- 1Department of Interventional Therapy, Affiliated Hospital of Qinghai University, Xining, China
- 2Department of Postgraduate, Qinghai University, Xining, China
- 3Department of Orthopedics, Sichuan Provincial People’s Hospital, Chengdu, China
- 4Department of Postgraduate, Chengdu Medical College, Chengdu, China
- 5Department of Hepatopancreatobiliary Surgery, Affiliated Hospital of Qinghai University, Xining, China
- 6Qinghai Province Key Laboratory of Hydatid Disease Research, Xining, China
Rupture of HCC (rHCC) is a life-threatening complication of hepatocellular carcinoma (HCC), and rHCC may lead to a high rate of peritoneal dissemination and affect survival negatively. Treatment for rHCC mainly includes emergency surgery, interventional therapies, and palliative treatment. However, the management of rHCC should be carefully evaluated. For patients with severe bleeding, who are not tolerant to open surgery, quick hemostatic methods such as rupture tissue ablation and TAE/TACE can be performed. We described clinical presentation, prognosis, complication, interventional management, and current evidence of rHCC from the perspective of interventional radiologists. Overall, our review summarized that interventional therapies are necessary for most patients with rHCC to achieve hemostasis, even in some patients with Child–Pugh C. Moreover, TAE/TACE followed by staged hepatectomy is a beneficial treatment for rHCC according to current clinical evidence. TAE/TACE is the first choice for most patients with rHCC, and appropriate interventional treatment may provide staged surgery opportunities for those who are not tolerant to emergency surgery to reach an ideal prognosis.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most diagnosed cancer and the fourth leading cause of cancer-related death worldwide (1). Hepatitis C virus (HCV) infections and hepatitis B virus (HBV) infections account for most cases of HCC, and the rest mainly comprise people with excessive alcohol consumption, nonalcoholic fatty hepatitis, liver cirrhosis, and a family history of HCC (2, 3). Spontaneous rupture of HCC (rHCC) is a life-threatening complication with an estimated incidence of 3%–26% in patients with HCC (4, 5) and a mortality rate ranging from 25% to 75% (6–9). Furthermore, even if the hemorrhage is completely controlled, rHCC may lead to a high rate of peritoneal dissemination and affect patient survival negatively (10). Therefore, rHCC is significantly associated with high risk of recurrence and poor prognosis (11). The primary aim remains the prevention of hypovolemic shock and the stabilization of the condition of patients (12, 13); thus, therapeutic options should be discussed according to the condition of the patient, tumor stage, liver function, and feasibility of resection. The treatment options for spontaneous rHCC mainly include emergency surgery, interventional therapy, and palliative treatment. However, the choice of emergency surgery is still controversial because some studies believed that patients with spontaneous rHCC should be managed by non-operative treatment initially (14). Moreover, emergency surgery has a high risk for elderly patients or patients with poor physical condition (15, 16). Emergency surgery may increase the risk of intraperitoneal hemorrhage, liver failure, abdominal infection, and bile leakage. According to the American Joint Committee on Cancer, all patients with spontaneous rHCC are assigned a grade of T4, even if the tumor is small, and there is no vascular invasion (17), indicating the poor prognosis of rHCC; thus, whether those with rHCC are tolerant to emergency surgery should be carefully evaluated (18, 19). Another controversial point is that emergency surgery may prolong the overall survival time for some patients, but it may increase the risk of tumor metastasis for others (18, 20). After the first 12 h of clinical management without significant improvement, supportive care could be chosen in situations of acute uncontrolled bleeding in moribund patients (21). Initial management of rHCC includes a multidisciplinary and comprehensive approach with a primary aim of patient survival, rather than HCC treatment.
Interventional therapy, including transarterial chemoembolization (TACE), transarterial embolization (TAE), transarterial radioembolization (TARE), microwave ablation (MWA), and radiofrequency ablation (RFA), remains a minimally invasive treatment for HCC, even suitable for some patients who have extrahepatic metastases or recurrence (4). TACE/TAE could embolize the blood supply of rHCC via the hepatic artery and other extrahepatic arteries. The application of interventional therapy is still broader than open surgery, as interventional therapy can be applied in some cases with Child–Pugh C (22). Furthermore, for patients with severe bleeding, who are not tolerant to open surgery, quick hemostatic methods such as rupture tissue ablation and TAE/TACE can be performed (23). This review will aim to clarify the clinical presentation, prognosis, complication, interventional management, and current evidence of rHCC from the perspective of interventional radiologists.
Pathophysiology and Risk Factors of HCC Rupture
The mechanism and prognosis of rHCC have not been completely investigated. Studies have also reported that tumor location, tumor size, hypertension, hepatic parenchyma, and liver cirrhosis were associated with rHCC.
The overlying liver parenchyma plays an important role in rHCC. As tumor progresses, when the tumor invades the hepatic arteries or portal vein, the pressure inside the liver may increase and lead to rHCC (24–26). Moreover, a study found that there was almost no overlying liver parenchyma on the ruptured tumor, neither was it shown by imaging examination nor found in operation (27).
The relationship between tumor size and HCC rupture is controversial. Although the size of tumor is associated with the pressure inside the tumor and vessel, Li et al. (27) reported that tumor size is not a significant risk factor in rHCC. However, Chen et al. (25) and Zhu et al. (28) reported that HCC size of more than 5 cm is at high risk of rHCC. Undoubtedly, tumor size is an independent prognostic factor in evaluating overall survival and disease-free survival (29, 30). If the mass volume increases too fast and partial necrosis of the tumor occurs, it can lead to the collapse of the tumor surface and bleeding. Furthermore, researchers also found that an HCC as small as 2 cm has been reported to be ruptured (31). Tumor size is an important study-level factor of rHCC, but clinicians cannot predict rHCC simply by tumor size.
Previous studies have identified that vascular injury may be associated with rHCC. A widely accepted mechanism of rHCC is that hepatic arteries and veins are invaded or occluded by tumor cells, leading to high pressure within the tumor mass (32). As HCC is supplied by the tumor supply vessels, Zhu et al. (33) reported that the vascular injury was present more frequently in the patients with rHCC than that in the patients with non-ruptured HCC. Moreover, Zhu et al. (34) reported that the expression of the antigen–antibody complex including hepatitis B e1 antigen and complement C1q on vascular wall and vascular injuries was mainly presented in patients with rHCC. Their conclusion can also explain why it is more common to see patients with rHCC in Asia than in Europe, because Asia has the highest incidence rates of chronic HBV infection (especially in China) (35). In addition, due to the invasion and growth of tumor, the pressure of hepatic vein may increase, and the high pressure of hepatic vein may be associated with venous congestion, resulting in tumor ischemia, necrosis, and liquefaction. Hibi (36) reported that increased intraluminal pressure of the biliary system due to obstruction by the tumor thrombus is considered to have led to the rupture of the liver abscess into the bronchus, thus creating a bronchobiliary fistula; thus, intraluminal pressure of the biliary system may be associated with a series of risks including tumor rupture. As patients with HCC can be affected by various factors such as coagulopathy and abnormal liver function, all these factors lead to rHCC and intrableeding of tumor (12).
In addition, rHCC is associated with factors involving liver function, portal vein thrombus, location, and so on. Monroe et al. (37) reported that patients with a higher degree of liver dysfunction have an overall poor prognosis and a high possibility of rHCC. After the portal vein was embolized by tumor thrombus, dystrophic necrosis appeared in the peripheral part of the superficial tumor, and the rupture of superficial tumor may also be associated with rHCC (38); thus, the location of the tumor is a risk factor for rHCC. On the basis of the experience from our center, when the tumor is located at the superficial position of the hepatic encapsulation, it can be easily affected by external forces. The thin tumor encapsulation and the extremely fragile cancer tissue also cause rupture and bleeding.
Clinical Presentations and Diagnosis of Ruptured HCC
Although rHCC can present with various symptoms, acute abdominal pain can be observed in most cases (66%–100%) of rHCC (17). Shock is the second most common complication of rHCC, with an incidence range from 33% to 90% (7, 9, 20, 39–41). Liver failure occurs in 12%–42% of rHCC (12). Furthermore, in the study by Zhu et al. (28), patients with spontaneous rHCC can be asymptomatic, or experience abdominal discomfort and anemic symptoms, indicating that the presentation of rHCC varies from individuals. Even in some rare cases, patients presented with non-bleeding rHCC (42) or hemothorax (43).
Ultrasonography (US) and computed tomography (CT) are recommended for detecting rHCC, the location of ruptured tumor, and changes in hematoma density. The most direct evidence of rHCC from CT scans is hemoperitoneum (44), but hemoperitoneum cannot be observed in all cases. Moreover, highest-attenuating hematomas, active extravasation of contrast materials, contour protrusion of tumor, discontinuity of the hepatic surface, and the enucleation sign are various CT findings of usual manifestations of rHCC (25, 45–49). Furthermore, the location of highest-attenuation hematomas is close to the rHCC (50).
