- 1Clinical School, Weifang Medical University, Weifang, China
- 2Department of Radiation Oncology, Weifang People’s Hospital, Weifang, China
- 3Department of Pathology, Weifang People’s Hospital, Weifang, China
- 4Weifang Key Laboratory of Radiophysics and Oncological Radiobiology, Weifang, China
Background: Epithelial–myoepithelial carcinoma (EMCa) is a rare low-grade malignant tumor that most commonly occurs in the salivary glands, with approximately 320 cases having been reported worldwide. Here, we report the third case of EMCa occurring in the nasopharynx. Rare cases in the breast, pituitary gland, lacrimal gland, nose, paranasal sinus, nasal cavity, trachea and bronchus, lung, and even the pleura mediastinalis have also been reported. Histopathology and immunohistochemistry are useful for confirming the diagnosis of EMCa, which is characterized by biphasic tubular structures composed of inner ductal and outer clear myoepithelial cells and stains for different markers in each layer. However, because of the rarity of EMCa, the clinicopathological characteristics and treatment of these patients remain unclear.
Case presentation: We report a rare case of EMCa of the nasopharynx. A 51-year-old man presented with a 5-month history of pain while swallowing and aggravation accompanied by right ear tinnitus lasting for 1 month. Nasopharyngoscopy and magnetic resonance imaging (MRI) of the nasopharynx and neck revealed a 5.6 cm × 3.4 cm × 3.1 cm mass in the nasopharyngeal space, invasion of the right cavernous sinus, and lymph node enlargement in the right retropharyngeal space. On 17 April 2019, based on the histopathological and immunohistochemical features, a final diagnosis of EMCa of the right nasopharynx was made. The patient underwent concurrent chemoradiotherapy (CCRT), and his symptoms were relieved after treatment. On 10 January 2022, nasopharynx MRI and biopsy revealed local recurrence, but chest and abdominal computed tomography (CT) showed no obvious signs of metastasis. The local recurrence-free survival (LRFS) period was 33 months.
Conclusion: To the best of our knowledge, this is the third reported case of EMCa in the nasopharynx and the only case of EMCa in the nasopharynx treated with CCRT, and a partial response was achieved. Therefore, to improve the quality of life and prognosis of patients with unresectable tumors, we believe that CCRT is a suitable option. Further clinical observations are required to elucidate the pathophysiology and prognosis of EMCa.
Background
Epithelial–myoepithelial carcinoma (EMCa) is a rare low-grade malignant epithelial neoplasm composed of variable proportions of ductular cells with large, clear cytoplasmic myoepithelial cells arranged around the periphery of the ducts (1–4). EMCa predominantly arises from the parotid gland, accounting for less than 1% of all salivary gland tumors and approximately 2% of malignant salivary gland neoplasms (3–12). Of all the types of nasopharyngeal malignancies treated at our center, the incidence of EMCa is 1/315 (0.3%) as of 2021. This tumor can occur in unusual sites, such as the breast (13–15), pituitary gland (16), paranasal sinus (9, 17, 18), lacrimal gland (19–22), nasal cavity (1, 3, 6, 23–25), trachea and bronchus (26, 27), lung (28–34), nasopharynx (4, 5), and even the pleura mediastinalis (35). EMCa was first reported by Donath in 1972, and 8 patients have been reported with a salivary gland tumor that was termed EMCa (36). However, it was described in the literature as early as 1956 (2, 7, 37). Only approximately 320 cases have been reported thus far (2, 11, 38). The domestic and foreign literature mostly consist of case reports, with two cases of nasopharyngeal EMCa being reported by Imate et al. in 2000 (5) and Kim et al. in 2015 (4). Here, we report the third case of EMCa of the nasopharynx in a 51-year-old man who was treated with concurrent chemoradiotherapy (CCRT).
Case presentation
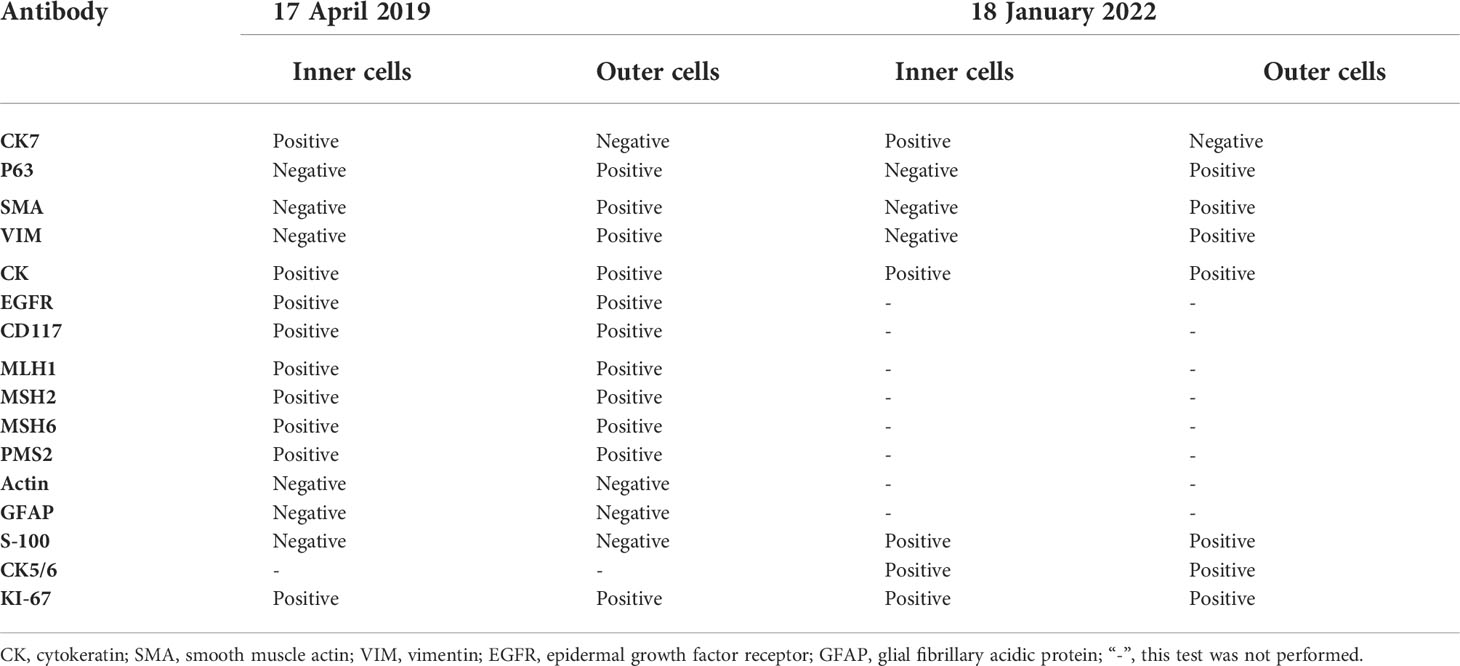
On 14 April 2019, a 51-year-old man presented with a 5-month history of pain while swallowing and aggravation accompanied by right ear tinnitus lasting for 1 month. He was admitted to the otolaryngology department of our hospital. Nasopharyngoscopy revealed a mass on the right nasopharyngeal wall, and a partial tissue sample was obtained via biopsy. Magnetic resonance imaging (MRI) of the nasopharynx and neck on 15 April 2019 revealed a 5.6 cm × 3.4 cm × 3.1 cm mass within the right parietal and lateral walls of the nasopharynx, accompanied by skull base bone erosion, and invasion of the oropharyngeal lateral wall and right parapharyngeal space, the right medial pterygoid muscle and musculus longus capitis, and the cavernous sinus and the posterior nostril. Simultaneously, lymph node enlargement was found in the right retropharyngeal space (Figure 1). A final diagnosis of EMCa was made based on the histological and immunohistochemical (IHC) features of nasopharyngeal biopsy on 17 April 2019. Hematoxylin and eosin staining showed a classical biphasic pattern with typical epithelial cells and myoepithelial cells (Figure 2) without nerve invasion or vascular cancer embolus. The patient was transferred to our department for further treatment. The tumor was staged as IVa (cT4N1M0) according to the 8th edition of the AJCC staging system (39).
