- 1Department of Radiation Oncology, The First Affiliated Hospital of China Medical University, Shenyang, China
- 2Department of Surgical Oncology and General Surgery, The First Affiliated Hospital of China Medical University, Shenyang, China
Purpose: To compare the lesion characteristics and radiotherapy efficacy of patients with single and multiple esophageal squamous cell carcinoma (ESCC), to evaluate the effect of multiple lesions on ESCC, and establish a nomogram survival prediction model for patients with synchronous multiple primary esophageal squamous cell carcinoma (SMPESCC) who received definitive radiotherapy.
Materials and methods:: The study enrolled 1,034 patients with ESCC who underwent definitive radiotherapy between 2010 and 2020. The efficacy of radiotherapy was compared between 101 patients with SMPESCC and 933 patients with single ESCC. Propensity score matching was used to control for potential confounders. For patients with SMPESCC, a nomogram prediction model was established based on the Cox regression model.
Results: The median OS was 30.00 (95% CI = 25.08-34.92) months for the single lesion group and 19.00 (95% CI = 15.51-22.48) months for the multiple cancer group respectively. Multivariate COX regression analysis showed that multiple cancer was an independent prognostic factor for ESCC patients (HR=1.89, 95%CI=1.49-2.38, P<0.001). Cox multivariate analysis of SMPESCC patients showed that T stage (P =0.002), chemotherapy (P =0.006), and lesion spacing (P =0.004) were independent prognostic factors associated with OS. The nomogram was established by combining T stage, chemotherapy, and lesion spacing, and Harrell’s C index was 0.711 after internal cross-validation. The calibration curve and decision curve analysis confirmed that the nomogram survival prediction model had a good predictive value for individual survival.
Conclusions: The survival rate of single esophageal cancer is significantly better than that of multiple lesions. Patients with SMPESCC exhibit worse survival than patients with single ESCC. Multiple lesions have a significant impact on the survival of patients with ESCC. The nomogram model established for SMPESCC patients can well predict the individual survival of patients.
Introduction
Esophageal cancer (EC) is the ninth most common cancer worldwide (1). More than 450000 people worldwide are infected by EC (2). In recent years, more advanced therapies have been used in clinics. This has increased the survival time of EC patients. Meanwhile, the incidence rate of multiple primary cancer developing in patients with EC is increasing, which has been reported in the literature between 5.81% and 32.2% (3–5). At present, the research on EC with multiple primary cancers mainly focuses on EC and other sites, such as head and neck cancer, stomach cancer, etc. However, the different locations in the esophagus are rarely studied. Multiple primary esophageal carcinoma is defined as two or more primary malignant tumors occurring simultaneously or successively in different parts of the esophagus. The second cancer occurring within 6 months is defined as simultaneous multiple primary esophageal carcinoma (SMPEC), and the second cancer occurring over 6 months is referred to as metachronous multiple primary esophageal carcinomas. Some studies have shown that smoking and drinking are closely related to the occurrence of the second primary cancer of EC (6, 7), but the risk factors for SMPEC remain unclear. At present, the reported 5-year survival rate of single EC is mostly between 15%-25% (8–10). However, there are few studies on the survival rate of SMPEC, and the results of existing studies vary widely, ranging from 7.7% to 28.9% (11, 12). The impact of multiple lesions on the prognosis of EC has not been determined. In addition, there is no standard treatment strategy for patients with SMPEC. Currently, radical treatment is mostly used to improve the survival rate.
Therefore, this study analyzed the characteristics and prognosis of synchronous multiple primary esophageal squamous cell carcinoma (SMPESCC) patients who received definitive radiotherapy in a single institution within 10 years, aiming to determine the risk factors for its occurrence and the impact of multiple lesions on the survival of EC, and to evaluate the efficacy and prognostic factors of radiotherapy, to provide a reference for clinical doctor’s treatment decisions.
Materials and methods
Patients
A total of 1034 patients with ESCC from January 2010 to June 2020 received definitive radiotherapy at the Department of Radiotherapy of the First Affiliated Hospital of China Medical University were recruited, resulting in 101 patients with SMPESCC, accounting for about 9.77% of the total. The diagnosis of multiple primary carcinomas is based on criteria proposed by Warren et al. in 1933 (13): (1) the tumors must be malignant on histologic examination; (2) the tumors must be separated by normal mucosa; and (3) the possibility that the second tumor is metastatic must be excluded. In addition, all lesions met the radiographic diagnostic criteria proposed by Xiao et al. (14).
The exclusion criteria were: (1) Having received surgery; (2) Accompanied by hypopharyngeal carcinoma or other local cancers; (3) The pathological type was adenocarcinoma or other types. The stage and location of a lesion of EC is defined according to the American Joint Commission on Cancer (AJCC) Tumor Node Metastasis (TNM) Classification of the esophageal tumor (8th Edition, 2017). T-stage and N-stage were determined by endoscopic ultrasound and chest enhanced CT. The length of the affected lesions was determined according to the length measured by gastrointestinal endoscopy before treatment. For esophageal stenosis, gastrointestinal barium X-ray imaging or chest enhanced CT was used to determine the length. Cervical lymph node involvement was determined by chest enhanced CT and cervical Doppler ultrasound.
This study was approved by Institutional Review Board of The First Hospital of China Medical University (AF-SOP-07-1.1-01), and all patients were enrolled in our department after written informed consent was obtained.
Treatment
All patients were treated with definitive radiotherapy and received the usual segmentation regimen: 1.8-2.5Gy each time and 5 times per week with a 6-mV linear accelerator. The radiotherapy dose was 50-66Gy/25-33fractions. Among them, 9 patients received three-dimensional conformal radiotherapy, and 92 patients received intensity-modulated radiotherapy. Fourteen of them discontinued/did not complete planned radiotherapy. 67 patients received synchronous/sequential chemotherapy, mainly 2-4 cycles of platinum.
Follow up
Overall survival (OS) was calculated from the date of the first diagnosis to the point of death or last follow-up. Patients were followed up until March 1, 2022, mainly by outpatient review and telephone. Patients who were lost to follow-up or survived at the last follow-up were recorded as censored data.
