, et al. DNA Methylation Regulates TMEM16A/ANO1 Expression Through Multiple CpG Islands in Head and Neck Squamous Cell Carcinoma. Sci Rep (2017) 7:15173. doi: 10.1038/s41598-017-15634-9
15. Hermida PF, Menéndez ST, Afanasiev PA, Diaz RG, Teijeiro SÁ., Villaronga MÁ., et al. Distinctive Expression and Amplification of Genes at 11q13 in Relation to HPV Status With Impact on Survival in Head and Neck Cancer Patients. J Clin Med (2018) 7:1–14. doi: 10.3390/jcm7120501
16. Shi ZZ, Shang L, Jiang YY, Hao JJ, Zhang Y, Zhang TT, et al. Consistent and Differential Genetic Aberrations Between Esophageal Dysplasia and Squamous Cell Carcinoma Detected by Array Comparative Genomic Hybridization. Clin Cancer Res (2013) 19:5867–78. doi: 10.1158/1078-0432.CCR-12-3753
17. Yu Y, Cao J, Wu WB, Zhu Q, Tang Y, Zhu CX, et al. Genome-Wide Copy Number Variation Analysis Identified ANO1 as a Novel Oncogene and Prognostic Biomarker in Esophageal Squamous Cell Cancer. Carcinogenesis (2019) 40:1198–208. doi: 10.1093/carcin/bgz077
18. Britschgi A, Bill A, Brinkhaus H, Rothwell C, Clay I, Duss S, et al. Calcium-Activated Chloride Channel ANO1 Promotes Breast Cancer Progression by Activating EGFR and CAMK Signaling. Proc Natl Acad Sci U.S.A. (2013) 110:E1026–34. doi: 10.1073/pnas.1217072110
19. Wu HZ, Guan S, Sun ML, Yu ZJ, Zhao L, He M, et al. Ano1/TMEM16A Overexpression Is Associated With Good Prognosis in PR-Positive or HER2-Negative Breast Cancer Patients Following Tamoxifen Treatment. PloS One (2015) 10:e0126128. doi: 10.1371/journal.pone.0126128
20. Fujimoto M, Kito H, Kajikuri J, Ohya S. Transcriptional Repression of Human Epidermal Growth Factor Receptor 2 by ClC-3 Cl (-) /H (+) Transporter Inhibition in Human Breast Cancer Cells. Cancer Sci (2018) 109:2781–91. doi: 10.1111/cas.13715
21. Wu HZ, Wang H, Guan S, Zhang J, Chen QC, Wang XD, et al. Cell-Specific Regulation of Proliferation by Ano1/TMEM16A in Breast Cancer With Different ER, PR, and HER2 Status. Oncotarget (2017) 8:84996–5013. doi: 10.18632/oncotarget.18662
22. Wang H, Yao F, Luo SY, Ma K, Liu MI, Bai LC, et al. A Mutual Activation Loop Between the Ca (2+)-Activated Chloride Channel TMEM16A and EGFR/STAT3 Signaling Promotes Breast Cancer Tumorigenesis. Cancer Lett (2019) 455:48–59. doi: 10.1016/j.canlet.2019.04.027
23. Liu ZT, Zhang SS, Hou F, Zhang CX, Gao JJ, Wang KW. Inhibition of Ca (2+) -Activated Chloride Channel ANO1 Suppresses Ovarian Cancer Through Inactivating PI3K/Akt Signaling. Int J Cancer (2019) 144:2215–26. doi: 10.1002/ijc.31887
24. Liu W, Lu M, Liu BG, Huang Y, Wang KW. Inhibition of Ca (2+)-Activated Cl (-) Channel ANO1/TMEM16A Expression Suppresses Tumor Growth and Invasiveness in Human Prostate Carcinoma. Cancer Lett (2012) 326:41–51. doi: 10.1016/j.canlet.2012.07.015
