- Department of Clinical Laboratory, Harbin Medical University Cancer Hospital, Harbin, China
Ovarian cancer is the most deadly gynecologic malignancy, and its incidence is gradually increasing. Despite improvements after treatment, the results are unsatisfactory and survival rates are relatively low. Therefore, early diagnosis and effective treatment remain two major challenges. Peptides have received significant attention in the search for new diagnostic and therapeutic approaches. Radiolabeled peptides specifically bind to cancer cell surface receptors for diagnostic purposes, while differential peptides in bodily fluids can also be used as new diagnostic markers. In terms of treatment, peptides can exert cytotoxic effects directly or act as ligands for targeted drug delivery. Peptide-based vaccines are an effective approach for tumor immunotherapy and have achieved clinical benefit. In addition, several advantages of peptides, such as specific targeting, low immunogenicity, ease of synthesis and high biosafety, make peptides attractive alternative tools for the diagnosis and treatment of cancer, particularly ovarian cancer. In this review, we focus on the recent research progress regarding peptides in the diagnosis and treatment of ovarian cancer, and their potential applications in the clinical setting.
1 Introduction
Ovarian cancer (OC) is a common gynecologic malignancy with 314,000 new cases and 207,000 deaths in 2020 year (1). Due to the early stage with unique symptoms such as pelvic/abdominal pain, abdominal distension, and frequent urination/urgency, and the lack of apparent diagnostic indicators, more than 70% of patients had advanced disease at presentation (2). OC treatment regimens are generally platinum- and paclitaxel-based chemotherapy regimens after initial cytoreductive surgery (3). Despite improvement after treatment, most patients experience relapse within 3 years due to the development of chemoresistance, leading to a significant decrease in survival (4). In addition, due to the lack of targeting, chemotherapy drugs not only kill cancer cells but also damage normal cells, which has certain toxic side effects on patients. Therefore, the development of specific early diagnostic indicators and the improvement of anti-tumor drug targeting are two issues that need to be addressed urgently.
The development of targeted therapy, including targeted anti-angiogenesis therapy, immune checkpoint inhibitor therapy and Poly-ADP-ribose polymerase (PARP) inhibitors therapy, brings hope to many OC patients (5). In targeted therapy, the selection of appropriate ligands is a crucial step. In the past, antibody-drug conjugate (ADC) was an advanced targeted drug delivery strategy for OC therapy, which could selectively deliver cytotoxic drugs to tumor cells. However, its application in vivo was limited by poor delivery due to the high molecular weight of antibodies and systemic toxicity caused by non-specific uptake of the reticuloendothelial system (6). In recent years, peptides have attracted a lot of attention in tumor diagnosis and treatment. They are naturally occurring compounds, usually 2-50 amino acid residues (AA) long, responsible for many biological functions. As targeting ligands, peptides exhibit high affinity and specificity for the corresponding receptors. Compared with antibodies and proteins, polypeptides have the characteristics of small molecular weight, low immunogenicity, and easy chemical modification, which makes the polypeptide have stronger cell, tissue penetration and metabolic stability (7). Moreover, combinatorial library chemistry and phage display strategies provide source safeguards for the development of various peptides with targeting potential (8).
Currently, peptide-based research is attracting numerous researchers for development and application. In addition to its use as a targeting ligand for therapeutic purposes it is also (I) widely used in diagnostic imaging due to its specific targeting and rapid renal clearance (9); (II) differential peptides secreted in body fluids are used as diagnostic markers (10); (III) some peptides are related to cancer cell growth, migration, invasion and other activities, and these differentially expressed or peptides acting on therapeutic targets inhibit the occurrence and development of tumors (11); (IV) peptide vaccines (12). Based on these properties, peptides may be promising candidates for tumor diagnosis and therapy. Some excellent reviews have reported the value of peptides in cancer diagnosis and treatment (13, 14); Here, we discuss the role of peptides in the diagnosis and treatment of ovarian cancer.
2 Peptide diagnosis in ovarian cancer
Peptide biomarkers can be widely divided into laboratory diagnosis and imaging diagnosis in diagnostic methods. In terms of laboratory diagnosis, some endogenous peptides can reveal the different pathology and heterogeneity of diseases by reflecting the expression of disease proteins or changes in the corresponding enzyme activities, which have the potential to become promising diagnostic markers (15). In terms of imaging, peptide receptors overexpressed in ovarian cancer have been used as molecular targets to localize tumors, and radiolabeled peptides can accurately localize primary tumor lesions (16).
2.1 Peptide-based laboratory diagnosis
In clinical laboratory diagnosis, the research focus is still on biological specimens such as blood, urine, saliva, and cerebrospinal fluid, which can be obtained by non-invasive or minimally invasive techniques (17). In general, abnormal proteins in body fluids such as CA125 and HE4 have been the first choice for ovarian cancer examination. However, detection sensitivity and specificity continue to fall short of the desired aim, and there are certain limits in early diagnosis (18). The exploration of peptide markers has attracted much attention in recent years and has potential clinical application prospects. Peptide markers can be relatively resistant to protease degradation, and small peptides may survive even if the protein is degraded by proteases in the tumor extracellular space or circulation (19). Therefore, it may provide more information than the precursor protein (Protein containing the target polypeptide sequence). Moreover, advances in technologies such as peptidomics, peptide microarray, and reaction monitoring (SRM) analysis support the detection of more low-molecular-weight, tumor-specific peptides in body fluids. In addition to high specificity, SRM analysis provides sensitivity and linearity, providing an opportunity for accurate quantification of peptides (20, 21).
The research has shown that peptides with different clinical features can be identified, quantified and compared using detection techniques, and these low molecular weight peptides are more likely to be markers for diagnosis. A prospective study about urinary micropeptides in patients with ovarian cancer has shown that they presenced in about 62.5% of patients, and were related to the pathological stage. Under 100% specificity, the detection sensitivity of CA125 was 23.2%, while that of urinary micropeptides was 62.5% (22). At the peptidome level, more peptides could be observed in serum samples compared to plasma. However, serum shows many strong artificial peptide signals that hinder the sensitive detection of endogenous peptides. Therefore, most investigators have turned their goals to plasma samples (23, 24). Wang and colleagues (20) used SRM method to detect peptide biomarkers and found that 11 (64.7%) of 17 patients with early ovarian cancer had positive scores of small peptides from peptidyl prolyl cis-trans isomerase A(PPIA), which may be a useful diagnostic marker for ovarian cancer. Lu’s group (18) isolated three promising predictive small peptides from plasma samples of 140 patients with epithelial ovarian cancer (EOC) and 158 patients with benign ovarian tumors (BOT), and their combination with CA125 increased the specificity (0.944) and AUC (0.904) of CA125 while maintaining an acceptable sensitivity of 0.804.
2.2 Peptide-based imaging diagnosis
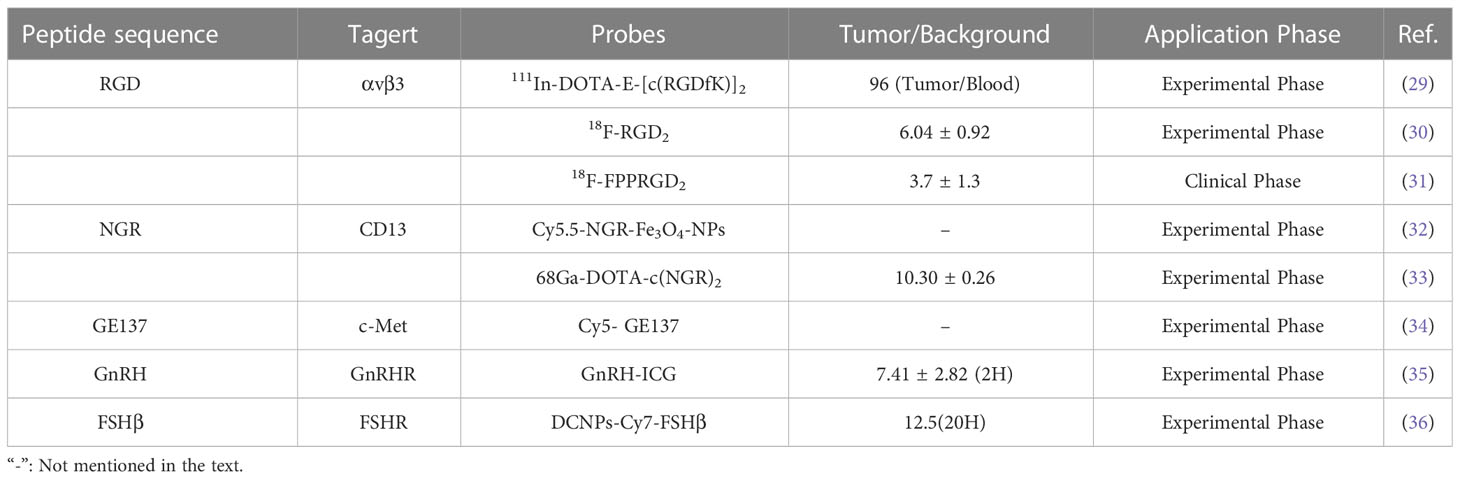
Traditional imaging techniques rely on non-specific imaging methods for disease examination, such as image contrast using anatomical, physiological, or metabolic abnormalities, and are often less efficient at targeting (25). In fact, molecular or biochemical alterations in disease often precede morphological and functional alterations and can more accurately characterize tumor properties or biological processes (26). Based on the observation that peptides can bind specifically to disease-associated receptors, coupling imaging molecules to peptides is a simple and accurate way to improve traditional imaging. In general, the size of the polypeptide can provide sufficient attachment sites for the chelating agent molecule, which is more suitable for linking with the chelating agent than the larger molecule, without losing the binding affinity with the receptor (27). The advantages of using radiopeptide imaging include the ease of synthesis and radiolabeling, as well as the ease of modification that enhances renal excretion and significantly improves kinetics (28). Furthermore, the small molecular weight of peptide allows for rapid blood clearance and renal excretion, which makes it among the key features of an ideal imaging probe. Many selected peptides or peptide analogues used to target OC-related molecules or receptors show high affinity and specificity for the receptors, such as RGD, NGR, etc (Table 1) (37, 38).
The RGD (Arg-Gly-Asp) peptide recognizes the highly expressed αvβ3 integrin in ovarian cancer cells and can specifically bind αvβ3 with high affinity after radiolabeling. Imaging probes based on RGD peptides show good imaging effects on ovarian cancer and are one of the most promising diagnostic probes (39). Janssen and colleagues (29) synthesized radiolabeled dimeric RGD peptide E- [c (RGDfK)]2, which showed specific targeting, good tumor uptake, and rapid renal excretion in the OVCAR-3 xenograft model. Yang’s group (30)found that 18F-RGD2 had a higher tumor/background ratio than 18F-FDG in SKOV-3 xenograft mice (6.04 ± 0.92 vs. 3.34 ± 0.79), and in addition, the anti-angiogenic effect was sensitively and reliably detected at low doses of paclitaxel. At present, imaging probes based on RGD peptides have entered preclinical or clinical trials. In a clinical study of ovarian and breast cancer, the PET/CT imaging analysis found that RGD-based imaging probes (18F-FPPRGD2) can provide independent information with 18F-FDG, although the foci uptake and tumor/background ratio of 18F-FPPRGD2 were lower than 18F-FDG, but 1 patient increased uptake after 1 week of anti-angiogenesis therapy. This is a potential benefit of RGD peptide-based PET tracers with the potential to predict anti-angiogenesis therapy early (31).
