- 1Department of Dermatology, Shanghai Institute of Dermatology, Huashan Hospital, Fudan University, Shanghai, China
- 2Huashan Hospital, Fudan University, Shanghai, China
Objective: This study provided a systematic analysis of the trend in incidence and incidence-based mortality for cutaneous squamous cell carcinoma (cSCC) on the lips in the USA using demographic characteristics from the Surveillance, Epidemiology, and End Results (SEER) database.
Methods: Patients diagnosed with cSCC on the lips between 2000 and 2019 from the 17 registries of the USA were identified. Incidence and incidence-based mortality rates were analyzed using SEER*Stat 8.4.0.1 software. This paper calculated incidence rates and incidence-based mortality rates by 100,000 person-years for sex, age, race, SEER registries, median household income ($/year), rural-urban distribution, and primary site. The annual percent changes (APC) in incidence and incidence-based mortality rates were then calculated using joinpoint regression software.
Results: Among 8,625 patients diagnosed with cSCC on the lips from 2000 to 2019, men (74.67%), white (95.21%), and 60–79 years old were the most common population, and 3,869 deaths from cSCC on the lips occurred. The overall incidence of cSCC on the lips was 0.516 per 100,000 person-years. cSCC on the lip incidence rates were highest among men, white, and patients aged 60–79 years old. cSCC on the lip incidence rates decreased by 3.210%/year over the study period. The incidence of cSCC on the lips has been decreasing in all sexes, ages, high- or low-income households, and urban or rural patients. The overall incidence-based mortality rate of cSCC on the lips during 2000–2019 was 0.235 per 100,000 person-years. cSCC on the lip incidence-based mortality rates were highest among men, whites, and people older than 80 years old. cSCC on the lip incidence-based mortality increased by 4.975%/year over the study period. cSCC on the lip incidence-based mortality rates increased for all sexes, races, ages, primary sites, high- or low-income households, and urban or rural patients during the study period.
Conclusion: Among patients in the USA diagnosed with cSCC on the lips from 2000 to 2019, the overall incidence decreased by 3.210% annually, and incidence-based mortality increased by 4.975%/year. These findings update and supplement the epidemiological information of cSCC on the lips in the USA.
1 Introduction
Cutaneous squamous cell carcinoma (cSCC) is a malignant proliferation of the skin epithelium that accounts for 20% to 50% of skin cancers and is the second most common nonmelanoma skin cancer (NMSC) or keratinocytic carcinoma (1–6). cSCC occurs mainly in the head and neck, and more than 50% of newly diagnosed lesions occur in this area (7).
During 2002–2007, the age-standardized incidence rate of cSCC in Europe was nine to 96 cases per 100,000 men and five to 68 cases per 100,000 women (8). According to statistics from 2002, the incidence rate of cSCC in Australia in 2011 was about 499 cases per 100,000 men and 291 cases per 100,000 women, and the mortality rate was two cases per 100,000 people (5). The incidence rate of CSCC in Spain is 40 cases per 100,000 person-years (8). Whereas, the US National Tumor Registry did not include cSCC, and exact US incidence and mortality rates cannot be derived.
The risk factors for cSCC include exposure to the sun, age, fair skin, and immunosuppression (3, 5, 9–11). cSCC is more common in white people and men, with a ratio of 3:1. The incidence rate increased with age, with the median age of onset over 60 years old. Despite a lower prevalence among Hispanic, black, and Asian patients, cSCC is the most common form of skin cancer in these populations. The mortality of cSCC in black patients can be as high as 18% due to delayed diagnosis and poor prognosis (2–5). Most cSCC can be successfully resolved by surgical operation, while some cSCC are at high risk for recurrence, metastasis, and death (12, 13). cSCC on the lips is considered a high-risk skin cancer with a metastasis rate of 11% compared to 1% for other sites. cSCC become a public health problem due to the adverse outcomes it causes.
Therefore, this study provided a systematic analysis of the trend in incidence and the incidence-based mortality for cSCC on the lips in the USA using demographic characteristics from the Surveillance, Epidemiology, and End Results (SEER) database.
2 Method
2.1 Data sources
This research used the SEER*Stat 8.4.0.1 software to obtain data on cSCC on the lip cases diagnosed during 2000–2019 from SEER-17 registries (“Incidence - SEER Research Data, 17 Registries, November 2021 Sub (2000–2019)”). The SEER-17 Incidence-Based Mortality database provides a convenient and intuitive mechanism for analyzing cSCC on the lip mortality from death certificates (14). In contrast to general mortality rates, incidence-based mortality allows for the classification of deaths based on variables related to cancer occurrence.
2.2 Analysis population
This research included patients who were diagnosed with cSCC on the lips between the years 2000 and 2019. Cases were determined by selecting squamous cell tumors (codes 8050-8089) and primary sites on the lips (codes C00.0-00.3, 00.6, 44.0). Demographic characteristics analyzed in this study includes the following variables: sex, race, age at diagnosis (0–19, 20–39, 40–59, 60–79, and 80 years or older) or age at death in the case of incidence-based mortality calculation, SEER registries, median household income ($/year), rural-urban distribution, and primary site.
2.3 Statistical analysis
The SEER*stat version 8.4.0.1 software was used to calculate the incidence and incidence-based mortality rates of cSCC on the lips. All rates were adjusted to the 2000 US standard population and expressed by 100,000 person-years. We then used the National Cancer Institute’s Joinpoint Regression program, version 4.9.1.0, to calculate the annual percentage changes (APCs) and 95% confidence intervals (CIs) (15). The Joinpoint Regression software analyzed rates over time and detected significant changes in APCs, then selected the best model with the minimum number of joinpoints and calculated p-values using t-tests. All statistical tests were two-sided, and p-values less than 0.05 were considered statistically significant.
3 Results
3.1 Baseline characteristics
Between the years 2000 and 2019, 8,625 cases diagnosed as cSCC on the lips in the states recorded by SEER-17 were included in the incidence analysis (Table 1). Most patients were men (74.67%), white (95.21%), 60–79 years old (47.14%), and with an external lower lip (80.60%). Incidence-based mortality analysis revealed that, from 2000 to 2019, 3,869 patients with cSCC on the lips died, of whom 2,870 (74.18%) cases were men, 3,773 (97.52%) were white patients, 3,588 (92.8%) were over 60 years old, and 3,115 (80.51%) were external lower lips.