Patients with rHCC usually presented with acute abdominal pain, and with the utilization of medical imaging, laboratory analyses, and history of patients, a diagnosis of rHCC can usually be made. However, in some cases of rHCC, the diagnosis of rHCC is still a challenge, as some cases are noncirrhotic and there is an absence of risk factors, indicating that other potential factors may also play an important role in carcinogenesis and rHCC (51, 52).
Abdominal paracentesis can be a choice when patients are suspected with rHCC (53). If the non-coagulable blood was sucked out by a syringe, it shows that there is visceral hemorrhage in the abdominal cavity, which is the most effective and routine examination method for the diagnosis of abdominal visceral hemorrhage (54). However, the coagulation function of HCC patients should be taken into account when abdominal paracentesis is performed. Moreover, diagnostic paracentesis is in vain when performed in some rare case presented with no bleeding (42). It is worth noting that no diagnostic study has been published to investigate the sensitivity and specificity of abdominal paracentesis for the diagnosis of rHCC.
Transarterial Embolization/Transarterial Chemoembolization for Rupture of HCC
Although the prognosis of rHCC is not ideal, survival benefits for interventional radiology and hepatectomy have been reported in patients with rHCC (55–57). Actually, several studies (55, 58, 59) reported that hepatectomy provided better survival benefits than TAE/TACE, reaching an in-hospital survival of 76.5% and a 1-month survival of 71%. However, TAE is the most frequent option to achieve hemostasis and stabilize the condition of patients. The hemostatic success rate from published studies is ideal as most patients can achieve hemostasis (Table 1) (29, 30, 58–79). A meta-analysis (5) involving 21 studies with 974 rHCC participants (485 participants treated with TACE/TAE and 489 participants treated with emergency surgery) reported that TAE/TACE was significantly superior to emergency surgery in terms of complications [OR = 0.36, 95% confidence interval (CI): 0.22–0.57] and in-hospital mortality (OR = 0.52, 95% CI: 0.29–0.94), and emergency surgery did not provide a more favorable effect on successful hemostasis (96.2% vs. 94.6%) and 1-year survival rate (47.6% vs. 48.7%) than TAE/TACE. However, all included studies of this meta-analysis were conducted in China, which will undoubtedly lead to bias. Another meta-analysis and systematic review regarding rHCC reported that among patients treated with TACE/TAE, the in-hospital and 1-month survival ranged from 30.3% to 66.7% and from 44.4% to 87.5% (80). Furthermore, Zhou et al. (59) reported that there are no differences in successful rate of hemostasis between TAE and hepatectomy. Furthermore, due to the abnormal coagulation function, poor hepatic function reserve, hemodynamic instability, cirrhosis, and other risk factors (58, 80, 81), many patients with rHCC are not well tolerant to open surgery. Interventional therapies involving TAE/TACE for rHCC can reach an ideal effect. TACE/TAE can be performed as a temporary treatment for patients waiting for staged hepatectomy. Even some cases with poor prognostic factors such as liver function deteriorated, and aforementioned interventional options may be employed in patients with hemodynamic instability or advanced underlying patients with rHCC.
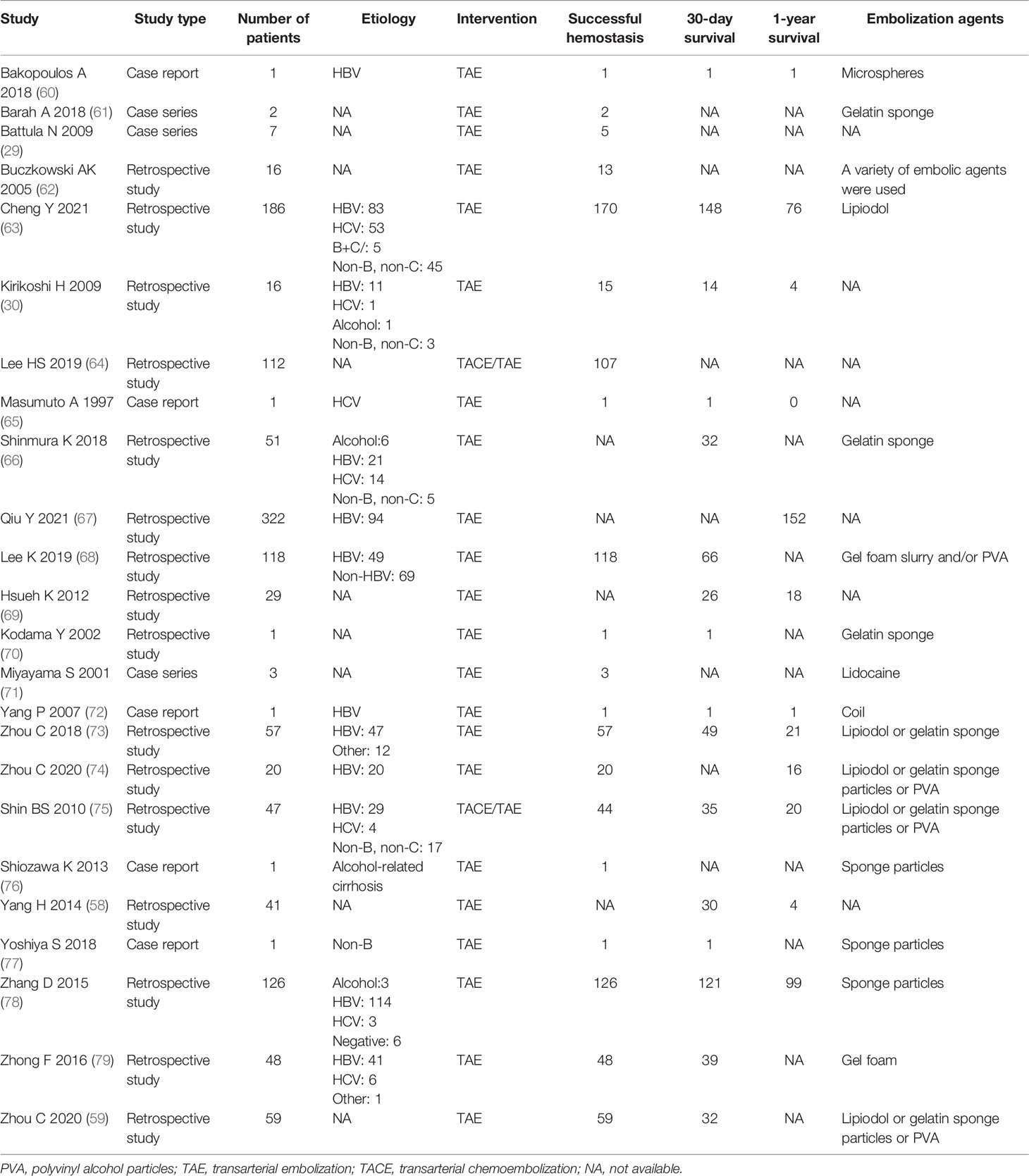
Table 1 Summary of clinical studies on successful hemostasis, survival, and embolization agents of TAE/TACE for rHCC.
The aim of interventional therapy is not only to achieve successful hemostasis, but also to prolong the survival of patients with rHCC. Lee et al. (64) and Hsueh et al. (69) reported that those who underwent staged hepatectomy after TACE/TAE had significantly higher overall survival than those who underwent TACE alone, indicating that staged hepatectomy is encouraged for post-TAE/TACE patients who are tolerant to hepatectomy. However, whether TAE/TACE is to be followed by staged hepatectomy depends on the recovery of liver function and a thorough investigation of the tumor characteristics. Moreover, whether HCC is ruptured, staged hepatectomy is recommended (12, 20, 82). It should be noted that a meta-analysis (83) reported that long-term staged hepatectomy might not be more beneficial than emergency hepatectomy. However, in this meta-analysis, we found that the authors did not mention how they acquire survival data to estimate hazard ratio from included studies and their results contradict the studies that they included; thus, their conclusion should be interpreted with caution.
Chen et al. (38) and Kirikoshi et al. (30) reported that the main cause of death is failed hemostasis after TAE and hepatic failure. Furthermore, Cheng et al. (63) reported that Child–Pugh classification, MELD score, BCLC stage, albumin, INR, and post-TAE total bilirubin in 7 days are independent risk factors for post TAE 30-day mortality and successful hemostasis in rHCC.
Interventional radiologists should also note that extrahepatic rupture of metastatic HCC has been reported as a rare complication of TAE (84). Suoh et al. (85) reported a case of hemothorax secondary to spontaneous rHCC metastasis to the chest wall in an 87-year-old man who was treated with TAE, and transcatheter treatment can achieve hemostasis and a favorable survival even in this setting. Nagao et al. (86) reported a case of ruptured chest wall metastasis of HCC that was controlled by TAE.