Aminopeptidase N (APN/CD13), a membrane protein and marker of tumor angiogenesis, is upregulated in various solid tumors, including melanoma, prostate, ovarian, lung, and breast cancers (40). NGR (Asn-Gly-Arg) peptide specifically recognizes aminopeptidase N on the surface of tumor cells. Radiolabeled NGR has been reported to have high uptake in ovarian tumors and is excreted through the kidneys (41). Meng and colleagues (32)prepared Cy5.5-labeled, NGR-conjugated iron oxide nanoparticles (Cy5.5-NGR-Fe3O4 NPs) as imaging nanoprobe. MR and NIRF dual-modality imaging showed that NGR targeting effectively improved tumor uptake and enhanced imaging contrast, in contrast, the control group image did not change significantly. In another work, NGR peptide-based imaging probes exhibited selective targeting and quick blood clearance of ES2 tumors, with high contrast tumor visualization occurring at 1 h and a maximal tumor/background ratio of 10.30 ± 0.26 (33).
In addition to radiolabeling, optical imaging of fluorescently labeled peptides may be advantageous because it may improve the detection of metastatic tumor deposits while reducing the risk of population radioactivity exposure (42, 43). Liu and colleagues (34) report the biological characterization of fluorescently labeled GE137 (c-Met targeting peptide) in ovarian cancer cells. The peptide demonstrated selective tumor uptake and a consistently high signal-to-noise ratio, allowing real-time observation of subcutaneous submillimeter SKOV3 tumor deposition in mice. Besides, some fluorescent probes can be used to detect ovarian cancer metastasis. In ovarian cancer mice, Liu’s group found that GnRHa-ICG was specifically localized in peritoneal tumors with high tumor-to-muscle and tumor-to-gut ratios and stable fluorescent signals compared to ICG injection alone (35). Intraoperative specific fluorescence imaging has been reported to improve tumor stage and size and improve prognosis. Wang’s team (36) designed in vivo assembly of NIR-II emitting down conversion nanoparticles (DCNPs) modified with DNA and FSHβ peptides. Imaging showed that the tumor background ratio remained at 12.5 for 20-28 hours after injection, the images could clearly distinguished the tumor margin, and HE staining demonstrated that even macroscopically invisible ultra-small tumors ≤ 1 mm could be detected and completely resected.
3 Peptide therapy in ovarian cancer
Peptide-based therapy can be divided into three main types, peptide ligand targeted therapy, anticancer peptide therapy, and peptide vaccines. Peptides are employed as ligands for targeted treatment, recognizing tumor markers and the ability to effectively deliver therapeutic drugs to tumor sites to enhance efficacy and minimize side effects (44). Anticancer peptides are proliferation inhibitors that exhibit antitumor activity by regulating the growth, apoptosis and other activities of various cancer cells in vitro and in vivo. Hence, long-term therapy with appropriate peptide analogs may be able to diminish or stop tumor growth in vivo (45). In advanced cancers, peptide-based vaccines have been used to deliver tumor antigen epitope antigens to T cells in order to elicit a cell-mediated anti-tumor response and improve overall patient survival (46).
3.1 Targeted therapy based on peptide ligands
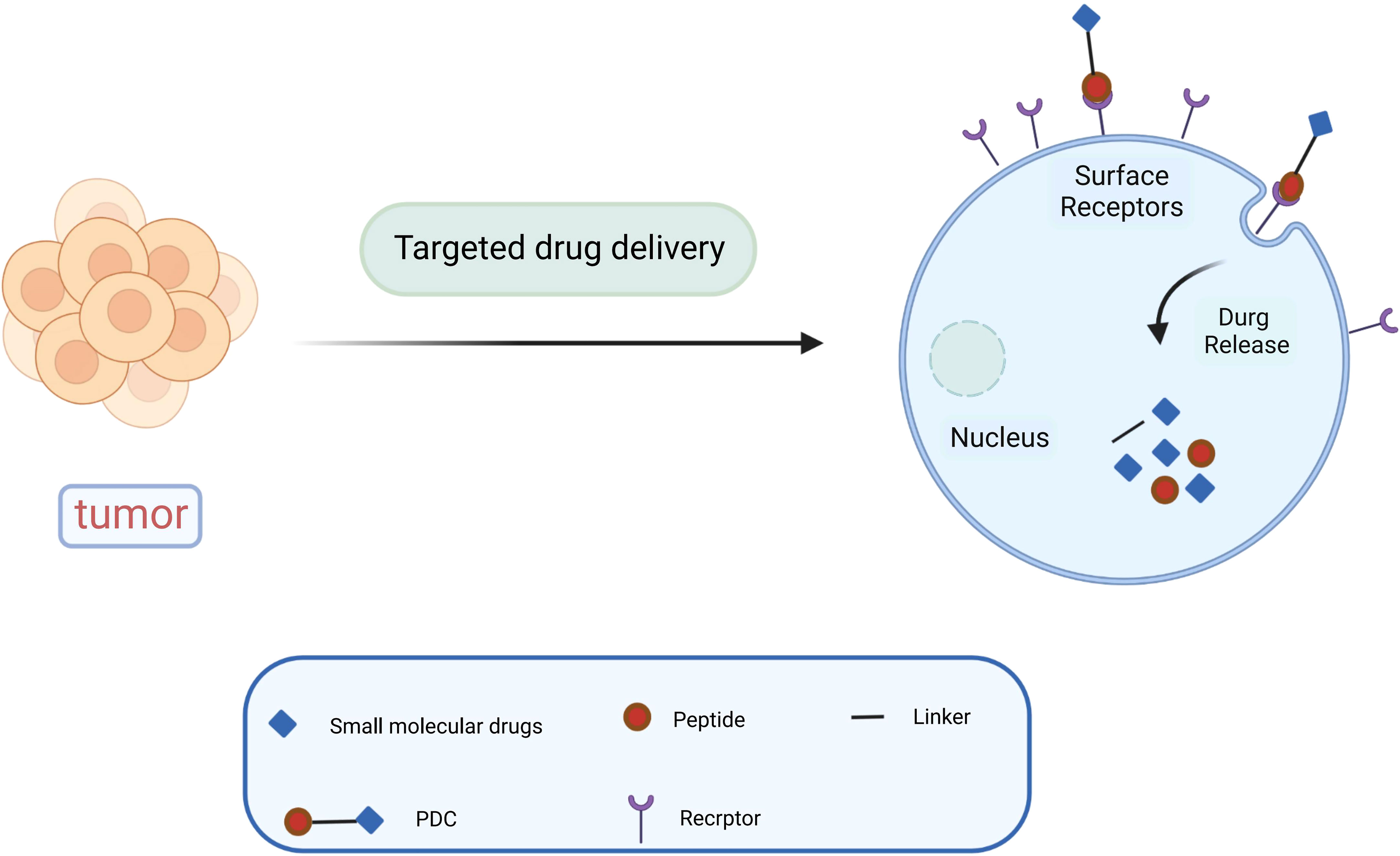
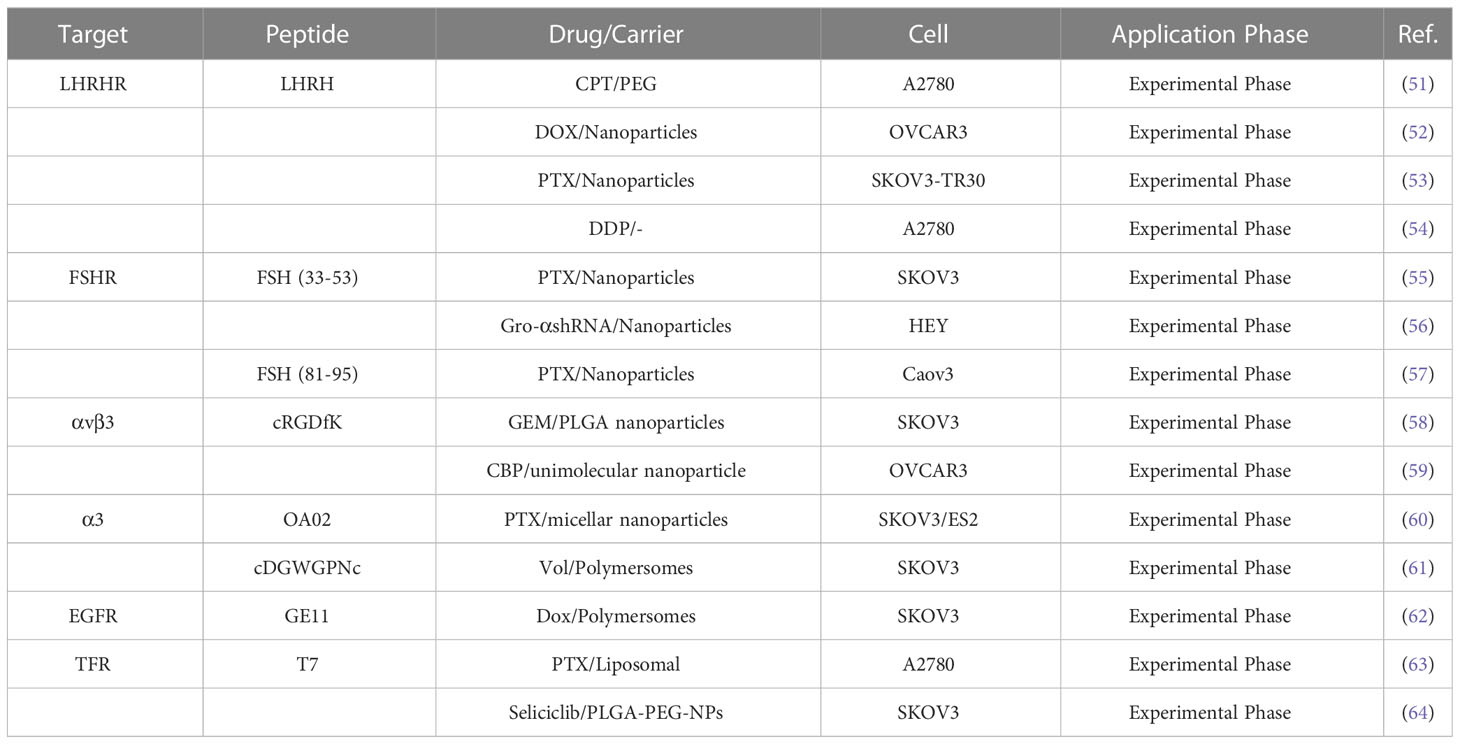
Paclitaxel combined with platinum-based chemotherapy is the first-line regimen for ovarian cancer. However, the low selectivity and short retention period of current chemotherapeutic agents limit their exposure time at the lesion site (47). Reasonable targeting can significantly improve the treatment effect of ovarian cancer, reduce adverse reactions and drug resistance, and enhance the bioavailability of chemotherapy drugs (48). Anticancer drugs are attached to peptide carriers via biodegradable linkers, and peptide are internalized by binding to specific receptors, which in turn deliver the drug to the target site for action (Figure 1). In specific tumor-targeted therapies, the use of polypeptides as targeted ligands exhibits a variety of advantages, including low toxicity, low or no immunogenicity, and high specificity (49). Peptides are also highly permeability because they are tiny and generally hydrophilic molecules, allowing for simple and quick access into tumor locations. Polypeptides coupled to the surface of nanoparticles can mediate active targeting, as a complementary strategy to enhance permeability and retention (EPR) effects, and to facilitate targeted cell uptake of drugs (50). A variety of peptide ligands including LHRH peptides, FSHR peptides, RGD peptides, OA02 peptides have been designed and explored for OC targeted therapy (Table 2), and drug delivery is achieved through various carrier systems such as liposomes, polymers, micellars, and nanoparticles.
