Abstract
This article presents a case of a 62-year-old Vietnamese woman with a history of Lynch syndrome (LS), who developed lung adenocarcinoma with EGFR L858R mutation. LS is an autosomal dominant cancer predisposition syndrome caused by a pathogenic germline variant in DNA mismatch repair genes, often leading to microsatellite instability. While LS is primarily associated with gastrointestinal, endometrial, ovarian, and urologic tract cancers, lung cancer accounts for less than 1% of LS-related cancers, with only six cases of LS-related lung cancer previously reported in the literature. The patient underwent multiple lines of treatment for her lung adenocarcinoma, including tyrosine kinase inhibitors, stereotactic body radiation therapy, pemetrexed and pembrolizumab, amivantamab, and fam-trastuzumab deruxtecan, but all resulted in only a partial response followed by a progressive disease. This case highlights the complex interplay of genetic cancer predisposition syndromes and the development of spontaneous driver mutations in the disease course and the subsequent management of tumors arising in these patients.
1 Introduction
Lynch syndrome (LS) is a genetically defined disease entity often associated with the clinical syndrome hereditary non-polyposis colon cancer, an autosomal dominant cancer predisposition syndrome. Patients with LS have an increased risk of a wide array of malignancies, most commonly gastrointestinal cancer, endometrial cancer, ovarian cancer, and urologic tract cancer (1). LS is caused by an autosomal dominant, pathogenic, germline variant in one of the DNA mismatch repair (MMR) genes (MSH2, MLH1, MSH6, or PMS2) or EPCAM, which leads to epigenetic silencing of MSH2 (2). Pathogenic variants in the MMR genes are relatively frequent, with an estimated prevalence of 1 in 279 (3), and thus LS represents one of the most prevalent cancer predisposition syndromes (4).
The resulting deficiency in MMR due to LS gene variants results in the accumulation of errors throughout the genome, including in short, repeated, microsatellite regions, a phenomenon termed microsatellite instability high (MSI-H). MSI-H is a hallmark of tumors associated with LS (5), and LS contributes to a significant proportion of MSI-H tumors across tumor types (6). Notably, there is significant heterogeneity in MSI prevalence between tumor types in LS patients, with a high MSI prevalence observed in ureteral, colorectal, and ovarian tumors (100%, 98%, and 94%, respectively) and a low MSI prevalence in tumors such as renal and primary brain tumors (25% and 0%, respectively) (7, 8). This MSI prevalence heterogeneity may have important treatment implications for LS-related tumors, as MSI-H tumors are more likely to respond to immune checkpoint therapy (7, 9), whereas the role of IC therapies in microsatellite-stable disease in LS patients is less clear (10).
Lung cancer is the most frequently diagnosed cancer and leading cause of cancer death (11), with cigarette smoking contributing significantly to the prevalence of the disease. While driver mutations are identified in tumors of both smokers and non-smokers, driver mutations are widely prevalent in the disease of non-smokers, occurring in 70% and 95% in cohorts of NSCLC and lung adenocarcinoma, respectively (12, 13). Among driver mutations in NSCLC, epidermal growth factor receptor (EGFR) mutations are the most common (13). Targeting EGFR mutations with tyrosine kinase inhibitors (TKIs) has revolutionized the therapeutic landscape of metastatic NSCLC. However, many patients eventually progress despite the initial good response and will receive chemotherapy-based second-line treatment. Despite the immune checkpoint blockade showing promising results in the second-line treatment of metastatic NSCLC (14, 15), those with EGFR mutations are unlikely to respond to immune checkpoint blockade (16, 17).
Less than 1% of lung cancer is associated with LS, and screening for LS is not recommended. Only six cases of lung cancer arising in patients with LS have been reported. EGFR mutation in LS-related lung cancer is even a rarer reported event. Here we report a case of incidentally discovered lung adenocarcinoma developing in a patient with a previous diagnosis of LS. Additionally, we review the leterature on lung cancer related to LS and the subset of this population with EGFR-mutated tumors.
