- 1Nutrition Research Center, School of Nutrition and Food Sciences, Shiraz University of Medical Sciences, Shiraz, Iran
- 2Department of Nutrition, School Of Public Health, International Campus, Iran University of Medical Sciences, Tehran, Iran
- 3The Ohio State University Interdisciplinary ph.D. program in Nutrition (OSUN), Columbus, United States
- 4The Ohio State University Comprehensive Cancer Center, Columbus, United States
- 5Department of Exercise Physiology, Faculty of Physical Education and Sport Sciences, Islamic Azad University, Central Tehran Branch, Tehran, Iran
- 6Aboozar Children’s Medical Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 7Department of Nutrition, Science and Research Branch, Islamic Azad University, Tehran, Iran
- 8Department of Nutrition, School of Allied Medical Sciences, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 9Cancer Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 10Department of Nutrition, Zanjan University of Medical Sciences, Zanjan, Iran
- 11Nutrition Sciences and Applied Food Safety Studies, Research Centre for Global Development, School of Sport and Health Sciences, University of Central Lancashire, Preston, United Kingdom
- 12Nursing and Midwifery School, Guilan University of Medical Sciences, Rasht, Iran
- 13Department of Community Nutrition, Faculty of Nutrition and Food Technology, National Nutrition and Food Technology Research Institute, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Background: The association between breast cancer (BC) and different indices of dietary fats has not been well-studied. Thus, this study aimed to investigate the association between BC and dietary fat quality (DFQ) indices in Iranian women.
Methods: This case–control study was conducted on 120 women with breast cancer and 240 healthy women in Tehran, Iran. Food Frequency Questionnaire and nutritionist IV software were used to assess the intake of dietary fats and to calculate the DFQ indices.
Results: The patients with BC had a higher total fat (TF) (P < 0.01) and a lower ratio of polyunsaturated fatty acids (PUFAs) omega-3 to PUFAs omega-6 (ω-3/ω-6) compared with the controls (P < 0.001). TF had a significant association with BC risk (OR: 1.16; 95% CI: 1.01–1.33, P < 0.001). No significant association was found between BC and PUFA/saturated fatty acid ratio or the ω-3/ω-6 ratio.
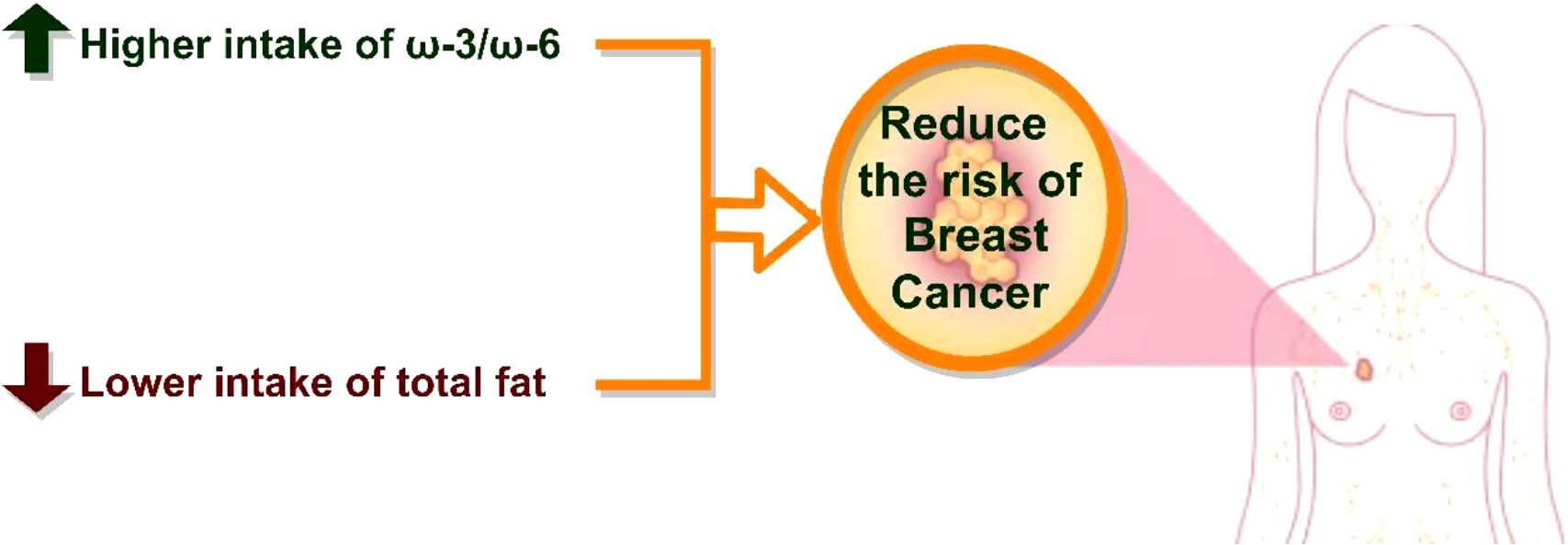
Conclusion: The patients with BC had a lower ω-3/ω-6 ratio and a higher total dietary fat intake than the healthy women. Total dietary fat intake was also directly associated with the risk of BC. Thus, low-fat diets may have beneficial effects for BC prevention. Further longitudinal studies are warranted.
Introduction
Cancer, which reduces life expectancy, is a leading cause of death worldwide. The World Health Organization (WHO, 2019) declared cancer as the leading cause of death before age 70 in 112 of 183 countries partly due to the sharp decline in mortality rates from stroke and coronary heart disease compared with cancer (1). Breast cancer (BC) has now surpassed lung cancer as the leading cause of cancer in 2020, with an estimated 2.3 million new cases, representing 11.7% of all cancer cases. In addition, BC is the fifth leading cause of cancer mortality, accounting for 685,000 deaths globally (2). BC affects one in every four women and kills one in every six women worldwide. BC has been reported to be the most common cancer in Iran. According to the latest Iranian national database, the age-standardized rate for BC is 33.21 per 100,000, with an overall 5-year survival rate of 72% in women and 60% in men. The mortality rate is 14.2 per 100,000, and the 5- and 10-year survival rate is 81% and 77%, respectively (3, 4).
