- 1Department of Radiology, Taichung Veterans General Hospital, Taichung, Taiwan
- 2Department of Radiology, Taipei Veterans General Hospital, Taipei, Taiwan
- 3Department of Radiology, National Taiwan University Hospital Hsin-Chu Branch, Hsinchu, Taiwan
- 4Department of Medical Imagine, National Cheng Kung University Hospital, Tainan, Taiwan
- 5Department of Radiology, Koo Foundation Sun Yat-Sen Cancer Center, Taipei, Taiwan
- 6Department of Radiology, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
- 7Department of Radiology, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
- 8Department of Radiology, Tungs’ Taichung Metroharbor Hospital, Taichung, Taiwan
- 9Department of Radiology, Cheng Hsin General Hospital, Taipei, Taiwan
- 10Department of Radiology, Chang−Gung Memorial Hospital, Taoyuan, Taiwan
- 11Department of Radiology, Chi Mei Medical Center, Tainan, Taiwan
- 12Department of Radiology, MacKay Memorial Hospital, Taipei, Taiwan
- 13Department of Radiology, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan
Developed in early 1980s, transarterial chemoembolization (TACE) with Lipiodol was adopted globally after large-scale randomized control trials and meta-analyses proving its effectiveness were completed. Also known as “conventional TACE” (cTACE), TACE is currently the first-line treatment for patients with unresectable intermediate stage hepatocellular carcinoma (HCC) and delivers both ischemic and cytotoxic effects to targeted tumors. Although new technology and clinical studies have contributed to a more comprehensive understanding of when and how to apply this widely-adopted therapeutic modality, some of these new findings and techniques have yet to be incorporated into a guideline appropriate for Taiwan. In addition, differences in the underlying liver pathologies and treatment practices for transcatheter embolization between Taiwan and other Asian or Western populations have not been adequately addressed, with significant variations in the cTACE protocols adopted in different parts of the world. These mainly revolve around the amount and type of chemotherapeutic agents used, the type of embolic materials, reliance on Lipiodol, and the degree of selectiveness in catheter positioning. Subsequently, interpreting and comparing results obtained from different centers in a systematic fashion remain difficult, even for experienced practitioners. To address these concerns, we convened a panel of experts specializing in different aspects of HCC treatment to devise modernized recommendations that reflect recent clinical experiences, as well as cTACE protocols which are tailored for use in Taiwan. The conclusions of this expert panel are described herein.
1 Introduction
Hepatocellular carcinoma (HCC) is the predominant form of liver cancer in many countries and is the third most common cause of cancer-related death in the Asia-Pacific region. Chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are the two chief contributors to cases of HCC in Asia, with 75% of the more than 350 million chronic HBV carriers in the world living in the Asia-Pacific region (1, 2). Since the virus is endemic to much of the Asia-Pacific region, and with vertical transmission being common, chronic HBV infection remains by far the largest single factor for developing liver cancer, accounting for more than 70% of new cases diagnosed annually (3, 4). In contrast to the rest of Asia, HCV-related infection is responsible for 80% to 90% of all HCC cases in Japan. However, over the past several decades, the prevalence of HCV has risen in many Asia-Pacific countries, with prominent increases in HCV-related HCC being found in Taiwan (5). In Taiwan, HCC is the second leading cause of cancer-related death, and the majority of cases are still associated with chronic HBV infection (6).
While surgical resection continues to be the mainstream treatment in well-selected patients, less-invasive techniques such as conventional chemoembolization (cTACE) have become globally accepted as an effective treatment for patients with unresectable HCC (7, 8). Previous studies have shown that HCC staging based on the tumor-node-metastasis (TNM) system and therapy selection via the Barcelona clinic liver cancer (BCLC) staging system are applicable for the Taiwanese population and demonstrate clear survival benefits (9–13). Initially developed in Japan in the early 1980s, cTACE has been established worldwide as the standard treatment for intermediate-stage HCC without portal vein invasion (14–17). At its core, cTACE involves the use of specialized catheters to localize arterial administration of one or more cytotoxic drugs to hepatic tumors. Common therapeutic agents include doxorubicin (18) and cisplatin (19), which are thoroughly mixed with ethiodized oil (Lipiodol, Guerbet, France) prior to injection. This may then be followed by embolization of tumor-feeding vessels with particulate embolic agents, such as gelatin sponge or polyvinyl alcohol (PVA) particles (16). cTACE operates on the principle of delivering high local concentrations of a chemotherapeutic drug into liver tumors via the hepatic arterial supply, followed by the embolization of these arteries with various types of particles in order to reduce arterial blood flow. The surrounding liver parenchyma is spared due to its preferential blood supply by the portal venous circulation. Consequently, systemic toxicity can be minimized, as most of the injected chemotherapeutic agent remains within the tumor bed rather than escaping into systemic circulation. Recently, Lencioni (2016) reported the results of a meta-analysis on the efficacy of cTACE, which included a total of 10,108 HCC patients treated by Lipiodol cTACE. The systematic review showed a median overall survival (OS) of 19.4 months and a 5-year OS of 32.4% (20). A survival analysis (2018) which compared OS between TACE with a tyrosine kinase inhibitor (orantinib) or cTACE with a placebo found that patients without portal vein invasion (PVI) reached a median OS of 32.3 months using cTACE alone, which was not significantly worse than cTACE with a target therapy (21).
Over the past 30 years, numerous studies have examined the application of cTACE in HCC treatment, with more standardized concepts in patient selection and treatment protocols evolving as interventional radiologists around the world developed a more comprehensive understanding of this therapeutic modality. However, although cTACE has been performed in Taiwan for more than 30 years and interventional techniques and devices have matured in recent decades, a set of standardized best practices (the specifics of preparing the Lipiodol and drug emulsion, its method of administration, as well as the embolic agents used) needs to be established in order to be widely integrated into a modern model for oncologic treatment of liver tumors.
To that end, a group of expert physicians was convened on September 9, 2018, to develop an up-to-date technical guideline on the use of cTACE for HCC treatment in Taiwan, based on their accumulated clinical experience and research data. The experts in this panel all had extensive experience in the field of interventional radiology, and their opinions reflect the best standards of practice at leading medical centers for HCC treatment in Taiwan. During the preparation of this manuscript, a consensus statement on the treatment of intermediate-stage HCC was established in 2019 during the 10th Annual Meeting of the Asia-Pacific Primary Liver Cancer Expert (APPLE), which was held in Sapporo, Japan (22). A virtual meeting of the panel members was later convened in October of 2021 to refine the statement of cTACE ineligibility. The following statements summarize the consensus recommendations of this multidisciplinary expert panel. Although they may serve as a set of general guidelines, each patient undergoing cTACE presents a unique combination of individual and tumor characteristics that must be carefully assessed and evaluated, and the best approach for any individual patient can only be decided by their treating interventional radiologist.
2 Patient selection
Statement 2.1 (evidence level: 1, recommendation: A, agreement: 100%)
cTACE is recommended as a first-line treatment for unresectable, multifocal or large HCCs without vascular invasion or extrahepatic spread
cTACE can also be selectively performed in early-stage patients for whom radiofrequency ablation is unsuitable, due to either tumor location or medical comorbidities (Asian Pacific Association for the study of the liver).
• Vascular invasion
Patients with subsegmental (Vp1) or segmental (Vp2) levels of portal thrombosis may still be treated by cTACE.
• Liver function
Pretreatment Child-Pugh classification score A is recommended, but superselective embolization may still be considered in Child-Pugh B patients with hypervascular HCC.
At present, cTACE is primarily indicated in patients who are unable to receive curative treatment via surgery, liver transplantation or percutaneous ablation. Based on consensus recommendations on HCC treatment by the Asian Pacific Association for the Study of the Liver, cTACE is recommended as a first-line treatment for unresectable, large or multifocal HCCs without vascular invasion or extrahepatic spread. The prerequisite for any cTACE procedure is a comprehensive assessment of the patient’s clinical condition, with particular attention paid to the underlying liver disease and performance status (ECOG). Rather than considering the absolute tumor size in relation to the liver, an assessment should be made based on a patient’s pre-treatment liver function and the percentage of functional liver parenchyma that would remain after cTACE. In general terms, a pre-treatment Child-Pugh classification score of A is recommended, but superselective embolization may still be considered in patients with Child Pugh class B of score 7 (8). Several clinical trials and a recent meta-analysis have shown that, cTACE is capable of improving survival for patients with intermediate-stage HCC, with a median survival of 28.7 months (18) and 1- and 2-year survival of 57% and 31% (19), as opposed to 17.9 months (18) or 32% and 11% (19) for untreated patients.
cTACE can also be selectively performed in early-stage HCC cases for which RFA is unsuitable, due to either tumor location or medical comorbidities (23). If a local cure is achieved through an initial application of TACE, the method can be referred to as curative TACE (17).
However, the presence of portal vein tumor thrombosis (PVTT) is still often considered a contraindication for transplantation, curative resection and TACE (23–25). Studies have demonstrated that interruptions to the hepatic arterial supply in patients whose portal venous supply is already compromised can potentially lead to widespread segmental liver necrosis (25, 26). If PVTT is shown on pre-treatment imaging, we recommend following the grading scheme developed by the Liver Cancer Study Group of Japan (27), which has five grades in total (Vp0 to Vp4) which correspond to progressively more central thrombosis of the portal venous branches (27).
