- 1Department of Radiation Oncology, Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, Miami, FL, United States
- 2Department of Otolaryngology, University of Miami Miller School of Medicine, Miami, FL, United States
- 3Department of Medicine, Florida International University, Miami, FL, United States
- 4Department of Public Health Sciences, Biostatistics and Bioinformatics Shared Resource, Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, Miami, FL, United States
- 5Department of Psychiatry, University of Miami Miller School of Medicine, Miami, FL, United States
Objectives: Radiation therapy (RT) is an integral part of treatment of head/neck cancer (HNC) but is associated with many toxicities. We sought to evaluate sociodemographic, pathologic, and clinical factors associated with emergency department (ED) visits, hospital admissions (HA), and RT breaks in HNC patients undergoing curative-intent RT.
Methods: We completed a Level 3 (Oxford criteria for evidence-based medicine) analysis of a cohort of HNC patients who underwent curative-intent RT at our institution from 2013 to 2017. We collected demographic characteristics and retrospectively assessed for heavy opioid use, ED visits or HA during RT as well as RT breaks. Treatment breaks were defined as total days to RT fractions ratio ≥1.6. Multivariable stepwise logistic regression analyses were done to determine the association of various sociodemographic, pathologic, and clinical characteristics with ED visits, HA and RT treatment breaks.
Results: The cohort included 376 HNC patients (294 male, 82 female, median age 61). On multivariable analysis, significant factors associated with ED visits during RT were heavy opioid use and black race. Receipt of concomitant chemotherapy was the only factor associated with hospital admissions during RT. Advanced age, lower socioeconomic class, glandular site, and receipt of chemotherapy were all independently associated with RT breaks. Lower cancer stage and lack of substance abuse history were independently associated with lack of treatment breaks.
Conclusion: HNC patients with factors such as heavy opioid use, Black race, receipt of concomitant chemotherapy, and lower socioeconomic class may require closer monitoring during RT.
Introduction
Head and neck cancer accounts for approximately 900,000 cases and over 400,000 deaths annually (GLOBOCAN 2020 data) (1). Radiation therapy (RT) is an important part of the multidisciplinary management of head and neck cancer. Unplanned hospitalizations and emergency department (ED) visits during the radiation treatment course can lead to treatment breaks, disproportionately affecting certain vulnerable populations and leading to a financial burden on patients and the healthcare system (2). Overall survival and cancer-specific survival is significantly decreased for head and neck cancer patients hospitalized during radiation therapy, with dehydration and fever the leading causes of admission (3). Moreover, adherence to the radiation treatment timeline is important as unplanned RT breaks and prolongation of the RT period is associated with worse survival and locoregional control of disease, possibly due to rapid repopulation (4–6).
Quality of life scores significantly decrease as patients experience oral complications during or after RT, with pain a major contributor (7). Pain can be prevalent throughout the radiation treatment course and, in some patients, persist 6-12 months post-RT (8). Painful sequelae such as oral mucositis during curative-intent RT for head and neck cancer is a common reason for hospitalizations during treatment (9). In order to optimize pain control amongst head and neck cancer patients, opioids are commonly prescribed (10, 11). Head and neck cancer patients treated with radiotherapy are at risk for long-term opioid use (12, 13), but predicting long-term opioid use is difficult (11).
Although pain and resulting opioid use play a large role in the treatment course of head and neck patients, prior studies evaluating risks for ED visits, unplanned hospitalizations, and treatment breaks in this population have failed to take these important components into account (11). In this study, we sought to analyze the occurrence of ED visits, unplanned hospitalizations, and radiation treatment breaks in head and neck cancer patients undergoing curative-intent radiation therapy in relation to pain and opioid use as well as other clinical, treatment and socioeconomic characteristics.
Materials and methods
This single-institution study was approved by the Institutional Review Board (IRB). We retrospectively identified a group of patients with head and neck cancer at our institution from the Tumor Registry who were treated with curative-intent external beam radiotherapy from 2013 to 2017. Additional inclusion criteria for the cohort included: (a) received RT at our institution, (b) did not have persistent disease or recurrent disease within 18 months, (c) had no history of chronic opioid use for non-cancer pain before cancer presentation or diagnosis, (d) received RT to the primary disease site and (e) had non-metastatic disease. Patients were excluded if they had received prior irradiation or other treatment not part of the current treatment course (indicating recurrent disease).