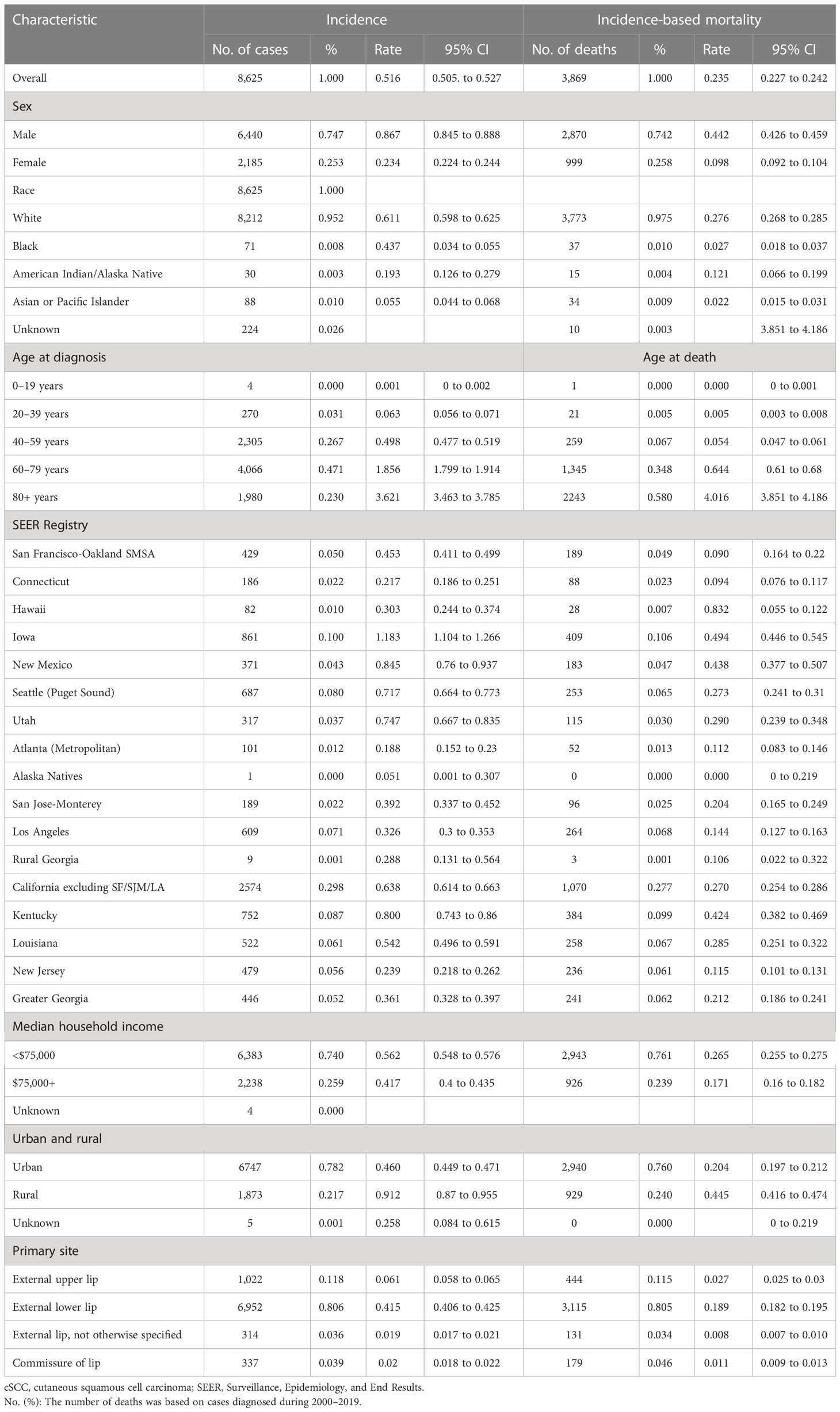
Table 1 cSCC on the lip incidence and incidence-based mortality (2000–2019): The SEER-17 registry database.
3.2 Incidence rates and trends over time
The overall incidence of cSCC on the lips during 2000–2019 was 0.516 (95% CI, 0.505 to 0.527) per 100,000 person-years. cSCC on the lip incidence rates were highest among men (0.867 (95% CI, 0.845 to 0.888)), white (0.437 (95% CI, 0.034 to 0.055)), patients 60–79 years old (1.856 (95% CI, 1.799 to 1.914)), and external lower lip patients (0.415 (95% CI, 0.406 to 0.425)). When compared to states in the SEER-17 registries, the incidence was the highest in Iowa (1.183 (95% CI, 1.104 to 1.266)) and the lowest in Alaska Natives (0.051 (95% CI, 0.001 to 0.307)) (Table 1).
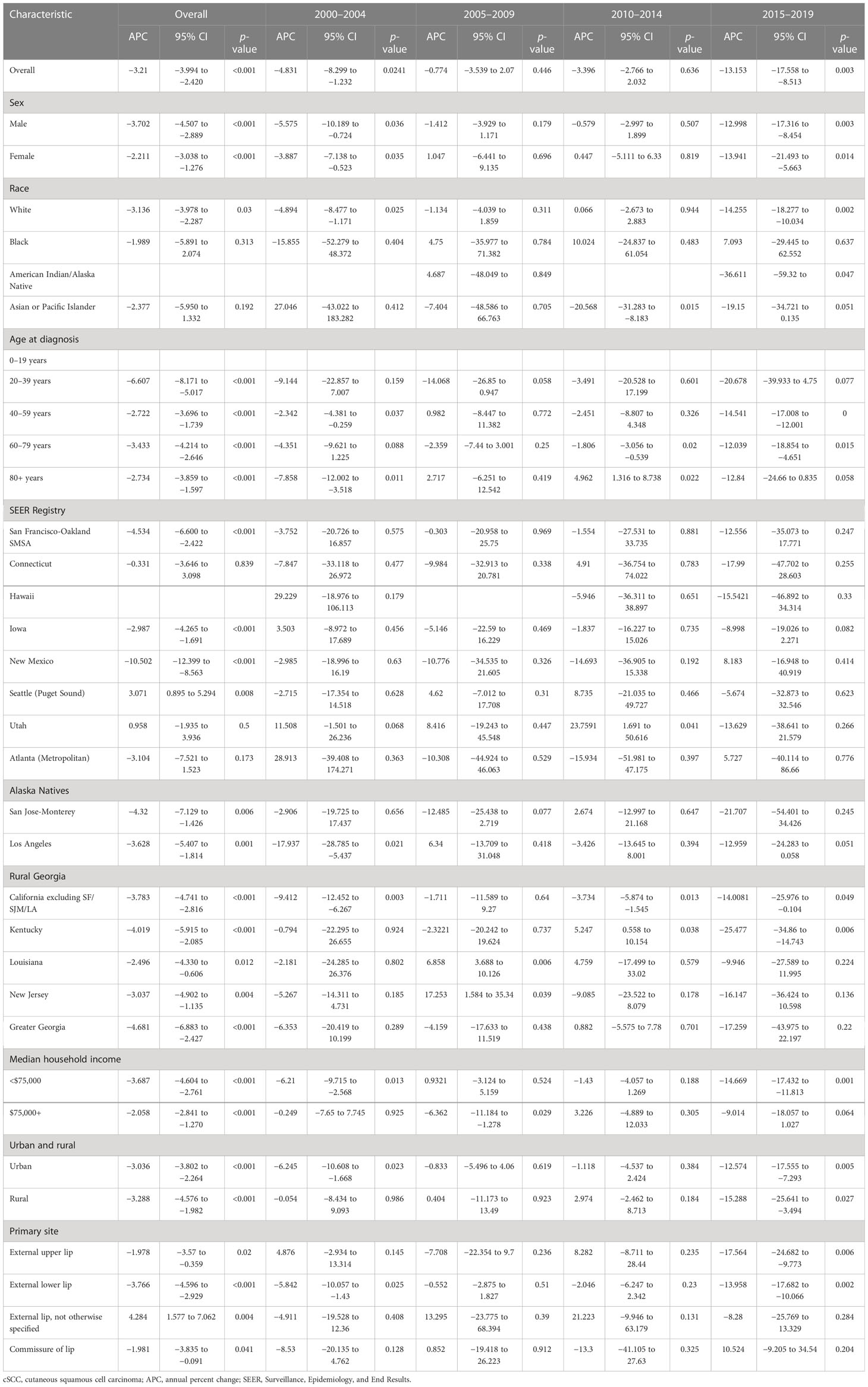
cSCC on the lip incidence rates decreased by −3.210% (95% CI, −3.994 to −2.420; p < 0.001) per year over the study period, with an incidence rate of 0.022 in 2000 and 0.294 in 2019 per 100,000 person-years (Table 2). Rates did not decrease significantly during 2005–2009 (APC = −0.774%, (95% CI, −3.539 to 2.07; p = 0.446)) and 2010–2014 (APC = −3.396% (95% CI, −2.766 to 2.032; p = 0.636)) but decreased by −13.153% (95% CI, −17.558 to −8.513; p = 0.003) per year during 2015–2019. The incidence of cSCC on the lips has been decreasing in all sex and ages (Figure 1). From 2000 to 2019, there was a significant downward trend in the incidence rates for high- or low-income