Nevertheless, there is evidence that certain patients with PVTT can still benefit from a modified form of cTACE, given good pre-treatment liver function and the existence of collateral blood flow around the site of portal vein obstruction (28, 29). Two recent studies have reported increased survival in HCC patients with PVTT after receiving cTACE. In the first, Luo et al. (10) found in their prospective nonrandomized study that median survival significantly improved in the cTACE group (n=84) as compared to the group that received conservative treatment (n=80), in either non-cirrhotic or Child A cirrhotic patients with PVTT. The authors reported median overall survival (OS), 1-year, and 2-year survival rates of 7.1 months, 30.9% and 9.2% respectively for the cTACE group, and 4.1 months, 3.8% and 0% for the conservative treatment group (p < 0.001) (10, 30). In the second study, Chung et al. (30) also reported significant improvements in the survival of HCC patients with Vp4 PVTT who were treated with cTACE (n = 83), as compared to supportive care alone (n = 42; median OS of 5.6 versus 2.2 months, p < 0.001).
While patients with Vp1 or Vp2 levels of portal thrombosis may still be treated by cTACE with modified methods as shown above, Vp3 patients have better received a combined regimen of both cTACE and radiotherapy, as some studies have shown an increase in patient survival using this method (31, 32).The presence of severe PVTT (Vp4) is, at present, a relatively stronger contraindication to TACE. In all cases with PVTT, other therapeutic modalities – such as Yttrium-90 (Y-90) (33), hepatic artery infusion chemotherapy (HAIC) (34), and immuno-target therapy (35) – should be considered in addition to cTACE.
In conclusion, HCC with portal or hepatic venous invasion should not be considered an absolute contraindication to cTACE.
3 Pre-treatment imaging
Statement 3.1 (evidence level: 2, recommendation: A, agreement: 100%)
Dynamic multiphase CT and MRI are recommended as the first-line modalities for diagnosis and staging of HCC. In order to evaluate whether there are appropriate indications for transcatheter embolization of HCC and set up tailored treatment plans, and to allow proper assessment of the number, size and location of hepatic tumors, multiphasic computed tomography (CT) or dynamic contrast-enhanced magnetic resonance imaging (MRI) of the liver must be performed and must include at least three phases, including unenhanced, arterial, and portal venous phases. Suitable contrast injection rates and timing of each phase of scanning are essential to providing the highest possible HCC detection rate. Additional imaging modalities are required to evaluate the existence of extrahepatic disease, either within the abdomen or in distant organs (36). Previous studies have found differences in the detection rates of small HCCs with different imaging modalities, which may influence physician choice. The sensitivity of contrast enhanced ultrasound (US), CT and MRI for 1-2 cm HCC was found to be 26%, 44%, and 44%, respectively, with 100% specificity (37). However, liver-specific MRI contrast agents such as Gadoxetic acid (Gd-EOB-DTPA) have been shown to provide higher sensitivity and overall accuracy in HCC detection when compared to conventional multiphasic CT (38). Thus, gadoxetic acid-enhanced and diffusion-weighted MR imaging may be considered in cases with atypical enhancement patterns on multiphasic CT (39).
Statement 3.2 (evidence level: 2, recommendation: B, agreement: 100%)
Late arterial phase is suggested for pretreatment imaging in the diagnosis of HCC
To improve the detection of hypervascular HCCs with multidetector computed tomography (MDCT), some investigators have previously recommended the use of double arterial phase imaging (40). However, Ichikawa et al. (2002) found no significant advantages when comparing double arterial phase imaging to single late arterial phase imaging for the detection of hypervascular HCC (41).
This panel recommends the use of MDCT with late arterial phase and portal phase imaging to optimize pre-treatment tumor detection. Late arterial phase images have been shown to maximize celiac axis attenuation, while portal venous phase images tend to demonstrate best attenuation of the portal vein and normal hepatic parenchyma. For hypovascular liver tumors, late arterial and portal venous phase images were shown to have superior tumor-to-parenchyma differences as compared to early arterial phase images. As for hypervascular tumors, late arterial phase images were also found to provide subjectively better tumor conspicuity compared to early arterial phase images (41–43).
4 Drug: lipiodol and chemo agents
Statement 4.1 (evidence level: 5, recommendation: D, agreement: 88.4%)
Mixtures of anticancer drugs and Lipiodol should be based on the principle of water-in-oil emulsion, with relative proportions varying according to tumor size and vascularity.
Prior to injection, the anticancer drug(s) selected for cTACE are mixed with Lipiodol to form an emulsion. A W/O emulsion is favored when the volume of aqueous drug solution is lower than the volume of Lipiodol, and this should be maintained under most circumstances. The recommended ratio of drug to Lipiodol in Japan is typically 1:2, although this must be adjusted for factors such as tumor vascularity and size (15).The ratio of epirubicin to Lipiodol can also be used to adjust desired viscosity in the resultant W/O emulsion. The use of large-droplet W/O emulsions in intraarterial hepatic injection has also been theorized to limit the lung uptake while increasing the tumor uptake of iodized oil (44). Adequate mixing of an aqueous chemotherapy solution and Lipiodol requires at least 20 pumping exchanges through a 3-way stopcock, and the first push should be from the chemotherapy drug towards Lipiodol. The pumping speed strongly influences internal phase droplet size (and thus emulsion viscosity), with greater pumping resulting in smaller droplet sizes (45).
Our recommended method of preparing cTACE emulsion is via the 3-way stopcock method, using polycarbonate or glass syringes and preferably a metal stopcock that can resist degradation by the emulsion. During the mixing process, the chemotherapy drug should be pumped into the syringe containing Lipiodol in the first push, so as to favor a water-in-oil emulsion. A minimum of 20 alternating pumping exchanges through the stopcock are needed to ensure that the final emulsion contains the appropriate size of aqueous chemotherapy droplets (46). Lastly, it should be noted that the emulsion must be prepared immediately prior to intra-arterial administration, and it should be used directly after preparation. If some time has passed between injections during a single cTACE session, the mixture can be re-homogenized using the above-mentioned stopcock method.
Statement 4.2 (evidence level: 1, recommendation: A, agreement: 100%)
● The most common single anticancer drugs are the anthracycline group agents, which include doxorubicin and epirubicin.
● The recommended dose of doxorubicin or epirubicin in a single session is 10-50 mg of body surface area based on tumor size.
A variety of cytotoxic anticancer drugs have been trialed for cTACE, and the most widely-used single-agent drugs in Taiwan are from the anthracycline group, which includes doxorubicin and epirubicin (47). According to European Association For The Study Of The Liver/European Organisation For Research And Treatment Of Cancer (EASL-EORTC) guidelines, there is at present insufficient evidence to recommend a single chemotherapeutical agent or combination of agents as being the best choice for cTACE (48). Nevertheless, a single-agent regimen involving either doxorubicin or cisplatin as a standard for fresh cases is recommended (49), with a common dosage of doxorubicin being approximately 10-50 mg of body surface area. The therapeutic effects can then be evaluated and a tailored re-treatment strategy formulated, with chemoembolization sessions scheduled 3–4 times per year during, which the anticancer agent may be changed or its dosage adjusted. It must be noted that there are currently no universally accepted criteria for determining the optimum dosage of chemotherapeutic agents in cTACE. Different authors have referenced various combinations of a patient’s body surface area, body weight, tumor burden or bilirubin level, and some have even use a fixed dose (50, 51). The dosing regimen reported also varies among different institutions in Taiwan, although the most common dose for doxorubicin tends to range 10-50 mg/m2 in a single session.
Statement 4.3 (evidence level: 1, recommendation: A, agreement: no agreement)
● Several combination drug regimens have been reported, the most popular being cisplatin, doxorubicin, and mitomycin C
● Recommended dose: doxorubicin 50mg + mitomycin C 10mg
Although combined regimens involving multiple chemotherapeutic agents for cTACE have shown some promising results (52), there is currently a lack of adequate experience with their use in Taiwan. As such, this panel could not establish an agreement on which anticancer drugs to use in combination, and what the relative dosage of each drug should be. Nevertheless, the most popular combination regimen that may be considered includes cisplatin, doxorubicin, and mitomycin C.
5 Embolic agents: gelfoam, PVA, microsphere
Statement 5.1 (evidence level: 3, recommendation: B, agreement: 94.2%)
• Both Gelatin sponge and non-resorbable calibrated microspheres can be used as embolic agents for cTACE, to be chosen based on the operator’s own judgement.
During each cTACE procedure, the infusion of a chemotherapeutic drug mixed with iodized oil is usually followed by the embolization of tumor-feeding arteries. This is done through the injection of gelatin sponge particles or other embolic agents, such as non-resorbable calibrated microspheres, and will significantly improve the cytotoxic effects of chemotherapy via additional ischemic effects. Gelatin particles, which may be purchased in pre-determined sizes or manually cut from larger pads, are the most commonly-used material for this purpose and have the main advantage of demonstrating complete vessel recanalization in 1–2 weeks after embolization (53). This provides temporary reduction in local blood flow to the tumor, which still allowing subsequent cTACE using the same tumor feeders. The size distribution of the gelatin sponge particles will differ according to the method used to make them. Reasonably uniform particle sizes are recommended and can be more consistently produced by cutting, rather than pumping (54).
