- 1Department of Hepatobiliary Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 2Department of Gastrointestinal Surgery, the First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 3The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 4Department of Urology Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
Background: Recent studies have shown that the fibrinogen to albumin ratio (FAR) is closely related to the prognosis of various cancers. The aim of this systematic review and meta-analysis was to investigate the prognostic value of FAR in malignancies based on the available evidence.
Method: To systematically search the Cochrane Library, Embase, PubMed, Google Scholar, Baidu scholars, CNKI and VIP databases for relevant studies published before April 1, 2022, and to evaluate the fibrinogen-to-albumin ratio (FAR) and survival of patients with malignant tumors through a meta-analysis relationship between the results. Results. This meta-analysis included 19 eligible studies involving 5926 cancer patients. We found that high FAR was associated with poor overall survival (HR=2.25, 95%CI 1.86-2.74, p<0.001), recurrence-free survival (HR=2.29, 95%CI 1.91-2.76, P<0.001), progression-free survival (HR: 2.10, 95%CI 1.58-2.79, p<0.001), disease-free survival (HR=1.52, 95%CI 1.17-1.96, p=0.001), and time to recurrence (HR: 1.555, 95%CI 1.031-2.346, P=0.035) was significantly correlated.
Conclusions: High FAR is significantly associated with poor clinical outcomes in cancer, suggesting that it may be an important predictor of prognosis in patients with malignancies.
Introduction
According to the latest data released by the American Cancer Society, 19.3 million new cancer cases and approximately 10 million deaths from cancer are expected worldwide in 2022 (1). As one of the leading causes of human death worldwide, how to prevent and treat cancer is a major issue in increasing the life expectancy of all human beings (2). Therefore, it is very important to find simple, effective and cheap markers to predict the prognosis of tumor patients. In recent years, many studies have shown that some biomarkers reflecting inflammation, nutritional status and immunity are related to the prognosis of cancer, including lymphocyte to monocyte ratio (LMR), neutrophil-to-lymphocyte ratio (NLR), fibrinogen to pre-albumin ratio (FPR), prognostic nutritional index (PNI) and albumin-to-alkaline phosphatase ratio (AAPR) (3–7).
Fibrinogen is a glycoprotein synthesized by liver cells and belongs to the acute phase positive protein (8). Fibrinogen levels are elevated during infection or inflammation and play an important role in clotting, cell attachment and thrombosis (9). During the tumor-associated inflammatory response, many events that promote tumor growth and metastasis often occur, including increased release of cytokines and inflammatory mediators, inhibition of apoptosis, and the exertion of immunosuppressive effects (10). Plasma fibrinogen levels have been reported to be associated with tumor progression and prognosis, such as colorectal, endometrial, hepatocellular and pancreatic cancers (11–14). Albumin is produced by the liver and is considered a negative acute phase protein (15). Plasma albumin plays an important role in regulating plasma osmotic pressure, antioxidant, capillary permeability and immune regulation (16, 17). A growing number of studies have shown an association between plasma albumin concentrations, inflammation, and tumorigenesis (18, 19). Available evidence suggests that hypoproteinemia is strongly associated with poor quality of life in cancer patients and is associated with poor prognosis in patients with various malignancies (20, 21). High fibrinogen and low albumin are associated with poor prognosis in cancer patients (12, 22). As a simple and effective new independent predictor, the fibrinogen-to-albumin ratio (FAR) has more prognostic value than high fibrinogen or low serum albumin. FAR has been reported as a potential predictor of adverse outcomes in various malignancies, such as esophageal squamous cell carcinoma, hepatocellular carcinoma, and non-small cell lung cancer (23–25).
A meta-analysis describing the relationship between fibrinogen and albumin ratio and cancer prognosis, exploring the effect of fibrinogen to albumin ratio (FAR) combined with albumin to fibrinogen (AFR) on cancer OS and DFS (26). However, this meta-analysis did not independently explore the effect of fibrinogen-to-albumin ratio (FAR) on cancer prognosis. Therefore, this study included 19 studies for meta-analysis, and evaluated the prognostic significance of FAR in human malignant tumors for the first time.
Materials and methods
Search strategy
