- 1Department of Otorhinolaryngology Head and Neck Surgery, People’s Hospital of Wanning, Wanning, Hainan, China
- 2Department of General Practice, Hainan General Hospital, Hainan Affiliated Hospital of Hainan Medical University, Haikou, Hainan, China
Background: Head and neck cancer (HNC) is the sixth most common malignant tumor worldwide and imposes a serious economic burden on society and individuals. Annexin has been implicated in multiple functions which are essential in HNC development, including cell proliferation, apoptosis, metastasis, and invasion. This study focused on the linkage between ANXA6 variants and HNC susceptibility in Chinese people.
Methods: Eight SNPs in ANXA6 from 139 HNC patients and 135 healthy controls were genotyped by the Agena MassARRAY platform. The correlation of SNPs with HNC susceptibility was evaluated using odds ratio and 95% confidence interval calculated by logistic regression using PLINK 1.9.
Results: Overall analysis results demonstrated that rs4958897 was correlated with an increased HNC risk (allele: OR = 1.41, p = 0.049; dominant: OR = 1.69, p = 0.039), while rs11960458 was correlated with reduced HNC risk (OR = 0.54, p = 0.030). In age ≤ 53, rs4958897 was related to reduce HNC risk. In males, rs11960458 (OR = 0.50, p = 0.040) and rs13185706 (OR = 0.48, p = 0.043) were protective factors for HNC, but rs4346760 was a risk factor for HNC. Moreover, rs4346760, rs4958897, and rs3762993 were also correlated with increased nasopharyngeal carcinoma risk.
Conclusions: Our findings suggest that ANXA6 polymorphisms are linked to the susceptibility to HNC in the Chinese Han population, indicating that ANXA6 may serve as a potential biomarker for HNC prognosis and diagnosis.
Introduction
Head and neck cancer (HNC) is the seventh most common malignant tumor worldwide, which is a squamous cell carcinoma that occurs in the lip, oral cavity, pharynx, and larynx (1). The global cancer burden using the GLOBOCAN 2020 estimation of cancer incidence and mortality produced by the International Agency for Research on Cancer (IARC) is estimated to be 931,931 new HNC cases and 467,125 HNC deaths in 2020. There will be approximately 148,344 new HNC cases and 78,554 HNC deaths in China in 2022 (2). The treatment regimens for HNC are complicated and bring a heavy burden to patients, often affecting their speech, swallowing, and respiratory functions (3). Therefore, it is necessary and urgent to explore the pathological mechanism of HNC.
HNC is a multifactorial disease that may be caused by complex factors, including environmental and genetic factors. Previous studies have indicated that tobacco smoking, excessive alcohol consumption and human papillomavirus (HPV) infection could contribute to the occurrence and development of HNC (3–5). In recent years, a study has demonstrated that individuals with a family history of HNC have an increased risk of HNC approximately two to three-fold (6). However, only a small proportion of individuals will eventually develop HNC. Genetic mutations such as single nucleotide polymorphisms (SNPs) may potentially alter the susceptibility of an individual to HNC. Several studies have identified that genetic polymorphisms of TCF19 (7), CYP2B6, HSD17B12 (8), GSTM1, and GSTT1 (9) are associated with HNC risk. Taken together, these findings reveal that genetic mutations play an important role in tumorigenesis and increase the risk of HNC.
Annexinis a kind of calcium ion-dependent phospholipid binding protein. A great deal of literature has reported that annexin plays a key role in multiple functions essential in cancer, including cell proliferation, apoptosis, chemosensitivity, metastasis, and invasion (10–13). Notably, the role of annexin in HNC development has attracted widespread attention. For example, Chen et al. have found that the overexpression of ANXA2 is correlated with a poor prognosis of HNC (14). Salom et al. have shown that ANXA9 and ANXA10 are abnormally expressed in HNC tissues and are related to the grade of tumor differentiation (15). A study has indicated that ANXA1 promotes nasopharyngeal carcinoma growth and metastasis via the binding and stabilization of EphA2 (16). ANXA6 has been reported to be closely associated with a variety of tumors and be involved in cancer cell growth, motility, invasion, and adhesion (17). Xin Sun et al. have showed that ANXA6 suppresses the tumorigenesis of cervical cancer through autophagy induction (18). ANXA6 induces gemcitabine resistance by inhibiting ubiquitination and degradation of EGFR in triple-negative breast cancer (19). Polymorphisms in the ANXA6 gene were significantly associated with the risk of osteonecrosis of the femoral head (ONFH) (20), systemic lupus erythematosus (21). However, there is a lack of data on ANXA6 polymorphisms in the occurrence and development of HNC.
Therefore, this study was planned to explore whether ANXA6 gene polymorphisms affect the susceptibility to HNC in the Chinese Han population. Eight SNPs in the ANXA6 gene were screened to evaluate the linkage between ANXA6 variants and HNC susceptibility from 139 patients with HNC and 135 healthy controls. Our results may provide new ideas for the diagnosis and treatment of HNC.
Materials and methods
Study population
In total, 274 individuals from People’s Hospital of Wanning were recruitedin this study, including 139 HNC patients and 135 healthy controls. All patients were histologically diagnosed with HNC by two pathologists. Patients who had received chemotherapy or radiotherapy and had a history or family history of cancer were excluded. The inclusion criteria for the control group were: individuals without a history of cancer or chronic diseases.
SNP selection and genotyping
A total of eight SNPs (rs11960458, rs4958892, rs78243462, rs4346760, rs4958897, rs3762993, rs9324677, and rs13185706) were screened from the ANXA6 gene and then genotyped using the Agena MassARRAY system (Agena, San Diego, CA, U.S.A.) as described previously (22, 23). These SNPs had a minor allele frequency (MAF) >5% in the Chinese Han Beijing (CHB) population from the 1000 Genomes Project. Total DNA was extracted from peripheral blood using a DNA Extraction Kit (GoldMag, Xi’an, China). The concentration and purity of DNA were measured by NanoDrop 2000 (Thermo Scientific, USA). Data management was conducted by Agena Typer 4.0 software.