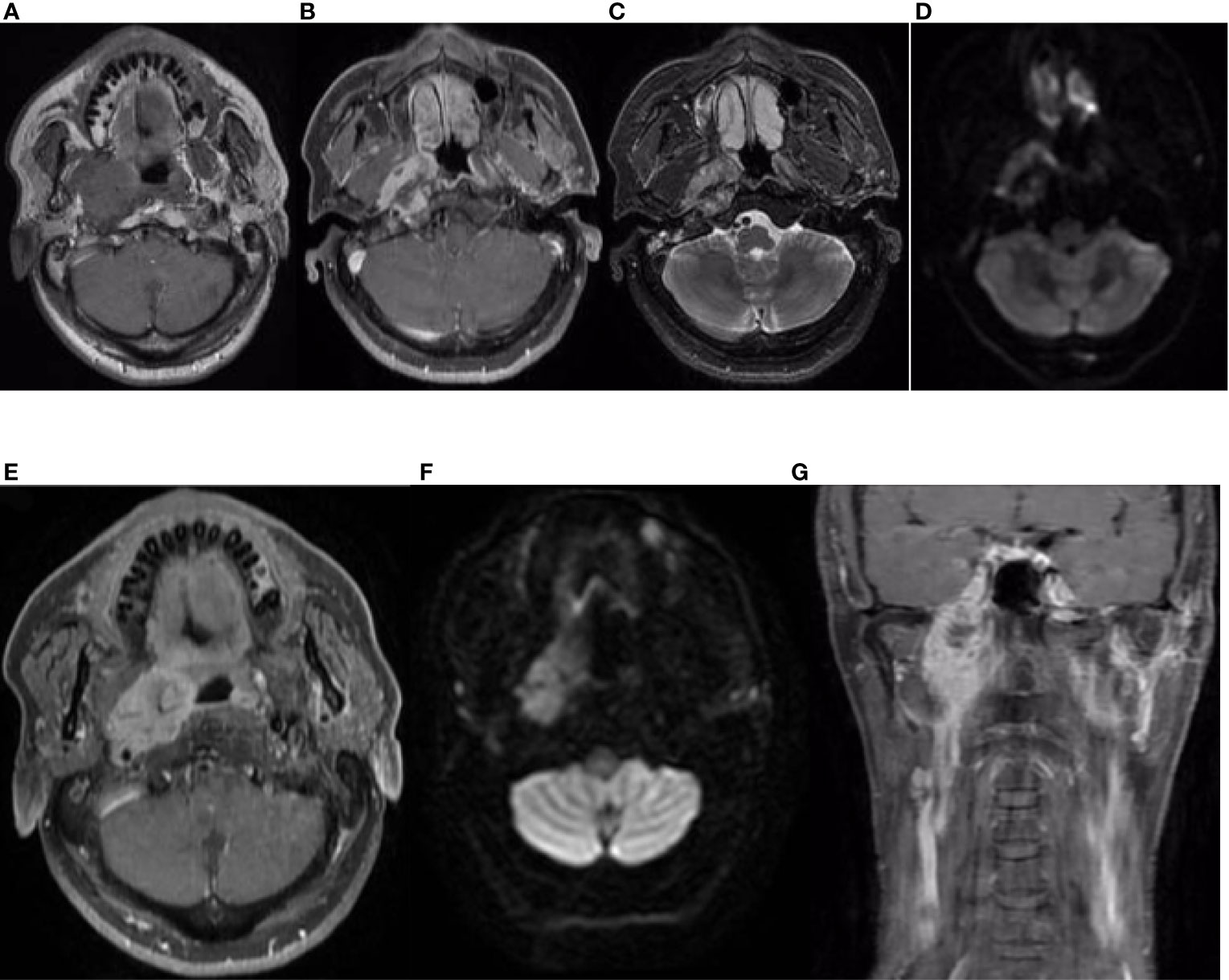
Figure 1 Nasopharynx and neck MRI findings from 15 April 2019. (A–D) The axial planes of T1-weighted, enhanced T1-weighted, T2-weighted, and diffusion-weighted imaging (DWI) MRI of the nasopharynx, showing a 5.6 cm × 3.4 cm × 3.1 cm mass within the right parietal and lateral walls of the nasopharynx, accompanied by skull base bone invasion, invasion of the oropharyngeal lateral wall and right parapharyngeal space, the right medial pterygoid muscle, and the musculus longus capitis. (E, F) The axial planes of enhanced T1-weighted DWI MRI revealed enlargement of the right retropharyngeal lymph node. (G) The coronal plane of enhanced T1-weighted imaging showed tumor involvement with the cavernous sinus.
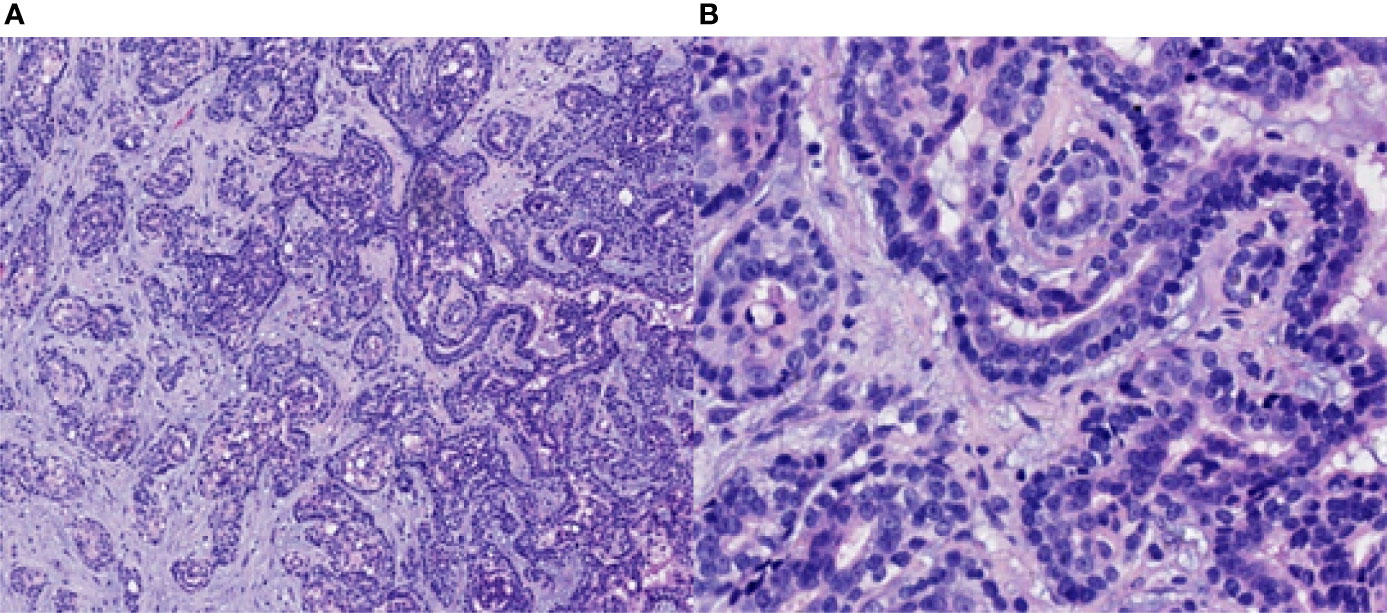
Figure 2 Pathological findings of the nasopharyngeal biopsy obtained on 17 April 2019. The EMCa of the nasopharynx was mainly composed of an inner layer of epithelial cells and an outer layer of clear cytoplasmic myoepithelial cells. H&E staining; magnification, ×10 (A) and ×40 (B). EMCa, epithelial–myoepithelial carcinoma; H&E, hematoxylin and eosin.