2 Case presentation
A 62-year-old Vietnamese woman, non-smoker, presented with dysphagia and odynophagia. She had a history of colon cancer that was treated with hemicolectomy and adjuvant chemotherapy at age 53 and stage I right upper lobe lung adenocarcinoma that was treated with lobectomy at age 57 in Vietnam. The family’s oncologic history was significant for colon cancer in her paternal grandmother and aunt. She was found to have left tongue squamous cell carcinoma by biopsy. During the staging of tongue squamous cell carcinoma, she was found to have one 1-cm right upper lobe nodule and one 1-cm left upper lobe nodule. The biopsy of the right upper lobe nodule revealed lung adenocarcinoma (Figure 1). Molecular testing showed EGFR L858R mutation—negative for ALK rearrangement, BRAF, and MET mutations. PD-L1 by 22C3 pharmDx was negative. A positron emission tomography/computed tomography scan showed moderate fluorodeoxyglucose uptake in both lesions, which are concerning for malignancy. Her family history was significant for colon cancer in her son at age 35 who was later found to carry the pathogenic germline MLH1 variant. Due to the patient’s history of multiple malignancies and her family history, genetic testing was performed, which revealed a pathogenic germline MLH1 variant, and the patient was subsequently diagnosed with LS.
Figure 1

Cytologic preparations of initial pleural effusion which show groups and single malignant cells with morphologic and immunophenotypic findings supportive of lung adenocarcinoma. (A) DiffQuik-stained cytospin preparation at ×400, (B) Pap-stained cytospin preparation at ×400, (C) hematoxylin and eosin-stained cell block preparation at ×400, and (D) TTF1 immunohistochemical stain at ×400.
She underwent left partial glossectomy and lymph node dissection for her stage II tongue squamous cell carcinoma. She then completed stereotactic body radiation therapy (SBRT, 50 Gy in five fractions) to both the left and right upper lobe lung lesions. However, she was found to have enlarged left lung nodules and new pleural effusion on surveillance scans 2 years after her SBRT treatment. The sampled pleural fluid contained cytologic features of adenocarcinoma. A ctDNA analysis by Guardant360 (18) was performed, but it only revealed BRCA2 variance of unknown significance. Given the EGFR mutation status on the initial tissue biopsy, she was started on osimertinib. She had stable disease for 6 months but then developed worsening pleural effusion and new bone lesions (Figures 2A, B). A repeat ctDNA was performed, and it showed ERBB2 (G778_P780dup) and TP53 (M237I) mutations at a very low frequency. Her treatment was switched to pemetrexed and pembrolizumab doublet. Platinum chemotherapy was omitted given her history of ischemic stroke and performance status. During the period following an initial partial response, she also received elective total hysterectomy and bilateral salpingo-oophorectomy for risk reduction. At 7 months after the initiation of the pemetrexed and pembrolizumab doublet, her disease progressed. The patient was then started on the EGFR/MET bispecific antibody amivantamab (Rybrevant), again showing partial response followed by a progressive disease after 7 months. Another ctDNA was conducted, and it revealed ERBB2 and TP53 mutations at low frequency. Notably, none of the ctDNA tests showed MSI-H disease but was significant for a MLH1 A681T mutation at approximately 50% allele frequency (47.8%), which was likely from her LS-defining germline heterozygosity. The patient was subsequently started on fam-trastuzumab deruxtecan (Enhertu) for three cycles. At 1 month following the final cycle of fam-trastuzumab deruxtecan, the patient developed fatal acute respiratory failure secondary to pulmonary edema (Figure 3).