Statistical analysis
We utilized t-test and χ2 test to analyze differencesin age and gender between cases and controls. Hardy-Weinberg equilibrium (HWE) of the control group was evaluated by χ2 test. Besides, odds ratio (OR) and 95% confidence interval (CI) were used to assess the linkage between ANXA6 variants and HNC risk under the five genetics models (allele, genotypes, dominant, recessive and additive model)via logistic regression analysis using PLINK 1.9. One SNP has two alleles (A/a), and there are three genotypes (AA, Aa and aa). If “a” is regarded as a risk allele, in the additive model, a frequency is counted as long as there is one “a” in the genotype, that is, when the genotype is AA, Aa, or aa, the frequency is 0, 1, or 2, respectively. In the dominant model, the frequency is calculated once as long as there is one “a” without taking into account the quantity of “a”, similar to the qualitative method, that is, when the genotype is AA, Aa, or aa, the frequency is 0, 1, or 1, respectively. In the recessive model, the frequency is calculated only if there are two “a”s, that is, when the genotype is AA, Aa, or aa, the frequency is 0, 0, or 1, respectively. Multi-factor dimensionality reduction (MDR) was used to assess the effect of potential SNP-SNP interactions on HNC risk. P < 0.05 was considered to be statistically significant.
Results
Study population
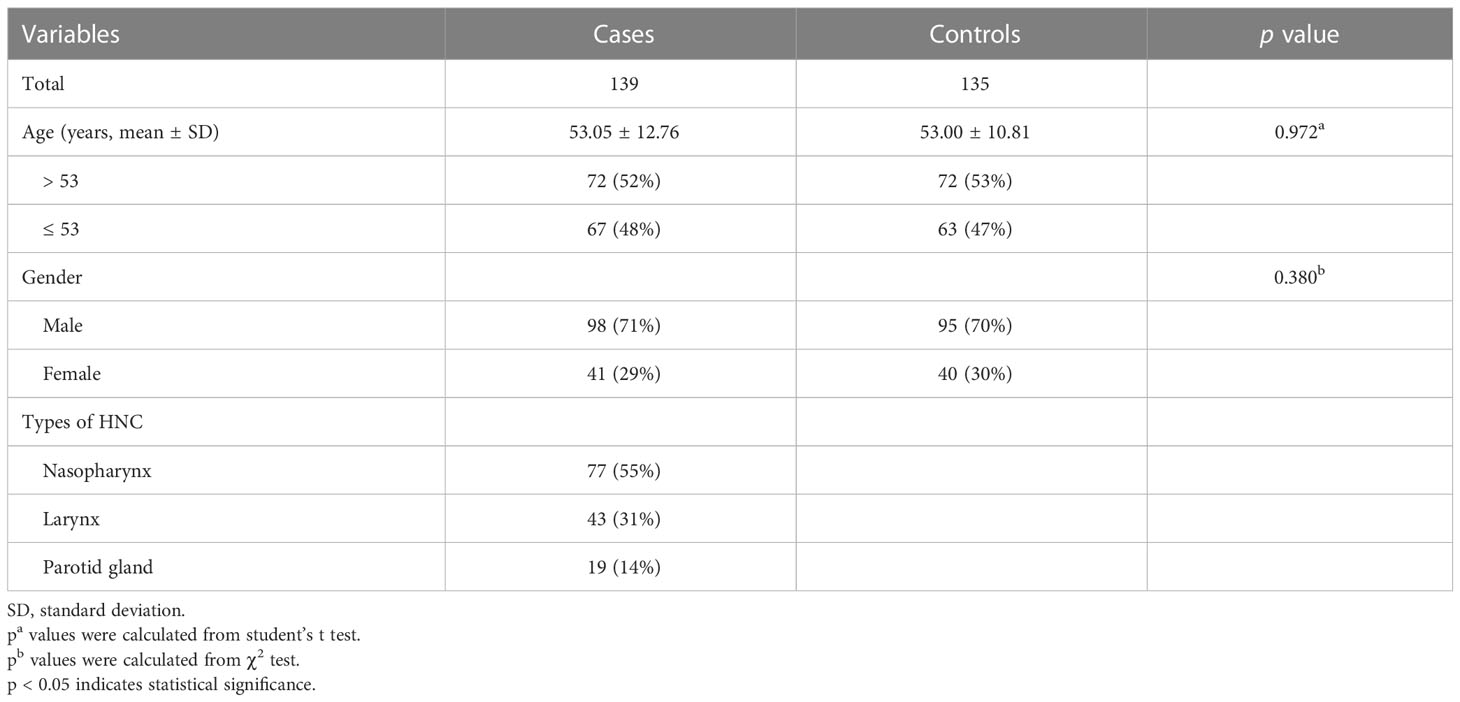
This study included 139 patients with HNC (98 men and 41 women) and 135 healthy controls (95 men and 40 women). The mean age of the control group was 53.00 ± 10.81 years, and that of the case group was 53.05 ± 12.76 years (Table 1). No significant differences were observed in age (p = 0.972) and gender stratification between the case and control groups (p = 0.380).