households, urban or rural patients, and external lower lip patients (APC = −3.766% (95% CI, −4.596 to −2.929; p < 0.001)). Among low-income households, the incidence rate decreased significantly from 2000 to 2004 (APC = −6.210% (95% CI, −9.715 to −2.568; p = 0.013]) and among high-income households, from 2005 to 2009 (APC = −6.362% (95% CI, −11.184 to −1.278; p = 0.029)). In urban areas, there was a significant decrease in prevalence in 2000–2004 (APC = −6.245% (95% CI, −10.608 to −1.668; p = 0.023)) and 2015–2019 (APC = −12.574% (95% CI, −17.555 to −7.293; p = 0.005)); in rural areas in 2015–2019 (APC = −15.288% (95% CI, −25.641 to −3.494; p = 0.027)), the incidence rate also decreased significantly. The incidence has been decreasing in most subgroups of SEER registries except for Seattle (Puget Sound) and Utah. However, it has not changed recently among blacks and Asians or Pacific Islanders and decreased significantly among whites (APC = −3.136% (95% CI, −3.978 to −2.287; p = 0.030)).
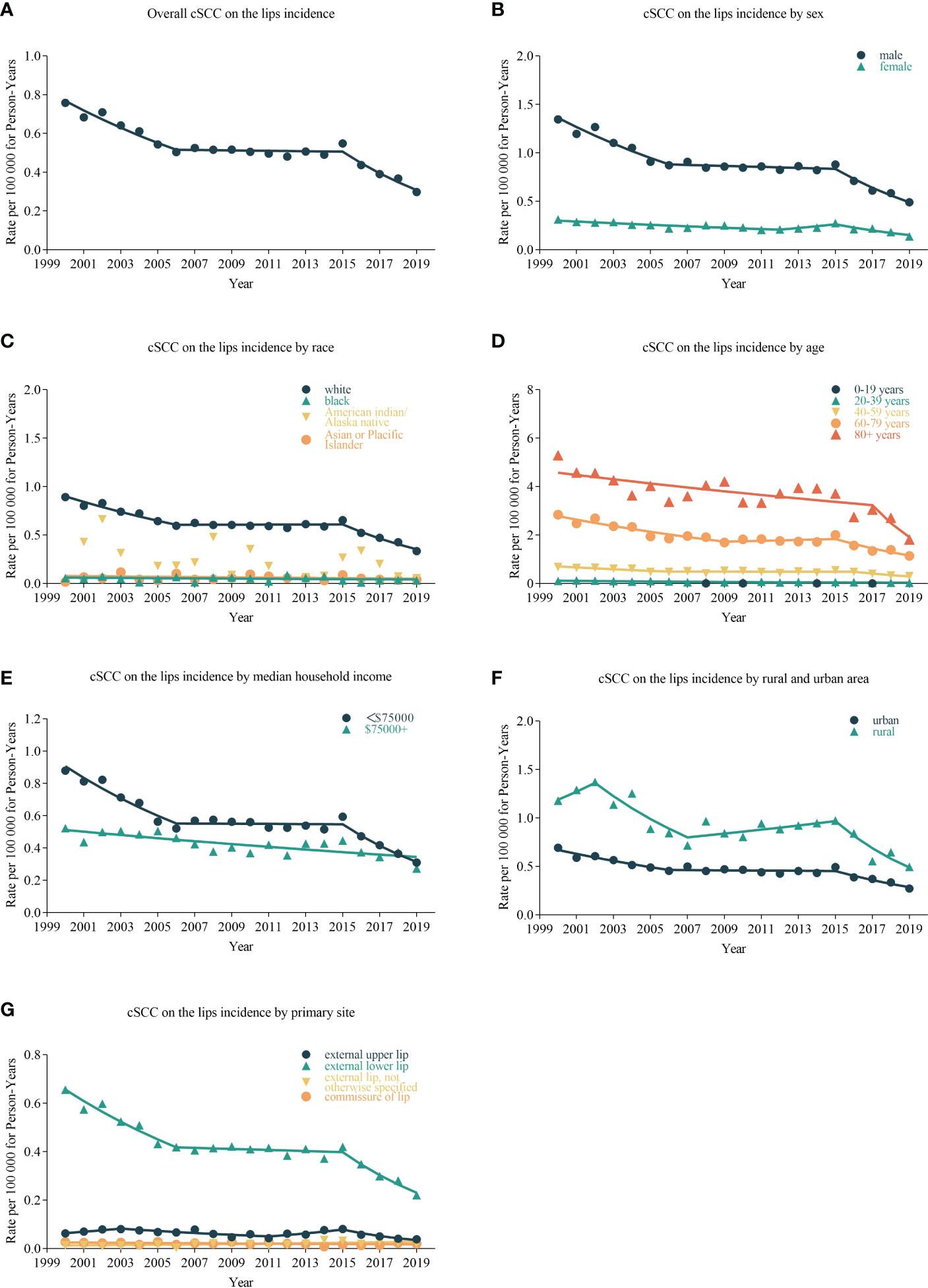
Figure 1 Trends in annual cSCC on the lip incidence rates. Data markers delegate observed incidence rates (per 100,000 person-years). The slope of the line indicates the annual percentage change (APC). (A) The overall incidence and trend of cSCC on the lips patients; the line graphs are classified into different groups, including sex (B), race (C), age (D), median household income (E), urban and rural area (F), and primary site (G).