Kodama et al. (70) reported of a case with rHCC supplied by the right renal capsular artery and Child–Pugh C liver function, indicating that TAE may be chosen for poor liver function when tumor feeders are only extrahepatic collateral vessels. However, Bassi et al. (87) reported that the mortality of patients with Child–Pugh C liver function is exceedingly high in the early post-TAE period, but only three patients with Child–Pugh C liver function received TAE in this study. TAE is a palliative procedure that is used when the liver function is compromised or, in the case of multifocal-bilobar HCC, when the post-treatment mortality of poor liver function is very high regardless of treatment type. In Shin’s study (75), the overall median survival time of ten patients with Child–Pugh class C who underwent TAE/TACE was 5 days.
HCC can recruit extrahepatic collateral vessels from a wide range of vessels such as gastroduodenal artery, superior mesenteric artery, and suprarenal arteries (88–90); thus, incomplete embolization of those vessels may also raise the risk of rebleeding. The estimated prevalence of extrahepatic arteries recruited by HCC ranged from 17% to 27% (91–94), indicating that some cases with extrahepatic collateral arteries cannot be successfully embolized because those vessels may originate from other organs such as stomach and duodenum. Moreover, due to the incomplete embolization of extrahepatic collateral arteries, recurrent bleeding and local tumor recurrence should be noted by interventional radiologists in clinical practice. In rHCC with celiac axis stenosis, Barah et al. (61) reported that the pancreaticoduodenal was used as a salvage alternative route for emergency TAE of hepatic arteries. However, in situations such as extensive stenosis or occlusion of the origin of the celiac axis, inaccessibility to hepatic arteries through the celiac axis is a highly challenging situation in which the technical success depends on the experience of interventional radiologists.
It is worth noting that TAE/TACE-led injuries of portal vein or hepatic vein may lead to recurrent bleeding and an unsatisfactory hemostatic effect (95, 96), as HCC can be ruptured after TAE/TACE or during TAE/TACE (97). Furthermore, some patients are at high risk of rupture because of a lack of protective surrounding parenchymal tissue, or tumor progression resulting from revascularization after embolization (98). Moreover, TACE may lead to a series of post-chemoembolization syndrome including pain and fever. Most patients who underwent chemoembolization may experience post-chemoembolization syndrome, but the data are lacking. Moreover, TACE may also be related to acute renal failure, upper gastrointestinal bleeding, and other serious complications (99–101). This may be the reason why TAE is much more frequently performed for patients with rHCC than TACE. Interestingly, Hidaka et al. reported that TACE for those with huge HCC (>10 cm) may also lead to rHCC (102), and a post-TACE huge HCC rupture can be successfully treated using interventional procedures. Tu et al. (103) reported that liver rupture can be observed in 6 of 1,120 patients who received conventional TAE/TACE therapy. According to aforementioned studies, we infer that patients with a history of TACE/TAE or a tumor diameter that is greater than 10 cm may have a higher incidence of rHCC. However, this hypothesis should be verified by further studies.
Various embolization materials can influence the patient prognosis, and the effect of different embolic agents is not completely known. Koçyiğit et al. (104) reported that the use of different embolic agents for TACE had no significant effect on survival on patients with HCC. Marelli et al. (105) reported that there is no evidence of the benefit of lipiodol, and gelatin sponge is the most used embolic agent, but PVA particles may be better. However, in clinical practice, in general, a variety of embolic agents were used, depending on tumor location, degree of bleeding, and interventional radiologist experience. Furthermore, no study has investigated the effect and safety of different embolic agents in the treatment of rHCC.
Discussing the level of embolization, Lee et al. (68) reported that selective or subsegmental TAE was performed if the tumor is solitary and/or a bleeding source was well delineated by CT and/or angiography. In our opinion, the primary aim remains the prevention of hypovolemic shock and stabilization of the condition of patients, and the secondary aim is to embolize the tumor as much as possible when feasible.
Another issue that should be noted is that aforementioned observational studies, non-randomized studies, case reports, and cluster-randomized trials have a known limitation as these studies will force us to focus more on benefits than harms (106).
Furthermore, the overall survival rate of patients with rHCC who underwent TAE/TACE is lower than those who underwent radical resection; hence, the long-term effect of TAE/TACE is still questioned (12).
Ablation for Rupture of HCC
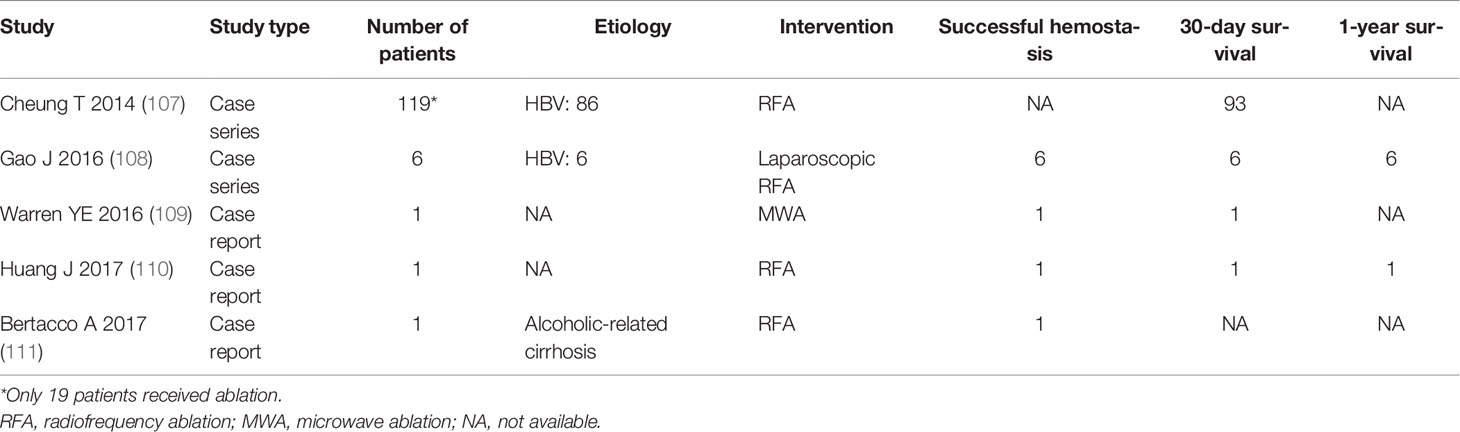
Ablation is another interventional therapy for rHCC, but the efficacy and safety are only reported in a few studies (Table 2) (107–111). Although ablation involves radiofrequency ablation therapy (RFA), cryoablation, MWA, and irreversible electroporation (IRE), RFA is the main choice in the treatment of HCC, which can be performed using a percutaneous, laparoscopical, or laparotomic approach. However, the safety of the percutaneous approach is not clear, which is associated with the risk of intrahepatic bleeding, bile leakage, and liver injury (108); thus, Livraghi et al. (112) suggested that the open or laparoscopic method is a safer approach in the treatment of subcapsular tumors.
Gao et al. (108) reported 10 patients [size: 6.6 ± 2.2 cm (4.0–10.1 cm)] with rHCC treated with laparoscopic RFA, with a 3-year survival rate of 70%. Sasaki et al. (113) reported that ruptured peritoneal metastases of HCC can also be controlled by RFA. Sun et al. (114) reported a case of a giant HCC with a maximum diameter of 14 cm with unstable circulation (Child–Pugh C). During the emergency laparotomy, the authors found that surgical excision was impossible due to the size and location of the tumor. Then, the patient received RFA as a salvage solution. Finally, patient follow-up for at least 56 months revealed a high quality of life, indicating that a giant rHCC can be successfully controlled by RFA. Chen et al. (115) reported that RFA can also be performed in a case of liver metastasis from rectal cancer and a case of liver metastasis from gastric sarcoma, reaching a beneficial survival time of 36 months and 17 months, respectively.
Among these studies, image-guided percutaneous ablation is commonly used, as it can be minimally invasive, and most cases can achieve hemostasis. Because only limited cases were reported, the benefit of ablation is not completely understood. Three studies (107, 108, 110) reported that direct puncturing of the bleeding site was performed. Similar to TAE/TACE, the primary aim of ablation remains the prevention of hypovolemic shock and the stabilization of the condition, and the secondary aim is to achieve tumor elimination when feasible.
