- 1Department of Hepatobiliary Surgery, Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College, Hangzhou, China
- 2Affiliated Mental Health Centre & Hangzhou Seventh People’s Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
- 3School of Medicine, Zhejiang Chinese Medical University, Zhejiang Shuren College, Hangzhou, China
- 4Jinan Microecological Biomedicine Shandong Laboratory, Jinan, China
Background: Hepatocellular carcinoma (HCC) is a primary malignant tumor responsible for approximately 90% of all liver cancers in humans, making it one of the leading public health problems worldwide. The gut microbiota is a complex microbial ecosystem that can influence tumor formation, metastasis, and resistance to treatment. Therefore, understanding the potential mechanisms of gut microbiota pathogenesis is critical for the prevention and treatment of HCC.
Materials and methods: A search was conducted in the Web of Science Core Collection (WoSCC) database for English literature studies on the relationship between gut microbiota and HCC from 2011 to 2022. Bibliometric analysis tools such as VOSviewer, CiteSpace, and R Studio were used to analyze global trends and research hotspots in this field.
Results: A total of 739 eligible publications, comprising of 383 articles and 356 reviews, were analyzed. Over the past 11 years, there has been a rapid increase in the annual number of publications and average citation levels, especially in the last five years. The majority of published articles on this topic originated from China (n=257, 34.78%), followed by the United States of America (n=203, 27.47%), and Italy (n=85, 11.50%). American scholars demonstrated high productivity, prominence, and academic environment influence in the research of this subject. Furthermore, the University of California, San Diego published the most papers (n=24) and had the highest average citation value (value=152.17) in the study of the relationship between gut microbiota and HCC. Schnabl B from the USA and Ohtani N from Japan were the authors with the highest number of publications and average citation value, respectively.
Conclusion: In recent years, research on the gut microbiota’s role in HCC has made rapid progress. Through a review of published literature, it has been found that the gut microbiota is crucial in the pathogenesis of HCC and in oncotherapy.
Introduction
Liver cancer is a common digestive malignant cancer worldwide (1). According to accumulated data, every year approximately 841,000 new cases of liver cancer are diagnosed and nearly 1 million people die from diseases related to liver cancer (1, 2). Hepatocellular carcinoma (HCC) with high fatality rate is the most prevalent clinicopathological type of liver cancer (3). Most patients with HCC in China are at intermediate-advanced stages when diagnosed, in which effective treatment strategies are limited and with negative results, with an extremely poor prognosis (4). Therefore, uncovering the potential biological mechanisms of hepatocellular carcinogenesis may be useful for cancer comprehensive therapy and is urgently required.
In recent years, several studies have focused on the connection between intestinal flora and diseases (5). Regulating the intestinal flora and improving gut barrier function has gradually become a novel and exciting method for the preventing and curing some diseases (6). As the hepatic portal vein and bile secretion systems directly links the liver to the gut, this association might be relevant to find specific associations between liver diseases and intestinal flora. Hepatocytes are unremittingly exposed to a wide range of intestinal flora and microbe-associated molecular patterns that influence the stability of hepatic microenvironments, thereby driving the initiation and progression of liver disease, including nonalcoholic fatty liver disease (NAFLD) (7). It has been previously established that intestinal microbiota depletion by administration of broad-spectrum antibiotics suppress experimental nonalcoholic steatohepatitis (NASH), which is one of common causes of HCC in developed countries (8, 9). In contrast, the regulation of distinct intestinal flora can influence anti-tumor immunotherapy responses, including HCC (9). Also, the dynamic gut microbiota variation characteristics may be exploited as a potent biomarker to early predictions immunotherapy outcomes in HCC, which is critical for HCC surveillance and cancer immunotherapy decision-making (9). So, it is crucial to explore a relationship between gut microbiota and hepatocarcinogenesis that is beneficial to understand both the molecular mechanisms of tumor formation and tumor treatment.
Currently, numerous scientific methods are used to systematically understand the current status of an academic field, of which bibliometric analysis using mathematical and statistical methods has become the most prevalent tool to acquire overall knowledge structure and research priorities in an academic domain (10, 11). In recent years, with the deepening of intestinal flora in tumorigenesis and cancer treatment, studies have revealed that the unbalance of intestinal flora is closely correlated to HCC (5, 9). Although the research on the intestinal flora were previously explored in HCC based on numerous experiments, no systematic analysis of global research trends in this specific academic fields. Bibliometrics can not only describe the current research status but also assess research prospects and predict new tendencies in current academic research, which cannot be achieved using other methods including meta-analyses, conventional reviews or experimental studies. Hence, in the present study, we first used bibliometric methods to analyze the current status and future research directions of the intestinal flora research in HCC domain.
Materials and methods
Ethics statement
This study was based on published papers from January 01, 2011 to October 26, 2022 available in the public domain and did not involve human or animal studies or experiments. So, this research did not require ethical approval.
Data source and retrieval strategies
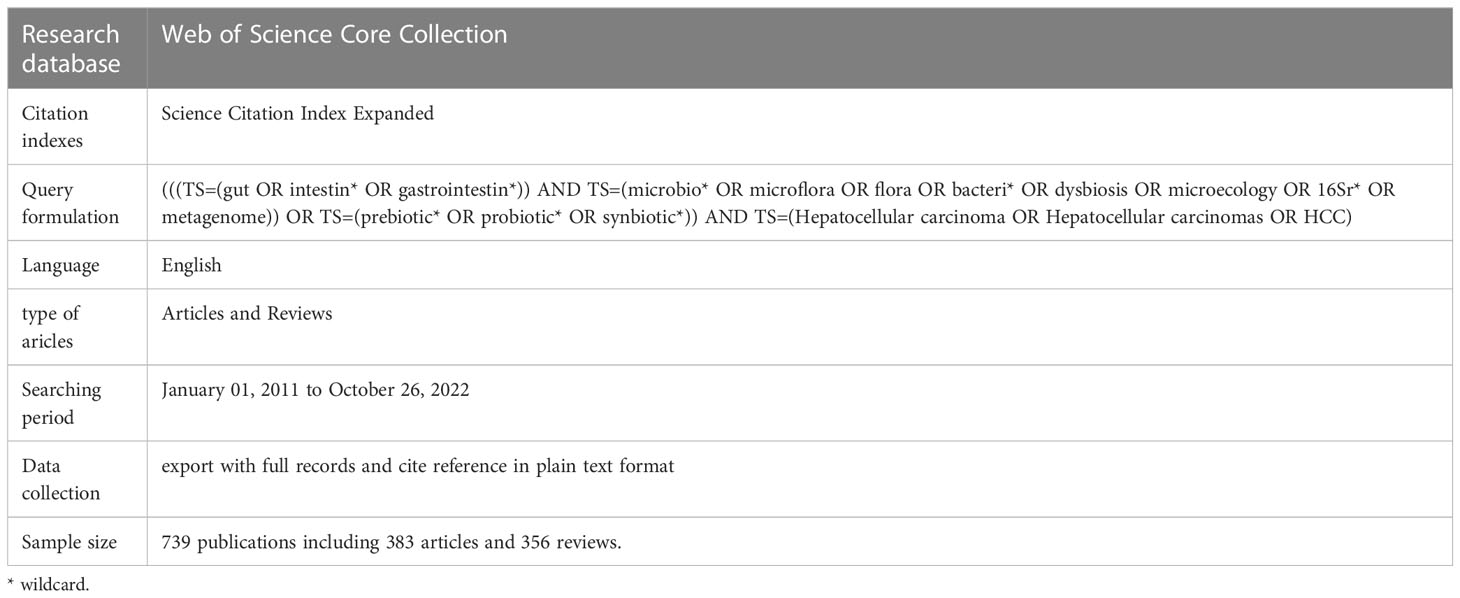
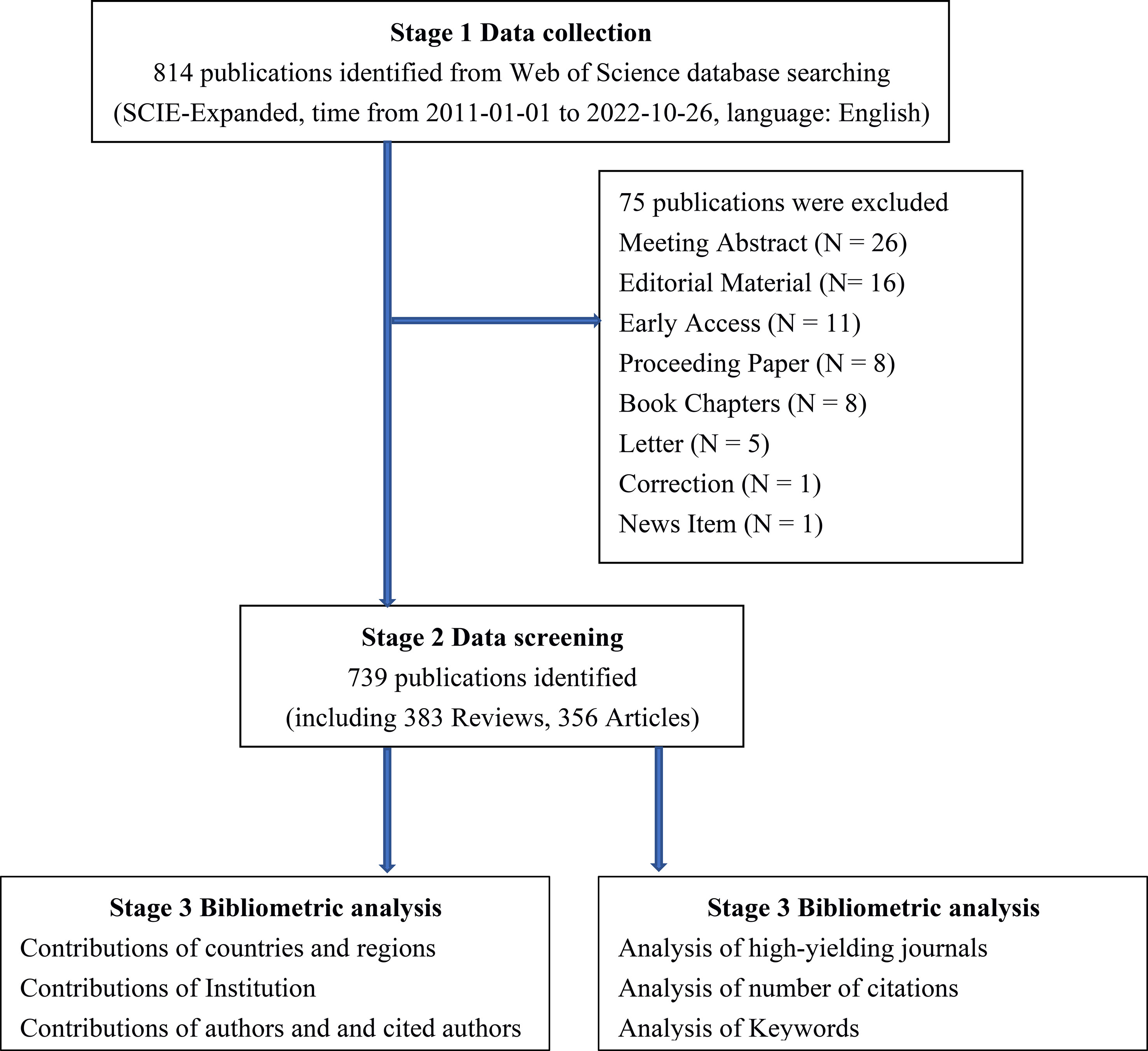
We conducted a comprehensive literature search for all included studies of gut microbiota in HCC in Web of Science Core Collection (WoSCC, https://www.webofscience.com/) database. The search was independently searched and extracted by coauthors (ZC and CD) and excluded those that did not fit according to the following inclusion and exclusion criteria: (1) The publications were from the WoSCC Science Citation Index Expanded (SCI-E) databases. (2) Language restriction for the publication is in English. (3) The types of publications are restricted to original articles and reviews. (4) Excluding other types of publications, including meeting abstract, editorial material, early access, proceeding paper, book chapters, letter, correction, news item. (5) The retrieval time range is from January 01, 2011 to October 26, 2022. (6) The subjects of the publication were HCC patients, HCC animal models, and HCC cellular models, and the studies must also assess the correlation between subjects and gut microbiota. The detailed WoSCC database search strategy is summarized in Table 1. Finally, 739 eligible publications, including 383 articles and 356 reviews, were included in the subsequent analysis (Figure 1).