Many factors could be associated with BC (5, 6). Among the various risk factors identified, diet plays an important role in the incidence of BC (7–9). Despite being extensively studied, only a few dietary components associated with the incidences of BC have been identified (6)—for instance, dietary patterns characterized by a low intake of fruits, vegetables, and whole grains and high in red meats, saturated fat, and sodium are reported to be associated with a higher risk of developing BC (10). The underlying mechanisms of dietary components and their mechanistic role in the molecular as well as cellular pathways associated with the BC pathway are yet to be elucidated (6).
The results on the association between dietary fat intake and BC risk were controversial. High intakes of dietary fat have been linked to an increased risk of BC in adult women (7, 8). However, some prospective cohort studies (4), meta-analysis studies (11), and observational studies have reported a weak (12–14) or a non-significant relationship between BC and dietary fats (7, 15–17). Furthermore, the association between the type of fat consumed and the development of BC is not yet clear. Farvid et al. (18), as well as Tayyem et al. (8), reported that a higher intake of saturated fatty acids (SFA) and monounsaturated fatty acids (MUFAs) was associated with an increased risk of BC. In contrast, two observational studies (19, 20) reported a protective effect of MUFAs on the development of BC. In addition, several studies reported that polyunsaturated fatty acid (PUFA) intake increased the risk of BC (7, 8, 12, 21). However, this result was not confirmed by a case–control study (22) and a meta-analysis (11). Since different dietary fatty acids are metabolized through common pathways and the amount of intake of one group of fatty acids may be effective on the metabolism of other fatty acids (11), it is possible that considering them separately does not present the correct result in terms of their connection with BC. Hence, this study aimed to investigate the association between BC and different types of dietary fat quality indices in Iranian women.
Methods
Participants
This case–control study was performed in September 2020 on 120 women with BC and 240 healthy age-matched women referred to the cancer clinic of Shohadaye Tajrish Hospital, Tehran, Iran. A 1:2 case‐to‐control ratio was used in this matched case–control study due to concern for sufficient numbers in the stratified analysis and increase in power given the expected prevalence of exposure among the controls. The inclusion criteria for the case group were as follows: women with BC, age between 35 and 65 years, no more than 1 month after the diagnosis of BC, no diseases affecting food intake, and no dietary fat or fatty acid supplement intake. The inclusion criteria for the control group were as follows: age between 35 and 65 years, no disease affecting food intake, no use of dietary fat or fatty acid supplements, and do not have any type of cancer. The exclusion criteria were the inability to collect the required information and any conditions that may affect the diet during the last year. Written informed consent forms were obtained from all participants before the study.
Data on age, number of pregnancies, breastfeeding duration, number of abortion cases, family history of BC, and physical activity were collected. Anthropometric indices including weight, height, body mass index (BMI), and waist circumstance were collected.
Dietary fat quality indices
A validated semi-quantitative Food Frequency Questionnaire (FFQ) was used to assess the dietary intake over the past year through face-to-face interviews with a trained dietitian (12). The amounts of intake of different types of dietary fatty acids were assessed using Nutritionist IV software (version 3.5.2, Axxya. Systems, Redmond, WA, USA), modified for Iranian foods.
Energy percentage from total fat (TF) was calculated by dividing the average obtained energy from fat (kcals) by the average daily energy intake. Furthermore, other dietary fat quality (DFQ) indices were calculated as follows: PUFA-to-SFA ratio (PSR) was calculated by the amount of dietary intake of PUFAs divided by the amount of dietary intake of SFAs. The ω-3/ω-6 ratio was also calculated by the amount of dietary intake of omega-3 fatty acids divided by the amount of dietary intake of ω-6 fatty acids (13).
Statistical analyses
Data analyses were conducted using version 26 of Statistical Package Software for Social Sciences (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov’s test and a histogram chart were used to test the normality of the data. Baseline characteristics and dietary intakes were expressed as mean ± SD or median (interquartile range, IQR) for quantitative variables with normal and skewed distribution, respectively. Independent sample t-test and Pearson chi-square test were applied to compare the quantitative and qualitative variables between the groups, respectively. A comparison of the medians between the two groups was done using the Mann–Whitney U-test. Binary logistic regression was then utilized to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) adjusted for multiple covariates in different models. The significance level was determined as P <0.05.