Furthermore, another issue of ablation that should be noted is the “heat sink” effect, which is defined as a phenomenon whereby flowing blood adjacent to or within tissues being targeted for ablation results in relative tissue cooling due to heat transfer by convection (116), leading to a bad prognosis. On the other hand, rHCC usually has a large volume and rich blood supply; thus, the “heat sink” effect may have a significant impact on RFA for a ruptured giant HCC. Interestingly, several studies found that the “heat sink” effect has a significant impact on RFA but not MWA in combination with embolization, indicating that the “heat sink” effect plays a more important role in RFA than in MWA (117–119). Puza et al. (120) reported that the “heat sink” effect appears to have a more minor impact on MWA in a rabbit model and the effect of ablation can be affected when performed adjacent to major vessels. On the basis of these studies, we infer that RFA is much more sensitive in the treatment of rHCC with a smaller diameter and MWA is the optimal ablation for a huge rHCC. However, the conclusion is limited because only several well-documented cases are reported.
Interventional Combination Therapies for Rupture of HCC
The application of interventional combination therapies is used in some settings. However, interventional combination therapies for rHCC are only reported in several studies (Table 3) (121, 122). Baimas-George et al. (121) reported that TAE or TACE followed by laparoscopic MWA and washout may offer an advantage in the treatment of rHCC. It not only achieves hemostasis but also could have an oncologic benefit by targeting local tumor and decreasing peritoneal carcinomatosis risk, reaching a median survival of 431 days in 15 patients, but the 30-day mortality was 6/15. Takao et al. (122) reported that a case of hemobilia from HCC can be controlled by TAE followed by MWA; the patient died of liver failure with no recurrence of hemobilia. However, the prognosis of patients who received TAE/TACE followed by MWA should be evaluated by more high-quality clinical evidence. It is too early to draw final conclusions about the impact of interventional combination therapies on rHCC.
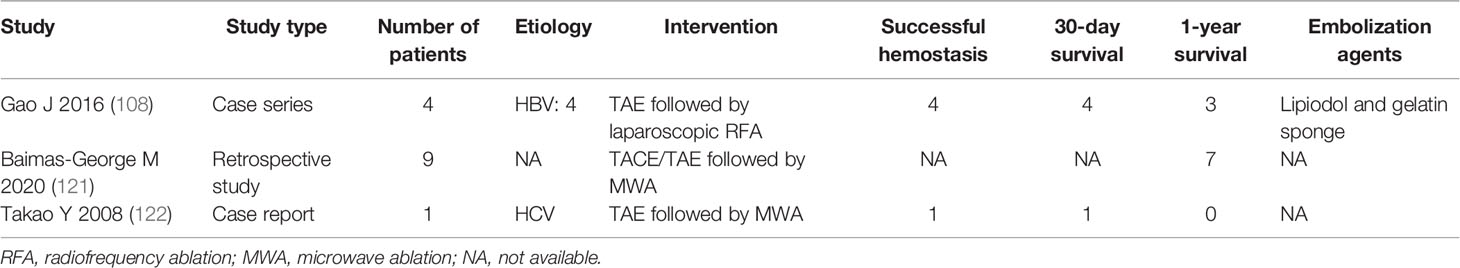
Table 3 Summary of clinical studies on successful hemostasis, survival, and embolization agents of interventional combination therapies for rHCC.
Recently, the interventional combination therapies for HCC include TACE plus brachytherapy and transarterial ethanol ablation combined with TACE (123–125). Moreover, immune checkpoint inhibitors and tyrosine kinase inhibitors are adopted in clinical practice, but there is no evidence to investigate the relationship between rHCC and these drugs (126). Although such treatments are not reported in the treatment of rHCC, those studies prompted us to consider that interventional combination therapies may improve the prognosis of patients with rHCC in the future.
Conclusion
In conclusion, the clinical management of rHCC remains a challenge. Our review summarized that interventional therapies are necessary for most patients with rHCC to achieve hemostasis, even in some patients with Child–Pugh C. Furthermore, TAE/TACE followed by staged hepatectomy is the most beneficial treatment for rHCC according to current clinical evidence. However, the efficacy and safety of ablation are still questioned because only a few well-documented studies were reported. Interventional radiologists should also keep in mind that TAE/TACE is the first choice for most patients with rHCC, and appropriate interventional treatment may provide staged surgery opportunities for those who are not tolerant to emergency surgery to reach an ideal prognosis.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was supported by Project of Science and Technology Department of Qinghai Province (2020-ZJ-Y01).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer (2020) 9(6):682–720. doi: 10.1159/000509424
2. El-Serag HB. Epidemiology of Viral Hepatitis and Hepatocellular Carcinoma. Gastroenterology (2019) 156(2):477–491.e1. doi: 10.1053/j.gastro.2018.08.065
3. Yang JD, Roberts LR. Hepatocellular Carcinoma: A Global View. Nat Rev Gastroenterol Hepatol (2010) 7(8):448–58. doi: 10.1038/nrgastro.2010.100
4. Ren A, Luo S, Ji L, Yi X, Liang J, Wang J, et al. Peritoneal Metastasis After Emergency Hepatectomy and Delayed Hepatectomy for Spontaneous Rupture of Hepatocellular Carcinoma. Asian J Surg (2019) 42(2):464–9. doi: 10.1016/j.asjsur.2018.09.006
5. Xu X, Chen C, Liu Q, Huang X. A Meta-Analysis of TAE/TACE Versus Emergency Surgery in the Treatment of Ruptured HCC. Cardiovasc Intervent Radiol (2020) 43(9):1263–76. doi: 10.1007/s00270-020-02514-5
6. Liu CL, Fan ST, Lo CM, Tso WK, Poon RT, Lam CM, et al. Management of Spontaneous Rupture of Hepatocellular Carcinoma: Single-Center Experience. J Clin Oncol (2001) 19(17):3725–32. doi: 10.1200/JCO.2001.19.17.3725
7. Ong GB, Taw JL. Spontaneous Rupture of Hepatocellular Carcinoma. Br Med J (1972) 4:146–9. doi: 10.1136/bmj.4.5833.146
8. Cherqui D, Panis Y, Rotman N, Fagniez PL. Emergency Liver Resection for Spontaneous Rupture of Hepatocellular Carcinoma Complicating Cirrhosis. Br J Surg (1993) 80(6):747–9. doi: 10.1002/bjs.1800800631
9. Leung KL, Lau WY, Lai PB, Yiu RY, Meng WC, Leow CK. Spontaneous Rupture of Hepatocellular Carcinoma: Conservative Management and Selective Intervention. Arch Surg (1999) 134(10):1103–7. doi: 10.1001/archsurg.134.10.1103
10. Yunoki Y, Takeuchi H, Makino Y, Murakami I, Yasui Y, Tanakaya K, et al. Intraperitoneal Seeding of Ruptured Hepatocellular Carcinoma: Case Report. Abdom Imaging (1999) 24(4):398–400. doi: 10.1007/s002619900522
11. Portolani N, Baiocchi GL, Gheza F, Molfino S, Lomiento D, Giulini SM. Parietal and Peritoneal Localizations of Hepatocellular Carcinoma: Is There a Place for a Curative Surgery? World J Surg Oncol (2014) 12:298. doi: 10.1186/1477-7819-12-298
12. Lai EC, Lau WY. Spontaneous Rupture of Hepatocellular Carcinoma: A Systematic Review. Arch Surg (2006) 141(2):191–8. doi: 10.1001/archsurg.141.2.191
13. Bassi N, Caratozzolo E, Bonariol L, Bridda A, Padoan L, Antoniuttu M, et al. Management of Ruptured Hepatocellular Carcinoma: Implications of Therapy. World J Gastroenterol (2010) 16:1221–5. doi: 10.3748/wjg.v16.i10.1221
14. Wu JJ, Zhu P, Zhang ZG, Zhang BX, Shu C, Mba'nbo-Koumpa AA, et al. Spontaneous Rupture of Hepatocellular Carcinoma: Optimal Timing of Partial Hepatectomy. Eur J Surg Oncol (2019) 45(10):1887–94. doi: 10.1016/j.ejso.2019.02.033
15. Lin XJ. The Efficacy and Prognosis Analysis of Transarterial Chemoembolization Versus Surgery in the Treatment of Spontaneous Rupture of Hepatocellular Carcinoma. J Med Theor Pract (2018) 31(24):3697–9. doi: 10.19381/j.issn.1001-7585.2018.24.030
16. Xu KY, Huang YH, Tao CL. The Outcome of Patients With Spontaneous Ruptured Hepatocellular Carcinoma: Comparing Surgical Resection and Tanscatheter Aterial Embolization for Therapy. J Hepato Pancreato Biliary Surg (2014) 26(1):9–11.