25. Cha JY, Wee JW, Jung JY, Jang YW, Lee B, Hong GS, et al. Anoctamin 1 (TMEM16A) is Essential for Testosterone-Induced Prostate Hyperplasia. Proc Natl Acad Sci U.S.A. (2015) 112:9722–7. doi: 10.1073/pnas.1423827112
26. Seo Y, Ryu K, Park J, Jeon DK, Jo S, Lee HK, et al. Inhibition of ANO1/TMEM16A Chloride Channel by Idebenone and Its Cytotoxicity to Cancer Cell Lines. PloS One (2015) 10:e0133656. doi: 10.1371/journal.pone.0133656
27. Song Y, Gao J, Guan LZ, Chen XL, Gao JJ, Wang KW. Inhibition of ANO1/TMEM16A Induces Apoptosis in Human Prostate Carcinoma Cells by Activating TNF-Alpha Signaling. Cell Death Dis (2018) 9:703. doi: 10.1038/s41419-018-0735-2
28. Sui YJ, Sun MY, Wu F, Yang LF, Di WH, Zhang GZ, et al. Inhibition of TMEM16A Expression Suppresses Growth and Invasion in Human Colorectal Cancer Cells. PloS One (2014) 9:e115443. doi: 10.1371/journal.pone.0115443
29. Mokutani Y, Uemura M, Munakata K, Okuzaki D, Haraguchi N, Takahashi H, et al. Down-Regulation of microRNA-132 is Associated With Poor Prognosis of Colorectal Cancer. Ann Surg Oncol (2016) 23:599–608. doi: 10.1245/s10434-016-5133-3
30. Jiang YS, Cai YH, Shao WW, Li F, Zhou Y, Tang C, et al. MicroRNA144 Suppresses Aggressive Phenotypes of Tumor Cells by Targeting ANO1 in Colorectal Cancer. Oncol Rep (2019) 41:2361–70. doi: 10.3892/or.2019.7025
31. Park YR, Lee ST, Kim SL, Zhu SM, Lee MR, Kim SH. Down-Regulation of miR-9 Promotes Epithelial Mesenchymal Transition via Regulating Anoctamin-1 (ANO1) in CRC Cells. Cancer Genet (2019) 231-232:22–31. doi: 10.1016/j.cancergen.2018.12.004
32. Jia L, Liu W, Guan L, Lu M, Wang K. Inhibition of Calcium-Activated Chloride Channel ANO1/TMEM16A Suppresses Tumor Growth and Invasion in Human Lung Cancer. PloS One (2015) 10:e0136584. doi: 10.1371/journal.pone.0136584
33. Hu C, Zhang RG, Jiang DP. Tmem16a as a Potential Biomarker in the Diagnosis and Prognosis of Lung Cancer. Arch Iran Med (2019) 22:32–8.
34. Liu J, Liu Y, Ren YG, Kang L, Zhang LH. Transmembrane Protein With Unknown F Unction 16A Overexpression Promotes Glioma Formation Through the Nuclear Factor-Kappa B Signaling Pathway. Mol Med Rep (2014) 9:1068–74. doi: 10.3892/mmr.2014.1888
35. Lee YS, Lee JK, Bae Y, Lee BS, Kim E, Cho CH, et al. Suppression of 14-3-3gamma-Mediated Surface Expression of ANO1 Inhibits Cancer Progression of Glioblastoma Cells. Sci Rep (2016) 6:26413. doi: 10.1038/srep26413
36. Liu F, Cao Q-H, Lu DJ, Luo B, Lu X-F, Luo RC, et al. TMEM16A Overexpression Contributes to Tumor Invasion and Poor Prognosis of Human Gastric Cancer Through TGF-β Signaling. Oncotarget (2015) 6:11585–99. doi: 10.18632/oncotarget.3412
37. Cao QH, Liu F, Ji KY, Liu N, He Y, Zhang WH, et al. MicroRNA-381 Inhibits the Metastasis of Gastric Cancer by Targeting TMEM16A Expression. J Exp Clin Cancer Res (2017) 36:29. doi: 10.1186/s13046-017-0499-z
38. Sauter DRP, Novak I, Pedersen SF, Larsen EH, Hoffmann EK. ANO1 (TMEM16A) in Pancreatic Ductal Adenocarcinoma (PDAC). Pflugers Arch (2015) 467:1495–508. doi: 10.1007/s00424-014-1598-8