Results
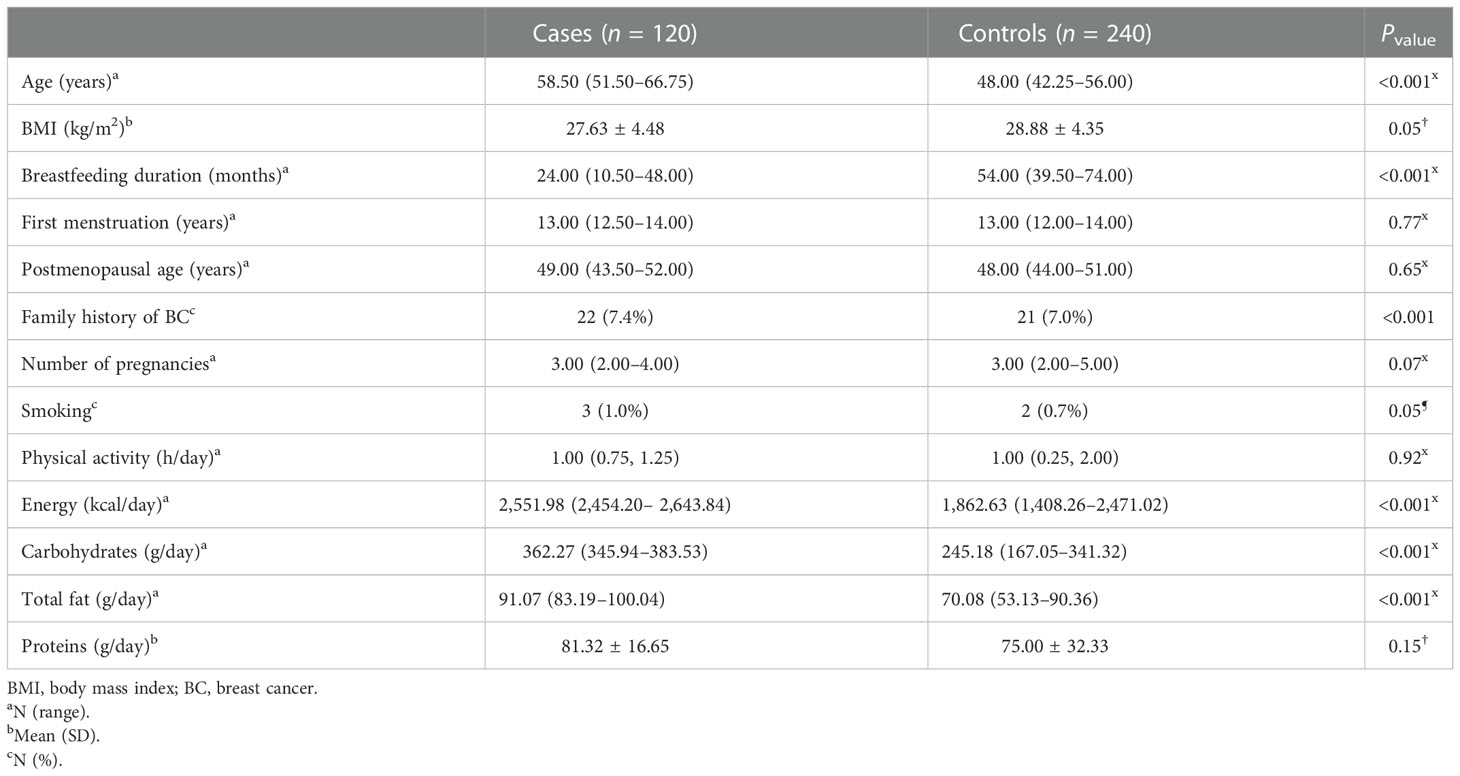
In this study, 360 participants (120 cases and 240 controls) were included. More than 93% of the participants (n = 335) were menopausal women. The general characteristics and the dietary intakes of the case and the control groups are presented in Table 1. The median (IQR) age of the participants in the cases and the controls was 58.50 (51.50, 66.75) and 48.00 (42.25, 56.00) years, respectively (P < 0.001). The two groups had significant differences in BMI (<0.05), breastfeeding duration (P < 0.001), and family history of BC (P < 0.001). Compared with the control group, BC patients consumed significantly more calories (P < 0.001), carbohydrates (P < 0.001), and total fat (P < 0.001).
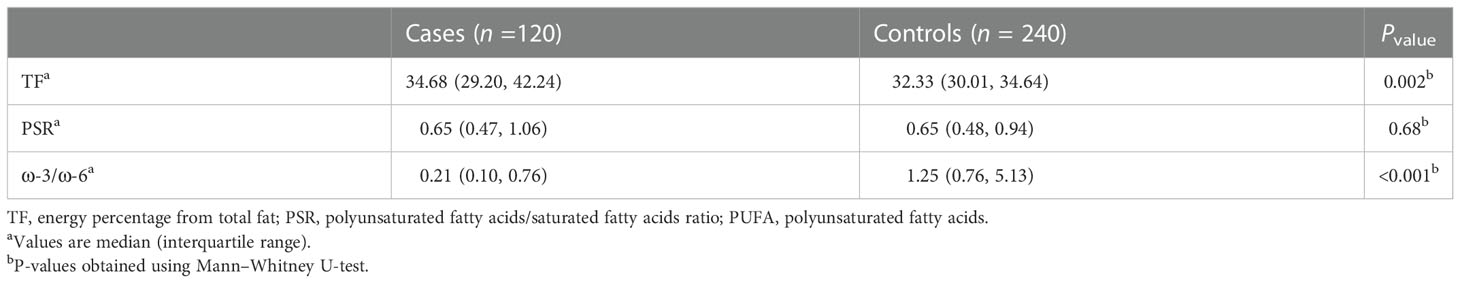