Data analyses and visualization
The WoSCC intrinsic toolkits were used to analyze general characteristics of eligible literatures, including the list of Web of Science subject categories, the number of annual publications, citations and the h-index. Data analysis and visualization, the mainstream bibliometric analysis tools include CiteSpace (12), VOSviewer (11), R software, SCI2, and HistCite. There is no consensus as to which approach of bibliometric analysis is the best. Therefore, the respective properties and advantages for these tools were combined together, we used VOSviewer, R software and CiteSpace at the same time for next analysis. VOSviewer (11) (Leiden University, Leiden, The Netherlands) is a powerful software for bibliometric network construction and visualization based on publications, countries, authors, journals and keywords. And the burst keywords for forecasting the possible hotspots was explored via CiteSpace (10) (Drexel University, Philadelphia, PA, USA). Meanwhile, the R package bibliometrix was used to output the collaboration map of countries in gut microbiota and HCC.
Results
Annual tendencies and annual citations
According to the search strategy (Table 1), a total of 814 eligible publications were collected from the time of January 01, 2011 to October 26, 2022 in WoSCC database. Subsequently, 75 literatures were extracted, including meeting abstract (N = 26), editorial material (N= 16), early access (N = 11), proceeding paper (N = 8), book chapters (N = 8), letter (N = 5), correction (N = 1), news item (N = 1). Finally, 739 eligible publications, including 383 articles and 356 reviews, were included in the subsequent analysis (Figure 1). The research on gut microbiota in HCC has continuously and steadily increased over the past 10 years, with annual growth rate 22.67% (Figure 2A). The number of published papers and the corresponding citations have exponentially increased year by year, and the number of both in 2021 was the largest (157 publications and 7215 citations), indicating that gut microbiota has gradually gained considerable attention in the field of HCC research (Figure 2A). In the field of gut microbiota in HCC research, the China and United States of America (USA) published the most research results (Figure 2B). As of October 26, 2022, the 739 publications were cited 28,227 times which is an average 38.2 citations per publication. Meanwhile, we found that these publications in “gut microbiota and HCC” domains cited 46,843 expanded literature from 4,640 different journals.
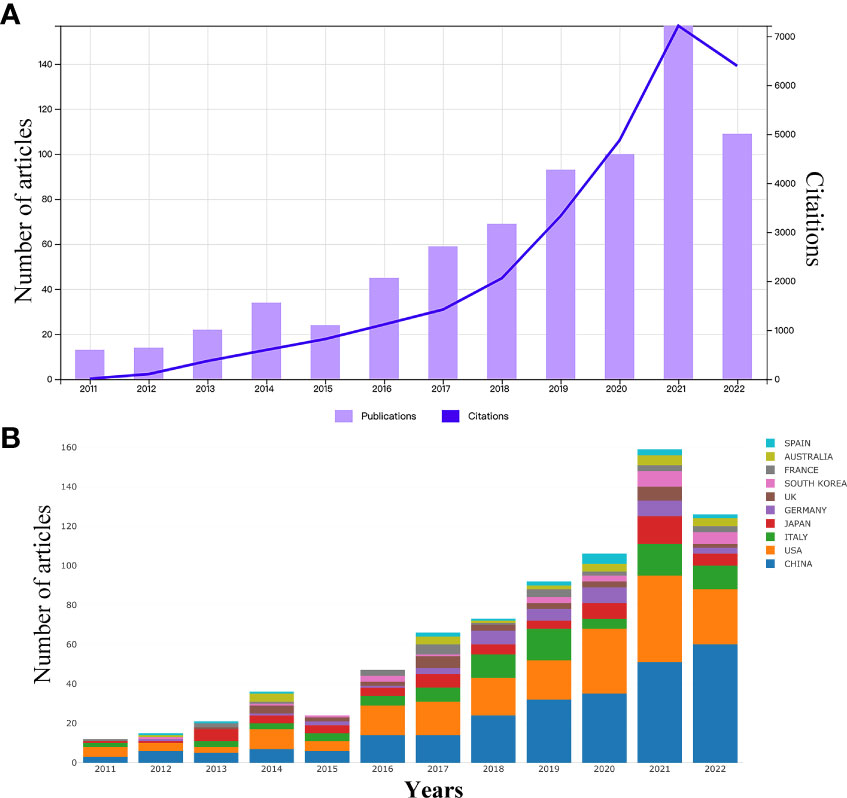
Figure 2 (A) The number of annual research publications and growth trends on the links of gut microbiota and HCC from 2011 to 2022. (B) Trends in the proportion of annual research publications in different countries.
Distribution of countries/regions
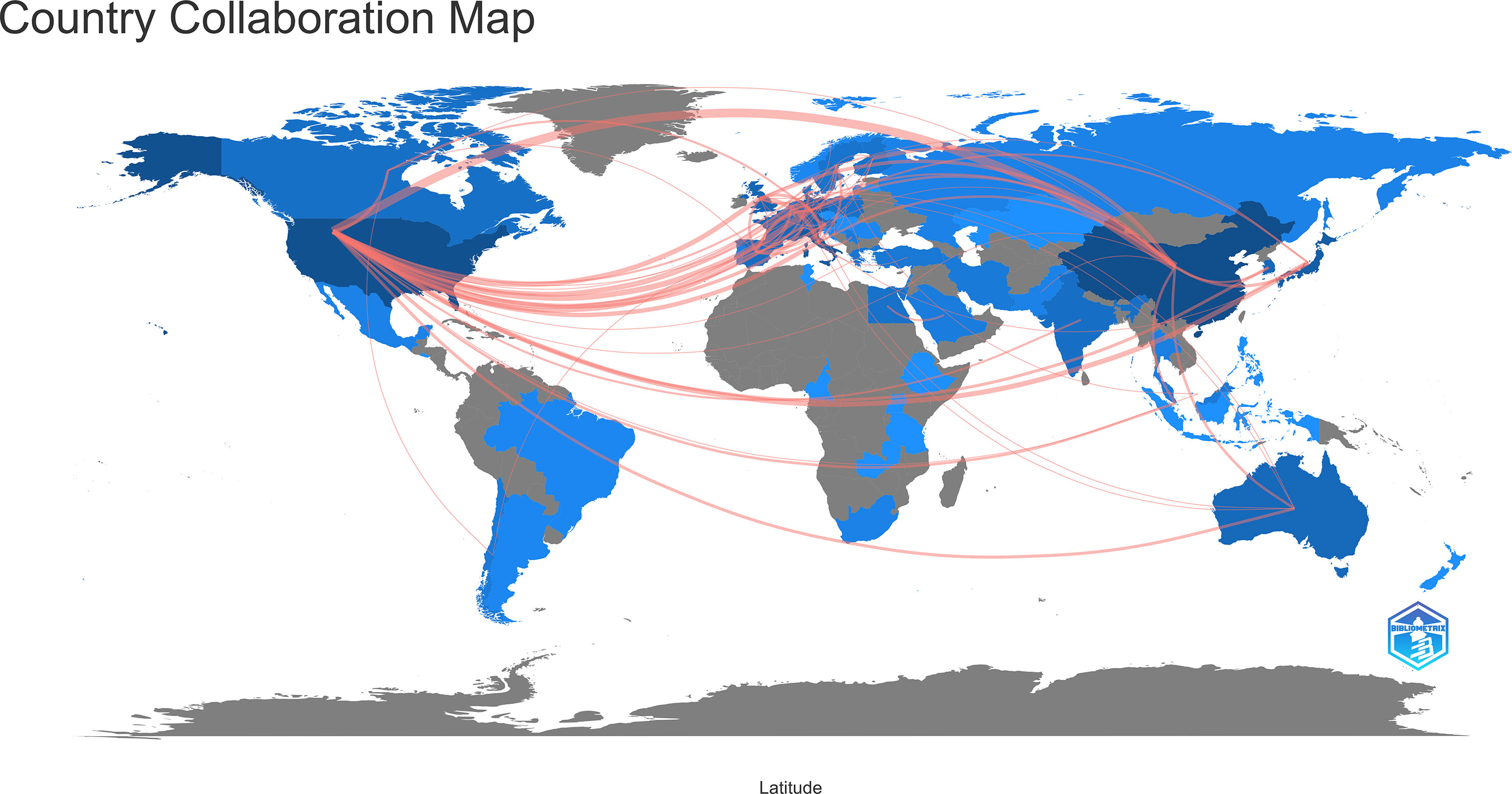
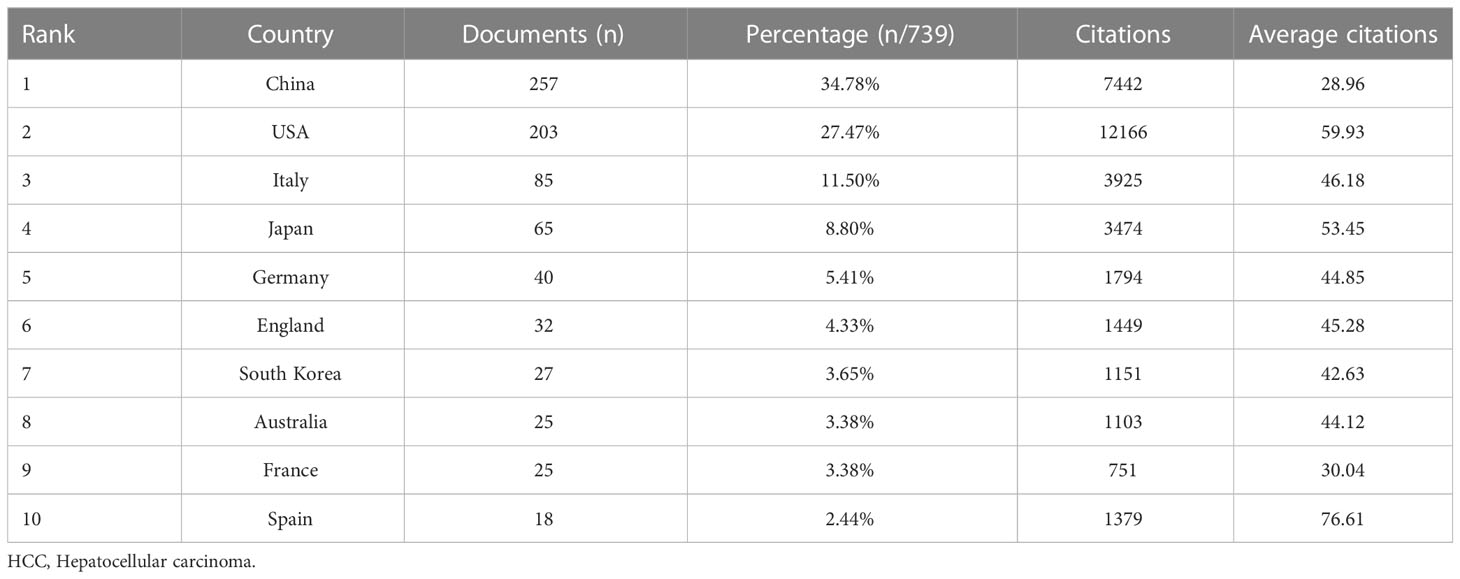
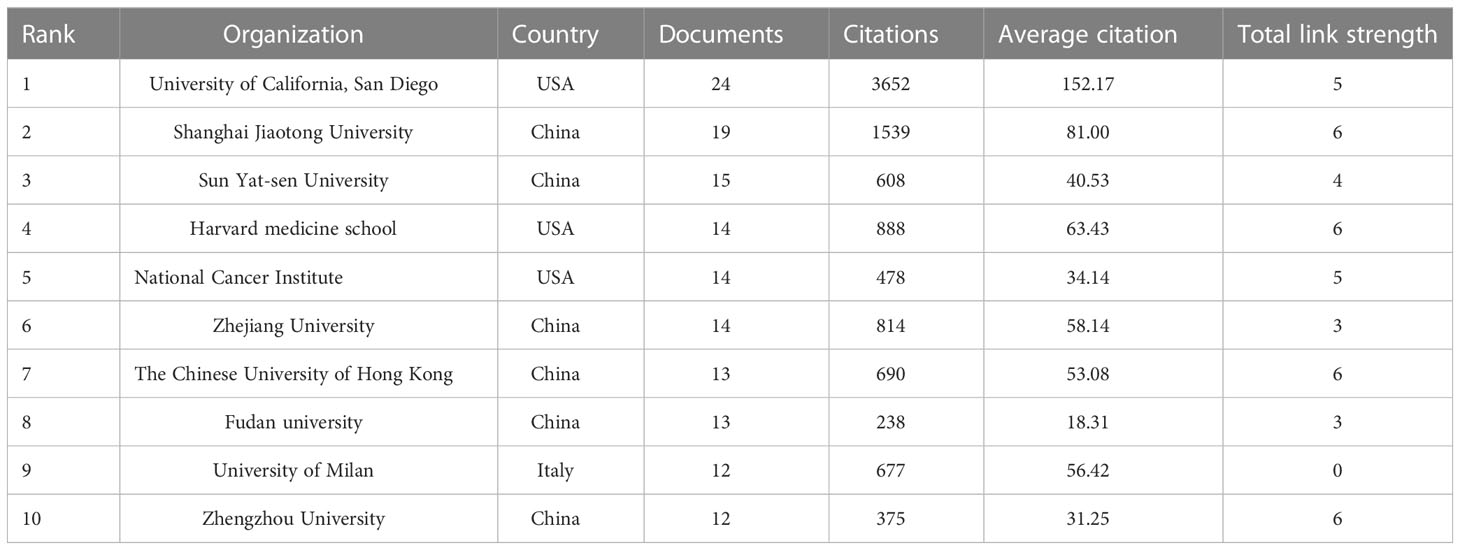
In the past 11 years, a total of 1,155 research institutions from 64 countries have published gut microbiota and HCC related publications. We found that these articles were from 334 journals and 4296 authors. Information from the published articles was used to create a world map illustrating the cooperative relationship of the research topic in different countries (Figure 3). The countries were ranked in relation to number of published articles, and only the top ten countries are presented. According to the Table 2, we found that the majority of published articles regarding this topic have their origin in the China (n = 257, 34.78%), followed by United States of America (n = 203, 27.47%), and Italy (n = 85, 11.50%). Looking at number of published papers, these three countries have made indelible contributions in the gut microbiota and HCC field. Based on the number of publications by researcher, one thing worth noting is that USA has the second number of publications in this field and the highest average citation score, indicating that research institutions in USA is important research forces in this field. Although China has the highest number of publications, but its average citation score is 28.96. Meanwhile, Figures 4, 5 present the collaboration map of countries in gut microbiota and HCC. The result shows that China largely cooperated with USA, England, Australia, Italy, and Japan. The result also reveals that scientific research respects no geographical boundaries, so international collaboration in conducting studies in the field will be important.