39. Crottès D, Lin YT, Peters CJ, Gilchrist JM, Wiita AP, Jan YN, et al. TMEM16A Controls EGF-Induced Calcium Signaling Implicated in Pancreatic Cancer Prognosis. Proc Natl Acad Sci U.S.A. (2019) 116:13026–35. doi: 10.1073/pnas.1900703116
40. Deng L, Yang JH, Chen HW, Ma B, Pan KM, Su CK, et al. Knockdown of TMEM16A Suppressed MAPK and Inhibited Cell Proliferation and Migration in Hepatocellular Carcinoma. Onco Targets Ther (2016) 9:325–33. doi: 10.2147/OTT.S95985
41. Zhang CT, Liu JX, Han ZY, Cui X, Peng D, Xing YF. Inhibition of TMEM16A Suppresses Growth and Induces Apoptosis in Hepatocellular Carcinoma. Int J Clin Oncol (2020) 25:1145–54. doi: 10.1007/s10147-020-01653-6
42. Gasparotto D, Sbaraglia M, Rossi S, Baldazzi D, Brenca M, Mondello A, et al. Tumor Genotype, Location, and Malignant Potential Shape the Immunogenicity of Primary Untreated Gastrointestinal Stromal Tumors. JCI Insight (2020) 5:1–17. doi: 10.1172/jci.insight.142560
43. Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discovery (2022) 12:31–46. doi: 10.1158/2159-8290.CD-21-1059
44. Kunzelmann K, Ousingsawat J, Benedetto R, Cabrita I, Schreiber R. Contribution of Anoctamins to Cell Survival and Cell Death. Cancers (Basel) (2019) 11:1–24. doi: 10.3390/cancers11030382
45. Basu A, Haldar S. The Relationship Between Bcl2, Bax and P53: Consequences for Cell Cycle Progression and Cell Death. Mol Hum Reprod (1998) 4:1099–109. doi: 10.1093/molehr/4.12.1099
46. Fernald K, Kurokawa M. Evading Apoptosis in Cancer. Trends Cell Biol (2013) 23:620–33. doi: 10.1016/j.tcb.2013.07.006
47. Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF Receptor Superfamilies: Integrating Mammalian Biology. Cell (2001) 104:487–501. doi: 10.1016/s0092-8674(01)00237-9
48. Cornejo PP, Gokhale A, Duran C, Cui YY, Xiao QH, Hartzell HC. Anoctamin 1 (Tmem16A) Ca2+-Activated Chloride Channel Stoichiometrically Interacts With an Ezrin-Radixin-Moesin Network. Proc Natl Acad Sci U.S.A. (2012) 109:10376–81. doi: 10.1073/pnas.1200174109
49. Seo Y, Jeong SB, Woo JH, Kwon OB, Lee S, Oh HI, et al. Diethylstilbestrol, a Novel ANO1 Inhibitor, Exerts an Anticancer Effect on Non-Small Cell Lung Cancer via Inhibition of ANO1. Int J Mol Sci (2021) 22:7100. doi: 10.3390/ijms22137100
50. Yan YF, Ding XY, Han CH, Gao JJ, Liu ZT, Liu YN, et al. Involvement of TMEM16A/ANO1 Upregulation in the Oncogenesis of Colorectal Cancer. Biochim Biophys Acta Mol Basis Dis (2022) 868:166370. doi: 10.1016/j.bbadis.2022.166370
51. Henry E. The Activated Complex and the Absolute Rate of Chemical Reactions. J Chem Phys (1935) 3:107–15. doi: 10.1063/1.1749604
52. Guan LZ, Song Y, Gao J, Gao JJ, Wang KW. Inhibition of Calcium-Activated Chloride Channel ANO1 Suppresses Proliferation and Induces Apoptosis of Epithelium Originated Cancer Cells. Oncotarget (2016) 7:78619–30. doi: 10.18632/oncotarget.12524