PSM
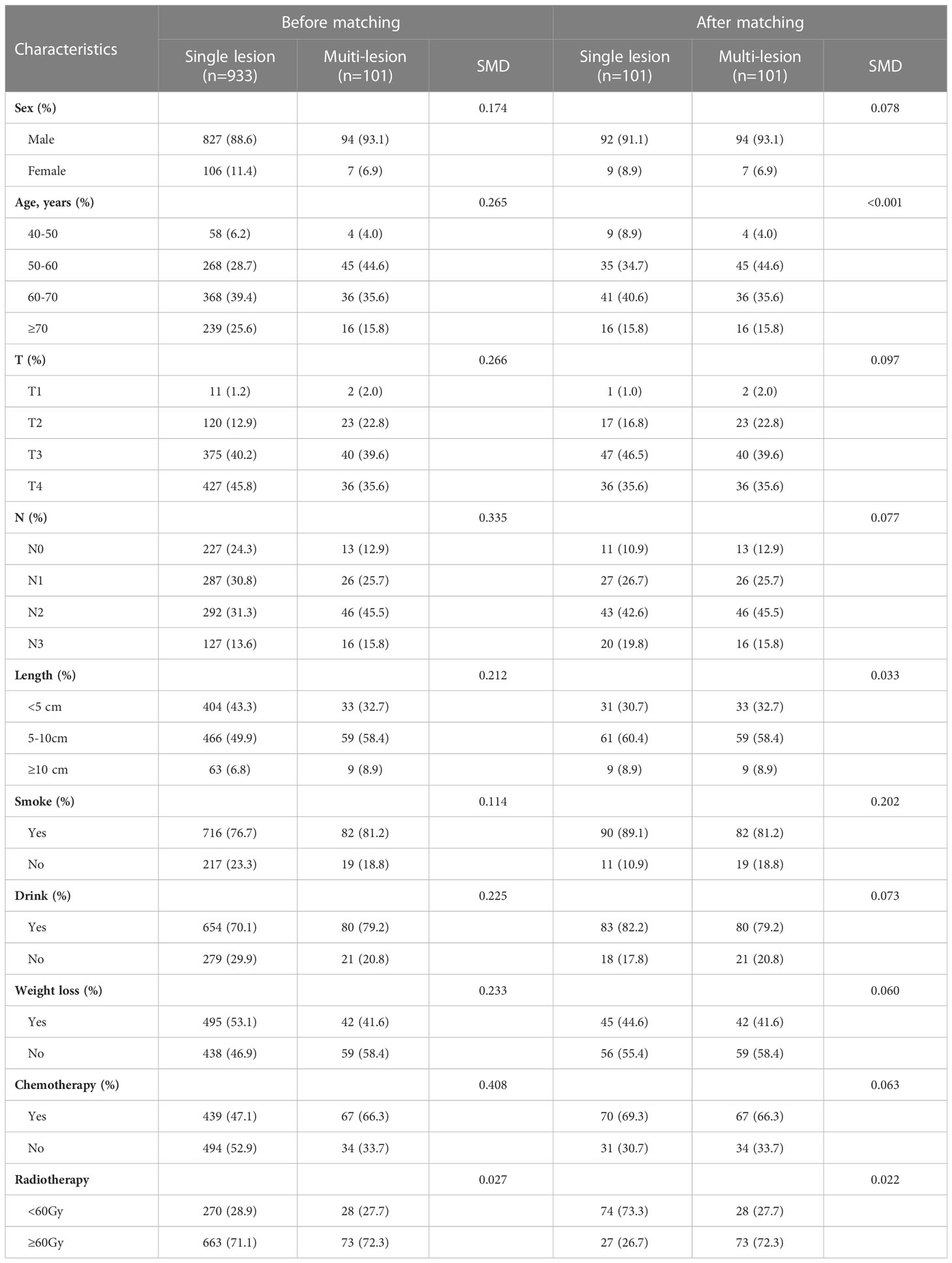
PSM is performed by the “MatchIt” package in R software (version 4.1.3). The general baseline data of the single and multiple lesion groups, including sex, age, lymph node metastases, history of alcohol and tobacco, chemotherapy and radiotherapy dose, were matched using a 1:1 nearest neighbor method, with a caliper value set to 0.05. Mean differences and equilibrium between all baseline covariates in exposed and control groups were assessed before and after matching to assess the degree of balance, with a 50% increase in equilibrium for a given covariable indicating good propensity score performance.
Statistical analysis
Statistical analysis was performed using SPSS 26.0 (Chicago, IL). A Chi-square test was used to compare the distribution of clinicopathological data in each group. Risk factors for multiple esophageal squamous cell carcinoma were determined by Logistic regression analysis. Cox regression analysis was used to evaluate whether multiple lesions were independent prognostic factors for esophageal squamous cell carcinoma patients who received definitive radiotherapy. Kaplan-Meier method and log-rank test were used to draw the survival curve. Univariate and multivariate Cox proportional risk regression models were used to assess prognostic factors in patients with SMPESCC undergoing definitive radiotherapy. All tests were two-sided, and P values less than 0.05 were considered statistically significant.
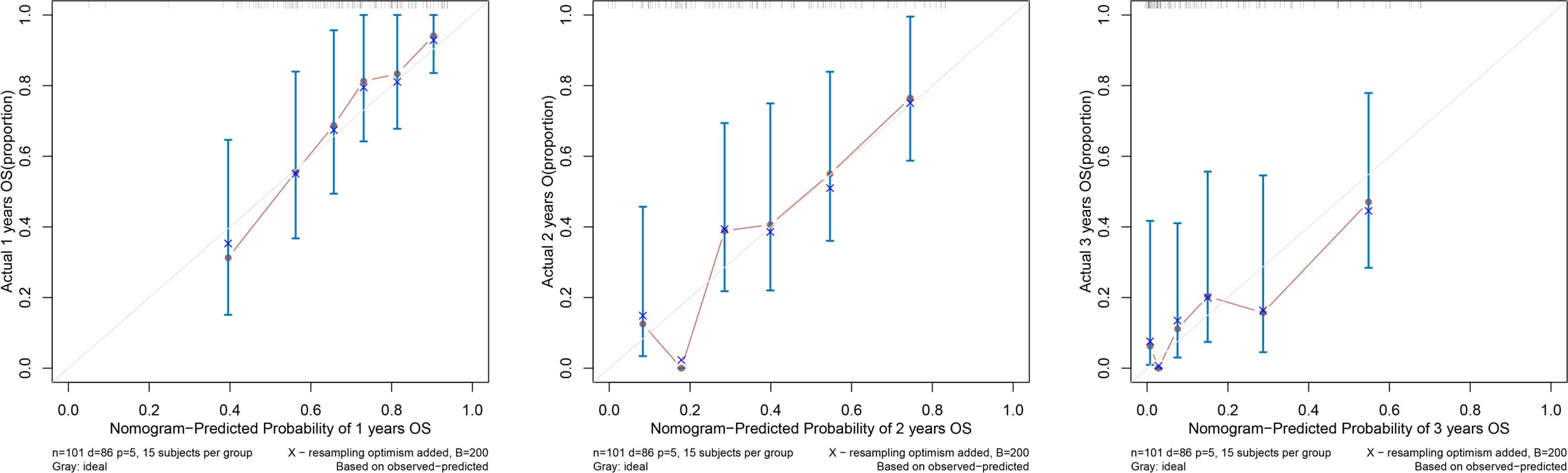
Based on the Cox regression model, the nomogram of OS-related prognostic factors in SMPESCC patients was established. Discrimination and calibration were used to evaluate the performance of the prognostic model. The Discrimination of the nomogram was measured by Harrell’s Consistency Index (C-index) and cross-validated with 1000 bootstrap samples to prevent overfitting of the model. The c-index ranges from 0.5 to 1, and a higher C-index indicated a more discriminative nomogram model. Calibration was generally assessed by plotting the predicted probability of survival (horizontal axis) against the actual probability of survival (vertical axis) from the nomogram model. The perfect and accurate calibration curve showed that all observation points fell on the diagonal line of 45 degrees. Decision curve analysis (DCA) was performed to determine the clinical net benefit of model reliability at different probability thresholds.
Results
Clinical characteristic
Of the 101 SMPESCC patients, the vast majority (93.07%) were male, aged 45-82 (median: 60 years). 96 patients had two lesions and 5 had three. Among the patients with double primary lesions, most patients (41/96) were synchronous middle thoracic segment and the lower thoracic segment. Among 5 patients with three primary esophageal carcinoma, 2 lesions were located in the lower thoracic segment, and the remaining 1 lesion was located in the neck segment (1/5) and the middle thoracic segment (4/5). The largest T stage in multiple lesions was considered as the total T stage of the patient. T3 stage (39.60%) and T4 stage (35.64%) accounted for the majority of patients. Cervical or supraclavicular lymph node metastasis was observed in 52 patients (51.49%), and mediastinal lymph node metastasis in 68 patients (67.33%). Compared with single esophageal cancer, the risk factors of multiple esophageal cancer were age (P=0.017) and weight loss (P=0.017).