Treatment and outcomes
The patient received CCRT in our department. Target volumes were delineated according to the consensus (40), and intensity-modulated radiation therapy (IMRT) was performed from 24 April 2019 to 10 June 2019. Over a total of 33 fractions, a dose of 7,000 cGy was delivered to the tumor, and a dose of 6,800 cGy was delivered to the metastatic lymph node in the right retropharyngeal space; the dose delivered to high-risk regions was 6,006 cGy, referred to as clinical target volume 1 (CTV1), and the dose delivered to low-risk regions was 5,775 cGy, referred to as CTV2. During radiation therapy, 80 mg/m2 cisplatin was administered every 3 weeks for up to 3 cycles. On 29 July 2019, 1.5 months after the end of treatment, nasopharynx and neck MRI revealed that the nasopharyngeal lesions on the right side had shrunk significantly, but there was a 4.8 cm × 3.0 cm × 2.7 cm residual area of low signal and no enhancement, which suggested necrosis (Figure 3). In September 2019, the patient suffered from massive nasopharyngeal hemorrhage at home, which improved after receiving symptomatic treatment such as hemostasis in a local hospital. Then, follow-up of the patient became irregular. On 7 July 2020, 1 year after the end of treatment, nasopharynx and neck MRI revealed that the nasopharyngeal lesions had subsided significantly, but there was still an area of inhomogeneous residual enhancement of the right nasopharynx and parapharyngeal space (Figure 4). The residual area of inhomogeneous enhancement had shrunk to a smaller size, and there were no obvious signs of recurrence or metastasis on reexamination on 27 April 2021 (Figure 5). The patient’s pain while swallowing was completely relieved, and his symptoms of right tinnitus were partially relieved. On 10 January 2022, the patient was examined at our department, and nasopharynx and neck MRI revealed that the nasopharyngeal enhancement area was more obvious than before, which was considered recurrence (Figure 6). Chest and abdominal computed tomography (CT) showed no obvious signs of metastasis. Nasopharyngeal biopsy was performed that same day, and the pathological (Figure 7) and IHC findings on 18 January 2022 suggested EMCa recurrence of the nasopharynx. In summary, the follow-up at 33 months revealed recurrence but no distant metastasis.

Figure 3 Nasopharynx and neck MRI findings from 29 July 2019. (A–D) The axial planes of T1-weighted, enhanced T1-weighted, T2-weighted, and DWI and (E) the coronal plane of enhanced T1-weighted MRI, showing that the nasopharyngeal lesions on the right side subsided significantly, but there was a 4.8 cm × 3.0 cm × 2.7 cm residual area of low signal and no enhancement, which suggested necrosis.

Figure 4 Nasopharynx and neck MRI findings from 7 July 2020. (A–D) The axial planes of T1-weighted, enhanced T1-weighted, T2-weighted, and DWI and (E) the coronal plane of enhanced T1-weighted MRI, showing that the nasopharyngeal lesions and the necrotic area had subsided significantly. However, there was still a residual area of inhomogeneous enhancement in the right nasopharynx and parapharyngeal space, demonstrating restricted diffusion on DWI.
Figure 5 Nasopharynx and neck MRI findings from 27 April 2021. (A–D) The axial planes of T1-weighted, enhanced T1-weighted, T2-weighted, and DWI and (E) the coronal plane of enhanced T1-weighted MRI, showing that the residual area of inhomogeneous enhancement had shrunk.
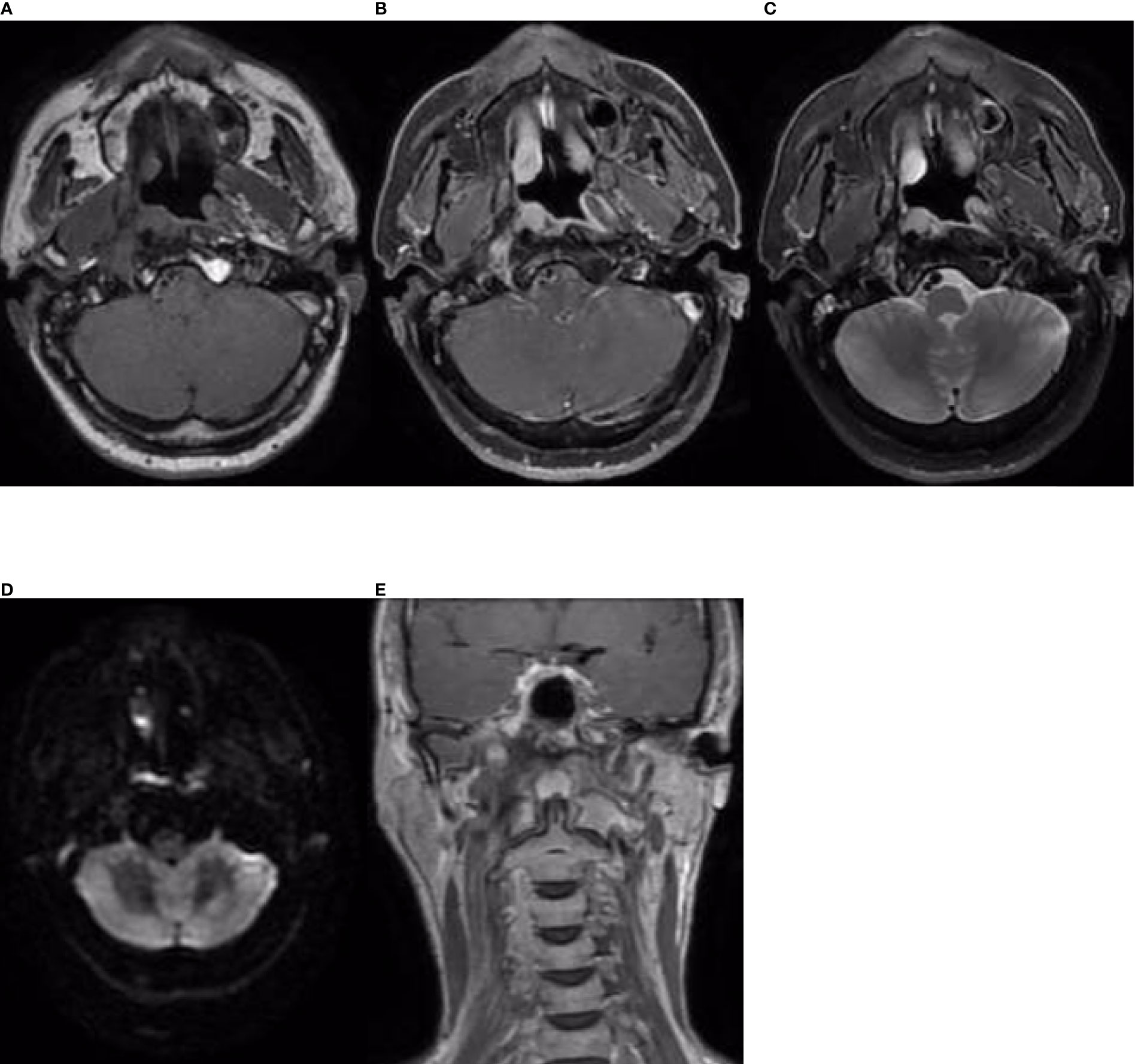
Figure 6 Nasopharynx and neck MRI findings from 10 January 2022. (A–D) The axial planes of T1-weighted, enhanced T1-weighted, T2-weighted, and DWI and (E) the coronal plane of enhanced T1-weighted MRI, showing an area of enhancement in the right nasopharyngeal and parapharyngeal space. DWI demonstrated slightly restricted diffusion, which was considered indicative of recurrence.