Association of ANXA6 SNPs with HNC risk
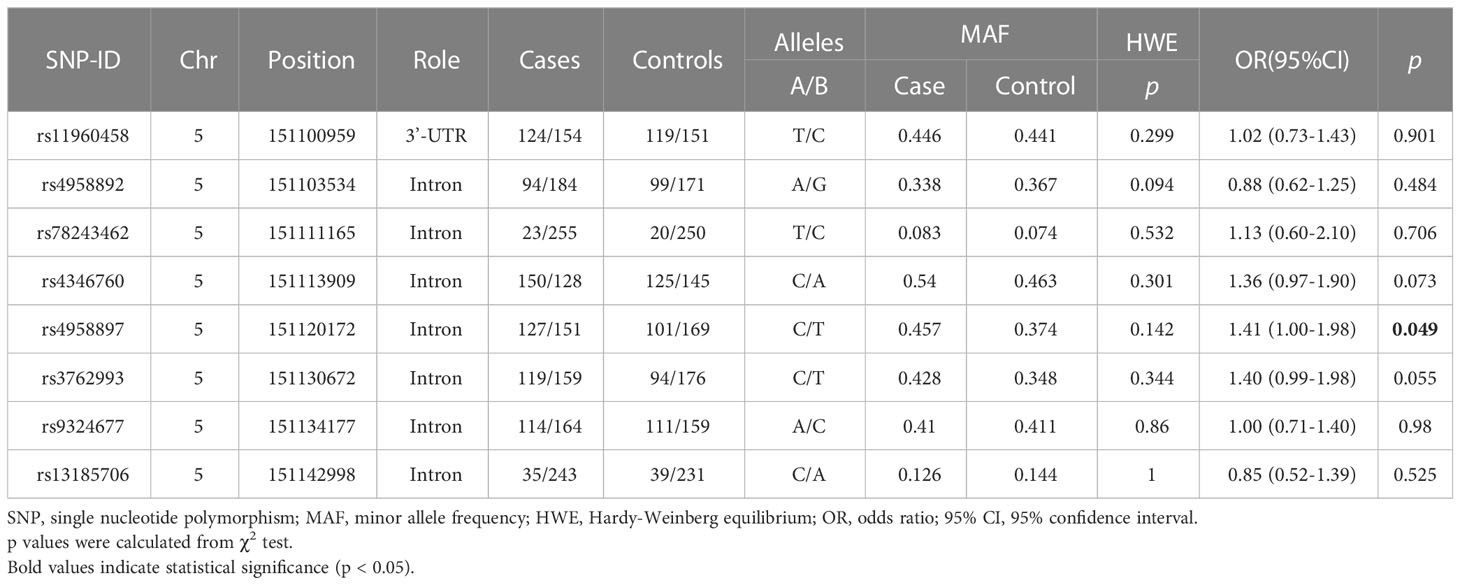
The primary information on ANXA6 SNPs is listed in Table 2, and all SNPs met HWE (p > 0.05). It was revealed that our study population was in a state of genetic balance, and the genotyping results were reliable, meeting the requirements of random sampling. This study results indicateed that the C allele of rs4958897 was correlated with an increased risk of HNC compared with the T allele (OR = 1.41, 95% CI = 1.00-1.98, p = 0.049). No correlation was observed between the other seven ANXA6 SNPs and susceptibility to HNC (p > 0.05).
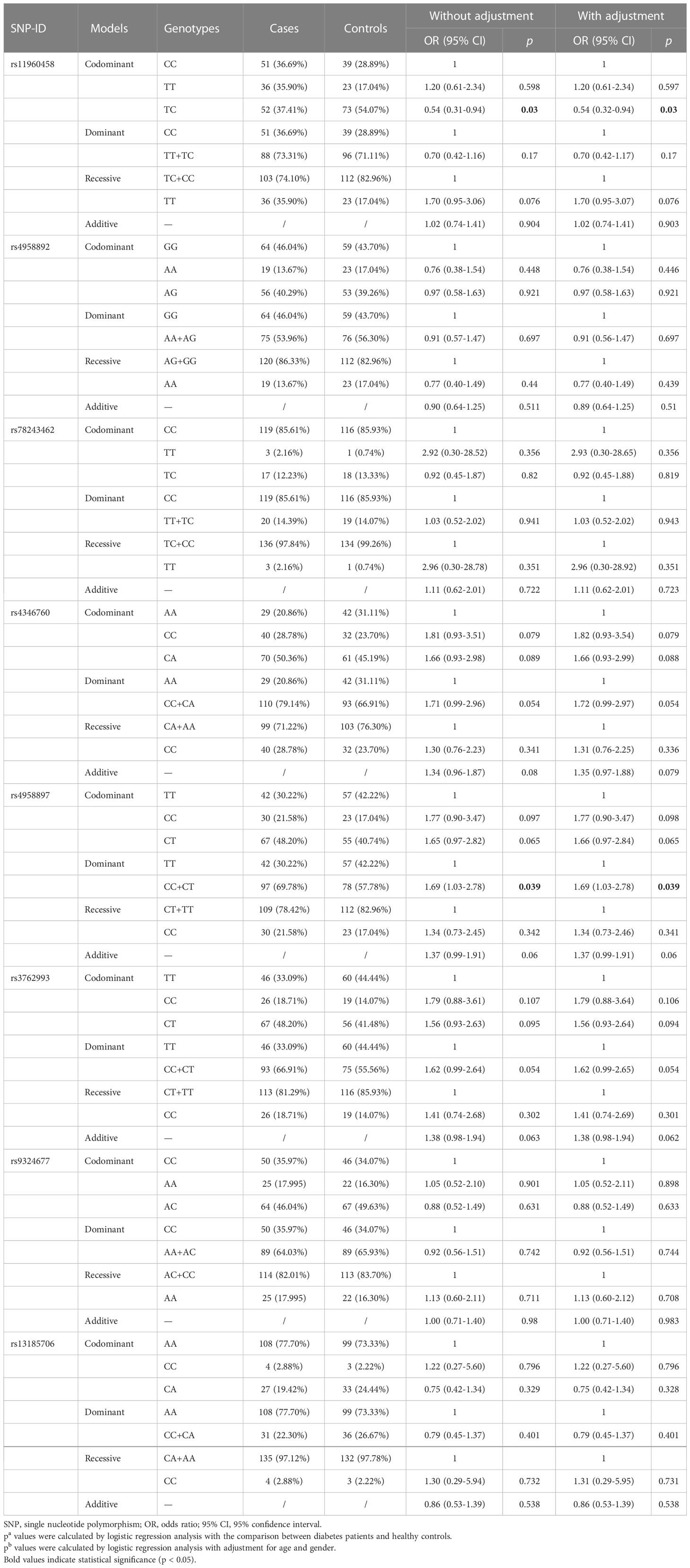
As illustrated in Table 3, the results of this study demonstrated that the TC genotype of rs11960458was correlated with reduced risk of HNC compared with TT genotype (adjusted OR = 0.54, 95% CI = 0.31-0.94, p = 0.030). The CC+CT genotype of rs4958897 was found to be asociated with an increased HNC risk compared with the TT genotype (adjusted OR = 1.69, 95% CI = 1.03-2.78, p = 0.039).