Impact of multiple lesions on survival of ESCC
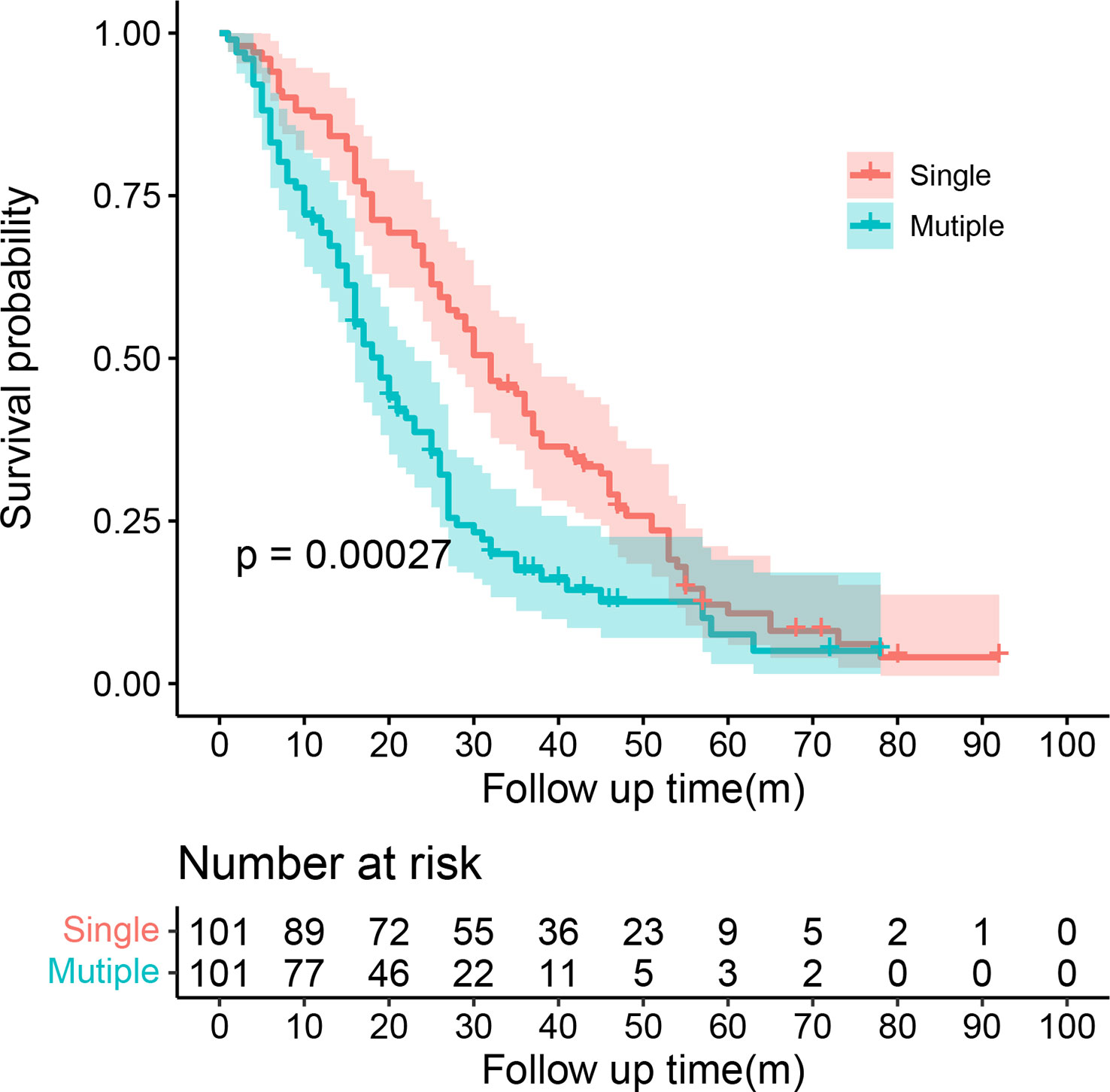
SMPESCC patients were matched with single lesion ESCC patients undergoing definitive radiotherapy at the same time. The distribution of patients before and after propensity score matching is shown in Figure 1A. Finally, 101 patients with isolated ESCC lesions and 101 patients with multiple ESCC lesions were matched (Figure 1B, Table 1). The equilibrium test showed that the mean difference of the overall distance between the two groups decreased from 0.5897 to 0.0007, and equilibrium increased by 99.78%. The equilibrium of all variables increased by more than 50%, indicating a good matching effect. The median OS was 30.00 (95% CI = 25.08-34.92) months for the single lesion group and 19.00 (95% CI = 15.51-22.48) months for the multiple lesions group respectively. Figure 2 shows the K-M survival curve between the two groups. Patients with multiple lesions had a significantly shorter survival time than those with single lesions (P<0.001). Multivariate COX regression analysis showed that multiple cancer was an independent prognostic factor for ESCC patients (HR=1.89, 95%CI=1.49-2.38, P<0.001). Other prognostic factors for ESCC patients were T stage (P<0.001) and chemotherapy (P<0.001) (Table 2).