53. Bill A, Gutierrez A, Kulkarni S, Kemp C, Bonenfant D, Voshol H, et al. ANO1/TMEM16A Interacts With EGFR and Correlates With Sensitivity to EGFR-Targeting Therapy in Head and Neck Cancer. Oncotarget (2015) 6:9173–88. doi: 10.18632/oncotarget.3277
54. Navas C, Porras IH, Schuhmacher AJ, Sibilia M, Guerra C, Barbacid M. EGF Receptor Signaling is Essential for K-Ras Oncogene-Driven Pancreatic Ductal Adenocarcinoma. Cancer Cell (2012) 22:318–30. doi: 10.1016/j.ccr.2012.08.001
55. Ardito CM, Grüner BM, Takeuchi KK, Martellato CL, Teichmann N, Mazur PK, et al. EGF Receptor is Required for KRAS-Induced Pancreatic Tumorigenesis. Cancer Cell (2012) 22:304–17. doi: 10.1016/j.ccr.2012.07.024
56. Betto G, Cherian OL, Pifferi S, Cenedese V, Boccaccio A, Menini A. Interactions Between Permeation and Gating in the TMEM16B/anoctamin2 Calcium-Activated Chloride Channel. J Gen Physiol (2014) 143:703–18. doi: 10.1085/jgp.201411182
57. Ferrera L, Caputo A, Ubby I, Bussani E, Moran OZ, Ravazzolo R, et al. Regulation of TMEM16A Chloride Channel Properties by Alternative Splicing. J Biol Chem (2009) 284:33360–8. doi: 10.1074/jbc.M109.046607
58. Zhang X, Zhang GH, Zhao ZJ, Xiu RL, Jia J, Chen PP, et al. Cepharanthine, a Novel Selective ANO1 Inhibitor With Potential for Lung Adenocarcinoma Therapy. Biochim Biophys Acta Mol Cell Res (2021) 1868:119132. doi: 10.1016/j.bbamcr.2021.119132
59. Vite JAC, Rangel SC, Jesús-Pérez JJD, Figueroa IAA, Menchaca AAR, Cornejo PP, et al. Revealing the Activation Pathway for TMEM16A Chloride Channels From Macroscopic Currents and Kinetic Models. Pflugers Arch (2016) 468:1241–57. doi: 10.1007/s00424-016-1830-9
60. Carpenter G. The EGF Receptor: A Nexus for Trafficking and Signaling. Bioessays (2020) 22:697–707. doi: 10.1002/1521-1878(200008)22:8<697::AID-BIES3>3.0.CO;2-1
61. Hodeify R, Yu F, Courjaret R, Nader N, Dib M, Sun L, et al. Regulation and Role of Store-Operated Ca 2+ Entry in Cellular Proliferation. Calcium Entry Channels Non-Excitable Cells (2018) 12:1–18. doi: 10.1201/9781315152592-12
62. Kunzelmann K. Tmem16, Lrrc8a, Bestrophin: Chloride Channels Controlled by Ca(2+) and Cell Volume. Trends Biochem Sci (2015) 40:535–43. doi: 10.1016/j.tibs.2015.07.005
63. Agell N, Bachs O, Rocamora N, Villalonga P. Modulation of the Ras/Raf/MEK/ERK Pathway by Ca (2+), and Calmodulin. CellSignal (2002) 14:649–54. doi: 10.1016/s0898-6568(02)00007-4
64. Darling NJ, Cook SJ. The Role of MAPK Signaling Pathways in the Response to Endoplasmic Reticulum Stress. Biochim Biophys Acta (2014) 1843:2150–63. doi: 10.1016/j.bbamcr.2014.01.009
65. Peretti M, Angelini M, Savalli N, Florio T, Yuspa SH, Mazzanti M. Chloride Channels in Cancer: Focus on Chloride Intracellular Channel 1 and 4 (CLIC1 AND CLIC4) Proteins in Tumor Development and as Novel Therapeutic Targets. Biochim Biophys Acta (2015) 1848:2523–31. doi: 10.1016/j.bbamem.2014.12.012