In order to investigate the potential role of FAR in the prognosis of malignant tumors, this study followed the preferred reporting program of the systematic review and meta-analysis (PRISMA) guidelines (27) and searched the Cochrane Library, Embase, PubMed, Google Scholar, baidu scholar, CNKI and VIP databases. Search for relevant studies published no later than April 1, 2022, regardless of language. Combining the main keywords and free words, the complete search strategy is as follows: (“fibrinogen-to-albumin” OR “fibrinogen/albumin” OR “fibrinogen” OR “albumin”OR “FAR”) AND (“neoplasms” OR “carcinoma” OR “cancer” OR “tumor”) AND (“survival” or “recurrence” or “prognosis” or “progress”). In addition, the references of the retrieved publications are reviewed again to explore more potential relevant research.
Inclusion and exclusion criteria
The inclusion criteria are shown below:(1)the paper reports the relationship between fibrinogen-to-albumin ratio and long-term prognosis of patients with malignant tumor; (2)prognostic endpoints included overall survival (OS), progression-free survival (PFS), disease-free survival (DFS), recurrence-free survival (RFS) or time to recurrence (TTR);(3) hazard ratio (HR) and 95% confidence interval (CI) are provided or we can calculate it by Kaplan-Meier curve;(4) study patients were divided into two groups based on FAR. Exclusion criteria are listed:(1) hazard ratio (HR) and 95% confidence interval (CI) are not included; (2) outcomes—studies without primary or secondary results; (3) different articles published on the same cohort of patient data; (4) expert opinions, abstracts, case studies, letters or reviews.
Data extraction and quality assessment
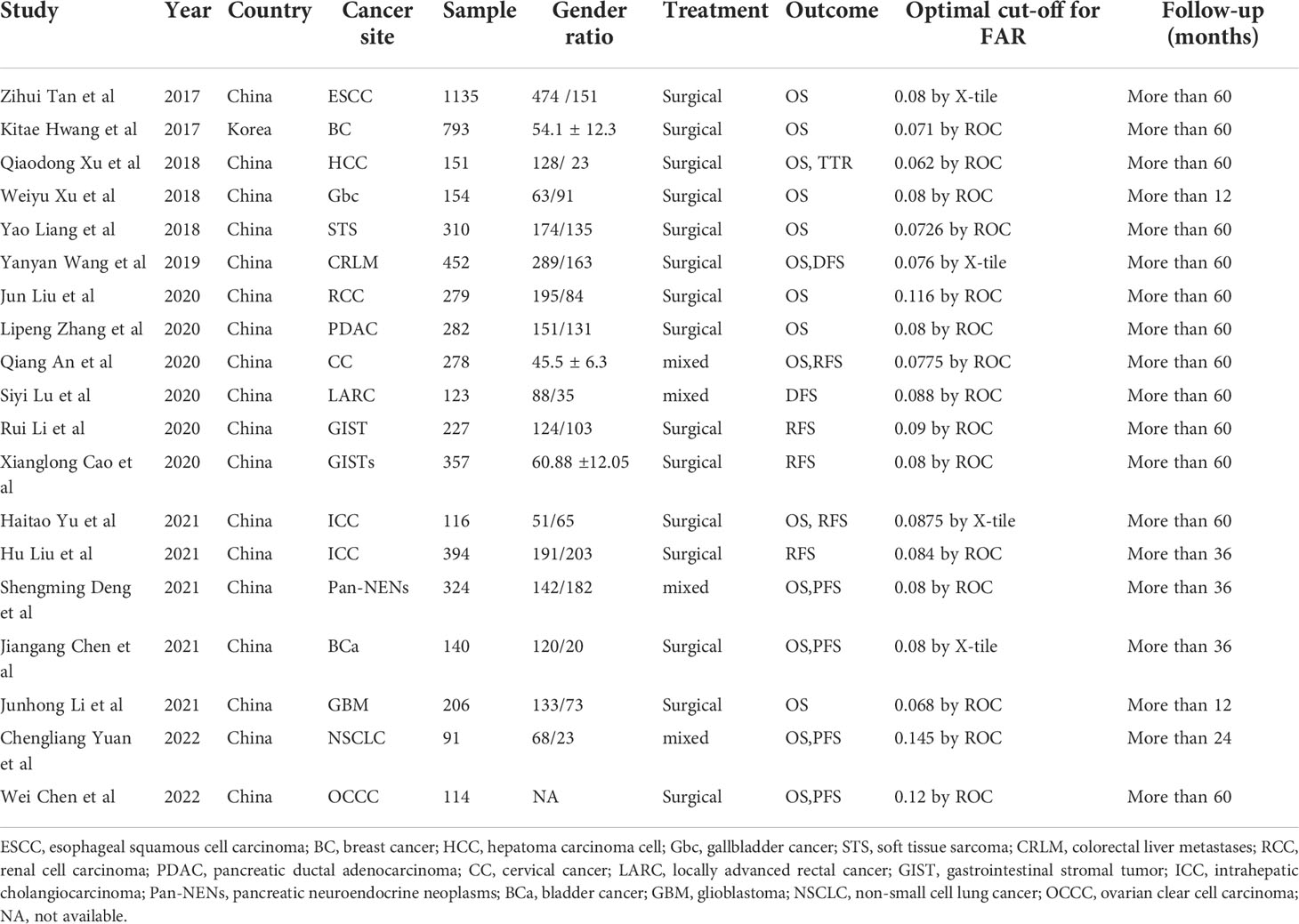
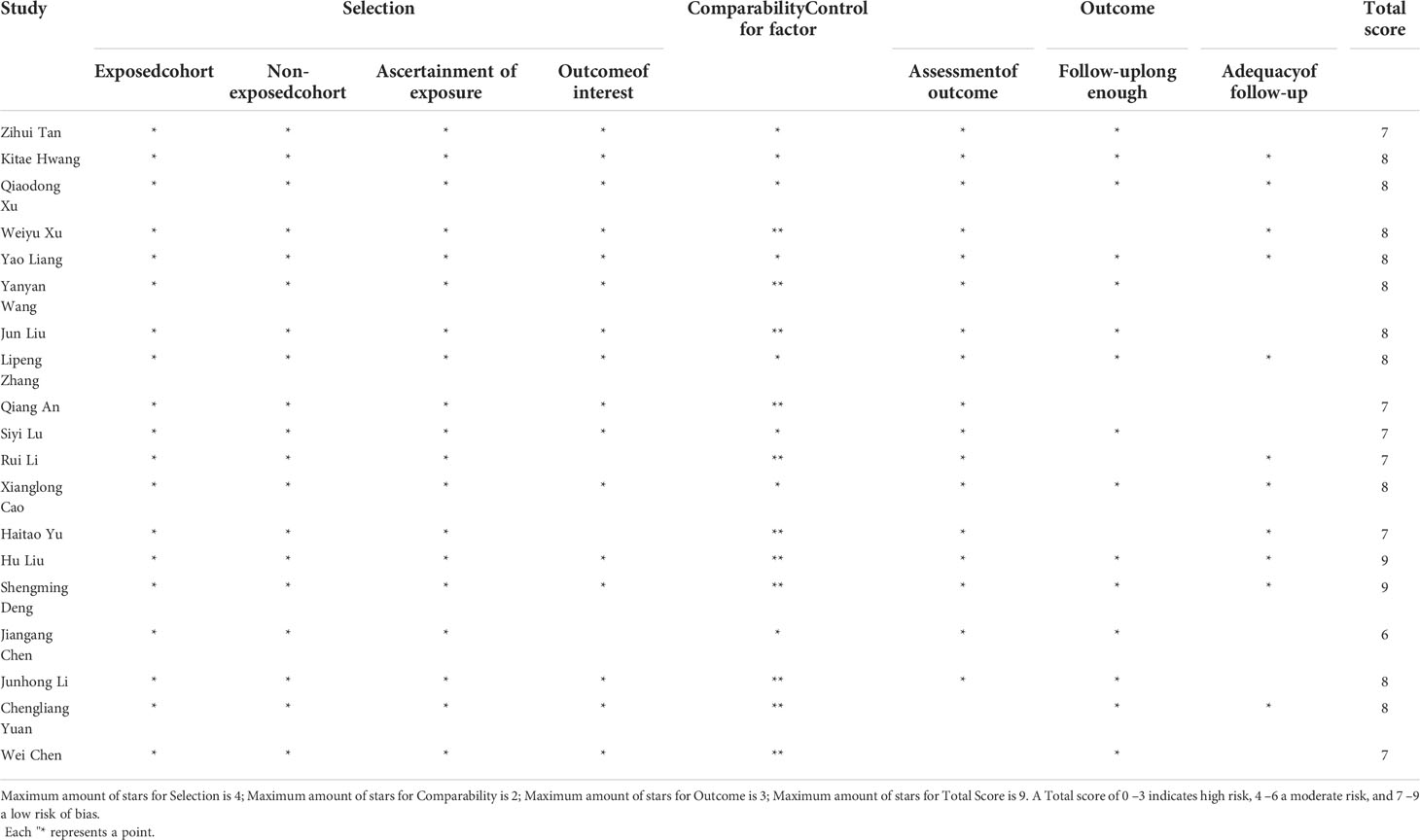
Two researchers (Baibei Li and Huachu Deng) used standardized forms to extract relevant data from the study independently, and if there was a disagreement, all the authors negotiated and resolved it. The basic information extracted included first author name, publication year, country, study type, tumor type, sample size, tumor stage, age, sex, treatment, cut-off, follow-up date, outcome indicators, hazard ratio (HR) and 95% confidence interval (CI). The researcher actively contacted the original author of the included literature to confirm the accuracy of the data and the process of data extraction by the original author. Each included study was scored according to the Newcastle-Ottawa Scale (NOS) (28) criteria to assess its quality. The total score of NOS ranged from 0 to 9, including patient selection (0-4), comparability (0-2) and outcome (0-3). If the NOS score was higher than 6, it was considered to be of high quality in methodology.
Statistical analysis
STATA (version 12) was used for data analysis, and the combined hazard ratio (HR) and 95% confidence interval (CI) were calculated. Cochran’s Q test and Higgins I2 test were used to evaluate the heterogeneity of each study. When I2 > 50% or P < 0.10, there was significant heterogeneity, so the random effect model was used. Otherwise, the fixed effect model is adopted. Subgroup analysis was used to evaluate the sources of heterogeneity, and sensitivity analysis was used to determine the reliability and stability of the results. Begg’s test and Egger’s were used to test whether there was publication bias. When P > 0.05, there was no publication bias, otherwise trim-and-fill method would be used for re-evaluation. In this study, all the tests were bilateral, and P < 0.05 was considered to be statistically significant.
Results
Study characteristics
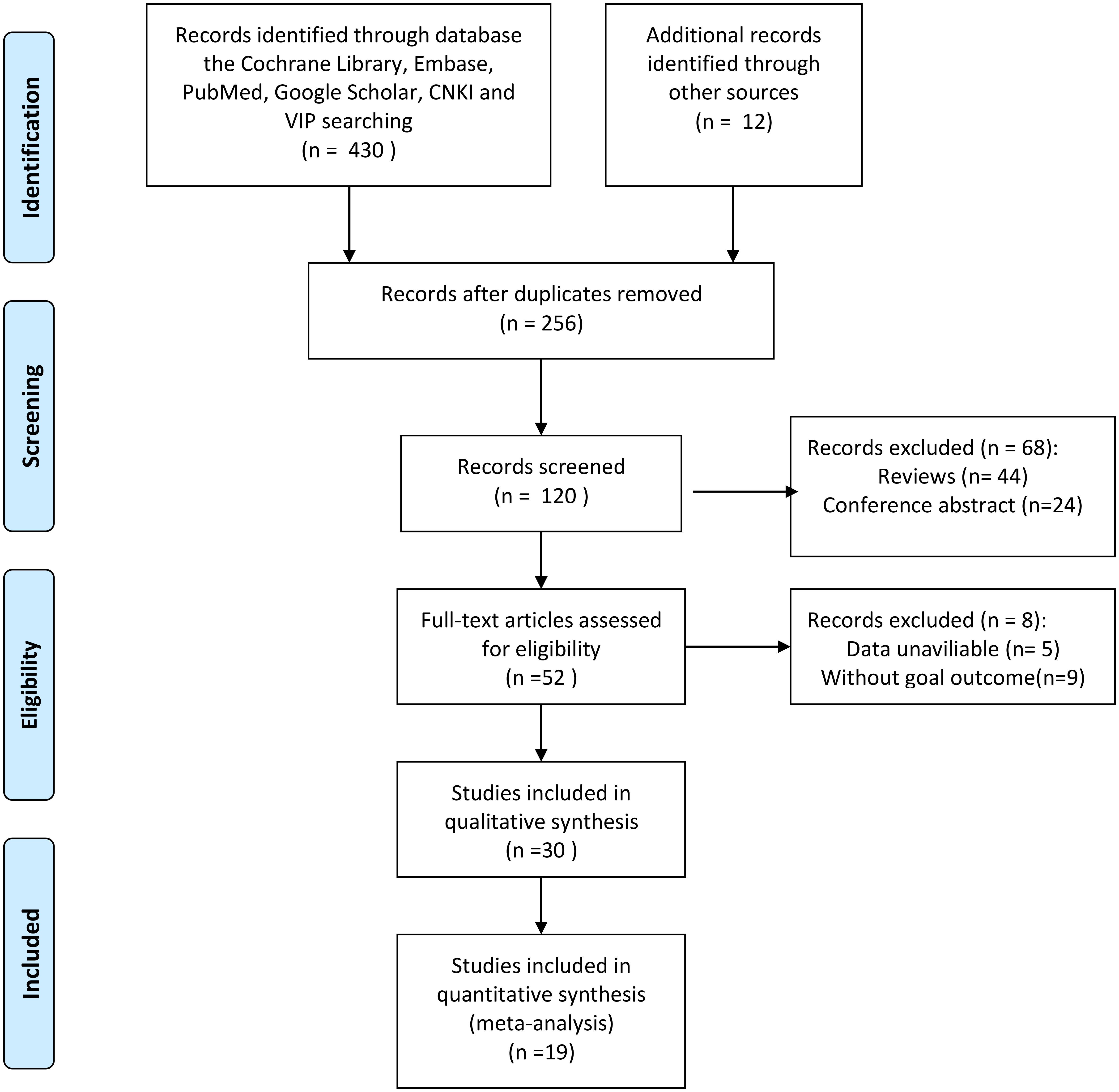
Figure 1 shows the screening process of the literature included in this study. In the aggregate, a total of 430 articles were retrieved through search strategies, and 12 articles were retrieved through additional records identified through other sources. After duplicates were removed, 256 articles remained. After reading the literature titles and abstracts, 44 reviews and 24 conference abstracts were deleted. Afterwards, we evaluated the full text of the articles and found that the data of 5 of them was of no value, and 9 of the articles did not mention the target result. Therefore, 19 studies involving 5926 cases were included in our meta-analysis (23–25, 29–44). Of the included studies, 18 were from China and one was from South Korea. Publication years are from 2017 to 2022. Sample sizes ranged from 91 to 1,135. In addition, 15 cohorts reported OS, 5 cohorts reported RFS, 4 cohorts reported PFS, 2 cohorts reported DFS, and 1 cohort reported TTR. The baseline information is shown in Table 1. The quality of each included article was evaluated by NOS, and the results showed that the scores of these studies were all≥6, indicating that the quality of the included study was high (Table 2).