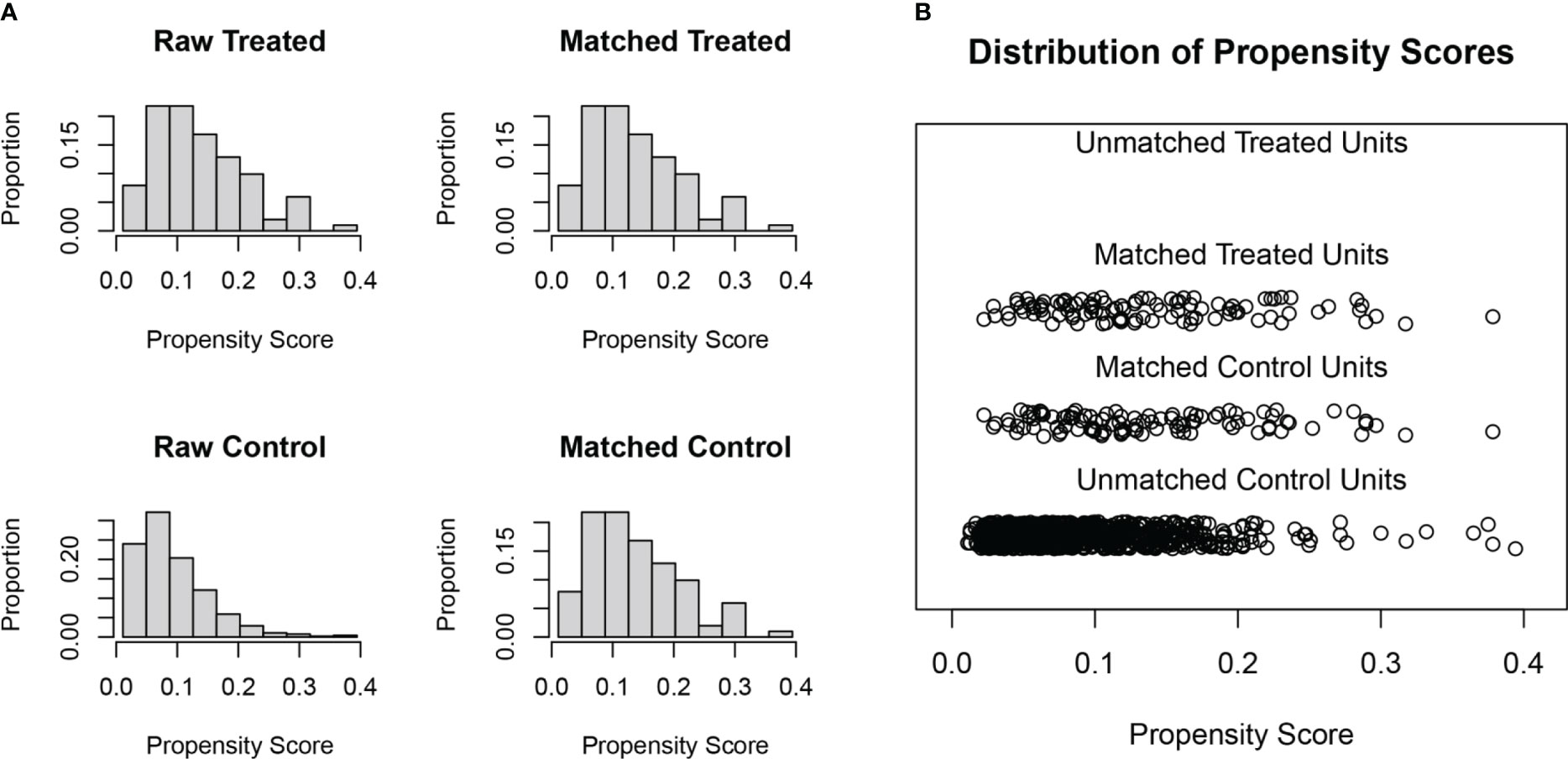
Figure 1 (A) Histogram of patients distribution before and after propensity score matching. (B) Distribution results of patients after propensity score matching.
Prognostic analysis of SMPESCC patients
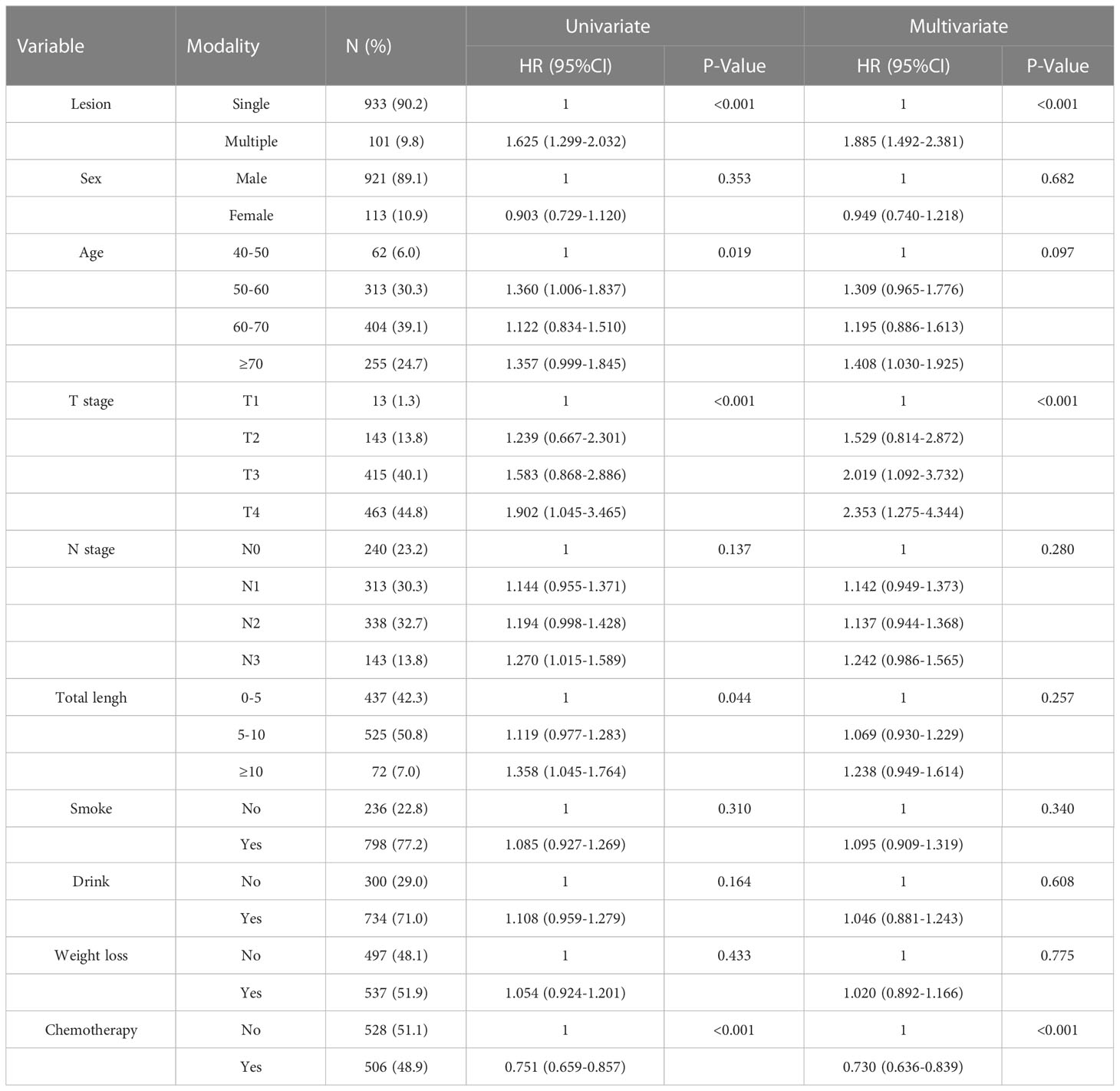
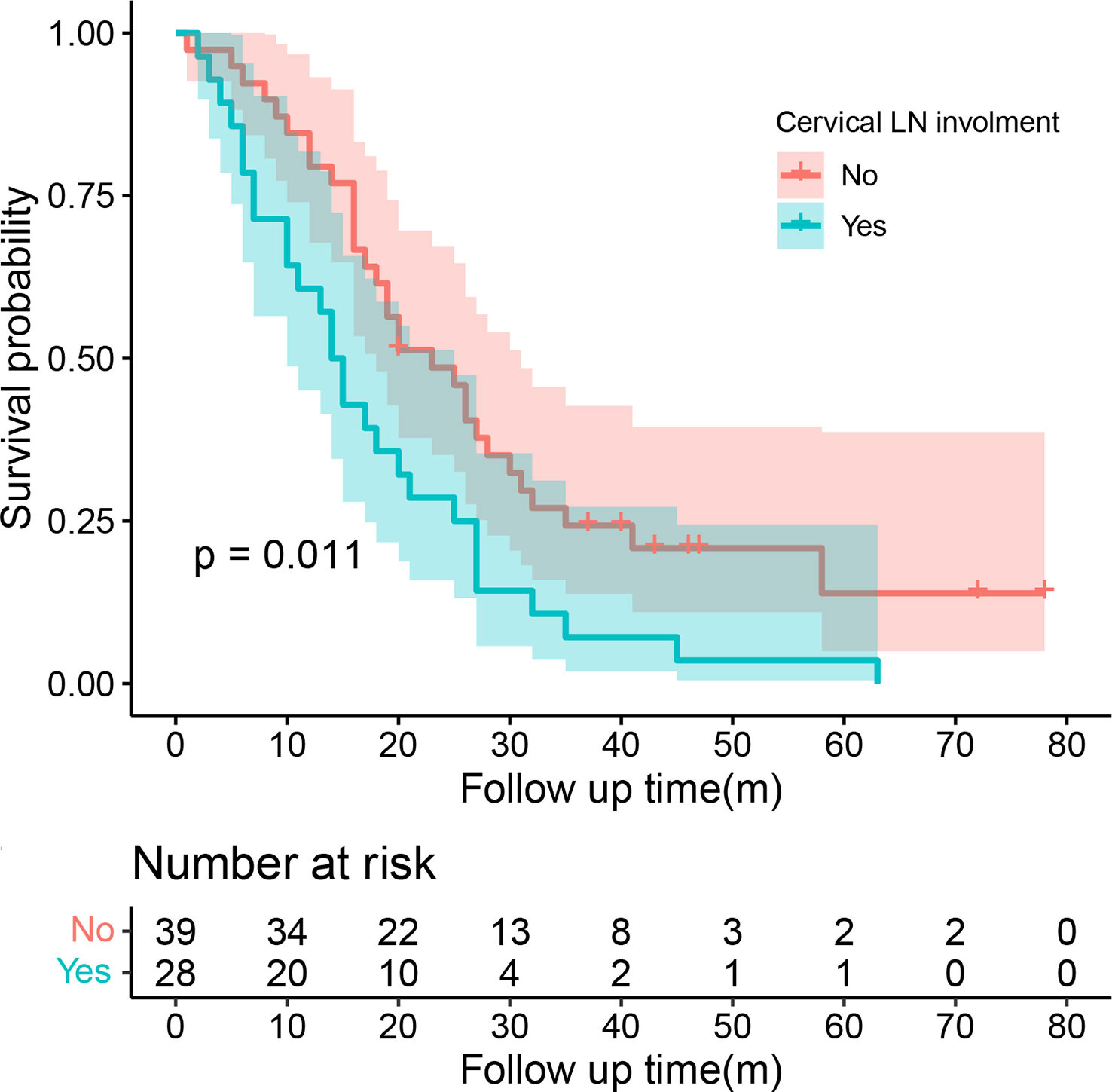
Univariate COX regression analysis showed that T stage (P=0.008), the distance between two lesions (HR=1.14, 95%CI=1.06-1.22, P<0.001), and chemotherapy (HR=0.64, 95%CI=0.41-0.99, P=0.048) may affect the survival of patients with SMPESCC (Table 3). Multivariate