Figure 2
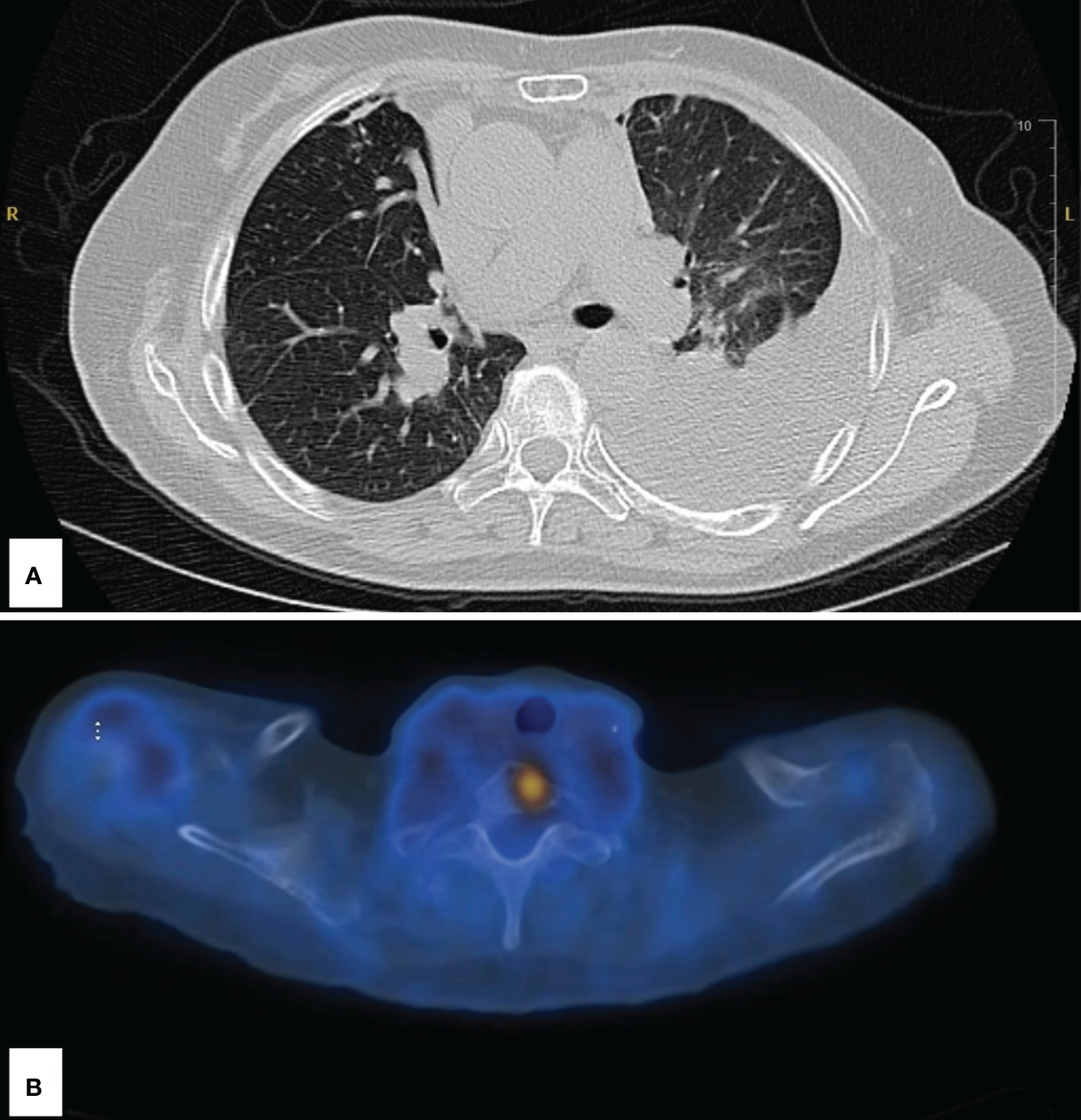
Imaging following disease progression at 2 years’ status post-stereotactic body radiation therapy. (A) Large left pleural effusion and left lower lobe atelectasis. (B) Abnormal fluorodeoxyglucose uptake in the upper spine; SUV of 5.5.