66. Wang H, Zou L, Ma K, Yu JK, Wu HZ, Wei MJ, et al. Cell-Specific Mechanisms of TMEM16A Ca (2+)-Activated Chloride Channel in Cancer. Mol Cancer (2017) 16:152. doi: 10.1186/s12943-017-0720-x
67. Wang L, Yu PW. miR-300 Promotes Proliferation and EMT-Mediated Colorectal Cancer Migration and Invasion by Targeting P53. Oncol Rep (2016) 36:3225–32. doi: 10.3892/or.2016.5193
68. Liu MJ, He XS, Xiao XJ, Wang YC. Overexpression of miR-422a Inhibits Cell Proliferation and Invasion, and Enhances Chemosensitivity in Osteosarcoma Cells. Oncol Rep (2016) 36:3371–8. doi: 10.3892/or.2016.5182
69. Chi YD, Zhou DM. MicroRNAs in Colorectal Carcinoma–From Pathogenesis to Therapy. J Exp Clin Cancer Res (2016) 35:43. doi: 10.1186/s13046-016-0320-4
70. Godse NR, Khan N, Yochum ZA, Gomez CR, Kemp C, Shiwarski DJ, et al. TMEM16A/ANO1 Inhibits Apoptosis Via Downregulation of Bim Expression. Clin Cancer Res (2017) 23):7324–32. doi: 10.1158/1078-0432.CCR-17-1561
71. Zhang AL, Yan XX, Li HL, Gu ZF, Zhang P, Zhang HL, et al. TMEM16A Protein Attenuates Lipopolysaccharide-Mediated Inflammatory Response of Human Lung Epithelial Cell Line A549. Exp Lung Res (2014) 40:237–50. doi: 10.3109/01902148.2014.905655
72. Fröbom R, Sellberg F, Xu C, Zhao A, Larsson C, Lui WO, et al. Biochemical Inhibition of DOG1/TMEM16A Achieves Antitumoral Effects in Human Gastrointestinal Stromal Tumor Cells. In Vitro. Anticancer Res (2019) 39:3433–42. doi: 10.21873/anticanres.13489
73. Filippou A, Pehkonen H, Karhemo PR, Väänänen J, Nieminen AI, Klefström J, et al. ANO1 Expression Orchestrates P27kip1/MCL1-Mediated Signaling in Head and Neck Squamous Cell Carcinoma. Cancers (Basel). (2021) 13):1170. doi: 10.3390/cancers13051170
74. Lippitz BE. Cytokine Patterns in Patients With Cancer: A Systematic Review. Lancet Oncol (2013) 14:e218–28. doi: 10.1016/S1470-2045(12)70582-X
75. Seo Y, Kim J, Chang J, Kim SS, Namkung W, Kim L. Synthesis and Biological Evaluation of Novel Ani9 Derivatives as Potent and Selective ANO1 Inhibitors. Eur J Med Chem (2018) 160:245–55. doi: 10.1016/j.ejmech.2018.10.002
76. Jo S, Yang E, Lee Y, Jeon D, Namkung W. Cinobufagin Exerts Anticancer Activity in Oral Squamous Cell Carcinoma Cells Through Downregulation of ANO1. Int J Mol Sci (2021) 22:12037. doi: 10.3390/ijms222112037
77. Seo Y, Ryu K, Park J, Jeon DK, Jo S, Lee HK, et al. Inhibition of ANO1 by Luteolin and its Cytotoxicity in Human Prostate Cancer PC-3 Cells. PloS One (2017) 12:e0174935. doi: 10.1371/journal.pone.0174935
78. Kim T, Cho S, Oh H, Hur J, Kim H, Choi YH, et al. Design of Anticancer 2,4-Diaminopyrimidines as Novel Anoctamin 1 (ANO1) Ion Channel Blockers. Molecules (2020) 25:5180. doi: 10.3390/molecules25215180
79. Shi S, Ma B, Sun F, Qu C, An HL. Theaflavin Binds to a Druggable Pocket of TMEM16A Channel and Inhibits Lung Adenocarcinoma Cell Viability. J Biol Chem (2021) 297:101016. doi: 10.1016/j.jbc.2021.10