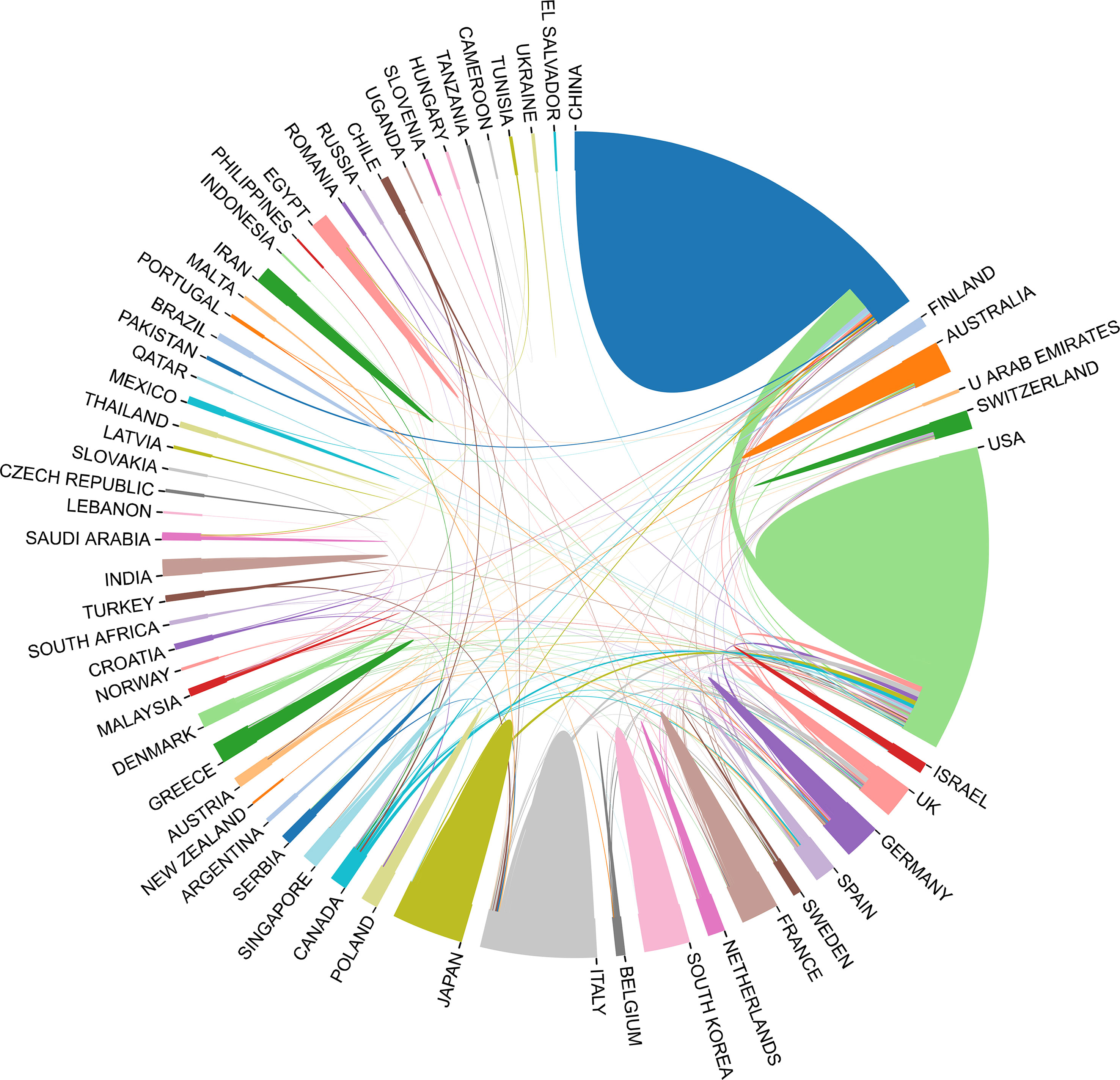
Figure 4 Cooperative relationships between different countries on the connection of gut microbiota and HCC generated with R software.
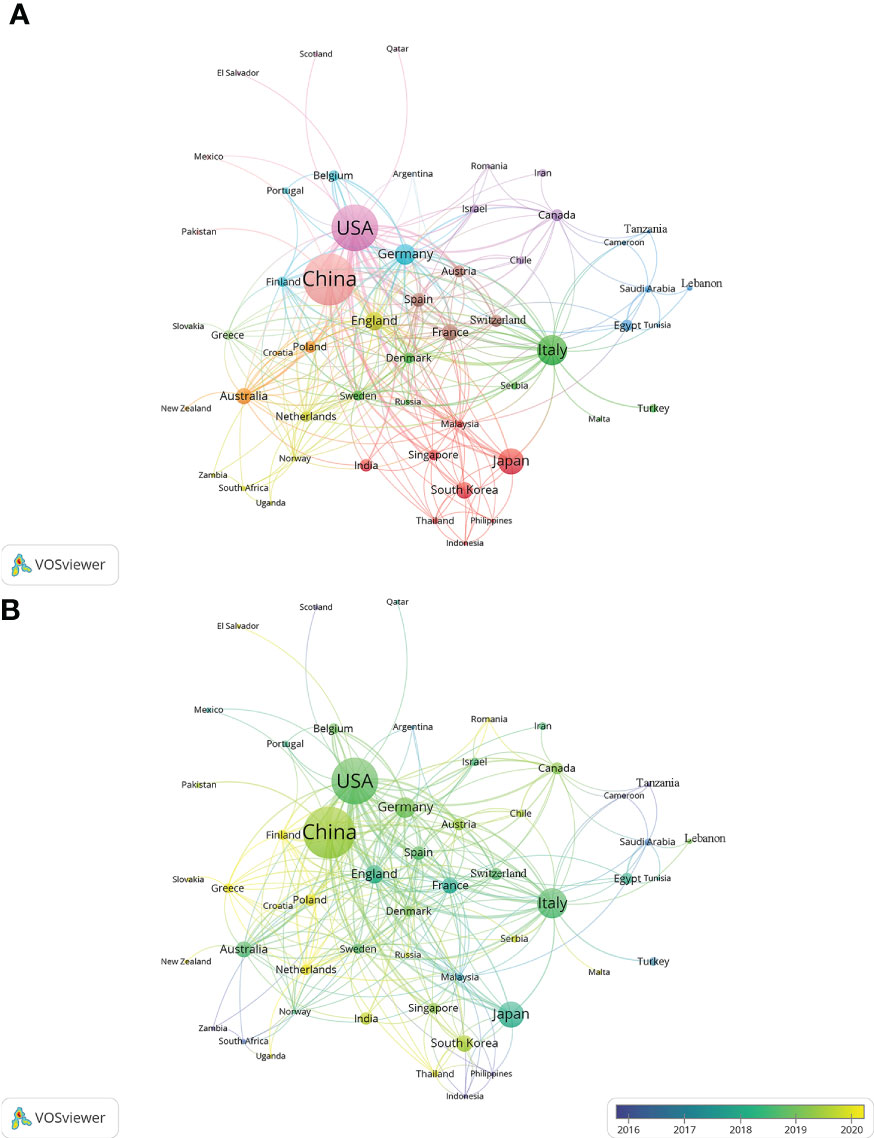
Figure 5 Cooperative relationships between different countries on the connection of gut microbiota and HCC generated with VOSviewer. (A) The network of different countries visualization. Each node represents one country. Circle size is based on the number of publications. Connecting lines represent collaboration between countries. Different colors represent different items. (B) Present the distribution of countries according to the average time of occurrence. Green and blue circles indicate more past publications, and yellow circles indicate more recent publications.
Distribution of institutions
VOSviewer was used to analyze and visualize the 1,155 research institutions that contributed to the field. Publications (the minimum number of documents used by an organization was defined as more than 5) were identified in the 71 institutions and visualized using VOSviewer (Figure 6). Table 3 shows the top ten contributing research institutions. There is a close cooperative relationship among these institutions. In the past 11 years, more than half of the top 10 research institutions from China (6/10), followed by the USA (3/10). The results indicating that institutions in China and USA have made outstanding contributions in gut microbiota and HCC field. Additionally, University of California, San Diego published the most papers (n = 24) and has the highest average citation value (value = 152.17), followed by Shanghai Jiaotong University with 19 publications and second average citation (value = 81.00). The low junction of institutions network indicates the lack of inter-agency collaboration and further emphasizes the importance of collaboration.