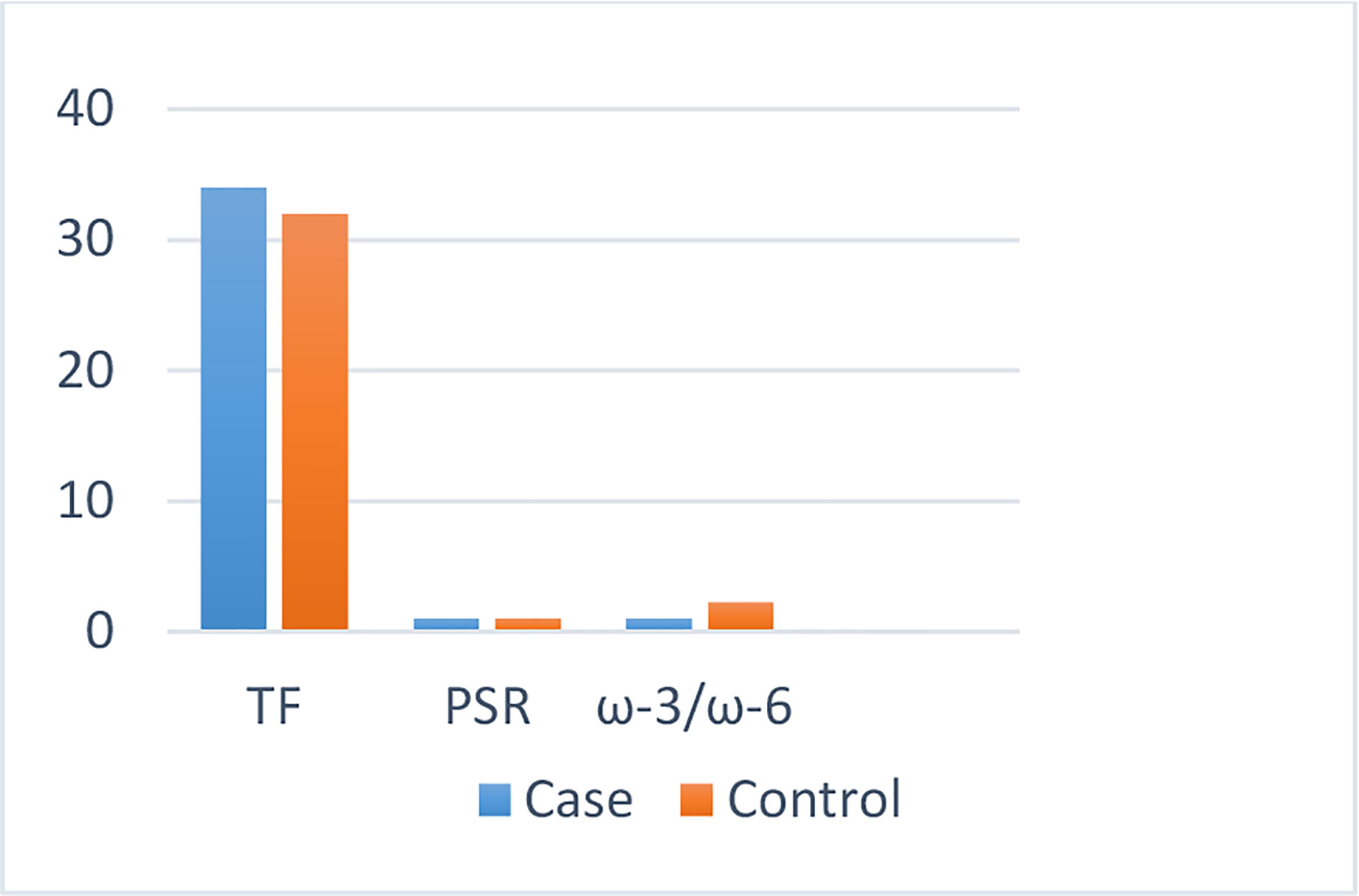
The dietary fat quality indices among the case and the control groups (Table 2) indicated that individuals with BC obtained higher amounts of energy from consuming fat (P < 0.01) and that their diet had a significantly lower level of ω-3/ω-6 compared with those of the controls (P < 0.001) (Figure 1).
The crude and adjusted ORs (95% CIs) for the underlying associations between TF, PSR, plus ω-3/ω-6 ratio, and BC risk are reported in Table 3. Individuals with a higher TF had greater odds for BC (OR: 1.16; 95% CI: 1.01–1.33), though no association between the PSR and ω-3/ω-6 ratio with BC risk was found (Figure 2).
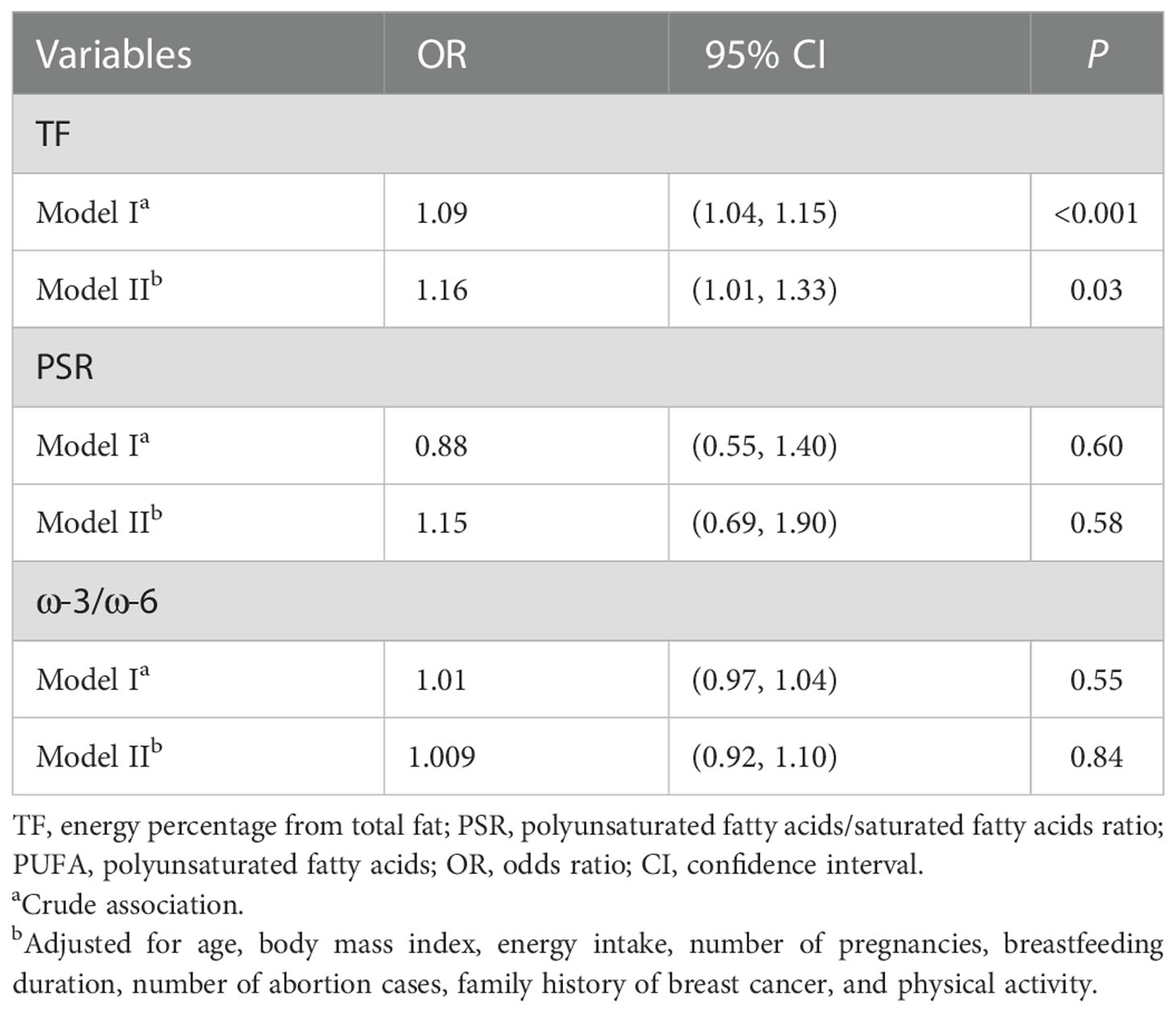
Table 3 Logistic regression of the association between dietary fat quality indices and breast cancer.