The electronic medical record (EMR) of eligible patients was reviewed and clinical data was collected in a Research Data Electronic Capture (REDCap) database. Sociodemographic, pathologic, and clinical factors were collected for each patient (Table 1). Additionally, we recorded opioid use, hospitalizations and ED visits within the radiation treatment period and total days to complete radiation therapy from the EMR. Each patient’s chart was reviewed for hospitalizations and ED visits in our hospital system as well as documentation of hospitalizations or ED visits outside of our hospital system. Documentation reviewed included weekly on-treatment notes during RT. Planned hospitalizations solely for chemotherapy infusion were excluded unless the hospitalization was extended beyond two days for supportive care related to RT side effects. ED visits leading to a hospital admission were counted as a hospitalization, but not an ED visit. We calculated the ratio of total days from start to completion of RT divided by the fractions completed to assess for a prolonged RT course. Substantial treatment breaks were defined as total days to RT fractions ratio ≥1.6. The ratio cutoff of ≥1.6 days/fraction was chosen based on work by Ho et al. demonstrating a survival significance at this days/fraction ratio (14). In certain cases where an opioid was prescribed, but the patient reported not using opioids, no opioid dose was recorded. Heavy opioid use was defined as >30 morphine milligram equivalents (MME) daily as used elsewhere in the literature (15, 16).
Statistical methods
Continuous variables were summarized with descriptive statistics and categorical variables were summarized using counts and proportions. Univariable and multivariable logistic regression analyses were performed to determine the association of socioeconomic class, race, ethnicity, age, marital status, gender, primary language, employment, living situation, chemotherapy, opioid use, non-opioid substance use, history of substance use, chronic pain condition, psychiatric disease, cancer site or cancer stage with ED visits, hospital admissions and RT treatment breaks. Odds ratios (OR) were collected and a p-value <0.05 was considered statistically significant. For the multivariable analysis, we used stepwise variable selection. Statistical analyses were performed with the statistical software package R, version 4.0.5 (R foundation for Statistical Computing).
Results
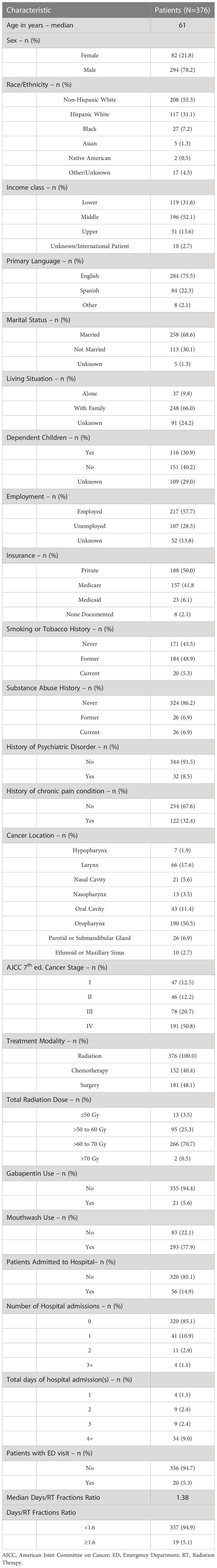
The institution tumor registry contained a total of 678 patients with head and neck cancer treated with external beam radiation therapy from 2013 to 2017. Our cohort included 376 patients after excluding patients who did not meet our inclusion and exclusion criteria. Table 1 contains patient characteristics of the cohort. The cohort consisted of 78.2% males and 21.8% females with a median age of 61 years. The patients included non-Hispanic white (55.3%), Hispanic white (31.1%), Black (7.2%), Asian (1.3%), Native American (0.5%), and other (4.5%). The majority of patients were married (68.6%), middle class (52.1%), English-speaking (75.5%), living with family members (66%), employed (57.7%), without history of substance abuse (86.2%) or history of psychiatric disorder (91.5%), and with locally advanced disease (71.5% stage III-IV). The most common primary cancer sites were oropharynx (50.5%) and larynx (17.6%). The median radiation therapy dose received was 66 Gy. Of the cohort, 40.4% of patients received chemotherapy during the treatment course and 48.1% of patients received radiation therapy pre- or post-operatively.
In the cohort, 14.9% of the patients had at least one unplanned hospital admission and 5.3% had at least one ED visit not leading to hospitalization. Of the patients who had at least one unplanned hospitalization, 41 (73.2%) patients had one admission, 11 (19.6%) patients had two admissions and 4 (7.1%) patients had three or more admissions. The majority of patients with unplanned hospital admissions had a length of stay greater than 3 days (60.7%). The reasons for unplanned hospital admissions included dehydration (51.8% of admissions), mucositis (17.8%), fever with or without neutropenia (17.8%), intractable nausea or vomiting (3.5%), non-opioid induced constipation (1.8%), diarrhea (1.8%), draining fistula (1.8%), chest pain (1.8%), and urinary tract infection (1.8%). For unplanned hospitalizations, 93% were due to treatment or cancer-related factors and 7% were non-cancer related. The diagnoses for ED visits included dehydration (40%), dysphagia (15%), neutropenic fever (10%), urinary retention (10%), shortness of breath (5%), irritation around PEG tube (5%), depression (5%), constipation (5%), and nephrolithiasis (5%). In total, 80% of ED visits were due to treatment or cancer-related factors and 20% were non-cancer related. The median days to RT fraction ratio was 1.38 days/fraction, with 19 (5.1%) of patients with a ratio ≥ 1.6 days/fraction.