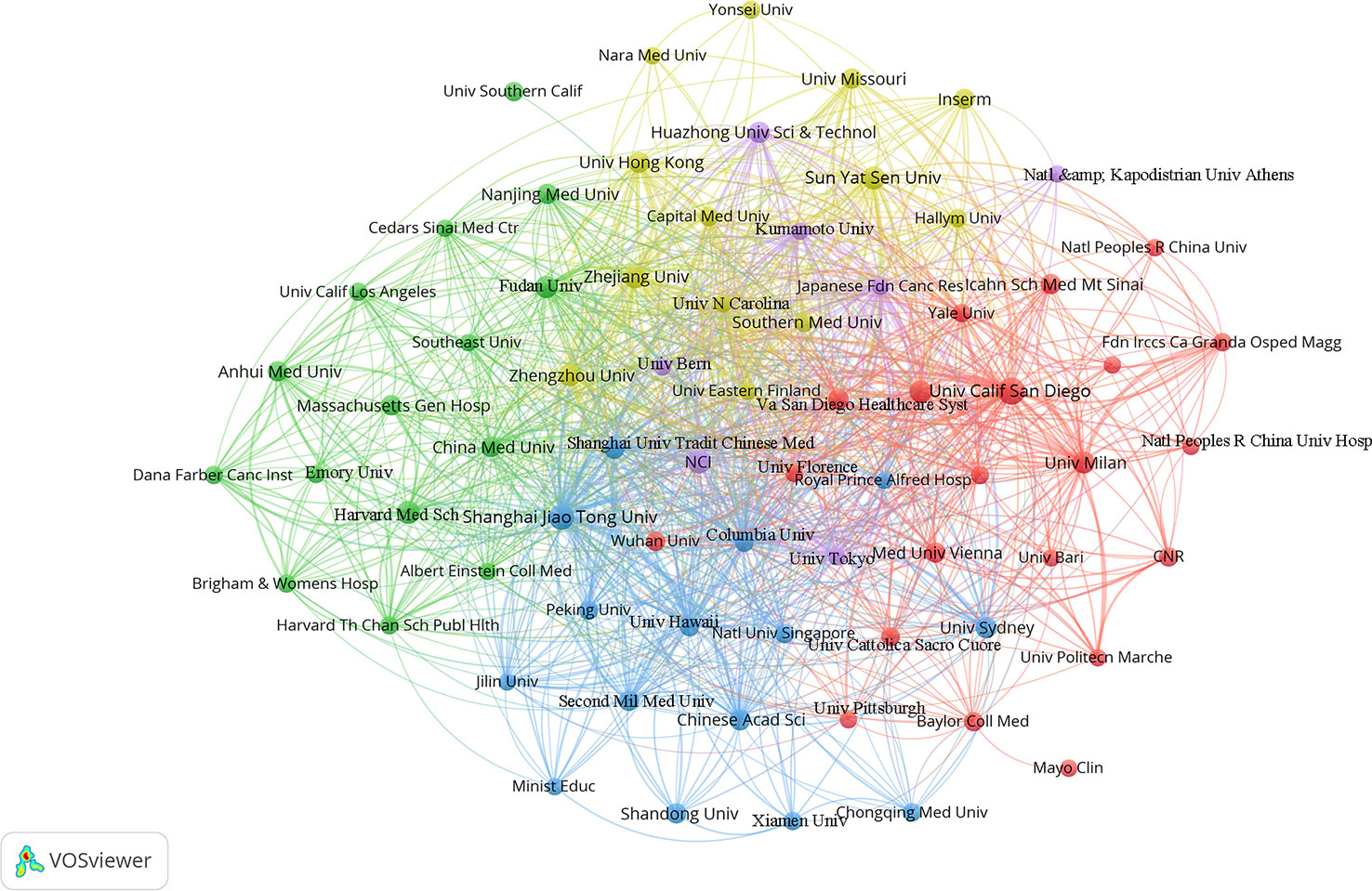
Figure 6 The co-authorship network visualization map of institutions. Each node represents one country. Circle size is based on the number of publications. Connecting lines represent collaboration between institutions, and the same color of node represent the same cluster.
Contributions of authors and cited authors
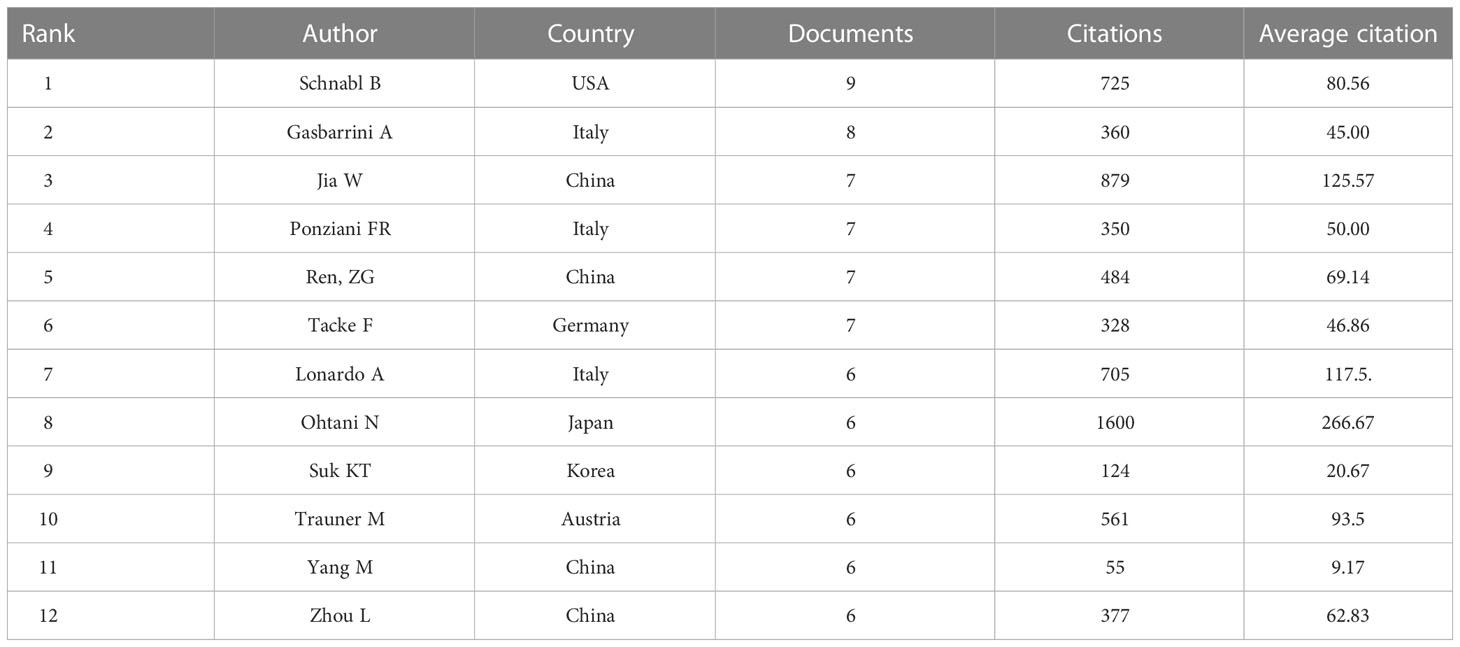
Authors (n = 27) with a minimum productivity of 5 publications were visualized using VOSviewer software and showed in Figure 7A. Meanwhile, the collaboration relationship among these authors were displayed. The top 12 authors with the most active published articles also were listed (Table 4), including Schnabl B (9 articles, 725 citations), Gasbarrini A(8 articles, 360 citations), Jia W (7 articles, 879 citations), Ponziani FR (7 articles, 350 citations), Ren, ZG (7 articles, 484 citations), Tacke F (7 articles, 328 citations), Lonardo A (6 articles, 705 citations), Ohtani N (6 articles, 1600 citations), Suk KT (6 articles, 124 citations), Trauner M (6 articles, 561 citations), Yang M (6 articles, 55 citations), Zhou L (6 articles, 377 citations). Among these authors, high cited authors are from Asia (China and Japan). Ohtani N from Osaka Metropolitan University Graduate School of Medicine School of Medicine, Japan, is the author with the highest average citation, indicating that he is academically highly influential with outstanding contributions in the field. Jia W with second average citation from School of Chinese Medicine, Hong Kong Baptist University, Kowloon Tong, Hong Kong, also has extremely high academic influence in the field. The co-citation analysis of authors was performed by VOSviewer software (Figure 7B). A total of 18 authors were selected with more than 100 citations.
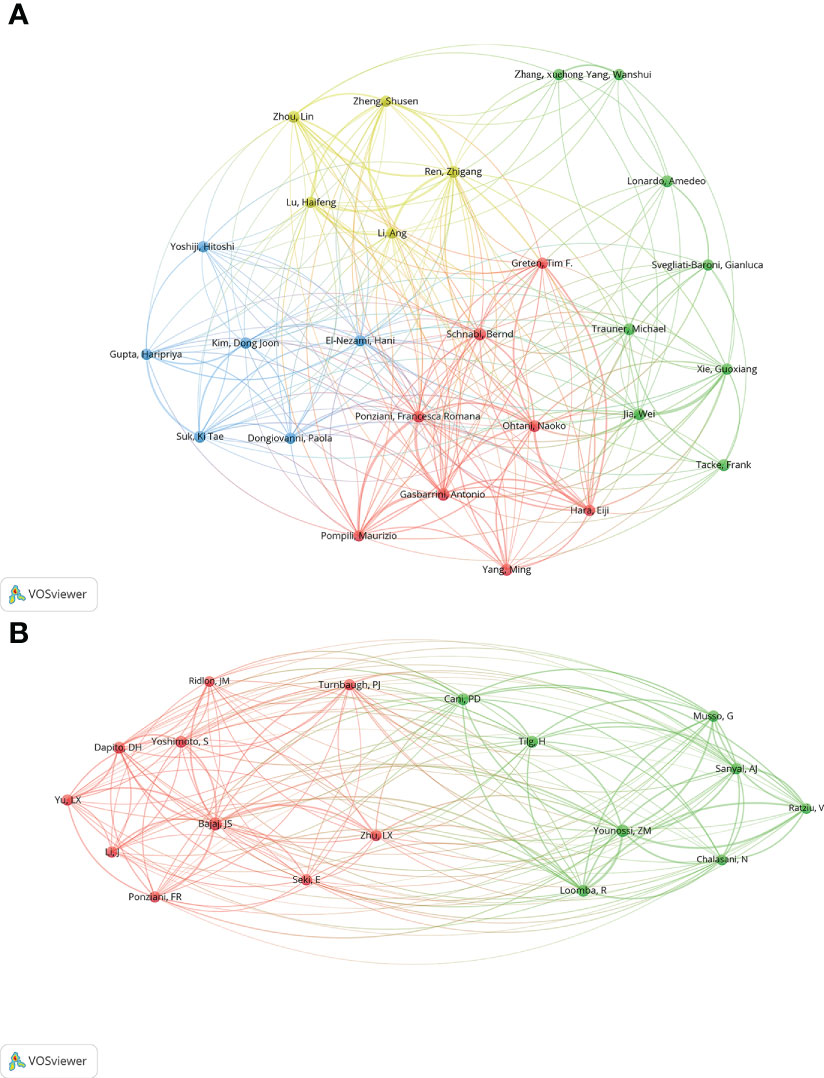
Figure 7 (A) Co-authorship network visualization map of authors. Circles represent the number of articles published. Connecting lines represent collaboration between authors. (B) Co-citation network visualization map of authors. The size of node and word reflects the co-occurrence frequencies, the link indicate the co-occurrence relationship, and the same color of node represent the same cluster.