Discussion
Despite the heavy interest in investigating the association between fat intake and BC, their relationship is still controversial. The aim of the current study was to explore the relationship between dietary fat quality and BC in Iranian women. In this study with 120 cases and 240 controls, it was observed that individuals with BC had a higher intake of total fat and a lower ω-3/ω-6 ratio compared with the controls, and the risk of developing BC was positively associated with the total fat intake. There was no association between BC risk and the polyunsaturated-fat-to-saturated-fat ratio or ω-3/ω-6 index.
Several case–control studies have reported a positive association between total fat intake and BC risk (14–17), while prospective cohort studies have not confirmed these observations (7, 18). In other words, previous studies have shown no association between total fat and BC, or this positive association has been trivial (23). These conflicting results may be due to the different assessment methods in different studies—for example, it has been suggested that the use of food records may provide a stronger correlation between dietary fat and BC risk compared with the FFQs (19, 20). Furthermore, several studies have reported that non-dietary risk factors such as menopausal status, a history of benign breast disease, and menopausal hormone therapy may affect the association of total fat and BC (7, 14, 21, 22, 24).
In our study, the polyunsaturated-fat-to-saturated-fat ratio was similar in both groups, while the ω-3-to-ω-6 ratio was higher in the cases than in the controls. Similar to our findings, some previous studies have found no association between BC and fat subtypes (25–27). However, contrary to the findings of our study, one study reported a significant positive association between fat subtypes and the risk of BC in postmenopausal women (17), and some other studies reported an association between BC and animal fat (a rich source of SFA) in premenopausal women (27–29). One of the reasons for the discrepancy observed in the results of different studies may be the difference in menopausal status. Moreover, the effect of dietary fat on the risk of BC may be influenced by the level of sex hormones.
Furthermore, the results of the present study indicated that the difference of the ω-3/ω-6 ratio between cases and controls was significant; still no association was found between this ratio and the risk of BC. According to previous studies, PUFAs may have different effects on the risk of BC based on the double bond position (30, 31). Some studies have established that PUFAs, particularly linoleic acid and arachidonic acid, promote while marine-derived ω-3 fatty acids inhibit mammary tumorigenesis (17, 31, 32). In addition, some case–control studies have found a positive association between ω-6 fatty acid intake and the risk of BC (33, 34). On the other hand, another study reported that serum ω-6 fatty acids were inversely related to BC risk (35). Based on the report of cohort studies, ω-3 fatty acids have protective effects on BC (36, 37). Furthermore, other studies showed an inverse relationship between ω-3 PUFAs and the risk of BC (38–40). However, in some other studies, ω-3 fatty acids did not affect BC (41–44). Because of these conflicting results, some researchers suggested that ω-3-to-ω-6 ratios should be used instead of a specific fatty acid (45). Two studies reported that the ω-3-to-ω-6 ratio is inversely related to BC (46–50). Some other studies of ω-3/ω-6 PUFAs and BC led to contradictory results (51, 52). The various results observed in different studies can be due to differences in the study population, dietary sources of specific fats, amounts of total fat and specific fatty acids, menopausal status, and methods used to measure fat intake. Thus, it is suggested that large-scale studies with different dietary patterns as well as well-designed trials be conducted to indicate the association between total fat and DFQ and the risk of BC.
This study suggested that energy percentage from total fat may be a more critical factor in determining the risk of BC. A large randomized, controlled trial reported that a low-fat diet might reduce the risk of developing BC in post-menopausal women (53). A meta-analysis study reported that the risk of BC is higher in post-menopausal women on a high-fat diet. At the same time, dietary fat may have protective effects in pre-menopausal women. Thus, capturing the menopausal status of people can also be a determining factor (54).
Several mechanisms have been proposed for the possible association between different types of dietary fats and BC. Previous studies have identified that SFAs may play a more significant role in determining the risk of BC than other types of fat (7, 25). SFAs may increase insulin resistance (55), where an association between plasma insulin concentration and the risk of BC has been reported (56). Dietary PUFAs usually contain a high proportion of linoleic acid, a precursor to prostaglandins (57). Arachidonic acid and prostaglandin E2 may play a role in inducing BC by affecting estrogen synthesis (48). It has been suggested that marine ω-3 may affect the BC risk, possibly through effects on BMI or related factors (insulin and adiponectin) (40). The mechanisms proposed for the protective effect of ω-3 PUFAs include suppressing the biosynthesis of arachidonic acid-derived eicosanoids, altering the estrogen metabolism, reducing the production of free radicals, and modifying insulin sensitivity (58). However, it should be noted that the association of ω-3 fatty acids with BC risk may be influenced by the availability of dietary antioxidants such as vitamins E and C (58). Moreover, ω-6 fatty acids are easily oxidized due to their high double bonds and thus contribute to cell damage. They may also increase the risk of BC by competing with ω-3 fatty acids in producing eicosanoids (33).
As in all case–control studies, the major limitation of the current study was recall bias. Women who are aware of a beneficial diet can often give overestimated amounts of products that have health-promoting effects. In addition, the evaluation of PUFAs with FFQ may differ on the actual content of the product, which may depend on different periods of the harvest of food products, storage time, or method of cooking (59). Furthermore, considering that the majority of the participants were menopausal, a comparison between menopausal and non-menopausal women was not possible. Future studies should attempt to determine the content of individual fatty acids in the patients’ foods and in their blood samples (60). The duration of breastfeeding in controls was also significantly higher than in the cases and in various studies; breastfeeding has been suggested as a protective factor against BC (61). There was a significant difference between the case and control groups regarding the family history of BC and genetic factors. Given the fact that the effects of these factors in the regression models were adjusted, the association between total fat and BC observed in this study is hence robust enough.