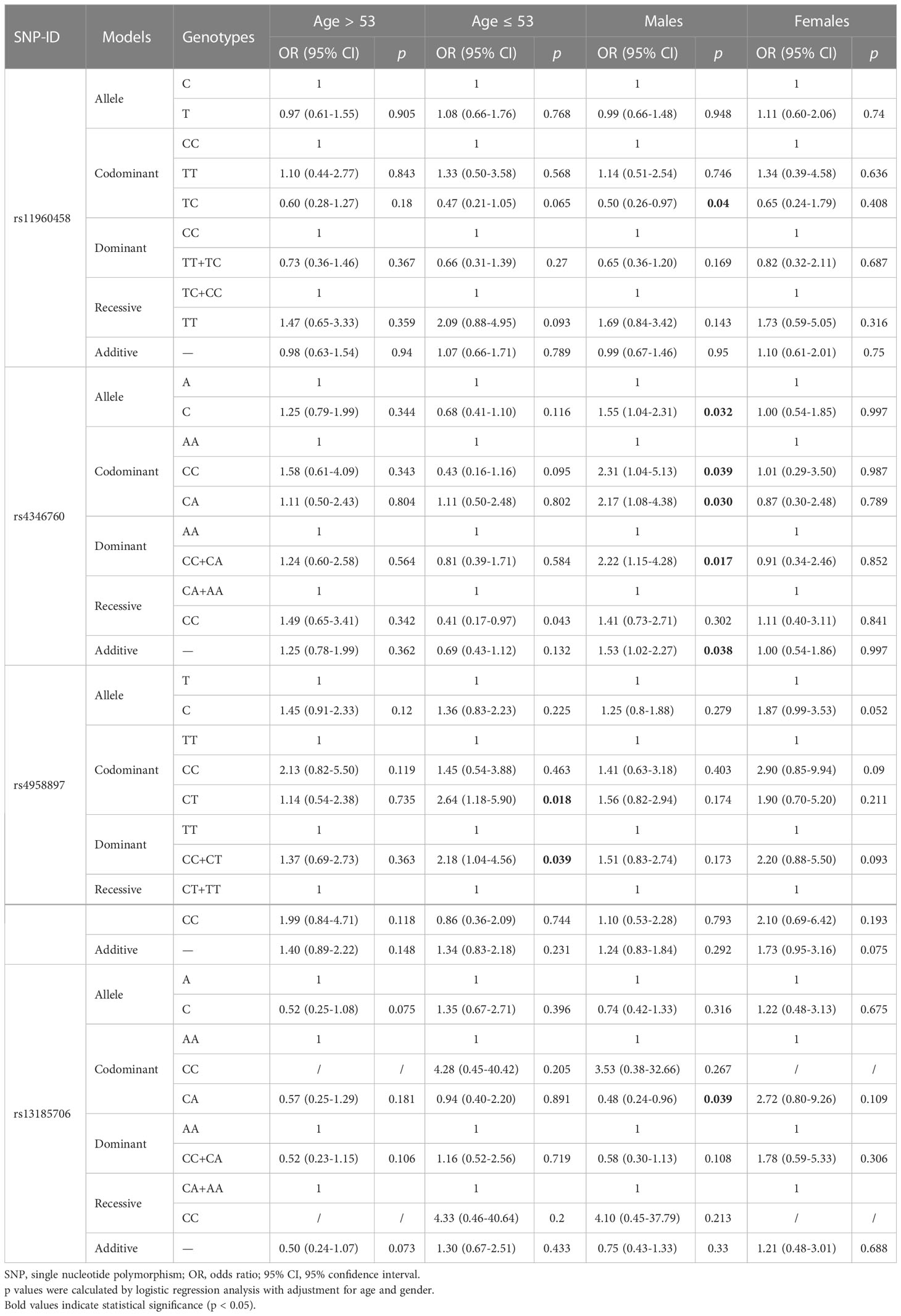
To further investigate the associations of ANXA6 SNPs with HNC risk, stratified analyses based on age, gender, and tumor sites were conducted. The results of age-stratification analysisshowed that rs4958897 was associated with an increased risk of HNC in individuals aged ≤ 53 years (CT vs. TT: OR = 2.64, 95% CI = 1.18-5.90, p = 0.018; CC+CT vs. TT: OR = 2.18, 95% CI = 1.04-4.56, p = 0.039), as shown in Table 4. The results of gender-stratification analysis indicated that the TC genotype of rs11960458 (TC vs. CC: OR = 0.50, 95% CI = 0.26-0.97, p = 0.040) and the CA genotype of rs13185706 (CA vs. CC: OR = 0.48, 95% CI = 0.24-0.96, p = 0.043) were associated with reduced HNC risk in males. However, rs4346760 was a risk factor for HNC in males (C vs. A: OR = 1.55, 95% CI = 1.04-2.31, p = 0.032; homozygous: OR = 2.31, 95% CI = 1.04-5.13, p = 0.039; heterozygous: OR = 2.17, 95% CI = 1.08-4.38, p = 0.030; additive: OR = 1.53, 95% CI = 1.02-2.27, p = 0.038), as shown in Table 4.