Analysis of high-yielding journals
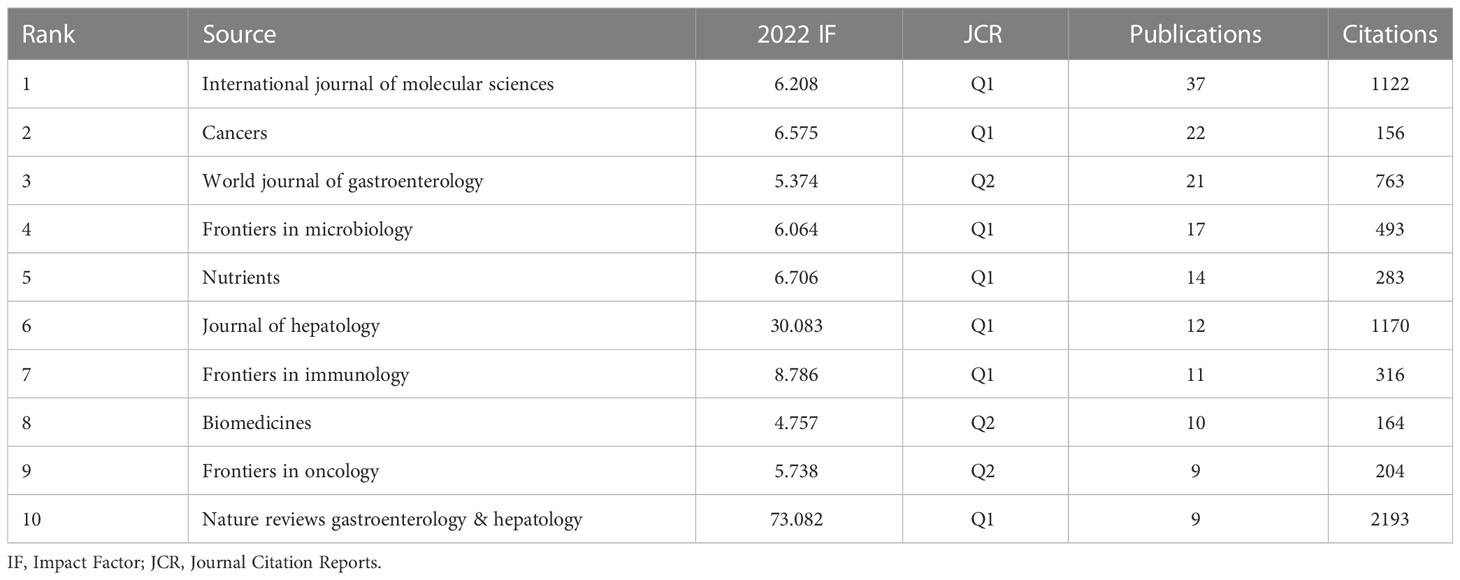
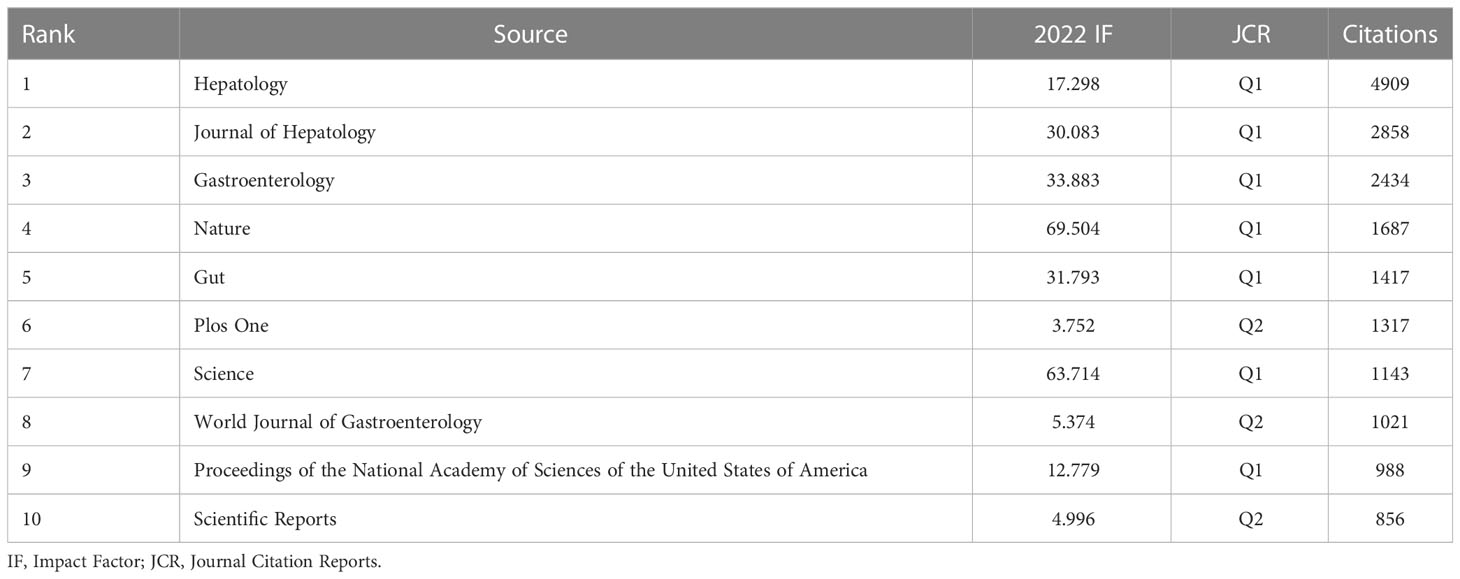
From the time of January 01, 2011 to October 26, 2022, 739 publications on research linked to gut microbiota and HCC were published in 334 journals, 31 journals of which contained at least 5 articles. The co-authorship analysis of journals was performed, and the network map was presented (Figure 8). We listed the top 10 journals with the most publications in this field (Table 5). Among the top ten journals, Nature reviews gastroenterology & hepatology has the highest impact factor (IF) with 73.082. Additionally, International journal of molecular sciences (2021 IF: 6.208), Cancers (2021 IF: 6.575), and World journal of gastroenterology (2021 IF: 5.374) are the journals with more than 20 publications each. Additionally, combined with the information provided by VOSviewer software and the Journal Citation Reports (JCR) assessment system, we also found that the top 10 journals are mainly concentrated in JCR of Q1(70%) and Q2 (30%), which indicates that these journals have a high degree of influence in the relevant domains. According to Table 6, Hepatology contributed the maximum quantity of publication citations with 4909. Journal of Hepatology was the second most cited journal, which had 2,858 citations. And it was followed by Gastroenterology, Nature, Gut.
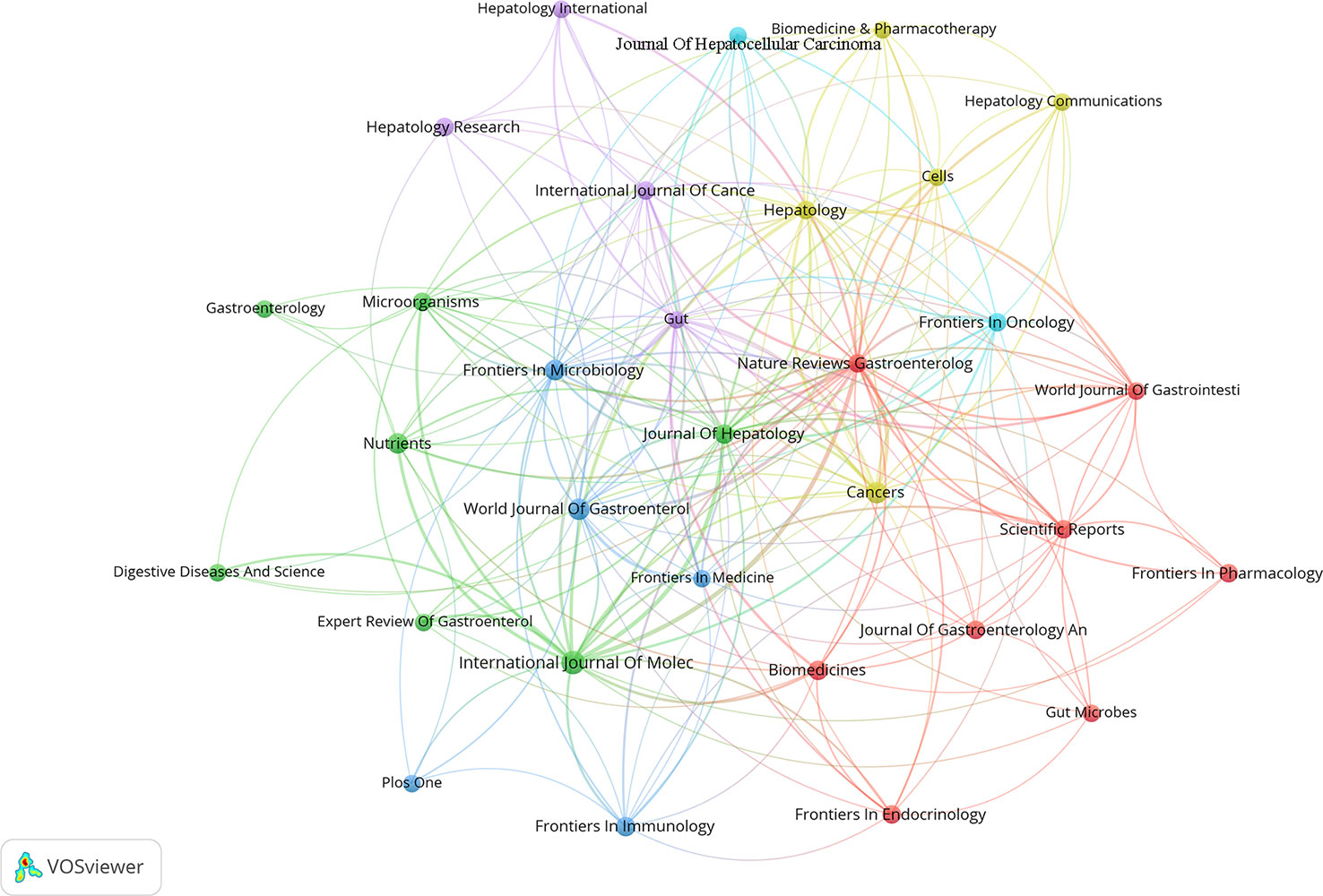
Figure 8 The co-authorship map of high-yielding journals. In the visualization map, one node represents a journal, and its size is proportional to the number of publications. Different colors represent different items. There are six items in the figure.
Analysis of number of citations
The number of citations is a measure of impact of a publication in a scientific field. These 739 publications were counted and ranked by number of citations, and the top 10 publications are shown in Table 7. The most cited article was Jorge Henao-Mejia et al. (13) of Yale University published in 2012 with a citation number of 1557. The second cited article was also published in Nature in 2013. They believed that obesity can change the intestinal microbiota through senescence-associated secretory phenotype (SASP), which plays an important role in the development of HCC in mice (14). Additionally, the third cited article by Dianne H Dapito et al. (15) believed that gut microbiota has a profound influence on hepatocarcinogenesis in the chronically injured liver cells. Therefore, they also found that targeting the gut microbiota and TLR4 might ultimately improve primary or secondary prevention of HCC. These publications demonstrated that gut microbiota is a double-edged sword and plays a key role in tumorigenesis, progression and oncotherapy of HCC.