3.3 Incidence-based mortality rates and trends over time
The overall incidence-based mortality rate of cSCC on the lips during 2000–2019 was 0.235 (95% CI, 0.227 to 0.242) per 100,000 person-years. cSCC on the lip incidence-based mortality rates were highest among men (0.442 (95% CI, 0.426 to 0.459%)), whites (0.276 (95% CI, 0.268 to 0.285)), people older than 80 years old (4.016 (95% CI, 3.851 to 4.186)), and external lower lip patients (0.189 (95% CI, 0.182 to 0.195)). When compared to states in the SEER-17 registries, incidence-based mortality was highest in Hawaii (0.832 (95% CI, 0.055 to 0.122)) and lowest in Alaska Natives (0 (95% CI, 0 to 0.219)).
cSCC on the lip incidence-based mortality increased by 4.975% (95% CI, 2.942 to 7.048; p < 0.001) per year over the study period (Table 3). cSCC on the lip incidence-based mortality rates increased for all sexes, races, and ages during the study period (Figure 2). High-income (APC = 4.782 [95% CI, 2.810–6.792, p < 0.001)), low-income (APC = 5.856 (95% CI, 3.471–8.296; p < 0.001)), urban (APC = 4.732 (95% CI, 2.663 to 6.843; p < 0.001)), rural (APC = 6.331 (95% CI, 3.877 to 8.842; p < 0.001]) patients, external upper lip (APC = 5.154 (95% CI, 2.362 to 8.022; p = 0.001)), and external lower lip (APC = 4.909 (95% CI, 2.911 to 6.945; p < 0.001)) showed a significant increase in mortality. In urban areas, there was a significant increase in morbidity-based mortality in 2000–2004 (APC = 37.693 (95% CI, 0.675 to 88.322; p = 0.047)) and a significant decrease in morbidity-based mortality in 2015–2019 (APC = −0.732 (95% CI, −1.244 to −0.218; p = 0.02)).
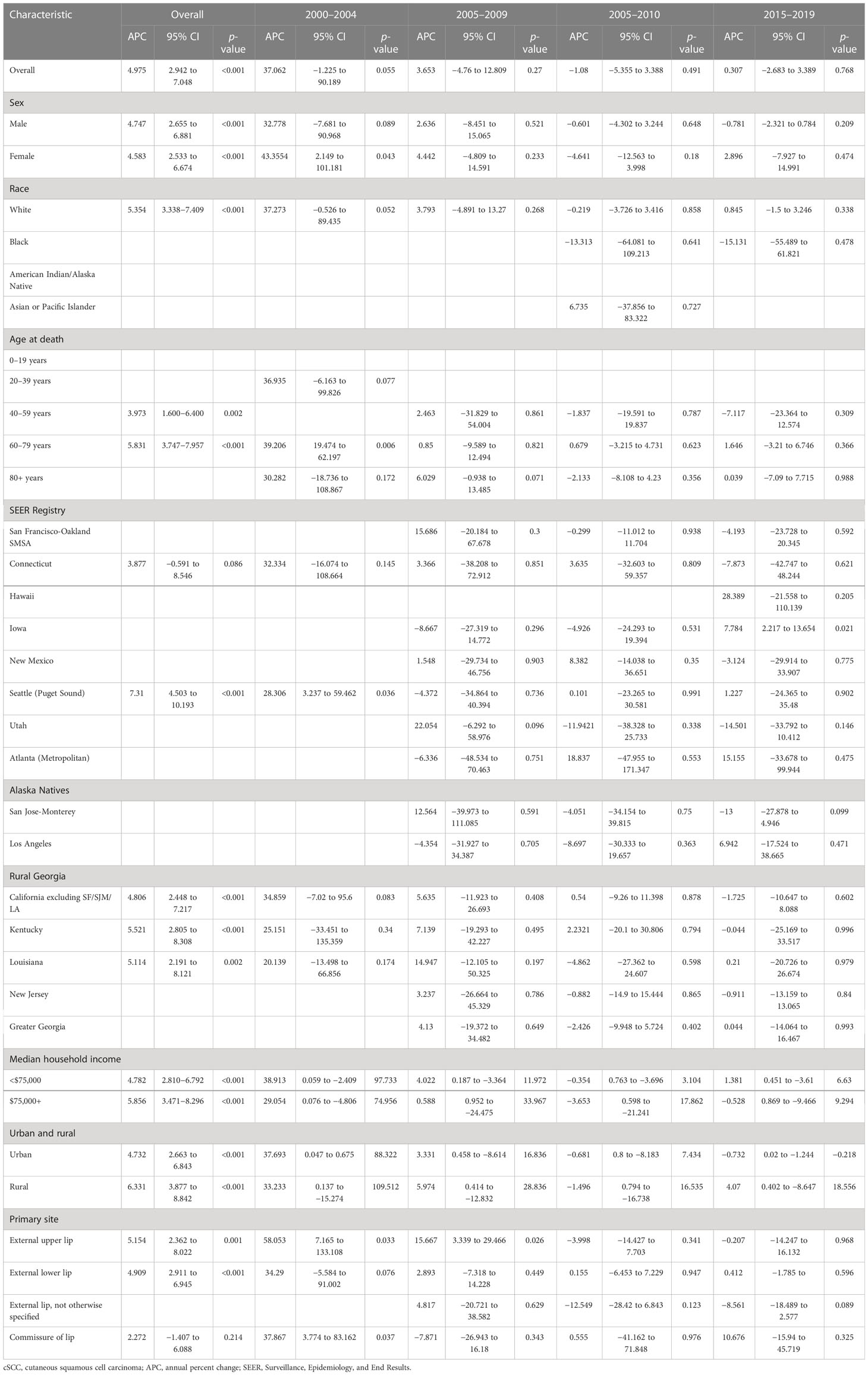
Table 3 Trends in cSCC on the lip incidence-based mortality rates (2000–2019): The SEER-17 registry database.