3.1.1 Targeted LHRH peptide receptor therapy
High gonadotropin levels are considered one of the main etiological assumptions for the development of ovarian cancer (65). In a retrospective study, 54.5% FSHR and 88.3% GnRHR expression were detected in 875 cases of high-grade serous ovarian cancer tissues (66). As a result, it has the potential to be a therapeutic target for boosting anti-tumor activities and decreasing adverse effects. LHRH peptides, as a targeted component, allow anticancer medications to be directed specifically to tumors while minimizing injury to normal tissues (51). Recent research indicated that various cytotoxic drugs, such as different combinations of platinum, Doxorubicin (DOX), camptothecin (CPT) with LHRH peptides, had sound drug delivery effects (52). Dharap and colleagues (52) targeted ovarian tumors by combining LHRH peptide with chymopapain (CPT, an apoptosis inducer). They discovered that using LHRH peptide as a targeting moiety improved chemotherapeutic effectiveness, increased tumor apoptosis induction, and reduced the negative effects of CPT on vital organs. Notably, the 2.5 mg/kg dose of CPT-PEG-LHRH in mice did not disrupt the circulating brain and pituitary barriers circulation. Many nanomaterials have been developed as drug carriers to enhance drug penetration and retention effects (67). However, their cellular targeting levels are limited, which limits the toxic effects of drugs (68). LHRH peptide-mediated multifunctional nanomaterials targeting tumors could significantly improve drug delivery and therapeutic efficacy against drug-resistant cancers (69). Pan and colleagues (53) found in a paclitaxel-resistant ST30 cell model that LHRH peptide targeting prolonged drug circulation and enhanced cytotoxicity compared to untargeted nano-delivery vehicles (IC50:3160nM vs 1285nM). LHRH peptide modification of DOX-loaded nanoparticles enhances the specific uptake and therapeutic efficiency of nanoparticles by OVCAR-3 cancer cells (54). In vitro studies show 80% cell growth inhibition within 72 hours; in vivo imaging results indicated that LHRH-HA-cys-ADOX/Cy5.5 nanoparticles could accumulate at the tumor site and exert long-lasting anti-tumor effects. A recent study discovered that LHRH peptides can directly functionalize novel platinum (IV), increasing platinum water solubility, and that this complex specifically targets the A2780 cell line with 5-8 times more cytotoxicity than LHRH receptor-negative cell lines (70).
3.1.2 Targeted FSHR peptide receptor therapy
Follicle stimulating hormone receptor (FSHR) is another specific hormone receptor for OC. It is expressed not only in plasmacytotic ovarian cancer but also in other particularly aggressive and chemoresistant histological types, such as clear cell and mucinous tumors (71). Peptide sequences on the FSHβ chain have been shown to act as targeting ligands for targeting cells with high expression of FSHR (55). Based on this, Modi’s team (72) designed nanocarriers of FSH (FSH33) peptide-coupled dendrimer molecules as a potential delivery platform. Their study showed that this new system could potentially be used to deliver chemotherapeutic drugs to malignant cells in the ovary while preserving healthy cells. It has been reported that the FSHβ (33-53) peptide has the highest affinity to the FSHR binding domain, and coupling this sequence to PEG-PLA nanoparticles as a paclitaxel (PTX) delivery system to target ovarian cancer cells could enhance the anticancer effects of chemotherapeutic drugs (56). In another work, FSH (33-53) peptide-coupled PEG-PEI copolymer that encapsulated shRNA to silence growth regulatory oncogene alpha (gro-α), successfully down-regulated gro-a expression in HEY cells (FSHR+), which dramatically suppressed cancer cell proliferation, invasion, and migration (57). To achieve higher tumor uptake, Zhang and colleagues (73) used a smaller molecular weight FSH (81-95) peptide as a FSHR-targeting ligand. At equivalent PTX doses, modification (active targeting) of FSH (81-95) peptide improved antitumor efficacy, resulting in a significant delay in tumor growth and 65.57% tumor volume inhibition.
3.1.3 Targeted integrin receptor therapy
The generation of new blood vessels provides oxygen and nutrients for tumor growth and is a hallmark of tumorigenesis and metastasis. It has been demonstrated that the high density of both developing and established vessels contributes to the poor prognosis of ovarian cancer patients (74). Integrins are associated with the process of neovascularization and help to strengthen the adhesion, invasion, and migration of cancer cells (75). Expression of integrin αv subunits was detected in 100% (30/30) solid tumors of ovarian cancer and malignant effusions in 93% (113/121) (76). As a ligand of αvβ3, RGD peptide selectively delivers therapeutic drugs to tumor cells through coupling with liposomes, micelles, nanoparticles and other drug carriers, which enhances the targeted uptake of drugs by tumor cells (58). Kulhari’s team (59) used cRGDfK to modify gemcitabine-containing nanoparticles, which reduced the nonspecific lytic cytotoxicity of the drug compared to unmodified, while enhancing the antiproliferative effect of the drug by modulating mitochondrial membrane potential (DΨm) and reactive oxygen species (ROS) levels. Wang ‘s group (77) similarly demonstrated that in OVCAR-3 ovarian cancer cells, cRGD-coupled nanoparticles exhibited higher cellular uptake (3.4-fold) than non-targeted, thereby leading to higher cytotoxicity. OA02 peptide and cDGWGPNc(A3) peptide are ovarian cancer-specific peptides screened by bacteriophage display technology, and are bound to α3 integrin (60, 78). OA02 peptide modified micellar nanoparticles based on PEG-dendritic bile acid (PEG5k-CA8), enhanced by 6- and 4-fold uptake in SKOV-3 and ES-2 cells, respectively. Moreover, mice treated with 30 mg/kg PTX-OA02-NPs showed the longest survival time, a significant improvement compared to PBS, Taxol, PTX-NPs (61). Polo-like kinase 1 (PLK1) inhibitor volasertib (Vol) is a molecular drug for the treatment of OC patients. A recent experiment conducted by Wang and colleagues (79) demonstrated that the α3 integrin ligand A3 peptide-targeted polymer (A3-Ps) enhanced deep tumor penetration and increased the cycle time of Vol, significantly improving tumor suppression (90%) (90%).
3.1.4 Targeted epidermal growth factor receptor therapy
Epidermal growth factor receptors (EGFRs) belong to the tyrosine kinase family and include four receptor subtypes. EGFR binds to ligands after dimerization, and triggers receptor enzyme activity through phosphorylation of the tyrosine residue at the end of the receptor C-terminal, which causes intracellular signaling to be activated, promotes cell proliferation, inhibits cell apoptosis, and enhances cell motility and angiogenesis (80, 81). EGFR overexpression has been reported in 30-98% of epithelial ovarian cancers (82). Small peptides with EGFR targeting such as LALLT, YHWYGYTPQNVI (GE11) and so on have been screened out in a variety of ways. As EGFR-targeted ligands, GE11 peptides are different from anti-EGFR antibodies and EGF in that they effectively bind to epidermal growth factor receptors without activating the EGFR-mediated signaling pathways (83). Rapid targeting of GE11 peptides can optimize the distribution of some drug carriers (liposomes) in internal tumor tissue to improve in vivo efficacy (62). Zou’s team (84) studied the therapeutic effect of GE11 peptide-modified reversibly cross-linked polymersomal doxorubicin (GE11-PS-Dox) on ovarian cancer. They found that GE11-PS-Dox exhibited higher tumor uptake and retention in vitro and in vivo, effectively inhibited SKOV3 tumor progression and reduced adverse effects in mice compared to control, which in turn significantly increased the survival rate by 100%. This suggests that GE11-guided drug targeting could be an alternative approach to treat EGFR overexpressing ovarian cancer.
3.1.5 Targeted transferrin receptor therapy
Ovarian tumor, particularly high-grade serous ovarian cancer (HGSOC), exhibit enhanced iron uptake and retention. Changes in iron metabolism may promote ovarian cancer cell proliferation and metastasis by manipulating mechanisms such as p53 inactivation and ROS, c-myc expression (85). Transferrin receptor (TFR) is a transmembrane glycoprotein that binds with its natural iron-rich ligand transferrin (TF) to engage in cellular iron transport. TFR1 expression has been reported to be higher in malignant ovarian tissues than in normal ovarian tissues (86). Targeting the TFR of ovarian cancer cells can improve therapeutic drug delivery while also blocking the receptor’s normal activity, resulting in cancer cell death. T7 peptide (HAIYPRH) binds specifically to TFR with high affinity. As a result of the difference in binding sites with TF, the uptake of T7 peptide modified carriers is not inhibited by competition from endogenous transferrin (87). More interestingly, Tf contributes to the targeting of T7 peptides to small cavities on the surface of TfR, which in turn translocate into the cell and increase its endocytosis rate (88).Wu and colleagues (63) reported that T7 peptide can enhance the uptake and penetration of A2780 with higher TFR expression levels to liposomes, thereby significantly enhancing the inhibitory effect of PTX on cells and 3D tumor spheroids. Seliciclib is an inhibitor of cell cycle protein-dependent kinases (CDKs) that plays a potential role in anticancer therapy. Compared to free seliclib, T7 peptide-modified NPs achieved better cellular uptake, effective delivery of seliclib to SKOV3 cells and enhanced cytotoxicity (64). The above results suggest that the improved uptake of drug delivery system by TfR overexpressing cancer cells is achieved by T7 peptide.