Conclusion
This study suggested an association between the amount of dietary fat and the risk of BC. Patients with BC had a lower ω-3/ω-6 ratio in their diet and a higher intake of total dietary fat than others. As a result, a lower intake of dietary fats may be beneficial in BC prevention. Further studies are warranted to confirm these results and to identify the underlying mechanisms of the association between dietary fat and BC.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
All patients signed an informed consent form at baseline. This study was approved by the ethical committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (code: IR.SBMU.CRC.REC.1398.006). The patients/participants provided their written informed consent to participate in this study.
Author contributions
FS, MG, SD, ZA, ST, MV and SS designed the study, involved in the data collection, analysis, and drafting of the manuscript. SD, MA, SM, BB, and MG were involved in the design of the study. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: 74859).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vahid F, Hatami M, Sadeghi M, Ameri F, Faghfoori Z, Davoodi SH. The association between the index of nutritional quality (INQ) and breast cancer and the evaluation of nutrient intake of breast cancer patients: A case-control study. Nutrition (2018) 45:11–6. doi: 10.1016/j.nut.2017.06.011
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A cancer journal for clinicians (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Dabbagh N, Riazi H, Khayamzadeh M, Negahi A, Akbari M, Akbari ME. The effect of neoadjuvant vs adjuvant chemotherapy on final outcome of patients with triple negative breast cancer. Med J Islam Repub Iran (2022) 36. doi: 10.47176/mjiri.36.61
4. Nafissi N, Khayamzadeh M, Zeinali Z, Pazooki D, Hosseini M, Akbari ME. Epidemiology and histopathology of breast cancer in Iran versus other middle Eastern countries. Middle East J Cancer (2018) 9(3):243–51. doi: 10.30476/mejc.2018.42130
5. Al Shahrani AS, Faraj S, Alhargan A, Aljumaid M, Gosty A, Al-Sugair M, et al. Factors affecting age at menopause among Saudi women in Riyadh, SA: a cross-sectional study. (2018) 2018(1):55–60. doi: 10.5114/fmpcr.2018.73704
6. Doaei S, Jarrahi SM, Moghadam AS, Akbari M, Kooshesh SJ, Badeli M, et al. The effect of rs9930506 FTO gene polymorphism on obesity risk: A meta-analysis. Biomolecular Concepts. (2019) 10(1):237–42. doi: 10.1515/bmc-2019-0025
7. Smith-Warner SA, Spiegelman D, Adami HO, Beeson WL, van den Brandt PA, Folsom AR, et al. Types of dietary fat and breast cancer: a pooled analysis of cohort studies. Int J Cancer (2001) 92(5):767–74. doi: 10.1002/1097-0215(20010601)92:5<767::AID-IJC1247>3.0.CO;2-0
8. Psaltopoulou T, Kosti RI, Haidopoulos D, Dimopoulos M, Panagiotakos DB. Olive oil intake is inversely related to cancer prevalence: A systematic review and a meta-analysis of 13,800 patients and 23,340 controls in 19 observational studies. Lipids Health Dis (2011) 10:127. doi: 10.1186/1476-511X-10-127
9. Chen M, Li S, Arora I, Yi N, Sharma M, Li Z, et al. Maternal soybean diet on prevention of obesity-related breast cancer through early-life gut microbiome and epigenetic regulation. The Journal of nutritional biochemistry (2022) 110:109119. doi: 10.1016/j.jnutbio.2022.109119
10. Dandamudi A, Tommie J, Nommsen-Rivers L, Couch S. Dietary patterns and breast cancer risk: A systematic review. Anticancer Res (2018) 38(6):3209–22. doi: 10.21873/anticanres.12586
11. Lottenberg AM, da Silva Afonso M, Lavrador MSF, Machado RM, Nakandakare ER. The role of dietary fatty acids in the pathology of metabolic syndrome. J Nutr Biochem (2012) 23(9):1027–40. doi: 10.1016/j.jnutbio.2012.03.004
12. Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr (2010) 13(5):654–62. doi: 10.1017/S1368980009991698
13. Ulbricht T, Southgate D. Coronary heart disease: Seven dietary factors. Lancet (1991) 338(8773):985–92. doi: 10.1016/0140-6736(91)91846-M
14. Thiébaut AC, Kipnis V, Chang S-C, Subar AF, Thompson FE, Rosenberg PS, et al. Dietary fat and postmenopausal invasive breast cancer in the national institutes of health–AARP diet and health study cohort. J Natl Cancer Institute (2007) 99(6):451–62. doi: 10.1093/jnci/djk094
15. Cho E, Spiegelman D, Hunter DJ, Chen WY, Stampfer MJ, Colditz GA, et al. Premenopausal fat intake and risk of breast cancer. J Natl Cancer Institute (2003) 95(14):1079–85. doi: 10.1093/jnci/95.14.1079
16. Howe GR, Hirohata T, Hislop TG, Iscovich JM, Yuan J-M, Katsouyanni K, et al. Dietary factors and risk of breast cancer: combined analysis of 12 case–control studies. JNCI: J Natl Cancer Institute (1990) 82(7):561–9. doi: 10.1093/jnci/82.7.561
17. Mozafarinia M, Sasanfar B, Toorang F, Salehi-Abargouei A, Zendehdel K. Association between dietary fat and fat subtypes with the risk of breast cancer in an Iranian population: a case-control study. Lipids Health Dis (2021) 20(1):1–11. doi: 10.1186/s12944-021-01557-y