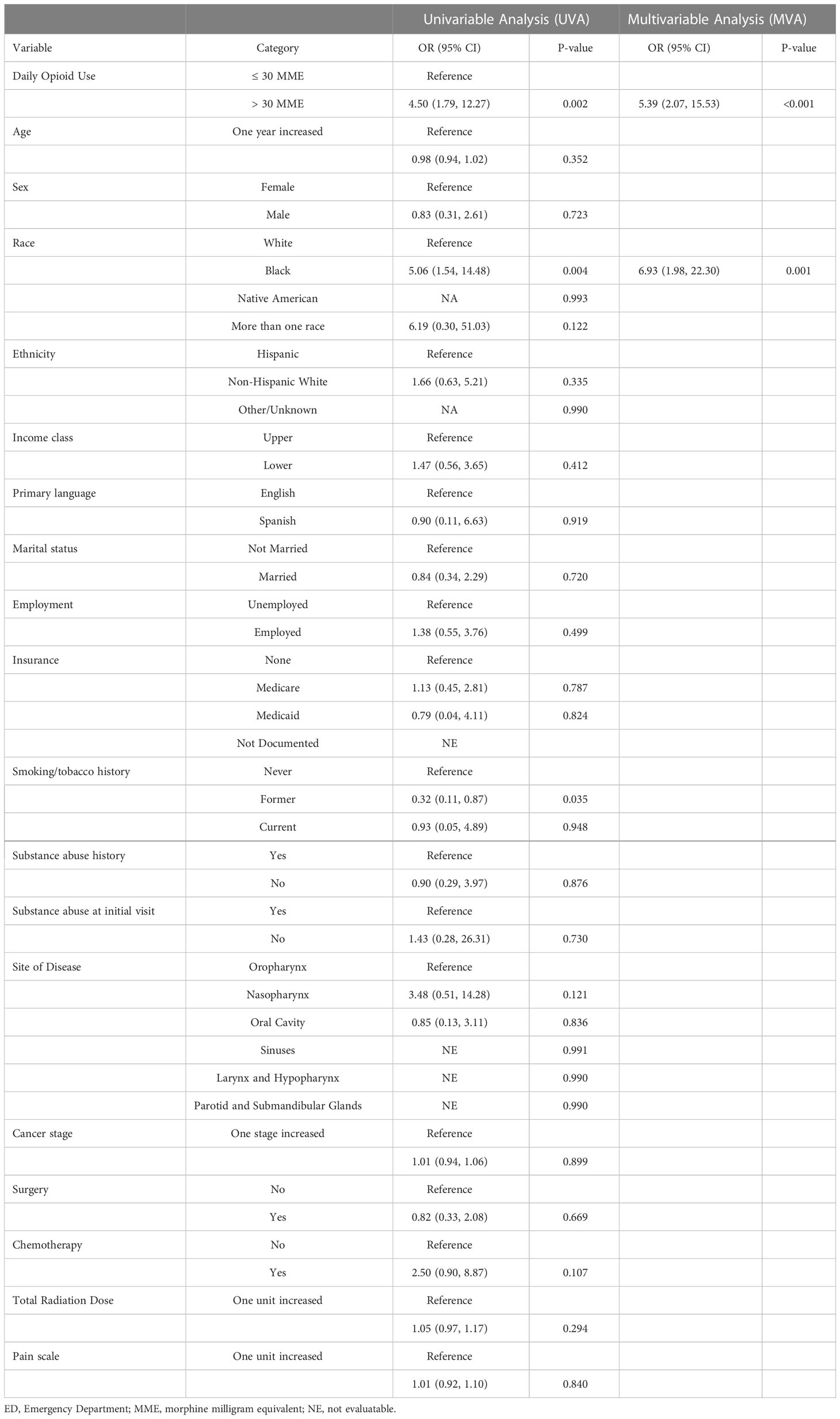
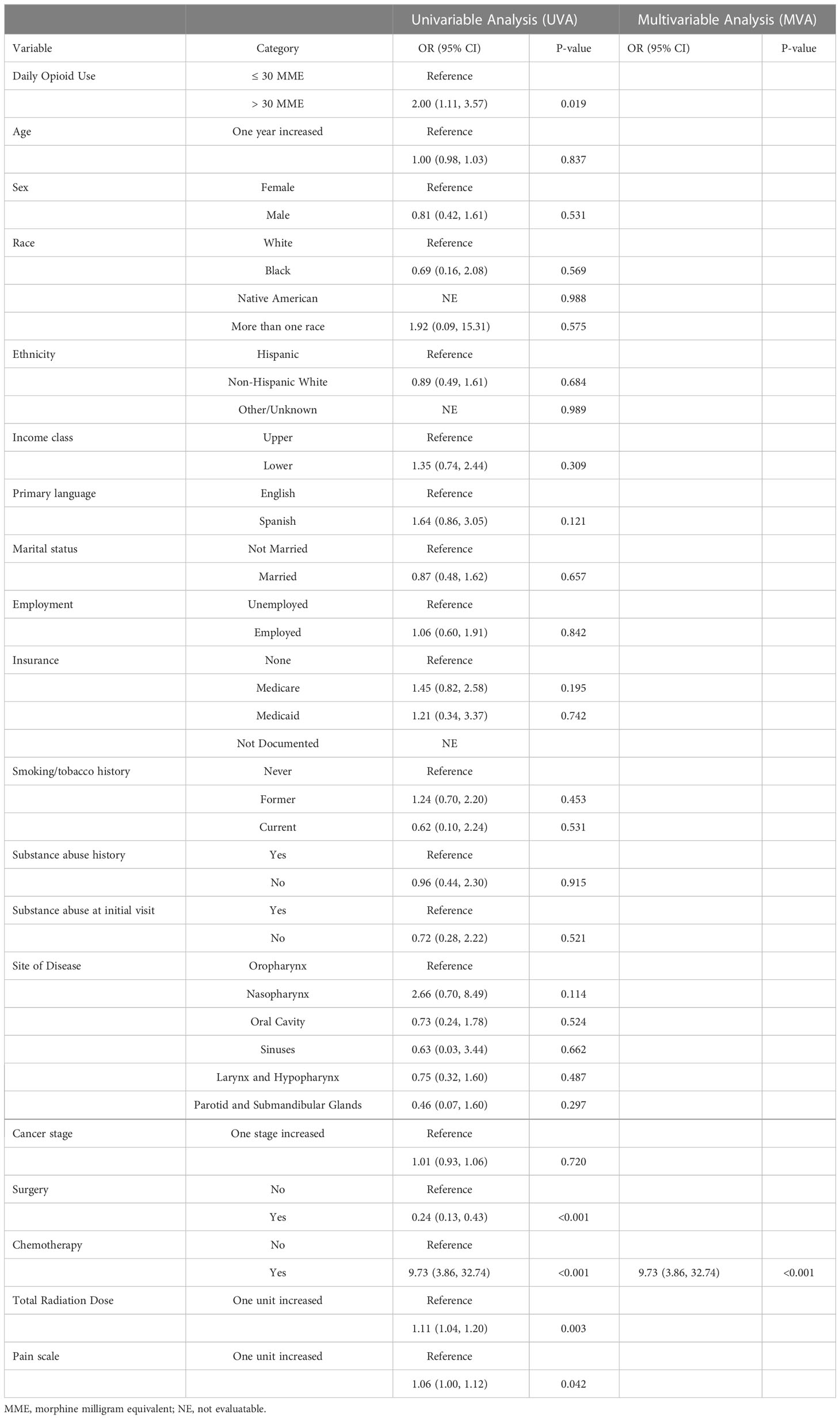
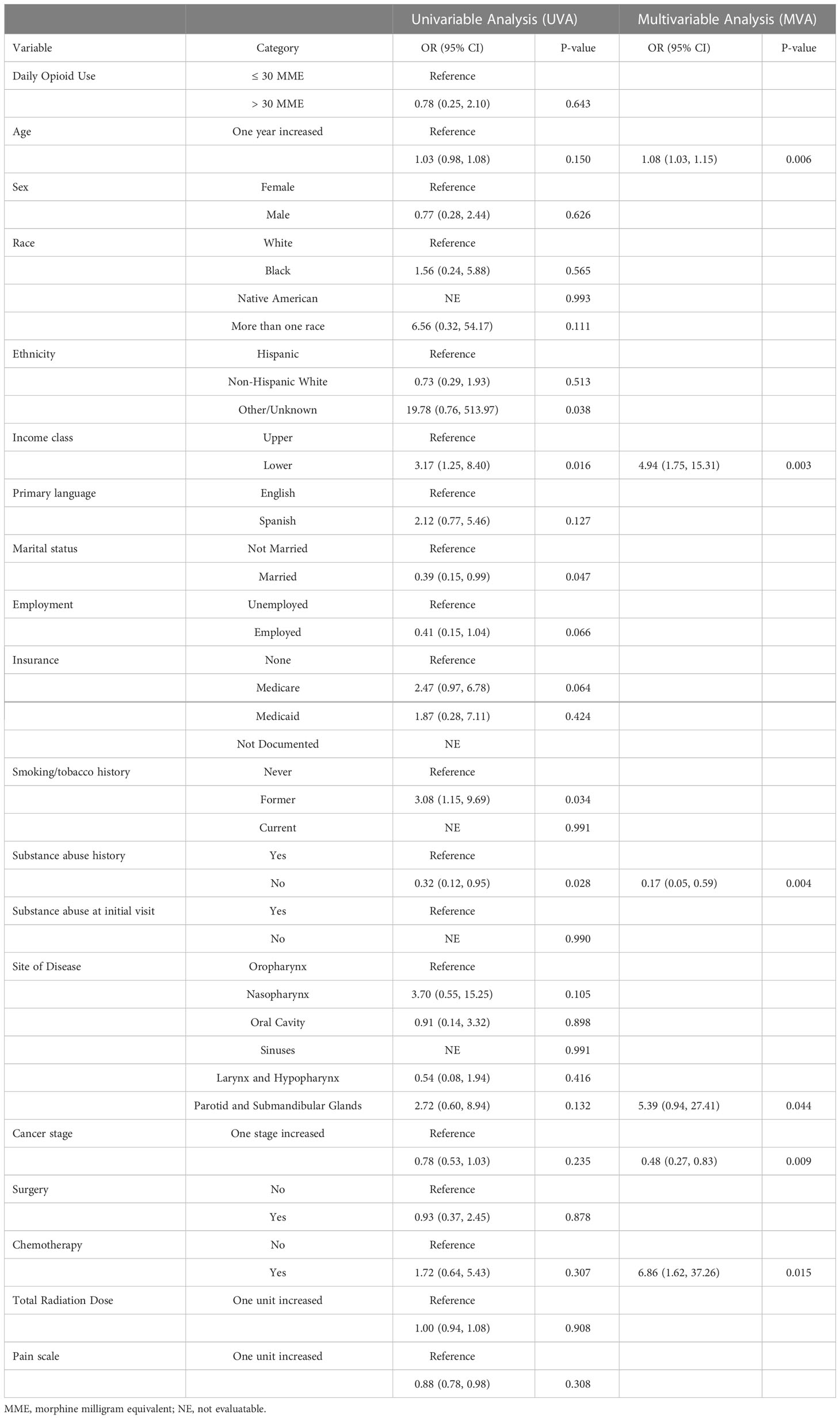
A univariable and multivariable analysis was completed to determine factors associated with ED visits, hospital admissions, and RT treatment breaks. On multivariable analysis, the factors independently associated with ED visits during RT were heavy opioid use (OR 5.39, p<0.01) and black race (OR 6.93, p<0.01) (Table 2). Unplanned hospital admissions during RT were only independently associated with the receipt of concomitant chemotherapy (OR 9.73, p<0.01) (Table 3). Heavy opioid use was associated with hospital admissions on univariable analysis (OR 2.00, p=0.019), but this was not significant on multivariable analysis. On the multivariable analysis, older age (OR 1.08, p<0.01), lower socioeconomic class (OR 4.94, p<0.01), primary salivary tumor site (OR 5.39, p=0.04), and receipt of chemotherapy (OR 6.86, p=0.01) were all independently