FAR and overall survival
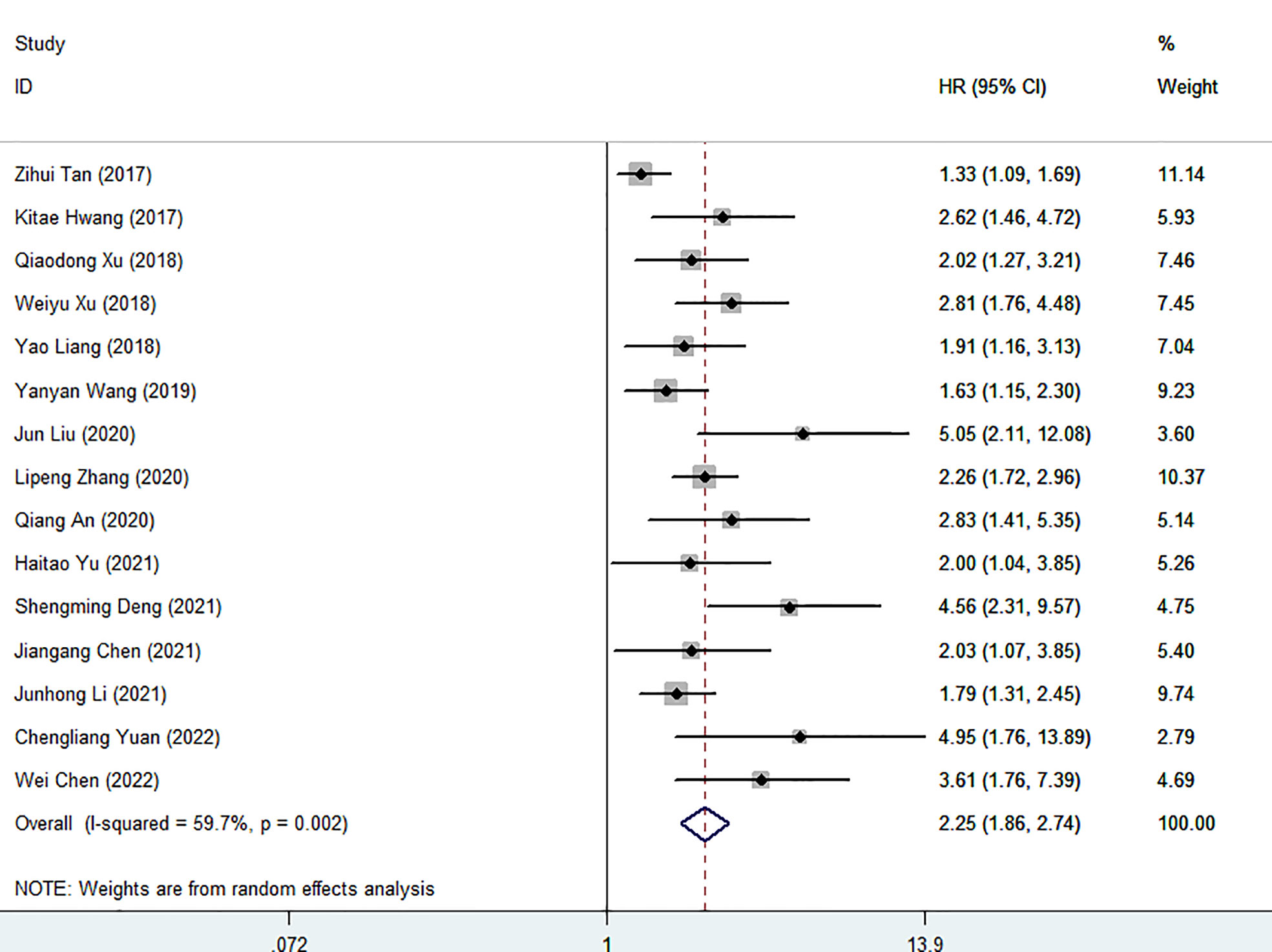
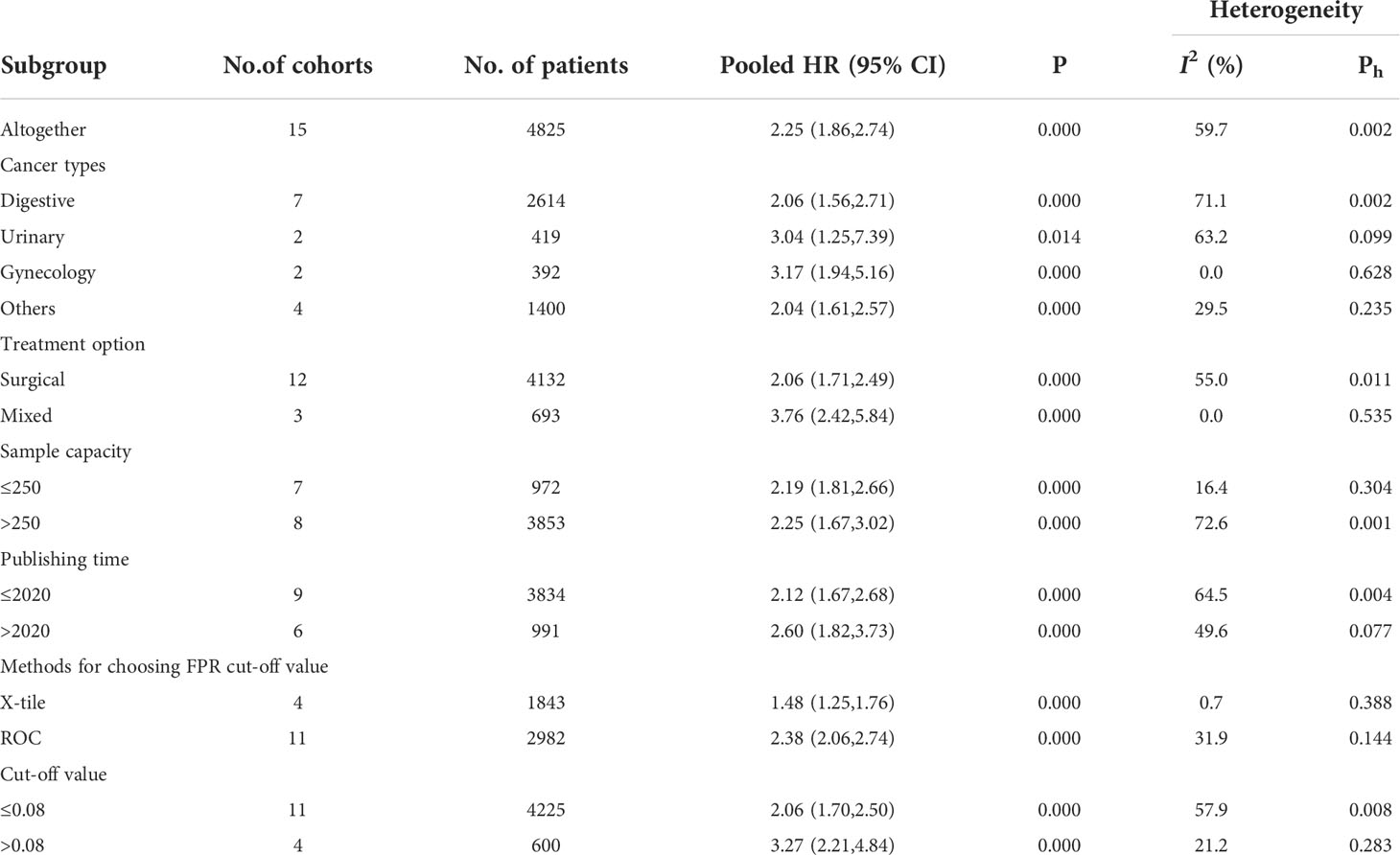
Fifteen cohort studies involving a total of 4825 patients revealed the prognostic effect of FAR levels on human malignant tumor OS. Our results show that the relatively high level of HRR before treatment is related to the decrease of OS (HR=2.25, 95%CI 1.85-2.74, p<0.001). Because of the significant heterogeneity, the random effect model is adopted (I2 = 59.7%, P=0.002) (Figure 2). Therefore, we performed a stratified subgroup analysis by cancer type, treatment option, sample capacity, publishing time, methods for choosing FPR cut-off value and cut-off value to explore the source of heterogeneity (Table 3). Despite the differences between groups, high FAR was significantly associated with poor OS. Furthermore, some subgroup heterogeneity was eliminated when we stratified according to factors such as “Gynecology”, “Mixed”, “Cut-off value > 0.08”, “Sample capacity ≤ 250” and “X-tile”. Further analysis of these results led us to suggest that the use of mixed treatment option and the large sample capacity may have contributed to the heterogeneity.