17. Yoshida H, Mamada Y, Taniai N, Uchida E. Spontaneous Ruptured Hepatocellular Carcinoma. Hepatol Res (2016) 46(1):13–21. doi: 10.1111/hepr.12498
18. Aoki T, Kokudo N, Matsuyama Y, Izumi N, Ichida T, Kudo M, et al. Prognostic Impact of Spontaneous Tumor Rupture in Patients With Hepatocellular Carcinoma: An Analysis of 1160 Cases From a Nationwide Survey. Ann Surg (2014) 259:532–42. doi: 10.1097/SLA.0b013e31828846de
19. Chan AC, Dai JW, Chok KS, Cheung TT, Lo CM. Prognostic Influence of Spontaneous Tumor Rupture on Hepatocellular Carcinoma After Interval Hepatectomy. Surgery (2016) 159:409–17. doi: 10.1016/j.surg.2015.07.020
20. Miyamoto M, Sudo T, Kuyama T. Spontaneous Rupture of Hepatocellular Carcinoma: A Review of 172 Japanese Cases. Am J Gastroenterol (1991) 86:67–71.
21. Schwarz L, Bubenheim M, Zemour J, Herrero A, Muscari F, Ayav A, et al. Bleeding Recurrence and Mortality Following Interventional Management of Spontaneous HCC Rupture: Results of a Multicenter European Study. World J Surg (2018) 42(1):225–32. doi: 10.1007/s00268-017-4163-8
22. Hiraoka A, Kumada T, Michitaka K, Toyoda H, Tada T, Ishikawa T, et al. Is There a Survival Benefit in Interventional Radiology for Hepatocellular Carcinoma in Patients With Child-Pugh C Liver Cirrhosis?: A Multicenter Study. Hepatol Res (2016) 46(6):521–8. doi: 10.1111/hepr.12583
23. Zheng YJ, Li DL, Luo D, Chen XP, Zhang B, Fang C, et al. Early Versus Delayed Hepatectomy for Spontaneously Ruptured Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. J Invest Surg (2021) 34(11):1214–22. doi: 10.1080/08941939.2020.1792009
24. Rossetto A, Adani GL, Risaliti A, Baccarani U, Bresadola V, Lorenzin D, et al. Combined Approach for Spontaneous Rupture of Hepatocellular Carcinoma. World J Hepatol (2010) 2:49–51. doi: 10.4254/wjh.v2.i1.49
25. Chen CY, Lin XZ, Shin JS, Lin CY, Leow TC, Chen CY, et al. Spontaneous Rupture of Hepatocellular Carcinoma. A Review of 141 Taiwanese Cases and Comparison With Nonrupture Cases. J Clin Gastroenterol (1995) 21:238–42. doi: 10.1097/00004836-199510000-00015
26. Yoshida H, Mamada Y, Taniai N, Mizuguchi Y, Kakinuma D, Ishikawa Y, et al. Long-Term Results of Elective Hepatectomy for the Treatment of Ruptured Hepatocellular Carcinoma. J Hepatobiliary Pancreat Surg (2008) 15:178–82. doi: 10.1007/s00534-007-1239-0
27. Li J, Huang L, Liu CF, Cao J, Yan JJ, Xu F, et al. Risk Factors and Surgical Outcomes for Spontaneous Rupture of BCLC Stages A and B Hepatocellular Carcinoma: A Case-Control Study. World J Gastroenterol (2014) 20(27):9121–7. doi: 10.3748/wjg.v20.i27.9121
28. Zhu Q, Li J, Yan JJ, Huang L, Wu MC, Yan YQ. Predictors and Clinical Outcomes for Spontaneous Rupture of Hepatocellular Carcinoma. World J Gastroenterol (2012) 18(48):7302–7. doi: 10.3748/wjg.v18.i48.7302
29. Battula N, Madanur M, Priest O, Srinivasan P, O’Grady J, Heneghan MA, et al. Spontaneous Rupture of Hepatocellular Carcinoma: A Western Experience. Am J Surg (2009) 197(2):164–7. doi: 10.1016/j.amjsurg.2007.10.016
30. Kirikoshi H, Saito S, Yoneda M, Fujita K, Mawatari H, Uchiyama T, et al. Outcomes and Factors Influencing Survival in Cirrhotic Cases With Spontaneous Rupture of Hepatocellular Carcinoma: A Multicenter Study. BMC Gastroenterol (2009) 9:29. doi: 10.1186/1471-230X-9-29
31. Clarkston W, Inciardi M, Kirkpatrick S, Mc Ewen G, Ediger S, Schubert T. Acute Hemoperitoneum From Rupture of a Hepatocellular Carcinoma. J Clin Gastroenterol (1988) 10:221–5. doi: 10.1097/00004836-198804000-00025
32. Zhu LX, Wang GS, Fan ST. Spontaneous Rupture of Hepatocellular Carcinoma. Br J Surg (1996) 83:602–7. doi: 10.1002/bjs.1800830507
33. Zhu L, Geng X, Fan S. Relationship Between Vascular Elasticity and Spontaneous Rupture of Hepatocellular Carcinoma. Chinese-German J Clin Oncol (2003) 01:21–25+62.
34. Zhu LX, Geng XP, Fan ST. Spontaneous Rupture of Hepatocellular Carcinoma and Vascular Injury. Arch Surg (2001) 136(6):682–7. doi: 10.1001/archsurg.136.6.682
35. Liu J, Liang W, Jing W, Liu M. Countdown to 2030: Eliminating Hepatitis B Disease, China. Bull World Health Organ (2019) 97(3):230–8. doi: 10.2471/BLT.18.219469
36. Hibi T, Sakamoto Y, Asamura H, Tochigi N, Ojima H, Shimada K, et al. Successful Resection of Hepatocellular Carcinoma With Bronchobiliary Fistula Caused by Repeated Transcatheter Arterial Embolizations: Report of a Case. Surg Today (2007) 37(2):154–8. doi: 10.1007/s00595-006-3351-1
37. Monroe EJ, Kogut MJ, Ingraham CR, Kwan SW, Hippe DS, Padia SA. Outcomes of Emergent Embolisation of Ruptured Hepatocellular Carcinoma in a Western Population. Clin Radiol (2015) 70(7):730–5. doi: 10.1016/j.crad.2015.03.007
38. Chen Y, Guo D, Li X, Xu C, Zhu Q. Predictors of Spontaneous Rupture of Hepatocellular Carcinoma and Clinical Outcomes Following Hepatectomy. Front Oncol (2022) 27:820867. doi: 10.3389/fonc.2022.820867
39. Dewar GA, Griffin SM, Ku KW, Lau WY, Li AK. Management of Bleeding Liver Tumours in Hong Kong. Br J Surg (1991) 78(4):463–6. doi: 10.1002/bjs.1800780424
40. Xu HS, Yan JB. Conservative Management of Spontaneous Ruptured Hepatocellular Carcinoma. Am Surg (1994) 60(8):629–33.
41. Muhammad I, Mabogunje O. Spontaneous Rupture of Primary Hepatocellular Carcinoma in Zaria, Nigeria. J R Coll Surg Edinb (1991) 36(2):117–20.
42. Islam M, Deka P, Kapur R, Ansari MA. Non-Bleeding Spontaneous Rupture of Hepatocellular Carcinoma. Niger J Surg (2013) 19(2):82–4. doi: 10.4103/1117-6806.119241
43. Ono F, Hiraga M, Omura N, Sato M, Yamamura A, Obara M, et al. Hemothorax Caused by Spontaneous Rupture of Hepatocellular Carcinoma: A Case Report and Review of the Literature. World J Surg Oncol (2012) 10:215. doi: 10.1186/1477-7819-10-215
44. Lubner M, Menias C, Rucker C, Bhalla S, Peterson CM, Wang L, et al. Blood in the Belly: CT Findings of Hemoperitoneum. Radiographics (2007) 27(1):109–25. doi: 10.1148/rg.271065042
45. Shanmuganathan K, Mirvis SE, Reaney SM. Pictorial Review: CT Appearances of Contrast Medium Extravasations Associated With Injury Sustained From Blunt Abdominal Trauma. Clin Radiol (1995) 50(3):182–7. doi: 10.1016/s0009-9260(05)83054-5
46. Choi BG, Park SH, Byun JY, Jung SE, Choi KH, Han JY. The Findings of Ruptured Hepatocellular Carcinoma on Helical CT. Br J Radiol (2001) 74(878):142–6. doi: 10.1259/bjr.74.878.740142
47. Kim PT, Su JC, Buczkowski AK, Schaeffer DF, Chung SW, Scudamore CH, et al. Computed Tomography and Angiographic Interventional Features of Ruptured Hepatocellular Carcinoma: Pictorial Essay. Can Assoc Radiol J (2006) 57(3):159–68.