associated with RT breaks (Table 4). Lower cancer stage (OR 0.48, p<0.01) and lack of substance abuse history (OR 0.17, p<0.01) were independently associated with lack of treatment breaks. Other socioeconomic, pathologic and clinical treatment variables analyzed in this study did not disclose significant associations.
Discussion
ED visits and unplanned hospitalizations during curative-intent RT for head and neck cancer can lead to significant resource utilization (9, 17)and treatment breaks, which result in worse locoregional disease control and poorer survival (5, 6). Despite the importance of minimizing unplanned hospital encounters and treatment breaks in this patient population, the literature on factors associated with these events is limited and without inclusion of pain or opioid use, both important, quantifiable factors of patient experience during RT. In this large cohort of 376 head and neck cancer patients treated with curative intent RT, heavy opioid use was independently associated with ED visits during RT (OR 5.39, p<0.001), but not unplanned hospitalizations or RT treatment breaks. Pain scores during RT were not independently associated with ED visits, unplanned hospitalizations or RT treatment breaks.
Other socioeconomic and treatment related factors were associated with these events in the cohort. Black race (OR 6.93, p= 0.001) was a significant, independent predictor of ED visits. At least one unexpected hospital admissions occurred in 14.9% of the patients in our cohort (n = 56). Unplanned hospital admissions had a significant association with receipt of concomitant chemotherapy (OR 9.73, p<0.001). 5.1% of patients in the cohort had a significant radiation treatment break. Advanced age, lower socioeconomic status, primary salivary tumor site, lower cancer stage, receipt of chemotherapy, and history of substance abuse were all independently associated with RT breaks (p<0.05).