Sensitivity analysis and publication bias for OS
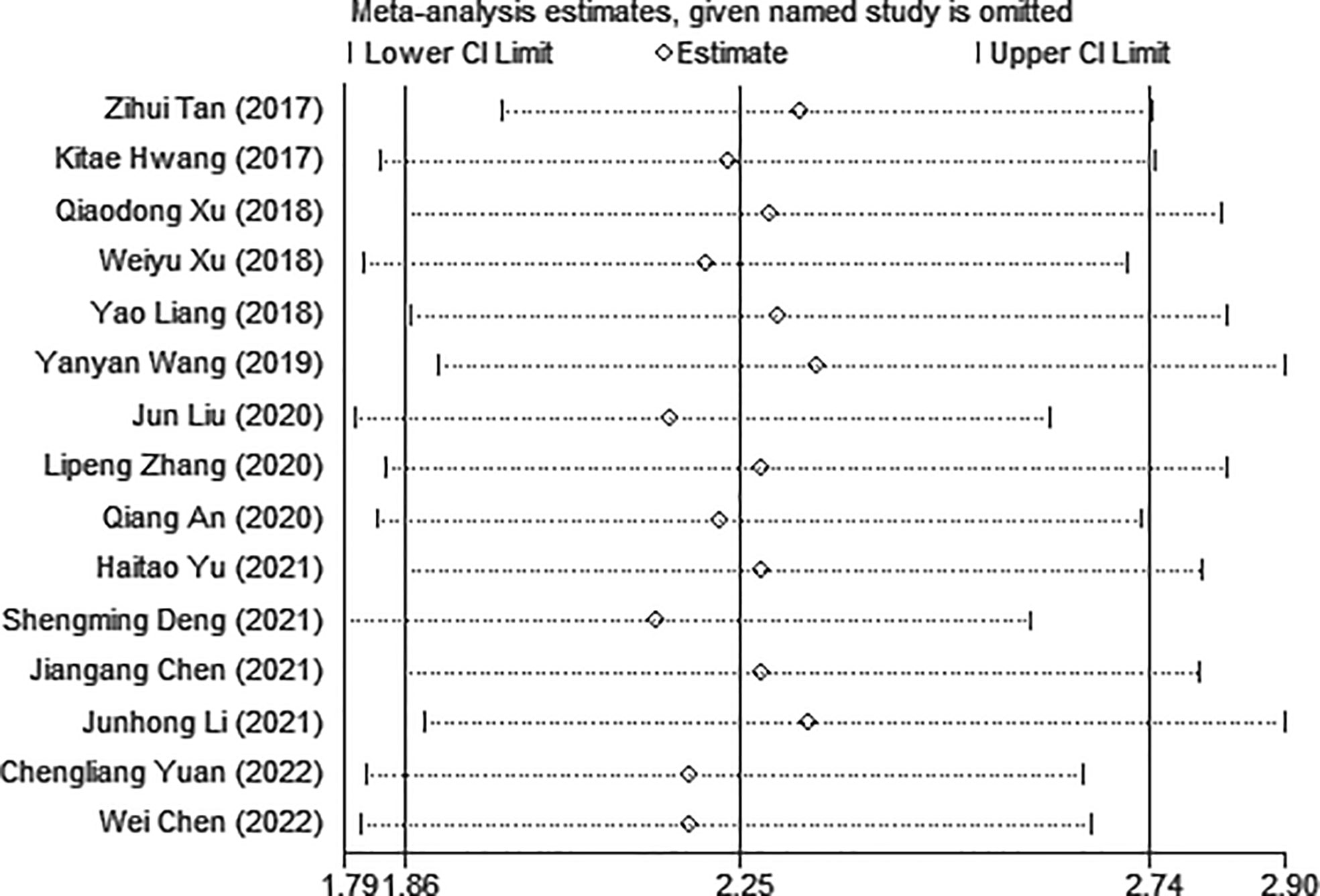
Sensitivity analysis was used to explore the potential impact of each study on the combined results. After we removed each study separately, we recalculated the combined HR and its 95%CI.The results show that ignoring any study will not significantly change the effect of FAR on OS joint meta-analysis, in other words, the comprehensive results of our meta-analysis are stable (Figure 3). In the meta-analysis of OS, Begg’s test (p = 0.006) and Egger’s test (p = 0.000) suggested potential publication bias. Therefore, the trim-and-fill method is further used for correction. The symmetrical funnel diagram is obtained by adding six studies, and the corrected HR is still significant (HR=1.853, 95%CI 1.522-2.257, p<0.001), indicating that our results are reliable (Figures 4A–C).
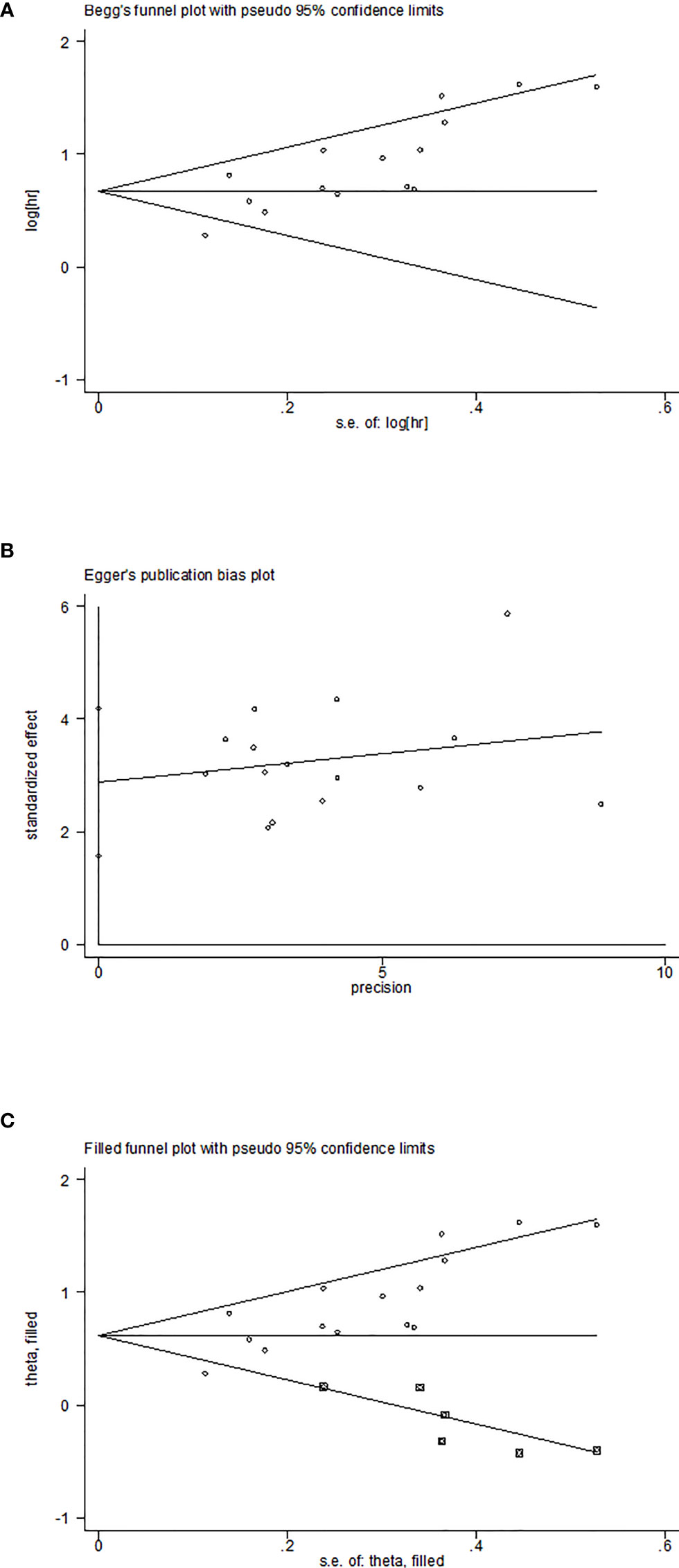
Figure 4 Plots for publication bias test in meta-analysis for overall survival. (A)Begg’s funnel plot; (B) Egger’s publication bias plot; (C) The trim-and-fill methods.