regression analysis showed the above factors were also independent prognostic factors for survival of SMPESCC patients, while N stage had no significant relationship with survival. However, when all the lesions of SMPESCC patients were located in the middle or lower thoracic segment, we found that the survival time of patients with cervical lymph node metastasis was shorter than that of those without lymph node metastasis. (Figure 3)
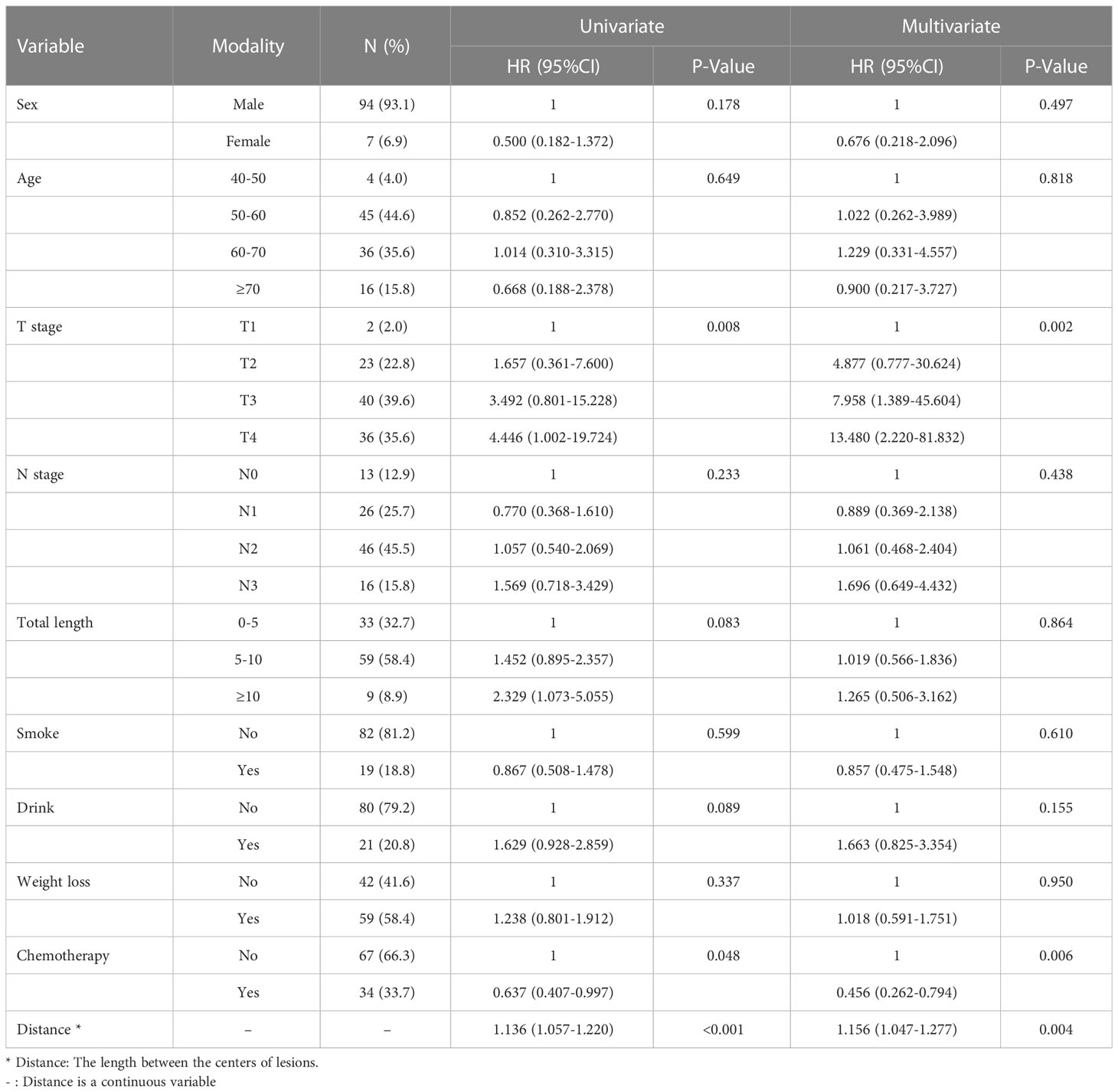
Table 3 Cox univariate and multivariate regression analysis for overall survival of SPESCC patients.