3.2 Anticancer peptide therapy
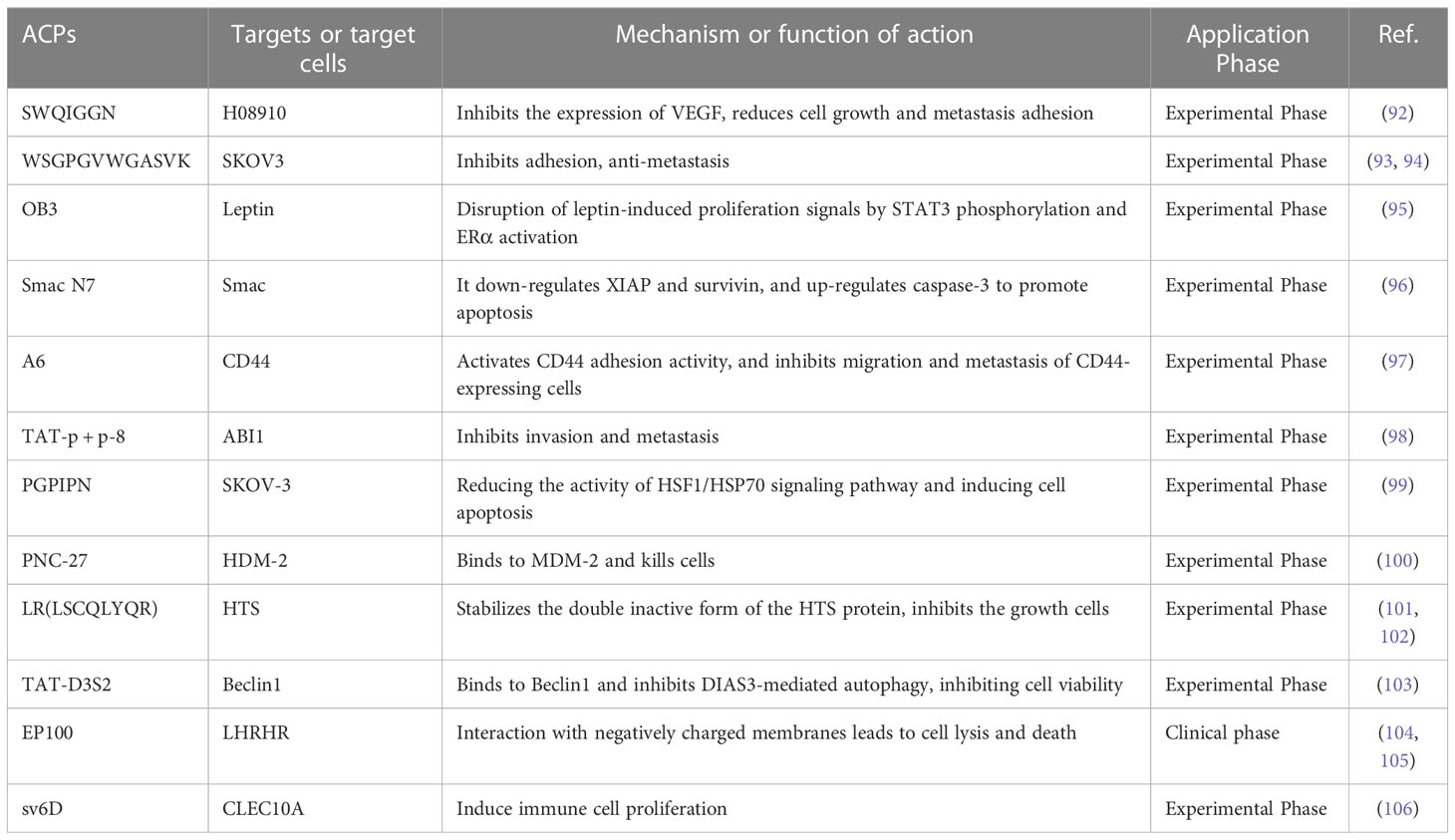
Inhibition of tumor growth and metastasis is a strategy for ovarian cancer treatment, which is crucial for the prognosis of patients. Compared with peptides used in drug delivery systems, anticancer peptides are a rising focus in anti-tumor research (89). Anticancer peptides (ACPs) act by inducing apoptosis and disrupting cell membrane structure. Large quantities of evidence signify that cancer cells have higher mobility and more abundant microvilli compared to healthy cells. When ACPs interact with cancer cells, they cause membrane instability, cytotoxicity, and cell lysis (90). In addition, ACPs can also inhibit the proliferation of cancer cells by changing the pH around or inside the cell (91). Small peptides with anticancer effects can be discovered by phage display or chemical synthesis of peptide libraries. We summarize the small peptides that inhibit the growth and metastasis of ovarian cancer cells (Table 3), some of which can directly act on ovarian cancer cells to exert anticancer effects. Zhou’s team (92) used a phage display peptide library to isolate the small peptide “SWQIGGN”, which can combine and internalize ovarian cancer cells. It not only inhibited the viability, migration, invasion and adhesion of ovarian cancer cells in vitro but also reduced the volume of ascites and controlled tumor growth and metastasis in vivo. Another research found the homologous peptide “WSGPGVWGASVK”, identified based on ovarian cancer cell-specific ligand (pc3-1), inhibits adhesion between SKOV-3 cells and HUVECs cells (93) and inhibits cancer cell invasion by down-regulating the active expression of MMP-2 and MMP-9 (94). Furthermore, several short inhibitory peptides, like as “OB3 peptide,” “Smac N7 peptide,” and “A6 peptide,” have been demonstrated to have anticancer effects by acting on ovarian cancer signaling pathways or bioprocess-related targets (95–98).
Unfortunately, due to tumor heterogeneity, drug-resistant phenotypes may emerge late in treatment, including resistance to pro-apoptotic stimuli and/or cytotoxic resistance to anticancer compounds (47). According to certain study findings, bioactive peptides may also have a function in increasing cancer cell susceptibility to chemotherapeutic medicines and reversing cancer cell resistance. Guo’s group (99)found that hexapeptide (PGPIPN), derived from bovine β-casein, significantly increased the sensitivity of DDP to human ovarian cancer cells inhibition of survival and induction of apoptosis in vitro. In an in vivo model of ovarian cancer, Alagkiozidis’s team (100)discovered that the anticancer peptide PNC-27 could target paclitaxel-resistant cells, and that the addition of PNC-27 to weekly paclitaxel injection greatly decreased tumor development. Similarly, some peptides can be designed to act on anticancer drug targets, and thus provide better inhibitory effect on drug-resistant phenotypes (107). For example, LR peptide (LSCQLYQR) is an anticancer peptide that acts on human thymidylate synthase (hTS), and active site inhibitors of hTS are commonly employed in chemotherapy (101). When LR was given to ovarian cancer cells, it could inhibit intracellular hTS expression. Also, inhibited the growth of approximately 50% of cisplatin-sensitive and resistant ovarian cancer cell lines at low micromolar concentrations (510 μM), more effectively than the same concentration of 5-FU (102).
It is worth noting that although some peptides have inhibitory effects on the growth, invasion and metastasis of ovarian cancer cells. However, due to its weak penetration capacity, it needs to be transported into cells in combination with cell penetrating peptides (CCP) or protein-specific systems to improve the bioavailability of anticancer drugs, and these delivery systems usually do not affect cell proliferation or cell cycle progression (108, 109). D3S2, an autophagy-inhibiting peptide based on the switch II region of DIRAS3, inhibition of autophagy can induce cancer cell death. D3S2 was linked to TAT (a cell-penetrating peptide) to act on OC cells to enhance penetration, and Tat-D3S2 treatment reduced the induction of autophagy in OC cell lines, as evidenced by a decrease in LC3I/LC3II conversion rate (103).EP-100 is composed of a natural ligand for GnRH linked to cleavage peptide (CLIP-71), which delivers the cleaved peptide to GnRH receptor-positive cancer cells, thereby exerting selective toxic effects (104). Kim and colleagues (105) found that EP-100 rapidly destroyed the cell membrane and increased PD-L1 levels after specific binding to ovarian cancer cells, and observed that EP-100 increased anti-tumor effector T cells and IL33 expression and induced anticancer activity in vivo when EP-100 and anti-PD-L1 antibodies were combined.
Previous reports have indicated that peptides are generally not toxic in normal host cells and thus may represent a powerful therapeutic tool. Moreover, the combination of anticancer peptides and more aggressive chemotherapeutic agents can improve efficacy, counteract chemotherapy-induced resistance, and reduce overall drug combination toxicity (110). For instance, sv6D acts as a multivalent anticancer peptide that specifically targets the C-type lectin A family 10 member (CLEC10A). In a mouse ovarian cancer model, the peptide sv6D significantly inhibited the formation of ascites when combined with the chemotherapeutic agent paclitaxel or an immunotherapeutic antibody against the receptor PD-1 (106).Combining EP-100 with PARP inhibitor (olaparib) significantly increased the number of nuclear foci of phosphorylated histone H2AX, leading to increased DNA damage in ovarian cancer cells (111). A phase II trial of paclitaxel in combination with EP-100 showed that a subset of patients with target liver lesions showed a greater overall response rate to the combination (69%) compared to paclitaxel alone (16%) (112).
3.3 Peptide vaccine treatment
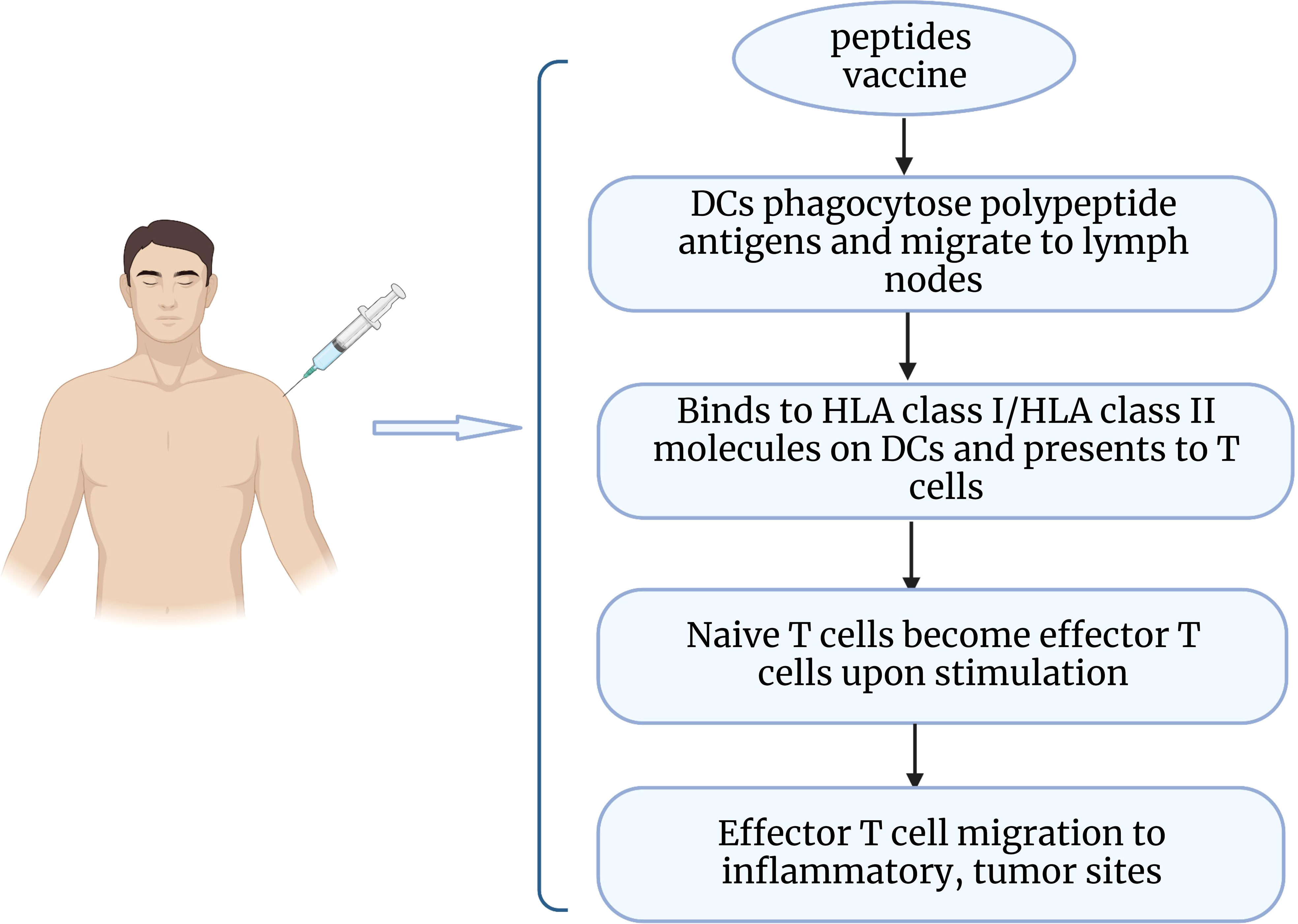
Epithelial ovarian cancer is considered to be an immunogenic tumor. The presence of tumor infiltrating lymphocytes (TIL) in the tumor microenvironment has been shown to increase the 5-year survival rate and improve clinical outcomes for patients (113). As a potential immunotherapeutic route, peptide vaccines are captured by antigen-presenting cells upon entry into the body and delivered to T cells, which then recognize and initiate an immune response that promotes the production of cytotoxic T cells and enhances the body’s ability to fight tumors (114) (Figure 2). Peptide vaccines generally consist of an immune adjuvant and a targeted peptide derived from a tumor-associated antigen (TAA) epitope. A retrospective analysis of vaccine treatments over 20 years in ovarian cancer found that peptide vaccines had the highest coverage rate (34.3%), which can be attributed to the fact that peptide vaccines are well tolerated and have fewer side effects (115). A number of peptide vaccines against ovarian cancer are currently in clinical trials (Table 4), such as NY-ESO-1 peptide vaccine (116), folic acid receptor α peptide vaccine (120), HER-2/neu peptide vaccine (117), P53 synthetic long peptide vaccine (p53-SLP) (118), and WT1 peptide vaccine (119). Among them, the study of folate receptor alpha peptide vaccines in gynecological tumors has attracted much interest to enhance immunity to folate receptors by producing T cells. E39(EIWTHSYKV) is an HLA-A2 restricted FR peptide that induced a significant, dose-dependent in vivo immune response and decreased the risk of recurrence in a clinical phase I/IIa research (124). A recent study identified the optimal vaccination dose of E39 peptide, and the 1000 μg dose significantly improved the 2-year disease-free survival of patients (77.9% at 1000μg vs 31.2% at < 1000μg) (120). However, some researchers believe that overstimulation of E39 may lead to activation induced cell death (AICD). Berry’s group (125) developed the attenuated peptide E39’ (EIWTFSTKV) by replacing two amino acids of E39 peptide and found to elicit an effective CTL response and possibly even avoid AICD caused by repeated stimulation with E39 peptide. The safety of E39’ was subsequently demonstrated in a phase Ib trial (121). This trial also showed that sequential inoculation of 3 times E39 and 3 times E39’ resulted in greater local response and DTH response.