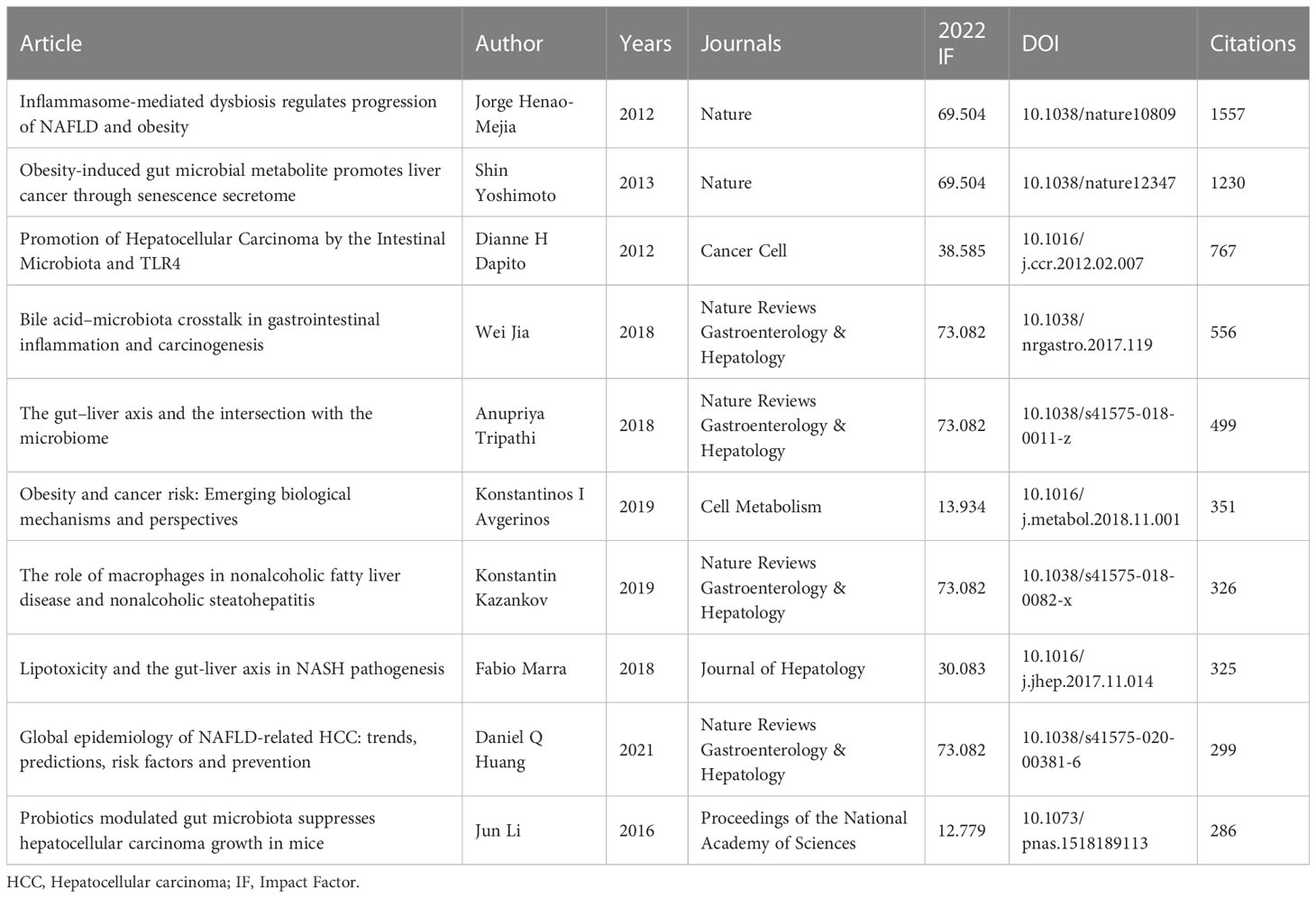
Table 7 The top 10 most cited articles in the connection of gut microbiota and HCC research from 2011 to 2022.
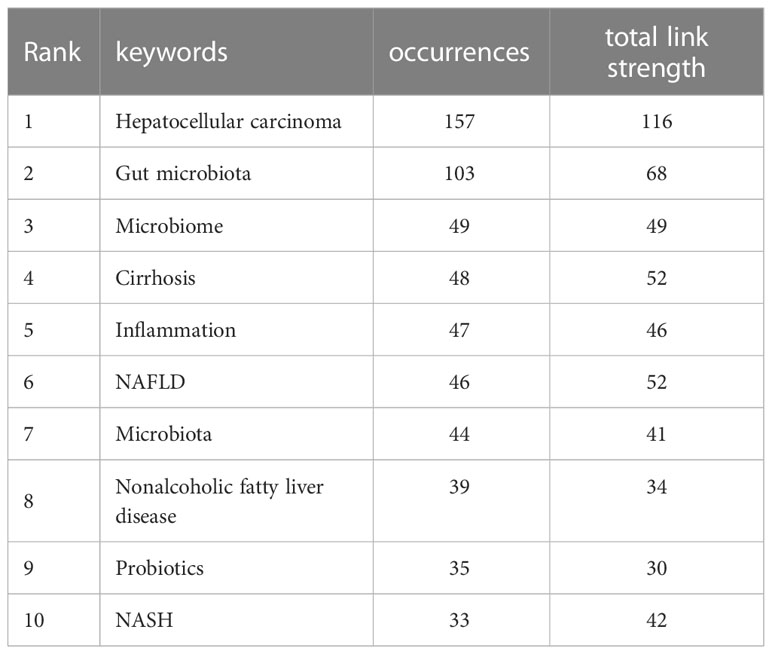
Analysis of keywords
Based on the literature keyword co-occurrence analysis, the detailed description of hotspots topics in the research field were presented. By analyzing the keywords of the 739 publications, a total of 1536 keywords were found, and VOSviewer identified 34 hotspots keywords that appeared at least 15 times and visualized connection with a network map (Figure 9A). Table 8 lists the top 10 hotspots keywords with the highest frequencies. Analysis of keywords with the strongest citation bursts “Burst words” represent keywords that are cited frequently over a period of time, thereby indicating the academic frontier in this field. In present study, the CiteSpace software was used to identified the keywords with strong citation bursts and 14 “Burst words” were presented. Figure 9B displays the top 14 “Burst words” as citations in the connection of HCC and gut microbiota during the period of 2011-2022. The red strip indicates the period that the burst keywords maintain. Amongst them, the most keywords with strong citation bursts, risk factor, began in 2013 and end in 2015 with highest strength of 4.47, which was associated with the hepatocarcinogenesis. The second strength of 4.44 was HCC, during 2019-2020 that was associated with the concept of the connection of gut microbiota and HCC. Bile acid, hepatitis B and dysbiosis were the latest keywords with strong citation bursts, indicating that these may be a hot topic in recent years.
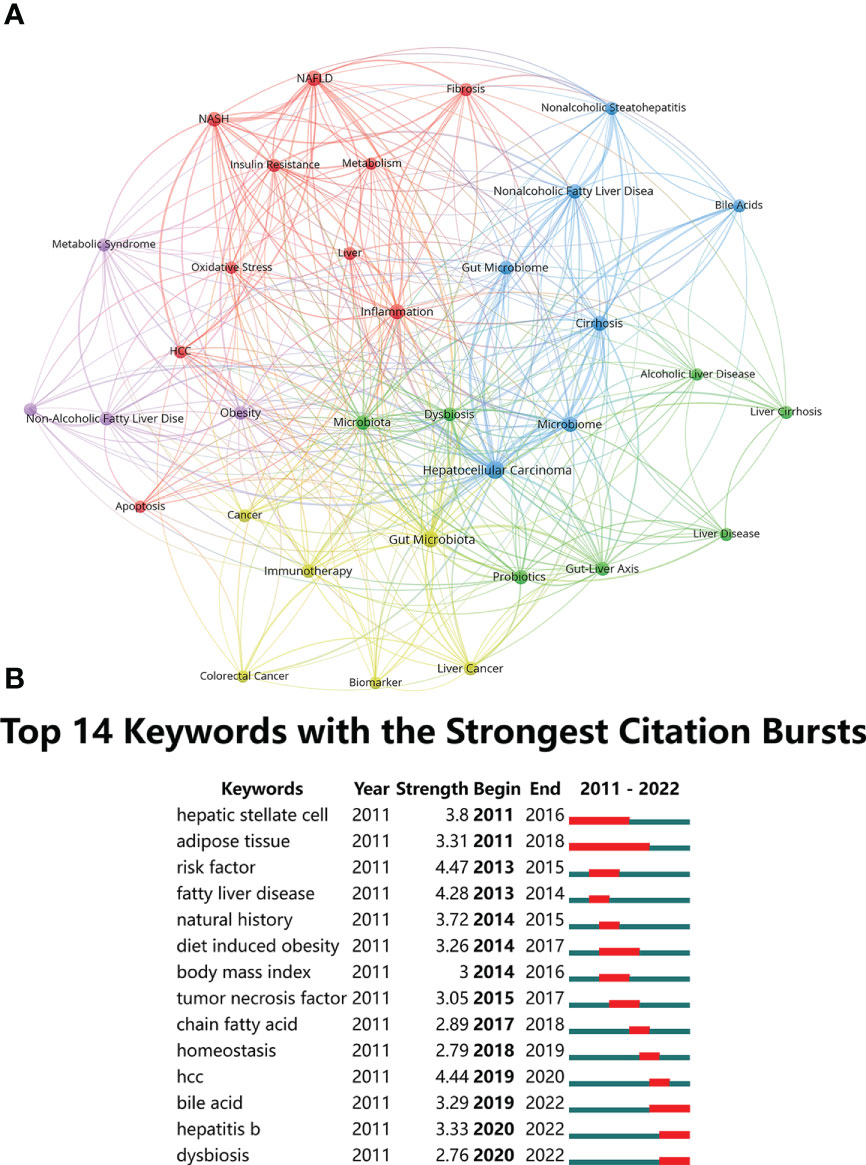
Figure 9 (A) The co-occurrence network of keywords. The lines between nodes represent co-occurrence between different keywords. Each node represents one keyword. Circle size is based on the number of occurrences. Different colors represent different items. There are five items in the figure. (B) Top 14 Keywords with the strongest citation bursts.
Discussion
Liver cancer ranks the second location among all causes inducing cancer-related death worldwide, and HCC is the commonest malignant tumor of primary liver cancer (1, 2). Hepatocarcinogenesis occurrence has various causes, involving factors including environmental factors, cell cycle metabolism, and the immune system, all of which are associated with activation of proto-oncogenes and inactivation in tumor suppressor genes (16). Hepatitis B virus (HBV) infection, Hepatitis C virus (HCV) infection, alcohol abuse and nonalcoholic fatty liver disease (NAFLD) is currently the most common cause of chronic hepatitis and HCC worldwide (17, 18). Some studies have found that the gut microbiota has significant influence in the development of liver cirrhosis, liver ischemic injury, NAFLD, HCC, and liver graft rejection (19–22). Additionally, the gut microbiotas of HCC patients are significantly different from those of normal subjects, and changes in the composition of gut microbiotas could reveal to grade severity or progression of illness in HCC (23, 24). Therefore, further exploration of gut microbiota promotes an understanding of the tumorigenesis and therapeutic strategies of HCC.
In present bibliometric analysis, we screened 739 publications including 383 articles and 356 reviews on the connection between gut microbiotas and HCC research after searching the WoSCC database. The number of studies related to gut microbiotas and HCC has been steadily increasing since 2011, with approximately ten times as many publications in 2021 as in 2011. The rapid increase in the number of publications over the past decade, indicating that the research of gut microbiota has attracted the attention of HCC research. There have been a number of studies on the involvement of gut microbiota in HCC, including the mechanism of hepatocellular carcinogenesis and tumor drug resistance, diagnostic markers, and treatment strategy (25, 26).
We first analyzed the contributions of countries, institutions, journals and authors in the research of the connection between gut microbiota and HCC. China and the USA are the two countries with the largest number of published papers in this field. Meanwhile, articles published by American scholars have the second average number of citations, only after Spain, which suggest that USA was the most influential country in gut microbiota and HCC research. These were closely related to the fact that the United States has the most advanced scientific research equipment and the largest number of scientific researchers in the world. Although China is far ahead of other countries except the USA in the number of articles published. However, the average citations of articles published by Chinese scholars are in the bottom of the top 10 publications productive countries. This means that Chinese scholars need to concentrate on more meaningful research results, rather than winning over other countries in terms of quantity. The University of California, San Diego in the United States and Shanghai Jiaotong University in China have the highest number of published papers and average citation. there have made tremendous contributions to the study of the gut microbiota in the occurrence and development of liver cancer. Their pioneering research has shed new light on the complex relationship between the microbiome and cancer, deepening our understanding of the mechanisms underlying this deadly disease. Their groundbreaking work represents a true testament to the power of scientific research and the immense potential for collaboration in tackling some of the most pressing health challenges of our time. However, there is very little collaboration between the two research institutions. It is hoped that different research institutions from different countries will have more collaboration in the future. Meanwhile, we found that a large number of publications are primarily distributed in economically developed countries and universities with high academic standing. These factors are closely related to the research resources, research culture, and international collaboration. Economically developed countries and universities with high academic standing have more research resources, invest significant funds to support scientific research, and foster research cultures that encourage innovation and exploration. Additionally, they have more opportunities for international exchange. Moreover, Ohtani N, a renowned scholar from Japan, holds the highest average citation record in his field. His unwavering commitment to uncovering the intricate interplay between the microbiome and liver carcinogenesis has brought to light a previously overlooked area of cancer biology (27, 28). His groundbreaking insights have paved the way for the development of novel strategies aimed at preventing and treating this devastating disease. The scientific community owes a great debt of gratitude to Ohtani N for his outstanding contributions to the field of cancer research.