FAR and recurrence−free survival
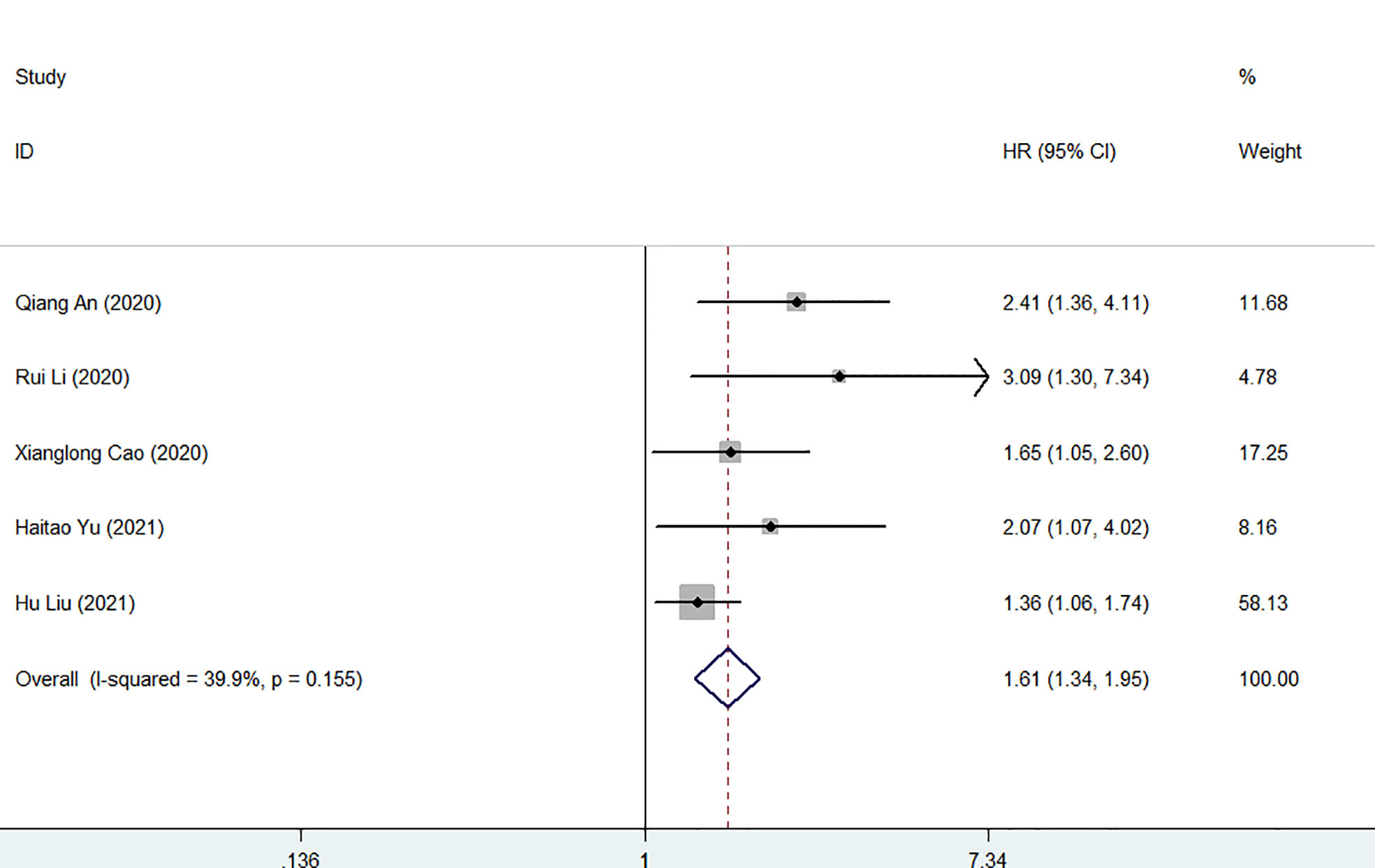
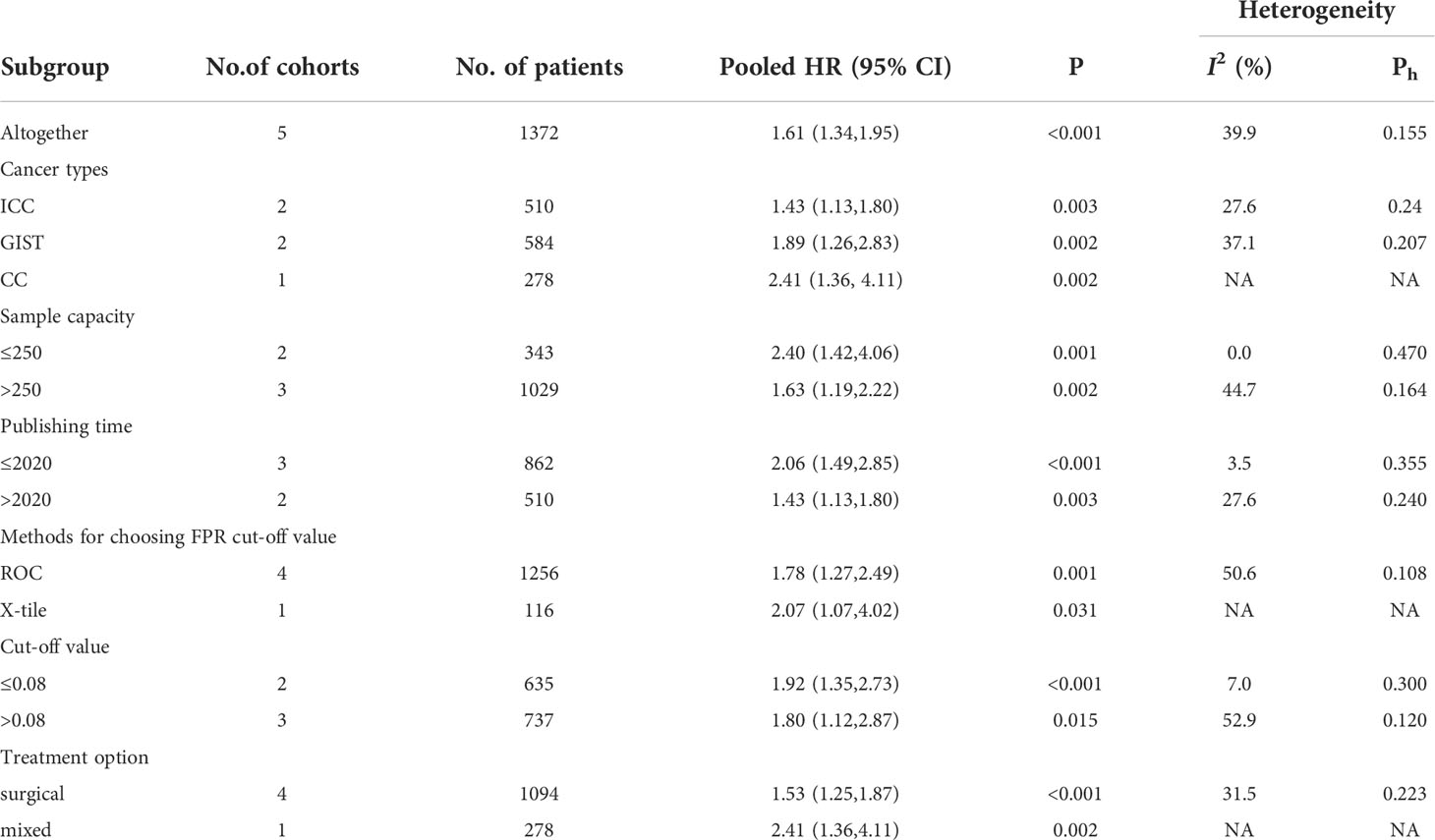
Five studies involving 1372 patients reported the association between FAR and postoperative recurrence-free survival in patients with malignancy. The comprehensive results showed that a high FAR was related to poor RFS in patients with malignant (HR = 1.61, 95% CI 1.34-1.95, p < 0.001). In the case of low heterogeneity (I2 = 39.9%, P = 0.155), we used a fixed-effect model (Figures 5). Furthermore, we performed subgroup analysis according to cancer type, sample capacity, publishing time, sample capacity, methods for choosing FPR cut-off value, sample capacity, cut-off value and treatment option. The results showed that FAR was an independent prognostic factor affecting RFS in each subgroup (Table 4).