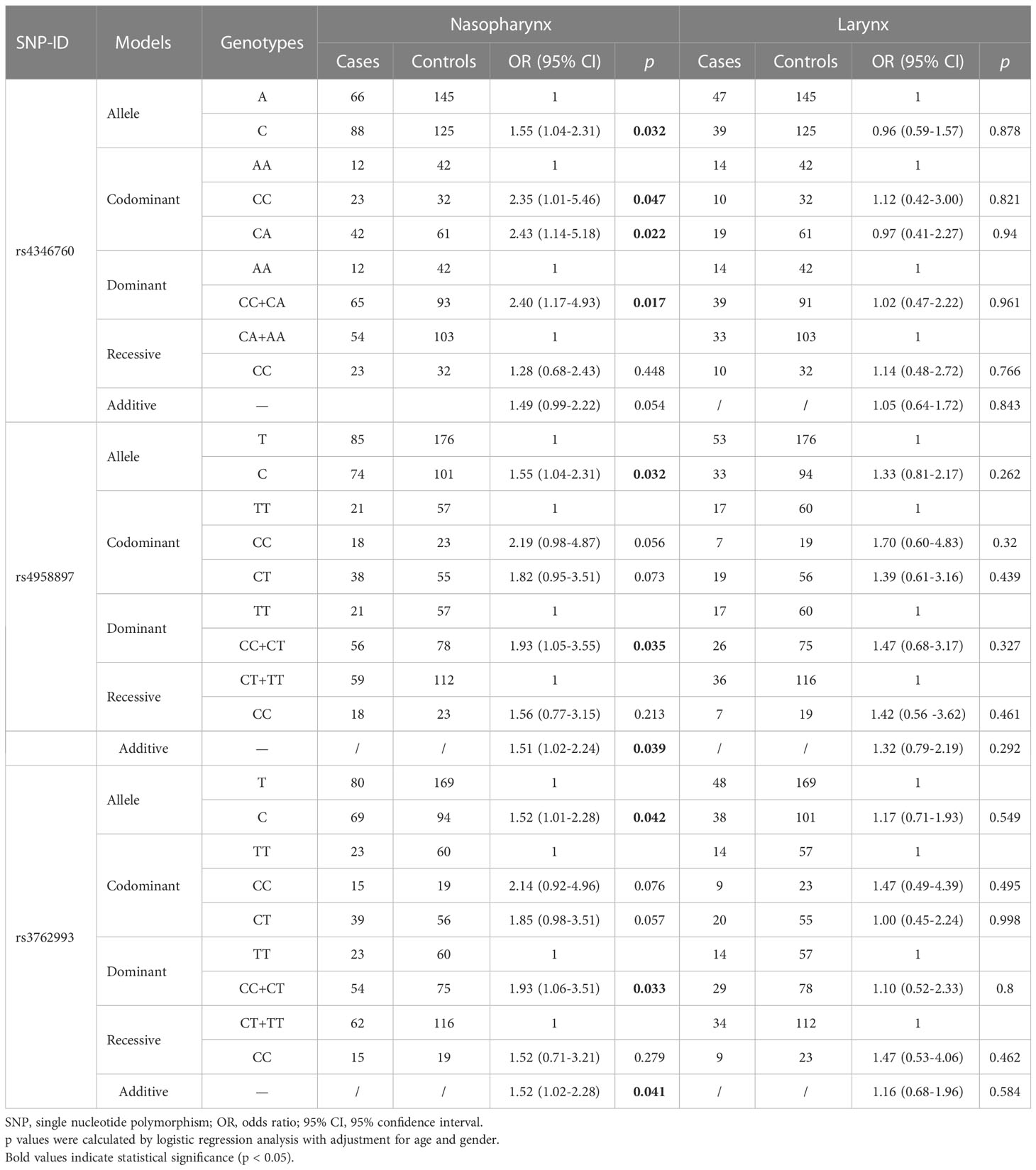
Furthermore, the results of tumor sites stratification analysis obsevered that rs4346760 was correlated with an increased risk of nasopharyngeal carcinoma (NPC) under the allele (OR = 1.55, 95% CI = 1.04-2.31, p = 0.032), homozygous (OR = 2.35, 95% CI = 1.01-5.46, p = 0.047), heterozygous (OR = 2.43, 95% CI = 1.14-5.18, p = 0.022), and dominant models (OR = 2.40, 95% CI = 1.17-4.93, p = 0.017). Moreover, rs4958897 (C vs. T: OR = 1.55, 95% CI = 1.04-2.31, p = 0.032; CC+CT vs. TT: OR = 1.93, 95% CI = 1.05-3.55, p = 0.035; additive: OR = 1.51, 95% CI = 1.02-2.24, p = 0.039) and rs3762993 (C vs. T: OR = 1.52, 95% CI = 1.01-2.28, p = 0.042; CC+CT vs. TT: OR = 1.93, 95% CI = 1.06-3.51, p = 0.033; additive: OR = 1.52, 95% CI = 1.01-2.28, p = 0.041) were also found to be associated with increased risk of NPC, as presented in Table 5.
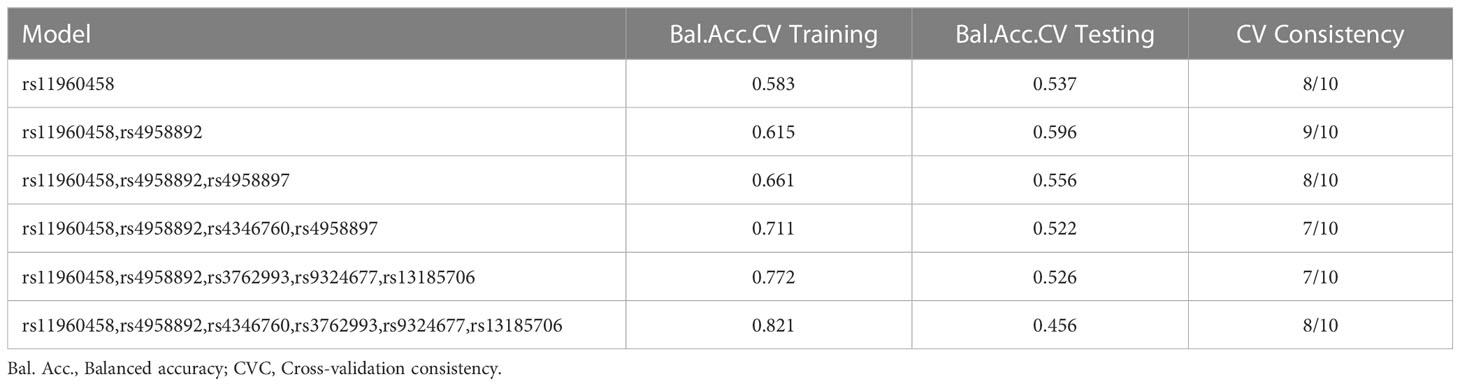
In addition, we used the MDR method to analyze the SNP-SNP interactions (Figure 1 and Table 6). These results revealed that rs11960458 and rs4958892 had a positive synergistic effect on increased HNC risk. However, rs11960458 and rs4958897 had a negative synergistic effect on HNC risk. The two-locus model (rs11960458 and rs4958892) had the highest Cross-validation (CV) consistency and balanced accuracy (Bal. Acc) testing. (CV Consistency: 9/10; Testing Bal. Acc.: 0.596).