Most peptide vaccines are designed based on long-standing tumor-associated antigens (TAA) and have poor immunogenicity. However, peptide vaccines can generate strong immunogenic responses by increasing antigenic epitopes. Multi-epitope vaccines can carry multiple epitopes at the same time and have the ability to be recognized and bound by MHC molecules of multiple genetic backgrounds, resulting in efficient presentation (126). In a phase I clinical trial study, Kalli and colleagues (122) used five putative HLA-A2 class binding peptides (FR30, FR56, FR76, FR113 and FR238) mixed with GM-CSF to form a multi-epitope FR peptide. This experiment showed that more than 90% of ovarian cancer patients produced T-cell immune enhancement and exhibited a stable high immune memory during the observation period. It has been reported that vaccination alone in the early stage of the tumor or under minimal change disease prevents relapse. In contrast, in patients with advanced disease, combination therapy may be more effective in preventing immune escape and gaining clinical benefit (127). Combining peptide-based cancer vaccines with checkpoint medications is a prominent example. Immune checkpoint blockade can restore the body’s immune response by preventing T cell depletion and promoting activation. Zamarin and colleagues (123) evaluated the efficacy of a multi-epitope FR vaccine (TPIV200) in combination with a PD-L1 inhibitor (durvalumab) in patients with advanced platinum-resistant OC. The trial was conducted in 27 patients in 28-day cycles, receiving Durvalumab intravenously on days 1 and 15 of cycles 1-12 and three TPIV200 intradermal injections on day 1 of cycles 1-6. All patients produced a robust FRα-specific T-cell response after treatment. Despite a low overall remission rate, the median overall survival was 21 months, suggesting that combination therapy could help prolong disease control and alter patient outcomes. Likewise, other potential combination therapies such as peptide vaccines and poly ADP ribose polymerase (PARP) inhibitors, anti-angiogenic drugs should be considered.
4 Conclusion and future challenges
Despite significant progress in developing new therapies, OC still suffers from adverse treatment reactions and frequent relapses, and there is a great need for new diagnostic and therapeutic approaches. Peptides have shown an important role in the ovarian cancer diagnosis. The differential expression of peptides in body fluids provides a new approach and useful tool used in the early diagnosis of ovarian cancer. The overexpression of some peptide receptors in cancer cells is a potential target for converting peptides into diagnostic tools such as radiolabeled imaging. In addition, surgical resection remains one of the primary treatment options for OC, and fluorescently labeled peptide probes can visualize cancer intraoperatively and provide real-time assessment of margin status imaging. This is critical for increasing surgical success and improving prognosis.
Currently, the field of peptides for tumor targeting and developing specific delivery methods into cancer cells is evolving and playing a strong role in advancing tumor therapy (128). For targeted therapy, peptides are very promising for the delivery of cytotoxic drugs, siRNAs, hormones, fluorophores, or nanoparticles into cells, opening new horizons for targeted therapy as well as combination therapy in metastatic, recurrent ovarian cancer. Although peptides have many advantages in targeted delivery, they also exhibit poor stability and short circulation times in solid tumors. Furthermore, targeted delivery is based on peptide receptor overexpression in tumors, whereas peptide receptor expression in cell lines does not always correspond to the peptide receptor profile in the corresponding human primary tumors and varies greatly between tissue subtypes of the same tumor. An in-depth understanding of ovarian cancer receptor pathogenesis would be beneficial. If we can figure out what controls and regulates the expression of peptide receptors, this could be a new opportunity for the treatment of ovarian cancer.
The discovery of insulin has driven the progress of peptide therapy. Anticancer peptides are characterized by ease of modification, low immunogenicity, and biological and chemical diversity. Peptide-based therapeutics are being tested in clinical trials and it approved by the FDA (129). However, natural peptides are unstable and easily degraded by peptidases in vitro and in vivo, therefore, some peptides with anticancer effects have limited uptake by tumor cells and usually require administration of large doses (equal to or higher than tolerated) before achieving a therapeutic response. This may cause adverse reactions such as allergy and hemolytic toxicity. Another major challenge faced by anticancer peptides in clinical translation is their poor absorption and low oral bioavailability due to their short half-life and sensitivity to different pH values in the gastrointestinal tract. The conjugation of anticancer peptides with non-structural proteins is thought to improve in vivo half-life, but the amino acid number of the peptides must also be considered when selecting conjugates, or effectiveness may be lost due to steric hindrance.
In ovarian cancer, various peptide vaccines have been studied in clinical trials. For chemotherapy-resistant patients, peptide vaccines can effectively improve chemotherapy resistance without serious side effects. In addition, researchers can design personalized peptide vaccines for individual antigenic epitopes. However, peptide vaccines still face some difficulties in clinical application, such as immunosuppressive tumor environment and low immunogenicity. Therefore, more research is needed to improve vaccine-host interactions and to understand the different immune responses to vaccine therapy.
In general, peptide targeted therapies, anticancer peptides and peptide vaccines are difficult to achieve efficacy alone, while combinations with conventional chemotherapeutic agents (carboplatin, docetaxel and doxorubicin), molecular chemotherapeutic agents (PARP inhibitors), and biotherapeutic agents (siRNA and proteins) may yield greater clinical benefit. Recently, the combination of two peptide vaccines targeting different antigenic epitopes on the surface of ovarian cancer has shown promising results. In addition, combinations of peptide vaccines and a number of anticancer peptides are being tested in phase I/II clinical trials. Considering that peptides have shown promising results in the diagnosis and treatment of ovarian cancer patients, combination therapies containing specific targeted peptides, anticancer peptides and peptide vaccines may be more tumor specific, stable, safe and efficacious in the near future. Meanwhile, with rapid advances in computational molecular modeling, machine learning and high-throughput screening technologies facilitating the discovery of new peptides for cancer diagnosis and treatment. In conclusion, peptides can be ideal tools for the diagnosis and treatment of ovarian cancer and are very promising for progressive translation from preclinical development to clinical application.
Author contributions
LG conceived the topic of the Review. LG, JW, NL, JC and YS contributed to discussions of the content. LG wrote the manuscript. LG, JW, NL, JC and YS corrected and reviewed the article before submission. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by the Harbin Medical University Cancer Hospital Haiyan Research Foundation [grant number JJZD2022-01].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Goff BA, Mandel LS, Drescher CW, Urban N, Gough S, Schurman KM, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer (2007) 109(2):221–7. doi: 10.1002/cncr.22371
3. Bristow RE, Montz FJ, Lagasse LD, Leuchter RS, Karlan BY. Survival impact of surgical cytoreduction in stage IV epithelial ovarian cancer. Gynecol Oncol (1999) 72(3):278–87. doi: 10.1006/gyno.1998.5145
4. Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2018) 29(Suppl 4):iv259. doi: 10.1093/annonc/mdy157