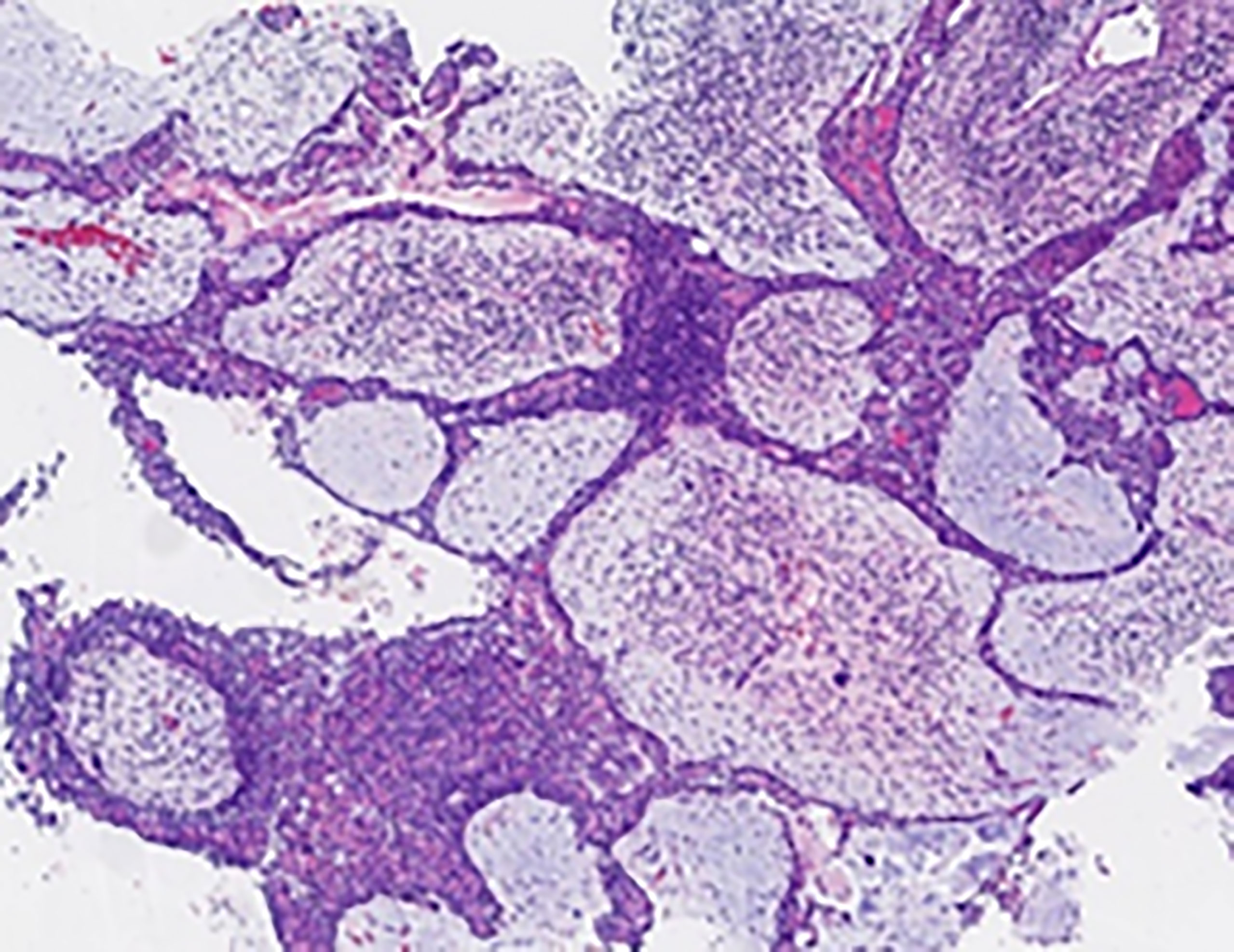
Figure 7 Pathological findings of the nasopharyngeal biopsy obtained on 12 January 2022. Nasopharyngeal EMCa recurrence was considered. H&E staining; magnification, ×4. EMCa, epithelial–myoepithelial carcinoma; H&E, hematoxylin and eosin.
IHC findings
On 17 April 2019, at first admission, IHC staining revealed that cytokeratin (CK) was widely positive, and the inner epithelial cells were positive for CK7, an epithelial cell marker. The outer myoepithelial cells were positive for P63, smooth muscle actin (SMA) and vimentin (VIM), consistent with a myoepithelial phenotype, confirming the diagnosis of EMCa. Epidermal growth factor receptor (EGFR) and CD117 staining was also positive, while S-100, actin, and glial fibrillary acidic protein (GFAP) staining was negative. The expression of programmed death ligand-1 (PD-L1) was less than 1% in tumor cells and 10% in stromal cells. The expression of MLH1, MSH2, MSH6, and PMS2 and all four mismatch repair (MMR) proteins was positive, which was interpreted as proficient mismatch repair (pMMR). Ki-67 was positive in 35% of the neoplastic cells (Figure 8).