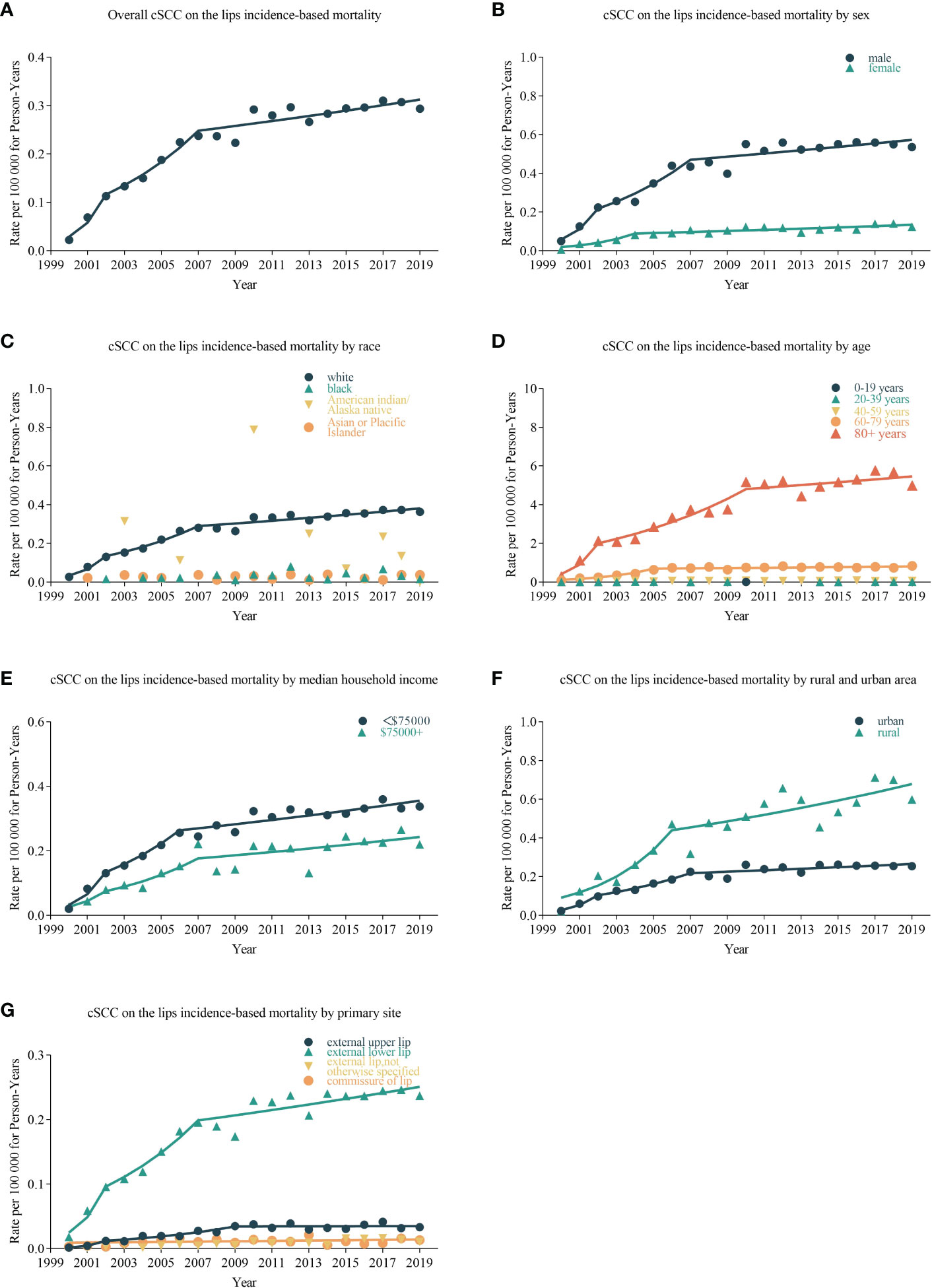
Figure 2 Trends in annual cSCC on the lip incidence-based mortality rates. Data markers represent observed incidence-based mortality rates (per 100,000 person-years). The slope of the line shows the annual percentage change (APC). (A) The overall incidence-based mortality and trend of cSCC on the lips patients; line graphs are divided into different groups, including sex (B), race (C), age (D), median household income (E), urban and rural area (F), and primary site (G).
4 Discussion
The lips are the anatomical junction of two distinct groups of cancers. Lip squamous cell carcinoma (lip SCC) is more aggressive than cSCC but less aggressive than oral mucosal squamous cell carcinoma (16). The external lower lip was the location with the highest risk of metastasis (17, 18). Therefore, this study was conducted to systematically analyze the trends in incidence and incidence-based mortality for cSCC on the lips using the SEER database.
Since the 1980s, researchers have demonstrated that UVR can cause skin damage and increase the risk of skin cancer. In 1992, the International Agency for Research on Cancer classified solar radiation as the first category of carcinogenic hazard, which is known to cause human cancer. In 2009, the World Health Organization classified UVR tanning devices as a Group I carcinogen based on evidence related to indoor tanning and an increased risk of skin cancer (19).
This study mainly found that the age-adjusted incidence rate of cSCC on the lips decreased significantly from 2000 to 2019, while the incidence-based mortality rate increased significantly. People with fair skin have less melanin and less protection against UVR (20–22). Consistent with the results of previous studies, cSCC on the lips was most common in white people (22) and less common in black people, Indians, and Asians (23, 24). It is more common in men than in women, with a male-to-female ratio of up to 4:1 (22, 25). The high incidence in men may be related to the fact that men were exposed to the sun for a relatively long time for working (26). Women have more knowledge of skincare and sun protection, use sunscreen lotion, and seek shade more frequently than men (27). The decrease in incidence in men may be due to the strengthening of sun protection education, the increase in the proportion of wearing sun protection clothing, and the increase in the number of people using sunscreen (28, 29).
The reduction of the incidence rate may also be influenced by other factors. Actinic keratosis (AK) is considered a precancerous lesion caused by prolonged exposure of the skin to UVR and is one of the most common causes of dermatologic treatment, which can progress to invasive cSCC (30, 31). Advanced age, fair skin, and prolonged sun exposure are major risk factors for AK (32, 33). The incidence of AK was increasing due to extended life expectancy and inappropriate sun exposure behaviors. Although the exact prevalence of AK is unknown, it affects 1%–44% of the adult population worldwide (32). There were more than 3,500 new cases of lip cancer in the USA, 90% of which were cSCC. A recent study showed that the prevalence of AC was 31.3% among people over 45 years old in northwestern Spain (34). The treatments for AK include cryotherapy, topical treatments with or without photodynamic therapy (PDT), and surgery and laser therapy which are less used (32). The actual definition of AK remains controversial; it is also defined as carcinoma in situ by some experts. AC (35) is a precursor of cSCC on the lips (36). Similar to AK, AC is a precancerous lesion caused by prolonged sun exposure or UVR that is most commonly on the lower lip along the vermilion border. Other risk factors include increasing age, especially over 60 years, working outdoors for more than 25 years, or a history of NMSC (37). The prevalence of AC is higher in people with fair skin and in areas with high UV exposure near the equator. Men have a higher incidence of AC than women, probably because they are exposed to relatively more sunlight and use less lip balm and cosmetics (38).