Figure 3

Timeline of the disease course and cfDNA trends. (A) Timeline of clinical course and treatment modalities. (B) cfDNA composition throughout the disease course.
3 Discussion
We report the case of a patient with LS who was diagnosed with NSCLC with EGFR mutation and had a short response to EGFR-targeted tyrosine kinase inhibitor and subsequent immune checkpoint inhibitor. To our knowledge, this is the second reported case of NSCLC with EGFR mutation in patients with LS and the first reported case with a long-term clinical outcome.
LS is known as one of the most common forms of inherited cancer predisposition (3). Although LS is classically associated with increased risks of a wide array of malignancies (1), NSCLC is not traditionally believed to be one of them. Sun et al. (19) analyzed the germline mutational status of 1,179 paired samples of lung cancer tumor tissue and normal lung tissue, and only six of 1,179 (0.5%) patients were found to have germline MMR gene pathogenic variants. Takamochi et al. (20) analyzed the MSI status in 366 patient samples, and only one tumor sample was found to have MSI-H, and this patient had no background of LS. A larger study by Warth et al. (21) also confirmed low MSI-H frequency (0.8%) in patients with lung adenocarcinoma. These studies indicate that lung cancer with MMR germline pathogenic variants or MSI-H is a rare and sporadic event, and screening for LS in patients newly diagnosed with lung cancer will be low-yield and likely be cost-ineffective.
However, there have been several case reports of lung cancer that developed in patients with LS (Table 1). Including our case, all cases were diagnosed with lung adenocarcinoma with low or negative PD-L1 expression. MSH2 germline mutation was the most common mutation, followed by MLH1 mutation. In total, five of seven (71.4%) patients were found to have loss of MMR expression on tumor tissues; none of these five patients had EGFR mutation, and three out of these five patients had either remission or stable disease as the best response to second-line immune checkpoint inhibitors. Moreover, two of seven (28.6%) patients had tumors that harbored EGFR mutation, but neither tumor had loss of MMR expression. In the study by Warth et al. (21), two of four (50%) patients who had MSI-H tumors also harbored EGFR mutations, and in the study by Sun et al. (19), two of six (33.3%) patients who had germline MMR mutation harbored EGFR mutations. The prevalence of EGFR mutations in lung cancer patients with LS from the case reports and studies mentioned above is 35.3% (6/17), which is higher than that of 10%–20% observed in Europe and North America populations (28).
Table 1
| Age/gender | Germline gene pathogenic variant |
Other malignancies |
Lung cancer histology | MMR (D/P) |
MSI | PD-L1 level | EGFR mutation | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| Canney et al. (22) | 59/M | MSH2 | Colon cancer | NSCLC | D* | Not reported | Not reported | Not reported | Not reported |
| Kawashima et al. (23) | 68/M | MSH2 | Colon, rectal, and prostate | NSCLC | D (loss of MSH2 and MSH6) | S | 3% | No | Long-lasting response to nivolumab >22 months |
| Masuzawa et al. (24) | 36/M | MLH1 | None | NSCLC | D (loss of MLH1 and PMS2) | H | 1%–24% | No | Long-lasting response to nivolumab >20 months |
| Nolan et al. (25) | 64/M | MSH2 | Colon and bladder | NSCLC | D (loss of MSH2)† | H | Not reported | No | Alive after surgery |
| Maccaroni et al. (26) | 74/F | MSH6 | Ovarian and rectal | NSCLC | D (loss of MSH6 on brain met) | H | Negative | No | 4 months of SD on pembrolizumab, disease progression and death at 6 months |
| Hissong et al. (27) | 66/F | MSH2 | Endometrial | NSCLC | P | S | 5% | EGFR L858A | Not reported |
| Our case | 62/F | MLH1 | Colon, tongue | NSCLC | Not reported | S | Negative |
EGFR
L858R |
Deceased following failure of multiple lines of therapy |
Summary of patients with Lynch syndrome who developed lung cancer.