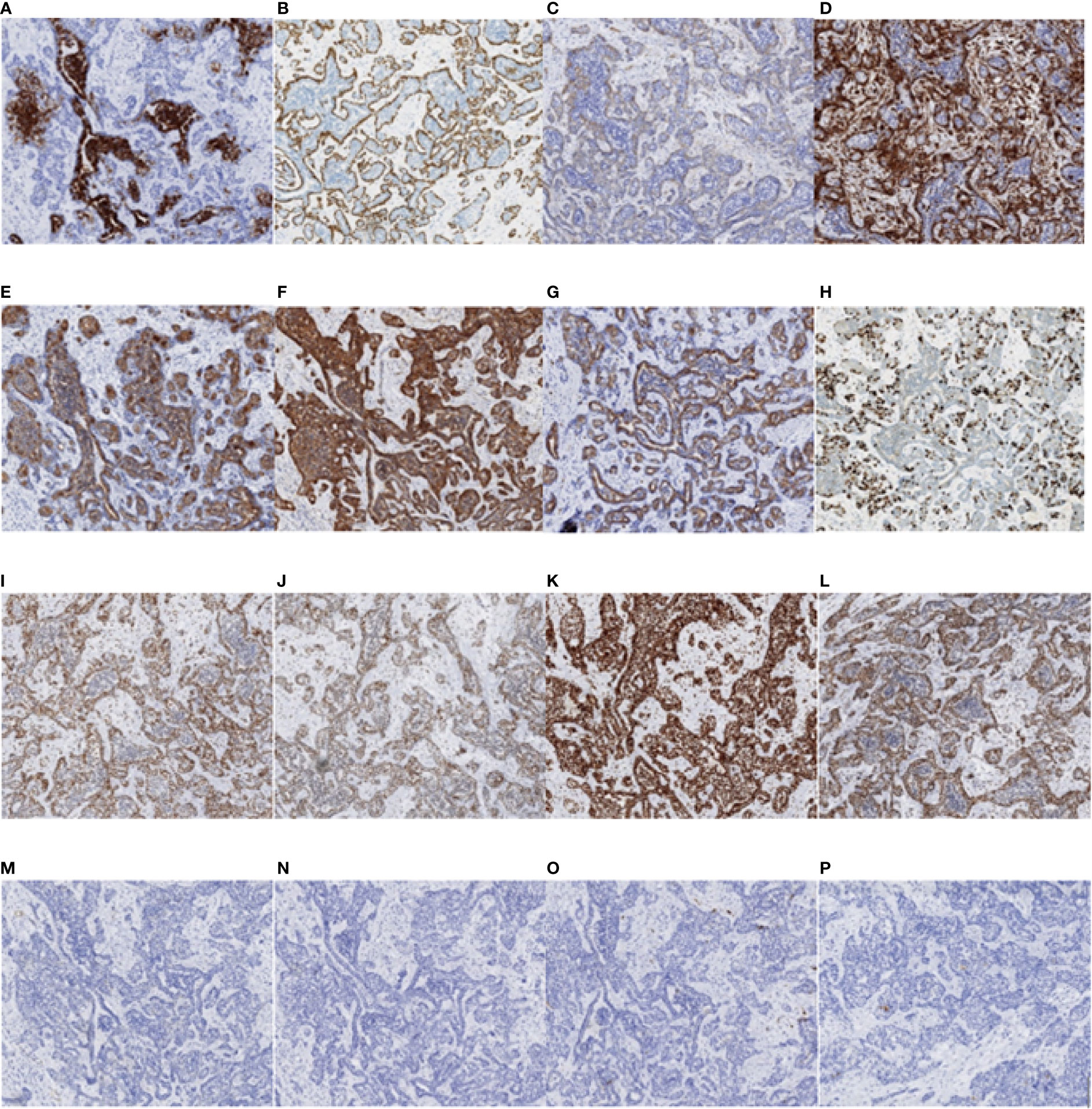
Figure 8 Immunohistochemistry test results of the nasopharyngeal biopsy obtained on 17 April 2019. (A) CK7 positivity was observed in epithelial cells. (B–D) P63, SMA, and VIM positivity was observed in myoepithelial cells. (E–G) CK, EGFR, and CD117 positivity was observed in tumor cells. (H) Ki-67 positivity was observed in 35% of the tumor cells. (I–L) MLH1, MSH2, MSH6, and PMS2 positivity was observed in tumor cells. (M–O) Actin, GFAP, and S-100 negativity was observed in tumor cells. (P) PD-L1 positivity was observed in less than 1% of tumor cells and in 10% of stromal cells (magnification, ×10). CK, cytokeratin; SMA, smooth muscle actin; VIM, vimentin; EGFR, epidermal growth factor receptor; GFAP, glial fibrillary acidic protein; PD-L1, programmed death ligand-1.
On 18 January 2022, at the second admission, IHC staining revealed that CK was wildly positive, and the inner epithelial cells were positive for CK7. The outer myoepithelial cells were positive for P63, SMA, and VIM. S-100 was positive in some cells, and CK5/6 was positive in most cells. Ki-67 was positive in 15% of the neoplastic cells (Figure 9). The IHC results of the inner epithelial cells and the outer myoepithelial cells are summarized in Table 1.
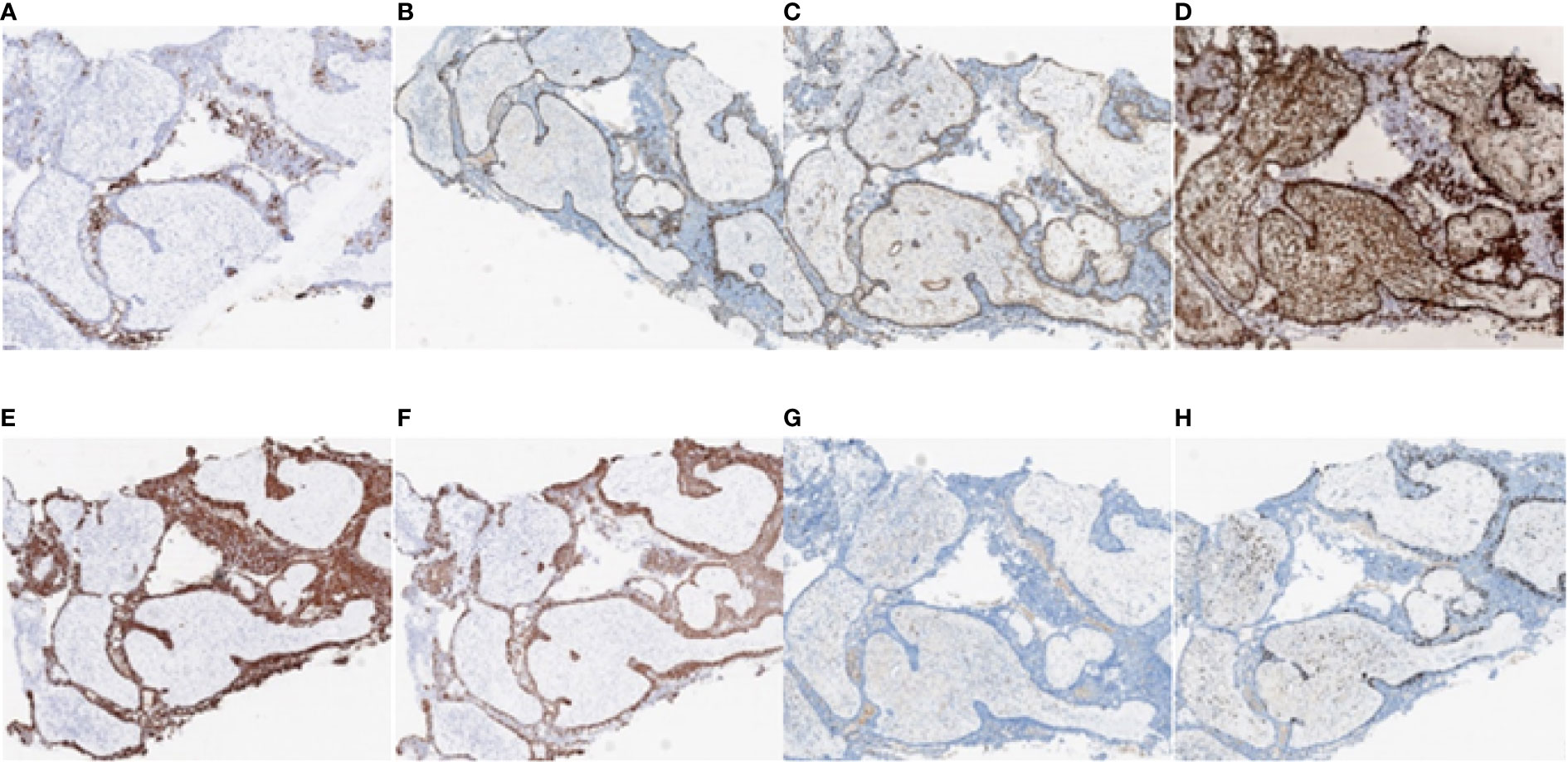
Figure 9 Immunohistochemistry test results of the nasopharyngeal biopsy obtained on 18 January 2022. (A) CK7 positivity was observed in epithelial cells. (B–D) P63, SMA, and VIM positivity was observed in myoepithelial cells. (E–G) CK, CK5/6, and S-100 positivity was observed in tumor cells. (H) Ki-67 positivity was observed in 15% of tumor cells (magnification, ×4). CK, cytokeratin; SMA, smooth muscle actin; VIM, vimentin.