Association between FAR and other outcomes
We also investigated the effect of FAR on PFS, DFS and TTR in human patients with malignant tumors. Four studies involving 669 medical records reported the prognostic impact of FAR on PFS. Due to the lack of heterogeneity, a fixed-effects model was used (I2 = 0.0%, P=0.779). Higher FAR was a prognostic factor for poor PFS in patients with human malignancies (HR: 2.10, 95% CI 1.58-2.79, p<0.001) (Figure 6A). Two studies involving a total of 575 patients reported the relationship between FAR and DFS in patients with malignancy, and the combined results showed that high FAR was an independent risk factor for DFS in patients with malignancy (HR=1.52, 95%CI 1.17-1.96, p= 0.001) (Figure 6B). Additionally, a study involving 151 medical records reported the prognostic effect of FAR on TTR, and FAR was also a prognostic factor for poor TTR in patients with human malignancies (HR: 1.555, 95% CI 1.031-2.346, P=0.035) (Figure 6C).
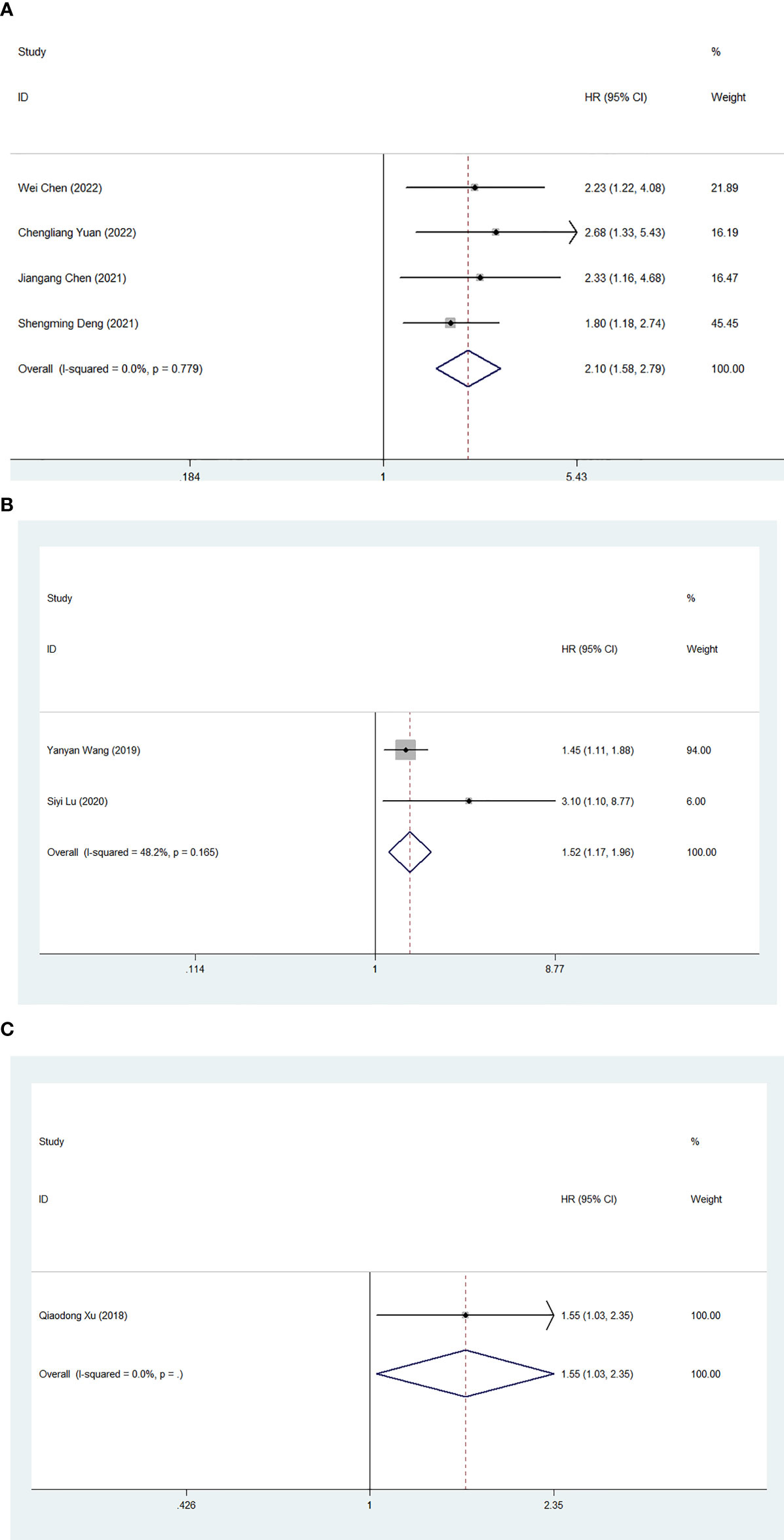
Figure 6 Forest plot for the association between FAR and progression-free survival (A)/disease-free survival (B)/time to recurrence (C).
Discussion
In recent years, there have been continuous studies to explore the relationship between FAR and the clinical outcome of human solid tumors (29, 35, 36, 39). FAR is expected to be a simple, inexpensive, and readily available biomarker for predicting clinical prognosis in patients with solid tumors. However, the underlying mechanisms of how FAR influences cancer prognosis remain unclear. As a composite indicator based on fibrinogen and albumin, FAR can explain its mechanism of action in cancer prognosis by studying the functions of its components.
When the body is under pathophysiological states such as tumor, surgery, infection, inflammation, trauma, etc., the level of fibrinogen increases to varying degrees (45). Studies have shown that fibrinogen plays a cytoskeleton role in tumor extracellular matrix, protecting tumor cells from being killed by immune cells (46, 47). Jay S Desgrosellier et al. showed that fibrinogen can act as a bridge between platelets and circulating tumor cells (CTCs), promote platelet adhesion to CTCs, and increase the metastatic potential of tumor cells (48). In addition, fibrinogen can also directly bind to the intercellular adhesion molecule-1 (ICAM-1) of endothelial cells to promote tumor cell adhesion, proliferation and migration (49). Many studies have shown that tumor cells can synthesize and secrete additional endogenous fibrinogen, and high fibrinogen promotes the synthesis of IL-6, thereby stimulating T and B cells to promote chronic validation responses (50, 51). In animal experiments, tumor cell metastasis and migration were significantly inhibited in fibrinogen-deficient mice (52).
Previous studies have shown that the nutritional status of cancer patients is related to the patient’s age, degree of disease progression and prognosis (53). Albumin, the most abundant circulating protein in plasma, not only reflects the nutritional status of the human body, but also participates in systemic inflammatory responses (54). Low albumin levels may lead to impaired immune function in tumor patients and promote tumor proliferation, invasion and migration (55). Studies have shown that albumin deficiency is closely related to postoperative complications, secondary operations and recurrence of malignant tumors (56). In addition, Christopher G Lis et al. reported that albumin may help stabilize DNA replication and cell growth, regulate body responses, enhance natural immunity, and prevent malignant diseases (57).
Many biomarkers have been shown to be associated with OS, PFS, etc. in cancer, including neutrophil-to-albumin ratio (NAR), neutrophil-to-lymphocyte ratio (NLR), and C-reactive protein-to-albumin ratio (58–60). Available evidence suggests that fibrinogen and albumin are considered independent prognostic indicators for human solid tumors (61, 62). The FAR value derived from the ratio of these two indicators may combine the predictive effects of these indicators, reflecting a mixed prognostic value (40). Therefore, FAR is not only related to systemic inflammation, but also to coagulation and nutritional status (23). A study of 273 patients with advanced gastric cancer showed that FAR was considered a valuable predictor of PFS and OS, and was superior to fibrinogen or albumin alone (63). Junhong Li et al. reported that FAR was better than fibrinogen and albumin in predicting the prognosis of glioblastoma patients (30).
Here, we included 19 studies involving 5926 patients with malignancies. Available evidence suggests that FAR is a sensitive predictor of prognosis in human malignancies, with patients with high FAR exhibiting worse OS than patients with low FPR. At the same time, we also performed subgroup analyses to explore the influence of various factors on our final conclusions. Despite clear differences between subgroups, high FAR was still significantly associated with poor prognosis. The stability of the meta-analysis was further verified by sensitivity analysis and publication bias. In addition, we further discuss the relationship between FAR and RFS. The combined results showed that FPR was an independent predictor of RFS in patients with malignancy. Meanwhile, subgroup analysis showed that despite differences in different subgroups such as cancer types, sample capacity, publishing time, sample capacity, methods for choosing FPR cut-off value, sample capacity, cut-off value and treatment option, high FAR still significantly associated with poorer RFS. In addition, we discuss the relationship of FPR to other prognostic indicators of malignancy, with higher FPR being associated with poor clinical outcomes in PFS, DFS and TTR. In conclusion, FAR can be considered as an important and practical clinical indicator for predicting the prognosis of patients with malignant tumors. In addition, the critical value of FAR in most of the included studies was 0.08, which provided a certain reference value for the application of FAR in clinical work.
However, the results of our meta-analysis should be interpreted with caution, considering some limitations. First, despite the use of subgroup analyses and sensitivity analyses, sources of heterogeneity could not be fully traced. Second, all the included studies were conducted in Asia, which may affect its applicability in other populations when applied on a large scale. More studies from other regions are needed in the future to confirm its applicability in the whole human race. Third, only aggregated data were included, and data from individual patients were not provided for analysis. Fourth, including only English-language studies may introduce some bias. Despite these limitations, based on the available evidence, we have meaningfully explored the prognostic value of preconditioning FAR in cancer patients.