We found a significant association between ED visits and heavy opioid use, but unplanned hospital admissions and RT breaks did not share this association. Pain was also not an independent risk factor. This may suggest patients requiring heavy opioids require extra counseling or alternative analgesics to prevent unnecessary ED visits. However, patients with heavy opioid use do not appear to be at higher risk of more serious complications leading to hospitalizations or treatment breaks.
The predictors of unplanned hospital encounters found in the present study should be taken into context with existing literature. Like the present study, chemotherapy in the treatment course of head and neck cancer patients has been previously shown to be associated with an increased number of hospital admissions (3, 18, 19). This association is not limited to head and neck patients (20). Our study is the first to report that Black race is associated with ED visits. Other studies have found treatment at a public hospital, comorbidities, radiation dose, smoking status all associated with unplanned hospitalizations (18, 21).
In our cohort, 14.9% of the patients had at least one unplanned hospital admission and 5.3% had at least one ED visit, which is consistent with existing literature showing 20%-36% of patients undergoing curative intent RT for head and neck cancer had at least one hospitalization (3) (18). Unplanned hospital encounters, in addition to treatment breaks and resulting worse cancer outcomes, lead to significant resource utilization (2, 3, 17). In the United States, a 2019 study showed hospitalizations of head and neck cancer patients had an average length of stay of 6.6 days for one admission with an average cost of $18,371 (17).
In the present study, advanced age, lower socioeconomic status, primary salivary tumor site, lower cancer stage, receipt of chemotherapy, and history of substance abuse were all independently associated with RT breaks (p<0.05). The literature reports various patient characteristics associated with treatment breaks (22–24). Age is associated with enteral feeding during RT for head and neck cancer and could explain this finding (25). Other studies have found treatment breaks are associated with lower socioeconomic status (23, 24), and this could explain the worse survival seen in head and neck patients with higher baseline financial burden undergoing RT (26). Treatment breaks could also be explained by insurance disparities described elsewhere, although this was not the case for the present study (27).
Nutritional status, hydration status, and feeding tube placement are important factors that can result in unplanned ED visits, hospital admissions and treatment breaks. Among the ED visits in this study, 60% were related to nutritional or hydration status and 52% of unplanned hospital admissions were related to nutritional or hydration status. Early PEG tube placement during head and neck radiotherapy is correlated with a reduction in weight loss and, as a result, hospitalizations for nutritional deficits (28). However, PEG tube placement comes with a variety of complications that could lead to unplanned ED visits or hospitalizations: 12% of all tubes require replacement, with infection rates of approximately 9% and significant pain in 6% of patients (29).
Our study includes several limitations in addition to biases inherent to respective studies. First, the data was collected from the EMR at a single institution. Although all available records were diligently reviewed, outside ED visits or hospitalizations may have been missed if they were not documented in the EMR at out institution. Additionally, the days of treatment to RT fractions ratio may have been affected by holidays, but given the cutoff of ≥1.6 this is unlikely to have much, if any, effect on the categorization of treatment breaks. Lastly, the cohort only included head and neck cancer patients who underwent curative-intent radiation therapy and excluded patients with persistent disease, making the data collected pertinent only to this subset of patients. This study is strengthened by a relatively large, homogenous number of patients with a robust number of variables collected.
Conclusion
In this analysis of 376 head and neck patients receiving curative-intent RT, we found heavy opioid use to be independently associated with ED visits during RT, but not unplanned hospitalizations or RT treatment breaks. Other factors independently, significantly associated included Black race with ED visits and receipt of chemotherapy with unplanned hospital admissions during RT. RT breaks were associated with advanced age, lower socioeconomic class, primary salivary tumor site, and concomitant chemotherapy. Lower cancer stage and lack of substance abuse history were independently associated with lack of treatment breaks. Head and neck cancer patients with these factors may require extra care during the RT course to prevent ED visits, hospitalizations and treatment breaks.
Data availability statement
The datasets presented in this article are not readily available because not available for public dissemination as per IRB. Requests to access the datasets should be directed toc2hhcmVlbi5wYXRlbEBtZWQubWlhbWkuZWR1.
Author contributions
SP, BR, GA, and MS contributed to conception and design of the study. SP, L-ES, MM organized the database. DK, RC and BR performed the statistical analysis. SP and BR wrote the first draft of the manuscript. MS and GA wrote sections of the manuscript. CW, MR-L, and ZS contributed to investigation. SS, LF, MS and MA contributed to supervision of study. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020 A Cancer Journal for Clinicians (2021). (71):3:209–249 doi: 10.3322/caac.21660.