Discussion
EMCa is a rare and unique tumor that most commonly occurs in the salivary glands (7, 11, 12). The average age at diagnosis is approximately 60 years old, and the incidence is higher in women than in men, with a ratio of 1.34-2:1 (2, 7). These tumors have a propensity for infiltration but low malignancy (4). Thus, they invade nerves, blood vessels, and bone, and local recurrence is often observed (0–56%) (5), but the mortality rate is low (11). The mean times to recurrence and metastases are 5 years (41) and 15 years (42), respectively. The median disease-free survival (DFS) time is 11.34 years, and the 5- and 10-year disease-specific survival (DSS) rates are 93.5% and 81.8%, respectively (2). Factors associated with overall survival include the following: tumor size < 4 cm, absence of regional nodal or distant metastases, patient age < 80 years at diagnosis, surgical treatment, and Ki-67 index less than 3.5% (7, 10, 43, 44). Age younger than 80 years was the only factor associated with a good prognosis in the case reported herein. Clinical manifestations of EMCa are usually nonspecific and can vary depending on the site of origin and extent of the tumor (10). Tumors involving the nasopharynx may cause symptoms such as nose blockage, epistaxis, tinnitus, hearing loss and pain in the ear (4, 5). In our case, the patient presented with pain while swallowing and tinnitus, for which the main cause was the lesion occupying the surrounding tissues.
The confirmative diagnosis of EMCa depends on the histopathological and IHC results (11). EMCa exhibits a biphasic histological morphology, with the inner layer being a single layer of cubic sacral epithelial cells and the peripheral layer being composed of clear cytoplasmic myoepithelial cells, with a duct-like structure and infiltrating margins (7, 11). Compared with the low specificity of radiologic imaging, immunohistochemistry is useful for distinguishing EMCa, as it can depict the characteristic biphasic epithelial–myoepithelial phenotype and the differential staining of markers in each layer; thus, it plays a major role in the final diagnosis (2, 10). IHC staining of glandular epithelial markers such as CK, epithelial membrane antigen (EMA), and CD117 (11) and myoepithelial markers such as SMA, S-100 protein, Actin, VIM, CK14, GFAP and Calponin, and P63 is observed (45–47). Kawahara et al. suggested that P63 is an especially useful marker of myoepithelial cells with naked nuclei in EMCa (48). Nevertheless, studies have shown that VIM and calponin, especially the latter, are sensitive markers for salivary myoepithelial tumor cells. SMA and P63 are relatively less sensitive than calponin (11, 49, 50). In our case, we observed positive CK7 staining in the inner epithelial cells along with P63, SMA, and VIM in the outer myoepithelial cells.
There is currently no consensus regarding the optimal treatment of this disease, largely due to its rarity (24). For other malignant tumors of the head and neck (51), surgery is the preferred method (11). Studies have shown that the true survival benefit of radiotherapy is unclear (7), but others have argued that it may be effective at preventing local recurrence, particularly for neoplasms with a diameter larger than 4 cm (6, 24, 52). A recent study suggested that the complete remission (CR) rate could be improved by consecutive radiotherapy, and if tumors are deemed primarily unresectable, definitive radiotherapy may be used with or without chemotherapy (44). To the best of our knowledge, two cases of EMCa in the nasopharynx reported in the literature were treated by surgical resection (5), and another was treated with CCRT followed by systemic chemotherapy (4), which resulted in a partial response. In our case, due to the anatomical location and the clinical stage of the patient being locally advanced, the tumor was unresectable; thus, CCRT was suggested.
Conclusion
Although it is a low-grade malignancy, EMCa should be treated aggressively, as it has a tendency for both local recurrence and distant metastasis. Although the optimal treatment strategy for EMCa remains poorly defined due to its rarity, the present study reports the only case of EMCa in the nasopharynx treated with CCRT, and a partial response was achieved. Therefore, to improve the quality of life and prognosis of patients with unresectable tumors, we believe that CCRT is a suitable option. Further accumulation of cases and long-term follow-up data are needed to elucidate the pathophysiology and prognosis of epithelial–myoepithelial carcinoma.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Weifang People’s Hospital. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WZ, X-XW, X-LW, and YZ drafted the manuscript and performed the literature review. X-FL and Y-XZ retrieved and analyzed pathological and immunohistochemistry information. Y-YC and F-RH performed the chemoradiotherapy in this case. YL and H-QR retrieved the imaging data, and WZ and F-RH performed patient follow-up. F-RH and Y-XZ conceived, designed, and supervised the study. This paper properly credits the meaningful contributions of all coauthors and coresearchers. All authors have been personally and actively involved in substantial work leading to the publication of this paper and take public responsibility for its content.