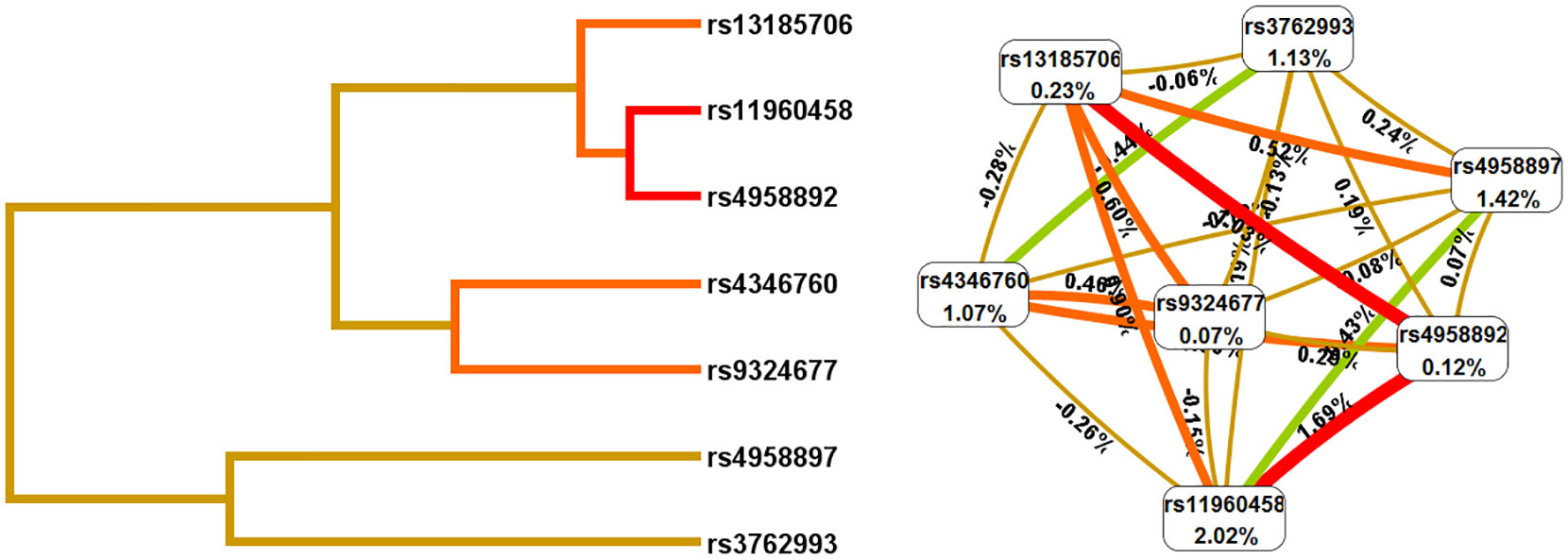
Figure 1 The SNP-SNP interaction analysis (Dendrogram and Fruchterman-Reingold). The colors represent synergy or redundancy. Green and blue with a negative correlation; red, orange and brown with a positive correlation.).
Discussion
This case-control study observed that rs4958897 was associated with an increased risk of HNC, while rs11960458 was linked to a reduced risk of HNC. Age and gender stratification results revealed that ANXA6 polymorphisms (rs11960458, rs4958897, rs4346760, and rs13185706) were significantly related to the susceptibility to HNC. Furthermore, rs4346760, rs4958897, and rs3762993 were found to be associated with the risk of nasopharyngeal carcinoma. These results highlighted the importance of the ANXA6 gene in the occurrence and development of HNC, and confirmed that ANXA6 might be a potential target for HNC prognosis and diagnosis.
Annexin is a calcium-dependent superfamily of proteins that can bind negatively charged membrane phospholipids and is a highly abundant protein. Annexin has been studied in laryngeal carcinoma, nasopharyngeal carcinoma and other head and neck tumors. For example, Luo et al. have uncovered that ANXA2 is highly expressed in laryngeal carcinoma and its expression is associated with tumor size, distant metastasis and clinical stage (24). Others have also illustrated that ANXA1 and ANXA2 could facilitate the progression of NPC (25, 26). As far as we know, the sequences of ANXA6 are highly similar to those of ANXA1 and ANXA2. ANXA6, a member of annexin superfamily, is located on human chromosome 5q33.1 and contains 26 exons with a length of about 60kbp. Some literatures have demonstrated that ANXA6 is involved in cell growth, differentiation, invasion, and motility in many cancers (27, 28). Furthermore, Chen et al. have observed that ANXA6 promotes autophagy through suppressing the PI3K/AKT/mTOR pathway, thereby upregulating radioresistance in NPC (29). These reports suggest that ANXA6 may play an important role in HNC and other malignant tumors. Nevertheless, there are few studies on the role of ANXA6 in HNC development at present.
In this study, the linkage between ANXA6 SNPs and HNC risk in the Chinese people was assessed. Overall analysis results indicated that the C allele and CC+CT genotypes of rs4958897 were associated with increased risk of HNC. However, individuals with the TC genotype of rs11960458 had lower risk of HNC compared with those with the TT genotype Rs11960458 is located in the 3’-UTR region of miRNA-binding site of the ANXA6 gene. Therefore, we speculated that rs11960458 affected the expression of ANXA6 and had a protective effect on HNC by maintaining mRNA stability and miRNA binding activity. However, our hypothesis requires functional studies to confirm.