The primary alternative to gelatin sponge particles is PVA microspheres with calibrated sizes of 100-300 μm. However, in patients with arterio-portal or arterio-venous shunt, larger particles (300-1000 μm) should be adopted. These come in two main types, one which is resorbable once infused into hepatic vessels and the other which is permanent. Since previous studies have not reported any additional benefit to using non-resorbable microspheres, use of the resorbable variant is recommended, in order to preserve the patency of tumor feeders and other arteries that may be valuable for subsequent cTACE treatment. To achieve optimal response in terms of tumor ischemia and shrinkage, as well as to ensure distal occlusion near the tumor vascular bed while preserving feeding segmental arteries, calibrated small microspheres are also recommended (55).Using microsphere sizes smaller than this increases the risk of shunting through hypervascular tumors and potential bile duct injury, while larger sizes may occlude feeding arteries without entering the vascular bed of the tumor (56).
6 Catheter positioning, embolization technique and end point
Statement 6.1 (evidence level: 4, recommendation: C, agreement: 100%)
● A superselective (i.e., segmental or subsegmental) approach should be used whenever possible using a microcatheter.
● A lobar approach may be considered for large multinodular or bilobar tumors, depending on the patient’s condition.
Yamakado et al. reported that selective hepatic arterial embolization in
HCC patients can significantly improve survival (57). When performing a selective segmental or subsegmental cTACE, a microcatheter should be used whenever possible, and all tumor-feeding vessels must be precisely located and treated (58), especially subphrenic HCC nodules, which are usually supplied through the phrenic artery. Besides easing access to smaller vessels, deploying microcatheters also helps reduce the incidence of vasospasms during emulsion delivery and ensures the forward flow of embolic materials. To prevent refluxing to non-target vessels during infusion, the operator should take care that sufficient flow exists around the catheter to carry the drug/Lipiodol mixture or embolic material forward.
In patients for whom selective embolization is not feasible (for example, if the tumor is very large and multinodular or present in bilateral lobes), a lobar approach may become acceptable. If this is the case, the operator should maneuver the catheter into position in the left or right hepatic artery in such a manner that as many tumor feeding arteries as possible are covered, while avoiding vessels that supply extrahepatic organs.
Statement 6.2 (evidence level: 4, recommendation: C, agreement: 100%)
Use of three-dimensional reconstructions obtained from CT or cone-beam CT is recommended, if available, to aid in the identification of tumor-feeding arteries.
“Targeted transarterial oily chemoembolization” is defined as superselective catheterization of a subsegmental or even more distal artery, followed by oily chemoembolization. The entire process is conducted with the assistance of a unified helical CT and real-time fluoroscopic system, usually referred to as C-arm cone-beam computed tomography (CBCT), which also allows intraoperative confirmation of embolization results via the CT component. Images produced by CBCT prior to superselective TACE can sometimes reveal subtle details of tumor-feeding arteries that may not be visible on digital subtraction angiography (DSA) alone. Thus, in addition to angiography of the hepatic and mesenteric artery, which is routinely obtained to identify liver arterial anatomy, major tumor feeders, and obvious portal or venous shunts, rotational angiography with C-arm CBCT may also be used to delineate tumor arterial supply with greater assurance. Additionally, the multiplanar reconstructions obtained during rotational imaging can be used to guide catheter tip positioning (59, 60).
By repeating selective arteriography with follow-up CBCT scans during catheter advancement through a segmental or subsegmental artery, a precisely targeted embolization of very distal tumor feeding branches may be achieved. This has the major advantage of strengthening the effect of chemoembolization on a target lesion while reducing damage done to surrounding healthy liver parenchyma. A reduction of up to two-thirds of the amount of anticancer agent and iodized oil used may be realized with this technique, while the need for gelatin sponge particles is also drastically reduced. Another merit of using a C-arm CBCT system, as mentioned above, is the ability to immediately confirm with CT images upon completion of cTACE whether targeted lesions were completely treated (61).
Statement 6.3 (evidence level: 3, recommendation: B, agreement: 100%)
Complete stasis up to the catheter tip when placed in subsegmental arteries.
• Lipiodol visualization in distal portal vein radicals of embolized areas, and completely occlusion of tumor feeding branch after gelatin sponge injection
The degree of portal vein visualization due to overflow of embolic materials has been divided into three grades by Miyayama et al. (62), as follows: Grade 0 = no obvious visualization of the portal vein or its branches (no visualization); Grade 1 = visualization of the portal vein adjacent to the tumor (slight visualization); and Grade 2 = marked visualization of the portal veins in the whole embolized area or extending into surrounding non-embolized areas (marked visualization). The optimal endpoint of a cTACE procedure can be recognized by portal vein visualization in the entire embolized area, along with complete blockage of tumor-feeding branch(es). Iodized oil injection should cease when portal veins extending to embolized area become visible, while the injection of gelatin sponge particles is deemed complete when tumor feeding branch(es) have been completely obstructed. So long as excessive overflow does not occur, the catheter tip should be kept in a subsegmental artery while injecting iodized oil, until complete vessel stasis is achieved. Injection of a small amount of gelatin sponge particles can subsequently be performed in the same location to complete the cTACE session (63).
Statement 6.4 (evidence level: 5, recommendation: C, agreement: 100%)
To identify residual tumors, post-embolization angiography or CBCT images may be obtained immediately after completion of a cTACE session.
The radiopaque nature of Lipiodol allows an operator to continuously visualize the flow of chemotherapeutic agents during infusion, which increases the safety and efficacy of cTACE. The Lipiodol-drug emulsion should be monitored in real-time via fluoroscopy until the vascular bed of the target tumor is completely saturated and stasis in peripheral branches next to the vascular bed is observed. Tumors with more complete Lipiodol opacification correlate with better treatment response, including lower rates of local recurrence (64) and higher rates of complete tumor necrosis (62). Therefore, we recommend repeating CBCT or angiography immediately after Lipiodol delivery in order to confirm tumor saturation and the occlusion of feeding vessels (59).
Statement 6.5 (evidence level: 2-3, recommendation: B, agreement: 100%)
6.5.1 Substasis angiographic endpoints during cTACE
6.5.1.1 Subjective angiographic chemoembolization endpoint scale levels II and III
6.5.2 Preservation of flow in the major lobar and segmental arteries when performing less-selective cTACE
6.5.2.1 A ‘‘tree-in-the winter’’ appearance
6.5.2.2 Occlusion of small tumor-feeding radicals
There is at present no global consensus on an optimal embolization endpoint for TACE (65), resulting in great variability between individual operators and institutions. However, the two primary goals of embolization are as follows: first, to induce ischemia and necrosis in the target tumor by reducing blood flow to the lesion, and second, to enhance the effects of administered cytotoxic agents (66, 67). An appropriate amount of embolization that is tailored to each target lesion is crucial, since both under- and over-embolization during cTACE can lead to undesirable effects; inadequate embolization can obviously result in poor treatment response (68, 69) and activate angiogenic growth factors that increase HCC progression (70–72), while over-embolization may increase liver toxicity (73). In addition, excessive embolization during cTACE may permanently block a tumor’s feeding vessels, hindering future cTACE procedures and potentially affecting patient survival. Patients with Child-Pugh class B or C disease require extra care during embolization, since baseline liver dysfunction tends to increase the risk of post-TACE liver failure (74).This is a wide-spread issue in HCC patients, since up to 80% have been found in autopsy to have underlying cirrhosis (75).
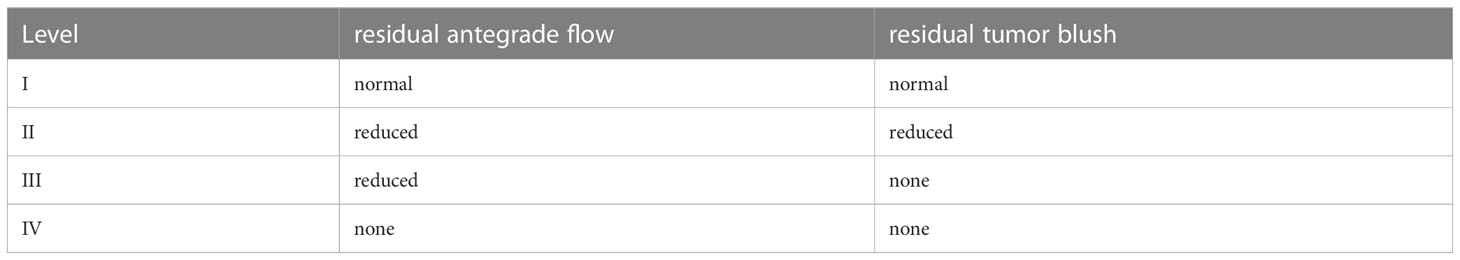
As the aforementioned findings show, a more consistent set of criteria for determining appropriate embolization endpoints is urgently needed, and some researchers have attempted to establish such a system. Jin et al. introduced the SACE scale to describe angiographic endpoints of cTACE (Table 1), which this panel found to be quite useful (65). Level I represents under-embolization, which is shown as minimal or no change in tumor blush or antegrade flow in feeding vessels on DSA images after cTACE. Level IV represents over-embolization, which is defined as the elimination of both tumor blush and antegrade blood flow after embolization. Jin et al. also demonstrated that embolization to an intermediate, sub-stasis endpoint (SACE levels II and III) during cTACE improves survival, when compared with embolization to a more complete stasis endpoint (level IV). Since the optimal end-point of embolization is the subtotal stasis of tumor blood flow, the interventional radiologist should aim to achieve a “tree-in-the winter’’ fluoroscopic appearance. This entails the occlusion of terminal tumor-feeding branches while preserving flow in the lobar and segmental hepatic arteries, which will allow repeat treatment through these vessels in the future (76, 77). Stopping embolization at SACE II-III (substasis) is especially important in patients with increased bilirubin levels and/or those with high likelihood to undergo repeated cTACE, both to preserve liver function and to facilitate future treatment. Complete stasis (SACE IV) may be relatively more acceptable in cases with normal bilirubin levels and/or a low likelihood to be retreated in the same targeted artery. In general, however, interventional radiologists should aim for intermediate, sub-stasis angiographic endpoints of cTACE for most patients.