48. Kanematsu M, Imaeda T, Yamawaki Y, Seki M, Goto H, Sone Y, et al. Rupture of Hepatocellular Carcinoma: Predictive Value of CT Findings. AJR Am J Roentgenol (1992) 158(6):1247–50. doi: 10.2214/ajr.158.6.1317090
49. Kim HC, Yang DM, Jin W, Park SJ. The Various Manifestations of Ruptured Hepatocellular Carcinoma: CT Imaging Findings. Abdom Imaging (2008) 33(6):633–42. doi: 10.1007/s00261-007-9353-7
50. Orwig D, Federle MP. Localized Clotted Blood as Evidence of Visceral Trauma on CT: The Sentinel Clot Sign. AJR Am J Roentgenol (1989) 153(4):747–9. doi: 10.2214/ajr.153.4.747
51. Tartaglia N, Di Lascia A, Cianci P, Fersini A, Pacilli M, Pavone G, et al. Hemoperitoneum Caused by Spontaneous Rupture of Hepatocellular Carcinoma in Noncirrhotic Liver. A Case Rep systematic Rev Open Med (Wars) (2020) 15(1):739–44. doi: 10.1515/med-2020-0202
52. Michelotti G, Machado M, Diehl A. NAFLD. NASH and Liver Cancer. Nat Rev Gastroenterol Hepatol (2013) 10(11):656–65. doi: 10.1038/nrgastro.2013.183
53. Wang B, Lu Y, Zhang XF, Yú L, Pan CE, Wu Z. Management of Spontaneous Rupture of Hepatocellular Carcinoma. ANZ J Surg (2008) 78(6):501–3. doi: 10.1111/j.1445-2197.2008.04543.x
54. Sahu SK, Chawla YK, Dhiman RK, Singh V, Duseja A, Taneja S, et al. Rupture of Hepatocellular Carcinoma: A Review of Literature. J Clin Exp Hepatol (2019) 9(2):245–56. doi: 10.1016/j.jceh.2018.04.002
55. Jin YJ, Lee JW, Park SW, Lee JI, Lee DH, Kim YS, et al. Survival Outcome of Patients With Spontaneously Ruptured Hepatocellular Carcinoma Treated Surgically or by Transarterial Embolization. World J Gastroenterol (2013) 19(28):4537–44. doi: 10.3748/wjg.v19.i28.4537
56. Zhang DZ, Zhang K, Wang XP, Cai H. Patients With Spontaneously Ruptured Hepatocellular Carcinoma Benefit From Staged Surgical Resection After Successful Transarterial Embolization. Asian Pac J Cancer Prev (2015) 16(1):315–9. doi: 10.7314/apjcp.2015.16.1.315
57. Zhang NN, Lu W, Cheng XJ, Liu JY, Zhou YH, Li F. High-Powered Microwave Ablation of Larger Hepatocellular Carcinoma: Evaluation of Recurrence Rate and Factors Related to Recurrence. Clin Radiol (2015) 70(11):1237–43. doi: 10.1016/j.crad.2015.06.092
58. Yang H, Chen K, Wei Y, Liu F, Li H, Zhou Z, et al. Treatment of Spontaneous Ruptured Hepatocellular Carcinoma: A Single-Center Study. Pak J Med Sci (2014) 30(3):472–6. doi: 10.12669/pjms.303.4001
59. Zhou C, Zu QQ, Liu XL, Wang B, Zhou CG, Shi HB, et al. Treatment Strategies and Prognosis for Initially Unresectable Ruptured Hepatocellular Carcinoma: A Single-Center Experience in 94 Patients. Diagn Interv Radiol (2020) 26(3):223–9. doi: 10.5152/dir.2019.19049
60. Bakopoulos A, Koliakos N, Tsilimigras DI, Schizas D, Moris D, Angelopoulos A, et al. Management of Ruptured Liver Segment IV Hepatocellular Carcinoma: Is Transarterial Embolization (TAE) Superior to Chemoembolization (TACE)?-The Jury is Still Out. Ann Transl Med (2018) 6(13):272. doi: 10.21037/atm.2018.06.01
61. Barah A, Omar A, El-Menyar A, Almokdad O, Sayedin A, Alsherbini A, et al. Pancreaticoduodenal Arcades as Salvage Route for Transarterial Embolization of Life-Threatening Hepatic Hemorrhage in Patients With Severe Celiac Axis Stenosis: Case Series. Int J Surg Case Rep (2018) 48:5–9. doi: 10.1016/j.ijscr.2018.03.043
62. Buczkowski AK, Kim PT, Ho SG, Schaeffer DF, Lee SI, Owen DA, et al. Multidisciplinary Management of Ruptured Hepatocellular Carcinoma. J Gastrointest Surg (2006) 10(3):379–86. doi: 10.1016/j.gassur.2005.10.012
63. Cheng YT, Teng W, Lui KW, Hsieh YC, Chen WT, Huang CH, et al. MELD Score is the Better Predictor for 30-Day Mortality in Patients With Ruptured Hepatocellular Carcinoma Treated by Trans-Arterial Embolization. Am J Cancer Res (2021) 11(7):3726–34.
64. Lee HS, Choi GH, Choi JS, Han KH, Ahn SH, Kim DY, et al. Staged Partial Hepatectomy Versus Transarterial Chemoembolization for the Treatment of Spontaneous Hepatocellular Carcinoma Rupture: A Multicenter Analysis in Korea. Ann Surg Treat Res (2019) 96(6):275–82. doi: 10.4174/astr.2019.96.6.275
65. Masumoto A, Motomura K, Uchimura K, Morotomi I, Morita K. Case Report: Haemothorax Due to Hepatocellular Carcinoma Rupture Successfully Controlled by Transcatheter Arterial Embolization. J Gastroenterol Hepatol (1997) 12(2):156–8. doi: 10.1111/j.1440-1746.1997.tb00399.x
66. Shinmura K, Choi YH, Shimohira M, Baba Y, Ikeda S, Hayashi S, et al. Comparison of Conservative Treatment Versus Transcatheter Arterial Embolisation for the Treatment of Spontaneously Ruptured Hepatocellular Carcinoma. Pol J Radiol (2018) 83:e311–8. doi: 10.5114/pjr.2018.77024
67. Qiu Y, Wang T, Yang X, Shen S, Yang Y, Wang W. Development and Validation of Artificial Neural Networks for Survival Prediction Model for Patients With Spontaneous Hepatocellular Carcinoma Rupture After Transcatheter Arterial Embolization. Cancer Manag Res (2021) 13:7463–77. doi: 10.2147/CMAR.S328307
68. Lee KH, Tse MD, Law M, Cheng AK, Wong HF, Yu ML, et al. Development and Validation of an Imaging and Clinical Scoring System to Predict Early Mortality in Spontaneous Ruptured Hepatocellular Carcinoma Treated With Transarterial Embolization. Abdom Radiol (NY) (2019) 44(3):903–11. doi: 10.1007/s00261-019-01895-7
69. Hsueh KC, Fan HL, Chen TW, Chan DC, Yu JC, Tsou SS, et al. Management of Spontaneously Ruptured Hepatocellular Carcinoma and Hemoperitoneum Manifested as Acute Abdomen in the Emergency Room. World J Surg (2012) 36(11):2670–6. doi: 10.1007/s00268-012-1734-6
70. Kodama Y, Shimizu T, Endo H, Hige S, Kamishima T, Holland GA, et al. Spontaneous Rupture of Hepatocellular Carcinoma Supplied by the Right Renal Capsular Artery Treated by Transcatheter Arterial Embolization. Cardiovasc Intervent Radiol (2002) 25(2):137–40. doi: 10.1007/s00270-001-0060-4
71. Miyayama S, Matsui O, Akakura Y, Yamamoto T, Nishida H, Yoneda K, et al. Hepatocellular Carcinoma With Blood Supply From Omental Branches: Treatment With Transcatheter Arterial Embolization. J Vasc Interv Radiol (2001) 12(11):1285–90. doi: 10.1016/s1051-0443(07)61553-x
72. Yang PW, Wang WY, Yang CH, Chou CC, Yen DH, Chou J. Treatment of Massive Retroperitoneal Hemorrhage From Adrenal Metastasis of Hepatoma. J Chin Med Assoc (2007) 70(3):126–31. doi: 10.1016/S1726-4901(09)70343-0
73. Zhou C, Zu QQ, Wang B, Zhou CG, Shi HB, Liu S. Efficacy and Prognostic Factors of Transarterial Embolization as Initial Treatment for Spontaneously Ruptured Hepatocellular Carcinoma: A Single-Center Retrospective Analysis in 57 Patients. Jpn J Radiol (2019) 37(3):255–63. doi: 10.1007/s11604-018-0799-z
74. Zhou C, Zhang C, Zu QQ, Wang B, Zhou CG, Shi HB, et al. Emergency Transarterial Embolization Followed by Staged Hepatectomy Versus Emergency Hepatectomy for Ruptured Hepatocellular Carcinoma: A Single-Center, Propensity Score Matched Analysis. Jpn J Radiol (2020) 38(11):1090–8. doi: 10.1007/s11604-020-01007-2
75. Shin BS, Park MH, Jeon GS. Outcome and Prognostic Factors of Spontaneous Ruptured Hepatocellular Carcinoma Treated With Transarterial Embolization. Acta Radiol (2011) 52(3):331–5. doi: 10.1258/ar.2010.100369
76. Shiozawa K, Watanabe M, Ikehara T, Matsukiyo Y, Ishii K, Igarashi Y, et al. Usefulness of Contrast-Enhanced Ultrasonography in the Diagnosis of Ruptured Hepatocellular Carcinoma. Clin J Gastroenterol (2013) 6(4):334–7. doi: 10.1007/s12328-013-0398-6
77. Yoshiya S, Iwaki K, Sakai A, Fujita S, Kawasaki T, Yoshizumi F, et al. Laparoscopic Left Hepatectomy for Ruptured Hepatocellular Carcinoma Controlled After Transcatheter Arterial Embolization: Case Report and Review of the Literature. In Vivo (2018) 32(3):659–62. doi: 10.21873/invivo.11290