5. Guan LY, Lu Y. New developments in molecular targeted therapy of ovarian cancer. Discovery Med (2018) 26(144):219–29.
6. Kobayashi H, Choyke PL, Ogawa M. Monoclonal antibody-based optical molecular imaging probes; considerations and caveats in chemistry, biology and pharmacology. Curr Opin Chem Biol (2016) 33:32–8. doi: 10.1016/j.cbpa.2016.05.015
7. Newman M, Benoit DJ. In vivo translation of peptide-targeted drug delivery systems discovered by phage display. Bioconjugate Chem (2018) 29(7):2161–9. doi: 10.1021/acs.bioconjchem.8b00285
8. Jaroszewicz W, Morcinek-Orlowska J, Pierzynowska K, Gaffke L, Wegrzyn G. Phage display and other peptide display technologies. FEMS Microbiol Rev (2022) 46(2):52–67. doi: 10.1093/femsre/fuab052
9. Wang W, Hu Z. Targeting peptide-based probes for molecular imaging and diagnosis. Adv Mater (2019) 31(45):e1804827. doi: 10.1002/adma.201804827
10. Chu LY, Zhou JY, Zhao YX, Ou YT, Yang T, Peng YH, et al. Serum CYR61 as a potential biomarker for the diagnosis of esophagogastric junction tumor. Biosci Rep (2021) 41(6):117–32. doi: 10.1042/BSR20204117
11. Huang X, Zhou J, Tang R, Han S, Zhou X. Potential significance of peptidome in human ovarian cancer for patients with ascites. Int J Gynecol Cancer (2018) 28(2):355–62. doi: 10.1097/IGC.0000000000001166
12. Ukai M, Yokoi A, Yoshida K, Suzuki S, Shibata K, Kikkawa F, et al. Extracellular miRNAs as predictive biomarkers for glypican-3-Derived peptide vaccine therapy response in ovarian clear cell carcinoma. Cancers (Basel) (2021) 13(3):550–72. doi: 10.3390/cancers13030550
13. Miao Y, Quinn T. Advances in receptor-targeted radiolabeled peptides for melanoma imaging and therapy. J Nucl Med (2021) 62(3):313–8. doi: 10.2967/jnumed.120.243840
14. Sonju J, Dahal A, Singh S, Jois S. Peptide-functionalized liposomes as therapeutic and diagnostic tools for cancer treatment. J Control Release (2021) 329:624–44. doi: 10.1016/j.jconrel.2020.09.055
15. Xiu L, Li N, Wang W, Chen F, Yuan G, Sun Y, et al. [Identification of serum peptide biomarker for ovarian cancer diagnosis by clin-TOF-II-MS combined with magnetic beads technology]. Zhonghua Zhong Liu Za Zhi (2021) 43(11):1188–95. doi: 10.3760/cma.j.cn112152-20210315-00229
16. Shahdeo D, Roberts A, Kesarwani V, Horvat M, Chouhan R, Gandhi S. Polymeric biocompatible iron oxide nanoparticles labeled with peptides for imaging in ovarian cancer. Biosci Rep (2022) 42(2):59–66. doi: 10.1042/BSR20212622
17. Carreras-Dieguez N, Glickman A, Munmany M, Casanovas G, Agusti N, Diaz-Feijoo B, et al. Comparison of HE4, CA125, ROMA and CPH-I for preoperative assessment of adnexal tumors. Diagnostics (Basel) (2022) 12(1):226–43. doi: 10.3390/diagnostics12010226
18. Lu X, Li Y, Xia B, Bai Y, Zhang K, Zhang X, et al. Selection of small plasma peptides for the auxiliary diagnosis and prognosis of epithelial ovarian cancer by using UPLC/MS-based nontargeted and targeted analyses. Int J Cancer (2019) 144(8):2033–42. doi: 10.1002/ijc.31807
19. Ding D, Chen M, Xiao X, Cao P, Li S. Novel serum peptide model revealed by MALDI-TOF-MS and its diagnostic value in early bladder cancer. Int J Biol Marker (2020) 35(3):59–66. doi: 10.1177/1724600820935473
20. Wang Q, Zhang M, Tomita T, Vogelstein J, Zhou S, Papadopoulos N, et al. Selected reaction monitoring approach for validating peptide biomarkers. P Natl Acad Sci USA (2017) 114(51):13519–24. doi: 10.1073/pnas.1712731114
21. Liljedahl L, Malmstrom J, Kristjansdottir B, Waldemarson S, Sundfeldt K. Targeted selected reaction monitoring verifies histology specific peptide signatures in epithelial ovarian cancer. Cancers (Basel) (2021) 13(22):71–89. doi: 10.3390/cancers13225713
22. Murgan S, Abd Elaziz F, Nasr A, Elfaki M, Khalil E. Ovarian cancer: tumor-specific urinary micro-peptides profiling as potential biomarkers for early diagnosis. Proteomes (2020) 8(4):32–46. doi: 10.3390/proteomes8040032
23. Tammen H, Schulte I, Hess R, Menzel C, Kellmann M, Mohring T, et al. Peptidomic analysis of human blood specimens: comparison between plasma specimens and serum by differential peptide display. Proteomics (2005) 5(13):3414–22. doi: 10.1002/pmic.200401219
24. Tammen H, Peck A, Budde P, Zucht HD. Peptidomics analysis of human blood specimens for biomarker discovery. Expert Rev Mol Diagn (2007) 7(5):605–13. doi: 10.1586/14737159.7.5.605
25. Tardif JC, Lesage F, Harel F, Romeo P, Pressacco J. Imaging biomarkers in atherosclerosis trials. Circ Cardiovasc Imaging (2011) 4(3):319–33. doi: 10.1161/CIRCIMAGING.110.962001
27. Mohtavinejad N, Shafiee Ardestani M, Khalaj A, Pormohammad A, Najafi R, Bitarafan-Rajabi A, et al. Application of radiolabeled peptides in tumor imaging and therapy. Life Sci (2020) 258:118206. doi: 10.1016/j.lfs.2020.118206
28. Charron CL, Hickey JL, Nsiama TK, Cruickshank DR, Turnbull WL, Luyt LG. Molecular imaging probes derived from natural peptides. Nat Prod Rep (2016) 33(6):761–800. doi: 10.1039/c5np00083a
29. Janssen M, Oyen W, Dijkgraaf I, Massuger L, Frielink C, Edwards D, et al. Tumor targeting with radiolabeled alpha(v)beta(3) integrin binding peptides in a nude mouse model. Cancer Res (2002) 62(21):6146–51.
30. Yang G, Nie P, Kong Y, Sun H, Hou G, Han J. MicroPET imaging of tumor angiogenesis and monitoring on antiangiogenic therapy with an (18)F labeled RGD-based probe in SKOV-3 xenograft-bearing mice. Tumour Biol (2015) 36(5):3285–91. doi: 10.1007/s13277-014-2958-x
31. Minamimoto R, Karam A, Jamali M, Barkhodari A, Gambhir SS, Dorigo O, et al. Pilot prospective evaluation of (18)F-FPPRGD2 PET/CT in patients with cervical and ovarian cancer. Eur J Nucl Med Mol Imaging (2016) 43(6):1047–55. doi: 10.1007/s00259-015-3263-7
32. Meng Y, Zhang Z, Liu K, Ye L, Liang Y, Gu W. Aminopeptidase n (CD13) targeted MR and NIRF dual-modal imaging of ovarian tumor xenograft. Mater Sci Eng C Mater Biol Appl (2018) 93:968–74. doi: 10.1016/j.msec.2018.09.002
33. Yang Y, Zhang J, Zou H, Shen Y, Deng S, Wu Y. Synthesis and evaluation of (68)Ga-labeled dimeric cNGR peptide for PET imaging of CD13 expression with ovarian cancer xenograft. J Cancer (2021) 12(1):244–52. doi: 10.7150/jca.49628
34. Liu S, Zheng Y, Volpi D, El-Kasti M, Klotz D, Tullis I, et al. Toward operative in vivo fluorescence imaging of the c-met proto-oncogene for personalization of therapy in ovarian cancer. Cancer (2015) 121(2):202–13. doi: 10.1002/cncr.29029
35. Liu Q, Zhou X, Feng W, Pu T, Li X, Li F, et al. Gonadotropin-releasing hormone receptor-targeted near-infrared fluorescence probe for specific recognition and localization of peritoneal metastases of ovarian cancer. Front Oncol (2020) 10:266. doi: 10.3389/fonc.2020.00266
36. Wang P, Fan Y, Lu L, Liu L, Fan L, Zhao M, et al. NIR-II nanoprobes in-vivo assembly to improve image-guided surgery for metastatic ovarian cancer. Nat Commun (2018) 9(1):2898. doi: 10.1038/s41467-018-05113-8
37. Xie Q, Liu T, Ding J, Zhou N, Meng X, Zhu H, et al. Synthesis, preclinical evaluation, and a pilot clinical imaging study of [(18)F]AlF-NOTA-JR11 for neuroendocrine neoplasms compared with [(68)Ga]Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging (2021) 48(10):3129–40. doi: 10.1007/s00259-021-05249-8
38. Chastel A, Vimont D, Claverol S, Zerna M, Bodin S, Berndt M, et al. (68)Ga-radiolabeling and pharmacological characterization of a kit-based formulation of the gastrin-releasing peptide receptor (GRP-r) antagonist RM2 for convenient preparation of [(68)Ga]Ga-RM2. Pharmaceutics (2021) 13(8):1160–18. doi: 10.3390/pharmaceutics13081160
39. Chen H, Niu G, Wu H, Chen X. Clinical application of radiolabeled RGD peptides for PET imaging of integrin alphavbeta3. Theranostics (2016) 6(1):78–92. doi: 10.7150/thno.13242
40. Guzman-Rojas L, Rangel R, Salameh A, Edwards JK, Dondossola E, Kim YG, et al. Cooperative effects of aminopeptidase n (CD13) expressed by nonmalignant and cancer cells within the tumor microenvironment. Proc Natl Acad Sci U.S.A. (2012) 109(5):1637–42. doi: 10.1073/pnas.1120790109
41. Zhu L, Ding Z, Li X, Wei H, Chen Y. Research progress of radiolabeled asn-Gly-Arg (NGR) peptides for imaging and therapy. Mol Imaging (2020) 19:1536012120934957. doi: 10.1177/1536012120934957
42. Moss J, Vāvere A, Azhdarinia A. Design of peptide imaging agents for whole-body and intraoperative molecular imaging. Curr Med Chem (2012) 19(20):3255–65. doi: 10.2174/092986712801215856
43. Shaw S, Liu W, Gómez Durán C, Schreiber C, Betancourt Mendiola M, Zhai C, et al. Non-covalently pre-assembled high-performance near-infrared fluorescent molecular probes for cancer imaging. Chemistry (2018) 24(52):13821–9. doi: 10.1002/chem.201801825
44. Ahmadi Bidakhvidi N, Goffin K, Dekervel J, Baete K, Nackaerts K, Clement P, et al. Peptide receptor radionuclide therapy targeting the somatostatin receptor: basic principles, clinical applications and optimization strategies. Cancers (Basel) (2021) 14(1):129–47. doi: 10.3390/cancers14010129
45. Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discovery Today (2015) 20(1):122–8. doi: 10.1016/j.drudis.2014.10.003
46. Nelde A, Rammensee HG, Walz JS. The peptide vaccine of the future. Mol Cell Proteomics (2021) 20:100022. doi: 10.1074/mcp.R120.002309
47. Song M, Cui M, Liu K. Therapeutic strategies to overcome cisplatin resistance in ovarian cancer. Eur J Med Chem (2022) 232:114205. doi: 10.1016/j.ejmech.2022.114205
48. Wang S, Wu W, Liu Y, Wang C, Xu Q, Lv Q, et al. in vitroTargeted peptide-modified oxidized mesoporous carbon nanospheres for chemo-thermo combined therapy of ovarian cancer. Drug Delivery (2022) 29(1):1947–52. doi: 10.1080/10717544.2022.2089298
49. Li F, Yuan Y, Dai Y, Cheng T, Cao H, Yan D, et al. M11: a tropism-modified oncolytic adenovirus arming with a tumor-homing peptide for advanced ovarian cancer therapies. Hum Gene Ther (2022) 33(5-6):262–74. doi: 10.1089/hum.2021.247
50. Gong Z, Liu X, Zhou B, Wang G, Guan X, Xu Y, et al. Tumor acidic microenvironment-induced drug release of RGD peptide nanoparticles for cellular uptake and cancer therapy. Colloids Surf B Biointerfaces (2021) 202:111673. doi: 10.1016/j.colsurfb.2021.111673
51. Deng X, Qiu Q, Ma K, Huang W, Qian H. Synthesis and in vitro anti-cancer evaluation of luteinizing hormone-releasing hormone-conjugated peptide. Amino Acids (2015) 47(11):2359–66. doi: 10.1007/s00726-015-2021-2
52. Dharap S, Wang Y, Chandna P, Khandare J, Qiu B, Gunaseelan S, et al. Tumor-specific targeting of an anticancer drug delivery system by LHRH peptide. P Natl Acad Sci USA (2005) 102(36):12962–7. doi: 10.1073/pnas.0504274102
53. Pan Q, Tian J, Zhu H, Hong L, Mao Z, Oliveira J, et al. Tumor-targeting polycaprolactone nanoparticles with codelivery of paclitaxel and IR780 for combinational therapy of drug-resistant ovarian cancer. ACS Biomater Sci Eng (2020) 6(4):2175–85. doi: 10.1021/acsbiomaterials.0c00163
54. Lin CJ, Kuan CH, Wang LW, Wu HC, Chen Y, Chang CW, et al. Integrated self-assembling drug delivery system possessing dual responsive and active targeting for orthotopic ovarian cancer theranostics. Biomaterials (2016) 90:12–26. doi: 10.1016/j.biomaterials.2016.03.005
55. Santa-Coloma T, Grasso P, Reichert LJ. Synthetic human follicle-stimulating hormone-beta-(1-15) peptide-amide binds Ca2+ and possesses sequence similarity to calcium binding sites of calmodulin. Endocrinology (1992) 130(3):1103–7. doi: 10.1210/endo.130.3.1537277
56. Zhang XY, Chen J, Zheng YF, Gao XL, Kang Y, Liu JC, et al. Follicle-stimulating hormone peptide can facilitate paclitaxel nanoparticles to target ovarian carcinoma in vivo. Cancer Res (2009) 69(16):6506–14. doi: 10.1158/0008-5472.CAN-08-4721
57. Hong SS, Zhang MX, Zhang M, Yu Y, Chen J, Zhang XY, et al. Follicle-stimulating hormone peptide-conjugated nanoparticles for targeted shRNA delivery lead to effective gro-alpha silencing and antitumor activity against ovarian cancer. Drug Delivery (2018) 25(1):576–84. doi: 10.1080/10717544.2018.1440667
58. Jin X, Zhou J, Zhang Z, Lv H. Doxorubicin combined with betulinic acid or lonidamine in RGD ligand-targeted pH-sensitive micellar system for ovarian cancer treatment. Int J Pharm (2019) 571:118751. doi: 10.1016/j.ijpharm.2019.118751
59. Kulhari H, Pooja D, Kota R, Reddy TS, Tabor RF, Shukla R, et al. Cyclic RGDfK peptide functionalized polymeric nanocarriers for targeting gemcitabine to ovarian cancer cells. Mol Pharm (2016) 13(5):1491–500. doi: 10.1021/acs.molpharmaceut.5b00935
60. Aina O, Marik J, Liu R, Lau D, Lam K. Identification of novel targeting peptides for human ovarian cancer cells using “one-bead one-compound” combinatorial libraries. Mol Cancer Ther (2005) 4(5):806–13. doi: 10.1158/1535-7163.MCT-05-0029
61. Xiao K, Li Y, Lee JS, Gonik AM, Dong T, Fung G, et al. “OA02” peptide facilitates the precise targeting of paclitaxel-loaded micellar nanoparticles to ovarian cancer in vivo. Cancer Res (2012) 72(8):2100–10. doi: 10.1158/0008-5472.CAN-11-3883
62. Tang H, Chen X, Rui M, Sun W, Chen J, Peng J, et al. Effects of surface displayed targeting ligand GE11 on liposome distribution and extravasation in tumor. Mol Pharm (2014) 11(10):3242–50. doi: 10.1021/mp5001718
63. Wu H, Yao L, Mei J, Li F. Development of synthetic of peptide-functionalized liposome for enhanced targeted ovarian carcinoma therapy. Int J Clin Exp Pathol (2015) 8(1):207–16.