The improvement of awareness of precancerous lesions, early diagnosis abilities, and more timely treatment methods can cure precancerous lesions at an early stage and prevent precancerous lesions from developing into cSCC. The reduced incidence may be associated with this. Immunosuppression is a high-risk factor for cSCC, which may lead to the development of invasive cancer and the metastasis of cSCC (10–13, 39–42). Patients with kidney or heart transplantation are 65 times more likely to develop cSCC than general populations (41). In recent years, the use of immunomodulators in the treatment of autoimmune diseases, allergic diseases, and cancer has become popular (43–46). This may also be one of the factors leading to the increase in mortality. The increase in mortality may also be related to the aging of the population and the prolongation of the lifespan of patients with chronic diseases (47). cSCC on the lips is considered a high-risk skin cancer with a metastasis probability of 11%, compared to 1% for other body sites (36), making it critical to identify and appropriately manage these potentially malignant precursor lesions.
cSCC on the lips is prevalent in people over 40 years of age, with the highest incidence in 60–79 years, and incidence-based morbidity increases over 80 years old. The elderly were relatively vulnerable, and the 5-year disease-specific survival rate of patients aged over 75 years old, especially those over aged 85 years old, is relatively poor (23). A study has found that old age is not a risk factor for poorer survival in metastatic cSCC on the lips, which might be limited to metastatic cSCC on the lips disease (48). The trend analysis in this study reflected the high morbidity of both metastatic and nonmetastatic disease in elderly patients. There were more risk factors in elderly patients that may lead to high incidence and mortality in elderly patients (25). Elderly patients are more likely to suffer from tumors with large tumor diameters, low differentiation, deep tumor invasion, and more lymph node metastasis than young people. The risk of postoperative complications is high in elderly patients, with large postoperative defects and difficult wound healing. The poor mobility and weakness of the elderly make regular follow-up more difficult and the prognosis worse (25, 49).
Incidence and incidence-based mortality rates were higher among low-income and rural dwellers than among high-income and urban dwellers. Skin cancer was also a major economic burden in the USA (19). The cost of treating melanoma and NMSC was estimated at $1.7 billion per year (50, 51). The cost of lost productivity was estimated to be $3.8 billion (52). Prevention and early detection are effective ways to reduce the burden of disease and the cost of treatment (19, 53). A study conducted in Minnesota, USA, found that compared with urban dwellers, rural dwellers spent more time outdoors, used sun lotion less frequently, and had less awareness of sun protection (54). There were higher standards of education and income in urban dwellers than rural dwellers (42). Whites and people with high education levels know more about skin cancer and sunscreen, including using sunscreen, looking for shade, and wearing sunscreen clothes (27, 42, 55).
There are several limitations to this study. Some clinical data, such as tobacco and alcohol intake (39), staging and grading information, tumor depth (56), immunosuppression, HPV status, and family history of skin cancer, were lacking in the SEER database, thus limiting the ability of our results to exactly reflect the national patient population. Therefore, there may be a bias in the clinicopathological characteristics of tumors and specific risk factors for a poorer prognosis. The distribution of cases throughout race, age, and geographic regions is not equally represented. These restrictions should be kept in mind when using data collected through these registries. Nevertheless, the SEER database provides powerful statistical capabilities in evaluating the demographic characteristics of cSCC on the lips. Multicentered effort and additional risk factors are needed to further understand the incidence and incidence-based mortality of cSCC on the lips.
This study analyzed trends in incidence and incidence-based mortality in patients with cSCC on the lips from 2000 to 2019 and update and supplemented the epidemiological information on this type of cutaneous cancer. The incidence and incidence-based mortality in patients with cSCC on the lips were decreasing, but the mortality rate was increasing. The decrease in incidence might be related to the improvement of sun protection awareness with the increase in income and education levels, as well as timely diagnosis and treatment of precancerous lesions, thus preventing further deterioration of cSCC. The decline in men’s incidence was related to the improvement in male sunscreen protection awareness. The increased mortality may be related to the aging of the population and the use of immunosuppressive drugs that lead to the development of cSCC into invasive cancer.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
JZ designed the study. JZ and QY contributed to data analysis. JZ wrote the initial draft of the manuscript. JZ, QY, JW, RY, YL, XZ, XC, BW, and NZ reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Key R&D Program of China (2021YFA1101100), the National Natural Science Foundation of China (Grant No. 8217081123), and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16040400).
Acknowledgments
The authors would like to thank the SEER database for the availability of the data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fu T, Aasi SZ, Hollmig ST. Management of high-risk squamous cell carcinoma of the skin. Curr Treat Options Oncol (2016) 17(7):34. doi: 10.1007/s11864-016-0408-2
2. Goyal U, Prabhakar NK, Davuluri R, Morrison CM, Yi SK. Role of concurrent systemic therapy with adjuvant radiation therapy for locally advanced cutaneous head and neck squamous cell carcinoma. Cureus (2017) 9(10):e1784. doi: 10.7759/cureus.1784
3. Brantsch KD, Meisner C, Schönfisch B, Trilling B, Wehner-Caroli J, Röcken M, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: A prospective study. Lancet Oncol (2008) 9(8):713–20. doi: 10.1016/s1470-2045(08)70178-5
4. Kallini JR, Hamed N, Khachemoune A. Squamous cell carcinoma of the skin: Epidemiology, classification, management, and novel trends. Int J Dermatol (2015) 54(2):130–40. doi: 10.1111/ijd.12553
5. Stang A, Khil L, Kajüter H, Pandeya N, Schmults CD, Ruiz ES, et al. Incidence and mortality for cutaneous squamous cell carcinoma: Comparison across three continents. J Eur Acad Dermatol Venereol (2019) 33 Suppl 8(Suppl 8):6–10. doi: 10.1111/jdv.15967
6. Dessinioti C, Pitoulias M, Stratigos AJ. Epidemiology of advanced cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol (2022) 36(1):39–50. doi: 10.1111/jdv.17709
7. Lergenmuller S, Rueegg CS, Perrier F, Robsahm TE, Green AC, Lund E, et al. Lifetime sunburn trajectories and associated risks of cutaneous melanoma and squamous cell carcinoma among a cohort of Norwegian women. JAMA Dermatol (2022). doi: 10.1001/jamadermatol.2022.4053
8. Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am (2019) 33(1):1–12. doi: 10.1016/j.hoc.2018.08.001
9. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol (2018) 78(2):237–47. doi: 10.1016/j.jaad.2017.08.059
10. Plasmeijer EI, Sachse MM, Gebhardt C, Geusau A, Bouwes Bavinck JN. Cutaneous squamous cell carcinoma (Cscc) and immunosurveillance - the impact of immunosuppression on frequency of cscc. J Eur Acad Dermatol Venereol (2019) 33 Suppl 8:33–7. doi: 10.1111/jdv.16025
11. Elghouche AN, Pflum ZE, Schmalbach CE. Immunosuppression impact on head and neck cutaneous squamous cell carcinoma: A systematic review with meta-analysis. Otolaryngol Head Neck Surg (2019) 160(3):439–46. doi: 10.1177/0194599818808511
12. Adams CC, Thomas B, Bingham JL. Cutaneous squamous cell carcinoma with perineural invasion: A case report and review of the literature. Cutis (2014) 93(3):141–4.