2. Noel CW, Forner D, Wu V, Enepekides D, Irish JC, Husain Z, et al. Predictors of surgical readmission, unplanned hospitalization and emergency department use in head and neck oncology: A systematic review. Oral Oncol (2020) 111:105039. doi: 10.1016/J.ORALONCOLOGY.2020.105039
3. Han HR, Hermann GM, Ma SJ, Iovoli AJ, Wooten KE, Arshad H, et al. Matched pair analysis to evaluate the impact of hospitalization during radiation therapy as an early marker of survival in head and neck cancer patients. Oral Oncol (2020) 109:104854. doi: 10.1016/J.ORALONCOLOGY.2020.104854
4. Russo G, Haddad R, Posner M, Machtay M. Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist (2008) 13(8):886–98. doi: 10.1634/THEONCOLOGIST.2008-0024
5. McCloskey SA, Jaggernauth W, Rigual NR, Hicks WL, Popat SR, Sullivan M, et al. Radiation treatment interruptions greater than one week and low hemoglobin levels (12 g/dL) are predictors of local regional failure after definitive concurrent chemotherapy and intensity-modulated radiation therapy for squamous cell carcinoma of the head and neck. Am J Clin Oncol (2009) 32(6):587–91. doi: 10.1097/COC.0B013E3181967DD0
6. Suntharalingam M, Haas ML, Van Echo DA, Haddad R, Jacobs MC, Levy S, et al. Predictors of response and survival after concurrent chemotherapy and radiation for locally advanced squamous cell carcinomas of the head and neck. Journal (2001). doi: 10.1002/1097-0142(20010201)91:3<548::AID-CNCR1033>3.0.CO;2-A
7. Bjordal K, Hammerlid E, Ahlner-Elmqvist M, De Graeff A. Quality of life in head and neck cancer patients: Validation of the European organization for research and treatment of cancer quality of life questionnaire-H&N35 Male cancer view project Utrecht symptom diary view project. Article J Clin Oncol (1999). 17:(3):1008–19. doi: 10.1200/JCO.1999.17.3.1008
8. Epstein JB, Stewart KH. Radiation therapy and pain in patients with head and neck cancer. Eur J Cancer Part B: Oral Oncol (1993) 29(3):191–9. doi: 10.1016/0964-1955(93)90022-7
9. Murphy BA, Beaumont JL, Isitt J, Garden AS, Gwede CK, Trotti AM, et al. Mucositis-related morbidity and resource utilization in head and neck cancer patients receiving radiation therapy with or without chemotherapy. J Pain Symptom Manage (2009) 38(4):522–32. doi: 10.1016/J.JPAINSYMMAN.2008.12.004
10. Sethi RKV, Panth N, Puram SV, Varvares MA. Opioid prescription patterns among patients with head and neck cancer. JAMA Otolaryngology–Head Neck Surg (2018) 144(4):382–3. doi: 10.1001/JAMAOTO.2017.3343
11. Rich BJ, Schumacher LED, Sargi ZB, Masforroll M, Kwon D, Zhao W, et al. Opioid use patterns in patients with head and neck cancer receiving radiation therapy: Single-institution retrospective analysis characterizing patients who did not require opioid therapy. Head Neck (2021) 43(10):2973–84. doi: 10.1002/HED.26785
12. Bollig CA, Kinealy BP, Gilley DR, Clark AD, Galloway TLI, Zitsch RP, et al. Implications of treatment modality on chronic opioid use following treatment for head and neck cancer. Otolaryngol - Head Neck Surg (United States) (2021) 164(4):799–806. doi: 10.1177/0194599820960137
13. Zhao L, Moon DH, Avkshtol V, Siropaides CH, Terauchi S, Day AT, et al. Long-term opioid use in patients treated with head and neck intensity-modulated radiotherapy. Supportive Care Cancer (2022) 30(9):7517–25. doi: 10.1007/S00520-022-07155-7
14. Ho AS, Kim S, Tighiouart M, Mita A, Scher KS, Epstein JB, et al. Quantitative survival impact of composite treatment delays in head and neck cancer. Cancer (2018) 124(15):3154–62. doi: 10.1002/cncr.31533
15. Denawa Y, Kurtz W, Conermann T. The social and functional implications of high-versus low-dose opioids on chronic non-cancer pain. Pain Physician (2019) 22(4):401–11
16. Fudin J, Raouf M, Wegrzyn EL, Schatman ME. Safety concerns with the centers for disease control opioid calculator. J Pain Res (2018) 11:1. doi: 10.2147/JPR.S155444
17. Boakye EA, Johnston KJ, Moulin TA, Buchanan PM, Hinyard L, Tobo BB, et al. Factors associated with head and neck cancer hospitalization cost and length of stay-a national study. Am J Clin Oncology: Cancer Clin Trials (2019) 42(2):172–8. doi: 10.1097/COC.0000000000000487