Two lung lesions were identified: one had loss of MSH2 and MSH6 expression, and the other one was MMR proficient.
Two lung lesions were identified: one had loss of MSH2 expression and was MSI-H, and the other one was MMR proficient.
Even though our patient harbored EGFR mutation, her response to osimertinib and immune checkpoint inhibitor with chemotherapy was short-lasting. Li et al. (29) reported the association between a stronger MLH1 expression and a higher EGFR mutation frequency. The authors predicted that the overexpression of MLH1 could be a potential marker for sensitivity to EGFR TKIs. If true, patients with LS with a loss of MLH1 expression would likely demonstrate a suboptimal response to EGFR TKIs. Moreover, although patients with LS are expected to have an increased response to immune checkpoint inhibitors, a decreased response to immune checkpoint inhibitors is observed in EGFR-mutated NSCLC at large (30) and likely contributed to the failure of immune checkpoint therapy in this patient. Furthermore, MMR deficiency in tumors arising in LS patients cannot be presumed, especially in non-typical LS tumor types, and therefore individualized testing of tumors may be warranted to guide the use of IC therapies in these patients.
This case also highlights the need to carefully weigh the decision to pursue risk reduction surgery, weighing the pathogenicity of different LS variants and the patient’s underlying comorbidities as recommended by the NCCN (31). Moreover, in patients with current and stable malignancies, the risks of exacerbating the disease through unrelated risk reduction surgeries must be considered (32, 33).
4 Conclusion
NSCLC is not among the malignancies that are commonly associated with LS. In patients with LS who developed NSCLC, EGFR mutation seems to be more prevalent and should be checked as in patients without LS. Despite the MSI-H status that is commonly seen in LS with an associated expected good response to immune checkpoint blockade, these patients with EGFR mutations and LS tend to have a poor response to immune checkpoint inhibitors.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AH, KS, and EB each contributed to data gathering, analysis, and manuscript preparation. KS and EB contributed to the clinical care of the patient. TS provided the pathology analysis. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to thank the patient and her family and the clinical team involved in patient’s care.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Watson P Vasen HFA Mecklin JP Bernstein I Aarnio M Järvinen HJ et al . The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer. (2008) 123(2):444–9. doi: 10.1002/ijc.23508
2
Rebuzzi F Ulivi P Tedaldi G . Genetic predisposition to colorectal cancer: how many and which genes to test? IJMS (2023) 24(3):2137. doi: 10.3390/ijms24032137
3
Win AK Jenkins MA Dowty JG Antoniou AC Lee A Giles GG et al . Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomarkers Prev (2017) 26(3):404–12. doi: 10.1158/1055-9965.EPI-16-0693
4
Peltomäki P Nyström M Mecklin JP Seppälä TT . Lynch syndrome genetics and clinical implications. Gastroenterology (2023) 164(5):783–99. doi: 10.1053/j.gastro.2022.08.058
5
Peltomäki P Lothe RA Aaltonen LA Pylkkänen L Nyström-Lahti M Seruca R et al . Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome. Cancer Res (1993) 53(24):5853–5.