7 Treatment schedule
Statement 7.1 (evidence level: 1, recommendation: A, agreement: 88.4%)
● On-demand cTACE procedures up to 3 or 4 times per year according to treatment response.
● At least two cTACE procedures should be performed before treatment is abandoned due to lack of response.
● Patients with types of HCC unsuitable for cTACE – including confluent multinodular type, massive or infiltrative type, poorly differentiated type, and intrahepatic multiple disseminated nodules and nodules that exceed the up-to-7 criteria – should first seek out systemic therapy (e.g., immuno-target therapy) and/or locoregional therapy (e.g., y-90 radioembolization and HAIC).
7.1.1 cTACE failure/refractoriness
We recommend that a minimum of two sequential cTACE procedures be performed in a treatment cycle, since a single chemoembolization may be insufficient to effectively treat intermediate stage HCC. While repeating cTACE is widely known to prolong overall patient survival (78), current guidelines do not specify the conditions and timing under which repeat treatment should be performed. In the opinion of this expert panel, ‘on-demand’ embolization (i.e. adjusting the number of treatment sessions based on tumor response) up to 3 or 4 times per year provides the most effective and flexible treatment option, while maintaining the patient’s quality of life (79–81). According to the “Management consensus guideline for HCC: 2016 updated by the Taiwan liver cancer association and the gastroenterological society of Taiwan”, TACE-refractory HCC should be considered when evidence of disease progression or viable components making up over 50% of a tumor are observed on CT or MR images, for target lesions which have been adequately treated in two or more sessions within a 6-month period (82). For refractory HCC cases, alternative therapeutic options should be sought in the absence of response after two cTACE sessions (83).
7.1.2 cTACE-unsuitability
Some clinical conditions may prevent HCC patients from experiencing survival benefits from cTACE (22), including (I) the unlikeliness to respond to cTACE due to confluent multinodular type, massive or infiltrative type, poorly differentiated type, or intrahepatic multiple disseminated nodules and (II) the likeliness to develop TACE failure/refractoriness and/or deterioration of liver function in nodules exceeding the up-to-7 criteria (84). Because survival does not differ between non-responders to cTACE and untreated patients, cTACE is not recommended to be repeated in cases in which OR was not achieved by prior cTACE (85), since repetition may lead to sarcomatous or biliary-mixed type changes and the development of an aggressive type of cancer that does not respond to cTACE (86, 87). Furthermore, repeated cTACE procedures might impair liver function, with the incidence higher in patients exceeding the up-to-7 criteria than in those within them (88).
Radiological assessment of tumor response and changes in Child-Pugh score are two common patient prognostic factors after cTACE, while Golfieri et al. proposed that tumor size and radiologic response be taken into account when considering further treatment (89).
In the current era of multi-molecular targeted agents and immune checkpoint inhibitors, lenvatinib has been the only first-line agent to demonstrate an OS benefit over cTACE (37.9 vs. 21.3 months) in TACE-naïve patients with tumor burdens exceeding the up-to-7 criteria (90). Approximately 70% of such patients have undergone cTACE after initial lenvatinib therapy; thereafter, initial lenvatinib therapy with subsequent selective cTACE has been proposed for intermediate-stage HCC with high tumor burden (91). Noting that the above conclusions come from a single retrospective propensity-score matched study (90), further prospective randomized control trial is warranted to confirm the effectiveness of this treatment strategy (91). In the 2019 APPLE consensus document (22), country-specific issues such as healthcare insurance or approval status were not considered, so it is necessary to make adjustments appropriate to each situation, especially to account for the highly-variable costs of medical treatments in each country.
8 Post-treatment care and imaging follow-up
Statement 8.1 (evidence level: 3, recommendation: B, agreement: 100%)
● Post-embolization syndrome consisting of fever, abdominal pain and increased serum white blood cell count is an expected side-effect of cTACE and should not be regarded as a complication.
● Pain relievers, prophylactic antibiotics, antiemetic therapy, and gastric protection should be provided according to standard institutional protocols
● Both the non-enhancing and Lipiodol retention areas should be considered necrotic before treatment is abandoned due to lack of response
Post-chemoembolization syndrome, which occurs in approximately 60% to 80% of patients who undergo cTACE, is typically associated with elevation of hepatic transaminases, transient abdominal pain, and fever. Routine monitoring of vital signs and conservative treatment are all that are required in most patients, as it is self-limiting and resolves itself within 3–4 days. Post-embolization hospitalization may be prolonged, however, in patients who develop these symptoms. Even though there is currently no solid evidence supporting the routine use of antibiotic prophylaxis, many physicians still recommend antibiotic treatment for 3–7 days after cTACE treatment, using appropriate agents that cover Gram-negative enteric pathogens (92, 93).
Statement 8.2 (evidence level: 4, recommendation: C, agreement: 100%)
Major complications such as liver infarction, biloma, surgical cholecystitis, gastrointestinal ulceration or hemorrhage, vascular dissection occur in less than 1% of procedures, while the incidence of liver failure and patient mortality in 30 days is less than 4%.
Complications may occur in an estimated 10% of cTACE patients. Severe adverse events, which include liver infarction, biloma, surgical cholecystitis, gastrointestinal ulceration or hemorrhage, and vascular dissection, each have an incidence rate of < 1%. Meanwhile, the expected rates of liver failure and patient death within 30 days post-TACE are 2.3% and 2-4%, respectively (94). Liver insufficiency leading to hepatic failure is the most common cause of death, although the overall mortality rate remains low at approximately 0.6% (20). This suggests that an accurate assessment of a patient’s baseline liver function parameters is the most important factor in lowering the risk of treatment-related mortality, as well as reducing overall liver-related adverse effects.
Statement 8.3 (evidence level: 5, recommendation: C, agreement: 100%)
● To evaluate tumor response and plan further therapy, serum alpha-fetoprotein (AFP) level and follow-up dynamic liver CT/MRI should be examined 8-10 weeks after a course of cTACE.
● If treatment of both lobes of the liver is planned, additional imaging between sessions may be performed based on operator preference.
After all hepatic tumors have been treated, follow-up dynamic liver CT or MRI should be arranged within 8-10 weeks. Additional imaging of the liver may also be considered if bilobar embolization is being planned. When imaging is unable to confirm or rule out the presence of residual tumors or necrotic/fibrotic tumor remnants, serum AFP or PIVKA II may be helpful in measuring the degree of treatment response (95).
Statement 8.4 (evidence level: 1, recommendation: A, agreement: 100%)
● The modified Response Evaluation Criteria in Solid Tumors (mRECIST) guideline is recommended for classification of HCC response to cTACE.
● For patients with no evidence of residual viable disease, imaging follow-up is recommended every 3 months.
The mRECIST criteria rely on assessments of tumor devascularization to classify treatment responses (96, 97), and have proven to be the most accurate system for evaluating cTACE in terms of predicting time to disease progression and overall patient survival (98). For patients without active disease upon initial follow-up after embolization, routine liver imaging should be arranged every 3 months.
Statement 8.5 (evidence level: 4, recommendation: B, agreement: 100%)
● When CT is used for disease evaluation, both non-enhancing areas and areas with iodized oil retention should be considered part of tumor necrosis.
● Lipiodol retention in treated lesions may cause difficulties in assessing residual or recurrent tumor using contrast-enhanced CT. In such cases, contrast-enhanced liver MRI may be chosen as an alternative modality.
Takayasu et al. demonstrated that CT is suitable for evaluating the efficacy of cTACE for HCC, based on the assumption that the portions of the tumor which retain iodized oil are necrotic. The authors showed that tumor size reduction measured on CT does not directly correlate with the therapeutic effect of chemoembolization, and that we should instead focus on the extent of tumor necrosis (99). Signs of tumor necrosis include Lipiodol uptake and the absence of arterial-phase enhancement in areas where it was present prior to chemoembolization. In certain situations, the high density of retained Lipiodol in already-treated lesions may mask the presence of residual or recurrent tumors in its vicinity when using contrast-enhanced CT. Dynamic contrast-enhanced MRI may be considered an alternative follow-up modality in these cases, since it is much less affected by Lipiodol-induced artifacts. In MR imaging, absent arterial enhancement in the areas of the tumor where it was present prior to therapy is the principal determinant of tumor necrosis (100).