Funding
This work was supported by Weifang Science and Technology Development Project (Grant NO. 2019YX003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jin X, Ding C, Chu Q. Epithelial-myoepithelial carcinoma arising in the nasal cavity: a case report and review of literature. Pathology (1999) 31(2):148–51. doi: 10.1080/003130299105340
2. Seethala R, Barnes E, Hunt J. Epithelial-myoepithelial carcinoma: a review of the clinicopathologic spectrum and immunophenotypic characteristics in 61 tumors of the salivary glands and upper aerodigestive tract. Am J Surg Pathol (2007) 31(1):44–57. doi: 10.1097/01.pas.0000213314.74423.d8
3. Yamanegi K, Uwa N, Hirokawa M, Ohyama H, Hata M, Yamada N, et al. Epithelial-myoepithelial carcinoma arising in the nasal cavity. Auris Nasus Larynx (2008) 35(3):408–13. doi: 10.1016/j.anl.2007.10.001
4. Kim S, Park S, Bae H, Song D, Oh H, Cho K, et al. Epithelial-myoepithelial carcinoma of the nasopharynx: A case report and review of the literature. Oncol Lett (2015) 10(2):927–30. doi: 10.3892/ol.2015.3314
5. Imate Y, Yamashita H, Endo S, Okami K, Kamada T, Takahashi M, et al. Epithelial-myoepithelial carcinoma of the nasopharynx. ORL J Oto-Rhino-Laryngology Its Related Specialties (2000) 62(5):282–5. doi: 10.1159/000027761
6. Park J, Jung C, Sun D, Kim M. An unusual presentation of aggressive epithelial-myoepithelial carcinoma of the nasal cavity with high-grade histology. J Laryngology Otology (2011) 125(12):1286–9. doi: 10.1017/s0022215111002222
7. Vázquez A, Patel T, D'Aguillo C, Abdou R, Farver W, Baredes S, et al. Epithelial-myoepithelial carcinoma of the salivary glands: An analysis of 246 cases. Otolaryngology–head Neck Surg Off J Am Acad Otolaryngology-Head Neck Surg (2015) 153(4):569–74. doi: 10.1177/0194599815594788
8. Sentani K, Ogawa I, Uraoka N, Ikeda M, Hayashi N, Hattori T, et al. High-grade epithelial-myoepithelial carcinoma of the parotid gland with mucous cell differentiation. Pathol Int (2015) 65(9):490–4. doi: 10.1111/pin.12315
9. Sunami K, Yamane H, Konishi K, Iguchi H, Takayama M, Nakai Y, et al. Epithelial-myoepithelial carcinoma: An unusual tumor of the paranasal sinus. ORL J Oto-Rhino-Laryngology Its Related Specialties (1999) 61(2):113–6. doi: 10.1159/000027652
10. Lee Y, Ha S, Paik S, Yang H, Jeon H, Park D, et al. Epithelial-myoepithelial carcinoma originating from a minor salivary gland in the nasal septum: A case report and literature review. Medicine (2020) 99(5):e19072. doi: 10.1097/md.0000000000019072
11. Wang F, Li B, Wang Y, Shen Y, Yang H. Clinical and pathological analysis of 10 cases of salivary gland epithelial-myoepithelial carcinoma. Medicine (2020) 99(41):e22671. doi: 10.1097/md.0000000000022671
12. Okuyama K, Michi Y, Kashima Y, Tomioka H, Hirai H, Yokokawa M, et al. Epithelial-myoepithelial carcinoma of the minor salivary glands: Case series with comprehensive review. Diagnostics (Basel Switzerland) (2021) 11(11):2124. doi: 10.3390/diagnostics11112124
13. Chen P, Chen C, Nicastri A, Wait R. Myoepithelial carcinoma of the breast with distant metastasis and accompanied by adenomyoepitheliomas. Histopathology (1994) 24(6):543–8. doi: 10.1111/j.1365-2559.1994.tb00573.x
14. Kakkar A, Jangra K, Kumar N, Sharma M, Mathur S, Deo S. Epithelial-myoepithelial carcinoma of the breast: A rare type of malignant adenomyoepithelioma. Breast J (2019) 25(6):1273–5. doi: 10.1111/tbj.13463
15. Baum J, Sung K, Tran H, Song W, Ginter P. Mammary epithelial-myoepithelial carcinoma: Report of a case with HRAS and PIK3CA mutations by next-generation sequencing. Int J Surg Pathol (2019) 27(4):441–5. doi: 10.1177/1066896918821182
16. Lavin V, Callipo F, Donofrio C, Ellwood-Thompson R, Metcalf R, Djoukhadar I, et al. Primary epithelial-myoepithelial carcinoma of the pituitary gland. Neuropathology Off J Japanese Soc Neuropathology (2020) 40(3):261–7. doi: 10.1111/neup.12628
17. Patra S, Panda N, Saikia U. Epithelial-myoepithelial carcinoma of the maxillary sinus: a rare case. Laryngoscope (2012) 122(7):1579–81. doi: 10.1002/lary.23310
18. Kuran G, Sagıt M, Akın I, Hucumenoglu S, Ocal B, Celık S. Bilateral epithelial-myoepithelial carcinoma: an extraordinary tumor of the paranasal sinuses. Skull base Off J North Am Skull Base Soc [et al] (2008) 18(2):145–50. doi: 10.1055/s-2007-1016955
19. Anjum S, Modaboyina S, Sen S, Sharma M, Meel R. Epithelial-myoepithelial carcinoma of the lacrimal gland: Case report of youngest patient. Ophthalmic Plast Reconstructive Surg (2020) 36(6):e141–e4. doi: 10.1097/iop.0000000000001663
20. Gonçalves A, de Lima P, Monteiro M. Epithelial-myoepithelial carcinoma of the lacrimal gland 14 years after en bloc resection of a pleomorphic lacrimal gland adenoma. Ophthalmic Plast Reconstructive Surg (2016) 32(2):e42–4. doi: 10.1097/iop.0000000000000225
21. Singh G, Sharma M, Agarwal S, Prasad G, Mishra S, Singh M, et al. Epithelial-myoepithelial carcinoma of the lacrimal gland: a rare case. Ann Diagn Pathol (2012) 16(4):292–7. doi: 10.1016/j.anndiagpath.2011.02.004
22. Sharma D, Neiweem A, Davis K, Prendes M, Chundury R, Illing E. Epithelial-myoepithelial carcinoma of the lacrimal sac and literature review of the lacrimal system. Allergy Rhinology (Providence RI) (2020) 11:2152656720920600. doi: 10.1177/2152656720920600
23. Nguyen S, Perron M, Nadeau S, Odashiro A, Corriveau M. Epithelial myoepithelial carcinoma of the nasal cavity: Clinical, histopathological, and immunohistochemical distinction of a case report. Int J Surg Pathol (2018) 26(4):342–6. doi: 10.1177/1066896917747732
24. Lee H, Kim A, Lee S. Epithelial-myoepithelial carcinoma of the nasal cavity. Eur Arch oto-rhino-laryngology Off J Eur Fed Oto-Rhino-Laryngological Societies (EUFOS) Affiliated German Soc Oto-Rhino-Laryngology - Head Neck Surg (2000) 257(7):376–8. doi: 10.1007/s004050000250
25. Harada H, Kashiwagi S, Fujiura H, Kusukawa J, Morimatsu M. Epithelial-myoepithelial carcinoma–report of a case arising in the nasal cavity. J Laryngology Otology (1996) 110(4):397–400. doi: 10.1017/s0022215100133754
26. Konoglou M, Cheva A, Zarogoulidis P, Porpodis K, Pataka A, Mpaliaka A, et al. Epithelial-myoepithelial carcinoma of the trachea-a rare entity case report. J Thorac Dis (2014) 6(S1):S194–9. doi: 10.3978/j.issn.2072-1439.2013.11.17
27. Song D, Choi I, Ha S, Han K, Han J, Kim T, et al. Epithelial-myoepthelial carcinoma of the tracheobronchial tree: the prognostic role of myoepithelial cells. Lung Cancer (Amsterdam Netherlands) (2014) 83(3):416–9. doi: 10.1016/j.lungcan.2014.01.005
28. Mori M, Hanagiri T, Nakanishi R, Ashikari S, Yasuda M, Tanaka F. Primary epithelial-myoepithelial carcinoma of the lung with cavitary lesion: A case report. Mol Clin Oncol (2018) 9(3):315–7. doi: 10.3892/mco.2018.1678
29. Hagmeyer L, Tharun L, Schäfer S, Hekmat K, Büttner R, Randerath W. First case report of a curative wedge resection in epithelial-myoepithelial carcinoma of the lung. Gen Thorac Cardiovasc Surg (2017) 65(9):535–8. doi: 10.1007/s11748-017-0796-7
30. Tajima S, Aki M, Yajima K, Takahashi T, Neyatani H, Koda K. Primary epithelial-myoepithelial carcinoma of the lung: A case report demonstrating high-grade transformation-like changes. Oncol Lett (2015) 10(1):175–81. doi: 10.3892/ol.2015.3169
31. Shen C, Wang X, Che G. A rare case of primary peripheral epithelial myoepithelial carcinoma of lung: Case report and literature review. Medicine (2016) 95(35):e4371. doi: 10.1097/md.0000000000004371
32. Kim C, Jeong J, Kim S, Lee Y. Endobronchial epithelial-myoepithelial carcinoma of the lung. Thorax (2018) 73(6):593–4. doi: 10.1136/thoraxjnl-2017-211155
33. Rosenfeld A, Schwartz D, Garzon S, Chaleff S. Epithelial-myoepithelial carcinoma of the lung: a case report and review of the literature. J Pediatr Hematology/Oncology (2009) 31(3):206–8. doi: 10.1097/MPH.0b013e3181978e62
34. Nguyen C, Suster S, Moran C. Pulmonary epithelial-myoepithelial carcinoma: a clinicopathologic and immunohistochemical study of 5 cases. Hum Pathol (2009) 40(3):366–73. doi: 10.1016/j.humpath.2008.08.009
35. Weissferdt A, Kalhor N, Moran C. Pleuromediastinal epithelial-myoepithelial carcinomas: A clinicopathologic and immunohistochemical study of two cases. Am J Clin Pathol (2016) 146(6):736–40. doi: 10.1093/ajcp/aqw199
36. Donath K, Seifert G, Schmitz R. [Diagnosis and ultrastructure of the tubular carcinoma of salivary gland ducts. Epithelial-myoepithelial carcinoma intercalated ducts] Virchows Archiv A Pathol Pathologische Anatomie (1972) 356(1):16–31.