18. Moore ZR, Pham NL, Shah JL, Nedzi L, Sumer BD, Day AT, et al. Risk of unplanned hospital encounters in patients treated with radiotherapy for head and neck squamous cell carcinoma. J Pain Symptom Manage (2019) 57(4):738–745.e3. doi: 10.1016/j.jpainsymman.2018.12.337
19. Eskander A, Krzyzanowska MK, Fischer HD, Liu N, Austin PC, Irish JC, et al. Emergency department visits and unplanned hospitalizations in the treatment period for head and neck cancer patients treated with curative intent: A population-based analysis. Oral Oncol (2018) 83(January):107–14. doi: 10.1016/j.oraloncology.2018.06.011
20. Waddle MR, Chen RC, Arastu NH, Green RL, Jackson M, Qaqish BF, et al. Unanticipated hospital admissions during or soon after radiation therapy: Incidence and predictive factors. Pract Radiat Oncol (2015) 5(3):e245–53. doi: 10.1016/J.PRRO.2014.08.004
21. Ling DC, Kabolizadeh P, Heron DE, Ohr JP, Wang H, Johnson J, et al. Incidence of hospitalization in patients with head and neck cancer treated with intensity-modulated radiation therapy. Head Neck (2015) 37(12):1750–5. doi: 10.1002/HED.23821
22. Patel UA, Thakkar KH, Holloway N. Patient compliance to radiation for advanced head and neck cancer at a tertiary care county hospital. Laryngoscope (2008) 118(3):428–32. doi: 10.1097/MLG.0B013E31815AE3D2
23. Ohri N, Rapkin BD, Guha D, Haynes-Lewis H, Guha C, Kalnicki S, et al. Predictors of radiation therapy noncompliance in an urban academic cancer center. Int J Radiat Oncology Biology Phys (2015) 91(1):232–8. doi: 10.1016/J.IJROBP.2014.09.030
24. Vera-Llonch M, Oster G, Hagiwara M, Sonis S. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer (2006) 106(2):329–36. doi: 10.1002/CNCR.21622
25. Sachdev S, Refaat T, Bacchus ID, Sathiaseelan V, Mittal BB. Age most significant predictor of requiring enteral feeding in head-and-neck cancer patients. Radiat Oncol (London England) (2015) 10(1). doi: 10.1186/S13014-015-0408-6
26. Ma SJ, Iovoli AJ, Attwood K, Wooten KE, Arshad H, Gupta V, et al. Association of significant financial burden with survival for head and neck cancer patients treated with radiation therapy. Oral Oncol (2021) 115:105196. doi: 10.1016/J.ORALONCOLOGY.2021.105196
27. Thomas K, Martin T, Gao A, Ahn C, Wilhelm H, Schwartz DL. Interruptions of head and neck radiotherapy across insured and indigent patient populations. J Oncol Pract (2017) 13(4):e319–28. doi: 10.1200/JOP.2016.017863
28. Rutter CE, Yovino S, Taylor R, Wolf J, Cullen KJ, Ord R, et al. Impact of early percutaneous endoscopic gastrostomy tube placement on nutritional status and hospitalization in patients with head and neck cancer receiving definitive chemoradiation therapy. Head Neck (2011) 33(10):1441–7. doi: 10.1002/HED.21624
Keywords: head/neck cancer, radiation therapy, hospital admissions, ED visits, treatment breaks, head and neck neoplasms, radiotherapy, hospital admissions
Citation: Patel S, Rich BJ, Schumacher L-ED, Sargi ZB, Masforroll M, Washington C, Kwon D, Rueda-Lara MA, Freedman LM, Samuels SE, Abramowitz MC, Samuels MA, Carmona R and Azzam GA (2023) ED visits, hospital admissions and treatment breaks in head/neck cancer patients undergoing radiotherapy. Front. Oncol. 13:1147474. doi: 10.3389/fonc.2023.1147474
Received: 18 January 2023; Accepted: 08 February 2023;
Published: 01 March 2023.
Edited by:
Giuseppe Carlo Iorio, University of Turin, ItalyReviewed by:
Emma D’Ippolito, University of Campania Luigi Vanvitelli, ItalyHuan Giap, Medical University of South Carolina, United States
Copyright © 2023 Patel, Rich, Schumacher, Sargi, Masforroll, Washington, Kwon, Rueda-Lara, Freedman, Samuels, Abramowitz, Samuels, Carmona and Azzam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gregory A. Azzam, Z3hhMzM4QG1lZC5taWFtaS5lZHU=
 Shareen Patel
Shareen Patel Benjamin J. Rich
Benjamin J. Rich Leif-Erik D. Schumacher
Leif-Erik D. Schumacher Zoukaa B. Sargi2
Zoukaa B. Sargi2 Deukwoo Kwon
Deukwoo Kwon Laura M. Freedman
Laura M. Freedman Stuart E. Samuels
Stuart E. Samuels Matthew C. Abramowitz
Matthew C. Abramowitz