78. Zhang DZ, Zhang K, Wang XP, Cai H. Patients With Spontaneously Ruptured Hepatocellular Carcinoma Benefit From Staged Surgical Resection After Successful Transarterial Embolization. Asian Pac J Cancer Prev (2015) 16(1):315–9. doi: 10.7314/apjcp.2015.16.1.315
79. Zhong F, Cheng XS, He K, Sun SB, Zhou J, Chen HM. Treatment Outcomes of Spontaneous Rupture of Hepatocellular Carcinoma With Hemorrhagic Shock: A Multicenter Study. Springerplus (2016) 5(1):1101. doi: 10.1186/s40064-016-2762-8
80. Moris D, Chakedis J, Sun SH, Spolverato G, Tsilimigras DI, Ntanasis-Stathopoulos I, et al. Management, Outcomes, and Prognostic Factors of Ruptured Hepatocellular Carcinoma: A Systematic Review. J Surg Oncol (2018) 117(3):341–53. doi: 10.1002/jso.24869
81. Kim JY, Lee JS, Oh DH, Yim YH, Lee HK. Transcatheter Arterial Chemoembolization Confers Survival Benefit in Patients With a Spontaneously Ruptured Hepatocellular Carcinoma. Eur J Gastroenterol Hepatol (2012) 24(6):640–5. doi: 10.1097/MEG.0b013e3283524d32
82. Tarantino L, Sordelli I, Calise F, Ripa C, Perrotta M, Sperlongano P. Prognosis of Patients With Spontaneous Rupture of Hepatocellular Carcinoma in Cirrhosis. Updates Surg (2011) 63(1):25–30. doi: 10.1007/s13304-010-0041-8
83. Zhang FQ, Li L, Huang PC, Fu YF, Xu QS. Transarterial Embolization With Hepatectomy for Ruptured Hepatocellular Carcinoma: A Meta-Analysis. Minim Invasive Ther Allied Technol (2021), 1–8. doi: 10.1080/13645706.2021.1986724
84. Seki S, Kitada T, Sakaguchi H, Nakatani K, Kamino T, Nakamura K, et al. Cardiac Tamponade Caused by Spontaneous Rupture of Mediastinal Lymph Node Metastasis of Hepatocellular Carcinoma. J Gastroenterol Hepatol (2001) 16(6):702–4. doi: 10.1046/j.1440-1746.2001.02375.x
85. Suoh M, Hagihara A, Kageyama K, Yamamoto A, Enomoto M, Tamori A, et al. Successful Transcatheter Arterial Embolization for Hemothorax From a Spontaneous Rupture of Hepatocellular Carcinoma Metastasis to the Chest Wall in an Elderly Patient. Intern Med (2021) 60(14):2223–8. doi: 10.2169/internalmedicine.6003-20
86. Nagao E, Hirakawa M, Soeda H, Tsuruta S, Sakai H, Honda H. Transcatheter Arterial Embolization for Chest Wall Metastasis of Hepatocellular Carcinoma. World J Radiol (2013) 5(2):45–8. doi: 10.4329/wjr.v5.i2.45
87. Bassi N, Caratozzolo E, Bonariol L, Ruffolo C, Bridda A, Padoan L, et al. Management of Ruptured Hepatocellular Carcinoma: Implications for Therapy. World J Gastroenterol (2010) 16(10):1221–5. doi: 10.3748/wjg.v16.i10.1221
88. Miyayama S, Matsui O, Taki K, Minami T, Ryu Y, Ito C, et al. Extrahepatic Blood Supply to Hepatocellular Carcinoma: Angiographic Demonstration and Transcatheter Arterial Chemoembolization. Cardiovasc Intervent Radiol (2006) 29(1):39–48. doi: 10.1007/s00270-004-0287-y
89. Moustafa AS, Abdel Aal AK, Ertel N, Saad N, DuBay D, Saddekni S. Chemoembolization of Hepatocellular Carcinoma With Extrahepatic Collateral Blood Supply: Anatomic and Technical Considerations. Radiographics (2017) 37(3):963–77. doi: 10.1148/rg.2017160122
90. Park SI, Lee DY, Won JY, Lee JT. Extrahepatic Collateral Supply of Hepatocellular Carcinoma by the Intercostal Arteries. J Vasc Interv Radiol (2003) 14(4):461–8. doi: 10.1097/01.rvi.0000064856.87207.1e
91. Llovet JM. Updated Treatment Approach to Hepatocellular Carcinoma. J Gastroenterol (2005) 40(3):225–35. doi: 10.1007/s00535-005-1566-3
92. Lau WY, Yu SC, Lai EC, Leung TW. Transarterial Chemoembolization for Hepatocellular Carcinoma. J Am Coll Surg (2006) 202(1):155–68. doi: 10.1016/j.jamcollsurg.2005.06.263
93. Lau K, Wong WW, Chan JK, Kan WK, Leung JL, Lee AS, et al. Emergency Transcatheter Embolization of Ruptured Hepatocellular Carcinomas With Tortuous Conventional or Aberrant Hepatic Vascular Anatomy, or Parasitic Supply. Australas Radiol (2007) 51(2):190–5. doi: 10.1111/j.1440-1673.2007.01704.x
94. Gish RC, Scott JA, Yu H. Transarterial Chemoembolization of Hepatocellular Carcinoma via Extrahepatic Collateral Artery From a Supraduodenal and Cystic Artery Trunk, Originating From the Gastroduodenal Artery: A Case Report. Radiol Case Rep (2021) 16(10):3064–3067. doi: 10.1016/j.radcr.2021.07.041
95. Micallef S, Cortis K, Magri C. Hepatic Necrosis After Trans-Arterial Embolization of Metastatic Neuroendocrine Tumour. Eur J Case Rep Intern Med (2020) 7(5):1530. doi: 10.12890/2020_001530
96. Gala K, Guardiola-Bright J, McGee S. Post-Transarterial Chemoembolization Tumor Rupture in a Patient With Autoimmune Hepatitis Cirrhosis and Hepatocellular Carcinoma. Cureus (2020) 12(4):e7750. doi: 10.7759/cureus.7750
97. Choi JH, Kim JH, Won JH, Kim YS, Goo DE, Choi DL, et al. Spontaneous Intratumoral Hemorrhage Into Hepatocellular Carcinoma During Transcatheter Arterial Embolization: A Case Report. J Korean Med Sci (2004) 19(6):895–7. doi: 10.3346/jkms.2004.19.6.895
98. Zhao W, Hou X, Li H, Guo J, Cai L, Duan Y, et al. Imaging Features and Outcomes in Patients With Ruptured Hepatocellular Carcinoma Following Transcatheter Arterial Chemoembolization: A Retrospective Clinical Study. J Int Med Res (2021) 49(4):3000605211007722. doi: 10.1177/03000605211007722
99. Huo TI, Wu JC, Lee PC, Chang FY, Lee SD. Incidence and Risk Factors for Acute Renal Failure in Patients With Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization: A Prospective Study. Liver Int (2004) 24:210–5. doi: 10.1111/j.1478-3231.2004.00911.x
100. Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL. A Prospective Study Regarding the Complications of Transcatheter Intraarterial Lipiodol Chemoembolization in Patients With Hepatocellular Carcinoma. Cancer (2002) 94:1747–52. doi: 10.1002/cncr.10407
101. Leung TK, Lee CM, Chen HC. Anatomic and Technical Skill Factor of Gastroduodenal Complication in Post-Transarterial Embolization for Hepatocellular Carcinoma: A Retrospective Study of 280 Cases. World J Gastroenterol (2005) 11:1554–7. doi: 10.3748/wjg.v11.i10.1554
102. Hidaka T, Anai H, Sakaguchi H, Sueyoshi S, Tanaka T, Yamamoto K, et al. Efficacy of Combined Bland Embolization and Chemoembolization for Huge (≥10 Cm) Hepatocellular Carcinoma. Minim Invasive Ther Allied Technol (2021) 30(4):221–8. doi: 10.1080/13645706.2020.1725580
103. Tu J, Jia Z, Ying X, Zhang D, Li S, Tian F, et al. The Incidence and Outcome of Major Complication Following Conventional TAE/TACE for Hepatocellular Carcinoma. Med (Baltimore) (2016) 95(49):e5606. doi: 10.1097/MD.0000000000005606
104. Koçyiğit A, Dicle O, Göktay Y, Astarcıoğlu I. The Effect of Using Different Embolic Agents on Survival in Transarterial Chemoembolization of Hepatocellular Carcinoma: Gelfoam Versus Polyvinyl Alcohol. Diagn Interv Radiol (2014) 20(4):323–9. doi: 10.5152/dir.2014.13462
105. Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, et al. Transarterial Therapy for Hepatocellular Carcinoma: Which Technique is More Effective? A Systematic Review of Cohort and Randomized Studies. Cardiovasc Intervent Radiol (2007) 30(1):6–25. doi: 10.1007/s00270-006-0062-3
106. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated Guidance for Trusted Systematic Reviews: A New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
107. Cheung TT, Poon RT, Chok KS, Chan AC, Tsang SH, Dai WC, et al. Management of Spontaneously Ruptured Hepatocellular Carcinomas in the Radiofrequency Ablation Era. PLoS One (2014) 9(4):e94453. doi: 10.1371/journal.pone.0094453
108. Gao J, Zhou Y, Zhang Q, Kong J, Ke S, Ding X, et al. Early Laparoscopic Radiofrequency Ablation for Spontaneous Rupture of Hepatocellular Carcinoma. J Laparoendosc Adv Surg Tech A (2016) 26(7):560–6. doi: 10.1089/lap.2016.0118
109. Warren YE, Kirks RC, Thurman JB, Vrochides D, Iannitti DA. Laparoscopic Microwave Ablation for the Management of Hemorrhage From Ruptured Hepatocellular Carcinoma. Hippokratia (2016) 20(2):169–71.