Age stratification results showed that rs4958897 was a risk factor for HNC in aged ≤ 53. Furthermore, the TC genotype of rs11960458 and CA genotype of rs13185706 were found to be associated with reduced HNC risk, while rs4346760 was related to increased risk of HNC in males. Three ANXA6 SNPs (rs4346760, rs4958897, and rs3762993) facilitated the occurrence of nasopharyngeal carcinoma. However, no association between eight SNPs in ANXA6 and risk of HNC was found in subgroups of those aged > 53, female, and with laryngeal carcinoma. These findings suggested that genetic susceptibility to HNC varied by age, gender and types of HNC. An epidemiological study indicated that the incidence of HNC differed among people of different sexes and ages, and is higher in males and the elderly (30). Males are much more susceptible to HNC than females, and this difference is mainly due to the discrepancies in the lower part of the upper aerodigestive tract, such as larynx and hypopharynx (31). Therefore, the importance of heterogeneity should be considered in the genetic association study of HNC risk.
In addition, SNP-SNP interaction results showed that rs11960458, rs4958892, rs4346760, and rs3762993 had positive synergistic effect on increased HNC risk. However, rs11960458 and rs4958897 had negative synergistic effect on HNC risk. These four SNPs (rs4346760, rs4958897, rs3762993, and rs13185706) are located in the intron region of the ANXA6 gene. Combining previous studies and database predictions, we hypothesized that AXAN6 intron SNPs could lead tochanges in ANXA6 expression and activity via influencing mRNA splicing, and ultimately affecting disease susceptibility. Further studies are needed to explore the specific role of these ANXA6 SNPs.
Although the association of ANXA6 with HNC susceptibility was detected in this study, there are still some limitations. Firstly, there are no supporting studies about these SNPs, but the good thing is this study isfirst to report the association between eight ANXA6 SNPs (rs11960458, rs4958892, rs78243462, rs4346760, rs4958897, rs3762993, rs9324677, and rs13185706) and risk of HNC in the Chinese Han population. Secondly, the subjects in this study were recruited from the same hospital, so there were geographic limitations on sample selection. Therefore, further studies with large samples are needed to validate our findings of ANXA6 as a biomarker for HNC.
Conclusions
In conclusion, these results demonstrate that polymorphisms (rs11960458, rs4346760, rs495889, rs3762993 and rs13185706) in the ANXA6 gene are related to the susceptibility to HNC in the Chinese Han population, indicating that ANXA6 may serve as a diagnostic and prognostic molecular biomarker for patients with HNC.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of People’s Hospital of Wanning (No. SL-2023-001). The patients/participants provided their written informed consent to participate in this study.
Author contributions
WX: drafted and revised important content. ZL and XZ: performed experiments. JC, ZC and XY: analyzed data. YD: conceived and designed experiments. All authors contributed to the article and approved the submitted version.
Acknowledgments
We sincerely thank People’s Hospital of Wanning for providing samples for our study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and united states, 2022: Profiles, trends, and determinants. Chin Med J (2022) 135(5):584–90. doi: 10.1097/CM9.0000000000002108
3. Samuel SR, Maiya AG, Fernandes DJ, Guddattu V, Saxena PUP, Kurian JR, et al. Effectiveness of exercise-based rehabilitation on functional capacity and quality of life in head and neck cancer patients receiving chemo-radiotherapy. Supportive Care Cancer (2019) 27(10):3913–20. doi: 10.1007/s00520-019-04750-z
4. Di Credico G, Polesel J, Dal Maso L, Pauli F, Torelli N, Luce D, et al. Alcohol drinking and head and neck cancer risk: The joint effect of intensity and duration. Br J Cancer (2020) 123(9):1456–63. doi: 10.1038/s41416-020-01031-z
5. Madathil S, Rousseau MC, Joseph L, Coutlée F, Schlecht NF, Franco E, et al. Latency of tobacco smoking for head and neck cancer among HPV-positive and HPV-negative individuals. Int J Cancer (2020) 147(1):56–64. doi: 10.1002/ijc.32708
6. Chulam TC, Bertonha FB, Villacis RAR, Filho JG, Kowalski LP, Rogatto SR. Epidemiological, clinical, and genomic profile in head and neck cancer patients and their families. Biomedicines (2022) 10(12):3278. doi: 10.3390/biomedicines10123278
7. Ji P, Chang J, Wei X, Song X, Yuan H, Gong L, et al. Genetic variants associated with expression of TCF19 contribute to the risk of head and neck cancer in Chinese population. J Med Genet (2022) 59(4):335–45. doi: 10.1136/jmedgenet-2020-107410
8. Liu H, Li G, Sturgis EM, Shete S, Dahlstrom KR, Du M, et al. Genetic variants in CYP2B6 and HSD17B12 associated with risk of squamous cell carcinoma of the head and neck. Int J Cancer (2022) 151(4):553–64. doi: 10.1002/ijc.34023
9. Surit R, Kumar S, Sinha DK, Shekhar R. Genomic pattern of GSTM1 and T1 gene null polymorphism of head and neck cancer patients in Eastern India. Asian Pacific J Cancer Prev APJCP (2022) 23(8):2655–9. doi: 10.31557/APJCP.2022.23.8.2655
10. Du R, Liu B, Zhou L, Wang D, He X, Xu X, et al. Downregulation of annexin A3 inhibits tumor metastasis and decreases drug resistance in breast cancer. Cell Death Dis (2018) 9(2):126. doi: 10.1038/s41419-017-0143-z