6
Latham A Srinivasan P Kemel Y Shia J Bandlamudi C Mandelker D et al . Microsatellite instability is associated with the presence of lynch syndrome pan-cancer. JCO (2019) 37(4):286–95. doi: 10.1200/JCO.18.00283
7
Therkildsen C Jensen LH Rasmussen M Bernstein I . An update on immune checkpoint therapy for the treatment of lynch syndrome. Clin Exp Gastroenterol (2021) 14:181–97. doi: 10.2147/CEG.S278054
8
Gylling AHS Nieminen TT Abdel-Rahman WM Nuorva K Juhola M Joensuu EI et al . Differential cancer predisposition in Lynch syndrome: insights from molecular analysis of brain and urinary tract tumors. Carcinogenesis (2008) 29(7):1351–9. doi: 10.1093/carcin/bgn133
9
Le DT Durham JN Smith KN Wang H Bartlett BR Aulakh LK et al . Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science (2017) 357(6349):409–13. doi: 10.1126/science.aan6733
10
Bari S Kim RD Wang X Matejcic M Muzaffar J . Outcomes of Lynch syndrome (LS) patients treated with immune checkpoint inhibitors (ICI). JCO (2020) 38(15_suppl):1548–8. doi: 10.1200/JCO.2020.38.15_suppl.1548
11
Bray F Ferlay J Soerjomataram I Siegel RL Torre LA Jemal A . Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
12
Mack PC Klein MI Ayers KL Zhou X Guin S Fink M et al . Targeted next-generation sequencing reveals exceptionally high rates of molecular driver mutations in never-smokers with lung adenocarcinoma. Oncologist. (2022) 27(6):476–86. doi: 10.1093/oncolo/oyac035
13
Sholl LM Aisner DL Varella-Garcia M Berry LD Dias-Santagata D Wistuba II et al . Multi-institutional oncogenic driver mutation analysis in lung adenocarcinoma: the lung cancer mutation consortium experience. J Thorac Oncol (2015) 10(5):768–77. doi: 10.1097/JTO.0000000000000516
14
Leighl NB Hellmann MD Hui R Carcereny E Felip E Ahn MJ et al . Pembrolizumab in patients with advanced non-small-cell lung cancer (KEYNOTE-001): 3-year results from an open-label, phase 1 study. Lancet Respir Med (2019) 7(4):347–57. doi: 10.1016/S2213-2600(18)30500-9
15
Jemielita T Li XN Piperdi B Zhou W Burke T Chen C . Overall survival with second-line pembrolizumab in patients with non-small-cell lung cancer: randomized phase III clinical trial versus propensity-adjusted real-world data. JCO Clin Cancer Inform. (2021) 5:56–65. doi: 10.1200/CCI.20.00099
16
Lee CK Man J Lord S Links M Gebski V Mok T et al . Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-A meta-analysis. J Thorac Oncol (2017) 12(2):403–7. doi: 10.1016/j.jtho.2016.10.007
17
Lisberg A Cummings A Goldman JW Bornazyan K Reese N Wang T et al . A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naïve patients with advanced NSCLC. J Thorac Oncol (2018) 13(8):1138–45. doi: 10.1016/j.jtho.2018.03.035
18
Aggarwal C Thompson JC Black TA Katz SI Fan R Yee SS et al . Clinical implications of plasma-based genotyping with the delivery of personalized therapy in metastatic non–small cell lung cancer. JAMA Oncol (2019) 5(2):173. doi: 10.1001/jamaoncol.2018.4305
19
Sun S Liu Y Eisfeld AK Zhen F Jin S Gao W et al . Identification of germline mismatch repair gene mutations in lung cancer patients with paired tumor-normal next generation sequencing: A retrospective study. Front Oncol (2019) 9:550. doi: 10.3389/fonc.2019.00550
20
Takamochi K Takahashi F Suehara Y Kitano S Sato E Kohsaka S et al . A microsatellite instability analysis using the promega panel in lung adenocarcinoma. Chest (2016) 150(4):715A. doi: 10.1016/j.chest.2016.08.810
21
Warth A Körner S Penzel R Muley T Dienemann H Schirmacher P et al . Microsatellite instability in pulmonary adenocarcinomas: a comprehensive study of 480 cases. Virchows Arch (2016) 468(3):313–9. doi: 10.1007/s00428-015-1892-7