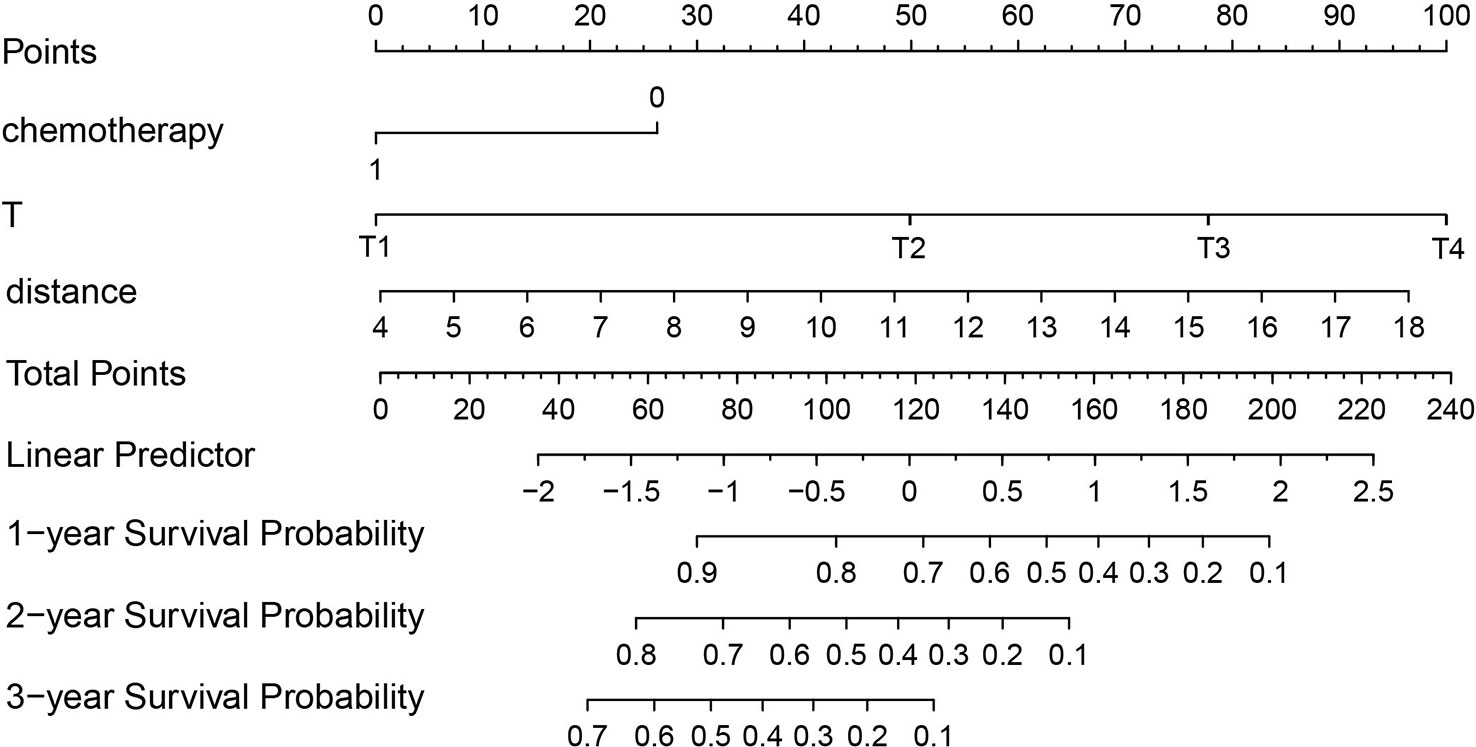
According to the results of the Cox regression model, the prognostic nomogram of OS was established by integrating the independent factors of OS. Nomogram according to T stage, the distance between two lesions, and the distribution point of chemotherapy (Figure 4). The axes of the individual covariates in the nomogram are plotted by ranking the effect estimates, with each covariate assigned a score on the scale. The total score for each covariate accumulation corresponded to the predicted probability of 1-year (AUC=0.764), 2-year (AUC=0.795), and 3-year survival (AUC=0.848). The bootstrap method was used to randomly select samples from the original data set. After 1000 repetitions, Harrell’s C index was 0.711, indicating the good performance of the model. The calibration curves for the predicted survival probability and the observed probability were close to the 45° diagonal (Figure 5). DCA was performed to determine the reliability of the prediction results based on nomograms under different decision thresholds. The DCA shows the positive net benefits of the nomogram model. Decision curves predicting the survival of patients with SMPESCC after 1 year, 2 years, and 3 years are shown in Figure 6.
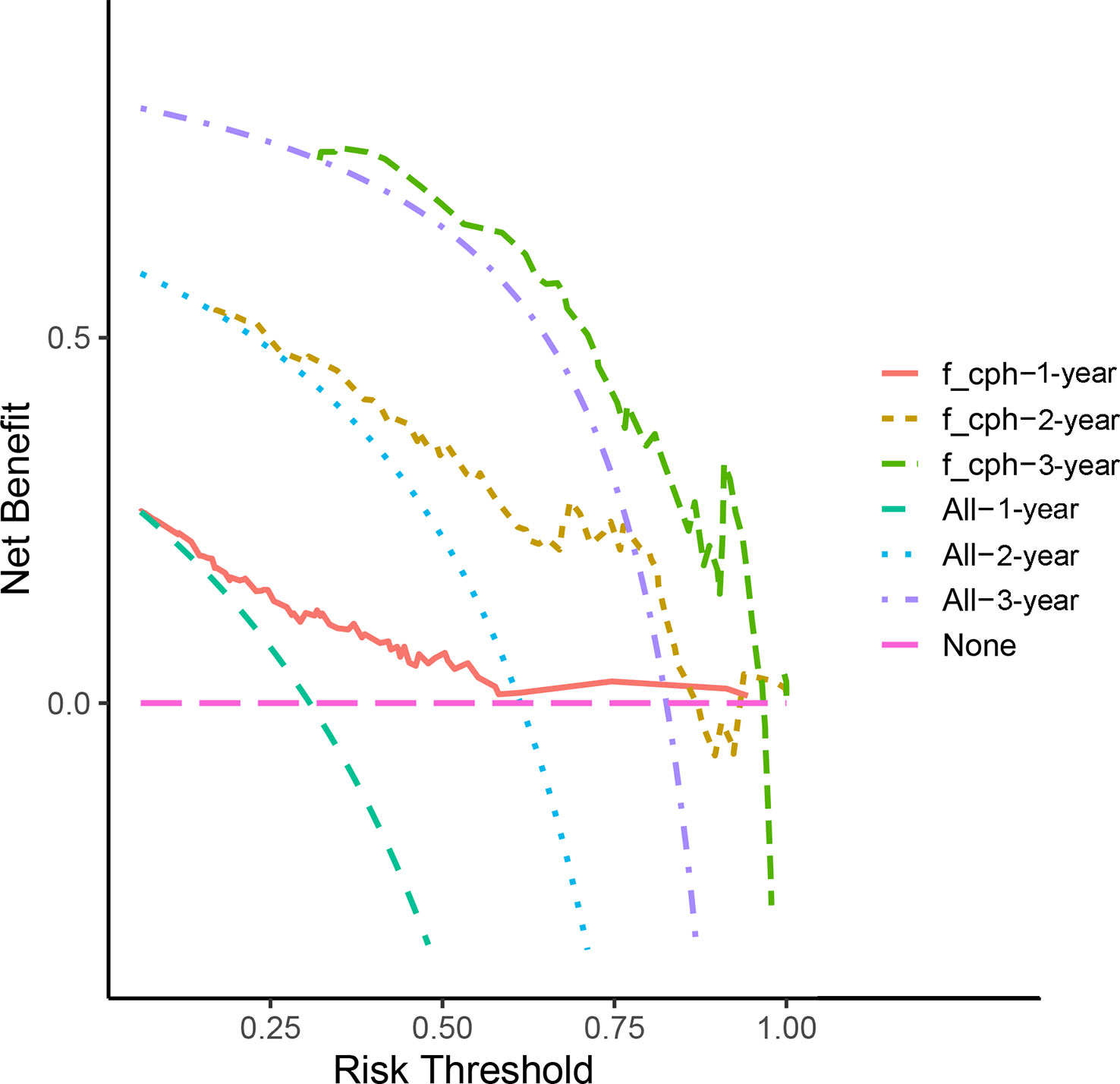
Figure 6 Decision curve analysis demonstrated positive net benefits by the nomogram model. The “f_cph-1,2,3-year” lines represent the net benefit of nomogram model, the “All-1,2,3-year” lines and “None” line represent that no patients and all patients would die, respectively.