Note:
Some controversies regarding the role of Lipiodol in cTACE exist in the literature. Before objectively discussing these controversies, we should establish a clear and precise nomenclature, as transarterial embolization terms have been inconsistently used across a multitude of studies:
1. Lp-TACE: lipiodol + anticancer agent + embolizer (gelfoam/PVA)
2. –Lp-TACE: anticancer agent + embolizer (gelfoam/PVA)
3. Lp-TAE: lipiodol + embolizer (gelfoam/PVA)
4. –Lp-TAE: embolizer alone (gelfoam/PVA)
Although lipiodol is widely adopted in TACE protocols, its exact role as a carrier vehicle, microembolizer, and/or both is still disputed. As a carrier, emulsifications of lipiodol and drugs are unstable, and the two components begin to separate as soon as they are injected into the hepatic arterial circulation (101, 102). Numerous studies have shown no differences between the injection of chemoagents as an intra-arterial infusion or as part of conventional TACE protocols, in terms of pharmacokinetic parameters or toxicity (103–107). Kobayashi et al. reported that there were no differences in plasma epirubicin concentration after injection (72) of whatever oil-in-water, water-in-oil, or suspension form of epirubicin-lipiodol emulsion. To date, there is also no evidence of the superiority of any chemotherapeutic agent, either alone or of monotherapy, versus combination therapy48.
3 RCTs compared the therapeutic effect of TACE versus TAE, with two trials favoring TAE (108, 109), and one favoring TACE (110). The 1- and 2-year survival rates in the two RCTs favoring TAE (Lp-TAE) were 74.4 & 51.3% vs 65.1 & 42.4%, P=.209 (108) and 72.5 & 39.5% vs 52.5 & 26.2% (P>.05) (109), while the median survivals were 25.3 months (-Lp-TAE) and 28.7 months (TACE), favoring the TACE trial (P>.05) (110). 2 non-randomized studies also reported that TACE had significantly superior survival than TAE, with 1- and 2-year survivals of 65% & 39% vs 39% & 13%, & 73% and 31% vs 39% & 10%, respectively (18, 111). However, in the latter three studies, TAE were all performed with gelfoam fragments only, without associated lipiodol injections (-LpTAE) (18, 110, 111). Moreover, Pelletier et al. and Bruix et al. both conducted RCT studies and found there was no survival benefit to treating unresectable HCC without lipiodol, by either chemoembolization with doxorubicin and gelfoam powder (-Lp-TACE) or gelfoam alone (-Lp-TAE) when compared to symptomatic treatment, with survival rates of 24% versus 31% at 12 months (P>0.05) in the former and 49% versus 50% at 24 months (P=.072) in the latter (112, 113).
From a large number of both randomized or nonrandomized studies and a systematic review in 2016 conducted by Lenioni et al, it is reasonable to conclude that “the key component of TACE is lipiodol … which is used both as a vehicle to carry and localize the chemotherapeutic agent inside the tumor and as a microembolic agent for tiny tumor vessels” (20). And the mechanism of TACE or TAE can be summarized as: 1) for TACE, the choice of bolus anticancer agents infusion play little role in the HCC treatment, insofar as no particular chemoagents have been proven to be more HCC-sensitive than any others and because of the early separation of the chemoagent from the emulsion, no matter the preparation; 2) small-droplet lipiodol oil act as an embolizer for tumor microvasculature per se, embolizing the distal hepatic arterioles and overflowing to the portal venules, thus playing the key role in TACE and in TAE, especially for small HCC less than 4-5 cm; 3) gelfoam particles of 1-2 mm act as an adjunct embolizer to occlude (sub)segmental hepatic arteries, delaying lipiodol washout in the subsegmental hepatic arteries and providing adequate lipiodol-retained duration for irreversible tumor cell ischemia and death.
Author contributions
Conceptualization: P-YC, R-CL, P-CL, Y-SL, VC, D-KW, Y-FC, J-IH, H-ST, C-HW, C-FH, R-HW, M-CC, H-MC, S-MC, C-LC and H-LL. Writing – original draft preparation: P-YC. Writing – review and editing: H-LL. Supervision: R-CL, P-CL, Y-SL, VC, D-KW, Y-FC, J-IH, H-ST, C-HW, C-FH, R-HW, M-CC, H-MC, S-MC, and C-LC. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Changchien C-S, Chen C-L, Yen Y-H, Wang J-H, Hu T-H, Lee C-M, et al. Analysis of 6381 hepatocellular carcinoma patients in southern Taiwan: prognostic features, treatment outcome, and survival. J Gastroenterol (2008) 43(2):159–70. doi: 10.1007/s00535-007-2134-9
2. Liaw Y-F, Chu C-M. Hepatitis b virus infection. Lancet (2009) 373(9663):582–92. doi: 10.1016/S0140-6736(09)60207-5
3. Lunn R-M, Zhang Y-J, Wang L-Y, Chen C-J, Lee P-H, Lee C-S, et al. P53 mutations, chronic hepatitis b virus infection, and alfatoxin exposure in hepatocellular carcinoma in Taiwan. Cancer Res (1997) 57(16):3471–7.
4. Lai C-L, Ratziu V, Yuen M-F, Poynard T. Viral hepatitis b. Lancet (2003) 362(9401):2089–94. doi: 10.1016/S0140-6736(03)15108-2
5. Zhu R-X, Seto W-K, Lai C-L, Yuen M-F. Epidemiology of hepatocellular carcinoma in the Asia-pacific region. Gut Liver (2016) 10(3):332. doi: 10.5009/gnl15257
6. Wu C-Y, Chen Y-J, Ho H-J, Hsu Y-C, Kuo K-N, Wu M-S, et al. Association between nucleoside analogues and risk of hepatitis b virus–related hepatocellular carcinoma recurrence following liver resection. Jama (2012) 308(18):1906–13. doi: 10.1001/2012.jama.11975
7. Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Canc (2014) 111(2):255. doi: 10.1038/bjc.2014.199
8. Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol (2010) 33(1):41–52. doi: 10.1007/s00270-009-9711-7
9. Ikeda M, Arai Y, Park S-J, Takeuchi Y, Anai H, Kim J-K, et al. Prospective study of transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: an Asian cooperative study between Japan and Korea. J Vasc Intervention Radiol (2013) 24(4):490–500. doi: 10.1016/j.jvir.2013.01.003
10. Luo J, Guo R-P, Lai E-C, Zhang Y-J, Lau W-Y, Chen M-S, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol (2011) 18(2):413–20. doi: 10.1245/s10434-010-1321-8
11. Pomfret E-A, Washburn K, Wald C, Nalesnik M-A, Douglas D, Russo M, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the united states. Liver Transplantat (2010) 16(3):262–78. doi: 10.1002/lt.21999
12. Chen C-H, Hu F-C, Huang G-T, Lee P-H, Tsang Y-M, Cheng A-L, et al. Applicability of staging systems for patients with hepatocellular carcinoma is dependent on treatment method–analysis of 2010 Taiwanese patients. Eur J Canc (2009) 45(9):1630–9. doi: 10.1016/j.ejca.2008.12.025
13. Wang C-K. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan liver cancer association and the gastroenterological society of Taiwan. J Formosa Med Assoc (2018) 117(5):381–403. doi: 10.1016/j.jfma.2017.09.007
14. Konno T, Maeda H, Iwai K, Tashiro S, Maki S, Morinaga T, et al. Effect of arterial administration of high-molecular-weight anticancer agent SMANCS with lipid lymphographic agent on hepatoma: a preliminary report. Eur J Canc (1983) 19(8):1053–61. doi: 10.1016/0277-5379(83)90028-7
15. Nakamura H, Hashimoto T, Oi H, Sawada S. Transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology (1989) 170(3):783–6. doi: 10.1148/radiology.170.3.2536946
16. Uchida H, Ohishi H, Matsuo N, Nishimine K, Ohue S, Nishimura Y, et al. Transcatheter hepatic segmental arterial embolization using lipiodol mixed with an anticancer drug and gelfoam particles for hepatocellular carcinoma. Cardiovasc Intervent Radiol (1990) 13(3):140–5. doi: 10.1007/BF02575465
17. Matsui O, Kadoya M, Yoshikawa J, Gabata T, Arai K, Demachi H, et al. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology (1993) 188(1):79–83. doi: 10.1148/radiology.188.1.8390073