18. Kushi LH, Sellers TA, Potter JD, Nelson CL, Munger RG, Kaye SA, et al. Dietary fat and postmenopausal breast cancer. JNCI: J Natl Cancer Institute (1992) 84(14):1092–9. doi: 10.1093/jnci/84.14.1092
19. Bingham SA, Luben R, Welch A, Wareham N, Khaw K-T, Day N. Are imprecise methods obscuring a relation between fat and breast cancer? Lancet (2003) 362(9379):212–4. doi: 10.1016/S0140-6736(03)13913-X
20. Freedman LS, Potischman N, Kipnis V, Midthune D, Schatzkin A, Thompson FE, et al. A comparison of two dietary instruments for evaluating the fat–breast cancer relationship. Int J Epidemiol (2006) 35(4):1011–21. doi: 10.1093/ije/dyl085
21. Velie E, Kulldorff M, Schairer C, Block G, Albanes D, Schatzkin A. Dietary fat, fat subtypes, and breast cancer in postmenopausal women: a prospective cohort study. J Natl Cancer Institute (2000) 92(10):833–9. doi: 10.1093/jnci/92.10.833
22. Toniolo P, Riboli E, Protta F, Charrel M, Cappa AP. Calorie-providing nutrients and risk of breast cancer. JNCI: J Natl Cancer Institute (1989) 81(4):278–86. doi: 10.1093/jnci/81.4.278
23. Key TJ, Appleby PN, Cairns BJ, Luben R, Dahm CC, Akbaraly T, et al. Dietary fat and breast cancer: comparison of results from food diaries and food-frequency questionnaires in the UK dietary cohort consortium. Am J Clin Nutr (2011) 94(4):1043–52. doi: 10.3945/ajcn.111.015735
24. Hunter DJ, Spiegelman D, Adami H-O, Beeson L, Van Den Brandt PA, Folsom AR, et al. Cohort studies of fat intake and the risk of breast cancer–a pooled analysis. New Engl J Med (1996) 334(6):356–61. doi: 10.1056/NEJM199602083340603
25. Holmes MD, Hunter DJ, Colditz GA, Stampfer MJ, Hankinson SE, Speizer FE, et al. Association of dietary intake of fat and fatty acids with risk of breast cancer. Jama (1999) 281(10):914–20. doi: 10.1001/jama.281.10.914
26. Cao Y, Hou L, Wang W. Dietary total fat and fatty acids intake, serum fatty acids and risk of breast cancer: A meta-analysis of prospective cohort studies. Int J Cancer (2016) 138(8):1894–904. doi: 10.1002/ijc.29938
27. Alexander DD, Morimoto LM, Mink PJ, Lowe KA. Summary and meta-analysis of prospective studies of animal fat intake and breast cancer. Nutr Res Rev (2010) 23(1):169–79. doi: 10.1017/S095442241000003X
28. Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC. Premenopausal dietary fat in relation to pre-and post-menopausal breast cancer. Breast Cancer Res Treat (2014) 145(1):255–65. doi: 10.1007/s10549-014-2895-9
29. Bertrand KA, Burian RA, Eliassen AH, Willett WC, Tamimi RM. Adolescent intake of animal fat and red meat in relation to premenopausal mammographic density. Breast Cancer Res Treat (2016) 155(2):385–93. doi: 10.1007/s10549-016-3679-1
30. Welsch CW. Relationship between dietary fat and experimental mammary tumorigenesis: A review and critique. Cancer Res (1992) 52(7 Supplement):2040s–8s.
31. Fay MP, Freedman LS, Clifford CK, Midthune DN. Effect of different types and amounts of fat on the development of mammary tumors in rodents: A review. Cancer Res (1997) 57(18):3979–88.
32. Calder PC. Dietary modification of inflammation with lipids. Proc Nutr Society (2002) 61(3):345–58. doi: 10.1079/PNS2002166
33. Wirfält E, Mattisson I, Gullberg B, Johansson U, Olsson H, Berglund G. Postmenopausal breast cancer is associated with high intakes of ω6 fatty acids (Sweden). Cancer Causes Control (2002) 13(10):883–93. doi: 10.1023/A:1021922917489
34. De Stefani E, Deneo-Pellegrini H, Mendilaharsu M, Ronco A. Essential fatty acids and breast cancer: A case-control study in Uruguay. Int J Cancer (1998) 76(4):491–4. doi: 10.1002/(SICI)1097-0215(19980518)76:4<491::AID-IJC8>3.0.CO;2-M
35. Rissanen H, Knekt P, Jarvinen R, Salminen I, Hakulinen T. Serum fatty acids and breast cancer incidence. Nutr Cancer (2003) 45(2):168–75. doi: 10.1207/S15327914NC4502_05
36. Wakai K, Tamakoshi K, Date C, Fukui M, Suzuki S, Lin Y, et al. Dietary intakes of fat and fatty acids and risk of breast cancer: A prospective study in Japan. Cancer Sci (2005) 96(9):590–9. doi: 10.1111/j.1349-7006.2005.00084.x
37. Gago-Dominguez M, Yuan J, Sun C, Lee H, Yu M. Opposing effects of dietary n-3 and n-6 fatty acids on mammary carcinogenesis: The Singapore Chinese health study. Br J Cancer (2003) 89(9):1686–92. doi: 10.1038/sj.bjc.6601340
38. Saadatian-Elahi M, Norat T, Goudable J, Riboli E. Biomarkers of dietary fatty acid intake and the risk of breast cancer: A meta-analysis. Int J Cancer (2004) 111(4):584–91. doi: 10.1002/ijc.20284
39. Gerber M. Omega-3 fatty acids and cancers: A systematic update review of epidemiological studies. Br J Nutr (2012) 107(S2):S228–S39. doi: 10.1017/S0007114512001614
40. Zheng J-S, Hu X-J, Zhao Y-M, Yang J, Li D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: meta-analysis of data from 21 independent prospective cohort studies. BMJ (2013) 346:f3706. doi: 10.1136/bmj.f3706
41. Petrek JA, Hudgins LC, Levine B, Ho M, Hirsch J. Breast cancer risk and fatty acids in the breast and abdominal adipose tissues. JNCI: J Natl Cancer Institute (1994) 86(1):53–6. doi: 10.1093/jnci/86.1.53