13. Rieth GE, Kocharyan A, Tamaki A, Thuener J, Johnson F. Poorly differentiated plasmacytoid squamous cell carcinoma: Case report of a rare malignancy. Am J Otolaryngol (2019) 40(1):124–8. doi: 10.1016/j.amjoto.2018.10.005
14. Chu KC, Miller BA, Feuer EJ, Hankey BF. A method for partitioning cancer mortality trends by factors associated with diagnosis: An application to female breast cancer. J Clin Epidemiol (1994) 47(12):1451–61. doi: 10.1016/0895-4356(94)90089-2
15. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the united states, 1974-2013. JAMA (2017) 317(13):1338–48. doi: 10.1001/jama.2017.2719
16. Stratigos A, Garbe C, Lebbe C, Malvehy J, del Marmol V, Pehamberger H, et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer (2015) 51(14):1989–2007. doi: 10.1016/j.ejca.2015.06.110
17. Knuutila JS, Riihilä P, Kurki S, Nissinen L, Kähäri VM. Risk factors and prognosis for metastatic cutaneous squamous cell carcinoma: A cohort study. Acta Derm Venereol (2020) 100(16)::adv00266. doi: 10.2340/00015555-3628
18. Brancaccio G, Briatico G, Pellegrini C, Rocco T, Moscarella E, Fargnoli MC. Risk factors and diagnosis of advanced cutaneous squamous cell carcinoma. Dermatol Pract Concept (2021) 11(Suppl 2):e2021166S. doi: 10.5826/dpc.11S2a166S
19. El Ghissassi F, Baan R, Straif K, Grosse Y, Secretan B, Bouvard V, et al. A review of human carcinogens–part d: Radiation. Lancet Oncol (2009) 10(8):751–2. doi: 10.1016/s1470-2045(09)70213-x
20. Saewan N, Jimtaisong A. Natural products as photoprotection. J Cosmet Dermatol (2015) 14(1):47–63. doi: 10.1111/jocd.12123
21. Rex M, Salawitch RJ, von der Gathen P, Harris NRP, Chipperfield MP, Naujokat B. Arctic Ozone loss and climate change. Geophysical Res Lett (2004) 31(4). doi: 10.1029/2003GL018844
22. Han AY, Kuan EC, Mallen-St Clair J, Alonso JE, Arshi A, St John MA. Epidemiology of squamous cell carcinoma of the lip in the united states: A population-based cohort analysis. JAMA Otolaryngol Head Neck Surg (2016) 142(12):1216–23. doi: 10.1001/jamaoto.2016.3455
23. Mehta NK, Nguyen SA, Chang BA, Nathan CA. Trend analysis of cutaneous squamous cell carcinoma of the external lip from 1975 to 2016. JAMA Otolaryngol Head Neck Surg (2021) 147(7):624–31. doi: 10.1001/jamaoto.2021.0760
24. Yu JB, Gross CP, Wilson LD, Smith BD. Nci seer public-use data: Applications and limitations in oncology research. Oncol (Williston Park) (2009) 23(3):288–95.
25. Unsal AA, Unsal AB, Henn TE, Baredes S, Eloy JA. Cutaneous squamous cell carcinoma of the lip: A population-based analysis. Laryngoscope (2018) 128(1):84–90. doi: 10.1002/lary.26704
26. Leiter U, Garbe C. Epidemiology of melanoma and nonmelanoma skin cancer–the role of sunlight. Adv Exp Med Biol (2008) 624:89–103. doi: 10.1007/978-0-387-77574-6_8
27. Watson M, Holman DM, Fox KA, Guy GP Jr., Seidenberg AB, Sampson BP, et al. Preventing skin cancer through reduction of indoor tanning: Current evidence. Am J Prev Med (2013) 44(6):682–9. doi: 10.1016/j.amepre.2013.02.015
29. Green A, Williams G, Neale R, Hart V, Leslie D, Parsons P, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: A randomised controlled trial. Lancet (1999) 354(9180):723–9. doi: 10.1016/s0140-6736(98)12168-2
30. Gupta AK, Paquet M, Villanueva E, Brintnell W. Interventions for actinic keratoses. Cochrane Database Syst Rev (2012) 12(12):Cd004415. doi: 10.1002/14651858.CD004415.pub2
31. Balcere A, Konrāde-Jilmaza L, Pauliņa LA, Čēma I, Krūmiņa A. Clinical characteristics of actinic keratosis associated with the risk of progression to invasive squamous cell carcinoma: A systematic review. J Clin Med (2022) 11(19). doi: 10.3390/jcm11195899
32. Moretta G, Samela T, Sampogna F, Ricci F, Carlesimo F, Panebianco A, et al. Attitudes among dermatologists regarding actinic keratosis treatment options. Dermatol Rep (2022) 14(3):9392. doi: 10.4081/dr.2022.9392
33. Werner RN, Stockfleth E, Connolly SM, Correia O, Erdmann R, Foley P, et al. Evidence- and consensus-based (S3) guidelines for the treatment of actinic keratosis - international league of dermatological societies in cooperation with the European dermatology forum - short version. J Eur Acad Dermatol Venereol (2015) 29(11):2069–79. doi: 10.1111/jdv.13180
34. Rodríguez-Blanco I, Flórez Á, Paredes-Suárez C, Rodríguez-Lojo R, González-Vilas D, Ramírez-Santos A, et al. Actinic cheilitis prevalence and risk factors: A cross-sectional, multicentre study in a population aged 45 years and over in north-West Spain. Acta Derm Venereol (2018) 98(10):970–4. doi: 10.2340/00015555-3014
35. Muse ME, Crane JS. Actinic cheilitis. In: Statpearls. Treasure Island (FL: StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC (2022).