18. Llovet J-M, Real M-I, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet (2002) 359(9319):1734–9. doi: 10.1016/S0140-6736(02)08649-X
19. Lo C-M, Ngan H, Tso W-K, Liu C-L, Lam C-M, Poon R-T-P, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology (2002) 35(5):1164–71. doi: 10.1053/jhep.2002.33156
20. Lencioni R, de Baere T, Soulen M-C, Rilling W-S, Geschwind JFH. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology (2016) 64(1):106–16. doi: 10.1002/hep.28453
21. Kudo M, Cheng A-L, Park J-W, Park J-H, Liang P-C, Hidaka H, et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): a randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterol Hepatol (2018) 3(1):37–46. doi: 10.1016/S2468-1253(17)30290-X
22. Kudo M, Han K-H, Ye S-L, Zhou J, Huang Y-H, Lin S-M, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-pacific primary liver cancer expert consensus statements. Liver Canc (2020) 9:245–60. doi: 10.1159/000507370
23. Omata M, Lesmana LA, Tateishi R, Chen P-J, Lin S-M, Yoshida H, et al. Asian Pacific association for the study of the liver consensus recommendations on hepatocellular carcinoma. Hepatol Int (2010) 4(2):439–74. doi: 10.1007/s12072-010-9165-7
24. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology (2011) 53(3):1020–2. doi: 10.1002/hep.24199
25. Jelic S, Sotiropoulos G, Group EGW. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2010) 21(suppl_5):v59–64. doi: 10.1093/annonc/mdq166
26. Lau W-Y, Sangro B, Chen P-J, Cheng S-Q, Chow P, Lee R-C, et al. Treatment for hepatocellular carcinoma with portal vein tumor thrombosis: the emerging role for radioembolization using yttrium-90. Oncology (2013) 84(5):311–8. doi: 10.1159/000348325
27. Ikai I, Arii S, Okazaki M, Okita K, Omata M, Kojiro M, et al. Report of the 17th nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res (2007) 37(9):676–91. doi: 10.1111/j.1872-034X.2007.00119.x
28. Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol: WJG (2006) 12(47):7561. doi: 10.3748/wjg.v12.i47.7561
29. Lee H-S, Kim J-S, Choi I-J, Chung J-W, Park J-H, Kim C-Y. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction: a prospective controlled study. Canc: Interdiscip Int J Am Cancer Soc (1997) 79(11):2087–94. doi: 10.1002/(SICI)1097-0142(19970601)79:11<2087::AID-CNCR5>3.0.CO;2-M
30. Chung G-E, Lee J-H, Kim H-Y, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology (2011) 258(2):627–34. doi: 10.1148/radiol.10101058
31. Yoon S-M, Lim Y-S, Won H-J, Hwang S-Y, Kim J-S, Chung J-W, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys (2012) 82(5):2004–11. doi: 10.1016/j.ijrobp.2011.03.019
32. Chu H-H, Kim J-H, Shim J-H, Yoon SM, Kim PH, Alrashidi I. Chemoembolization plus radiotherapy versus chemoembolization plus sorafenib for the treatment of hepatocellular carcinoma invading the portal vein: a propensity score matching analysis. Cancers (2020) 12. doi: 10.3390/cancers12051116
33. Abouchaleh N, Gabr A, Ali R, Asadi A-A, Mora R-A, Kallini J-R, et al. (90)y radioembolization for locally advanced hepatocellular carcinoma with portal vein thrombosis: long-term outcomes in a 185-patient cohort. J Nucl Med Off Publicat Soc Nucl Med (2018) 59:1042–8. doi: 10.2967/jnumed.117.199752
34. Li M-F, Liang H-L, Chiang C-L, Tsai W-L, Chen W-C, Tsai C-C, et al. New regimen of combining hepatic arterial infusion chemotherapy and lipiodol embolization in treating hepatocellular carcinoma with main portal vein invasion. J Personal Med (2023) 13:88. doi: 10.3390/jpm13010088
35. Cheng A-L, Qin S, Ikeda M, Galle P-R, Ducreux M, Kimet T-Y, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol (2022) 76:862–73. doi: 10.1016/j.jhep.2021.11.030
36. Burrel M, Llovet JM, Ayuso C, Iglesias C, Sala M, Miquel R, et al. MRI Angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology (2003) 38(4):1034–42. doi: 10.1002/hep.1840380430
37. Sangiovanni A, Manini M-A, Iavarone M, Romeo R, Forzenigo L-V, Fraquelli M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut (2010) 59(5):638–44. doi: 10.1136/gut.2009.187286
38. Liu X, Jiang H, Chen J, Zhou Y, Huang Z, Song B. Gadoxetic acid disodium–enhanced magnetic resonance imaging outperformed multidetector computed tomography in diagnosing small hepatocellular carcinoma: a meta-analysis. Liver Transplant (2017) 23(12):1505–18. doi: 10.1002/lt.24867
39. Kim H-S, Kim S-H, Kang T-W, Song K-D, Choi D, Park C-K. Value of gadoxetic acid-enhanced and diffusion-weighted MR imaging in evaluation of hepatocellular carcinomas with atypical enhancement pattern on contrast-enhanced multiphasic MDCT in patients with chronic liver disease. Eur J Radiol (2015) 84(4):555–62. doi: 10.1016/j.ejrad.2014.12.023
40. Murakami T, Kim T, Takamura M, Hori M, Takahashi S, Federle M-P, et al. Hypervascular hepatocellular carcinoma: detection with double arterial phase multi-detector row helical CT. Radiology (2001) 218(3):763–7. doi: 10.1148/radiology.218.3.r01mr39763
41. Ichikawa T, Kitamura T, Nakajima H, Sou H, Tsukamoto T, Ikenaga S, et al. Hypervascular hepatocellular carcinoma: can double arterial phase imaging with multidetector CT improve tumor depiction in the cirrhotic liver? Am J Roentgenol (2002) 179(3):751–8. doi: 10.2214/ajr.179.3.1790751
42. Francis I-R, Cohan R-H, McNulty N-J, Platt J-F, Korobkin M, Gebremariam A, et al. Multidetector CT of the liver and hepatic neoplasms: effect of multiphasic imaging on tumor conspicuity and vascular enhancement. Am J Roentgenol (2003) 180(5):1217–24. doi: 10.2214/ajr.180.5.1801217
43. Sultana S, Awai K, Nakayama Y, Nakaura T, Liu D, Hatemura M, et al. Hypervascular hepatocellular carcinomas: bolus tracking with a 40-detector CT scanner to time arterial phase imaging. Radiology (2007) 243(1):140–7. doi: 10.1148/radiol.2431060069
44. De Baere T, Zhang X, Aubert B, Harry G, Lagrange C, Ropers J, et al. Quantification of tumor uptake of iodized oils and emulsions of iodized oils: experimental study. Radiology (1996) 201(3):731–5. doi: 10.1148/radiology.201.3.8939223
45. Masada T, Tanaka T, Nishiofuku H, Fukuoka Y, Sato T, Tatsumoto S, et al. Techniques to form a suitable lipiodol-epirubicin emulsion by using 3-way stopcock methods in transarterial chemoembolization for liver tumor. J Vasc Intervention Radiol (2017) 28(10):1461–6. doi: 10.1016/j.jvir.2017.03.032
46. de Baere T, Dufaux J, Roche A, Berthault M-F, Denys A, Pappas P. Circulatory alterations induced by intra-arterial injection of iodized oil and emulsions of iodized oil and doxorubicin: experimental study. Radiology (1995) 194(1):165–70. doi: 10.1148/radiology.194.1.7997545
47. Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? a systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol (2007) 30(1):6. doi: 10.1007/s00270-006-0062-3
48. Dufour J-F. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Canc (2012) 2012(48(5)):599–641. doi: 10.1016/j.ejca.2011.12.021
49. Kasugai H, Kojima J, Tatsuta M, Okuda S, Sasaki Y, Imaoka S, et al. Treatment of hepatocellular carcinoma by transcatheter arterial embolization combined with intraarterial infusion of a mixture of cisplatin and ethiodized oil. Gastroenterology (1989) 97(4):965–71. doi: 10.1016/0016-5085(89)91505-9
50. Kawai S, Tani M, Okamura J, Ogawa M, Ohashi Y, Monden M, et al. Prospective and randomized trial of lipiodol-transcatheter arterial chemoembolization for treatment of hepatocellular carcinoma: a comparison of epirubicin and doxorubicin (second cooperative study). Cooperat Stud Group Liver Cancer Treat Japan. Semin Oncol (1997) 24(2 Suppl 6):S6–38-S6-45.