Increasing evidence has illustrated the association between gut microbiota and tumorigenesis, which is important for exploring the pathogenesis of hepatocarcinogenesis (29, 30). The microbiota-gut-liver axis is a physiological and anatomical communication system that integrates intestinal microbiota and immunological signals with gastrointestinal tract and liver (31, 32). Recent studies have demonstrated the stability of the gut microbiota and its metabolites is important factor for the maintenance of normal liver functions (33, 34). Exposure of the chronic hepatitis to the dysbiosis of the gut microbiota and their derived metabolites results in cirrhosis and ultimately to hepatocarcinogenesis (32, 35, 36). Various intestinal microorganisms, intestinal microbial-derived metabolites, and cellular components (peptidoglycans, flagellin, lipoteichoic acid, and lipopolysaccharide), intestinal epithelial cell exosomes are transported into the liver via the portal vein or biliary system and interact with intrahepatic immune cells, causing inflammatory immune response and inducing normal liver from pre-cirrhotic stages to cirrhosis, liver cancer (37) (Figure 10). LPS activates the TLR4 receptor of HCC cells through NF- κ B pathway induces epithelial mesenchymal transition (EMT), which is an important molecular mechanism of tumor cell proliferation and metastasis (38). Short-chain fatty acids (SCFAs) are generated in the colon by the fermentation of dietary fiber by gut microbiota. Hepatocytes can utilize SCFAs as a source of energy, which can lower glucose production by the liver and enhance insulin sensitivity (39, 40). SCFAs can also stimulate the secretion of intestinal hormones, including glucagon-like peptide-1 (GLP-1), which can further enhance insulin secretion and improve glucose metabolism (41). Indoleacetic acid (IAA) is a plant hormone that can be produced by certain bacteria in the gut microbiota. IAA exerts its effects by binding to specific receptors on the surface of bacterial cells, which can activate or inhibit various signaling pathways that control gene expression and metabolism (42). Additionally, IAA can stimulate the production of certain enzymes that break down complex carbohydrates, making them more accessible to other microbes (42). It can also influence the production of other hormones and signaling molecules that affect the growth and activity of other bacterial species (43). When the intestinal flora is damaged, the intestinal mucosal barrier and immune functions is destroyed, leading to intestinal endotoxemia (IETM), bacterial translocation, and excessive absorption of bacterial metabolites (44, 45). These factors contribute to the process of liver cell inflammation, damage, and repair, especially on the basis of the original liver disease, HCC can develop due to hepatocyte damage and repair during inflammation.
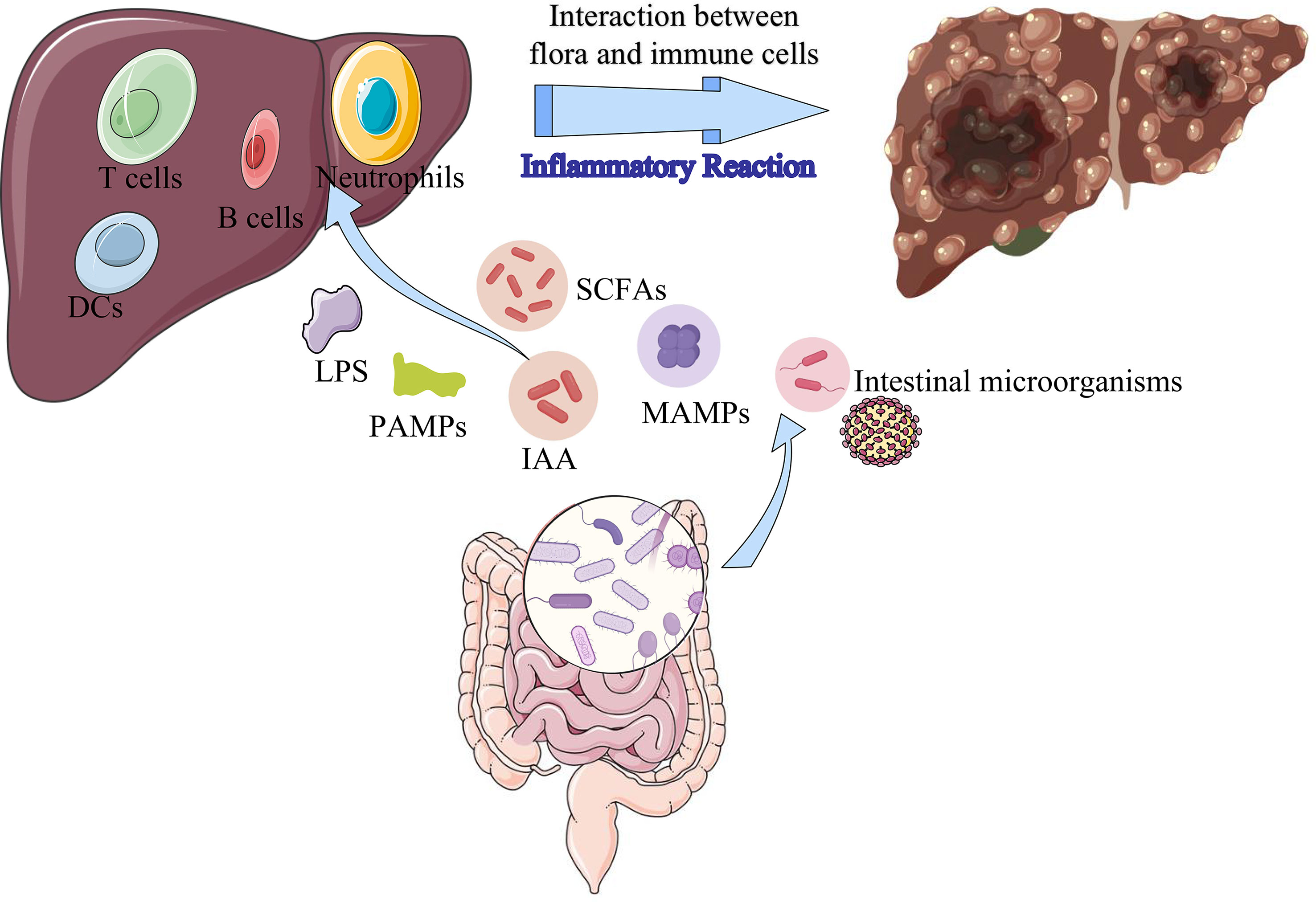
Figure 10 The mechanism of intestinal microbes and its metabolites influencing the formation of HCC. LPS, lipopolysaccharides; SCFAs, short-chain fatty acids; IAA, indoleacetic acid; MAMPs, microbe-associated molecular patterns; PAMPs, pathogen-associated molecular patterns.
There are also many studies on gut microbiota in the treatment of HCC (46, 47). Fessas P et al. found that in the mouse tumor model, the use of antibiotics will lead to the disorder of intestinal flora, which will cause changes in the composition of immune cells in the liver, thus affecting the growth of tumors in the liver (48). Probiotics are living microbes that confer beneficial effects to the host and help to maintain the stability of an organism as a whole. Probiotics can reduce toxic chemical induced hepatocarcinogenesis by rebuilding intestinal homeostasis and improving intestinal and liver inflammation (49). Research shows that dysbiosis of intestinal microbiota may be one cause of HCC, and probiotics that restore intestinal microbiota function are an effective and safe strategy to treat HCC (50, 51). Fecal microbiota transplantation (FMT) is the transplantation of fecal microbiota suspension from a healthy pre-screened donor to an individual with disease (52). Some studies have also found that FMT may effectively improve the immunotherapeutic response in HCC (53–55).
With the rapid development of high-throughput sequencing technologies such as single-cell sequencing, 16s RNA sequencing, genomics and spatial transcriptomics, numerous studies have been conducted to investigate the microbial diversity and biological functions of intestinal microbes in hepatocarcinogenesis. The crucial next step in exploring the influence of gut microbiota on the occurrence and progression of HCC is to integrate microbiome data, experimental results, and clinical data. By concentrating on the pathogenesis of HCC, we will search for new key targets that influence the development of HCC between the liver and the intestine. Meanwhile, future studies need to further explore the relationship between gut microbiota and HCC occurrence, development, and treatment and explore new strategies for regulating gut microbiota.
This study is the first to use bibliometric method to explore the connection between gut microbiotas and HCC research in the past decade. There are several limitations to this study that are worth noting. First, our publications were derived entirely from the WOSCC database and do not include publications of other databases, such as Google Scholar, PubMed and MEDLINE. Second, our research is mainly based on English-language literature, so we may be lost some high-quality literature published in other languages. We hope that those limitations can be elaborated in the future.
Conclusion
In present study, we explored the links between gut microbiota and HCC field with the method of bibliometric methods. The connection between gut microbiota and HCC field have always been enthusiastically investigated by researchers. Through bibliometric analysis, we found that gut microbiota is crucial in the pathogenesis and oncotherapy of HCC through published literature review.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
ZC and CD downloaded and analyzed the data. ZC, YG, QL designed the study. ZC, CD, and YH drafted the manuscript. QL, SZ, and YG reviewed and revised the manuscript. ZC and BC edited the figures and tables of the article. All authors contributed to the article and approved the submitted version. All authors met the ICMJE authorship criteria.
Funding
This work was funded by grants from the Jinan Microecological Biomedicine Shandong Laboratory (JNL-2022022C).