Discussion
As the survival rate of patients with EC improves, the second primary cancer will become an increasing concern for patients with EC. The pathogenesis of multiple primary EC remains unclear. Strong et al. (15) proposed the field cancerization hypothesis that the whole esophagus was the entirety of the cancerization and the regional tissues were in different stages of the cancerization. When various pathogenic factors accumulate to a certain stage, one or more cancer foci would appear successively or simultaneously in different parts of the esophagus. Kuwabara et al. (16) performed a genetic analysis of SMPEC and showed that SMPEC patients presented with alterations in one or more gene loci. The most commonly mutated genes were TP53 and D18S61, and there were great differences in gene mutations between different patients. In our study, the incidence of SMPESCC was 9.77%, and the majority of SMPESCC patients were male. Gender differences were also observed in patients with EC, for reasons that are unclear (17). Yingcai H et al. suggested that up-regulation of Y-BOX9 in the sex-determining region was associated with the development of ESCC (18). However, we did not find that EC patients of any gender were more likely to develop multiple cancer. It was reported that individuals diagnosed at 60–79 years old, with earlier stage and/or moderately differentiated EC were more likely to get EC-multiple cancer (3). In addition to the influence of internal factors, long-term and chronic stimulation of external carcinogenic factors such as tobacco and ethanol can lead to regional carcinogenesis of the upper airway and digestive tract (19, 20). Multiple studies had shown that long-term smoking and alcohol consumption were also associated with the emergence of multiple cancer (6, 21). In our study, smoking and alcohol consumption were not significantly associated with SMPESCC, while older patients and those with previous weight loss were more likely to develop SMPESCC.
The survival of multiple primary cancers varies greatly in different pieces of literature because it is affected by the location of the tumor, the time of tumor occurrence, and the tumor stage. A large study of patients with multiple primary cancer showed significantly better survival than patients with a single primary cancer (22). Possible explanations for this were that different patient populations had different tumor sites (mainly favorable sites) and/or disease stages (mainly early stages). For patients with SMPESCC, Yang et al. (23) suggested that there was no significant difference in survival between SMPESCC and single ESCC in the study of SMPESCC patients undergoing surgery. However, they also proposed that when the T stage was T3 or T4, the survival time of SMPESCC was shorter. In our study, after balancing baseline characteristics between the single and multiple groups, we found that ESCC with multiple lesions was significantly associated with poorer overall survival. Consistent with the above studies, most of patients in our study had high T stage. Furthermore, our study showed that multiple primary cancer could be an independent prognostic factor for survival of ESCC patients receiving definitive radiotherapy, which was consistent with the conclusions of Yoshifumi B et al. (6).
In a study of simultaneous multiple gastric cancers, the results showed that the primary and secondary lesions were located in the same vertical direction, and the size of the two lesions was positively and significantly correlated (24). In our study, most of the major lesions in SMPESCC patients were located in the middle thoracic segment (34.65%) and lower thoracic segment (51.49%), and the minor lesions were mainly located in the lower thoracic segment (60.40%). However, no significant correlation was observed between the vertical position of the two lesions (P=0.053). For patients with inoperable EC, the clinical staging was mostly used to evaluate the tumor status. According to the degree of CT invasion and the relationship between the lesions and surrounding tissues and organs, these SMPESCC patients performed clinical T staging. Most patients were in T3 stage and T4 stage. Univariate and multivariate analyses showed that patients with early T stage had a higher survival rate than those with advanced T stage, indicating that this clinical T stage can be used as an independent prognostic factor for SMPESCC patients receiving definitive radiotherapy. No significant correlation was observed between N stage and prognosis. But it does not mean that lymph node involvement has no effect on survival. We found that cervical lymph node involvement was indicative of a poor prognosis in SMPESCC patients whose lesions were located in the mid-chest or lower-chest segments. The effect of tumor length on the prognosis of EC had been controversial. For SMPEC patients, the study by Eloubeidi. M.A et al. pointed out that the shorter the tumor length, the longer the survival time (25). However, our results showed no significant association between total tumor length and survival, which means that more studies were needed to confirm the relationship in the future. We used the center of the lesion to judge the location of the lesion and included the spacing of the lesion in the multivariate regression model. The results showed that the distance between lesions significantly affected the survival time of patients with SMPESCC. The longer the distance, the shorter the survival time of patients. In inoperable locally advanced EC, concurrent chemoradiotherapy had been shown to significantly improve survival compared with radiotherapy alone (26). Multivariate analysis of this study showed that chemotherapy was an independent prognostic factor for SMPESCC patients who received definitive radiotherapy (P=0.006), suggesting that SMPESCC patients would also benefit from chemotherapy.
Nomograms can generate individual probabilities of clinical events by integrating different prognostic variables, thus fulfilling our need for clinical integration models. To more clearly demonstrate the ability of each variable to predict the survival of patients with SMPESCC, we constructed a nomogram based on the Cox regression model. The nomogram is based on tumor T stage, tumor spacing, and chemotherapy mapping. Internal cross-validation showed that the prediction model had a good prediction ability for OS (C-index=0.711). At the same time, the calibration curves for the predicted probabilities and the actual observed events of the 1-year, 2-year, and 3-year OS were close to a 45-degree diagonal. DCA also showed a positive net benefit, indicating a good calibration and clinical benefit of our model.
Limitation
This study is a retrospective article. Although multiple esophageal cancer is still a rare disease, we did not have enough cases to explore the prognosis, so there was no external verification after the nomogram was developed. Therefore, prospective studies with a larger sample size are needed to further elucidate the prognostic factors of SMPESCC patients and external validation of nomograms.