11. Zhao RR, Mao XR, Wang XF, Zheng Y, Wang YP, Zhou YN. Role of annexin a family in tumorigenesis and chemoresistance of gastric cancer. Neoplasma (2022) 69(2):251–63. doi: 10.4149/neo_2021_210629N872
12. Araújo TG, Mota STS, Ferreira HSV, Ribeiro MA, Goulart LR, Vecchi L. Annexin A1 as a regulator of immune response in cancer. Cells (2021) 10(9):2245. doi: 10.3390/cells10092245
13. Woodward A, Faria GNF, Harrison RG. Annexin A5 as a targeting agent for cancer treatment. Cancer Lett (2022) 547:215857. doi: 10.1016/j.canlet.2022.215857
14. Liu X, Ma D, Jing X, Wang B, Yang W, Qiu W. Overexpression of ANXA2 predicts adverse outcomes of patients with malignant tumors: a systematic review and meta-analysis. Med Oncol (Northwood Lond Engl) (2015) 32(1):392. doi: 10.1007/s12032-014-0392-y
15. Salom C, Álvarez-Teijeiro S, Fernández MP, Morgan RO, Allonca E, Vallina A, et al. Frequent alteration of annexin A9 and A10 in HPV-negative head and neck squamous cell carcinomas: Correlation with the histopathological differentiation grade. J Clin Med (2019) 8(2):229. doi: 10.3390/jcm8020229
16. Feng J, Lu SS, Xiao T, Huang W, Yi H, Zhu W, et al. ANXA1 binds and stabilizes EphA2 to promote nasopharyngeal carcinoma growth and metastasis. Cancer Res (2020) 80(20):4386–98. doi: 10.1158/0008-5472.CAN-20-0560
17. Qi H, Liu S, Guo C, Wang J, Greenaway FT, Sun MZ. Role of annexin A6 in cancer. Oncol Lett (2015) 10(4):1947–52. doi: 10.3892/ol.2015.3498
18. Sun X, Shu Y, Xu M, Jiang J, Wang L, Wang J, et al. ANXA6 suppresses the tumorigenesis of cervical cancer through autophagy induction. Clin Trans Med (2020) 10(6):e208. doi: 10.1002/ctm2.208
19. Li T, Tao Z, Zhu Y, Liu X, Wang L, Du Y, et al. Exosomal annexin A6 induces gemcitabine resistance by inhibiting ubiquitination and degradation of EGFR in triple-negative breast cancer. Cell Death Dis (2021) 12(7):684. doi: 10.1038/s41419-021-03963-7
20. Kim TH, Hong JM, Shin ES, Kim HJ, Cho YS, Lee JY, et al. Polymorphisms in the annexin gene family and the risk of osteonecrosis of the femoral head in the Korean population. Bone (2009) 45(1):125–31. doi: 10.1016/j.bone.2009.03.670
21. Zhang J, Zhang L, Zhang Y, Yang J, Guo M, Sun L, et al. Gene-based meta-analysis of genome-wide association study data identifies independent single-nucleotide polymorphisms in ANXA6 as being associated with systemic lupus erythematosus in Asian populations. Arthritis Rheumatol (Hoboken NJ) (2015) 67(11):2966–77. doi: 10.1002/art.39275
22. Xia P, Li B, Geng T, Deng Z, Dang C, Chang D, et al. FGFR2 gene polymorphisms are associated with breast cancer risk in the han Chinese population. Am J Cancer Res (2015) 5(5):1854–61.
23. Liang J, Kang X, Halifu Y, Zeng X, Jin T, Zhang M, et al. Secreted frizzled-related protein promotors are hypermethylated in cutaneous squamous carcinoma compared with normal epidermis. BMC Cancer (2015) 15:641. doi: 10.1186/s12885-015-1650-x
24. Luo S, Xie C, Wu P, He J, Tang Y, Xu J, et al. Annexin A2 is an independent prognostic biomarker for evaluating the malignant progression of laryngeal cancer. Exp Ther Med (2017) 14(6):6113–8. doi: 10.3892/etm.2017.5298
25. Chen CY, Lin YS, Chen CH, Chen YJ. Annexin A2-mediated cancer progression and therapeutic resistance in nasopharyngeal carcinoma. J Biomed Sci (2018) 25(1):30. doi: 10.1186/s12929-018-0430-8
26. Liao L, Yan WJ, Tian CM, Li MY, Tian YQ, Zeng GQ. Knockdown of annexin A1 enhances radioresistance and inhibits apoptosis in nasopharyngeal carcinoma. Technol Cancer Res Treat (2018) 17:1–10. doi: 10.1177/1533034617750309
27. Whalen DS, Widatalla SE, Korolkova OY, Nangami GS, Beasley HK, Williams SD, et al. Implication of calcium activated RasGRF2 in annexin A6-mediated breast tumor cell growth and motility. Oncotarget (2019) 10(2):133–51. doi: 10.18632/oncotarget.26512
28. Korolkova OY, Widatalla SE, Whalen DS, Nangami GN, Abimbola A, Williams SD, et al. Reciprocal expression of annexin A6 and RasGRF2 discriminates rapidly growing from invasive triple negative breast cancer subsets. PloS One (2020) 15(4):e0231711. doi: 10.1371/journal.pone.0231711
29. Chen Q, Zheng W, Zhu L, Yao D, Wang C, Song Y, et al. ANXA6 contributes to radioresistance by promoting autophagy via inhibiting the PI3K/AKT/mTOR signaling pathway in nasopharyngeal carcinoma. Front Cell Dev Biol (2020) 8:232. doi: 10.3389/fcell.2020.00232
30. Guo K, Xiao W, Chen X, Zhao Z, Lin Y, Chen G. Epidemiological trends of head and neck cancer: A population-based study. BioMed Res Int (2021) 2021:1738932. doi: 10.1155/2021/1738932
Keywords: head and neck cancer, ANXA6, single nucleotide polymorphism, case-control study, Chinese Han population
Citation: Xiong W, Li Z, Zeng X, Cui J, Cheng Z, Yang X and Ding Y (2023) The polymorphisms of ANXA6 influence head and neck cancer susceptibility in the Chinese Han population. Front. Oncol. 13:1100781. doi: 10.3389/fonc.2023.1100781
Received: 17 November 2022; Accepted: 28 February 2023;
Published: 14 March 2023.
Edited by:
Arun Khattri, Indian Institute of Technology (BHU), IndiaReviewed by:
Xianlu Zhuo, Guizhou Medical University, ChinaShailendra Shanker Maurya, Washington University in St. Louis, United States
John Morris, University of Cincinnati Medical Center, United States
Ruqia Baig, Pir Mehr Ali Shah Arid Agriculture University, Pakistan
Copyright © 2023 Xiong, Li, Zeng, Cui, Cheng, Yang and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yipeng Ding, eXBkaW5nQHllYWgubmV0
 Weihong Xiong1
Weihong Xiong1 Yipeng Ding
Yipeng Ding