110. Huang JH, Morelli JN, Ai F, Zou RH, Gu YK, Gao F, et al. Hydrochloric Acid-Enhanced Radiofrequency Ablation for Treating a Large Hepatocellular Carcinoma With Spontaneous Rapture: A Case Report. Chin J Cancer (2017) 36(1):1. doi: 10.1186/s40880-016-0161-8
111. Bertacco A, D'Amico F, Romano M, Finotti M, Vitale A, Cillo U. Liver Radiofrequency Ablation as Emergency Treatment for a Ruptured Hepatocellular Carcinoma: A Case Report. J Med Case Rep (2017) 11(1):54. doi: 10.1186/s13256-017-1199-1
112. Livraghi T, Meloni F, Solbiati L, Zanus G. Collaborative Italian Group Using AMICA System. Complications of Microwave Ablation for Liver Tumors: Results of a Multicenter Study. Cardiovasc Intervent Radiol (2012) 35(4):868–74. doi: 10.1007/s00270-011-0241-8
113. Sasaki N, Seyama Y, Ome Y, Doi M, Matsumura M, Imamura J, et al. A Resected Case of Ruptured Peritoneal Metastasis of Hepatocellular Carcinoma. Clin J Gastroenterol (2021) 14(4):1240–3. doi: 10.1007/s12328-021-01414-6
114. Sun WB, Ding XM, Ke S, Gao J, Zhang YF. Repeated Radiofrequency Ablation as Both Salvage Solution and Curative Treatment for Spontaneous Rupture of Giant Medial Lobe Hepatocellular Carcinoma. Chin Med J (Engl) (2009) 122(17):2067–70. doi: 10.3760/cma.j.issn.0366-6999.2009.17.020
115. Chen MH, Dai Y, Yan K, Yang W, Gao W, Wu W, et al. Intraperitoneal Hemorrhage During and After Percutaneous Radiofrequency Ablation of Hepatic Tumors: Reasons and Management. Chin Med J (Engl) (2005) 118(20):1682–7.
116. Yu NC, Raman SS, Kim YJ, Lassman C, Chang X, Lu DS. Microwave Liver Ablation: Influence of Hepatic Vein Size on Heat-Sink Effect in a Porcine Model. J Vasc Interv Radiol (2008) 19(7):1087–92. doi: 10.1016/j.jvir.2008.03.023
117. Bhardwaj N, Strickland AD, Ahmad F, Atanesyan L, West K, Lloyd DM. A Comparative Histological Evaluation of the Ablations Produced by Microwave, Cryotherapy and Radiofrequency in the Liver. Pathology (2009) 41(2):168–72. doi: 10.1080/00313020802579292
118. Pillai K, Akhter J, Chua TC, Shehata M, Alzahrani N, Al-Alem I, et al. Heat Sink Effect on Tumor Ablation Characteristics as Observed in Monopolar Radiofrequency, Bipolar Radiofrequency, and Microwave, Using Ex Vivo Calf Liver Model. Med (Baltimore) (2015) 94(9):e580. doi: 10.1097/MD.0000000000000580
119. Primavesi F, Swierczynski S, Klieser E, Kiesslich T, Jager T, Urbas R, et al. Thermographic Realtime-Monitoring of Surgical Radiofrequency and Microwave Ablation in a Perfused Porcine Liver Model. Oncol Lett (2018) 15(3):2913–20. doi: 10.3892/ol.2017.7634
120. Puza CJ, Wang Q, Kim CY. Evaluation of the Heat Sink Effect After Transarterial Embolization When Performed in Combination With Thermal Ablation of the Liver in a Rabbit Model. Cardiovasc Intervent Radiol (2018) 41(11):1773–8. doi: 10.1007/s00270-018-2034-9
121. Baimas-George M, Watson M, Murphy KJ, Sarantou J, Vrochides D, Martinie JB, et al. Treatment of Spontaneously Ruptured Hepatocellular Carcinoma: Use of Laparoscopic Microwave Ablation and Washout. HPB (Oxford) (2021) 23(3):444–50. doi: 10.1016/j.hpb.2020.08.001
122. Takao Y, Yoshida H, Mamada Y, Taniai N, Bando K, Tajiri T. Transcatheter Hepatic Arterial Embolization Followed by Microwave Ablation for Hemobilia From Hepatocellular Carcinoma. J Nippon Med Sch (2008) 75(5):284–8. doi: 10.1272/jnms.75.284
123. Yang B, You X, Yuan ML, Qin TQ, Duan LJ, He J, et al. Transarterial Ethanol Ablation Combined With Transarterial Chemoembolization for Hepatocellular Carcinoma With Portal Vein Tumor Thrombus. Hepat Mon (2016) 16(8):e37584. doi: 10.5812/hepatmon.37584
124. Song Z, Guo X, Yin C, Wang Y. Therapeutic Efficacy of TACE 125I Seed Implantation and its Combination With Intra-Tumor Injection of Cisplatin for the Treatment of Hepatocellular Carcinoma. Indian J Cancer (2021) 58(1):57–61. doi: 10.4103/ijc.IJC_635_18
125. Luo JJ, Zhang ZH, Liu QX, Zhang W, Wang JH, Yan ZP. Endovascular Brachytherapy Combined With Stent Placement and TACE for Treatment of HCC With Main Portal Vein Tumor Thrombus. Hepatol Int (2016) 10(1):185–95. doi: 10.1007/s12072-015-9663-8
Keywords: rupture of hepatocellular carcinoma, transarterial embolization (TAE), transarterial chemoembolization (TACE), ablation, prognosis, interventional radiology
Citation: Yan J, Li T, Deng M and Fan H (2022) Ruptured Hepatocellular Carcinoma: What Do Interventional Radiologists Need to Know? Front. Oncol. 12:927123. doi: 10.3389/fonc.2022.927123
Received: 23 April 2022; Accepted: 16 May 2022;
Published: 16 June 2022.
Edited by:
Duilio Pagano, Mediterranean Institute for Transplantation and Highly Specialized Therapies (ISMETT), ItalyReviewed by:
Kazuto Tajiri, University of Toyama University Hospital, JapanKakil Rasul, National Center for Cancer Care and Research, Qatar
Antonio Bottari, Università degli Studi di Messina, Italy
Copyright © 2022 Yan, Li, Deng and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingxin Yan, amluZ3hpbnlhbjE5OTdAMTI2LmNvbQ==; Haining Fan, ZmFuaGFpbmluZ0BtZWRtYWlsLmNvbS5jbg==
 Jingxin Yan
Jingxin Yan Ting Li
Ting Li Manjun Deng
Manjun Deng Haining Fan5,6*
Haining Fan5,6*