64. He GZ, Lin WJ. Peptide-functionalized nanoparticles-encapsulated cyclin-dependent kinases inhibitor seliciclib in transferrin receptor overexpressed cancer cells. Nanomater (Basel) (2021) 11(3):772–90. doi: 10.3390/nano11030772
65. Gründker C, Emons G. Role of gonadotropin-releasing hormone (GnRH) in ovarian cancer. Cells (2021) 10(2):65–77. doi: 10.3390/cells10020437
66. Feng Z, Wen H, Bi R, Ju X, Chen X, Yang W, et al. A clinically applicable molecular classification for high-grade serous ovarian cancer based on hormone receptor expression. Sci Rep (2016) 6:25408. doi: 10.1038/srep25408
67. Luo G, Chen W, Zeng X, Zhang X. Cell primitive-based biomimetic functional materials for enhanced cancer therapy. Chem Soc Rev (2021) 50(2):945–85. doi: 10.1039/D0CS00152J
68. Tan T, Wang Y, Wang J, Wang Z, Wang H, Cao H, et al. Targeting peptide-decorated biomimetic lipoproteins improve deep penetration and cancer cells accessibility in solid tumor. Acta Pharm Sin B (2020) 10(3):529–45. doi: 10.1016/j.apsb.2019.05.006
69. Kumar D, Moghiseh M, Chitcholtan K, Mutreja I, Lowe C, Kaushik A, et al. LHRH conjugated gold nanoparticles assisted efficient ovarian cancer targeting evaluated via spectral photon-counting CT imaging: a proof-of-concept research. J Mater Chem B (2023) 11(9):1916–28. doi: 10.1039/D2TB02416K
70. Yao H, Xu Z, Li C, Tse MK, Tong Z, Zhu G. Synthesis and cytotoxic study of a Platinum(IV) anticancer prodrug with selectivity toward luteinizing hormone-releasing hormone (LHRH) receptor-positive cancer cells. Inorg Chem (2019) 58(16):11076–84. doi: 10.1021/acs.inorgchem.9b01583
71. Perales-Puchalt A, Svoronos N, Rutkowski MR, Allegrezza MJ, Tesone AJ, Payne KK, et al. Follicle-stimulating hormone receptor is expressed by most ovarian cancer subtypes and is a safe and effective immunotherapeutic target. Clin Cancer Res (2017) 23(2):441–53. doi: 10.1158/1078-0432.CCR-16-0492
72. Modi D, Sunoqrot S, Bugno J, Lantvit D, Hong S, Burdette JJ. Targeting of follicle stimulating hormone peptide-conjugated dendrimers to ovarian cancer cells. Nanoscale (2014) 6(5):2812–20. doi: 10.1039/C3NR05042D
73. Zhang X, Chen J, Kang Y, Hong S, Zheng Y, Sun H, et al. Targeted paclitaxel nanoparticles modified with follicle-stimulating hormone beta 81-95 peptide show effective antitumor activity against ovarian carcinoma. Int J Pharm (2013) 453(2):498–505. doi: 10.1016/j.ijpharm.2013.06.038
74. Sopo M, Anttila M, Muukkonen OT, Yl AHS, Kosma VM, Keski-Nisula L, et al. Microvessels in epithelial ovarian tumors: high microvessel density is a significant feature of malignant ovarian tumors. Anticancer Res (2020) 40(12):6923–31. doi: 10.21873/anticanres.14716
75. Kobayashi M, Sawada K, Kimura T. Potential of integrin inhibitors for treating ovarian cancer: a literature review. Cancers (Basel) (2017) 9(7):83–94. doi: 10.3390/cancers9070083
76. Davidson B, Goldberg I, Reich R, Tell L, Dong HP, Trope’ CG, et al. αV- and β1-integrin subunits are commonly expressed in malignant effusions from ovarian carcinoma patients. Gynecol Oncol (2003) 90(2):248–57. doi: 10.1016/S0090-8258(03)00321-4
77. Wang Y, Wang L, Chen G, Gong S. Carboplatin-complexed and cRGD-conjugated unimolecular nanoparticles for targeted ovarian cancer therapy. Macromol Biosci (2017) 17(5):10–31. doi: 10.1002/mabi.201600292
78. Aina O, Marik J, Gandour-Edwards R, Lam K. Near-infrared optical imaging of ovarian cancer xenografts with novel alpha 3-integrin binding peptide _OA02_.pdf. Mol Imaging (2005) 4(4):439–47. doi: 10.2310/7290.2005.05169
79. Wang Z, Zhao S, Gu W, Dong Y, Meng F, Yuan J, et al. alpha3 integrin-binding peptide-functionalized polymersomes loaded with volasertib for dually-targeted molecular therapy for ovarian cancer. Acta Biomater (2021) 124:348–57. doi: 10.1016/j.actbio.2021.02.007
80. Kaufman NEM, Dhingra S, Jois SD, Vicente M. Molecular targeting of epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR). Molecules (2021) 26(4):1076–89. doi: 10.3390/molecules26041076
81. Bonello M, Sims AH, Langdon SP. Human epidermal growth factor receptor targeted inhibitors for the treatment of ovarian cancer. Cancer Biol Med (2018) 15(4):375–88. doi: 10.3390/molecules26041076
82. Gui T, Shen K. The epidermal growth factor receptor as a therapeutic target in epithelial ovarian cancer. Cancer Epidemiol (2012) 36(5):490–6. doi: 10.1016/j.canep.2012.06.005
83. Li Z, Zhao R, Wu X, Sun Y, Yao M, Li J, et al. Identification and characterization of a novel peptide ligand of epidermal growth factor receptor for targeted delivery of therapeutics. FASEB J (2005) 19(14):1978–85. doi: 10.1096/fj.05-4058com
84. Zou Y, Xia Y, Meng F, Zhang J, Zhong Z. GE11-directed functional polymersomal doxorubicin as an advanced alternative to clinical liposomal formulation for ovarian cancer treatment. Mol Pharm (2018) 15(9):3664–71. doi: 10.1021/acs.molpharmaceut.8b00024
85. Li L, Qiu C, Hou M, Wang X, Huang C, Zou J, et al. Ferroptosis in ovarian cancer: a novel therapeutic strategy. Front Oncol (2021) 11:665945. doi: 10.3389/fonc.2021.665945
86. Basuli D, Tesfay L, Deng Z, Paul B, Yamamoto Y, Ning G, et al. Iron addiction: a novel therapeutic target in ovarian cancer. Oncogene (2017) 36(29):4089–99. doi: 10.1038/onc.2017.11
87. Bi Y, Liu L, Lu Y, Sun T, Shen C, Chen X, et al. T7 peptide-functionalized PEG-PLGA micelles loaded with carmustine for targeting therapy of glioma. ACS Appl Mater Interfaces (2016) 8(41):27465–73. doi: 10.1021/acsami.6b05572
88. Li S, Wang R, Li J, Liu Y, Fu Y, Zhou J, et al. Revealing the dynamic mechanism by which transferrin promotes the cellular uptake of HAIYPRH peptide-conjugated nanostructures by force tracing. Mol Pharmaceut (2021) 18(3):1480–5. doi: 10.1021/acs.molpharmaceut.0c01119
89. Ng C, Lee S. The potential use of anticancer peptides (ACPs) in the treatment of hepatocellular carcinoma. Curr Cancer Drug Tar (2020) 20(3):187–96. doi: 10.2174/1568009619666191111141032
90. Harris F, Dennison SR, Singh J, Phoenix DA. On the selectivity and efficacy of defense peptides with respect to cancer cells. Med Res Rev (2013) 33(1):190–234. doi: 10.1002/med.20252
91. Ahmed S, Khan H, Fakhri S, Aschner M, Cheang WS. Therapeutic potential of marine peptides in cervical and ovarian cancers. Mol Cell Biochem (2022) 477(2):605–19. doi: 10.1007/s11010-021-04306-y
92. Zhou C, Kang J, Wang X, Wei W, Jiang W. Phage display screening identifies a novel peptide to suppress ovarian cancer cells in vitro and in vivo in mouse models. BMC Cancer (2015) 15:889. doi: 10.1186/s12885-015-1891-8
93. Ma C, Yin G, Yan D, He X, Zhang L, Wei Y, et al. A novel peptide specifically targeting ovarian cancer identified by in vivo phage display. J Pept Sci (2013) 19(12):730–6. doi: 10.1002/psc.2555
94. Pu X, Ma C, Yin G, You F, Wei Y. Cell adhesion and invasion inhibitory effect of an ovarian cancer targeting peptide selected via phage display in vivo. Biochem Biophys Res Commun (2014) 443(3):858–63. doi: 10.1016/j.bbrc.2013.12.058
95. Chin YT, Wang LM, Hsieh MT, Shih YJ, Nana AW, Changou CA, et al. Leptin OB3 peptide suppresses leptin-induced signaling and progression in ovarian cancer cells. J BioMed Sci (2017) 24(1):51. doi: 10.1186/s12929-017-0356-6
96. Mao HL, Pang Y, Zhang X, Yang F, Zheng J, Wang Y, et al. Smac peptide potentiates TRAIL- or paclitaxel-mediated ovarian cancer cell death in vitro and in vivo. Oncol Rep (2013) 29(2):515–22. doi: 10.3892/or.2012.2132
97. Piotrowicz RS, Damaj BB, Hachicha M, Incardona F, Howell SB, Finlayson M. A6 peptide activates CD44 adhesive activity, induces FAK and MEK phosphorylation, and inhibits the migration and metastasis of CD44-expressing cells. Mol Cancer Ther (2011) 10(11):2072–82. doi: 10.1158/1535-7163.MCT-11-0351
98. Yu X, Liang C, Zhang Y, Zhang W, Chen H. Inhibitory short peptides targeting EPS8/ABI1/SOS1 tri-complex suppress invasion and metastasis of ovarian cancer cells. BMC Cancer (2019) 19(1):878. doi: 10.1186/s12885-019-6087-1
99. Zhao M, Wei C, Yang X, Zhou J, Wang J, Gu F, et al. The milk-derived hexapeptide PGPIPN inhibits the invasion and migration of human ovarian cancer cells by regulating the expression of MTA1 and NM23H1 genes. Int J Oncol (2016) 48(4):1721–9. doi: 10.3892/ijo.2016.3390
100. Alagkiozidis I, Gorelick C, Shah T, Chen Y, Gupta V, Stefanov D, et al. Synergy between paclitaxel and anti-cancer peptide PNC-27 in the treatment of ovarian cancer. Ann Clin Lab Sci (2017) 47(3):271–81.