80. Seo Y, Lee HK, Park J, Jeon DK, Jo S, Jo M, et al. Ani9, A Novel Potent Small-Molecule ANO1 Inhibitor With Negligible Effect on ANO2. PloS One (2016) 11:e0155771. doi: 10.1371/journal.pone.0155771
81. Choi SH, Ryu SS, Sim K, Song C, Shin L, Kim SS, et al. Anti-Glioma Effects of 2-Aminothiophene-3-Carboxamide Derivatives, ANO1 Channel Blockers. Eur J Med Chem (2020) 208:112688. doi: 10.1016/j.ejmech.2020.112688
82. Mazzone A, Eisenman ST, Strege PR, Yao Z, Ordog T, Gibbons SJ, et al. Inhibition of Cell Proliferation by a Selective Inhibitor of the Ca (2+)-Activated Cl (-) Channel, Ano1. Biochem Biophys Res Commun (2012) 427:248–53. doi: 10.1016/j.bbrc.2012.09.022
83. Guo S, Chen YF, Shi S, Wang XZ, Zhang HL, Zhan Y, et al. Arctigenin, a Novel TMEM16A Inhibitor for Lung Adenocarcinoma Therapy. Pharmacol Res (2020) 155:104721. doi: 10.1016/j.phrs.2020.104721
84. Guo S, Chen YF, Pang CL, Wang XZ, Shi S, Zhang HL, et al. Matrine is a Novel Inhibitor of the TMEM16A Chloride Channel With Antilung Adenocarcinoma Effects. J Cell Physiol (2019) 234:8698–708. doi: 10.1002/jcp.27529
85. Zhang GH, Zhu L, Xue YC, Zhao ZJ, Li H, Niu ZY, et al. Benzophenanthridine Alkaloids Suppress Lung Adenocarcinoma by Blocking TMEM16A Ca 2+-Activated Cl - Channels. Pflugers Arch (2020) 472:1457–67. doi: 10.1007/s00424-020-02434-w
86. Zhang X, Zhang GH, Zhai WJ, Zhao ZJ, Wang S, Yi JF. Inhibition of TMEM16A Ca 2+-Activated Cl - Channels by Avermectins Is Essential for Their Anticancer Effects. Pharmacol Res (2020) 156:104763. doi: 10.1016/j.phrs.2020.104763
87. Bartel DP. Micronase: Genomics, Biogenesis, Mechanism, and Function. Cell (2004) 116:281–97. doi: 10.1016/s0092-8674(04)00045-5
88. Chen L, Luo L, Chen W, Xu HX, Chen F, Chen LZ, et al. MicroRNA-24 Increases Hepatocellular Carcinoma Cell Metastasis and Invasion by Targeting P53: miR-24 Targeted P53. BioMed Pharmacother (2016) 84:1113–8. doi: 10.1016/j.biopha.2016.10.051
89. Wang YF, Liu LL, Liu XQ, Zhang H, Liu JM, Feng B, et al. Shugoshin1 Enhances Multidrug Resistance of Gastric Cancer Cells by Regulating MRP1, Bcl-2, and Bax Genes. Tumor Biol (2013) 34:2205–14. doi: 10.1007/s13277-013-0758-3
Keywords: ANO1, cancer, protein network, signal pathway, tumor microenvironment, inhibitor, miRNA
Citation: Guo S, Zhang L and Li N (2022) ANO1: More Than Just Calcium-Activated Chloride Channel in Cancer. Front. Oncol. 12:922838. doi: 10.3389/fonc.2022.922838
Received: 18 April 2022; Accepted: 09 May 2022;
Published: 06 June 2022.
Edited by:
Jian-ye Zhang, Guangzhou Medical University, ChinaReviewed by:
Arjun Singh, Memorial Sloan Kettering Cancer Center, United StatesSenthilkumar Rajagopal, REVA University, India
Copyright © 2022 Guo, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Li, bGluYW9ubHk4MjhAMTYzLmNvbQ==
 Saisai Guo
Saisai Guo Linna Zhang
Linna Zhang