Conclusion
In conclusion, SMPESCC is a relatively rare but aggressive tumor. The appearance of multiple primary cancer makes the prognosis of esophageal cancer patients more pessimistic. Multivariate analysis showed that T stage, lesion spacing, and chemotherapy were independent prognostic factors, suggesting that SMPESCC patients who received definitive radiotherapy could benefit from chemotherapy. Based on the independent prognostic factors associated with OS, we established Nomogram models to predict 1-year, 2-year, and 3-year survival of SMPESCC patients, which can effectively predict individual survival time. In the meantime, our results need to be validated by prospective large clinical studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Research Ethics Committee of the First Affiliated Hospital of China Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
WW: Conceptualization, Methodology, Formal analysis, writing-Original Draft; XL: Data Curation, Visualization; JD: Writing-Review & Editing. GL: Validation, Supervision, Writing-Review & Editing. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank Dr.li and Dr Gao for their advice and guidance on the content of the paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet (2017) 390(10110):2383–96. doi: 10.1016/S0140-6736(17)31462-9
2. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet (2013) 381(9864):400–12. doi: 10.1016/s0140-6736(12)60643-6
3. Hu Z, Zhang M, Wang Z, Song J, Jiang W, Li L, et al. An observational study on the clinical features of esophageal cancer followed by multiple primary cancers. Future Oncol (2019) 15(6):601–10. doi: 10.2217/fon-2018-0621
4. Shibuya H, Takagi M, Horiuchi J, Suzuki S, Kamiyama R. Carcinomas of the esophagus with synchronous or metachronous primary carcinoma in other organs. Acta Radiol Oncol (1982) 21(1):39–43. doi: 10.3109/02841868209133982
5. Koide N, Komatsu D, Hiraga R, Kitazawa M, Suzuki A, Miyagawa S. Esophageal cancer associated with other primary cancers–historical comparison of clinicopathologic features in 359 esophageal cancer patients. Hepatogastroenterology (2010) 57(99-100):513–8. doi: 10.1016/j.apradiso.2004.03.084
6. Baba Y, Yoshida N, Kinoshita K, Iwatsuki M, Yamashita Y-I, Chikamoto A, et al. Clinical and prognostic features of patients with esophageal cancer and multiple primary cancers: A retrospective single-institution study. Ann Surg (2018) 267(3):478–83. doi: 10.1097/SLA.0000000000002118
7. Tabuchi T, Ito Y, Ioka A, Nakayama T, Miyashiro I, Tsukuma H. Tobacco smoking and the risk of subsequent primary cancer among cancer survivors: A retrospective cohort study. Ann Oncol (2013) 24(10):2699–704. doi: 10.1093/annonc/mdt279
8. Yang J, Liu X, Cao S, Dong X, Rao S, Cai K. Understanding esophageal cancer: The challenges and opportunities for the next decade. Front Oncol (2020) 10:1727. doi: 10.3389/fonc.2020.01727
9. Uhlenhopp DJ, Then EO, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: Update in global trends, etiology and risk factors. Clin J Gastroenterol (2020) 13(6):1010–21. doi: 10.1007/s12328-020-01237-x
10. Zeng H, Zheng R, Guo Y, Zhang S, Zou X, Wang N, et al. Cancer survival in China, 2003-2005: A population-based study. Int J Cancer (2015) 136(8):1921–30. doi: 10.1002/ijc.29227
11. Li M, Lin ZX. Characteristics and prognostic factors of synchronous multiple primary esophageal carcinoma: A report of 52 cases. Thorac Cancer (2014) 5(1):25–30. doi: 10.1111/1759-7714.12047
12. Chen JY, Zhang SS, Fu XY, Wen J, Yang H, Zhang YJ, et al. The characteristics and prognostic significance of esophageal squamous cell carcinoma with synchronous multiple lesions: Over 10-year experience. Esophagus (2021) 18(4):851–60. doi: 10.1007/s10388-021-00856-8
13. Warren S. Multiple primary malignant tumors. a survey of the literature and a statistical study. Am J Cancer (1932) 16:1358–414. doi: 10.1016/0016-5085(87)90440-9
14. Xiao Z, Yang Z, Wang M. Radiotherapy of multi-focal esophageal carcinoma. Chin J Radiat Oncol (1989) 4(4):222–4.
15. Strong MS, Incze J, Vaughan CW. Field cancerization in the aerodigestive tract–its etiology, manifestation, and significance. (1984) 13(1):1–6. doi: 10.1017/S0022215100148406
16. Kuwabara T, Hiyama T, Tanaka S, Yoshihara M, Arihiro K, Chayama K. Genetic pathways of multiple esophageal squamous cell carcinomas. Oncol Rep (2011) 25(2):453–9. doi: 10.3892/or.2010.1110
17. Huang J, Koulaouzidis A, Marlicz W, Lok V, Chu C, Ngai CH, et al. Global burden, risk factors, and trends of esophageal cancer: An analysis of cancer registries from 48 countries. Cancers (Basel) (2021) 13(1):141. doi: 10.3390/cancers13010141
18. Hong Y, Chen W, Du X, Ning H, Chen H, Shi R, et al. Upregulation of sex-determining region y-box 9 (Sox9) promotes cell proliferation and tumorigenicity in esophageal squamous cell carcinoma. Oncotargets (2015) 6(31):31241. doi: 10.18632/oncotarget.5160
19. Matsumoto A, Watanabe M, Shigaki H, Nishida K, Mine S, Sano T, et al. Efficacy of staged treatment strategy for patients with synchronous double cancers of the esophagus and head and neck: A retrospective study. World J Surg (2016) 40(2):388–94. doi: 10.1007/s00268-015-3276-1
20. Yokoyama A, Muramatsu T, Ohmori T, Makuuchi H, Higuchi S, Matsushita S, et al. Multiple primary esophageal and concurrent upper aerodigestive tract cancer and the aldehyde dehydrogenase-2 genotype of Japanese alcoholics. cancer (1996) 77(10):1986–90. doi: 10.1002/(SICI)1097-0142(19960515)77:10<1986::AID-CNCR4>3.0.CO;2-F
21. Yoshida N, Eto K, Kurashige J, Izumi D, Sawayama H, Horinouchi T, et al. Comprehensive analysis of multiple primary cancers in patients with esophageal squamous cell carcinoma undergoing esophagectomy. Ann Surg (2020) 276(2):305-11. doi: 10.1097/sla.0000000000004490
22. Amer MH. Multiple neoplasms, single primaries, and patient survival. Cancer Manag Res (2014) 6:119–34. doi: 10.2147/CMAR.S57378
23. Yang Y, Tang P, Ma M, Zhang H, Wang H, Zhu K, et al. Comparison of clinicopathological features and prognostic significance between synchronous multiple primary and solitary esophageal squamous cell carcinomas. BMC Cancer (2022) 22(1):1191. doi: 10.1186/s12885-022-10283-2
24. Kim JH, Jeong SH, Yeo J, Lee WK, Chung DH, Kim KO, et al. Clinicopathologic similarities of the main and minor lesions of synchronous multiple early gastric cancer. J Korean Med Sci (2016) 31(6):873–8. doi: 10.3346/jkms.2016.31.6.873
25. Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Wilcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the us: The importance of tumor length and lymph node status. Cancer (2002) 95(7):1434–43. doi: 10.1002/cncr.10868
Keywords: esophageal cancer, multiple primary cancer, definitive radiotherapy, survival, nomogram
Citation: Wang W, Liu X, Dang J and Li G (2023) Survival and prognostic factors in patients with synchronous multiple primary esophageal squamous cell carcinoma receiving definitive radiotherapy: A propensity score-matched analysis. Front. Oncol. 13:1132423. doi: 10.3389/fonc.2023.1132423
Received: 27 December 2022; Accepted: 13 March 2023;
Published: 24 March 2023.
Edited by:
Mingzhou Guo, People’s Liberation Army General Hospital, ChinaReviewed by:
Martin Leu, University Medical Center Göttingen, GermanyZhenzhou Yang, Chongqing Medical University, China
Copyright © 2023 Wang, Liu, Dang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guang Li, TEcxMzgwNDA1ODYxNkAxNjMuY29t
†These authors have contributed equally to this work
‡ORCID: Guang Li, orcid.org/0000-0001-5436-1491
 Wenyi Wang1†
Wenyi Wang1† Jun Dang
Jun Dang Guang Li
Guang Li