Acknowledgments
The authors acknowledge the support of the open-access resources.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HCC, Hepatocellular carcinoma; WoSCC, Web of Science Core Collection; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; JCR, Journal Citation Reports; IF, impact factor; SASP, senescence-associated secretory phenotype; HBV, Hepatitis B virus; HCV, Hepatitis C virus; EMT, epithelial mesenchymal transition; IETM, intestinal endotoxemia; FMT, Fecal microbiota transplantation.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology (2021) 73 Suppl 1(Suppl 1):4–13. doi: 10.1002/hep.31288
3. Villanueva A. Hepatocellular carcinoma. N Engl J Med (2019) 380(15):1450–62. doi: 10.1056/NEJMra1713263
4. Yu HC, Hung MH, Chen YL, Chu PY, Wang CY, Chao TT, et al. Erlotinib derivative inhibits hepatocellular carcinoma by targeting CIP2A to reactivate protein phosphatase 2A. Cell Death Dis (2014) 5(7):e1359. doi: 10.1038/cddis.2014.325
5. Li Z, Zhang Y, Hong W, Wang B, Chen Y, Yang P, et al. Gut microbiota modulate radiotherapy-associated antitumor immune responses against hepatocellular carcinoma Via STING signaling. Gut Microbes (2022) 14(1):2119055. doi: 10.1080/19490976.2022.2119055
6. Kim J, Ahn SW, Kim JY, Whon TW, Lim SK, Ryu BH, et al. Probiotic lactobacilli ameliorate alcohol-induced hepatic damage via gut microbial alteration. Front Microbiol (2022) 13:869250. doi: 10.3389/fmicb.2022.869250
7. Macpherson AJ, Heikenwalder M, Ganal-Vonarburg SC. The liver at the nexus of host-microbial interactions. Cell Host Microbe (2016) 20(5):561–71. doi: 10.1016/j.chom.2016.10.016
8. Schneider KM, Bieghs V, Heymann F, Hu W, Dreymueller D, Liao L, et al. CX3CR1 is a gatekeeper for intestinal barrier integrity in mice: Limiting steatohepatitis by maintaining intestinal homeostasis. Hepatology (2015) 62(5):1405–16. doi: 10.1002/hep.27982
9. Zheng Y, Wang T, Tu X, Huang Y, Zhang H, Tan D, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer (2019) 7(1):193. doi: 10.1186/s40425-019-0650-9
10. Chen C, Song M. Visualizing a field of research: A methodology of systematic scientometric reviews. PloS One (2019) 14(10):e0223994. doi: 10.1371/journal.pone.0223994
11. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics (2010) 84(2):523–38. doi: 10.1007/s11192-009-0146-3
12. Chen C, Hu Z, Liu S, Tseng H. Emerging trends in regenerative medicine: A scientometric analysis in CiteSpace. Expert Opin Biol Ther (2012) 12(5):593–608. doi: 10.1517/14712598.2012.674507
13. Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature (2012) 482(7384):179–85. doi: 10.1038/nature10809
14. Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature (2013) 499(7456):97–101. doi: 10.1038/nature12347
15. Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell (2012) 21(4):504–16. doi: 10.1016/j.ccr.2012.02.007
16. Liu JJ, Li Y, Chen WS, Liang Y, Wang G, Zong M, et al. Shp2 deletion in hepatocytes suppresses hepatocarcinogenesis driven by oncogenic β-catenin, PIK3CA and MET. J Hepatol (2018) 69(1):79–88. doi: 10.1016/j.jhep.2018.02.014
17. Shokouhian B, Aboulkheyr Es H, Negahdari B, Negahdari B, Tamimi A, Shahdoust M, et al. Hepatogenesis and hepatocarcinogenesis: Alignment of the main signaling pathways. J Cell Physiol (2022) 237(11):3984–4000. doi: 10.1002/jcp.30862
18. Zoller H, Tilg H. Nonalcoholic fatty liver disease and hepatocellular carcinoma. Metabolism (2016) 65(8):1151–60. doi: 10.1016/j.metabol.2016.01.010
19. Beyoğlu D, Idle JR. The gut microbiota - a vehicle for the prevention and treatment of hepatocellular carcinoma. Biochem Pharmacol (2022) 204:115225. doi: 10.1016/j.bcp.2022.115225
20. Manzoor R, Ahmed W, Afify N, Memon M, Yasin M, Memon H, et al. Trust your gut: The association of gut microbiota and liver disease. Microorganisms (2022) 10(5):1045. doi: 10.3390/microorganisms10051045
21. Liu J, Yang D, Wang X, Asare PT, Zhang Q, Na L, et al. Gut microbiota targeted approach in the management of chronic liver diseases. Front Cell Infect Microbiol (2022) 12:774335. doi: 10.3389/fcimb.2022.774335
22. Wang T, Guo XK, Xu H. Disentangling the progression of non-alcoholic fatty liver disease in the human gut microbiota. Front Microbiol (2021) 12:728823. doi: 10.3389/fmicb.2021.728823
23. Das BK. Altered gut microbiota in hepatocellular carcinoma: Insights into the pathogenic mechanism and preclinical to clinical findings. Apmis (2022) 130(12):719–40. doi: 10.1111/apm.13282
24. Schneider KM, Mohs A, Gui W, Galvez EJC, Candels LS, Hoenicke L, et al. Imbalanced gut microbiota fuels hepatocellular carcinoma development by shaping the hepatic inflammatory microenvironment. Nat Commun (2022) 13(1):3964. doi: 10.1038/s41467-022-31312-5
25. Wu Z, Zhang S, Li L, Huang Z, Huang D, Hu Y. The gut microbiota modulates responses to anti-PD-1 and chemotherapy combination therapy and related adverse events in patients with advanced solid tumors. Front Oncol (2022) 12:887383. doi: 10.3389/fonc.2022.887383
26. Watahiki T, Okada K, Miura I, To K, Tanaka S, Warabi E, et al. Antioxidative self-assembling nanoparticles attenuate the development of steatohepatitis and inhibit hepatocarcinogenesis in mice. Antioxid (Basel) (2022) 11(10):1939. doi: 10.3390/antiox11101939
27. Huu Hoang T, Sato-Matsubara M, Yuasa H, Matsubara T, Thuy LTT, Ikenaga H, et al. Cancer cells produce liver metastasis via gap formation in sinusoidal endothelial cells through proinflammatory paracrine mechanisms. Sci Adv (2022) 8(39):eabo5525. doi: 10.1126/sciadv.abo5525
28. Okina Y, Sato-Matsubara M, Kido Y, Urushima H, Daikoku A, Kadono C, et al. Nitric oxide derived from cytoglobin-deficient hepatic stellate cells causes suppression of cytochrome c oxidase activity in hepatocytes. Antioxid Redox Signal (2022) 38(7-9):463–79. doi: 10.1089/ars.2021.0279
29. McQuade JL, Daniel CR, Helmink BA, Wargo JA. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol (2019) 20(2):e77–91. doi: 10.1016/s1470-2045(18)30952-5
30. Kanmani P, Suganya K, Kim H. The gut microbiota: How does it influence the development and progression of liver diseases. Biomedicines (2020) 8(11). doi: 10.3390/biomedicines8110501
31. Kanmani P, Suganya K, Kim H. The gut microbiota: How does it influence the development and progression of liver diseases. Biomedicines (2020) 8(11):501. doi: 10.3390/biomedicines8110501
32. Orci LA, Lacotte S, Delaune V, Slits F, Oldani G, Lazarevic V, et al. Effects of the gut-liver axis on ischaemia-mediated hepatocellular carcinoma recurrence in the mouse liver. J Hepatol (2018) 68(5):978–85. doi: 10.1016/j.jhep.2017.12.025
33. Wieland A, Frank DN, Harnke B, Bambha K. Systematic review: Microbial dysbiosis and nonalcoholic fatty liver disease. Aliment Pharmacol Ther (2015) 42(9):1051–63. doi: 10.1111/apt.13376
34. Schwimmer JB, Johnson JS, Angeles JE, Behling C, Belt PH, Borecki I, et al. Microbiome signatures associated with steatohepatitis and moderate to severe fibrosis in children with nonalcoholic fatty liver disease. Gastroenterology (2019) 157(4):1109–22. doi: 10.1053/j.gastro.2019.06.028
35. Yu LX, Schwabe RF. The gut microbiome and liver cancer: Mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol (2017) 14(9):527–39. doi: 10.1038/nrgastro.2017.72
36. Schwabe RF, Greten TF. Gut microbiome in HCC - mechanisms, diagnosis and therapy. J Hepatol (2020) 72(2):230–8. doi: 10.1016/j.jhep.2019.08.016
37. Schwabe RF, Tabas I, Pajvani UB. Mechanisms of fibrosis development in nonalcoholic steatohepatitis. Gastroenterology (2020) 158(7):1913–28. doi: 10.1053/j.gastro.2019.11.311
38. Wen Z, Ji X, Tang J, Lin G, Xiao L, Liang C, et al. Positive feedback regulation between transglutaminase 2 and toll-like receptor 4 signaling in hepatic stellate cells correlates with liver fibrosis post schistosoma japonicum infection. Front Immunol (2017) 8:1808. doi: 10.3389/fimmu.2017.01808
39. May KS, den Hartigh LJ. Gut microbial-derived short chain fatty acids: Impact on adipose tissue physiology. Nutrients (2023) 15(2):272. doi: 10.3390/nu15020272
40. Deng M, Li X, Li W, Gong J, Zhang X, Ge S, et al. Short-chain fatty acids alleviate hepatocyte apoptosis induced by gut-derived protein-bound uremic toxins. Front Nutr (2021) 8:756730. doi: 10.3389/fnut.2021.756730
41. Si X, Shang W, Zhou Z, Shui G, Lam SM, Blanchard C, et al. Gamma-aminobutyric acid enriched rice bran diet attenuates insulin resistance and balances energy expenditure via modification of gut microbiota and short-chain fatty acids. J Agric Food Chem (2018) 66(4):881–90. doi: 10.1021/acs.jafc.7b04994
42. Park JW, Kim SE, Lee NY, Kim JH, Jung JH, Jang MK, et al. Role of microbiota-derived metabolites in alcoholic and non-alcoholic fatty liver diseases. Int J Mol Sci (2021) 23(1). doi: 10.3390/ijms23010426
43. Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol (2020) 72(3):558–77. doi: 10.1016/j.jhep.2019.10.003
44. Pezzino S, Sofia M, Faletra G, Mazzone C, Litrico G, La Greca G, et al. Gut-liver axis and non-alcoholic fatty liver disease: A vicious circle of dysfunctions orchestrated by the gut microbiome. Biol (Basel) (2022) 11(11):1622. doi: 10.3390/biology11111622
45. Brandt A, Baumann A, Hernández-Arriaga A, Jung F, Nier A, Staltner R, et al. Impairments of intestinal arginine and NO metabolisms trigger aging-associated intestinal barrier dysfunction and 'inflammaging'. Redox Biol (2022) 58:102528. doi: 10.1016/j.redox.2022.102528
46. Li J, Sung CY, Lee N, Ni Y, Pihlajamäki J, Panagiotou G, et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci U.S.A. (2016) 113(9):E1306–15. doi: 10.1073/pnas.1518189113
47. Fukui H. Improve gut microbiome: A new horizon of cancer therapy. Hepatobil Surg Nutr (2017) 6(6):424–8. doi: 10.21037/hbsn.2017.08.04
48. Fessas P, Naeem M, Pinter M, Marron TU, Szafron D, Balcar L, et al. Early antibiotic exposure is not detrimental to therapeutic effect from immunotherapy in hepatocellular carcinoma. Liver Cancer (2021) 10(6):583–92. doi: 10.1159/000519108
49. Zhang HL, Yu LX, Yang W, Tang L, Lin Y, Wu H, et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J Hepatol (2012) 57(4):803–12. doi: 10.1016/j.jhep.2012.06.011
50. Qin H, Yuan B, Huang W, Wang Y. Utilizing gut microbiota to improve hepatobiliary tumor treatments: Recent advances. Front Oncol (2022) 12:924696. doi: 10.3389/fonc.2022.924696
51. van Baarlen P, Wells JM, Kleerebezem M. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol (2013) 34(5):208–15. doi: 10.1016/j.it.2013.01.005
52. Wang JW, Kuo CH, Kuo FC, Wang YK, Hsu WH, Yu FJ, et al. Fecal microbiota transplantation: Review and update. J Formos Med Assoc (2019) 118 Suppl 1:S23–s31. doi: 10.1016/j.jfma.2018.08.011
53. Delaune V, Orci LA, Lacotte S, Peloso A, Schrenzel J, Lazarevic V, et al. Fecal microbiota transplantation: A promising strategy in preventing the progression of non-alcoholic steatohepatitis and improving the anti-cancer immune response. Expert Opin Biol Ther (2018) 18(10):1061–71. doi: 10.1080/14712598.2018.1518424
54. Chen D, Wu J, Jin D, Wang B, Cao H. Fecal microbiota transplantation in cancer management: Current status and perspectives. Int J Cancer (2019) 145(8):2021–31. doi: 10.1002/ijc.32003
Keywords: gut microbiota, hepatocellular carcinoma, bibliometric analysis, publications, average citation
Citation: Chen Z, Ding C, Gu Y, He Y, Chen B, Zheng S and Li Q (2023) Association between gut microbiota and hepatocellular carcinoma from 2011 to 2022: Bibliometric analysis and global trends. Front. Oncol. 13:1120515. doi: 10.3389/fonc.2023.1120515
Received: 10 December 2022; Accepted: 20 March 2023;
Published: 30 March 2023.
Edited by:
Francesca Romana Ponziani, Internal Medicine, Gastroenterology, Hepatology Fondazione Policlinico Universitario Agostino Gemelli IRCCS, ItalyReviewed by:
Tingting Huang, Guangxi Medical University, ChinaSidharth Prasad Mishra, University of South Florida, United States
Copyright © 2023 Chen, Ding, Gu, He, Chen, Zheng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiyong Li, bGlxaXlvbmdAemp1LmVkdS5jbg==; Shusen Zheng, c2h1c2VuemhlbmdAemp1LmVkdS5jbg==
 Zhitao Chen
Zhitao Chen Chenchen Ding2
Chenchen Ding2 Shusen Zheng
Shusen Zheng Qiyong Li
Qiyong Li