101. Cardinale D, Guaitoli G, Tondi D, Luciani R, Henrich S, Salo-Ahen OM, et al. Protein-protein interface-binding peptides inhibit the cancer therapy target human thymidylate synthase. Proc Natl Acad Sci U.S.A. (2011) 108(34):E542–549. doi: 10.1073/pnas.1104829108
102. Cannazza G, Cazzato AS, Marraccini C, Pavesi G, Pirondi S, Guerrini R, et al. Internalization and stability of a thymidylate synthase peptide inhibitor in ovarian cancer cells. J Med Chem (2014) 57(24):10551–6. doi: 10.1021/jm501397h
103. Sutton MN, Huang GY, Liang X, Sharma R, Reger AS, Mao W, et al. DIRAS3-derived peptide inhibits autophagy in ovarian cancer cells by binding to Beclin1. Cancers (Basel) (2019) 11(4):557–66. doi: 10.3390/cancers11040557
104. Ma S, Pradeep S, Villar-Prados A, Wen Y, Bayraktar E, Mangala LS, et al. GnRH-R-Targeted lytic peptide sensitizes BRCA wild-type ovarian cancer to PARP inhibition. Mol Cancer Ther (2019) 18(5):969–79. doi: 10.1158/1535-7163.MCT-18-0770
105. Kim M, Ma S, Chelariu-Raicu A, Leuschner C, Alila H, Lee S, et al. Enhanced immunotherapy with LHRH-r targeted lytic peptide in ovarian cancer. Mol Cancer Ther (2020) 19(11):2396–406. doi: 10.1158/1535-7163.MCT-20-0030
106. Eggink LL, Roby KF, Cote R, Kenneth Hoober J. An innovative immunotherapeutic strategy for ovarian cancer: CLEC10A and glycomimetic peptides. J Immunother Cancer (2018) 6(1):28. doi: 10.1186/s40425-018-0339-5
107. Pela M, Saxena P, Luciani R, Santucci M, Ferrari S, Marverti G, et al. Optimization of peptides that target human thymidylate synthase to inhibit ovarian cancer cell growth. J Med Chem (2014) 57(4):1355–67. doi: 10.1021/jm401574p
108. Kristensen M, Birch D, Mørck Nielsen H. Applications and challenges for use of cell-penetrating peptides as delivery vectors for peptide and protein cargos. Int J Mol Sci (2016) 17(2):185–97. doi: 10.3390/ijms17020185
109. Kong X, Xu J, Yang X, Zhai Y, Ji J, Zhai G. Progress in tumour-targeted drug delivery based on cell-penetrating peptides. J Drug Target (2022) 30(1):46–60. doi: 10.1080/1061186X.2021.1920026
110. Engel J, Tinneberg H, Rick F, Berkes E, Schally A. Targeting of peptide cytotoxins to LHRH receptors for treatment of cancer. Curr Drug Targets (2016) 17(5):488–94. doi: 10.2174/138945011705160303154717
111. Ma S, Pradeep S, Villar-Prados A, Wen Y, Bayraktar E, Mangala L, et al. BRCAGnRH-R-Targeted lytic peptide sensitizes wild-type ovarian cancer to PARP inhibition. Mol Cancer Ther (2019) 18(5):969–79. doi: 10.1158/1535-7163.MCT-18-0770
112. Chelariu-Raicu A, Nick A, Urban R, Gordinier M, Leuschner C, Bavisotto L, et al. A multicenter open-label randomized phase II trial of paclitaxel plus EP-100, a novel LHRH receptor-targeted, membrane-disrupting peptide, versus paclitaxel alone for refractory or recurrent ovarian cancer. Gynecol Oncol (2021) 160(2):418–26. doi: 10.1016/j.ygyno.2020.11.013
113. Lheureux S, Braunstein M, Oza AJ. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA-Cancer J Clin (2019) 69(4):280–304. doi: 10.3322/caac.21559
114. Liu W, Tang H, Li L, Wang X, Yu Z, Li J. Peptide-based therapeutic cancer vaccine: current trends in clinical application. Cell Prolif (2021) 54(5):e13025. doi: 10.1111/cpr.13025
115. Dafni U, Martin-Lluesma S, Balint K, Tsourti Z, Vervita K, Chenal J, et al. Efficacy of cancer vaccines in selected gynaecological breast and ovarian cancers: a 20-year systematic review and meta-analysis. Eur J Cancer (2021) 142:63–82. doi: 10.1016/j.ejca.2020.10.014
116. Odunsi K, Qian F, Matsuzaki J, Mhawech-Fauceglia P, Andrews C, Hoffman E, et al. Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. P Natl Acad Sci USA (2007) 104(31):12837–42. doi: 10.1073/pnas.0703342104
117. Gritzapis AD, Mahaira LG, Perez SA, Cacoullos NT, Papamichail M, Baxevanis CN. Vaccination with human HER-2/neu (435-443) CTL peptide induces effective antitumor immunity against HER-2/neu-expressing tumor cells in vivo. Cancer Res (2006) 66(10):5452–60. doi: 10.1158/0008-5472.CAN-05-4018
118. Leffers N, Vermeij R, Hoogeboom BN, Schulze UR, Wolf R, Hamming IE, et al. Long-term clinical and immunological effects of p53-SLP(R) vaccine in patients with ovarian cancer. Int J Cancer (2012) 130(1):105–12. doi: 10.1002/ijc.25980
119. Nishida S, Morimoto S, Oji Y, Morita S, Shirakata T, Enomoto T, et al. Cellular and humoral immune responses induced by an HLA class I-restricted peptide cancer vaccine targeting WT1 are associated with favorable clinical outcomes in advanced ovarian cancer. J Immunother (2022) 45(1):56–66. doi: 10.1097/CJI.0000000000000405
120. Brown TA, Byrd K, Vreeland TJ, Clifton GT, Jackson DO, Hale DF, et al. Final analysis of a phase I/IIa trial of the folate-binding protein-derived E39 peptide vaccine to prevent recurrence in ovarian and endometrial cancer patients. Cancer Med (2019) 8(10):4678–87. doi: 10.1002/cam4.2378
121. Vreeland TJ, Litton JK, Qiao N, Philips AV, Alatrash G, Hale DF, et al. Phase ib trial of folate binding protein (FBP)-derived peptide vaccines, E39 and an attenuated version, E39’: an analysis of safety and immune response. Clin Immunol (2018) 192:6–13. doi: 10.1016/j.clim.2018.03.010
122. Kalli KR, Block MS, Kasi PM, Erskine CL, Hobday TJ, Dietz A, et al. Folate receptor alpha peptide vaccine generates immunity in breast and ovarian cancer patients. Clin Cancer Res (2018) 24(13):3014–25. doi: 10.1158/1078-0432.CCR-17-2499
123. Zamarin D, Walderich S, Holland A, Zhou Q, Iasonos AE, Torrisi JM, et al. Safety, immunogenicity, and clinical efficacy of durvalumab in combination with folate receptor alpha vaccine TPIV200 in patients with advanced ovarian cancer: a phase II trial. J Immunother Cancer (2020) 8(1):829–50. doi: 10.1136/jitc-2020-000829
124. Jackson D, Byrd K, Vreeland T, Hale D, Herbert G, Greene J, et al. Interim analysis of a phase I/IIa trial assessing E39+GM-CSF, a folate binding protein vaccine, to prevent recurrence in ovarian and endometrial cancer patients. Oncotarget (2017) 8(9):15912–23. doi: 10.18632/oncotarget.13305
125. Berry J, Vreeland T, Hale D, Jackson D, Trappey A, Greene J, et al. Evaluation of attenuated tumor antigens and the implications for peptide-based cancer vaccine development. J Cancer (2017) 8(7):1255–62. doi: 10.7150/jca.16450
126. Gül A, Döşkaya M, Can H, Karakavuk M, Anıl-İnevi M, Sağlam-Metiner P, et al. Immunogenicity of a xenogeneic multi-epitope HER2 breast cancer DNA vaccine targeting the dendritic cell restricted antigen-uptake receptor DEC205. VACCINE (2022) 40(16):2409–19. doi: 10.1016/j.vaccine.2022.03.014
127. Brentville V, Metheringham R, Daniels I, Atabani S, Symonds P, Cook K, et al. Combination vaccine based on citrullinated vimentin and enolase peptides induces potent CD4-mediated anti-tumor responses. J J Immunother Cancer (2020) 8(1):560–86. doi: 10.1136/jitc-2020-000560
128. Jiang Z, Guan J, Qian J, Zhan C. Peptide ligand-mediated targeted drug delivery of nanomedicines. Biomater Sci-UK (2019) 7(2):461–71. doi: 10.1039/C8BM01340C
Keywords: peptides, ovarian cancer, diagnosis, targeted therapy, peptide vaccine
Citation: Guo L, Wang J, Li N, Cui J and Su Y (2023) Peptides for diagnosis and treatment of ovarian cancer. Front. Oncol. 13:1135523. doi: 10.3389/fonc.2023.1135523
Received: 01 January 2023; Accepted: 24 April 2023;
Published: 05 May 2023.
Edited by:
Yong Sang Song, Seoul National University, Republic of KoreaReviewed by:
Firas Hamdan, University of Helsinki, FinlandAnna Lucia Tornesello, G. Pascale National Cancer Institute Foundation (IRCCS), Italy
Xiaoyan Zhang, Fudan University, China
Copyright © 2023 Guo, Wang, Li, Cui and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yajuan Su, c3V5YWp1YW4xOTc3QHNpbmEuY29t
 Ling Guo
Ling Guo Jing Wang
Jing Wang