Conclusion
In summary, the outcomes of this meta-analysis shed light on that a higher level of pre-treatment FAR was positively associated with OS, RFS, PFS, DFS and TTR, indicating that it could be an independent prognostic factor in human solid tumors.
Future perspectives
Due to limited research, it restricted our in-depth investigation of the role of FAR. Hence, larger samples with higher quality randomized controlled trials were required to verify our findings. FAR consists of fibrinogen and albumin, both of which are commonly used laboratory tests that are easy and inexpensive to obtain. In the future, if FAR can be used in clinical treatment, it will improve the efficiency of diagnosis and reduce the cost of treatment for cancer patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
BBL and HCD contributed equally to this work. BBL and HCD designed this research. BL and LJC performed the statistical analysis. XYZ and DRS performed the data extraction, and drafted and revised the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank the researchers and study participants for their contributions.
Conflict of interest
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin (2022) 72(5):409–36. doi: 10.3322/caac.21731
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Qi F, Zhou X, Wang Y, Wang Y, Wang Y, Zhang Q, et al. Pre-treatment prognostic nutritional index may serve as a potential biomarker in urinary cancers: a systematic review and meta-analysis. Cancer Cell Int (2018) 18(1):11–12. doi: 10.1186/s12935-018-0708-7
4. Liu K, Zhao K, Wang L, Sun E. The prognostic values of tumor-infiltrating neutrophils, lymphocytes and neutrophil/lymphocyte rates in bladder urothelial cancer. Pathol - Res Pract (2018) 214(8):1074–80. doi: 10.1016/j.prp.2018.05.010
5. Xie H, Wei L, Tang S, Gan J, Essa MM. Prognostic value of pretreatment albumin-to-Alkaline phosphatase ratio in cancer: A meta-analysis. BioMed Res Int (2020) 2020:1–9. doi: 10.1155/2020/6661097
6. Li B, Deng H, Zhou Z, Tang B. The prognostic value of the fibrinogen to pre-albumin ratio in malignant tumors of the digestive system: a systematic review and meta-analysis. Cancer Cell Int (2022) 22(1):22. doi: 10.1186/s12935-022-02445-w
7. Hutterer GC, Stoeckigt C, Stojakovic T, Jesche J, Eberhard K, Pummer K, et al. Low preoperative lymphocyte-monocyte ratio (LMR) represents a potentially poor prognostic factor in nonmetastatic clear cell renal cell carcinoma. Urol Oncol (2014) 32(7):1041–8.
8. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med (1999) 340(6):448–54. doi: 10.1056/NEJM199902113400607
9. Lee SE, Lee JH, Ryu KW, Nam B-H, Cho SJ, Lee JY, et al. Preoperative plasma fibrinogen level is a useful predictor of adjacent organ involvement in patients with advanced gastric cancer. J Gastric Cancer (2012) 12(2):81. doi: 10.5230/jgc.2012.12.2.81
10. Balkwill F, Mantovani A. Inflammation and cancer: back to virchow? Lancet (9255) 2001:539–45:357. doi: 10.1016/S0140-6736(00)04046-0
11. Gan W, Yi Y, Fu Y, Huang J, Lu Z, Jing C, et al. Fibrinogen and c-reactive protein score is a prognostic index for patients with hepatocellular carcinoma undergoing curative resection: a prognostic nomogram study. J Cancer (2018) 9(1):148–56. doi: 10.7150/jca.22246
12. Tang L, Liu K, Wang J, Wang C, Zhao P, Liu J. High preoperative plasma fibrinogen levels are associated with distant metastases and impaired prognosis after curative resection in patients with colorectal cancer. J Surg Oncol (2010) 102(5):428–32. doi: 10.1002/jso.21668
13. Seebacher V, Polterauer S, Grimm C, Husslein H, Leipold H, Hefler-Frischmuth K, et al. The prognostic value of plasma fibrinogen levels in patients with endometrial cancer: a multi-centre trial. Br J Cancer (2010) 102(6):952–6. doi: 10.1038/sj.bjc.6605547
14. Wang H, Gao J, Bai M, Liu R, Li H, Deng T, et al. The pretreatment platelet and plasma fibrinogen level correlate with tumor progression and metastasis in patients with pancreatic cancer. Platelets (2013) 25(5):382–7. doi: 10.3109/09537104.2013.827782
15. Spinella R, Sawhney R, Jalan R. Albumin in chronic liver disease: structure, functions and therapeutic implications. Hepatol Int (2016) 10(1):124–32. doi: 10.1007/s12072-015-9665-6
16. Vlasov H, Juvonen T, Hiippala S, Suojaranta R, Peltonen M, Schramko A, et al. Effect and safety of 4% albumin in the treatment of cardiac surgery patients: study protocol for the randomized, double-blind, clinical ALBICS (ALBumin in cardiac surgery) trial. Trials (2020) 21(1):8–10. doi: 10.1186/s13063-020-4160-3
17. Garcia-Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: Pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology (2013) 58(5):1836–46. doi: 10.1002/hep.26338
18. Al-Shaiba R, McMillan DC, Angerson WJ, Leen E, McArdle CS, Horgan P. The relationship between hypoalbuminaemia, tumour volume and the systemic inflammatory response in patients with colorectal liver metastases. Br J Cancer (2004) 91(2):205–7. doi: 10.1038/sj.bjc.6601886
19. Dupré A, Malik HZ. Inflammation and cancer: What a surgical oncologist should know. Eur J Surg Oncol (2018) 44(5):566–70. doi: 10.1016/j.ejso.2018.02.209
20. Fan Z, Fan K, Gong Y, Huang Q, Yang C, Cheng H, et al. The CRP/Albumin ratio predicts survival and monitors chemotherapeutic effectiveness in patients with advanced pancreatic cancer. Cancer Manage Res (2019) 11:8781–8. doi: 10.2147/CMAR.S211363
21. Murakawa M, Yamamoto N, Kamioka Y, Kamiya M, Kobayashi S, Ueno M, et al. Clinical implication of pre-operative c-reactive protein-albumin ratio as a prognostic factor of patients with pancreatic ductal adenocarcinoma: A single-institutional retrospective study. In Vivo (2020) 34(1):347–53. doi: 10.21873/11780
22. Sohda M, Sakai M, Yamaguchi A, Watanabe T, Nakazawa N, Ubukata Y, et al. Pre-treatment CRP and albumin determines prognosis for unresectable advanced oesophageal cancer. In Vivo (2022) 36(4):1930–6. doi: 10.21873/12914
23. Tan Z, Zhang M, Han Q, Wen J, Luo K, Lin P, et al. A novel blood tool of cancer prognosis in esophageal squamous cell carcinoma: the Fibrinogen/Albumin ratio. J Cancer (2017) 8(6):1025–9. doi: 10.7150/jca.16491
24. Yuan C, Huang M, Wang H, Jiang W, Su C, Zhou S. Pretreatment fibrinogen-albumin ratio (FAR) associated with treatment response and survival in advanced non-small cell lung cancer patients treated with first-line anti-PD-1 therapy plus platinum-based combination chemotherapy. Cancer Manage Res (2022) Volume 14:377–86. doi: 10.2147/CMAR.S347547
25. Xu Q, Yan Y, Gu S, Mao K, Zhang J, Huang P, et al. A novel inflammation-based prognostic score: The Fibrinogen/Albumin ratio predicts prognoses of patients after curative resection for hepatocellular carcinoma. J Immunol Res (2018) 2018:1–11. doi: 10.1155/2018/4925498
26. Zhang Y, Xiao G. Prognostic significance of the ratio of fibrinogen and albumin in human malignancies: a meta-analysis. Cancer Manage Res (2019) Volume 11:3381–93. doi: 10.2147/CMAR.S198419
27. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj (2021) 2021:n71. doi: 10.1136/bmj.n71
28. Stang A.Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
29. Cao X, Cui J, Yu T, Li Z, Zhao G. Fibrinogen/Albumin ratio index is an independent prognosis predictor of recurrence-free survival in patients after surgical resection of gastrointestinal stromal tumors. Front Oncol (2020) 10. doi: 10.3389/fonc.2020.01459
30. Li J, Zhou X, Xiang Y, Zhang S, Feng W, Yuan Y, et al. Clinical significance of preoperative fibrinogen to albumin ratio in patients with glioblastoma: A singe center experience. Cancer Manage Res (2021) 13:3259–69. doi: 10.2147/CMAR.S305025
31. Wang Y-Y, Liu Z-Z, Xu D, Liu M, Wang K, Xing B-C. Fibrinogen–albumin ratio index (FARI): A more promising inflammation-based prognostic marker for patients undergoing hepatectomy for colorectal liver metastases. Ann Surg Oncol (2019) 26(11):3682–92. doi: 10.1245/s10434-019-07586-3