51. Watanabe S, Nishioka M, Ohta Y, Ogawa N, Ito S, Yamamoto Y. Prospective and randomized controlled study of chemoembolization therapy in patients with advanced hepatocellular carcinoma. Cancer Chemother Pharmacol (1994) 33(1):S93–6. doi: 10.1007/BF00686676
52. Gaba R-C. Chemoembolization practice patterns and technical methods among interventional radiologists: results of an online survey. Am J Roentgenol (2012) 198(3):692–9. doi: 10.2214/AJR.11.7066
53. Louail B, Sapoval M, Bonneau M, Wasseff M, Senechal Q, Gaux J. A new porcine sponge material for temporary embolization: an experimental short-term pilot study in swine. Cardiovasc Intervent Radiol (2006) 29(5):826–31. doi: 10.1007/s00270-004-0299-7
54. Katsumori T, Kasahara T. The size of gelatin sponge particles: differences with preparation method. Cardiovasc Intervent Radiol (2006) 29(6):1077–83. doi: 10.1007/s00270-006-0059-y
55. Suh C, Shin J, Yoon H, Yoon H-K, Ko G-Y, Gwon D-I, et al. Angiographic evaluation of hepatic arterial injury after cisplatin and gelfoam–based transcatheter arterial chemoembolization for hepatocellular carcinoma in a 205 patient cohort during a 6-year follow-up. Br J Radiol (2014) 87(1041):20140054. doi: 10.1259/bjr.20140054
56. Brown K-T. Fatal pulmonary complications after arterial embolization with 40–120-μm tris-acryl gelatin microspheres. J Vasc Intervent Radiol (2004) 15(2):197–200. doi: 10.1097/01.RVI.0000109400.52762.1F
57. Yamakado K, Miyayama S, Hirota S, Mizunuma K, Nakamura K, Inaba Y, et al. Hepatic arterial embolization for unresectable hepatocellular carcinomas: do technical factors affect prognosis? Japanese J Radiol (2012) 30(7):560–6. doi: 10.1007/s11604-012-0088-1
58. Basile A, Carrafiello G, Ierardi AM, Tsetis D, Brountzos E. Quality-improvement guidelines for hepatic transarterial chemoembolization. Cardiovasc Intervent Radiol (2012) 35(4):765–74. doi: 10.1007/s00270-012-0423-z
59. Virmani S, Ryu R-K, Sato K-T, Lewandowski R-J, Kulik L, Mulcahy M-F, et al. Effect of c-arm angiographic CT on transcatheter arterial chemoembolization of liver tumors. J Vasc Intervention Radiol (2007) 18(10):1305–9. doi: 10.1016/j.jvir.2007.07.006
60. Miyayama S, Yamashiro M, Okuda M, Yoshie Y, Sugimori N, Igarashi S, et al. Usefulness of cone-beam computed tomography during ultraselective transcatheter arterial chemoembolization for small hepatocellular carcinomas that cannot be demonstrated on angiography. Cardiovasc Intervent Radiol (2009) 32(2):255–64. doi: 10.1007/s00270-008-9468-4
61. Takayasu K, Muramatsu Y, Maeda T, Iwata R, Furukawa H, Muramatsu Y, et al. Targeted transarterial oily chemoembolization for small foci of hepatocellular carcinoma using a unified helical CT and angiography system: analysis of factors affecting local recurrence and survival rates. Am J Roentgenol (2001) 176(3):681–8. doi: 10.2214/ajr.176.3.1760681
62. Miyayama S, Matsui O, Yamashiro M, Ryu Y, Kaito K, Ozaki K, et al. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Intervention Radiol (2007) 18(3):365–76. doi: 10.1016/j.jvir.2006.12.004
63. de Baere T, Arai Y, Lencioni R, Geschwind J-F, Rilling W, Salem R, et al. Treatment of liver tumors with lipiodol TACE: technical recommendations from experts opinion. Cardiovasc Intervent Radiol (2016) 39(3):334–43. doi: 10.1007/s00270-015-1208-y
64. Miyayama S, Mitsui T, Zen Y, Sudo Y, Yamashiro M, Okuda M, et al. Histopathological findings after ultraselective transcatheter arterial chemoembolization for hepatocellular carcinoma. Hepatol Res (2009) 39(4):374–81. doi: 10.1111/j.1872-034X.2008.00465.x
65. Jin B, Wang D, Lewandowski R-J, Riaz A, Ryu R-K, Sato K-T, et al. Chemoembolization endpoints: effect on survival among patients with hepatocellular carcinoma. Am J Roentgenol (2011) 196(4):919–28. doi: 10.2214/AJR.10.4770
66. Coldwell D-M, Stokes K-R, Yakes W-F. Embolotherapy: agents, clinical applications, and techniques. Radiographics (1994) 14(3):623–43. doi: 10.1148/radiographics.14.3.8066276
67. Brown D-B, Pilgram T-K, Darcy M-D, Fundakowski C-E, Lisker-Melman M, Chapman W-C, et al. Hepatic arterial chemoembolization for hepatocellular carcinoma: comparison of survival rates with different embolic agents. J Vasc Intervent Radiol (2005) 16(12):1661–6. doi: 10.1097/01.RVI.0000182160.26798.A2
68. Ikeda M, Maeda S, Shibata J, Ashihara H, Tanaka M, Fujiyama S, et al. Transcatheter arterial chemotherapy with and without embolization in patients with hepatocellular carcinoma. Oncology (2004) 66(1):24–31. doi: 10.1159/000076331
69. Maeda S, Fujiyama S, Tanaka M, Ashihara H, Hirata R, Tomita K. Survival and local recurrence rates of hepatocellular carcinoma patients treated by transarterial chemolipiodolization with and without embolization. Hepatol Res (2002) 23(3):202–10. doi: 10.1016/S1386-6346(01)00174-7
70. Xiong Z-P, Yang S-R, Liang Z-Y, Xiao E-H, Yu X-P, Zhou S-K, et al. Association between vascular endothelial growth factor and metastasis after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int (2004) 3(3):386–90.
71. Rhee T-K, Young J-Y, Larson A-C, Haines G-K, Sato K-T, Salem R, et al. Effect of transcatheter arterial embolization on levels of hypoxia-inducible factor-1α in rabbit VX2 liver tumors. J Vasc Intervention Radiol (2007) 18(5):639–45. doi: 10.1016/j.jvir.2007.02.031
72. Kobayashi N, Ishii M, Ueno Y, Kisara N, Chida N, Iwasaki T, et al. Co-Expression of bcl-2 protein and vascular endothelial growth factor in hepatocellular carcinomas treated by chemoembolization. Liver (1999) 19(1):25–31. doi: 10.1111/j.1478-3231.1999.tb00005.x
73. Geschwind J-F-H, Ramsey D-E, Cleffken B, Wal B-C-H, Kobeiter H, Juluru K, et al. Transcatheter arterial chemoembolization of liver tumors: effects of embolization protocol on injectable volume of chemotherapy and subsequent arterial patency. Cardiovasc Intervent Radiol (2003) 26(2):111–7. doi: 10.1007/s00270-002-2524-6
74. Bruix J, Sherman M, Llovet J, Beaugrand M, Lencioni R, Burroughs A-K, et al. Clinical management of hepatocellular carcinoma. conclusions of the Barcelona-2000 EASL conference. J Hepatol (2001) 35(3):421–30. doi: 10.1016/S0168-8278(01)00130-1
75. Simonetti R-G, Cammà C, Fiorello F, Politi F, D'Amico G, Pagliaro L. Hepatocellular carcinoma. Digest Dis Sci (1991) 36(7):962–72. doi: 10.1007/BF01297149
76. Mauro M-A, Murphy K-P, Thomson K-R, Venbrux A-C, Morgan R-A. Image-guided interventions: expert radiology series (Expert consult-online and print). Netherlands: Elsevier Health Sciences (2013).
77. Kandarpa K, Machan L. Handbook of interventional radiologic procedures. United states: Lippincott Williams & Wilkins (2011).
78. Grieco A, Marcoccia S, Miele L, Marmiroli L, Caminiti G, Ragazzoni E, et al. Transarterial chemoembolization (TACE) for unresectable hepatocellular carcinoma in cirrhotics: functional hepatic reserve and survival. Hepato-gastroenterology (2003) 50(49):207–12.
79. Bolondi L, Burroughs A, Dufour J-F, Galle P-R, Mazzaferro V, Piscaglia F, et al. Heterogeneity of patients with intermediate (BCLC b) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin liver Dis (2012) 32(4):348–59. doi: 10.1055/s-0032-1329906
80. Raoul J-L, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev (2011) 37(3):212–20. doi: 10.1016/j.ctrv.2010.07.006
81. Venook A-P, Ferrell L-D, Roberts J-P, Emond J, Frye J-W, Ring E, et al. Liver transplantation for hepatocellular carcinoma: results with preoperative chemoembolization. Liver Transplant Surg (1995) 1(4):242–8. doi: 10.1002/lt.500010409
82. Cheng A-L, Amarapurkar D, Chao Y, Chen P-J, Geschwind J-F, Goh K-L, et al. Re-evaluating transarterial chemoembolization for the treatment of hepatocellular carcinoma: consensus recommendations and review by an international expert panel. Liver Int (2014) 34(2):174–83. doi: 10.1111/liv.12314
83. Facciorusso A, Licinio R, Muscatiello N, Di Leo A, Barone M. Transarterial chemoembolization: evidences from the literature and applications in hepatocellular carcinoma patients. World J Hepatol (2015) 7(16):2009. doi: 10.4254/wjh.v7.i16.2009
84. Peck-Radosavljevic M, Kudo M, Raoul JL, Lee H-C, Decaens T, Heo J, et al. Outcomes of patients (pts) with hepatocellular carcinoma (HCC) treated with transarterial chemoembolization (TACE): global OPTIMIS final analysis. J Clin Oncol (2018) 36:4018. doi: 10.1200/JCO.2018.36.15_suppl.4018
85. Llovet J-M, Real M-I, Montaña X, Planas R, Coll S, Aponte J, et al. Barcelona Liver cancer group. arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet (2002) 359(9319):1734–9. doi: 10.1016/S0140-6736(02)08649-X
86. Kojiro M, Sugihara S, Kakizoe S, Nakashima O, Kiyomatsu K. Hepatocellular carcinoma with sarcomatous change: a special reference to the relationship with anticancer therapy. Cancer Chemother Pharmacol (1989) 23(Suppl):S4–8. doi: 10.1007/BF00647229
87. Zen C, Zen Y, Mitry R-R, Corbeil D, Karbanová J, O'Grady J, et al. Mixed phenotype hepatocellular carcinoma after transarterial chemoembolization and liver transplantation. Liver Transplant (2011) 17(8):943–54. doi: 10.1002/lt.22314
88. Cheng A-L, Raoul J-L, Lee H-C. Acute and chronic deterioration in liver function after transarterial chemoembolization (TACE) in patients with hepatocellular carcinoma (HCC): the final analysis of OPTIMIS, in: ILCA, 12th Annual Conference, United Kingdom.