22
Canney A Sheahan K Keegan D Tolan M Hyland J Green A . Synchronous lung tumours in a patient with metachronous colorectal carcinoma and a germline MSH2 mutation. J Clin Pathol (2009) 62(5):471–3. doi: 10.1136/jcp.2008.063008
23
Kawashima Y Nishikawa S Ninomiya H Yoshida R Takano N Oguri T et al . Lung adenocarcinoma with lynch syndrome and the response to nivolumab. Intern Med (2019) 58(10):1479–84. doi: 10.2169/internalmedicine.1673-18
24
Masuzawa K Asakura T Ikemura S Yasuda H Kawada I Takaoka S et al . Long-lasting response to nivolumab for a patient with lynch syndrome–associated lung adenocarcinoma. JCO Precis Oncol (2020) 4):74–8. doi: 10.1200/PO.19.00156
25
Nolan L Eccles D Cross E Crawford G Beck N Bateman A et al . First case report of Muir-Torre syndrome associated with non-small cell lung cancer. Fam Cancer. (2009) 8(4):359–62. doi: 10.1007/s10689-009-9247-7
26
Maccaroni E Lenci E Agostinelli V Cognigni V Giampieri R Mazzanti P et al . Lynch syndrome-associated lung cancer: pitfalls of an immunotherapy-based treatment strategy in an unusual tumor type. Exploration of Targeted Anti-tumor Therapy (2021). Available at: https://www.explorationpub.com/Journals/etat/Article/100244.
27
Hissong E Baek I Costa V Beneck D Saxena A Solomon JP et al . Identification of a microsatellite stable, EGFR-mutant lung adenocarcinoma developing in a patient with lynch syndrome. JCO Precis Oncol (2020) 4:818–22. doi: 10.1200/PO.20.00074
28
Melosky B Kambartel K Häntschel M Bennetts M Nickens DJ Brinkmann J et al . Worldwide prevalence of epidermal growth factor receptor mutations in non-small cell lung cancer: A meta-analysis. Mol Diagn Ther (2022) 26(1):7–18. doi: 10.1007/s40291-021-00563-1
29
Li M Zhang Q Liu L Lu W Wei H Li RW et al . Expression of the mismatch repair gene hMLH1 is enhanced in non-small cell lung cancer with EGFR mutations. PloS One (2013) 8(10):e78500. doi: 10.1371/journal.pone.0078500
30
Hastings K Yu HA Wei W Sanchez-Vega F DeVeaux M Choi J et al EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small-cell lung cancer. Ann Oncol (2019) 30(8):1311–20. doi: 10.1093/annonc/mdz141
31
National Comprehensive Cancer Network . Genetic/familial high-risk assessment: colorectal. Available at: https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf.
32
Ceelen W Pattyn P Mareel M . Surgery, wound healing, and metastasis: Recent insights and clinical implications. Crit Rev Oncology/Hematology. (2014) 89(1):16–26. doi: 10.1016/j.critrevonc.2013.07.008
33
Tohme S Simmons RL Tsung A . Surgery for cancer: A trigger for metastases. Cancer Res (2017) 77(7):1548–52. doi: 10.1158/0008-5472.CAN-16-1536
Summary
Keywords
Lynch syndrome, NSCLC, MLH1, immune checkpoint therapy, ctDNA
Citation
Hodges A, Sun K, Sheu TG and Bernicker EH (2023) Lung adenocarcinoma in a patient with Lynch syndrome: a case report and literature review. Front. Oncol. 13:1193503. doi: 10.3389/fonc.2023.1193503
Received
25 April 2023
Accepted
18 September 2023
Published
13 October 2023
Volume
13 - 2023
Edited by
Yiming Meng, China Medical University, China
Reviewed by
Gianluca Tedaldi, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), Italy; Grigoriy Yanus, N.N. Petrov National Medical Research Center of Oncology, Russia
Updates
Copyright
© 2023 Hodges, Sun, Sheu and Bernicker.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eric H. Bernicker, ehbernick@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.