36. Barrado Solís N, Molés Poveda P, Lloret Ruiz C, Pont Sanjuan V, Velasco Pastor M, Quecedo Estébanez E, et al. Ingenol mebutate gel treatment for actinic cheilitis: Report of four cases. Dermatol Ther (2015) 28(2):79–82. doi: 10.1111/dth.12188
37. Vasilovici A, Ungureanu L, Grigore L, Cojocaru E, Şenilă S. Actinic cheilitis - from risk factors to therapy. Front Med (Lausanne) (2022) 9:805425. doi: 10.3389/fmed.2022.805425
38. Lucena IM, Santos IDS, Daroit NB, Salgueiro AP, Cavagni J, Haas AN, et al. Sun protection as a protective factor for actinic cheilitis: Cross-sectional population-based study. Oral Dis (2022) 28(7):1802–10. doi: 10.1111/odi.13837
39. Bota JP, Lyons AB, Carroll BT. Squamous cell carcinoma of the lip-a review of squamous cell carcinogenesis of the mucosal and cutaneous junction. Dermatol Surg (2017) 43(4):494–506. doi: 10.1097/dss.0000000000001020
40. Manyam BV, Garsa AA, Chin RI, Reddy CA, Gastman B, Thorstad W, et al. A multi-institutional comparison of outcomes of immunosuppressed and immunocompetent patients treated with surgery and radiation therapy for cutaneous squamous cell carcinoma of the head and neck. Cancer (2017) 123(11):2054–60. doi: 10.1002/cncr.30601
41. Jensen P, Hansen S, Møller B, Leivestad T, Pfeffer P, Geiran O, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol (1999) 40(2 Pt 1):177–86. doi: 10.1016/s0190-9622(99)70185-4
42. Allanson BM, Low TH, Clark JR, Gupta R. Squamous cell carcinoma of the external auditory canal and temporal bone: An update. Head Neck Pathol (2018) 12(3):407–18. doi: 10.1007/s12105-018-0908-4
43. Yang Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J Clin Invest (2015) 125(9):3335–7. doi: 10.1172/jci83871
44. Dobler CC. Biologic agents and tuberculosis. Microbiol Spectr (2016) 4(6). doi: 10.1128/microbiolspec.TNMI7-0026-2016
45. Nardone B, Orrell KA, Vakharia PP, West DP. Skin cancer associated with commonly prescribed drugs: Tumor necrosis factor alpha inhibitors (Tnf-Ais), angiotensin-receptor blockers (Arbs), phosphodiesterase type 5 inhibitors (Pde5is) and statins -weighing the evidence. Expert Opin Drug Saf (2018) 17(2):139–47. doi: 10.1080/14740338.2018.1400530
46. Fortina AB, Piaserico S, Caforio AL, Abeni D, Alaibac M, Angelini A, et al. Immunosuppressive level and other risk factors for basal cell carcinoma and squamous cell carcinoma in heart transplant recipients. Arch Dermatol (2004) 140(9):1079–85. doi: 10.1001/archderm.140.9.1079
47. Rodríguez-Blanco I, Flórez Á, Paredes-Suárez C, Rodríguez-Lojo R, González-Vilas D, Ramírez-Santos A, et al. Actinic cheilitis: Analysis of clinical subtypes, risk factors and associated signs of actinic damage. Acta Derm Venereol (2019) 99(10):931–2. doi: 10.2340/00015555-3237
48. Smith JA, Virk S, Palme CE, Low TH, Ch'ng S, Gupta R, et al. Age is not a predictor of prognosis in metastatic cutaneous squamous cell carcinoma of the head and neck. ANZ J Surg (2018) 88(4):E273–e7. doi: 10.1111/ans.13757
49. Moreno-Ramírez D, Silva-Clavería F, Fernández-Orland A, Eiris N, Ruiz de Casas A, Férrandiz L. Surgery for cutaneous squamous cell carcinoma and its limits in advanced disease. Dermatol Pract Concept (2021) 11(Suppl 2):e2021167S. doi: 10.5826/dpc.11S2a167S
50. Guy GP, Ekwueme DU. Years of potential life lost and indirect costs of melanoma and non-melanoma skin cancer: A systematic review of the literature. Pharmacoeconomics (2011) 29(10):863–74. doi: 10.2165/11589300-000000000-00000
51. Guy GP Jr, Ekwueme DU, Tangka FK, Richardson LC. Melanoma treatment costs: A systematic review of the literature, 1990-2011. Am J Prev Med (2012) 43(5):537–45. doi: 10.1016/j.amepre.2012.07.031
52. Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E, et al. The burden of skin diseases: 2004 a joint project of the American academy of dermatology association and the society for investigative dermatology. J Am Acad Dermatol (2006) 55(3):490–500. doi: 10.1016/j.jaad.2006.05.048
53. Grossman DC, Curry SJ, Owens DK, Barry MJ, Caughey AB, Davidson KW, et al. Behavioral counseling to prevent skin cancer: Us preventive services task force recommendation statement. Jama (2018) 319(11):1134–42. doi: 10.1001/jama.2018.1623
54. Vogel RI, Jewett PI, Ahmed RL, Lazovich D. Comparison of sun exposure and protection behaviors between urban and rural residents without a history of melanoma in the Midwestern united states. J Am Acad Dermatol (2022) 86(1):229–32. doi: 10.1016/j.jaad.2021.01.095
55. Demko CA, Borawski EA, Debanne SM, Cooper KD, Stange KC. Use of indoor tanning facilities by white adolescents in the united states. Arch Pediatr Adolesc Med (2003) 157(9):854–60. doi: 10.1001/archpedi.157.9.854
Keywords: cutaneous squamous cell carcinoma (cSCC), cSCC on the lips, annual percent changes (APC), incidence, incidence-based mortality
Citation: Zhang J, Yang Q, Wu J, Yuan R, Zhao X, Li Y, Cheng X, Wu B and Zhu N (2023) Trends in cutaneous squamous cell carcinoma on the lip incidence and mortality in the United States, 2000–2019. Front. Oncol. 13:1111907. doi: 10.3389/fonc.2023.1111907
Received: 30 November 2022; Accepted: 13 March 2023;
Published: 17 April 2023.
Edited by:
Ravi Prakash Sahu, Wright State University, United StatesReviewed by:
Florentia Dimitriou, University Hospital Zürich, SwitzerlandMojgan Alaeddini, Tehran University of Medical Sciences, Iran
Copyright © 2023 Zhang, Yang, Wu, Yuan, Zhao, Li, Cheng, Wu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ningwen Zhu, emh1bmluZ3dlbkBmdWRhbi5lZHUuY24=
 Jin Zhang
Jin Zhang Quyang Yang1
Quyang Yang1 Jinyan Wu
Jinyan Wu Baojin Wu
Baojin Wu