32. Liang Y, Wang W, Que Y, Guan Y, Xiao W, Fang C, et al. Prognostic value of the fibrinogen/albumin ratio (FAR) in patients with operable soft tissue sarcoma. BMC Cancer (2018) 18(1):2–10. doi: 10.1186/s12885-018-4856-x
33. Yu H, Wang M, Wang Y, Yang J, Deng L, Bao W, et al. The prognostic value of sarcopenia combined with preoperative fibrinogen–albumin ratio in patients with intrahepatic cholangiocarcinoma after surgery: A multicenter, prospective study. Cancer Med (2021) 10(14):4768–80. doi: 10.1002/cam4.4035
34. Liu H, Qiu G, Hu F, Wu H. Fibrinogen/albumin ratio index is an independent predictor of recurrence-free survival in patients with intrahepatic cholangiocarcinoma following surgical resection. World J Surg Oncol (2021) 19(1):3–10. doi: 10.1186/s12957-021-02330-2
35. Chen W, Shan B, Zhou S, Yang H, Ye S. Fibrinogen/albumin ratio as a promising predictor of platinum response and survival in ovarian clear cell carcinoma. BMC Cancer (2022) 22(1):4–10. doi: 10.1186/s12885-022-09204-0
36. Deng S, Fan Z, Xia H, Gong Y, Qian Y, Huang Q, et al. Fibrinogen/Albumin ratio as a promising marker for predicting survival in pancreatic neuroendocrine neoplasms. Cancer Manage Res (2021) Volume 13:107–15. doi: 10.2147/CMAR.S275173
37. Liu J, Gan Y, Song H, Zhu K, Zhang Q. The predictive value of the preoperative fibrinogen-albumin ratio on the postoperative prognosis of renal cell carcinoma. Trans Andrology Urol (2020) 9(3):1053–61. doi: 10.21037/tau-19-873
38. Zhang L-P, Ren H, Du Y-X, Wang C-F. Prognostic value of the preoperative fibrinogen-to-albumin ratio in pancreatic ductal adenocarcinoma patients undergoing R0 resection. World J Gastroenterol (2020) 26(46):7382–404. doi: 10.3748/wjg.v26.i46.7382
39. Chen J, Hao L, Zhang S, Zhang Y, Dong B, Zhang Q, et al. Preoperative fibrinogen–albumin ratio, potential prognostic factors for bladder cancer patients undergoing radical cystectomy: A two-center study. Cancer Manage Res (2021) Volume 13:3181–92. doi: 10.2147/CMAR.S300574
40. Hwang K-T, Chung JK, Roh EY, Kim J, Oh S, Kim YA, et al. Prognostic influence of preoperative fibrinogen to albumin ratio for breast cancer. J Breast Cancer (2017) 20(3):254. doi: 10.4048/jbc.2017.20.3.254
41. An Q, Liu W, Yang Y, Yang B. Preoperative fibrinogen-to-albumin ratio, a potential prognostic factor for patients with stage IB-IIA cervical cancer. BMC Cancer (2020) 20(1):4–8. doi: 10.1186/s12885-020-07191-8
42. Xu W-Y, Zhang H-H, Xiong J-P, Yang X-B, Bai Y, Lin J-Z, et al. Prognostic significance of the fibrinogen-to-albumin ratio in gallbladder cancer patients. World J Gastroenterol (2018) 24(29):3281–92. doi: 10.3748/wjg.v24.i29.3281
43. Lu S, Liu Z, Zhou X, Wang B, Li F, Ma Y, et al. et al: Preoperative fibrinogen-albumin ratio index (FARI) is a reliable prognosis and chemoradiotherapy sensitivity predictor in locally advanced rectal cancer patients undergoing radical surgery following neoadjuvant chemoradiotherapy. Cancer Manage Res (2020) Volume 12:8555–68. doi: 10.2147/CMAR.S273065
44. Li R, Song S, He X, Shi X, Sun Z, Li Z, et al. Relationship between fibrinogen to albumin ratio and prognosis of gastrointestinal stromal tumors: A retrospective cohort study. Cancer Manage Res (2020) 12:8643–51. doi: 10.2147/CMAR.S271171
45. Pieters M, Wolberg AS. Fibrinogen and fibrin: An illustrated review. Res Pract Thromb Haemost (2019) 3(2):161–72. doi: 10.1002/rth2.12191
46. Zheng S, Shen J, Jiao Y, Liu Y, Zhang C, Wei M, et al. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci (2009) 100(5):859–65. doi: 10.1111/j.1349-7006.2009.01115.x
47. Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood (2005) 105(1):178–85. doi: 10.1182/blood-2004-06-2272
48. Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer (2010) 10(1):11. doi: 10.1038/nrc2748
49. Roche Y, Pasquier D, Rambeaud J-J, Seigneurin D, Duperray A. Fibrinogen mediates bladder cancer cell migration in an ICAM-1-dependent pathway. Thromb Haemost (2003) 89(6):1089–97. doi: 10.1055/s-0037-1613412
50. Sahni A, Khorana AA, Baggs RB, Peng H, Francis CW. FGF-2 binding to fibrin(ogen) is required for augmented angiogenesis. Blood (2006) 107(1):126–31. doi: 10.1182/blood-2005-06-2460
51. Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, et al. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, c-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation (2012) 126(23):2739–48. doi: 10.1161/CIRCULATIONAHA.112.122556
52. Palumbo JS, Kombrinck KW, Drew AF, Grimes TS, Kiser JH, Degen JL, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood (2000) 96(10):3302–9. doi: 10.1182/blood.V96.10.3302
53. Mantzorou M, Koutelidakis A, Theocharis S, Giaginis C. Clinical value of nutritional status in cancer: What is its impact and how it affects disease progression and prognosis? Nutr Cancer (2017) 69(8):1151–76. doi: 10.1080/01635581.2017.1367947
54. Caraceni P, Tufoni M, Bonavita ME. Clinical use of albumin. Blood Transfus (2013) 11 Suppl 4:s18–25. doi: 10.2450/2013.005s
55. Hu W-H, Eisenstein S, Parry L, Ramamoorthy S. Preoperative malnutrition with mild hypoalbuminemia associated with postoperative mortality and morbidity of colorectal cancer: a propensity score matching study. Nutr J (2019) 18(1):33. doi: 10.1186/s12937-019-0458-y
56. Jones CH, Akbani H, Croft DC, Worth DP. The relationship between serum albumin and hydration status in hemodialysis patients. J Ren Nutr (2002) 12(4):209–12. doi: 10.1053/jren.2002.35295
57. Lis CG, Gupta D, Lammersfeld CA, Markman M, Vashi PG. Role of nutritional status in predicting quality of life outcomes in cancer–a systematic review of the epidemiological literature. Nutr J (2012) 11:27. doi: 10.1186/1475-2891-11-27
58. Ferro M, Babă D-F, de Cobelli O, Musi G, Lucarelli G, Terracciano D, et al. Neutrophil percentage-to-albumin ratio predicts mortality in bladder cancer patients treated with neoadjuvant chemotherapy followed by radical cystectomy. Future Sci OA (2021) 7(7):FSO709. doi: 10.2144/fsoa-2021-0008
59. Mouchli M, Reddy S, Gerrard M, Boardman L, Rubio M. Usefulness of neutrophil-to-lymphocyte ratio (NLR) as a prognostic predictor after treatment of hepatocellular carcinoma.” review article. Ann Hepatol (2021) 22:100249. doi: 10.1016/j.aohep.2020.08.067
60. Yue L, Lu Y, Li Y, Wang Y. Prognostic value of c-reactive protein to albumin ratio in gastric cancer: A meta-analysis. Nutr Cancer (2021) 73(10):1864–71. doi: 10.1080/01635581.2020.1817510
61. Perisanidis C, Psyrri A, Cohen EE, Engelmann J, Heinze G, Perisanidis B, et al. Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev (2015) 41(10):960–70. doi: 10.1016/j.ctrv.2015.10.002
62. Yang H, Wang K, Liang Z, Guo S, Zhang P, Xu Y, et al. Prognostic role of pre-treatment serum albumin in patients with nasopharyngeal carcinoma: A meta-analysis and systematic review. Clin Otolaryngol (2019) 45(2):167–76. doi: 10.1111/coa.13454
Keywords: fibrinogen to albumin ratio, prognosis, meta-analysis, malignant tumor, biomarker
Citation: Li B, Deng H, Lei B, Chen L, Zhang X and Sha D (2022) The prognostic value of fibrinogen to albumin ratio in malignant tumor patients: A meta-analysis. Front. Oncol. 12:985377. doi: 10.3389/fonc.2022.985377
Received: 03 July 2022; Accepted: 12 September 2022;
Published: 29 September 2022.
Edited by:
Felice Crocetto, Federico II University Hospital, ItalyReviewed by:
Biagio Barone, University of Naples Federico II, ItalyCiro Imbimbo, University of Naples Federico II, Italy
Copyright © 2022 Li, Deng, Lei, Chen, Zhang and Sha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dingran Sha, WUZZMTA4Mjc2QHNyLmd4bXUuZWR1LmNu
†These authors have contributed equally to this work
 Baibei Li1†
Baibei Li1† Biao Lei
Biao Lei Dingran Sha
Dingran Sha