89. Golfieri R, Renzulli M, Mosconi C, Forlani L, Giampalma E, Piscaglia F, et al. Hepatocellular carcinoma responding to superselective transarterial chemoembolization: an issue of nodule dimension? J Vasc Intervention Radiol (2013) 24(4):509–17. doi: 10.1016/j.jvir.2012.12.013
90. Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-To-Seven criteria and child-pugh a liver function: a proof-Of-Concept study. Cancers (Basel) (2019) 11(8):11. doi: 10.3390/cancers11081084
91. Kudo M. A new treatment option for intermediate-stage hepatocellular carcinoma with high tumor burden: initial lenvatinib therapy with subsequent selective TACE. Liver Cancer (2019) 8(5):299–311. doi: 10.1159/000502905
92. Ryan J-M, Ryan B-M, Smith T-P. Antibiotic prophylaxis in interventional radiology. J Vasc Intervention Radiol (2004) 15(6):547–56. doi: 10.1097/01.RVI.000024942.58200.5E
93. Castells A, Bruix J, Ayuso C, Brú C, Montanyà X, Boix L, et al. Transarterial embolization for hepatocellular carcinoma. antibiotic prophylaxis and clinical meaning of postembolization fever. J Hepatol (1995) 22(4):410–5. doi: 10.1016/0168-8278(95)80103-0
94. Brown D-B, Nikolic B, Covey A-M, Nutting C-W, Saad W-E-A, Salem R, et al. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Intervention Radiol (2012) 23(3):287–94. doi: 10.1016/j.jvir.2011.11.029
95. Yang W, Johnson P. Monitoring response to treatment in liver tumours. Best Pract Res Clin Gastroenterol (1999) 13(4):637–54. doi: 10.1053/bega.1999.0053
96. Ronot M, Bouattour M, Wassermann J, Bruno O, Dreyer C, Larroque B, et al. Alternative response criteria (Choi, European association for the study of the liver, and modified response evaluation criteria in solid tumors [RECIST]) versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncol (2014) 19(4):394–402. doi: 10.1634/theoncologist.2013-0114
97. Lencioni R, Llovet J-M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis (2010) 30(1):52–60. doi: 10.1055/s-0030-1247132
98. Raoul J-L, Gilabert M, Piana G. How to define transarterial chemoembolization failure or refractoriness: a European perspective. Liver Canc (2014) 3(2):119–24. doi: 10.1159/000343867
99. Takayasu K, Arii S, Matsuo N, Yoshikawa M, Ryu M, Takasaki K, et al. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. Am J Roentgenol (2000) 175(3):699–704. doi: 10.2214/ajr.175.3.1750699
100. Kubota K, Hisa N, Nishikawa T, Fujiwara Y, Murata Y, Itoh S, et al. Evaluation of hepatocellular carcinoma after treatment with transcatheter arterial chemoembolization: comparison of lipiodol-CT, power Doppler sonography, and dynamic MRI. Abdominal Imag (2001) 26(2):184–90. doi: 10.1007/s002610000139
101. Higuchi T, Kikuchi M, Okazaki M. Hepatocellular carcinoma after transcatheter hepatic arterial embolization. a histopathologic study of 84 resected cases. Cancer (1994) 73(9):2259–67. doi: 10.1002/1097-0142(19940501)73:9<2259::aid-cncr2820730905>3.0.co;2-p
102. Poggi G, Quaretti P, Minoia C, Bernardo G, Bonora M-R, Gaggeri R, et al. Transhepatic arterial chemoembolization with oxaliplatin-eluting microspheres (OEM-TACE) for unresectable hepatic tumors. Anticancer Res (2008) 28(6B):3835–42. doi: 10.1002/1097-0142(19940501)73:9<2259::aid-cncr2820730905>3.0.co;2-p
103. Johnson P-J, Kalayci C, Dobbs N, Raby N, Metivier E-M, Summers L, et al. Pharmacokinetics and toxicity of intraarterial adriamycin for hepatocellular carcinoma: effect of coadministration of lipiodol. J Hepatol (1991) 13(1):120–7. doi: 10.1016/0168-8278(91)90873-A
104. Dodds H-M, Walpole E-T, Rivory L-P, Strong R-W, Pond S-M. Disposition of epirubicin after intraarterial administration in lipiodol to patients with hepatocellular carcinoma. Ther Drug Monitor (1996) 18(5):537–43.
105. Hong K, Khwaja A, Liapi E, Torbenson M-S, Georgiades C-S, Geschwind J-F-H New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res (2006) 12(8):2563–7. doi: 10.1158/1078-0432.CCR-05-2225
106. Tzeng W-S, Wu R-H, Chang S-C, Chou C-K, Lin C-Y, Chen J-J, et al. Ionic versus nonionic contrast media solvents used with an epirubicin-based agent for transarterial chemoembolization of hepatocellular carcinoma. J Vasc Intervention Radiol (2008) 19(3):342–50. doi: 10.1016/j.jvir.2007.10.021
107. He M-K, Zou R-H, Wei W, Shen J-X, Zhao M, Zhang Y-F, et al. Comparison of stable and unstable ethiodized oil emulsions for transarterial chemoembolization of hepatocellular carcinoma: results of a single-center double-blind prospective randomized controlled trial. J Vasc Intervention Radiol (2018) 29(8):1068–1077.e1062. doi: 10.1016/j.jvir.2018.03.027
108. Kawai S, Okamura J, Ogawa M, Ohashi Y, Tani M, Inoue J, et al. Prospective and randomized clinical trial for the treatment of hepatocellular carcinoma–a comparison of lipiodol-transcatheter arterial embolization with and without adriamycin (first cooperative study). Cancer Chemother Pharmacol (1992) 31(1):S1–6. doi: 10.1007/BF00687096
109. Chang J-M, Tzeng W-S, Pan H-B, Yang C-F, Lai K-H. Transcatheter arterial embolization with or without cisplatin treatment of hepatocellular carcinoma. a randomized controlled study. Cancer (1994) 74(9):2449–53.
110. Aoyama K, Tsukishiro T, Okada K, Tsuchida T, Aiba N, Nambu S, et al. Evaluation of transcatheter arterial embolization with epirubicin-lipiodol emulsion for hepatocellular carcinoma. Cancer Chemother Pharmacol (1992) 31(1):S55–9. doi: 10.1007/BF00687106
111. Horiguchi Y, Itoh M, Takagawa H, Imai H, Kamei A, Sekoguchi B, et al. Assessment of chemoembolization therapy for primary liver cancer using a stabilized adriamycin-lipiodol suspension. Cancer Chemother Pharmacol (1992) 31(1):S60–4. doi: 10.1007/BF00687107
112. Pelletier G, Roche A, Ink O, Anciaux M-L, Derhy S, Rougier P, et al. A randomized trial of hepatic arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Hepatol (1990) 11(2):181–4. doi: 10.1016/0168-8278(90)90110-D
113. Bruix J, Llovet J-M, Castells A, Montañá X, Brú C, Ayuso C-M, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology (1998) 27(6):1578–83. doi: 10.1002/hep.510270617
Keywords: chemoembolization, cTACE, lipiodol, hepatocellular carcinoma, consensus
Citation: Chang P-Y, Lee R-C, Liang P-C, Liu Y-S, Chuang VP, Wu D-K, Cheng Y-F, Huang J-I, Tseng H-S, Hung C-F, Wu R-H, Chern M-C, Cheng H-M, Wu C-H, Cheng S-M, Chiang C-L and Liang H-L (2023) Multidisciplinary Taiwan consensus for the use of conventional TACE in hepatocellular carcinoma treatment. Front. Oncol. 13:1186674. doi: 10.3389/fonc.2023.1186674
Received: 15 March 2023; Accepted: 05 June 2023;
Published: 20 June 2023.
Edited by:
Vishal G. Shelat, Tan Tock Seng Hospital, SingaporeReviewed by:
Mingyue Cai, The Second Affiliated Hospital of Guangzhou Medical University, ChinaRobert Damm, University Hospital Magdeburg, Germany
Copyright © 2023 Chang, Lee, Liang, Liu, Chuang, Wu, Cheng, Huang, Tseng, Hung, Wu, Chern, Cheng, Wu, Cheng, Chiang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huei-Lung Liang, aGxsaWFuZ0B2Z2hrcy5nb3YudHc=
 Pi-Yi Chang
Pi-Yi Chang Rheun-Chuan Lee2
Rheun-Chuan Lee2 Yi-Sheng Liu
Yi-Sheng Liu Hua-Ming Cheng
Hua-Ming Cheng Huei-Lung Liang
Huei-Lung Liang