42. London SJ, Sacks FM, Stampfer MJ, Henderson IC, Maclure M, Tomita A, et al. Fatty acid composition of the subcutaneous adipose tissue and risk of proliferative benign breast disease and breast cancer. JNCI: J Natl Cancer Institute (1993) 85(10):785–93. doi: 10.1093/jnci/85.10.785
43. Chajès V, Torres-Mejía G, Biessy C, Ortega-Olvera C, Angeles-Llerenas A, Ferrari P, et al. ω-3 and ω-6 polyunsaturated fatty acid intakes and the risk of breast cancer in Mexican women: impact of obesity status. Cancer Epidemiol Prev Biomark (2012) 21(2):319–26. doi: 10.1158/1055-9965.EPI-11-0896
44. Holmes MD, Colditz GA, Hunter DJ, Hankinson SE, Rosner B, Speizer FE, et al. Meat, fish and egg intake and risk of breast cancer. Int J Cancer (2003) 104(2):221–7. doi: 10.1002/ijc.10910
45. Rose DP. Diet, hormones, and cancer. Annu Rev Public Health (1993) 14(1):1–17. doi: 10.1146/annurev.pu.14.050193.000245
46. Simonson N, van’t Veer P, Strain J, Martin-Moreno J, Huttunen J, Navajas J, et al. Adipose tissue omega-3 and omega-6 fatty acid content and breast cancer in the EURAMIC study. Am J Epidemiol (1998) 147:342–52. doi: 10.1093/oxfordjournals.aje.a009456
47. Dydjow-Bendek D, Zagoźdźon P. Total dietary fats, fatty acids, and omega-3/Omega-6 ratio as risk factors of breast cancer in the polish population–a case-control study. In Vivo (2020) 34(1):423–31. doi: 10.21873/invivo.11791
48. Nindrea RD, Aryandono T, Lazuardi L, Dwiprahasto I. Association of dietary intake ratio of n-3/n-6 polyunsaturated fatty acids with breast cancer risk in Western and Asian countries: A meta-analysis. Asian Pacif J Cancer Prevent: APJCP (2019) 20(5):1321. doi: 10.31557/APJCP.2019.20.5.1321
49. Pouchieu C, Chajès V, Laporte F, Kesse-Guyot E, Galan P, Hercberg S, et al. Prospective associations between plasma saturated, monounsaturated and polyunsaturated fatty acids and overall and breast cancer risk–modulation by antioxidants: A nested case-control study. PloS One (2014) 9(2):e90442. doi: 10.1371/journal.pone.0090442
50. Sczaniecka AK, Brasky TM, Lampe JW, Patterson RE, White E. Dietary intake of specific fatty acids and breast cancer risk among postmenopausal women in the VITAL cohort. Nutr Cancer (2012) 64(8):1131–42. doi: 10.1080/01635581.2012.718033
51. Murff HJ, Shu XO, Li H, Yang G, Wu X, Cai H, et al. Dietary polyunsaturated fatty acids and breast cancer risk in Chinese women: a prospective cohort study. Int J Cance. (2011) 128(6):1434–41. doi: 10.1002/ijc.25703
52. Engeset D, Alsaker E, Lund E, Welch A, Khaw KT, Clavel-Chapelon F, et al. Fish consumption and breast cancer risk. the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer (2006) 119(1):175–82. doi: 10.1002/ijc.21819
53. De Cicco P, Catani MV, Gasperi V, Sibilano M, Quaglietta M, Savini I. Nutrition and breast cancer: A literature review on prevention, treatment and recurrence. Nutrients (2019) 11(7):1514. doi: 10.3390/nu11071514
54. Turner LB. A meta-analysis of fat intake, reproduction, and breast cancer risk: An evolutionary perspective. Am J Hum Biol (2011) 23(5):601–8. doi: 10.1002/ajhb.21176
55. Riccardi G, Giacco R, Rivellese A. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr (2004) 23(4):447–56. doi: 10.1016/j.clnu.2004.02.006
56. Kaaks R ed. (2005). Nutrition, insulin, IGF-1 metabolism and cancer risk: a summary of epidemiological evidence. InNovartis Foundation symposium 2004 Nov 10 (pp. 247-264). Chichester; New York; John Wiley; 1999.
57. Aylsworth CF, Jone C, Trosko JE, Meites J, Welsch CW. Promotion of 7, 12-dimethylbenz [a] anthracene-induced mammary tumorigenesis by high dietary fat in the rat: possible role of intercellular communication. J Natl Cancer Institute (1984) 72(3):637–45.
58. Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n– 3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am J Clin Nutr (2004) 79(6):935–45. doi: 10.1093/ajcn/79.6.935
59. Echarte M, Zulet MA, Astiasaran I. Oxidation process affecting fatty acids and cholesterol in fried and roasted salmon. J Agric Food Chem (2001) 49(11):5662–7. doi: 10.1021/jf010199e
60. Arab L. Biomarkers of fat and fatty acid intake. J Nutr (2003) 133(3):925S–32S. doi: 10.1093/jn/133.3.925S
Keywords: breast cancer, dietary fats, fatty acids, cancer, fat
Citation: Shafie F, Tajadod S, Aslany Z, Allahyari P, Vahdat M, Shekari S, Mohseni GK, Gholamalizadeh M, Mohammadi S, Bahar B, Shafaei H and Doaei S (2023) Breast cancer and dietary fat quality indices in Iranian women: A case–control study. Front. Oncol. 12:993397. doi: 10.3389/fonc.2022.993397
Received: 13 July 2022; Accepted: 30 December 2022;
Published: 20 January 2023.
Edited by:
Michael Gnant, Medical University of Vienna, AustriaReviewed by:
Pruteanu Lavinia, Technical University of Cluj-Napoca, RomaniaEduardo Perez-Salazar, Instituto Politécnico Nacional de México (CINVESTAV), Mexico
Copyright © 2023 Shafie, Tajadod, Aslany, Allahyari, Vahdat, Shekari, Mohseni, Gholamalizadeh, Mohammadi, Bahar, Shafaei and Doaei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saeid Doaei, RG9hZWlAZ3Vtcy5hYy5pcg==
 Fatemeh Shafie1
Fatemeh Shafie1 Maryam Gholamalizadeh
Maryam Gholamalizadeh Saeid Doaei
Saeid Doaei