37. Angiero F, Sozzi D, Seramondi R, Valente M. Epithelial-myoepithelial carcinoma of the minor salivary glands: immunohistochemical and morphological features. Anticancer Res (2009) 29(11):4703–9.
38. Shinozaki A, Nagao T, Endo H, Kato N, Hirokawa M, Mizobuchi K, et al. Sebaceous epithelial-myoepithelial carcinoma of the salivary gland: clinicopathologic and immunohistochemical analysis of 6 cases of a new histologic variant. Am J Surg Pathol (2008) 32(6):913–23. doi: 10.1097/PAS.0b013e318160852a
39. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. New York: Springer (2017). doi: 10.1007/978-3-319-40618-3
40. Lee A, Ng W, Pan J, Poh S, Ahn Y, AlHussain H, et al. International guideline for the delineation of the clinical target volumes (CTV) for nasopharyngeal carcinoma. Radiotherapy Oncol J Eur Soc Ther Radiol Oncol (2018) 126(1):25–36. doi: 10.1016/j.radonc.2017.10.032
41. Arora S, Sharma N, Bhardwaj M. Epithelial myoepithelial carcinoma of head and neck region. Indian J Otolaryngol Head Neck Surg Off Publ Assoc Otolaryngologists India (2013) 65:163–6. doi: 10.1007/s12070-011-0414-4
42. Cho K, el-Naggar A, Ordonez N, Luna M, Austin J, Batsakis J. Epithelial-myoepithelial carcinoma of salivary glands. a clinicopathologic, DNA flow cytometric, and immunohistochemical study of ki-67 and HER-2/neu oncogene. Am J Clin Pathol (1995) 103(4):432–7. doi: 10.1093/ajcp/103.4.432
43. Wakasaki T, Kubota M, Nakashima Y, Tomonobe E, Mihara T, Fukushima J. Invasive myoepithelial carcinoma ex pleomorphic adenoma of the major salivary gland: two case reports. BMC Cancer (2016) 16(1):827. doi: 10.1186/s12885-016-2871-3
44. Witte H, Gebauer N, Lappöhn D, Umathum V, Riecke A, Arndt A, et al. Prognostic impact of PD-L1 expression in malignant salivary gland tumors as assessed by established scoring criteria: Tumor proportion score (TPS), combined positivity score (CPS), and immune cell (IC) infiltrate. Cancers (2020) 12(4):873. doi: 10.3390/cancers12040873
45. Foschini M, Scarpellini F, Gown A, Eusebi V. Differential expression of myoepithelial markers in salivary, sweat and mammary glands. Int J Surg Pathol (2000) 8(1):29–37. doi: 10.1177/106689690000800108
46. Alos L, Carrillo R, Ramos J, Baez J, Mallofre C, Fernandez P, et al. High-grade carcinoma component in epithelial-myoepithelial carcinoma of salivary glands clinicopathological, immunohistochemical and flow-cytometric study of three cases. Virchows Archiv an Int J Pathol (1999) 434(4):291–9. doi: 10.1007/s004280050344
47. Teixeira L, Janner É, Teixeira T, Passador-Santos F, Martinez E, Demasi A, et al. Comparison of p63/p40 expression with myoepithelial markers in minor salivary gland tumors. Int J Surg Pathol (2019) 27(4):360–71. doi: 10.1177/1066896918813678
48. Kawahara A, Harada H, Yokoyama T, Kage M. p63 expression of clear myoepithelial cells in epithelial-myoepithelial carcinoma of the salivary gland: a useful marker for naked myoepithelial cells in cytology. Cancer (2005) 105(4):240–5. doi: 10.1002/cncr.21095
49. Hornick J, Fletcher C. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol (2003) 27(9):1183–96. doi: 10.1097/00000478-200309000-00001
50. el-Naggar A, Batsakis J, Luna M, Goepfert H, Tortoledo M. DNA Content and proliferative activity of myoepitheliomas. J laryngology otology (1989) 103(12):1192–7. doi: 10.1017/s0022215100111326
51. Mikaelian D, Contrucci R, Batsakis J. Epithelial-myoepithelial carcinoma of the subglottic region: a case presentation and review of the literature. Otolaryngology–head Neck Surg Off J Am Acad Otolaryngology-Head Neck Surg (1986) 95(1):104–6. doi: 10.1177/019459988609500120
Keywords: epithelial–myoepithelial carcinoma, nasopharynx, immunohistochemistry, concurrent chemoradiotherapy, case report
Citation: Zhang W, Wang X-x, Wang X-l, Zhang Y, Li X-f, Li Y, Cai Y-y, Ren H-q, Zhang Y-x and Hao F-r (2022) Epithelial–myoepithelial carcinoma of the nasopharynx: A case report and review of the literature. Front. Oncol. 12:923579. doi: 10.3389/fonc.2022.923579
Received: 19 April 2022; Accepted: 30 June 2022;
Published: 05 August 2022.
Edited by:
Ira Ida Skvortsova, Innsbruck Medical University, AustriaReviewed by:
Maria Elena Samar, National University of Cordoba, ArgentinaSisir Kumar Patra, LLH Hospital, United Arab Emirates
Copyright © 2022 Zhang, Wang, Wang, Zhang, Li, Li, Cai, Ren, Zhang and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fu-rong Hao, aGtjNTE1QDE2My5jb20=; Yun-xiang Zhang, emhhbmdiaW5nMTk5NTkyQDE2My5jb20=
 Wei Zhang1
Wei Zhang1 Yun-xiang Zhang
Yun-xiang Zhang Fu-rong Hao
Fu-rong Hao